Abstract
Individuals with high heart rate variability tend to have better emotional well-being than those with low heart rate variability, but the mechanisms of this association are not yet clear. In this paper, we propose the novel hypothesis that by inducing oscillatory activity in the brain, high amplitude oscillations in heart rate enhance functional connectivity in brain networks associated with emotion regulation. Recent studies using daily biofeedback sessions to increase the amplitude of heart rate oscillations suggest that high amplitude physiological oscillations have a causal impact on emotional well-being. Because blood flow timing helps determine brain network structure and function, slow oscillations in heart rate have the potential to strengthen brain network dynamics, especially in medial prefrontal regulatory regions that are particularly sensitive to physiological oscillations.
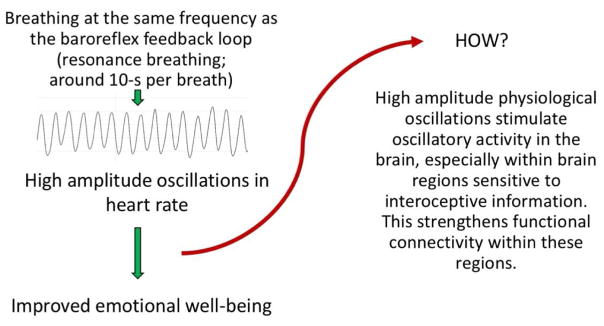
Having high heart rate variability (HRV) is associated with higher emotional well-being [1–3], including being correlated with lower levels of worry and rumination [4], lower anxiety [5], and generally more regulated emotional responding [6]. Thus, individuals with higher HRV appear to be better at regulating their emotions. However, it is not clear from these correlational studies if HRV is simply an output measure of regulatory brain health, or whether it somehow increases prefrontal regulation effectiveness. In healthy individuals, high HRV is not simply the result of random variability. Instead, much of the variability is due to the heart responding to physiological oscillatory signals such as breathing and blood pressure feedback, such that heart rate slows down and speeds up in a rhythmic fashion at certain frequencies. In this paper, we review findings that suggest that such oscillations in heart rate play a causal role in improving emotion regulation processes. Furthermore, we propose that high amplitude oscillations in heart rate modulate brain oscillatory activity, especially in brain regions associated with emotion regulation, and that daily episodes of synchronized activity within these networks can lead to enhanced functional connectivity strength in these emotion regulation networks even when HRV is not high.
Links Between HRV and Brain Regions Involved in Emotion Regulation
Emerging research indicates that emotion regulation and HRV are associated via the common brain regions involved in both systems [7]. For instance, in a meta analysis, HRV was significantly associated with regional cerebral blood flow in ventromedial prefrontal cortex (including anterior cingulate regions) and the amygdala [7]. In both younger and older adults scanned while at rest, higher HRV (measured used the root mean square successive differences; RMSSD) was associated with higher medial prefrontal cortex and amygdala functional connectivity [8; see also 9], a pattern associated with emotion regulation [10]. In addition, among younger and older adults, greater structural thickness in prefrontal regions was associated with greater HRV [11; see also 12; 13].
