Abstract
Background
Efavirenz is a potent, safe and tolerable non-nucleoside reverse transcriptase inhibitor (NNRTI) recommended as initial therapy. Recently, several new antiretroviral drugs, including second generation NNRTIs, protease-inhibitors, an integrase-inhibitor and a CCR5 inhibitor, have become or will be shortly available.
Objective
This article will review relevant efficacy and safety data of efavirenz compared to these novel agents or certain common alternate drugs currently used as initial therapy in treatment-naïve patients.
Methods
Published articles and conference presentations pertaining to efavirenz and/or the newer antiretroviral agents were evaluated.
Results/Conclusions
Efavirenz will continue to be preferred initial therapy for now. If longer-term studies of integrase inhibitors and second-generation NNRTIs confirm initial findings, they will eventually supplant efavirenz as preferred first-line agents.
Keywords: efavirenz, treatment naïve, novel antiretroviral agents
1. Introduction
Highly-active combination antiretroviral therapy has dramatically reduced HIV-related morbidity and mortality. Nevertheless, current treatment regimens are limited by intolerance, short- and long-term toxicities and emergence of drug resistance. Typical regimens include two nucleoside reverse transcriptase inhibitors (NRTIs) and either a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI). Efavirenz, an NNRTI FDA- approved in 1998, quickly became widely-used in developed countries. Current guidelines recommend efavirenz with two NRTIs, either abacavir/lamivudine or emtricitabine/tenofovir, as preferred first-line regimens for treatment-naïve patients1.
Efavirenz has a low pill burden, once daily dosing, absence of some adverse effects associated with PIs, and a long half-life allowing for relatively stable plasma concentrations and some forgiveness for doses not taken exactly on schedule. It is available in combination with emtricitabine and tenofovir as a single tablet, which is an ideal regimen for treatment-naïve patients. Drawbacks of efavirenz include neuropsychiatric adverse effects, teratogenicity, many clinically significant drug interactions, and a low genetic barrier to the development of drug-resistant viral mutants.
2. Efavirenz
2.1 Chemistry
Efavirenz is available in the U.S. alone (Sustiva®) or co-formulated with emtricitabine and tenofovir (Atripla™). Sustiva® is marketed in the U.S. as 50 mg and 200 mg capsules as well as 600 mg film-coated tablets, all for oral administration. Atripla™ is manufactured in the U.S. as film-coated tablets containing 600 mg efavirenz, 200 mg emtricitabine, and 300 mg tenofovir disoproxil fumarate.
Efavirenz is an HIV-1 specific, non-nucleoside reverse transcriptase inhibitor. The chemical name is (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one, with a molecular formula of C14H9ClF3NO2 and a molecular mass of 315.68 (Figure 1)2.
Figure 1.
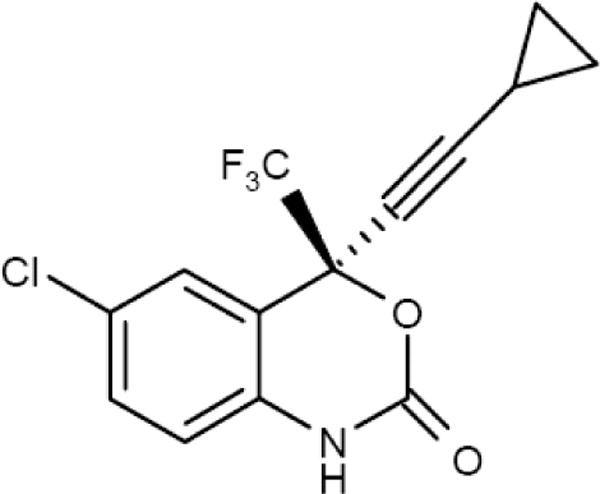
The chemical structure of efavirenz.
Efavirenz non-competitively inhibits wild-type HIV-1 reverse transcriptase. However, it does not inhibit human cellular DNA polymerases α, β, γ and δ3. In vitro activity of efavirenz against laboratory adapted and clinical isolates of HIV-1 demonstrate 90 – 95% inhibitory concentrations of 0.5 – 8 ng/mL. Efavirenz displayed additive antiviral activity when combined with other NRTIs, NNRTIs and PIs, except for atazanavir, where it showed additive to antagonistic activity2. Efavirenz is active against HIV-1 subtypes A, AE, AG, C, D, F, G, J, and N, but it is not active against HIV-2, simian immunodeficiency virus, and has reduced activity against group O viruses4.
2.2 Pharmacokinetics, Metabolism and Interactions
Efavirenz bioavailability is 40 – 45 % without food, with maximum concentrations (Cmax) reached within 2 – 5 hours. Food increases efavirenz AUC and Cmax by 15 – 30% and 40 – 80%, respectively. Standard doses of 600 mg by mouth once daily reach steady-state in 1 – 2 weeks, with maximum and minimum concentrations of 3 – 4.3 mcg/mL and ~ 1.5 mcg/mL, respectively5, 6. Mean efavirenz AUC, oral clearance and apparent volume of distribution are 55 – 60 mcg•hr/mL, 10 – 12 L/hr, and 280 – 500 L, respectively5–7. Efavirenz is extensively protein-bound (>99%), primarily by albumin. It has a long terminal half-life, 35 – 50 hours. Because of this long half-life, efavirenz may persist at low concentrations for several days to weeks after stopping treatment potentially allowing viral resistance to develop. Some experts recommend stopping efavirenz 1 – 2 weeks prior to stopping the rest of the antiretrovirals; however, the optimal time sequence for stopping each component of antiretroviral therapy is unknown.
