Abstract
Auxin triggers diverse responses in plants, and this is reflected in quantitative and qualitative diversity in the auxin signaling machinery.
Auxin acts as a general coordinator of plant growth and development, transferring information over both long and short ranges. Auxin famously appears to be extraordinarily multifunctional, with different cells responding very differently to changes in auxin levels. There has been considerable progress over recent years in understanding how this complexity is encoded in the cellular auxin response machinery. Of central importance is an elegantly short but versatile signaling pathway through which auxin triggers changes in gene expression. However, it is increasingly clear that this pathway is not sufficient to explain all auxin responses, and other auxin signaling systems are emerging.
AUXIN BY ANALOGY
Apparently, I have been writing reviews about auxin for 20 years (Leyser, 1997), and certainly I have been in very good company. Auxin is a much-written-about molecule. Entire books are dedicated to it (Estelle et al., 2011). From all these column inches, there is a clear consensus that auxin is extremely important, that it is involved in virtually every aspect of plant biology, and that this baffling array of functions makes the task of understanding auxin daunting to say the least.
Many very helpful analogies have been developed in an attempt to provide an intellectual framework within which auxin biology can be adequately encapsulated. A major driver for these analogies is to move away from the constraints of auxin as a specific instructive signal triggering specific and universal outcomes (Weyers and Paterson, 2001). This idea is clearly inappropriate as far as auxin is concerned, but nonetheless somehow inveigles its way into the field due to prevalent paradigms in signaling biology in general and hormone biology in particular. For auxin, it is increasingly clear that the specificity in the system is not in the signal but in the cells that perceive it. Auxin does not instruct cells to do anything in particular, but rather it influences the behavior of cells according to their preexisting identity. For example, I have previously suggested that auxin is like the baton wielded by the conductor of an orchestra: “When the auxin baton points your way, it’s your turn to play whatever musical instrument you happen to be holding” (Leyser, 2005, p. 821).
This analogy is of course deficient in many ways. In particular, it brings into sharp relief the second and perhaps more important challenge that must be embraced to understand auxin. Although auxin can trigger very specific changes in cells, this seldom involves step changes in auxin concentration. The question of how auxin works is not the question of what happens when some auxin arrives at a cell. There is always auxin. The absolute and relative amount of auxin at any one location in the plant varies over time, and this tunes and retunes the balance within a set of interlocking feedback loops operating at subcellular, cellular, tissue, organ, and whole-plant scales. Within the orchestral analogy, maybe some of this can be captured by considering the dynamics of the music. The baton is not a play-don’t play switch but can also be used to modulate how loudly to play. But even with this addition, the analogy does not allow sufficiently for feedback, with the baton being modulated by the players. Maybe if there is an unruly player in the orchestra, the baton might become a little more insistent, and certainly an orchestral performance is a collaboration between the conductor and the players, with information flow mediated by the baton and its behavior. The same musical score can be interpreted differently by different conductor-orchestra combinations. Nonetheless, in this scenario, there is no doubt who is in charge. It’s the conductor. In a plant, there is no central control. Rather, all aspects of auxin biology are characterized by emergent self-organizing properties that allow distributed rather than centralized decision making.
These considerations have led to another prevalent analogy or, rather, a useful comparison (Leyser, 2016). Many of the functions performed by the auxin network in plants are fulfilled by the nervous system in animals. Here, a very nonspecific signal, an electrical impulse, carries information through the organism modulating diverse outputs depending on the receiving tissue. There is extensive feedback, and in the brain in particular, dynamic self-organization with competition and reinforcement rewiring neural connections depending on their frequency of use. However, there is also the obvious and glaring difference, already mentioned above, of central versus distributed processing. This difference is often discussed in the context of the heterotrophic versus autotrophic life styles of animals versus plants. While heterotrophy is supported by locomotion allowing long distance foraging for food, autotrophy on land requires large surface areas for acquisition of dilute resources such as light, carbon dioxide, and water. This latter involves large underground surface areas, precluding locomotion. As a consequence, plants are unable to escape predation by locomotion and so must be robust to loss of body parts. Central processing—a brain—is poorly suited to this requirement and instead plants typically have no unique parts.