Inducing High Amplitude Oscillations in Heart Rate Improves Emotional Well-Being
High HRV could be associated with better emotion regulation simply because the same brain regions are involved in regulation of both systems, allowing HRV to serve as an indicator of the functioning of brain regulatory systems. However, recent findings [for review see 14] suggest that HRV itself influences brain and emotional function. In these studies, participants are taught to increase their HRV by breathing at around 10 s per breath. This .1 Hz frequency is a “resonance” frequency at which paced breathing induces oscillations in heart rate at an especially high amplitude [15]. Figure 1 shows an example of heart rate at rest in a healthy individual (panel A) followed by heart rate during paced breathing at their resonance pace (panel B). In resonance breathing HRV biofeedback studies, participants get feedback on how successfully they are increasing heart rate oscillations [15]. They typically engage in HRV biofeedback for at least 20 minutes a day for several weeks. A recent meta-analysis of 24 studies revealed that HRV biofeedback reduced self-reported stress and anxiety with a large effect size [16]. For instance, in one study, basketball players scoring high on anxiety were randomly assigned to HRV-biofeedback during resonance paced breathing, to an active control, or a no-contact control condition [17]. Participants completed sessions 10 days in a row for 20 minutes in each session and were tested before and after the 10-day intervention and again a month later. The intervention reduced state anxiety, and increased performance on standardized tests of basketball dribbling, passing and shooting. HRV biofeedback also has other positive effects on emotions. Coronary artery disease patients randomly assigned to HRV biofeedback during resonance breathing instead of a wait-list control showed decreased expressive and suppressive hostility and these effects were maintained a month after the 6-week intervention ended [18]. Likewise, veterans with post-traumatic stress disorder randomly assigned to HRV-biofeedback during resonance breathing showed reduced symptoms after 8 weeks of HRV-biofeedback whereas those assigned to treatment as usual did not show significant reductions in symptoms [19]. In addition, patients with post-stroke depression randomly assigned to treatment-as-usual in addition to HRV biofeedback during resonance breathing showed greater reductions in some indices associated with depression than those not assigned to HRV biofeedback [20]. Thus, in these studies, a series of HRV-biofeedback sessions using resonance paced breathing enhanced emotional outcomes.
Figure 1.
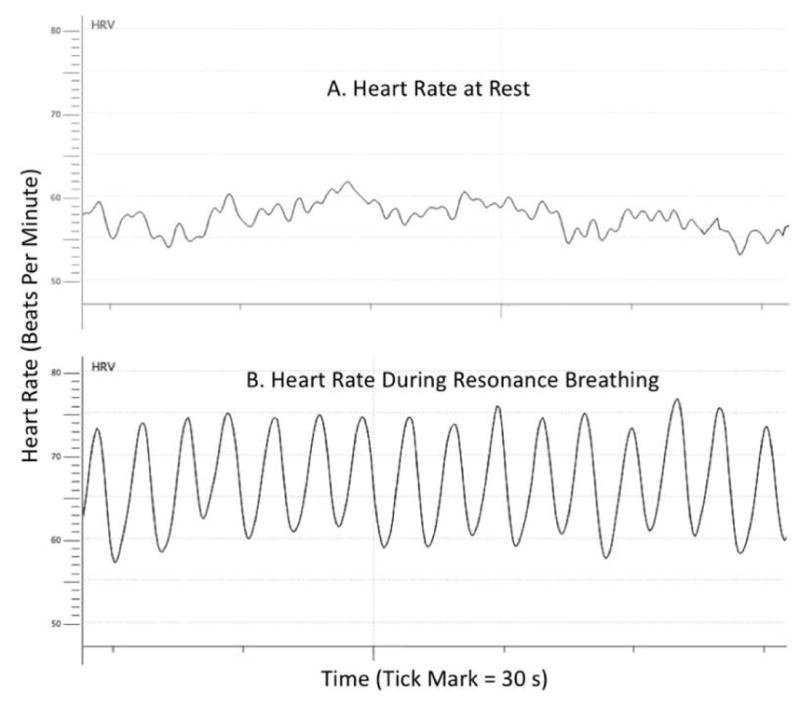
A. An example of heart rate variability during about a 2.5 minute time period during quiet rest. B. The same person’s heart rate during resonance breathing during another 2.5 minute time period.
Why Resonance Breathing Increases the Amplitude of Heart Rate Oscillations
The studies reviewed above using HRV biofeedback during paced breathing take advantage of the fact that two physiological rhythms that have a strong influence over the heart rate can be coordinated to induce high amplitude heart rate oscillations. The first of these physiological rhythms is the baroreflex. The vascular branch of the baroreflex has a lag time of approximately 10 seconds [21]. When vessels are stretching, baroreceptors signal via the brainstem to the heart to slow down the pace of heartbeats. There is a few-second delay in this feedback loop [between 4–6.5 s; 22] that creates oscillations in heart rate that take twice as long as the delay (from 0.075–0.12 Hz, depending on the individual) to complete a full oscillatory cycle [14; 22]. The second major influence over HRV is breathing. As we breathe in, heart rate tends to increase and as we breathe out, heart rate tends to decrease [23], although with a phase delay [24]. We usually breathe at a faster frequency (between .15 and .4 Hz) than the baroreflex. However, unlike the baroreflex, which has a fixed frequency, we can alter the pace of our own breathing. When breathing is slowed down to the same frequency as the baroreflex feedback loop, this creates resonance, a non-linear effect that is greater than an additive effect of the two influences. Thus, at an individual’s resonance frequency, there is the potential for high amplitude oscillations in heart rate.