Efavirenz is rapidly hydroxylated by cytochrome P450 (CYP) 2B6, and more slowly by CYP 3A4, to metabolites that are glucuronidated and eliminated. Patients with hepatic impairment or hepatitis B or C infection should be monitored carefully when starting efavirenz. Since < 1% of efavirenz is eliminated in the urine, no dose adjustment is necessary in renal impairment. Efavirenz induces CYP 3A4 metabolic activity in vivo, causing decreased exposure to drugs metabolized by this enzyme. Efavirenz exposure may also be affected by medications which greatly alter CYP 3A4 activity, since this is a secondary pathway of efavirenz metabolism2. Some efavirenz drug interactions may be overcome by altering doses of the affected drugs. Also, efavirenz has fewer significant drug interactions than a ritonavir-boosted regimen, as ritonavir induces and inhibits to a larger extent a wider variety of CYP enzymes. An allelic variant of CYP 2B6 (T/T genotype at position 516) is associated with higher efavirenz concentrations and increased frequency of central nervous system toxicities8. Therapeutic drug monitoring of efavirenz may be warranted, and the suggested mid-dosing interval plasma concentrations should fall within 1 – 4 mcg/mL9–12. Dose reductions in patients with high efavirenz concentrations may attenuate exposure-related adverse effects. One difficulty of individualizing an efavirenz dose is that the pill burden may increase if the patient needs to switch to a dose other than 600 mg daily.
2.3 Resistance
High level resistance to efavirenz can develop with a single mutation3. The K103N mutation is frequently the first resistance mutation selected during treatment with efavirenz, and confers up to 100 fold loss of potency. With continued treatment, most patients develop additional viral mutations such as L100I, V106M, V108I, Y181C/I, Y188L, G190A/S, and P225H13, 14. Because of polymorphisms in the viral reverse transcriptase gene between HIV subtypes, patients with clade C virus may develop different resistance mutations (K103E, V179D, and Y188C/H, for example); these also confer high-level resistance15–17. Cross-resistance occurs between nevirapine, efavirenz and delavirdine, but does not occur with NRTIs or second generation NNRTIs. In fact, some NRTI mutations confer hypersusceptibility to NNRTI (118I, 208Y, 215Y)18–20, though the clinical relevance has not been fully elucidated. Resistance mutations to NNRTI are genotypically detectible in most patients more than a year after stopping the drug. Mutations likely persist indefinitely in archived quasispecies even when they below the genotype level of detection. Furthermore, patients failing an initial NNRTI-based regimen consistently have a greater number viral resistance mutations than patients failing an initial PI-based therapy21. Hence, patients who fail an NNRTI-based regimen will have fewer treatment options for future regimens.
Transmission of resistant virus is of growing concern. The percentage of treatment-naïve subjects already harboring NNRTI-resistant virus varies among different geographic regions, but has been generally increasing to the current rates of 10 – 15 % in Europe and the United States22, 23. Rates of primary resistance as high as 20% have been noted in the United Kingdom23. With continued widespread use of efavirenz as first-line therapy, these rates are expected to continue to increase, and the first-generation NNRTIs will be effective in a much smaller subset of treatment-naïve patients.
3. Safety and Tolerability
The adverse effect profile of efavirenz is well-characterized, including dermatologic, gastrointestinal, hepatic, lipidemic, and central nervous system manifestations (Table 1)24–26. A mild-to-moderate maculopapular rash occurs in 2 – 10% of patients, usually within the first few weeks and resolving within a month without discontinuing therapy. Severe skin rash is rare, and efavirenz should be discontinued if it occurs. Nausea, diarrhea and vomiting occur in up to 13% of patients, and liver enzyme increases are seen in 2 – 7%. Efavirenz can increase HDL-cholesterol, total cholesterol and triglycerides, although to a lesser extent than PIs.
Table 1.
Patients with Adverse Events of Moderate or Severe Intensity Reported
| Adverse Event | Squires25,a | 514240, 41,b | Markowitz43,a | Merit44,a | ||||
|---|---|---|---|---|---|---|---|---|
| EFV ZDV/3TC (N=401) |
ATV ZDV/3TC (N=404) |
EFV 2 NRTI (N=250) |
LPV 2 NRTI (N=253) |
EFV TDF/3TC (N=38) |
RLT TDF/3TC (N=160) |
EFV ZDV/3TC (N=361) |
MVC ZDV/3TC (N=360) |
|
|
| ||||||||
| Clinical Adverse Event Grade ≥ 2, n (%) | ||||||||
|
| ||||||||
| Headache | 25 (6) | 23 (6) | N/A | N/A | 9 (24) | 14 (9) | (~ 25) | (~ 25) |
| Dizziness | 24 (6) | 8 (2)c | N/A | N/A | 11 (29) | 14 (9) | (~ 30) | (~ 13) |
| Diarrhea | 10 (2) | 5 (1) | N/A | N/A | 4 (11) | 10 (6) | (~ 23) | (~ 18) |
| Vomiting | 27 (7) | 17 (4) | N/A | N/A | N/A | N/A | (~ 15) | (~ 12) |
| Nausea | 51 (13) | 57 (14) | N/A | N/A | 5 (13) | 18 (11) | (~ 33) | (~ 35) |
|
| ||||||||
| Laboratory Adverse Event | ||||||||
|
| ||||||||
| AST Grade ≥3, n (%) | 8 (2) | 7 (2) | (4) | (4) | N/A | N/A | 11 (3.2) | 12 (3.4) |
| ALT Grade ≥3, n (%) | 10 (3) | 15 (4) | (3) | (5) | 2 (5) | 6 (4) | 11 (3.2) | 11 (3.1) |
| Mean change in cholesterol, mg/dL (%) | (+21) | (+2)c | +33 | +33d | ~20 | ~0 | +27 | +1c,d |
| Mean change in triglycerides, mg/dL (%) | (+23) | (−9)c | 14 | 44d | ~50 | ~0 | +10 | −4c,d |
ATZ: atazanavir; EFV: efavirenz; LPV/r: lopinavir/ritonavir; NRTI; nucleoside reverse transcriptase inhibitor; TDF: tenofovir disoproxil fumarate; RLT: raltegravir; ZDV: zidovudine; 3TC; lamivudine
Week 48 results
Week 96 results
P<0.05
median values
Neuropsychiatric adverse events occur in up to half of patients within the first few days to weeks of efavirenz therapy8, 27–29. Symptoms include abnormal dreams, insomnia, somnolence, hallucinations, dizziness and impaired concentration. These resolve spontaneously in most patients, with < 5% requiring discontinuation due to severe adverse effects (aggressive behavior, severe depression, suicidal thoughts, and paranoia). These self-limited neuropsychiatric side effects are the main reason that efavirenz is typically dosed at bedtime instead of in the morning. Mild-to-moderate neuropsychiatric symptoms do persist in some patients for months or even years after starting efavirenz28, 29.