Addressing these issues in their review, Stewart and Nemhauser argue that auxin is a cellular currency: “…think of it as money. Auxin does not have much intrinsic value—it stores very little energy or raw materials. However, like paper currency, it has great symbolic value, as an easily circulated means of facilitating transactions in the dynamic economy of plant life” (Stewart and Nemhauser, 2010, p. 1). Like auxin, money does everything, but what it does depends on who gives it to whom and under what circumstances. And the economics analogy certainly offers plenty of potential for feedback and complex self-organizing dynamics.
The money analogy is also interesting from an evolutionary perspective. Money was invented as a proxy for the things people really need or want, to allow diverse goods and services to be more readily exchanged in more complex and less direct ways than a one-on-one swap, for example, of eggs for bread. This is potentially an interesting way to think about auxin. It is metabolically close to an amino acid, Trp, and amino acid availability via limitations in nitrogen supply is an important constraint on plant growth (Elser et al., 2007). Auxin could plausibly have evolved from a mechanism directly linking amino acid availability to growth. In the context of the increasing complexity of multicellular plant form, with division of labor between different cell and tissue types, a direct local link between resource availability and growth does not allow the necessary coordination and prioritization of growth across the organism. For example, in most seed plants, shoots capture carbon and roots capture mineral nutrients such as nitrate. Growth of the root and shoot systems must therefore be prioritized depending on the C:N ratio in the plant, rather than the local availability of either fixed carbon or nitrogen. The requirement to coordinate growth, not just regulate it, necessitates dedicated signaling systems that can operate both systemically and locally. As a general description, auxin is one such growth coordinator—regulating where, when, how much, and what sort of growth should occur. We have previously argued that the apparent diversity of auxin action makes sense in this context (Bennett and Leyser, 2014). An early origin as a general growth coordinator could be followed by cooption to modulate wider coordinated activities. Once money has been invented, it can be used and reused to buy things that did not previously exist. In this review, I will focus on how auxin as a currency is traded at the cellular level, or put rather less fancifully, how cells recognize and use information about changes in the amount of auxin present.
AUXIN AND THE REGULATION OF TRANSCRIPTION
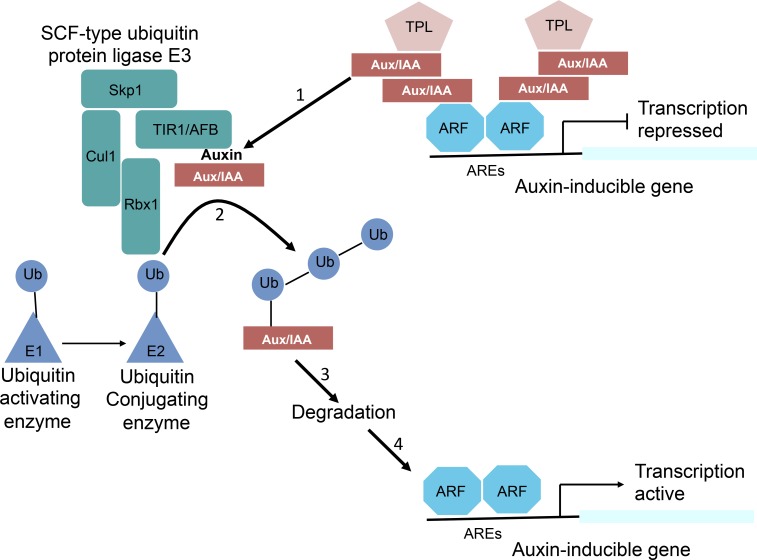
The major mechanism by which changes in auxin levels are converted into cellular responses is via changes in transcription. Hundreds of genes change their expression rapidly in response to exogenous auxin supply (Paponov et al., 2008). Auxin regulates transcription via an elegantly short signal transduction pathway, which has been extensively reviewed (Chapman and Estelle, 2009; Salehin et al., 2015) and is illustrated in Figure 1. In brief, auxin acts as molecular glue bringing together F-box proteins of the TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX (TIR1/AFB) family and members of the Aux/IAA transcriptional repressor family (Tan et al., 2007). F-box proteins are the substrate selection subunit of SCF-type ubiquitin protein ligase complexes, named after three of their subunits: Skp1, Cullin, and an F-box protein (Smalle and Vierstra, 2004). The F-box itself is a motif at the N-terminal end of F-box proteins through which