Abstract
Nanomaterials represent a promising and versatile platform for the delivery of therapeutics to the brain. Treatment of brain tumors has been a long-standing challenge in the field of neuro-oncology. The current standard of care – a multimodal approach of surgery, radiation and chemotherapy – yields only a modest therapeutic benefit for patients with malignant gliomas. A major obstacle for treatment is the failure to achieve sufficient delivery of therapeutics at the tumor site. Recent advances in local drug delivery techniques, along with the development of highly effective brain-penetrating nanocarriers, have significantly improved treatment and imaging of brain tumors in preclinical studies. The major advantage of this combined strategy is the ability to optimize local therapy, by maintaining an effective and sustained concentration of therapeutics in the brain with minimal systemic toxicity. This review highlights some of the latest developments, significant advancements and current challenges in local delivery of nanomaterials for the treatment of brain tumors.
Keywords: Glioblastoma, Local therapy, Nanocarriers, Convection-enhanced delivery
Graphical Abstract
Introduction
Despite recent advances in drug delivery, treatment of glioblastoma (GBM), the most prevalent and aggressive form of high-grade glioma, remains a paramount challenge. The prognosis for individuals with GBM is poor and has remained essentially unchanged over the past few decades, with a median survival of 15 months. Hallmarks of GBM include diffuse infiltration, necrosis, genomic instability, drug resistance, and nearly universal recurrence [1]. Effective treatment is hindered by the presence of the blood-brain barrier (BBB), which limits the entry into the brain of most hydrophilic molecules and chemotherapeutics that are administered systemically. In overcoming these challenges, various strategies that bypass the BBB have gained momentum in the past 10 years, with an increasing understanding that enhancing tumor penetration and intracranial distribution of therapeutics are crucial for improved outcome.
Some studies suggest that BBB in tumors is ‘leaky’ due to increased angiogenesis and formation of abnormal vessels that result in a dysfunctional blood-brain tumor barrier (BBTB) [11]. However, outside of the GBM tumor core, the BBB mostly remains intact and functional, preventing the passage of therapeutics as observed in the healthy brain [11]. These studies suggest that the changes in BBB may not be sufficient to enhance penetration of into tumors. The failure of systemically delivered agents to provide therapeutic benefit is likely due to their inability to physically cross the BBB, as well as other complex factors that contribute to their inefficacy.
Numerous ongoing studies are investigating local delivery strategies to circumvent the BBB and enhance accumulation at the tumor site. These include implantable or injectable systems with sustained drug release properties, with the goal of eliminating infiltrative GBM cells that cannot be surgically resected [1]. Unlike other delivery strategies, such as focused ultrasound [2], direct intracranial drug delivery is advantageous because it avoids interference with or disruption of the BBB. Additionally, systemic toxicity or side effects can be minimized with local drug delivery. Some of the earliest strategies included diffusion-based delivery mechanisms that involved direct injection of chemotherapeutics into the tumor resection cavity [3] or implantable polymers such as Gliadel® [4]. While these approaches provide the advantages of bypassing the BBB and minimizing systemic toxicity, the depth of distribution that can be achieved is very small, reaching only a few millimeters beyond the injection site [5] Multiple injections can be performed, but with the increased risk of neurotoxicity and local side effects such as hemorrhage. Local delivery methods to overcome the limitation of poor brain penetration have been studied extensively and evaluated in various clinical trials. The following sections highlight key studies that utilize local delivery in combination with nanomaterials, and discuss current limitations as well as potential strategies to improve therapeutic outcome.
Convection-enhanced delivery
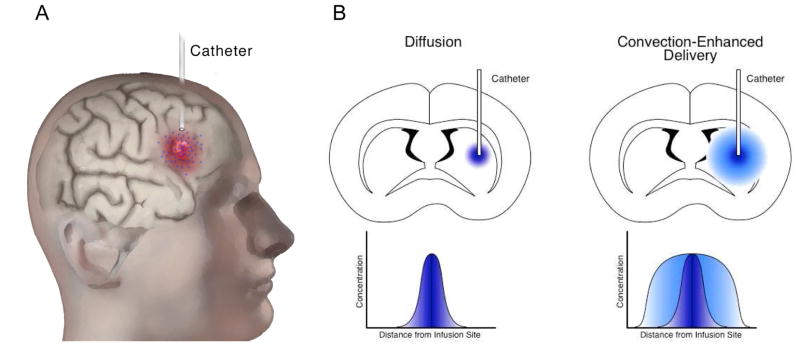
Convection-enhanced delivery (CED) has been shown to produce larger distribution volumes of agents infused into the brain parenchyma (Figure 1). Unlike diffusion-based methods, CED utilizes a continuous pressure gradient to drive bulk flow of agents which are infused directly into the tumor resection cavity, enabling large distribution of high drug concentrations, while avoiding systemic toxicity [6,7]. Importantly, CED can be used to distribute agents of various molecular weights and overcome the challenges of poor brain penetration. Compounds that do not penetrate the BBB, such as large molecular weight and/or hydrophilic compounds, are better candidates for CED because they remain in the brain parenchyma for a prolonged period after infusion, whereas small molecular weight and/or lipophilic agents can be readily eliminated through systemic circulation [8].
Figure 1.
Illustration of the CED method. A) The catheter is inserted into the tumor (shown in red) or the cavity created after tumor resection, and the therapeutic agent (blue) is continuously infused using convection. B) Diffusion relies on a concentration gradient, whereas CED utilizes bulk flow kinetics, resulting in a larger distribution of agents in the surrounding tissue.
The distribution volume that can be achieved with CED depends on several parameters, including the volume and rate of infusion, physical characteristics of the infusate, and catheter design. Clinical trials involving CED have been carried out with various agents, including conventional chemotherapeutics, monoclonal antibodies, targeted ligand-toxin conjugates, and liposomal formulations [7].
Nanomaterials for local drug delivery
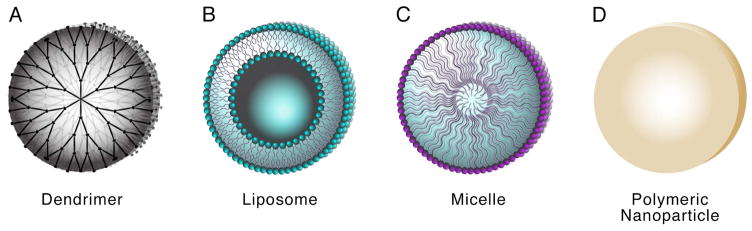
In addition to local delivery strategies, nanomaterials have been proposed as delivery systems to facilitate transport of therapeutic agents to the brain. These nanocarriers vary widely in composition, size and shape, with the ability to encapsulate either hydrophobic or hydrophilic molecules, including drugs, genetic material, radionuclides, and imaging agents [6]. Common examples of nanocarriers include liposomes, nanoparticles, dendrimers and micelles (Figure 2), many of which have been evaluated in combination with CED. Nanoencapsulation of therapeutic agents offers several advantages; it protects active molecules against degradation, reduces systemic toxicity, enables controlled or prolonged drug release, and provides the possibility of tumor targeting. The ideal nanocarrier should be capable of achieving high drug loading, with physicochemical characteristics—such as size, surface charge, and drug release profile—that are optimized for their intended use.
Figure 2.
Schematic of nanocarriers used in combination with CED including dendrimers (A), liposomes (B), micelles (C), and polymeric nanoparticles (D).
Systemic delivery of nanocarriers has been investigated to determine its potential for brain penetration. Ease of administration and the possibility of repeated dosage regimens are its main benefits. However, even with the best reported strategies to enhance transport across the BBB, intracranial accumulation of agents has been low, with about 1% of the injected dose reported to accumulate in the tumor in some cases [9,10]. Furthermore, systemic administration of nanocarriers inevitably results in abundant delivery to other tissues, such as the liver and lung, thereby increasing the risk of toxicity and undesired side effects.