Efavirenz is teratogenic to laboratory animals, with severe neural tube defects in primates receiving efavirenz early in pregnancy2. A case report documented that an infant exposed to efavirenz in utero had a neural tube defect30. The Antiretroviral Pregnancy Registry has prospectively documented 8 birth defects (not neural tube defects) in 321 live births, and retrospectively reported 5 cases of neural tube defects2. Efavirenz is pregnancy category D, and should not be used in early pregnancy31.
4. Clinical Efficacy
Historically, efavirenz-based regimens have proven superior to triple NRTI therapy32, and equivalent or modestly superior to nevirapine26. Several recent reviews provide a detailed description of these findings33–35. In recent comparative trials of new antiretroviral agents from multiple drug classes efavirenz continues to demonstrate excellent efficacy (Table 2). However, several drawbacks to efavirenz include a low genetic barrier to resistance, adverse neuropsychiatric effects and increased effects on total cholesterol and triglycerides compared to these newer agents (Table 1). Additionally, efavirenz is teratogenic and may not be an appropriate first-line agent for women of child-bearing age, especially considering that half of all pregnancies in the U.S. are unintended (unplanned), and women in resource-poor settings often do not have access or rights to effective contraception.
Table 2.
Viral and Immunologic Efficacy
| Study | Drug dose (mg) | Drug dose (mg) | Other agents | Study period (weeks) | Number of subjects | Baseline HIV RNA, median (log10 copies/ml) | Percent undetectable at last visit [HIV RNA < 400 (50)] | CD4 increase, mean (cells/mm3) |
|---|---|---|---|---|---|---|---|---|
| Squires25, a | EFV qd (600) |
— | ZDV/3TC | 48 | 401 | 4.91 | 64 (37) | 160b |
| — | ATV qd (400) |
ZDV/3TC | 48 | 404 | 4.87 | 70 (32) | 176 | |
| 514240,c | EFV qd (600) | — | 3TC/NRTI | 96 | 250 | 5 | 76 (89)b | 241b,d |
| — | LPV/r bid (400/100) | 3TC/NRTI | 96 | 253 | 5 | 67 (77) | 285 | |
| Markowitz43,a | EFV qd (600) |
— | TDF/3TC | 48 | 38 | 4.8e | 87 (87) | 170 |
| — | RLT bid (100 to 600) |
TDF/3TC | 48 | 160 | 4.6 - 4.8c | 85-98 (83-88) | 144-221 | |
| Merit44 | EFV qd (600) |
— | ZDV/3TC | 48 | 361 | 4.88 | 73.1 (69.3)b | 142b |
| — | MVC bid (300) |
ZDV/3TC | 48 | 360 | 4.86 | 70.6 (65.3) | 169 |
ATZ: atazanavir; EFV: efavirenz; LPV/r: lopinavir/ritonavir; NRTI; nucleoside reverse transcriptase inhibitor; TDF: tenofovir disoproxil fumarate; RLT: raltegravir; ZDV: zidovudine; 3TC; lamivudine
For the intent-to-treat analysis in this study, missing values were treated as failures
P<0.05 compared to comparator arm
For the intent-to-treat analysis in this study, missing values were ignored
median values
mean values
4.1 Efavirenz vs. Protease-Inhibitors in Treatment-Naïve Patients
Viral load reduction in treatment-naïve patients taking efavirenz is superior to that seen with unboosted indinavir and nelfinavir36, 37. However, similar rates of viral suppression were observed between efavirenz and the newer unboosted PI, atazanavir25. After 48 weeks, the proportion of subjects achieving viral suppression was similar for those receiving atazanavir 400 mg once-daily or efavirenz (below 400 copies/mL: 70% vs. 64%; below 50 copies/mL: 32% vs. 37%%). While rates of treatment discontinuation due to intolerance were similar between efavirenz and atazanavir (20% vs. 16%, respectively), subjects taking efavirenz had a higher incidence of rash and dizziness while atazanavir was associated with increased jaundice. Further, efavirenz-treated subjects had significantly greater increases from baseline for total cholesterol (mean change: +21% vs. +2.0%; P<0.001) and triglycerides (+23% vs. −9.0%; P<0.001).