A fascinating relevant phenomenon is that, in many meditative and religious chanting practices, breathing slows to around a 10-s (.1 Hz) rate and heart rate oscillates at this frequency [25–29]. For instance, reciting either the rosary Ave Maria prayer or a yoga mantra leads to breathing at a 10-s/breath rate and increased blood pressure and heart rate oscillations at that .1 Hz resonance frequency [25]. Hypotheses about the mechanisms of the positive effects of meditative practices typically focus on the role of attentional training and body awareness [30]. In contrast, there has been little focus on the role of physiological rhythms induced by the meditative practice.
High Amplitude Heart Rate Oscillations Should Promote Functional Connectivity, Especially in Brain Regions Involved in Emotion Regulation
We propose that episodes of high amplitude oscillations in heart rate (like those observed during meditative practice or HRV biofeedback) promote functional connectivity between certain brain regions, in particular among brain regions involved in emotion regulation. Why might this be the case?
First of all, brain activity is fueled by oxygen transported by blood, and so should be affected by oscillations in blood flow. Indeed, heart rate contributes to blood-oxygen level dependent (BOLD) fluctuations during functional magnetic resonance imaging (fMRI) [31–33]. Strong phase coupling of heart rate interval and BOLD oscillations have been observed in the mid cingulate and posterior cingulate regions at around the .1 Hz frequency [34]. These hemodynamics are likely to impact neural activity. In particular, oscillations in blood flow may lead to oscillations in the sensitivity of local cortical circuits to sensory stimuli [35].
Different brain regions vary in how long it takes for blood to reach them, with distant brain regions sometimes having similar vascular delays. Estimating vascular delay times for each voxel in an fMRI image and then running independent component analyses reveals components that resemble commonly identified resting state networks [36]. Resting state networks reflect brain regions that activate in correlated fashion at slow frequencies [<.1 Hz; 37]. It is intriguing that just knowing the vascular delays of different brain regions provides enough information to partially reconstruct resting state networks [36]. Indeed, part of what may lead some brain regions to develop coordinated network activity with each other may be their similar timing of blood delivery. Increasing the amplitude of blood flow oscillations via resonance breathing increases the impact of these coordinated vascular activities and thereby further stimulates networks that were shaped in part by blood flow patterns. Repeated brief episodes of coordinated activity within a network can strengthen its internal pathways, promoting greater functional connectivity during rest [e.g., 38; 39].
In addition to timing, regional differences in blood flow volume are also associated with functional connectivity [40]. Brain regions with high resting cerebral blood flow also show high functional connectivity with other brain regions, and the correlation between functional connectivity strength and blood flow is higher for measures of long-range than for short-range functional connectivity [40]. Brain regions showing strong cross-subject correlations between functional connectivity strength and blood flow include medial prefrontal cortex, anterior and posterior cingulate, and insula [40]. Thus, brain regions associated with emotion regulation are among those that experience high regional blood flow and serve as hub regions for brain functional connectivity. Thus, oscillations in blood flow are especially likely to impact these brain regions.
Breathing also influences brain rhythms. Breathing volume and pace help determine arterial CO2, which is a cerebral vasodilator and is expelled during breath exhalation. Brain regions differ in the timing and strength of their responses to these CO2 fluctuations, likely related to their proximity to large vessels, with strong correlations seen in insula and midline cingulate regions [41; 42]. Breathing causes respiration-synched oscillations across much of the neocortex and gamma power waxes and wans depending on the respiratory oscillation phase [43]. Breathing through one’s nose synchronizes oscillations in olfactory cortex as well as the amygdala and hippocampus [44]. In these limbic regions, nostril breathing entrains higher frequency oscillations in the delta, theta and beta ranges to the respiratory phase [44]. Thus, the breathing component of resonance breathing biofeedback practice should also contribute to neural oscillatory activity, especially in the limbic regions during nostril breathing.