For local delivery to the brain, nanocarriers must be less than 100 nm in size to facilitate penetration through the brain extracellular matrix (ECM) [12,13], neutral or negatively charged to limit non-specific binding [14] and provide sufficient tissue retention without undergoing rapid elimination through the blood capillaries. Cationic nanocarriers, which are widely utilized as gene delivery vectors, are prone to aggregation and poor penetration due to interactions with negatively charged brain parenchyma. Size has been shown to be a critical factor in nanoparticle distribution in the brain [12], while surface properties significantly affect cellular uptake [15]. The physiological differences between brain tumors versus normal tissue pose additional challenges for delivery. The increase in extracellular space, tortuosity, and dense cellular patterns pose significant barriers for diffusion in gliomas [16]. In addition, tumors exhibit high interstitial fluid pressure, which can result in inefficient uptake of therapeutics [17], while significant changes in ECM composition, volume and structure in tumor tissue can impair diffusion [18]. Thus, it is critical to consider these tumor-specific barriers for optimal nanocarrier design. Nanoparticle penetration in the brain can be enhanced by co-delivery of osmotic agents or enzymes that alter ECM [19], although it is not clear that these approaches can be translated into clinical practice. Bypassing the BBB may not be the only required criteria, as there are additional challenges to treatment, such as drug resistance, poor retention, and insufficient cellular uptake.
Newer and improved formulations developed within the last few years have often been described as “brain-penetrating,” with specific features that enhance distribution after CED or add new functions. Nanoparticles with ‘stealth’ properties, such as those coated with polyethylene glycol (PEG) or hyperbranched polyglycerol (HPG) have shown to enhance nanoparticle diffusion [20] or intracranial distribution by reducing interactions with proteins in the ECM [15,21]. However, despite efficient distribution, therapeutic efficacy of heavily PEGylated nanoparticles can be hindered; the “stealthy” character of these nanoparticles prevents uptake into tumor cells [15]. But other surface functionalizations of nanoparticles—particularly the addition of certain bioadhesive end groups—restores cellular uptake after CED, while providing a similar volume of distribution. Notably, nanoparticle-cell association rates measured in vitro were shown to predict the cellular tropism of nanoparticles in vivo, demonstrating that the interactions that occur in the brain environment can be modeled to some degree using cell culture systems [15].
In developing and evaluating nanomedicines for treatment of brain tumors, multiple criteria need to be considered. Survival benefits observed in animal models have not been readily translatable to human GBM patients in clinical trials, suggesting that therapeutic efficacy is dependent on a combination of different factors. The dose and frequency of treatment required to achieve therapeutic effect in animal models may not be relevant or feasible in humans. Importantly, nanocarriers should be designed to minimize toxicity and inflammation in normal cells in the brain, such as neurons, microglia and astrocytes. Strategies to enhance cellular specificity and facilitate tumor uptake, for example with the addition of peptides or surface coatings, are currently being investigated and hold great potential [15,22].
Nanocarriers encapsulating therapeutic agents
A wide array of nanocarrier formulations for local drug delivery have been investigated extensively over the past few years (Table 1). Earlier efforts focused on nanoencapsulation of conventional chemotherapeutics that cannot cross the BBB and exhibit short half-lives when administered in their free form. For instance, encapsulation of doxorubicin in liposomes has been shown to enhance its intracranial distribution by reducing its tissue affinity [21]. Highly effective combinations of therapeutic agents and nanocarriers include the encapsulation of hydrophobic, unstable, or toxic agents in polymeric nanoparticles or liposomes. Nanoencapsulation can protect these molecules from degradation, reduce toxicity, provide sustained release, and enhance tissue retention. For example, camptothecin and carboplatin have been loaded in poly(lactic-co-glycolic acid) (PLGA) nanoparticles [23,24], and irinotecan (CPT-11) in nanoliposomes [25]. These formulations provided significant anti-tumor effects and survival benefits in animal models. Nanoliposomal irinotecan, when combined with radiation, resulted in further improvement of survival benefit [26]. CED of nanodiamonds complexed with doxorubicin enhanced tissue retention, localized its toxicity and provided prolonged survival in rats with intracranial tumors [27]. Nano-micelles loaded with panobinostat, a strongly hydrophobic drug that is unstable when delivered in its unmodified form, prolonged survival of tumor-bearing rats [28]. Lipidoid nanoparticles encapsulating truncated diphtheria toxin were delivered by CED and inhibited brain tumor growth [29]. PLGA nanoparticles loaded with dithiazanine iodide, a compound that inhibited proliferation and self-renewal in brain cancer stem cells, produced significant improvements in median survival of tumor-bearing rats after administration by CED [12].
Table 1.
Examples of nanocarriers loaded with therapeutic or imaging agents for CED
| Nanocarrier | Material | Encapsulated agent | Significant results | Ref |
|---|---|---|---|---|
| Nanoparticle | PLGA | Camptothecin | Improved median survival (22 days vs. 15 days) with 30% long-term survivors in 9L rat model | [23] |
| PLGA | Carboplatin | Increased tumor cytotoxicity, reduced neurotoxicity, increased retention in rat and porcine models | [24] | |
| PLGA | Dithiazanine iodide | Brain-penetrating NPs inhibited growth of brain cancer stem cells and provided survival benefit in rats | [12] | |
| PEG-PLGA | Paclitaxel | PEGylated NPs provided enhanced distribution and tumor growth suppression in 9L rat model | [30] | |
| PEG-poly(aspartic acid) | Cisplatin | Reduced neurotoxicity of cisplatin with controlled release, and significantly enhanced survival (80% long-term survivors) | [31] | |
| Squalenoyl-PEG | Gemcitabine | PEGylated squalene NPs provided radiosensitization and survival benefit in RG2 rat model | [32] | |
| Poly(β-aminoester) | HSV thymidine kinase DNA | Achieved intratumoral transfection with and extended survival in 9L rat model | [35] | |
| PEG-poly-L-lysine | DNA | PEGylation of NPs improved distribution and efficient transfection in vivo | [38] | |
| Fe(Salen) | - | Combination with alternating magnetic field exposure provided greater anti-tumor effect (combined hyperthermia-chemotherapy) | [41] | |
| PLGA | SPIO | Enabled visualization of NP distribution in the rat brain by MRI, with long-lasting signal attenuation | [49] | |
| PEG-PLA-PCL | Temozolomide, magnetite | Multifunctional NPs were used to monitor NP distribution with MRI and prolonged survival of mice with U87 intracranial tumors | [52] | |
| Iron oxide | EGFRvIII antibody | Provided selective MRI contrast enhancement of tumor cells and targeted therapy in mouse model | [101] | |
| Liposome | Dioleoyl lecithin, cholesterol, PEG2000 PE | Gadolinium | Enabled in vivo monitoring of complete tumor coverage and co-infusion with Doxil | [46] |
| DSPC, cholesterol, PEG2000 PE | Irinotecan | Showed greater anti-tumor activity and survival benefit with CED of liposomal irinotecan plus radiation in mouse model | [26] | |
| DSPC, cholesterol | Rhenium-186 | Real-time visualization by SPECT; brachytherapy resulted in enhanced median survival (126 days vs. 49 days) in rat model | [39] | |
| DOPC, cholesterol, PEG-DSG | Gadolinium | CED and real-time MRI monitoring allowed visualization of distribution in non-human primate brain | [47] | |
| PEG-transferrin | Sodium borocaptate, lomeprol | Targeted boron to tumor tissue and enabled real-time CT imaging, with signal lasting over 72h | [54] | |
| Lipidoid telodendrimer | Lipid-polymer hybrid | Truncated diphtheria toxin | Improved intratumoral distribution; achieved delivery of active proteins and tumor growth inhibition in mice with U87 tumors | [29] |
| Lipid nanocapsule | Chitosan | siRNAs against Galectin-1 and EGFR | Combination therapy of siRNA-LNCs and TMZ enhanced survival of mice with U87 tumors | [36] |
| Lipoid | Rhenium-188 | Internal radiotherapy enhanced survival in mice witih orthotopic GBM tumors (50% long-term survivors) | [40] | |
| Nanodiamond | Diamond | Doxorubicin | Enhanced DOX retention, localized toxicity and prolonged survival in rat model | [27] |
| Micelle | Poloxamer407 | Panobinostat | 100% survival after a single infusion in F89 rat model | [28] |
| PEG-poly(glutamic acid) | SN-38 | Enhanced distribution and median survival in rat models, with minimal neurotoxicity | [53] |
As described above, the addition of PEG to the surface of nanoparticles reduces adhesive interactions, thereby improving brain penetration in some cases. PEGylated nanoparticles loaded with paclitaxel [30], cisplatin [31], and gemcitabine [32] have been demonstrated to enhance drug distribution, prolong release, and enhance anti-tumor effects in animal models. CED of PLA-PEG nanoparticles loaded with VE822, a DNA repair inhibitor, provided effective radiosensitization and survival benefit in animals treated with fractionated radiation therapy [33]. It is important to note that PEGylation—which can facilitate penetration of certain nanoparticle compositions—can also diminish cell uptake, so the effect of these competing factors must be considered.