Comparisons between efavirenz- and lopinavir/ritonavir-based regimens have been less clear-cut. In one study, virologic response was similar between efavirenz and lopinavir/ritonavir. Interestingly, CD4+ cell count recovery was greater in the lopinavir/ritonavir group38, which is consistent with other reports of improved immune recovery with PI- versus efavirenz-based regimens39. The most definitive study to date comparing efavirenz to lopinavir/ritonavir is A514240. This prospective study randomized 753 treatment-naïve patients to either lopinavir/ritonavir (400/100 mg twice- daily) or efavirenz (600 mg once-daily) combined with lamivudine and a second NRTI, or a NRTI-sparing regimen of lopinavir/ritonavir (533/133 mg twice-daily) and efavirenz (600 mg once-daily) for 96 weeks. The co-primary endpoints of the study were to compare, pairwise between the three treatment arms, the time to virologic failure (lack of suppression of plasma HIV-1 RNA by 1 log10 or rebound before Week 32, or failure to suppress to < 200 copies/mL or rebound after Week 32) and the time to regimen completion (either virologic failure or toxicity-related discontinuation). The adjusted significance level for multiple comparisons between arms and an interim analysis was α=0.016. At Week 96, the treatment failure rate for efavirenz was significantly lower than for lopinavir (24% vs. 33%; P=0.006). Further, a greater proportion of patients in the efavirenz arm had HIV RNA levels < 50 copies/mL at Week 96 than the lopinavir group (89% vs. 77%; P=0.003, respectively). However, increases in CD4+ cell count were greater for the lopinavir/ritonavir group (+268 vs. +241 at 96 weeks, p=0.01). Also, the efavirenz-treated subjects who failed therapy had a greater number of resistance mutations than lopinavir/ritonavir-treated subjects who experienced virologic failure. Lopinavir/ritonavir-treated subjects had greater increases in total cholesterol than efavirenz-treated subjects (47 mg/dL vs. 14 mg/dL, P<0.01). In contrast, efavirenz-treated subjects had a higher proportion of protocol-defined lipoatrophy than the lopinavir/ritonavir group (32% vs. 18%, P<0.01)41.
Darunavir is a PI, recently approved for treatment-experienced patients with extensive HIV drug resistance42. Although no comparative trials with efavirenz have been conducted, darunavir holds strong potential as a new therapeutic option for treatment- naïve patients. The ARTEMIS trial randomized 689 treatment-naïve subjects to darunavir/ritonavir (800/100mg once-daily) or lopinavir/ritonavir (400/100 mg twice-daily or 800/200 mg once-daily) plus tenofovir/emtricitabine for 48 weeks. Darunavir-treated patients (N=343) had a similar proportion with viral suppression to the twice-daily lopinavir/ritonavir (N=267) arm (HIV RNA below 50 copies/mL: 84% vs. 81%, respectively), and a significantly greater response than once-daily lopinavir/ritonavir (N=52) treated subjects (HIV RNA below 50 copies/mL: 84% vs. 71%, respectively; P<0.05). Compared to the combined lopinavir/ritonavir arm, darunavir-treated subjects had significantly fewer grade 2 to 4 total cholesterol gains (23% vs. 13%) and fewer grade 2 to 4 triglyceride gains (11% vs. 3%). Comparative trials of darunavir with efavirenz in treatment-naïve patients are warranted.
4.2 Efavirenz versus Raltegravir in Treatment-Naïve Patients
Raltegravir is the first agent approved in the new drug target class of strand-transfer inhibitors of HIV-1 integrase (integrase inhibitors), and was evaluated in 160 treatment-naïve subjects at doses ranging from 100 mg to 600 mg twice daily in combination with tenofovir/lamivudine, and compared to 38 treatment-naïve subjects randomized to standard dose efavirenz plus tenofovir/lamivudine over 48 weeks43. The primary endpoint was the proportion of patients achieving plasma HIV-1 RNA < 400 copies/mL at week 24. Of note, this study was not powered to perform formal efficacy comparisons between raltegravir and efavirenz. No difference was seen between groups for the primary endpoint. Likewise, 85 – 95% of subjects achieved viral loads < 50 copies/mL in the raltegravir arms, and 92% met that goal in the efavirenz arm. These reductions in viral load persisted through 48 weeks of therapy for 83 – 98%, again with no difference between groups. Virologic failure occurred in 3% of subjects taking raltegravir and in 3% of efavirenz-arm subjects. Mean increases in CD4+ T-cell counts were similar across groups, at 144 – 221 cells/mm3.
The notable differences between raltegravir and efavirenz were in the safety profiles and the rapidity of treatment response. Patients taking any dose of raltegravir achieved a viral load < 50 copies/mL earlier than patients taking efavirenz (p < 0.05). While the incidence of serious adverse events was similar with raltegravir arms (5% overall) and the efavirenz arm (5%), drug-related adverse events were less common in the raltegravir arms than in the efavirenz arm. Total cholesterol, low-density lipoprotein and triglyceride levels were unchanged over 48 weeks in the raltegravir groups. By contrast, all three of these increased in the efavirenz subjects. High-density lipoprotein increased in all subjects, with larger increases in those treated with efavirenz.