Heartbeats also cause EEG responses known as heartbeat-evoked potentials that are particularly prominent in brain regions associated with interoceptive sensation and emotion, including medial prefrontal cortex, cingulate cortex, insula, and amygdala [45; 46]. Thus, heartbeats should be especially likely to influence brain rhythms in these brain regions. Consistent with this, BOLD activity in the ventromedial prefrontal cortex covaries with heart rate more than does activity other brain regions [47], and as already reviewed, in general, medial prefrontal/anterior cingulate regions show activity associated with HRV [7].
In summary, resonance breathing stimulates high amplitude oscillations that can influence brain rhythms via several channels, including fluctuations in blood flow, CO2 levels, and sensory input from breathing and from heartbeats. These channels each are especially likely to modulate activity in brain regions associated with emotion regulation networks [48].
Slow Oscillations Can Modulate Faster Frequencies of Neural Activity
In the previous section, we laid out the case that resonance breathing is likely to lead to oscillations in brain activity. Here we argue that, in addition to provoking oscillations at the same frequency, resonance breathing should also modulate faster oscillatory activity. The power density of EEG is inversely proportional to frequency, such that more powerful and widespread slow oscillations can modulate weaker but faster local oscillations [49; 50]. Slow oscillations are also critical for brain networks, because the limited number and speed of neuronal connections connecting distant regions mean that large-scale brain networks can only oscillate in tandem during slow oscillations [50].
The ability of lower frequency oscillations to modulate the phase of higher frequency oscillations leads to a hierarchical structure for EEG. For instance, in awake macaque monkeys, delta phase (1–4 Hz) modulates theta phase (4–10 Hz) and theta modulates gamma phase (20–50 Hz) amplitude, with these oscillations controlling baseline excitability and leading to phasic oscillations in responsiveness to stimuli [51]. Activity at one frequency is especially likely to modulate activity at other frequencies that are multiples of that frequency, a phenomenon known as harmonic frequency. Indeed, one speculative proposal is that the heart rate is the basic frequency and scaling factor for EEG frequency domains [52]. EEG is categorized into a set of different frequency bands (delta, theta, alpha, beta, gamma). The center frequency of each of these frequency bands (estimated at 2.5, 5, 10, 20 and 40 Hz, respectively) is twice as high than the previous lower frequency [52]. If the harmonic sequence of EEG frequency bands is extended down from delta to slower oscillations, the next lower one is 1.25 Hz, which, at 75 beats per minute, is close to the average resting heart rate [e.g., 53], suggesting that heart rate may be a basic frequency that serves as a scaling factor (depending on individual differences in average heart rate) for the EEG frequency domains [52]. Furthermore, if one continues going down to the subharmonic frequencies, one of the frequencies overlaps with high frequency HRV range influenced by breathing and another overlaps with the low frequency HRV range influenced by baroreflex feedback [52]. Consistent with these harmonic relationships, during sleep high frequency HRV shows synchronization with each of the different EEG frequency bands [54]. Furthermore, during wakefulness, the EEG spectral peak frequency is correlated with heart rate and this correlation decreases as sleep depth increases [55].
Thus, low frequency oscillations induced by resonance breathing should be able to promote functional connectivity between non-adjacent brain regions while also modulating oscillations at higher EEG frequency bands. Studies examining the oscillatory properties of brain activity are consistent with this notion that oscillations in the resonance frequency range help organize and modulate activity at higher frequencies. For instance, oscillations in prefrontal oxyhemoglobin in the frequency band between .07 and .13 Hz were coupled with EEG alpha and/or beta power oscillations [56]. Intracranial recordings from human posteromedial cortex revealed that the magnitude of cross frequency theta-to-gamma modulation fluctuated at around a 0.1 Hz frequency [57]. Thus, oscillations in brain activity at around the resonance frequency can modulate interactions among faster frequency signals [see also 58].