Cationic polymers are by far the most widely used non-viral gene delivery vectors, due to their ability to efficiently condense the genetic material, enable intracellular delivery and facilitate endosomal escape. Poly(β-amino ester) nanoparticles have been utilized for the delivery of DNA, either alone or in combination with a prodrug that enables tumor-specific toxicity [34,35]. CED of chitosan-lipid nanocapsules loaded with small interfering RNA (siRNA) against Galectin-1 and EGFR has been shown to reduce drug resistance and enhance survival of mice with intracranial tumors [36]. Intratumoral delivery of antisense miRNAs (anti-miRs) has been described as promising new strategies for treatment of GBM [37]. Another study described PEGylated poly-L-lysine gene vectors that improve distribution and transfection efficiency [38].
Radionuclides have been successfully incorporated into nanocarrier formulations. CED of liposomes loaded with rhenium-186, followed by radiation, provided significant enhancement in median survival [39]. In a more recent study, rhenium-188-loaded lipid nanocapsules were utilized for “nanovectorized radiotherapy” which enables internal radiation as well as monitoring of distribution [40]. Notably, these nanocapsules were retained in the brain 24 hours after infusion, with no signs of toxicity to healthy tissue, and resulted in 50% cure rate in tumor-bearing mice, whereas external radiotherapy did not produce any long-term survivors.
An approach for combined hyperthermia-chemotherapy was demonstrated using magnetic Fe(Salen) nanoparticles, which can generate heat with exposure to an alternating magnetic field [41]. Hyperthermia increases sensitivity of cancer cells to chemotherapeutic agents, but the difficulty of targeting the exposure only to tumor cells without damaging normal tissue is a major limitation of this approach. This study demonstrated both in vitro and in vivo anti-tumor activity of locally delivered Fe(Salen) nanoparticles in combination with alternating magnetic field exposure.
In addition to injections into the brain parenchyma, CED is also being evaluated for delivery of therapeutics into the brainstem for treatment of diffuse pontine gliomas (DIPG) [42,43]. Image-guided CED of gadolinium-DTPA (diethylenetriamine penta-acetic acid) and gemcitabine to the brainstem has been successfully performed [44]. Another study that evaluated CED of liposomal doxorubicin to the brainstem of mice reported severe toxicity, resulting in the failure to reach a therapeutic window [45]. Because these tumors are universally fatal, and in locations that limit surgical options, additional research on nanomaterial-assisted CED and other local delivery approaches are needed.
Nanocarriers for imaging
Nanocarriers can be loaded with fluorescent markers, radiotracers, or contrast agents that allow for real-time imaging and/or post-procedural monitoring of infusion [6]. Fluorescent tracers have been used to evaluate distribution of nanocarriers in animal models, while radiotracers have been useful for determining the rate of degradation. Perhaps the most therapeutically relevant approach is the incorporation of magnetic resonance imaging (MRI) contrast agents, which enables real-time imaging during CED. In earlier studies, liposomes loaded with gadolinium were administered by CED into the brains of tumor-bearing rats [46] and non-human primates [47,48]. Used in conjunction with CED, in vivo MRI enabled direct and accurate visualization of nanocarrier distribution and tumor coverage in real time. Nanoparticles with magnetic cores have been widely utilized for MRI. For example, superparamagnetic iron oxide (SPIO)-loaded PLGA nanoparticles were administered by CED and the distribution detected using MRI, which showed signal retention in the brain for over one month after infusion [49]. Additionally, PLGA nanoparticles have facilitated non-invasive, quantitative positron emission tomography (PET) imaging to monitor distribution after CED [12].
Nanocarriers for multifunctional and combination therapies
Nanomaterials enable the co-delivery of multiple functionalities and different treatment modalities, which might lead to enhanced treatment effects on tumors [50]. Recently, multifunctional or theranostic nanocarriers, which combine therapeutic and diagnostic agents into a single platform, have generated much interest. For example, multifunctional magnetic iron oxide nanoparticles conjugated to a monoclonal antibody and EGFR inhibitor, cetuximab, targeted the nanoparticles to GBM cells, inhibited EGFR cell signaling, and provided therapeutic effect in three rodent GBM models [22]. Another study demonstrated the efficacy of methotrexate-loaded maghemite nanoparticles in providing large distribution and slow clearance after CED [51]. Therapeutic efficacy and in vivo imaging of infusion have been evaluated in animal models with the delivery of nanoparticles co-loaded with magnetite and temozolomide [52]. While the Vd/Vi was less than one, which may indicate insufficient tumor coverage, real-time visualization was demonstrated. More recently, polymeric micelles incorporating SN-38, a biologically active metabolite of irinotecan hydrochloride, were co-infused with gadolinium by CED. MRI evaluation showed a large distribution profile, and therapeutic efficacy was achieved in orthotopic brain tumor models [53].
Tumor-selective therapeutic effects have been demonstrated using boron neutron capture therapy, in which transferrin-conjugated PEG liposomes encapsulating sodium borocaptate and Iomeprol were administered by CED. Incorporation of real-time computed tomography (CT) showed targeted delivery of boron to tumor tissue [54]. Intratumoral thermotherapy has also been evaluated in clinical trials in which iron oxide nanoparticles were administered in conjunction with fractionated external beam radiotherapy to patients with recurrent GBM [55]. This study reported a substantial increase in median survival to 23.3 months, compared to 14.6 months in the reference group.
Nanocarriers loaded with radionuclides serve the dual purpose of targeted radiotherapy and noninvasive imaging. Rhenium-186-loaded liposomes delivered by CED enabled visualization and optimization of infusion with single-photon-emission computed tomography (SPECT) and CT, in addition to providing survival benefit in tumor-bearing rats [39].
Challenges with convection-enhanced delivery
Although local delivery by CED is a promising strategy that facilitates distribution of agents in the brain, many clinical trials have yielded disappointing outcomes. The unique pathological hallmarks of GBM tumors present significant challenges for CED. First, GBM tumors are often characterized by the presence of necrotic regions, hemorrhage and fibrin clots, making them naturally heterogeneous structures [56]. Second, the increased vascular permeability in tumors and surrounding regions can cause rapid clearance of infused drugs. Additionally, tumors often have increased interstitial pressure and heterogeneous pressure gradient, caused by elevated fluid and serum protein levels [57]. The high pressure within brain tumors can lead to reflux or backflow of the infusate up through the catheter. These features can significantly alter drug distribution, making consistent drug delivery difficult.