4.3 Efavirenz versus Maraviroc in Treatment-Naïve Patients
Maraviroc selectively and reversibly binds to the human chemokine receptor CCR5, preventing the interaction of HIV gp120 and the entry of CCR5-tropic HIV-1 into cells. The Merit study was a phase 3, prospective study of maraviroc in antiretroviral-naïve patients with R5 virus44. Subjects were randomized to either efavirenz (600mg once-daily or maraviroc (300mg twice-daily) in combination with zidovudine/lamivudine for 48 weeks. In the primary analysis, patients receiving efavirenz (n=361) had greater rates of viral suppression (HIV RNA <50 copies/mL) compared to patients treated with maraviroc (n=360; 69.3% versus 65.3%, respectively). Despite a greater percentage of virally-suppressed subjects in the efavirenz arm, those receiving maraviroc experienced significantly greater CD4 recovery at 48 weeks (mean CD4 gain: 169 vs. 142 cells/mm3; difference of 27, 95% CI 7 – 46). In addition, the rate of discontinuation due to adverse events was less in the maraviroc (4.2%) than efavirenz-treated subjects (13.6%). Of note, a coreceptor tropism assay must be performed prior to starting a CCR5 antagonist such as maraviroc, which is an added expense compared to other antiretrovirals.
4.4 Second Generation Non-Nucleoside Reverse Transcriptase Inhibitors
Second-generation NNRTIs are active against virus resistant to first-generation NNRTIs. Additionally, they require multiple mutations before their activity is reduced. Etravirine (TMC125) is currently indicated only in treatment-experienced HIV-infected adults at a dose of 200 mg twice daily. A clinical trial evaluating etravirine 400 mg once daily in treatment-naïve patients is ongoing. Etravirine is metabolized by cytochrome P450 enzymes (3A4, 2C9 and 2C19). It induces the activity of 3A4, and inhibits 2C9 and 2C19, and has the potential for significant drug interactions. Rash and nausea/vomiting are the most commonly-reported adverse effects in treatment-experienced patients taking etravirine45, 46. Other side effects occurred at rates similar to placebo, suggesting a lower propensity for central nervous system side effects and lipid alterations. Animal studies have not shown reproductive toxicity with etravirine, but no human data are available yet. No comparative studies with efavirenz are available.
Rilpivirine (TMC278) is an investigational second-generation NNRTI being studied in treatment-experienced and treatment-naïve subjects. Interim week 48 results of a 3-year comparative study of rilpivirine versus efavirenz in treatment-naïve subjects showed similar viral load reductions and CD4 cell count changes between the two groups47. Nausea rates were similar with the two agents, while central nervous system side effects and rash were significantly more common with efavirenz than with rilpivirine. Also, total and LDL cholesterol did not change in rilpivirine subjects while they increased in efavirenz-treated subjects. Unlike efavirenz, rilpivirine has not displayed teratogenic effects in studies conducted to date.
5. Conclusion
Efavirenz is an attractive first-line agent in treatment-naïve HIV-infected patients. It is dosed once daily, and comes in combination with NRTIs. The common adverse effects, rash and neuropsychiatric symptoms, are generally mild-to-moderate, and for many subjects, decrease over the first few weeks of continued therapy.
Extensive literature supports the virologic and immunologic efficacy of efavirenz regimens. Efavirenz is more potent than unboosted PIs, and has even shown superiority in some studies over lopinavir/ritonavir in viral load reduction. However, immunologic recovery in these studies was better in the lopinavir/ritonavir arms. The overall viral load reduction and CD4+ T-cell count recovery are similar, and efavirenz is considered to have similar efficacy to boosted PIs, with a better tolerability profile.
Toxicities of concern with long-term, widespread use of efavirenz include an adverse impact on total cholesterol, LDL and triglycerides. Another concern for use in women of child-bearing age is the documented teratogenicity in humans. This has limited efavirenz use worldwide in resource-poor settings. Even in developed countries, unintended pregnancies occur frequently, and efavirenz may not be an ideal choice in any woman of child-bearing age. Finally, efavirenz is a substrate of multiple cytochrome P450 enzymes, and a modulator of P450 enzyme activity; therefore, it is prone to many significant drug interactions.
A well-known characteristic of first-generation NNRTIs is the low genetic barrier to resistance development. A single mutation can drastically reduce the effectiveness of these agents. This can be an argument in favor of using these agents first-line. Once resistance develops, other PI-based regimens will still be active. Integrase inhibitors and second-line NNRTIs can then also be used sequentially after a first-generation NNRTI-based regimen fails, which provides more options for treatment-experienced patients. However, patients who fail an NNRTI-based initial regimen often have a greater number of viral resistance mutations as compared to patients failing a PI-based initial regimen, leaving fewer future treatment options for those who started with an NNRTI. Additionally, with the widespread use of NNRTIs over the past several years, transmission of resistant virus has increased, limiting even the initial treatment options for some patients.
An alternative approach being studied is to use these newer agents instead of efavirenz as first-line therapy in treatment-naïve patients. For raltegravir, week 24 and 48 virologic and immunologic efficacy were similar to efavirenz in treatment-experienced patients, although raltegravir appears to suppress viral replication more quickly than efavirenz. Furthermore, the side effect profile of raltegravir seems to lack adverse lipid and neuropsychiatric effects. Results from on-going treatment-naïve studies are needed to confirm that raltegravir is as potent as efavirenz, but more tolerable.