they interact with Skp1, which also interacts with a dimer of Cullin and RBX1. This dimer transfers activated ubiquitin from a ubiquitin activating enzyme and conjugates it to target proteins. The target protein is brought to the SCF by its interaction with the C-terminal domain of the F-box protein. In the case of TIR1/AFBs, this consists of Leu-rich repeats including an auxin binding pocket (Tan et al., 2007). The binding of auxin in this pocket is greatly stabilized by the docking of the Aux/IAA protein across the pocket mouth, mediated by a short protein motif in the Aux/IAA known as domain II (Tan et al., 2007). For this reason, TIR1/AFB- Aux/IAA pairs can be considered as coreceptors for auxin. The auxin-mediated binding of Aux/IAAs to TIR1/AFBs brings them to the SCF, allowing their ubiquitination and subsequent degradation (Gray et al., 2001; Maraschin et al., 2009). In this way, changes in auxin levels are converted into changes in Aux/IAA levels.
Figure 1.
The main pathway for regulation of transcription by auxin. Auxin-inducible genes have AREs in their promoters, which are bound by dimers of the ARF protein family. Gene expression is prevented by recruitment of Aux/IAA transcriptional repressors to these promoters via their interaction with the ARFs. Aux/IAAs recruit TPL family corepressors, which in turn recruit chromatin modifying enzymes (not shown) that stabilize the repressed state. The steps in the auxin response pathway are indicated by the numbered arrows. 1, Auxin acts as a molecular glue bringing together Aux/IAAs and F-box proteins of the TIR1/AFB family. 2, These F-box proteins are part of an SCF-type E3 ubiquitin protein ligase complex that transfers activated ubiquitin (Ub) from an E1/E2 enzyme system. 3, Polyubiquitination of the Aux/IAAs results in their degradation. 4, This releases repression at ARE-containing promoters.
There are 29 Aux/IAAs in Arabidopsis (Arabidopsis thaliana; Paponov et al., 2008). Their half-lives and the extent to which their half-lives reduce in response to applied auxin vary greatly (Dreher et al., 2006). An important determinant of these characteristics is the sequence of domain II. This sequence acts as a degron and is sufficient to confer auxin-triggered destruction on heterologous proteins (Zenser et al., 2001). Aux/IAA half-lives also depend on sequences outside the degron and the AFB with which the Aux/IAA interacts (Moss et al., 2015). There are six AFBs in Arabidopsis (Dharmasiri et al., 2005; Prigge et al., 2016). Different TIR1/AFB-Aux/IAA pairs have very different affinities for each other and for different auxins, contributing to the wide range of Aux/IAA half-lives and their auxin sensitivities (Calderón Villalobos et al., 2012). Reconstitution of the auxin response pathway in yeast demonstrates the diversity in dynamics that can be achieved through this mechanism, such that the same change in auxin input can result in a wide range of changes in Aux/IAA output (Havens et al., 2012). There is evidence that this variation is functionally significant in planta. For example, in Arabidopsis the Aux/IAA protein IAA14 is involved in the early stages of lateral root development. Stabilized auxin-insensitive versions of IAA14 are well known to block lateral root emergence completely. Point mutations in this protein have been engineered to produce variants, which in partnership with TIR1 have approximately 10 and 100 times lower affinity for auxin (Guseman et al., 2015). This increases their half-lives in response to auxin addition from approximately 20 min to more than 2 h. When these variants are expressed in plants, they delay lateral root emergence in proportion to their half-lives (Guseman et al., 2015).