Distribution of polymer nanoparticles after CED into brain tumors has been shown to be affected by the presence of tumors; nanoparticle distribution depends on the size and properties of the tumor, and appears to be heterogeneous and asymmetric [58]. These findings suggest that tumor size and characteristics are important criteria that should be considered when determining infusion site and parameters for CED. Further, since intracranial distribution is determined by the properties of the infusate, the choice of the nanomaterial is important for the successful outcome of CED. A variety of properties of nanoparticle formulations have been shown to influence their penetration through brain tissues, including size, charge, and extent of PEGylation [12,30,34]. Among the parameters that affect distribution volume after CED is tissue affinity of the infusate [21].
There are technical challenges associated with cannula positioning, infusion parameters, and properties of the infusion catheters. Backflow of infusate can be induced by tissue disruption, high infusion rates, and large catheter diameter [59]. The disappointing outcome of the PRECISE trial, which compared the efficacy of CED of citredekin besudotox with Gliadel® wafers, has been attributed to inadequate distribution likely due to suboptimal catheter placement [60–62]. Considerable progress is being made in the area of catheter design, especially the development of multi-port catheters [7] and implantable systems that can be used for multiple infusions [63]. Most of the current work with nanomaterials has used catheter systems developed for infusion of drug solutions, and it is possible that infusion of nanocarrier suspensions might be facilitated by even more specialized catheter designs. Currently, cannulas with flexible catheters are most often used in clinical trials. Other proposed designs include rounded-tip cannulas which can decrease tissue pressure, balloon-tip cannulas which increase surface area, and step cannulas which have smaller distal tips and can enable higher infusion rates [57,64]. These may be useful in reducing reflux or backflow during CED, but additional studies are needed for clinical application.
Finally, neurotoxicity is still a concern that warrants further investigation. CED does not ensure that only tumor cells are exposed to the therapeutic agent. Therefore, even though systemic toxicity is greatly reduced with CED, the agent must demonstrate an adequate safety profile against normal brain cells to permit local administration.
Other local drug delivery strategies
Local delivery devices such as drug-loaded gels or polymer-based films, disks, rods, or wafers can be implanted or injected into the resection cavity. The FDA-approved implant, Gliadel®, exhibits slow release of bis-chloroethylnitrosourea (BCNU), is well tolerated and provides a survival benefit in patients with newly diagnosed and recurrent GBM [65]. Other implant-based drug delivery systems have been developed and evaluated in animal models. For instance, one study described the controlled-release of a novel small molecule, n-butylidenephthalide (BP), loaded into a polyanhydride polymer wafer, which was shown to reduce tumor migration and invasion, and prolong survival of rats with intracerebral tumors [66]. Also, an injectable matrix scaffold with docetaxel-loaded polylysine-based nanoparticles exhibited better drug penetration in tumor and prolonged retention, resulting in inhibition of tumor growth and prolonged survival [67].
Numerous hydrogel formulations, loaded with hydrophilic or hydrophobic drugs, proteins or DNA, have been utilized as controlled release drug delivery systems for GBM treatment [68]. Hydrogels are three-dimensional polymeric networks that are capable of absorbing large amounts of water without dissolution [3]. They can be injected intratumorally or placed in the resection cavity after craniotomy. For example, an injectable PEG-dimethacrylate (PEG-DMA) hydrogel, which can be photopolymerized in situ, provided sustained release of temozolomide [69]. Some hydrogels have incorporated drug-loaded nanocarriers, including lipid nanocapsules loaded with lauroyl-gemcitabine [68], PLGA-PEG loaded with paclitaxel [70], and PLGA microspheres encapsulating camtothecin or vincristine [71].
Intranasal delivery of nanocarriers has been investigated as a non-invasive approach for drug delivery to the brain, utilizing the direct pathway from the nasal cavity to the CNS [72]. For example, chitosan nanoparticles loaded with an siRNA molecule targeting Galectin-1 were delivered by intranasal administration and showed intratumoral distribution and knockdown of Gal-1 expression [73]. Reduction of Galectin-1 expression displays synergistic effects with temozolomide or immunotherapy, suggesting the possibility of combination therapy [74]. Intraarterial delivery of liposomes has been proposed as a method of reducing nonspecific uptake. In contrast to nanocarriers delivered by CED, larger (200 nm) cationic liposomes have been shown to exhibit higher retention and more efficient glioma targeting compared to smaller (80 nm) neutral formulations [75].
Novel therapeutic agents for treatment of brain tumors
Local delivery of genetic materials such as DNA, siRNA, and microRNA (miRNA) is a promising strategy for the treatment of brain tumors. Tumor-suppressive miRNA or inhibitors of oncogenic miRNAs have been recognized in recent years for their therapeutic potential, but there is much debate about their clinical feasibility [76]. One of the most extensively studied oncomiR known to be overexpressed in GBM is miR-21, whose downstream protein targets include PTEN, PDCD4 and RECK [77,78]. Inhibition of miR-21 or other oncomiRs can suppress GBM cell viability while sparing normal cells. Other miRNAs implicated in GBM include miR-221, miR-10b and miR-26a [79]. Some miRNAs have tumor-suppressive properties. For example, forced expression or restoration of let-7 miRNAs, which are highly expressed in normal tissues and act to inhibit oncogenes such as K-Ras, has been shown to reduce GBM proliferation [80]. Delivery of siRNA results in the knockdown of a specific gene target, whereas miRNAs affect multiple genes and pathways. Thus, a major advantage of miRNA-based therapeutics is the ability to simultaneously target multiple pathways involved in GBM pathogenesis. However, a potential drawback is that off-target effects of miRNAs could be higher than siRNA, which can be designed to inhibit very specific targets.
Questions remain about whether adequate delivery of miRNA inhibitor can be achieved and whether that will be sufficient to induce a therapeutic effect. Delivery remains the biggest hurdle for miRNA-based therapies; because of the blood-brain barrier, systemically administered miRNA therapeutics are unlikely to reach the tumor, making local delivery a necessity. A recent finding that may have important therapeutic implications is that GBM cells undergo a process termed “microvesicle transfer” in which cells bud off and deliver their cytoplasmic contents to nearby cells, effectively sharing their miRNAs, proteins, and other molecules [81]. This phenomenon may improve chances for success of miRNA-based therapeutics by reducing the delivery threshold required for therapeutic efficacy.
Other agents are gaining attention as potential drug candidates for treatment of brain tumors. These include EGFR inhibitors (cetuximab, erlotinib, gefitinib), PI3K/mTOR inhibitors (everolimus, tacrolimus, sirolimus), pan-histone deacetylase inhibitor (panobinostat), and monoclonal antibodies such as those against VEGF (bevacizumab) [82]. Panobinostat, a strongly hydrophobic drug with poor BBB permeability, has been shown to be much more effective when loaded into nano-micelles [28]. Nanoencapsulation and local delivery of these agents could dramatically improve their activity against GBM tumors.
Immunotherapy has the potential to revolutionize cancer therapy, yet so far there are few published works describing the use of nanomaterials in combination with immunotherapy for GBM treatment. For treatment of other cancers, nanocarriers have been used to deliver antigens or adjuvants, either encapsulated or on the surface of the particle, in order to elicit adaptive immune responses [83]. Most of these have been administered systemically, and most frequently evaluated in melanoma or lymphoma tumor models. Immunotherapeutics for GBM treatment face the same physiological barriers that limit the effectiveness of standard therapy, as well as the added challenge of local immunosuppression present in the tumor microenvironment [84]. Nanocarriers offer a versatile platform to enhance the benefits of immunotherapy. For instance, encapsulation in nanocarriers can protect the antigen from degradation and increase its half-life in vivo, reduce systemic toxicity, and promote specific delivery to antigen presenting cells [83]. Antigens delivered by nanoparticles have been shown to trigger much greater immune responses compared to soluble antigens [85].