Etravirine and rilpivirine are also being studied as first-line therapy in treatment-naïve patients. Efavirenz could remain the NNRTI of choice even after these study results are reported and the agents approved for use in treatment-naïve patients. The rationale would be to use efavirenz in first, and then still have the option of a salvage NNRTI regimen if the efavirenz regimen fails. However, using etravirine after an initial efavirenz or nevirapine-based regimen is not as effective as using a boosted PI in the second regimen48, so the newer NNRTIs will likely only be used as a second regimen in NNRTI-experienced patients when PI regimens cannot be tolerated.
Initial results suggest second-generation NNRTIs have improved adverse effect profiles over efavirenz, again lacking the neuropsychiatric and lipid effects. Further, with the higher genetic barrier to resistance, etravirine and rilpivirine could be more durable in suppressing viral replication than first-generation NNRTIs. If longer-term studies indeed show similar efficacy to and improved tolerability over efavirenz, with or without improved durability of viral suppression, then these agents will likely supplant efavirenz as the NNRTI of choice of treatment-naïve patients. If these second-line agents also show no teratogenicity and no hepatic failure, their uptake worldwide to replace nevirapine, particularly for prevention of mother-to-child transmission of HIV, would be a natural consequence if their cost is not prohibitive.
6. Expert Opinion
Is efavirenz still the drug of choice for treatment-naïve patients? Yes. Efavirenz will remain the drug of choice in treatment-naïve patients in developed countries for the next several years. The vast amount of literature documenting efavirenz’s safety and efficacy will be difficult for newer agents to rapidly overcome. The ease of adherence, with a low pill burden and once-daily dosing, are further advantages of efavirenz. However, if the initial study results of integrase inhibitors and second-generation NNRTIs continue to show similar efficacy to efavirenz and less toxicity in the longer-term studies, these agents will become very attractive first-line agents and will probably supplant efavirenz in developed countries.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (K23 AI066901) and the California State University-wide AIDS Research Program (CH05-SD-607-005).
Abbreviations
- AUC
area under the curve
- CYP
cytochrome P450
- FDA
Food & Drug Administration
- HDL
high density lipoprotein
- HIV
human immunodeficiency virus
- NRTI
nucleoside reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- U.S.
United States
Contributor Information
Brookie M. Best, Assistant Professor of Pharmacy and Pediatrics, Division of Pharmacology and Drug Discovery, University of California, San Diego
Miguel Goicoechea, Assistant Professor of Medicine, University of California, San Diego, Antiviral Research Center, 150 West Washington Street, Suite 100, San Diego, CA 92103
Bibliography
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Jan 29, 2008. Panel on Antiretroviral Guidelines for Adolescents and Adults; pp. 1–128. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed May 14, 2008. [Google Scholar]
- 2.Bristol-Myers Squib Company. Sustiva® Prescribing Information. Princeton NJ: Mar, 2008. [cited 2008 May 14]; Available from: www.sustiva.com. [Google Scholar]
- 3.Young SD, Britcher SF, Tran LO, Payne LS, Lumma WC, Lyle TA, et al. L-743, 726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1995 Dec;39(12):2602–5. doi: 10.1128/aac.39.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther. 2004 Feb;9(1):57–65. [PubMed] [Google Scholar]
- 5.Veldkamp AI, Harris M, Montaner JS, Moyle G, Gazzard B, Youle M, et al. The steady-state pharmacokinetics of efavirenz and nevirapine when used in combination in human immunodeficiency virus type 1-infected persons. J Infect Dis. 2001 Jul 3;184(1):37–42. doi: 10.1086/320998. [DOI] [PubMed] [Google Scholar]
- 6.Villani P, Regazzi MB, Castelli F, Viale P, Torti C, Seminari E, et al. Pharmacokinetics of efavirenz (EFV) alone and in combination therapy with nelfinavir (NFV) in HIV-1 infected patients. Br J Clin Pharmacol. 1999 Nov;48(5):712–5. doi: 10.1046/j.1365-2125.1999.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfister M, Labbe L, Hammer SM, Mellors J, Bennett KK, Rosenkranz S, et al. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398. Antimicrob Agents Chemother. 2003 Jan;47(1):130–7. doi: 10.1128/AAC.47.1.130-137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004 Dec 3;18(18):2391–400. [PubMed] [Google Scholar]
- 9.Kappelhoff BS, Crommentuyn KM, de Maat MM, Mulder JW, Huitema AD, Beijnen JH. Practical guidelines to interpret plasma concentrations of antiretroviral drugs. Clin Pharmacokinet. 2004;43(13):845–53. doi: 10.2165/00003088-200443130-00002. [DOI] [PubMed] [Google Scholar]
- 10.Leth FV, Kappelhoff BS, Johnson D, Losso MH, Boron-Kaczmarska A, Saag MS, et al. Pharmacokinetic parameters of nevirapine and efavirenz in relation to antiretroviral efficacy. AIDS Res Hum Retroviruses. 2006 Mar;22(3):232–9. doi: 10.1089/aid.2006.22.232. [DOI] [PubMed] [Google Scholar]