Another Potential Pathway of Action of Resonance Frequency Heart Rate Oscillations on the Brain
Stimulation of the baroreflex with resonance paced breathing is also likely to modulate brainstem arousal pathways. As already touched upon, as part of their feedback loop, baroreceptors project to the nucleus of the solitary tract and stimulate both sympathoinhibitory and a vagal cardioinhibitory pathways that decrease heart rate [59]. In addition to its effects on the heart, the baroreflex pathway also interacts bidirectionally with brainstem and forebrain regions that regulate arousal [59]. Breathing also stimulates brainstem arousal centers and breathing and blood pressure signals interact in affecting sympathetic activity [e.g., 60]. Thus, resonance breathing should modulate arousal. Most likely, these effects will involve oscillatory influences. Consistent with this possibility, during slow breathing, muscle sympathetic nerve activity decreases in a phasic fashion, reaching the same peak level as in control conditions but showing phasic segments of suppression [61–63]. If inducing high amplitude oscillations in heart rate phasically suppresses sympathetic action while stimulating parasympathetic action, this could help explain the stress- and anxiety-reducing effects of resonance breathing [16].
Conclusions
Past research has focused on heart rate variability as a downstream measure, rather than something that itself affects emotion regulation. For instance, the Neurovisceral Integration Model proposed that the medial PFC along with a core set of neural structures integrates information from different system to regulate the heart, and that HRV provides an index of the effectiveness of this “core integration” system [7]. Furthermore, previous research has not distinguished whether it is random noise or increases in the amplitude of oscillatory activity that is the key component of HRV that is associated with better emotional outcomes. The findings we outlined in this paper suggest that heart rate oscillations can enhance emotion by entraining brain rhythms in ways that enhance regulatory brain networks.
Figure 2 (Article Summary Figure).
How resonance breathing could lead to improved emotional well-being by stimulating functional connectivity of emotion regulation networks within the brain.
Highlights.
Breathing at a 10-s (.1 Hz) rate typically increases amplitude of heart rate oscillations.
Daily practice increasing heart rate oscillations improves emotional well-being.
Physiological oscillations stimulate oscillatory activity in brain regions involved in emotion regulation.
Slow (~.1 Hz) oscillations can also modulate interactions among faster neural frequencies.
Heart rate oscillations thereby have potential to strengthen regulatory brain networks.
Acknowledgments
Work on this review was supported by NIH grant R01AG057184.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mara Mather, University of Southern California.
Julian Thayer, Ohio State University.
References
- 1.Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology. 2015;98(2):338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Kemp AH, Quintana DS. The relationship between mental and physical health: Insights from the study of heart rate variability. International Journal of Psychophysiology. 2013;89(3):288–296. doi: 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Frontiers in psychology. 2014:5. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. 2016;142(3):231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- 5.Chalmers JA, Quintana DS, Abbott MJ, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Frontiers in psychiatry. 2014;5:80. doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of general psychology. 2006;10(3):229. [Google Scholar]
- 7.Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Sakaki M, Yoo HJ, Nga L, Lee TH, Thayer JF, Mather M. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage. 2016;139:44–52. doi: 10.1016/j.neuroimage.2016.05.076. For both younger and older adults, those with higher heart rate variability had stronger amygdala-medial prefrontal cortex functional connectivity, indicating that functional connectivity within an emotion regulation circuit is related to heart rate variability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennings JR, Sheu LK, Kuan DCH, Manuck SB, Gianaros PJ. Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high-frequency heart rate variability. Psychophysiology. 2016;53(4):444–454. doi: 10.1111/psyp.