Key molecules of interest for immunotherapy include immune checkpoint inhibitors, which are novel immune modulatory agents that can facilitate the anti-tumor immune response [86]. Specifically, they act by blocking immunosuppressive receptors that inhibit effector T-cells. Among these receptors is programmed death 1 (PD-1), a cell surface receptor that has been elucidated as a key molecule involved in immune resistance [87]. Blockade of PD-1 with anti-PD-1 antibody, combined with localized radiation, resulted in improved survival of mice with intracranial gliomas [88]. Clinical trials involving the use of anti-PD-1 antibody, nivolumab, either alone or in combination with temozolomide and radiation, are ongoing [89,90]. Interestingly, the ligand for PD-1, programmed death ligand 1 (PD-L1), has been shown to be highly expressed in GBM tumors as well as in the tumor-infiltrating macrophages and microglia [91]. Loss of PTEN, a frequently occurring alteration in GBM, has been hypothesized to induce PD-L1 expression [92]. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) is a receptor expressed only on T-cells, and its blockage facilitates T-cell activation [93]. CTLA4-blocking antibodies, ipilimumab and tremelimumab, have been evaluated in clinical trials for treatment of metastatic melanoma [93], and highlight the potential for their utility in treating other cancers including GBM. Another target for immunotherapy is CD133, a cell surface protein present on GBM stem cells but absent on normal cells, which can be exploited to facilitate specific delivery of therapeutics to tumors [94]. Synergistic effects of immunotherapy with chemoradiation [95] suggests that it could be part of a multi-modal treatment approach using nanocarriers to augment the immune response.
Future prospects
New approaches and techniques are being investigated to address the causes of failures in clinical trials and improve the outcome for GBM patients. Emerging evidence suggests therapeutic efficacy is closely related to, and therefore can be improved by, enhanced drug distribution and tumor coverage. The feasibility of chronic and continuous CED has been demonstrated and may be beneficial, with some studies reporting that larger regions of the brain can be perfused with prolonged infusion [96].
Use of real-time image-guided CED that allows for visualization of drug distribution during infusion may enable better catheter placement and optimization of infusion parameters [97]. Reliable computer modeling that can accurately predict drug distribution after CED [98,99] would also be helpful. Using nanocarriers with contrast agents, MRI-guided catheter placement can be achieved with real-time image feedback.
Finally, a better understanding of the cellular processes that affect nanoparticle internalization could help improve design and efficiency. For instance, computational modeling has been used to quantify rates of various cellular processes involved in nanoparticle uptake [100]. Combination of CED with nanotechnology and novel targeted therapies holds great potential for GBM treatment, but the challenges and shortcomings associated with local delivery should be addressed in order to improve chances for success in clinical trials.
Conclusions
Failure of most therapeutics in treating GBM results from poor brain penetration and rapid degradation. Combination of local delivery methods such as CED with nanocarriers can greatly improve drug distribution and retention in the brain to ensure complete coverage of the tumor mass and infiltrating cells. The half-life of drugs can be extended by encapsulation, which prevents drugs from rapid degradation or elimination. As highlighted above, CED of various nanocarrier formulations has been successfully used to enhance intracranial distribution and provide therapeutic efficacy with minimal toxicity, though several challenges remain unresolved. An increasing understanding of the fate of nanoparticles after CED has highlighted the potential to improve therapeutic efficacy by optimizing their surface properties and incorporating specific features. The main conclusions from the studies reviewed here converge on the need for personalized treatment, with patients receiving individualized therapeutic regimen and drug delivery protocols for successful outcomes.
Highlights.
Convection-enhanced delivery (CED) of nanocarriers can significantly enhance intracranial distribution and facilitate sustained delivery of agents for the treatment of brain tumors.
Nanocarriers are effective delivery platforms for a wide variety of therapeutic and imaging agents, and enable combinations of multiple functionalities or different treatment modalities.
Modification of the nanocarrier surface with specific brain-penetrating features can enhance diffusion, improve cellular uptake, or provide tumor specificity.
Challenges with CED—including insufficient tumor coverage, backflow and neurotoxicity—must be addressed to improve therapeutic efficacy and achieve clinical success.
Several promising nanocarrier formulations are designed to target the immunological features of GBM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juratli TA, Schackert G, Krex D. Current status of local therapy in malignant gliomas--a clinical review of three selected approaches. Pharmacol Ther. 2013;139(3):341–358. doi: 10.1016/j.pharmthera.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Timbie KF, Afzal U, Date A, Zhang C, Song J, Wilson Miller G, Suk JS, Hanes J, Price RJ. Mr image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J Control Release. 2017 doi: 10.1016/j.jconrel.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastiancich C, Danhier P, Preat V, Danhier F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J Control Release. 2016;243:29–42. doi: 10.1016/j.jconrel.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 5.Fung LK, Ewend MG, Sills A, Sipos EP, Thompson R, Watts M, Colvin OM, Brem H, Saltzman WM. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58(4):672–684. [PubMed] [Google Scholar]
- 6.Allard E, Passirani C, Benoit J-P. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30(12):2302–2318. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Healy AT, Vogelbaum MA. Convection-enhanced drug delivery for gliomas. Surg Neurol Int. 2015;6(Suppl 1):S59–67. doi: 10.4103/2152-7806.151337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lonser RR, Sarntinoranont M, Morrison PF, Oldfield EH. Convection-enhanced delivery to the central nervous system. J Neurosurg. 2015;122(3):697–706. doi: 10.3171/2014.10.JNS14229. [DOI] [PubMed] [Google Scholar]
- 9.Saucier-Sawyer JK, Deng Y, Seo YE, Cheng CJ, Zhang J, Quijano E, Saltzman WM. Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. J Drug Target. 2015;23(7–8):736–749. doi: 10.3109/1061186X.2015.1065833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, et al. Spherical nucleic acid nanoparticle conjugates as an rnai-based therapy for glioblastoma. Sci Transl Med. 2013;5(209):209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Patel TR, Sirianni RW, Strohbehn G, Zheng MQ, Duong N, Schafbauer T, Huttner AJ, Huang Y, Carson RE, Zhang Y, et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc Natl Acad Sci U S A. 2013;110(29):11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103(14):5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacKay JA, Deen DF, Szoka FC., Jr Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035(2):139–153. doi: 10.1016/j.brainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- ••15.Song E, Gaudin A, King AR, Seo YE, Suh HW, Deng Y, Cui J, Tietjen GT, Huttner A, Saltzman WM. Surface chemistry governs cellular tropism of nanoparticles in the brain. Nat Commun. 2017;8(15322):15322. doi: 10.1038/ncomms15322. This study demonstrated how surface properties of nanoparticles affect cellular tropism in the tumor-bearing or healthy rat brain and showed that in vitro association rates can be used to predict in vivo internalization by different cell populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargova L, Homola A, Zamecnik J, Tichy M, Benes V, Sykova E. Diffusion parameters of the extracellular space in human gliomas. Glia. 2003;42(1):77–88. doi: 10.1002/glia.10204. [DOI] [PubMed] [Google Scholar]