Aux/IAA proteins act as transcriptional repressors (Ulmasov et al., 1997). They contain a conserved EAR domain through which they can recruit corepressors of the TOPLESS (TPL) family to promoters (Tiwari et al., 2004; Szemenyei et al., 2008). In turn, TPLs can recruit chromatin remodeling proteins that stabilize transcriptional repression (Szemenyei et al., 2008). The Aux/IAAs do not themselves bind DNA, but they can dimerize with transcription factors of the AUXIN RESPONSE FACTOR (ARF) family. Dimerization occurs through C-terminal PB1 domains shared by both protein families (Guilfoyle, 2015). The PB1 domain has an acidic and a basic interaction surface through which dimerization can occur. This arrangement can support Aux/IAA oligomerization, like a stack of Lego bricks (Korasick et al., 2014; Nanao et al., 2014; Dinesh et al., 2015). Point mutations along one face of IAA19 that allow Aux/IAA dimerization with ARFs but do not support subsequent oligomerization with additional Aux/IAAs are nonfunctional in some assays, suggesting that these higher order complexes can contribute to effective transcriptional repression (Korasick et al., 2014). However, transcription can be repressed without such higher order complex formation (Pierre-Jerome et al., 2016).
ARF proteins can also homodimerize through their N-terminal B3 domains, and it is through this homotypic dimerization that they cooperatively bind DNA (Boer et al., 2014). ARFs bind to specific Auxin Response Elements (AREs) with the consensus sequence TGTCTC in the promoters of auxin-regulated genes (Abel and Theologis, 1996; Mironova et al., 2014). A subset of ARFs include a Q-rich middle domain between the B3 and PB1 domains, and these ARFs can act as transcriptional activators through recruitment of chromatin remodeling enzymes (Ulmasov et al., 1999; Wu et al., 2015). Oligomerization with Aux/IAAs prevents this activation. Auxin modulates the level of expression from ARF-bound promoters by triggering Aux/IAA degradation.
Since the ability of Aux/IAAs to modulate transcription is entirely dependent on ARFs, the presence of different ARF complements in different cells can also contribute to auxin signaling specificity (Rademacher et al., 2012; Bargmann et al., 2013). There are 23 different ARFs in Arabidopsis and evidence for differential affinities between specific ARFs and Aux/IAAs (Vernoux et al., 2011). Different homo and hetero oligomerizations of Aux/IAAs at the promoter may add an additional mechanism for auxin response diversity (Knox et al., 2003; Rademacher et al., 2012; Bargmann et al., 2013). For palindromic AREs, there is also evidence for different affinities between different dimerized ARFs and ARE-containing promoters, based on the spacing of the ARE palindrome (Boer et al., 2014). Interestingly, many ARFs lack the Q-rich region (Ulmasov et al., 1999). They bind the same AREs, and in some cases there is good evidence that they act as transcriptional repressors (Ulmasov et al., 1999). It is likely that these repressor ARFs compete with activator ARFs for occupancy of the same promoters (Vert et al., 2008). The complement of repressive ARFs in a cell therefore provides an additional mechanism by which different cells might respond differently to auxin. It is also possible that these various alternative protein-DNA and protein-protein interactions at the promoters of auxin-regulated genes are further influenced by protein-protein interactions off the promoter. For example, off-promoter Aux/IAA oligomerizations could sequester specific Aux/IAAs, preventing their interactions with ARFs.