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nature Reviews Neuroscience. 2015;16(11):693. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- 11.Yoo HJ, Thayer JF, Greening SG, Lee TH, Ponzio A, Min J, … Koenig J. Brain structural concomitants of resting state heart rate variability in the young and old: Evidence from two independent samples. Brain Structure & Function. doi: 10.1007/s00429-017-1519-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkelmann T, Thayer JF, Pohlack S, Nees F, Grimm O, Flor H. Structural brain correlates of heart rate variability in a healthy young adult population. Brain Structure and Function. 2016:1–8. doi: 10.1007/s00429-016-1185-1. [DOI] [PubMed] [Google Scholar]
- 13.Woodward SH, Kaloupek DG, Schaer M, Martinez C, Eliez S. Right anterior cingulate cortical volume covaries with respiratory sinus arrhythmia magnitude in combat veterans. Journal of Rehabilitation Research and Development. 2008;45(3):451–464. doi: 10.1682/jrrd.2007.06.0082. [DOI] [PubMed] [Google Scholar]
- 14.Lehrer PM, Gevirtz R. Heart rate variability biofeedback: how and why does it work? Frontiers in psychology. 2014;5:756. doi: 10.3389/fpsyg.2014.00756. This review is an excellent source for further information on the mechanisms of how breathing rate interacts with heart rate and blood pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehrer P, Vaschillo B, Zucker T, Graves J, Katsamanis M, Aviles M, Wamboldt F. Protocol for heart rate variability biofeedback training. Biofeedback. 2013;41(3):98–109. [Google Scholar]
- 16.Goessl V, Curtiss J, Hofmann S. The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychological Medicine. 2017:1–9. doi: 10.1017/S0033291717001003. This meta-analysis of 24 studies found that HRV biofeedback training reduced self-reported stress and anxiety with a large effect size. Treatment efficacy was not moderated by the presence of an anxiety disorder, suggesting it had benefits even for those without clinical levels of anxiety. [DOI] [PubMed] [Google Scholar]
- 17.Paul M, Garg K. The effect of heart rate variability biofeedback on performance psychology of basketball players. Applied Psychophysiology and Biofeedback. 2012;37(2):131–144. doi: 10.1007/s10484-012-9185-2. [DOI] [PubMed] [Google Scholar]
- 18.Lin IM, Fan SY, Lu HC, Lin TH, Chu CS, Kuo HF, … Lu Y-H. Randomized controlled trial of heart rate variability biofeedback in cardiac autonomic and hostility among patients with coronary artery disease. Behaviour Research and Therapy. 2015;70:38–46. doi: 10.1016/j.brat.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Tan G, Dao TK, Farmer L, Sutherland RJ, Gevirtz R. Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): A pilot study. Applied Psychophysiology and Biofeedback. 2011;36(1):27–35. doi: 10.1007/s10484-010-9141-y. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Zhang T, Song LP, Zhang Y, Zhang GG, Xing CX, Chen H. Effects of heart rate variability biofeedback therapy on patients with poststroke depression: A case study. Chinese Medical Journal. 2015;128(18):2542. doi: 10.4103/0366-6999.164986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum M, Race D. Frequency-response characteristics of vascular resistance vessels. American Journal of Physiology--Legacy Content. 1968;215(6):1397–1402. doi: 10.1152/ajplegacy.1968.215.6.1397. [DOI] [PubMed] [Google Scholar]
- 22.Vaschillo EG, Vaschillo B, Lehrer PM. Characteristics of resonance in heart rate variability stimulated by biofeedback. Applied Psychophysiology and Biofeedback. 2006;31(2):129–142. doi: 10.1007/s10484-006-9009-3. [DOI] [PubMed] [Google Scholar]
- 23.Yasuma F, Hayano J-i. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125(2):683–690. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- 24.Angelone A, Coulter NA. Respiratory sinus arrhythmia: a frequency dependent phenomenon. Journal of Applied Physiology. 1964;19(3):479–482. doi: 10.1152/jappl.1964.19.3.479. [DOI] [PubMed] [Google Scholar]
- 25.Bernardi L, Sleight P, Bandinelli G, Cencetti S, Fattorini L, Wdowczyc-Szulc J, Lagi A. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. BMJ. 2001;323(7327):1446–1449. doi: 10.1136/bmj.323.7327.1446. This study demonstrates that the classic rosary prayer (the Ave Maria in Latin) and a typical yoga mantra (“om-mani-padme-om”) both take about 10 seconds to chant out loud and result in .1 Hz (10-s cycle) oscillations in heart rate and blood pressure in the person chanting. This suggests that these classic chants may have endured as calming practices because they stabilize the respiratory rate at the .1 Hz frequency that stimulates particularly high oscillations in heart rate and blood pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman EH, Coats AJ. Neurobiology of exaggerated heart rate oscillations during two meditative techniques. International Journal of Cardiology. 2000;73(2):199. doi: 10.1016/s0167-5273(00)00214-x. [DOI] [PubMed] [Google Scholar]