- 17.Heldin C-H, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure [mdash] an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 18.Zamecnik J. The extracellular space and matrix of gliomas. Acta Neuropathologica. 2005;110(5):435–442. doi: 10.1007/s00401-005-1078-5. [DOI] [PubMed] [Google Scholar]
- 19.Neeves KB, Sawyer AJ, Foley CP, Saltzman WM, Olbricht WL. Dilation and degradation of the brain extracellular matrix enhances penetration of infused polymer nanoparticles. Brain Res. 2007;1180:121–132. doi: 10.1016/j.brainres.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4(149):149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito R, Krauze MT, Noble CO, Tamas M, Drummond DC, Kirpotin DB, Berger MS, Park JW, Bankiewicz KS. Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: Implications for local drug delivery. Journal of Neuroscience Methods. 2006;154(1–2):225–232. doi: 10.1016/j.jneumeth.2005.12.027. [DOI] [PubMed] [Google Scholar]
- •22.Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget. 2015;6(11):8788–8806. doi: 10.18632/oncotarget.3554. Conjugation of cetuximab to iron-oxide nanoparticles resulted in targeted therapeutic effect against both GSCs and GBM non-stem cells. These multifunctional nanoparticles also enabled direct imaging by MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawyer AJ, Saucier-Sawyer JK, Booth CJ, Liu J, Patel T, Piepmeier JM, Saltzman WM. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv Transl Res. 2011;1(1):34–42. doi: 10.1007/s13346-010-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arshad A, Yang B, Bienemann AS, Barua NU, Wyatt MJ, Woolley M, Johnson DE, Edler KJ, Gill SS. Convection-enhanced delivery of carboplatin plga nanoparticles for the treatment of glioblastoma. PLoS One. 2015;10(7):e0132266. doi: 10.1371/journal.pone.0132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble CO, Krauze MT, Drummond DC, Yamashita Y, Saito R, Berger MS, Kirpotin DB, Bankiewicz KS, Park JW. Novel nanoliposomal cpt-11 infused by convection-enhanced delivery in intracranial tumors: Pharmacology and efficacy. Cancer Res. 2006;66(5):2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 26.Chen PY, Ozawa T, Drummond DC, Kalra A, Fitzgerald JB, Kirpotin DB, Wei KC, Butowski N, Prados MD, Berger MS, Forsayeth JR, et al. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro Oncol. 2013;15(2):189–197. doi: 10.1093/neuonc/nos305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xi G, Robinson E, Mania-Farnell B, Vanin EF, Shim KW, Takao T, Allender EV, Mayanil CS, Soares MB, Ho D, Tomita T. Convection-enhanced delivery of nanodiamond drug delivery platforms for intracranial tumor treatment. Nanomedicine. 2014;10(2):381–391. doi: 10.1016/j.nano.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Singleton WG, Collins AM, Bienemann AS, Killick-Cole CL, Haynes HR, Asby DJ, Butts CP, Wyatt MJ, Barua NU, Gill SS. Convection enhanced delivery of panobinostat (lbh589)-loaded pluronic nano-micelles prolongs survival in the f98 rat glioma model. Int J Nanomedicine. 2017;12:1385–1399. doi: 10.2147/IJN.S125300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Wang X, Bodman A, Shi C, Guo D, Wang L, Luo J, Hall WA. Tunable lipidoid-telodendrimer hybrid nanoparticles for intracellular protein delivery in brain tumor treatment. Small. 2016;12(31):4185–4192. doi: 10.1002/smll.201601234. A lipodoid-polymer hybrid nanocarrier facilitated intratumoral distribution and efficient delivery of truncated diphtheria toxin, resulting in tumor growth inhibition in mice with intracranial U87 tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nance E, Zhang C, Shih TY, Xu Q, Schuster BS, Hanes J. Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration. ACS Nano. 2014;8(10):10655–10664. doi: 10.1021/nn504210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Nance EA, Mastorakos P, Chisholm J, Berry S, Eberhart C, Tyler B, Brem H, Suk JS, Hanes J. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J Control Release. 2017 doi: 10.1016/j.jconrel.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudin A, Song E, King AR, Saucier-Sawyer JK, Bindra R, Desmaele D, Couvreur P, Saltzman WM. Pegylated squalenoyl-gemcitabine nanoparticles for the treatment of glioblastoma. Biomaterials. 2016;105:136–144. doi: 10.1016/j.biomaterials.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •33.King AR, Corso CD, Chen EM, Song E, Bongiorni P, Chen Z, Sundaram RK, Bindra RS, Saltzman WM. Local DNA repair inhibition for sustained radiosensitization in high grade gliomas. Mol Cancer Ther. 2017 doi: 10.1158/1535-7163.MCT-16-0788. CED of nanoparticles loaded with DNA repair inhibitors provided effective radiosensitization and survival benefit in tumor-bearing rats, demonstrating the feasibility of a new multi-modal approach to treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •34.Mastorakos P, Song E, Zhang C, Berry S, Park HW, Kim YE, Park JS, Lee S, Suk JS, Hanes J. Biodegradable DNA nanoparticles that provide widespread gene delivery in the brain. Small. 2016;12(5):678–685. doi: 10.1002/smll.201502554. This study described the development of brain-penetrating PBAE nanoparticles that can facilitate widespread distribution and high transgene expression in the rat striatum following CED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••35.Mangraviti A, Tzeng SY, Kozielski KL, Wang Y, Jin Y, Gullotti D, Pedone M, Buaron N, Liu A, Wilson DR, Hansen SK, et al. Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano. 2015;9(2):1236–1249. doi: 10.1021/nn504905q. CED of PBAE nanoparticles loaded with HSVtk DNA resulted in effective tumor transfection with widespread coverage, and provided significant survival benefit when combined with systemic administration of ganciclovir. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danhier F, Messaoudi K, Lemaire L, Benoit JP, Lagarce F. Combined anti-galectin-1 and anti-egfr sirna-loaded chitosan-lipid nanocapsules decrease temozolomide resistance in glioblastoma: In vivo evaluation. Int J Pharm. 2015;481(1–2):154–161. doi: 10.1016/j.ijpharm.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 37.Kim DG, Kim KH, Seo YJ, Yang H, Marcusson EG, Son E, Lee K, Sa JK, Lee HW, Nam DH. Anti-mir delivery strategies to bypass the blood-brain barrier in glioblastoma therapy. Oncotarget. 2016;7(20):29400–29411. doi: 10.18632/oncotarget.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry S, Mastorakos P, Zhang C, Song E, Patel H, Suk JS, Hanes J. Enhancing intracranial delivery of clinically relevant non-viral gene vectors. RSC Adv. 2016;48(6):41665–41674. doi: 10.1039/C6RA01546H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips WT, Goins B, Bao A, Vargas D, Guttierez JE, Trevino A, Miller JR, Henry J, Zuniga R, Vecil G, Brenner AJ. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro Oncol. 2012;14(4):416–425. doi: 10.1093/neuonc/nos060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •40.Cikankowitz A, Clavreul A, Tetaud C, Lemaire L, Rousseau A, Lepareur N, Dabli D, Bouchet F, Garcion E, Menei P, Couturier O, et al. Characterization of the distribution, retention, and efficacy of internal radiation of 188re-lipid nanocapsules in an immunocompromised human glioblastoma model. J Neurooncol. 2017;131(1):49–58. doi: 10.1007/s11060-016-2289-4. Fractionated internal radiotherapy by 188re-lipid nanocapsules following CED resulted in 50% cure rate in an orthotopic model of human GBM. [DOI] [PubMed] [Google Scholar]
- 41.Ohtake M, Umemura M, Sato I, Akimoto T, Oda K, Nagasako A, Kim JH, Fujita T, Yokoyama U, Nakayama T, Hoshino Y, et al. Hyperthermia and chemotherapy using fe(salen) nanoparticles might impact glioblastoma treatment. Sci Rep. 2017;7:42783. doi: 10.1038/srep42783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandberg DI, Edgar MA, Souweidane MM. Convection-enhanced delivery into the rat brainstem. J Neurosurg. 2002;96(5):885–891. doi: 10.3171/jns.2002.96.5.0885. [DOI] [PubMed] [Google Scholar]