This system allows extremely rapid changes in transcription in response to auxin. Changes in transcript abundance can be detected within 3 to 5 min of auxin treatments (McClure et al., 1989; Abel and Theologis, 1996). The half-life of many Aux/IAAs is very short even in the absence of exogenously applied auxin (Abel et al., 1994), and the genes encoding Aux/IAA proteins are themselves rapidly up-regulated in response to auxin (Abel and Theologis, 1996). Thus, cells continuously make and degrade Aux/IAAs with the flux of Aux/IAAs through this cycle modulated by auxin, potentially reequilibrating the system at different steady states depending on the auxin concentration. Consistent with this behavior, auxin signaling is particularly sensitive to mutation in apparently generic parts of the protein degradation and protein synthesis machinery (del Pozo et al., 2002; Gray et al., 2003; Hellmann et al., 2003; Chuang et al., 2004; Stirnberg et al., 2012). This powerfully illustrates the mismatch between auxin signaling and the classical on-off switch paradigm. Auxin modulates transcription through retuning the equilibrium in a highly dynamic, feedback-regulated network. The system is capable of acting as a bistable switch, but it has a much wider range of possible behaviors, and even stable high and low expression states are dependent on flux through the Aux/IAA synthesis-degradation cycle (Bridge et al., 2012).
Another important feature of this system is that auxin acts by degrading a non-DNA binding inhibitor of transcription. From an evolutionary perspective, this immediately suggests the hypothesis that the ability of auxin to regulate transcription could have been added on to an existing system of transcriptional regulation. A completely auxin-independent system, consisting only of activator and repressor ARFs competing for the same promoters with some mechanism for changing their relative abundance, could provide a way to modulate transcription from multiple genes, for example, tuning growth in response to environmental factors. Adding in Aux/IAA-mediated transcriptional repression and auxin-triggered Aux/IAA degradation would make these genes auxin responsive in the presence of activator ARFs. Some evidence to support this order of events in the evolution of auxin signaling comes from recent work on basal land plant species: the liverwort Marchantia polymorpha and the moss Physcomitrella patens.
The Marchantia genome has only three ARFs, one Aux/IAA, and one TIR1/AFB. Evidence to date supports the idea that this system works in the same way as in angiosperms, described above (Flores-Sandoval et al., 2015; Kato et al., 2015). The ARFs fall into three distinct clades covering both activator and repressor ARFs, all three of which are represented in angiosperm genomes (Kato et al., 2015). This three-clade structure is therefore the likely ancestral state for land plants. Analysis of gain-of-function and reduced function mutants in these components has identified diverse auxin-regulated aspects of growth and development such as cell expansion in dorsal epidermal tissues of the Marchantia thallus (Flores-Sandoval et al., 2015; Kato et al., 2015).
In P. patens, there are 16 ARFs, distributed across the three clades described above, and three Aux/IAAs (Prigge et al., 2010). Recently, a Physcomitrella line completely lacking all these Aux/IAAs has been generated (Lavy et al., 2016). These plants lack any detectable transcriptional response to auxin and phenotypically resemble moss plants treated with high levels of auxin. The analysis of this line arguably provides some support for the idea that auxin signaling evolved as a refinement of a system based on environmental control of the ratio of repressive and activating ARFs. First, overexpression of a repressive ARF in the triple Aux/IAA mutant background suppresses its constitutive high auxin phenotype, and indeed confers a phenotype similar to that conditioned by expression of stabilized auxin-resistant Aux/IAA protein variants (Lavy et al., 2016). This suggests that repressive and activating ARFs do indeed compete for access to the same promoters, and shifting the ratios between them can coordinately regulate transcription from these promoters.