- 27.Lehrer P, Sasaki Y, Saito Y. Zazen and cardiac variability. Psychosomatic Medicine. 1999;61(6):812–821. doi: 10.1097/00006842-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Peng CK, Mietus JE, Liu Y, Khalsa G, Douglas PS, Benson H, Goldberger AL. Exaggerated heart rate oscillations during two meditation techniques. International Journal of Cardiology. 1999;70(2):101–107. doi: 10.1016/s0167-5273(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 29.Peng CK, Henry IC, Mietus JE, Hausdorff JM, Khalsa G, Benson H, Goldberger AL. Heart rate dynamics during three forms of meditation. International Journal of Cardiology. 2004;95(1):19–27. doi: 10.1016/j.ijcard.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 31.Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44(3):857–869. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Munck JC, Gonçalves SI, Faes TJC, Kuijer JPA, Pouwels PJW, Heethaar RM, Lopes da Silva FH. A study of the brain’s resting state based on alpha band power, heart rate and fMRI. Neuroimage. 2008;42(1):112–121. doi: 10.1016/j.neuroimage.2008.04.244. [DOI] [PubMed] [Google Scholar]
- 33.Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38(2):306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfurtscheller G, Schwerdtfeger AR, Seither-Preisler A, Brunner C, Stefan Aigner C, Brito J, … Andrade A. Brain–heart communication: Evidence for “central pacemaker” oscillations with a dominant frequency at 0.1 Hz in the cingulum. Clinical Neurophysiology. 2017;128(1):183–193. doi: 10.1016/j.clinph.2016.10.097. [DOI] [PubMed] [Google Scholar]
- 35.Moore CI, Cao R. The hemo-neural hypothesis: On the role of blood flow in information processing. Journal of Neurophysiology. 2008;99(5):2035–2047. doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong Y, Hocke LM, Fan X, Janes A, Frederick B. Can apparent resting state connectivity arise from systemic fluctuations? Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00285. These authors found that a significant portion of BOLD fMRI slow oscillations (~.1 Hz) are associated with the vascular low frequency oscillations that can be detected in peripheral regions such as fingertips and toes. These systemic low frequency oscillations reach different brain regions at different time delays depending on local cerebral vasculature. The authors computed the delay time for these signals to reach each voxel in an functional magnetic resonance scan. Simulation data using just these voxel delay times in an independent components analysis revealed a number of the well-known resting-state network patterns in brain activity, indicating that such networks are related to systemic vascular fluctuations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, … Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma L, Narayana S, Robin DA, Fox PT, Xiong J. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage. 2011;58(1):226–233. doi: 10.1016/j.neuroimage.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martínez K, Solana AB, Burgaleta M, Hernández-Tamames JA, Álvarez-Linera J, Román FJ, … Quiroga MA. Changes in resting-state functionally connected parietofrontal networks after videogame practice. Human Brain Mapping. 2013;34(12):3143–3157. doi: 10.1002/hbm.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceedings of the National Academy of Sciences. 2013;110(5):1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang C, Glover GH. Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. Neuroimage. 2009;47(4):1381–1393. doi: 10.1016/j.neuroimage.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di X, Kannurpatti SS, Rypma B, Biswal BB. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cerebral Cortex. 2013;23:255–263. doi: 10.1093/cercor/bhs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Rezaie R, Freeman WJ, … Kozma R. Breathing as a fundamental rhythm of brain function. Frontiers in Neural Circuits. 2017;10(115) doi: 10.3389/fncir.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, Gottfried JA. Nasal respiration entrains human limbic oscillations and modulates cognitive function. Journal of Neuroscience. 