- 43.Goodwin CR, Xu R, Iyer R, Sankey EW, Liu A, Abu-Bonsrah N, Sarabia-Estrada R, Frazier JL, Sciubba DM, Jallo GI. Local delivery methods of therapeutic agents in the treatment of diffuse intrinsic brainstem gliomas. Clin Neurol Neurosurg. 2016;142:120–127. doi: 10.1016/j.clineuro.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Murad GJ, Walbridge S, Morrison PF, Szerlip N, Butman JA, Oldfield EH, Lonser RR. Image-guided convection-enhanced delivery of gemcitabine to the brainstem. J Neurosurg. 2007;106(2):351–356. doi: 10.3171/jns.2007.106.2.351. [DOI] [PubMed] [Google Scholar]
- •45.Sewing ACP, Lagerweij T, van Vuurden DG, Meel MH, Veringa SJE, Carcaboso AM, Gaillard PJ, Peter Vandertop W, Wesseling P, Noske D, Kaspers GJL, et al. Preclinical evaluation of convection-enhanced delivery of liposomal doxorubicin to treat pediatric diffuse intrinsic pontine glioma and thalamic high-grade glioma. J Neurosurg Pediatr. 2017;19(5):518–530. doi: 10.3171/2016.9.PEDS16152. CED of liposomal doxorubicin to the braistem and thalamus of mice was performed to evaluate the efficacy of this approach in treating DIPG. The results showed reduction in tumor growth in the thalamus but severe toxicity in the brainstem, suggesting that careful consideration of anatomical location is crucial for local delivery of doxorubicin. [DOI] [PubMed] [Google Scholar]
- 46.Saito R, Bringas JR, McKnight TR, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewiez KS. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Research. 2004;64(7):2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 47.Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Hong K, et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196(2):381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Krauze MT, McKnight TR, Yamashita Y, Bringas J, Noble CO, Saito R, Geletneky K, Forsayeth J, Berger MS, Jackson P, Park JW, et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res Brain Res Protoc. 2005;16(1–3):20–26. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Strohbehn G, Coman D, Han L, Ragheb RR, Fahmy TM, Huttner AJ, Hyder F, Piepmeier JM, Saltzman WM, Zhou J. Imaging the delivery of brain-penetrating plga nanoparticles in the brain using magnetic resonance. J Neurooncol. 2015;121(3):441–449. doi: 10.1007/s11060-014-1658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ediriwickrema A, Saltzman WM. Nanotherapy for cancer: Targeting and multifunctionality in the future of cancer therapies. ACS Biomater Sci Eng. 2015;1(2):64–78. doi: 10.1021/ab500084g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corem-Salkmon E, Ram Z, Daniels D, Perlstein B, Last D, Salomon S, Tamar G, Shneor R, Guez D, Margel S, Mardor Y. Convection-enhanced delivery of methotrexate-loaded maghemite nanoparticles. Int J Nanomedicine. 2011;6:1595–1602. doi: 10.2147/IJN.S23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernal GM, LaRiviere MJ, Mansour N, Pytel P, Cahill KE, Voce DJ, Kang S, Spretz R, Welp U, Noriega SE, Nunez L, et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine. 2014;10(1):149–157. doi: 10.1016/j.nano.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang R, Saito R, Mano Y, Sumiyoshi A, Kanamori M, Sonoda Y, Kawashima R, Tominaga T. Convection-enhanced delivery of sn-38-loaded polymeric micelles (nk012) enables consistent distribution of sn-38 and is effective against rodent intracranial brain tumor models. Drug Deliv. 2016;23(8):2780–2786. doi: 10.3109/10717544.2015.1081994. [DOI] [PubMed] [Google Scholar]
- 54.Miyata S, Kawabata S, Hiramatsu R, Doi A, Ikeda N, Yamashita T, Kuroiwa T, Kasaoka S, Maruyama K, Miyatake S. Computed tomography imaging of transferrin targeting liposomes encapsulating both boron and iodine contrast agents by convection-enhanced delivery to f98 rat glioma for boron neutron capture therapy. Neurosurgery. 2011;68(5):1380–1387. doi: 10.1227/NEU.0b013e31820b52aa. discussion 1387. [DOI] [PubMed] [Google Scholar]
- 55.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103(2):317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito R, Tominaga T. Convection-enhanced delivery of therapeutics for malignant gliomas. Neurol Med Chir (Tokyo) 2017;57(1):8–16. doi: 10.2176/nmc.ra.2016-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jahangiri A, Chin AT, Flanigan PM, Chen R, Bankiewicz K, Aghi MK. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J Neurosurg. 2017;126(1):191–200. doi: 10.3171/2016.1.JNS151591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saucier-Sawyer JK, Seo YE, Gaudin A, Quijano E, Song E, Sawyer AJ, Deng Y, Huttner A, Saltzman WM. Distribution of polymer nanoparticles by convection-enhanced delivery to brain tumors. J Control Release. 2016;232:103–112. doi: 10.1016/j.jconrel.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. Fabrication and characterization of microfluidic probes for convection enhanced drug delivery. J Control Release. 2006;111(3):252–262. doi: 10.1016/j.jconrel.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, Sampson JH, Ram Z, Gutin PH, Gibbons RD, Aldape KD, et al. Direct intracerebral delivery of cintredekin besudotox (il13-pe38qqr) in recurrent malignant glioma: A report by the cintredekin besudotox intraparenchymal study group. J Clin Oncol. 2007;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 61.Sampson JH, Archer G, Pedain C, Wembacher-Schroder E, Westphal M, Kunwar S, Vogelbaum MA, Coan A, Herndon JE, Raghavan R, Brady ML, et al. Poor drug distribution as a possible explanation for the results of the precise trial. J Neurosurg. 2010;113(2):301–309. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 62.Mehta AI, Linninger A, Lesniak MS, Engelhard HH. Current status of intratumoral therapy for glioblastoma. J Neurooncol. 2015;125(1):1–7. doi: 10.1007/s11060-015-1875-1. [DOI] [PubMed] [Google Scholar]
- 63.Barua NU, Hopkins K, Woolley M, O’Sullivan S, Harrison R, Edwards RJ, Bienemann AS, Wyatt MJ, Arshad A, Gill SS. A novel implantable catheter system with transcutaneous port for intermittent convection-enhanced delivery of carboplatin for recurrent glioblastoma. Drug Delivery. 2016;23(1):167–173. doi: 10.3109/10717544.2014.908248. [DOI] [PubMed] [Google Scholar]
- 64.Tosi U, Marnell CS, Chang R, Cho WC, Ting R, Maachani UB, Souweidane MM. Advances in molecular imaging of locally delivered targeted therapeutics for central nervous system tumors. Int J Mol Sci. 2017;18(2):351. doi: 10.3390/ijms18020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (bcnu) wafers (gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yen SY, Chen SR, Hsieh J, Li YS, Chuang SE, Chuang HM, Huang MH, Lin SZ, Harn HJ, Chiou TW. Biodegradable interstitial release polymer loading a novel small molecule targeting axl receptor tyrosine kinase and reducing brain tumour migration and invasion. Oncogene. 2016;35(17):2156–2165. doi: 10.1038/onc.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •67.Xu HL, Mao KL, Lu CT, Fan ZL, Yang JJ, Xu J, Chen PP, ZhuGe DL, Shen BX, Jin BH, Xiao J, et al. An injectable acellular matrix scaffold with absorbable permeable nanoparticles improves the therapeutic effects of docetaxel on glioblastoma. Biomaterials. 2016;107:44–60. doi: 10.1016/j.biomaterials.2016.08.026. Docetaxel-loaded nanoparticles incorporated into an injectable scaffold exhibited slower drug release and longer retention, enabled tumor penetration, and resulted in suppression of glioma growth in rats after intratumoral administration. [DOI] [PubMed] [Google Scholar]
- 68.Bastiancich C, Vanvarenberg K, Ucakar B, Pitorre M, Bastiat G, Lagarce F, Preat V, Danhier F. Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J Control Release. 2016;225:283–293. doi: 10.1016/j.jconrel.2016.01.054. [DOI] [PubMed] [Google Scholar]