- 11.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001 Jan 5;15(1):71–5. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 12.Nunez M, Gonzalez de Requena D, Gallego L, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Higher efavirenz plasma levels correlate with development of insomnia. J Acquir Immune Defic Syndr. 2001 Dec 1;28(4):399–400. doi: 10.1097/00126334-200112010-00015. [DOI] [PubMed] [Google Scholar]
- 13.Bacheler LT, Anton ED, Kudish P, Baker D, Bunville J, Krakowski K, et al. Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrob Agents Chemother. 2000 Sep;44(9):2475–84. doi: 10.1128/aac.44.9.2475-2484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joly V, Descamps D, Peytavin G, Touati F, Mentre F, Duval X, et al. Evolution of human immunodeficiency virus type 1 (HIV-1) resistance mutations in nonnucleoside reverse transcriptase inhibitors (NNRTIs) in HIV-1-infected patients switched to antiretroviral therapy without NNRTIs. Antimicrob Agents Chemother. 2004 Jan;48(1):172–5. doi: 10.1128/AAC.48.1.172-175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner B, Turner D, Oliveira M, Moisi D, Detorio M, Carobene M, et al. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS. 2003 Jan 3;17(1):F1–5. doi: 10.1097/00002030-200301030-00001. [DOI] [PubMed] [Google Scholar]
- 16.Loemba H, Brenner B, Parniak MA, Ma’ayan S, Spira B, Moisi D, et al. Polymorphisms of cytotoxic T-lymphocyte (CTL) and T-helper epitopes within reverse transcriptase (RT) of HIV-1 subtype C from Ethiopia and Botswana following selection of antiretroviral drug resistance. Antiviral Res. 2002 Nov;56(2):129–42. doi: 10.1016/s0166-3542(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 17.Loemba H, Brenner B, Parniak MA, Ma’ayan S, Spira B, Moisi D, et al. Genetic divergence of human immunodeficiency virus type 1 Ethiopian clade C reverse transcriptase (RT) and rapid development of resistance against nonnucleoside inhibitors of RT. Antimicrob Agents Chemother. 2002 Jul;46(7):2087–94. doi: 10.1128/AAC.46.7.2087-2094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tozzi V, Zaccarelli M, Narciso P, Trotta MP, Ceccherini-Silberstein F, De Longis P, et al. Mutations in HIV-1 reverse transcriptase potentially associated with hypersusceptibility to nonnucleoside reverse-transcriptase inhibitors: effect on response to efavirenz-based therapy in an urban observational cohort. J Infect Dis. 2004 May 1;189(9):1688–95. doi: 10.1086/382960. [DOI] [PubMed] [Google Scholar]
- 19.Shulman NS, Bosch RJ, Mellors JW, Albrecht MA, Katzenstein DA. Genetic correlates of efavirenz hypersusceptibility. AIDS. 2004 Sep 3;18(13):1781–5. doi: 10.1097/00002030-200409030-00006. [DOI] [PubMed] [Google Scholar]
- 20.Clark SA, Shulman NS, Bosch RJ, Mellors JW. Reverse transcriptase mutations 118I, 208Y, and 215Y cause HIV-1 hypersusceptibility to non-nucleoside reverse transcriptase inhibitors. AIDS. 2006 Apr 24;20(7):981–4. doi: 10.1097/01.aids.0000222069.14878.44. [DOI] [PubMed] [Google Scholar]
- 21.von Wyl V, Yerly S, Boni J, Burgisser P, Klimkait T, Battegay M, et al. Emergence of HIV-1 drug resistance in previously untreated patients initiating combination antiretroviral treatment: a comparison of different regimen types. Arch Intern Med. 2007 Sep 10;167(16):1782–90. doi: 10.1001/archinte.167.16.1782. [DOI] [PubMed] [Google Scholar]
- 22.Lazzari S, de Felici A, Sobel H, Bertagnolio S. HIV drug resistance surveillance: summary of an April 2003 WHO consultation. AIDS. 2004 Jun;18(Suppl 3):S49–53. doi: 10.1097/00002030-200406003-00010. [DOI] [PubMed] [Google Scholar]
- 23.Pillay D. Current patterns in the epidemiology of primary HIV drug resistance in North America and Europe. Antivir Ther. 2004 Oct;9(5):695–702. [PubMed] [Google Scholar]
- 24.Bartlett JA, Johnson J, Herrera G, Sosa N, Rodriguez A, Liao Q, et al. Long-term results of initial therapy with abacavir and Lamivudine combined with Efavirenz, Amprenavir/Ritonavir, or Stavudine. J Acquir Immune Defic Syndr. 2006 Nov 1;43(3):284–92. doi: 10.1097/01.qai.0000243092.40490.26. [DOI] [PubMed] [Google Scholar]
- 25.Squires K, Lazzarin A, Gatell JM, Powderly WG, Pokrovskiy V, Delfraissy JF, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004 Aug 15;36(5):1011–9. doi: 10.1097/00126334-200408150-00003. [DOI] [PubMed] [Google Scholar]
- 26.van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004 Apr 17;363(9417):1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 27.Clifford DB, Evans S, Yang Y, Acosta EP, Goodkin K, Tashima K, et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005 Nov 15;143(10):714–21. doi: 10.7326/0003-4819-143-10-200511150-00008. [DOI] [PubMed] [Google Scholar]
- 28.Fumaz CR, Munoz-Moreno JA, Molto J, Negredo E, Ferrer MJ, Sirera G, et al. Long-term neuropsychiatric disorders on efavirenz-based approaches: quality of life, psychologic issues, and adherence. J Acquir Immune Defic Syndr. 2005 Apr 15;38(5):560–5. doi: 10.1097/01.qai.0000147523.41993.47. [DOI] [PubMed] [Google Scholar]