2016;36(49):12448–12467. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babo-Rebelo M, Richter CG, Tallon-Baudry C. Neural Responses to Heartbeats in the Default Network Encode the Self in Spontaneous Thoughts. The Journal of Neuroscience. 2016;36(30):7829–7840. doi: 10.1523/JNEUROSCI.0262-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park H-D, Bernasconi F, Salomon R, Tallon-Baudry C, Spinelli L, Seeck M, … Blanke O. Neural Sources and Underlying Mechanisms of Neural Responses to Heartbeats, and their Role in Bodily Self-consciousness: An Intracranial EEG Study. Cerebral Cortex. 2017:1–14. doi: 10.1093/cercor/bhx136. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler G, Dahnke R, Yeragani VK, Bär KJ. The relation of ventromedial prefrontal cortex activity and heart rate fluctuations at rest. European Journal of Neuroscience. 2009;30(11):2205–2210. doi: 10.1111/j.1460-9568.2009.07008.x. [DOI] [PubMed] [Google Scholar]
- 48.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends in Cognitive Sciences. 2010;14(11):506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 51.Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. Journal of Neurophysiology. 2005;94(3):1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- 52.Klimesch W. An algorithm for the EEG frequency architecture of consciousness and brain body coupling. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00766. Brain activity measured using an electroencephalogram (EEG) is categorized into frequency bands (δ, θ, α, β, γ). Klimesch points out that the center frequency of each of these bands is twice as high than its next lower neighbor, making them a series of harmonic frequencies. He suggests that heart rate may be the basic frequency that serves as the scaling factor for all other frequency and speculates about how this may make heart rate variability adaptive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramaekers D, Ector H, Aubert AE, Rubens A, Van de Werf F. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? European Heart Journal. 1998;19(9):1334–1341. doi: 10.1053/euhj.1998.1084. [DOI] [PubMed] [Google Scholar]
- 54.Dumont M, Jurysta F, Lanquart JP, Migeotte PF, van de Borne P, Linkowski P. Interdependency between heart rate variability and sleep EEG: linear/non-linear? Clinical Neurophysiology. 2004;115(9):2031–2040. doi: 10.1016/j.clinph.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Lechinger J, Heib DPJ, Gruber W, Schabus M, Klimesch W. Heartbeat-related EEG amplitude and phase modulations from wakefulness to deep sleep: Interactions with sleep spindles and slow oscillations. Psychophysiology. 2015;52(11):1441–1450. doi: 10.1111/psyp.12508. [DOI] [PubMed] [Google Scholar]
- 56.Pfurtscheller G, Daly I, Bauernfeind G, Müller-Putz GR. Coupling between intrinsic prefrontal HbO2 and central EEG beta power oscillations in the resting brain. Plos One. 2012;7(8):e43640. doi: 10.1371/journal.pone.0043640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foster BL, Parvizi J. Resting oscillations and cross-frequency coupling in the human posteromedial cortex. Neuroimage. 2012;60(1):384–391. doi: 10.1016/j.neuroimage.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):5053–5057. doi: 10.1073/pnas.0305375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silvani A, Calandra-Buonaura G, Benarroch EE, Dampney RAL, Cortelli P. Bidirectional interactions between the baroreceptor reflex and arousal: an update. Sleep Medicine. 2015;16(2):210–216. doi: 10.1016/j.sleep.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Eckberg DL, Nerhed C, Wallin B. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. The Journal of Physiology. 1985;365(1):181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mozer M, Fadel P, Johnson C, Wallin B, Charkoudian N, Drobish J, … Wehrwein E. Acute slow-paced breathing increases periods of sympathetic nervous system quiescence (1170.12) The FASEB Journal. 2014;28(1 Supplement) [Google Scholar]
- 62.Oneda B, Ortega KC, Gusmão JL, Araújo TG, Mion D. Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertension Research. 2010;33(7):708–712. doi: 10.1038/hr.2010.74. [DOI] [PubMed] [Google Scholar]
- 63.Raupach T, Bahr F, Herrmann P, Luethje L, Heusser K, Hasenfuß G, … Andreas S. Slow breathing reduces sympathoexcitation in COPD. European Respiratory Journal. 2008;32(2):387–392. doi: 10.1183/09031936.00109607. [DOI] [PubMed] [Google Scholar]