- •69.Fourniols T, Randolph LD, Staub A, Vanvarenberg K, Leprince JG, Preat V, des Rieux A, Danhier F. Temozolomide-loaded photopolymerizable peg-dma-based hydrogel for the treatment of glioblastoma. J Control Release. 2015;210:95–104. doi: 10.1016/j.jconrel.2015.05.272. An injectable PEG-DMA-based hydrogel loaded with temozolomide and capable of in situ photopolymerization was shown to exhibit sustained drug release, good biocompability in the brain, and a potent in vivo anti-tumor effect. [DOI] [PubMed] [Google Scholar]
- 70.Vellimana AK, Recinos VR, Hwang L, Fowers KD, Li KW, Zhang Y, Okonma S, Eberhart CG, Brem H, Tyler BM. Combination of paclitaxel thermal gel depot with temozolomide and radiotherapy significantly prolongs survival in an experimental rodent glioma model. J Neurooncol. 2013;111(3):229–236. doi: 10.1007/s11060-012-1014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ozeki T, Kaneko D, Hashizawa K, Imai Y, Tagami T, Okada H. Improvement of survival in c6 rat glioma model by a sustained drug release from localized plga microspheres in a thermoreversible hydrogel. Int J Pharm. 2012;427(2):299–304. doi: 10.1016/j.ijpharm.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1):146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 73.Van Woensel M, Wauthoz N, Rosiere R, Mathieu V, Kiss R, Lefranc F, Steelant B, Dilissen E, Van Gool SW, Mathivet T, Gerhardt H, et al. Development of sirna-loaded chitosan nanoparticles targeting galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J Control Release. 2016;227:71–81. doi: 10.1016/j.jconrel.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 74.Van Woensel M, Mathivet T, Wauthoz N, Rosiere R, Garg AD, Agostinis P, Mathieu V, Kiss R, Lefranc F, Boon L, Belmans J, et al. Sensitization of glioblastoma tumor micro-environment to chemo- and immunotherapy by galectin-1 intranasal knock-down strategy. Sci Rep. 2017;7(1):1217. doi: 10.1038/s41598-017-01279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joshi S, Cooke JR, Chan DK, Ellis JA, Hossain SS, Singh-Moon RP, Wang M, Bigio IJ, Bruce JN, Straubinger RM. Liposome size and charge optimization for intraarterial delivery to gliomas. Drug Deliv Transl Res. 2016;6(3):225–233. doi: 10.1007/s13346-016-0294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Purow B. The elephant in the room: Do microrna-based therapies have a realistic chance of succeeding for brain tumors such as glioblastoma? J Neurooncol. 2011;103(3):429–436. doi: 10.1007/s11060-010-0449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shea A, Harish V, Afzal Z, Chijioke J, Kedir H, Dusmatova S, Roy A, Ramalinga M, Harris B, Blancato J, Verma M, et al. Micrornas in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016;5(8):1917–1946. doi: 10.1002/cam4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krichevsky AM, Gabriely G. Mir-21: A small multi-faceted rna. J Cell Mol Med. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M. A systematic review of microrna in glioblastoma multiforme: Micro-modulators in the mesenchymal mode of migration and invasion. Mol Neurobiol. 2013;47(1):131–144. doi: 10.1007/s12035-012-8349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee ST, Chu K, Oh HJ, Im WS, Lim JY, Kim SK, Park CK, Jung KH, Lee SK, Kim M, Roh JK. Let-7 microrna inhibits the proliferation of human glioblastoma cells. J Neurooncol. 2011;102(1):19–24. doi: 10.1007/s11060-010-0286-6. [DOI] [PubMed] [Google Scholar]
- 81.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport rna and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 83.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9(1):16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 84.Ung N, Yang I. Nanotechnology to augment immunotherapy for the treatment of glioblastoma multiforme. J Neurooncol. 2015;123(3):473–481. doi: 10.1007/s11060-015-1814-1. [DOI] [PubMed] [Google Scholar]
- 85.Leleux J, Roy K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: An immunological and materials perspective. Adv Healthc Mater. 2013;2(1):72–94. doi: 10.1002/adhm.201200268. [DOI] [PubMed] [Google Scholar]
- 86.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M, Kurscheid S, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17(8):1064–1075. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishikawa E, Yamamoto T, Matsumura A. Prospect of immunotherapy for glioblastoma: Tumor vaccine, immune checkpoint inhibitors and combination therapy. Neurol Med Chir (Tokyo) 2017 doi: 10.2176/nmc.ra.2016-0334. advpub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, et al. Anti-pd-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sampson JH, Vlahovic G, Desjardins A, Friedman HS, Baehring JM, Hafler D, Rollin L, Coric V, Perez SN, Reardon DA. Randomized phase iib study of nivolumab (anti-pd-1; bms-936558, ono-4538) alone or in combination with ipilimumab versus bevacizumab in patients (pts) with recurrent glioblastoma (gbm) Journal of Clinical Oncology. 2014;32(15_suppl):TPS2101–TPS2101. [Google Scholar]
- 90.Sampson JH, Omuro AMP, Preusser M, Lim M, Butowski NA, Cloughesy TF, Strauss LC, Latek RR, Paliwal P, Weller M, Reardon DA. A randomized, phase 3, open-label study of nivolumab versus temozolomide (tmz) in combination with radiotherapy (rt) in adult patients (pts) with newly diagnosed, o-6-methylguanine DNA methyltransferase (mgmt)-unmethylated glioblastoma (gbm): Checkmate-498. Journal of Clinical Oncology. 2016;34(15_suppl):TPS2079–TPS2079. [Google Scholar]
- 91.Miyazaki T, Ishikawa E, Matsuda M, Akutsu H, Osuka S, Sakamoto N, Takano S, Yamamoto T, Tsuboi K, Matsumura A. Assessment of pd-1 positive cells on initial and secondary resected tumor specimens of newly diagnosed glioblastoma and its implications on patient outcome. J Neurooncol. 2017:1–9. doi: 10.1007/s11060-017-2451-7. [DOI] [PubMed] [Google Scholar]
- 92.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, et al. Loss of tumor suppressor pten function increases b7-h1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 93.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choy W, Nagasawa DT, Trang A, Thill K, Spasic M, Yang I. Cd133 as a marker for regulation and potential for targeted therapies in glioblastoma multiforme. Neurosurg Clin N Am. 2012;23(3):391–405. doi: 10.1016/j.nec.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 95.Patel MA, Kim JE, Ruzevick J, Li G, Lim M. The future of glioblastoma therapy: Synergism of standard of care and immunotherapy. Cancers (Basel) 2014;6(4):1953–1985. doi: 10.3390/cancers6041953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fan X, Nelson BD, Ai Y, Stiles DK, Gash DM, Hardy PA, Zhang Z. Continuous intraputamenal convection-enhanced delivery in adult rhesus macaques. J Neurosurg. 2015;123(6):1569–1577. doi: 10.3171/2015.1.JNS132345. [DOI] [PubMed] [Google Scholar]
- 97.Han SJ, Bankiewicz K, Butowski NA, Larson PS, Aghi MK. Interventional mri-guided catheter placement and real time drug delivery to the central nervous system. Expert Rev Neurother. 2016;16(6):635–639. doi: 10.1080/14737175.2016.1175939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raghavan R, Brady M. Predictive models for pressure-driven fluid infusions into brain parenchyma. Phys Med Biol. 2011;56(19):6179–6204. doi: 10.1088/0031-9155/56/19/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sampson JH, Raghavan R, Brady ML, Provenzale JM, Herndon JE, 2nd, Croteau D, Friedman AH, Reardon DA, Coleman RE, Wong T, Bigner DD, et al. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro Oncol. 2007;9(3):343–353. doi: 10.1215/15228517-2007-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •100.Bishop CJ, Majewski RL, Guiriba TR, Wilson DR, Bhise NS, Quinones-Hinojosa A, Green JJ. Quantification of cellular and nuclear uptake rates of polymeric gene delivery nanoparticles and DNA plasmids via flow cytometry. Acta Biomater. 2016;37:120–130. doi: 10.1016/j.actbio.2016.03.036. This work describes the development of a quantitative flow cytometry-based assay and a four- compartment first order mass-action kinetics model, which can be used to assess cellular and nuclear uptake rates of non-viral gene delivery nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H. Egfrviii antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70(15):6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]