- 29.Lochet P, Peyriere H, Lotthe A, Mauboussin JM, Delmas B, Reynes J. Long-term assessment of neuropsychiatric adverse reactions associated with efavirenz. HIV Med. 2003 Jan;4(1):62–6. doi: 10.1046/j.1468-1293.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 30.Fundaro C, Genovese O, Rendeli C, Tamburrini E, Salvaggio E. Myelomeningocele in a child with intrauterine exposure to efavirenz. AIDS. 2002 Jan 25;16(2):299–300. doi: 10.1097/00002030-200201250-00025. [DOI] [PubMed] [Google Scholar]
- 31.Chersich MF, Urban MF, Venter FW, Wessels T, Krause A, Gray GE, et al. Efavirenz use during pregnancy and for women of child-bearing potential. AIDS Res Ther. 2006;3:11. doi: 10.1186/1742-6405-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, Meyer WA, 3rd, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004 Apr 29;350(18):1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 33.Goicoechea M, Best B. Efavirenz/emtricitabine/tenofovir disoproxil fumarate fixed-dose combination: first-line therapy for all? Expert Opin Pharmacother. 2007 Feb;8(3):371–82. doi: 10.1517/14656566.8.3.371. [DOI] [PubMed] [Google Scholar]
- 34.Maggiolo F. Efavirenz. Expert Opin Pharmacother. 2007 Jun;8(8):1137–45. doi: 10.1517/14656566.8.8.1137. [DOI] [PubMed] [Google Scholar]
- 35.Vrouenraets SM, Wit FW, van Tongeren J, Lange JM. Efavirenz: a review. Expert Opin Pharmacother. 2007 Apr;8(6):851–71. doi: 10.1517/14656566.8.6.851. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht MA, Bosch RJ, Hammer SM, Liou SH, Kessler H, Para MF, et al. Nelfinavir, efavirenz, or both after the failure of nucleoside treatment of HIV infection. N Engl J Med. 2001 Aug 9;345(6):398–407. doi: 10.1056/NEJM200108093450602. [DOI] [PubMed] [Google Scholar]
- 37.Staszewski S, Morales-Ramirez J, Tashima KT, Rachlis A, Skiest D, Stanford J, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999 Dec 16;341(25):1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 38.Manfredi R, Calza L, Chiodo F. First-line efavirenz versus lopinavir-ritonavir-based highly active antiretroviral therapy for naive patients. AIDS. 2004 Nov 19;18(17):2331–3. doi: 10.1097/00002030-200411190-00017. [DOI] [PubMed] [Google Scholar]
- 39.Waters L, Stebbing J, Jones R, Michailidis C, Sawleshwarkar S, Mandalia S, et al. A comparison of the CD4 response to antiretroviral regimens in patients commencing therapy with low CD4 counts. J Antimicrob Chemother. 2004 Aug;54(2):503–7. doi: 10.1093/jac/dkh329. [DOI] [PubMed] [Google Scholar]
- 40.Riddler SA, Haubrich R, DiRienzo G, Peeples L, Powderly WG, Klingman KL, et al. A prospective, randomized, Phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV-1 infection - ACTG 5142. XVI International AIDS Conference; 2006; Toronto, Canada. [Google Scholar]
- 41.Haubrich RRS, DiRienzo G, Komarrow L, Powderly W, Garren K, George T, Rooney J, Mellors J, Havlir D, the ACTG 5142 Team Metabolic Outcomes of AVTG 5142: A Prospective, Randomized, Phase III Trial of NRTI-, PI-, and NNRTI-sparing Regimens for Initial Treatment of HIV-1 Infection. 14th Conference on Retroviruses and Opportunistic Infections; 2007 February 25-28; Los Angeles, USA. [Google Scholar]
- 42.Clumeck NVLJ, Chiliade P, et al. ARTEMIS: Efficacy and safety of lopinavir (BID vs QD) and darunavir (QD) in antiretroviral-naive patients. European AIDS Conference; 2007 October 24-27; Madrid, Spain. [Google Scholar]
- 43.Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, et al. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):125–33. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 44.Saag MSIP, Heera J, et al. A multicenter, randomized, double-blind, comparative trial of a novel CCR5 antagonist, maraviroc versus efavirenz, both in combination with Combivir (zidovudine/lamivudine), for the treatment of antiretroviral naive subjects infected with R5 HIV-1: week 48 results of the MERIT study. 4th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; 2007 July 22-25; Sydney, Australia. [Google Scholar]
- 45.Lazzarin A, Campbell T, Clotet B, Johnson M, Katlama C, Moll A, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007 Jul 7;370(9581):39–48. doi: 10.1016/S0140-6736(07)61048-4. [DOI] [PubMed] [Google Scholar]
- 46.Madruga JV, Cahn P, Grinsztejn B, Haubrich R, Lalezari J, Mills A, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007 Jul 7;370(9581):29–38. doi: 10.1016/S0140-6736(07)61047-2. [DOI] [PubMed] [Google Scholar]
- 47.Pozniak A, Morales-Ramirez JO, Mohapi L, Santoscoy M, Chetchotisakd P, Hereygers M, et al. 48-week primary analysis of trial TMC278-C204: TMC278 demonstrates potent and sustained efficacy in ART-naive patients. 14th Conference on Retroviruses and Opportunistic Infections; 2007; Los Angeles, CA. [Google Scholar]
- 48.Woodfall B, Vingerhoets J, Peeters M, Peeters I, De Smedt G, Miralles G, et al. Impact of NNRTI and NRTI resistance on the response to the regimen of TMC 125 plus two NRTIs in study TMC125-C227. 8th International Congress on Drug Therapy in HIV Infection; November 12-16, 2006; Glasgow, Scotland. Abstract no. 483. [Google Scholar]