Second, examination of the auxin-Aux/IAA-ARF-regulated transcriptome in moss shows that the gene set down-regulated by auxin and loss of Aux/IAAs is enriched for genes involved in light responses, photosynthesis, and carbon fixation, while the up-regulated gene set is enriched for genes involved in transcription and biosynthesis (Lavy et al., 2016). This correlates with the ability of auxin to suppress the production of the chloroplast-rich chloronemal moss filaments and promote the production of relatively chloroplast-poor caulonemal filaments, which extend the moss colony. Auxin can therefore be seen as switching the moss colony from prioritizing energy and carbon capture to prioritizing growth and expansion. This might be associated with differences in the C:N ratio, and could ancestrally have been driven, for example, by light regulation of ARFs, perhaps modulated in some way by nitrogen availability. Consistent with this speculative idea, like auxin, high light prioritizes caulonemal over chloronemal growth (Thelander et al., 2005). However, unlike light, auxin is potentially mobile in the plant and hence can coordinate growth systemically. As described above, an early origin as a general growth coordinator could be followed by cooption to modulate and coordinate a wider range of activities across the plant body, consistent with the lethality often observed in higher plant auxin signaling mutants, for example, lacking multiple members of the TIR1/AFB family (Dharmasiri et al., 2005).
In this context, the recent discovery of an additional ARF-dependent auxin-sensing mechanism is of particular interest. ETTIN is a noncanonical ARF that lacks the PB1 Aux/IAA interaction domain. Its best characterized role is in regulating patterning in the developing gynoecium (Sessions et al., 1997; Nemhauser et al., 2000). Here, ETTIN dimerizes with the basic helix-loop-helix transcription factor INDEHISCENT (IND) to regulate transcription of key target genes (Simonini et al., 2016). This dimerization is affected by auxin binding, and this correlates with auxin-dependent changes in the association of ETTIN with the promoters of target genes and auxin-dependent changes in the expression of these targets (Simonini et al., 2016). This ability of auxin to modulate the interaction between ETTIN and partner transcription factors extends beyond IND, and indeed could be quite widespread (Simonini et al., 2016). It will be interesting to explore the evolutionary origin of this mechanism for auxin-regulated transcription based on a noncanonical ARF.
IS THAT ALL THERE IS?
The TIR1/AFB-Aux/IAA-ARF signaling system provides plenty of scope for diversity in auxin response, and the dramatic phenotypes of mutants compromised in this pathway attest to its importance in the coordination of plant growth and development.
This begs the question as to whether this is the only auxin detection system in plants. Several arguments have been put forward in support of the existence of additional auxin response systems. However, many of these can easily be accommodated by the TIR1/AFB-Aux/IAA-ARF system. One argument that there are multiple systems comes from the very different auxin sensitivities of different auxin responses, both with respect to their dose-response kinetics and their specificity for different auxins. For example, a much-studied classical response to auxin is its ability to drive cell elongation in hypocotyls, epicotyls, and coleoptiles, all of which must elongate rapidly during early seedling establishment after seed germination. This response is complex. In pea (Pisum sativum), the natural auxin indole-3-acetic acid (IAA) has a biphasic dose response curve with maxima in the 1 μm and 1 mm ranges (Yamagami et al., 2004). Meanwhile, the dose response curve for the synthetic auxin 1-naphthaleneacetic acid (NAA) has only one maximum in the 10 μm range (Yamagami et al., 2004). Of these response maxima, only the high-affinity IAA response is strongly sensitive to Ca2+ availability, while the others are not (Yamagami et al., 2004). This kind of diversity has been used to argue for two distinct perception mechanisms with different affinities for IAA and NAA. However, the diversity in auxin sensitivity of TIR1/AFB-Aux/IAA pairs could account for these phenomena given the wide range of Kms described for different auxins and different AFB-Aux/IAA pairs (Calderón Villalobos et al., 2012). The basis for the differences in Ca2+ sensitivity is less straightforward to accommodate through the AFB-Aux/IAA system.
While the downstream effectors driving these elongation responses are a matter for debate, there is substantial evidence supporting a role for various ion fluxes across the plasma membrane (Rück et al., 1993). Because the activity of diverse membrane transporters is regulated posttranscriptionally, this is a second argument that has fueled speculation about additional response pathways and, specifically, nontranscriptional auxin effects. For example, auxin-induced elongation is associated with stimulation of the plasma membrane proton-pumping ATPase (PM H+ ATPase), thereby acidifying the apoplast (Hager, 2003; Takahashi et al., 2012). According to the acid growth hypothesis, this in turn affects cell wall extensibility leading to turgor-driven elongation (Kutschera, 1994). This response is quite slow and so could reasonably result from the very rapid changes in gene expression triggered by auxin (Badescu and Napier, 2006). Indeed, recent data support this idea. In particular, there is mounting evidence that auxin stimulates the activity of the PM H+ ATPase by up-regulating the transcription of members of the SMALL AUXIN UP-REGULATED RNA (SAUR) gene family via the canonical TIR1/AFB-Aux/IAA-ARF pathway (Chae et al., 2012; Spartz et al., 2012).
As their name suggests, the SAUR genes were originally identified because many of them are rapidly up-regulated in response to auxin (Abel and Theologis, 1996). The promoters of the auxin-up-regulated SAUR family members include the classical ARE ARF binding motif, and changes in SAUR transcript abundance can be detected within 5 min of auxin application (McClure et al., 1989; Abel and Theologis, 1996). SAUR genes are plant-specific and typically present as multigene families, making genetic analysis difficult. However, the use of artificial microRNAs to target multiple SAURs simultaneously, together with gain-of-function studies, has demonstrated that in Arabidopsis the SAUR19-SAUR24 subfamily are positive regulators of cell expansion in diverse tissues, including the hypocotyl (Spartz et al., 2012).
This work has been greatly helped by the discovery that fusion proteins between these SAURs and a wide range of tags result in the stabilization of the proteins, providing overexpression lines (Spartz et al., 2012). Arabidopsis lines overexpressing tagged SAUR19 have long hypocotyls due to increased cell expansion. This phenotype is associated with reduced apoplastic pH, which is significantly attenuated in PM H+ ATPase mutant backgrounds (Spartz et al., 2014). Consistent with these results, the SAUR19 overaccumulating lines show constitutive activity of the PM H+ ATPase, associated with increased phosphorylation of Thr- 947 within the pump’s C-terminal autoinhibitory domain. Phosphorylation at this site is known to activate the PM H+ ATPase by driving recruitment of a 14-3-3 protein, which binds to the domain, alleviating its inhibitory influence (Fuglsang et al., 1999; Kanczewska et al., 2005). Fluorescent tags demonstrate that SAUR19 can localize to the plasma membrane (Spartz et al., 2012). Furthermore, SAUR19 and other SAUR family members interact directly with type 2C protein phosphatase D (PP2C-D), inhibiting its activity, and this PP2C-D interacts with and inhibits the activity of the PM H+ ATPase (Spartz et al., 2014; Sun et al., 2016). These results support a clear chain of events through which auxin regulates PM H+ ATPase activity via transcriptional up-regulation of SAUR19, which directly inhibits PP2C-D activity, leading to the accumulation of active Thr-947 phosphorylated PM H+ ATPase, apoplast acidification, cell wall loosening, and growth. In support of this model, Arabidopsis SAUR19 and SAUR9 can inhibit tomato PP2C-Ds, and overexpression of Arabidopsis SAUR19 in tomato bypasses the requirement for auxin addition to trigger increased cell wall extensibility and elongation in excised hypocotyl segments (Spartz et al., 2017). Consistent with these results, acid growth in Arabidopsis hypocotyls is dependent on the TIR1/AFB-Aux/IAA-ARF signaling system (Fendrych et al., 2016).
These results provide direct evidence that the TIR1/AFB-Aux/IAA-ARF transcriptional pathway for auxin response regulates PM H+ ATPase activity and that this contributes to the ability of auxin to promote elongation. As mentioned above, the response times for acidification and growth in these tissues are in the order of 10 to 30 min, concordant with this idea. However, in other situations much more rapid changes in ion fluxes and growth are observed.
A particularly convincing example comes from high spatiotemporal resolution analyses of root responses to auxin. Addition of physiologically relevant concentrations of auxin to Arabidopsis roots results in a dose-dependent influx of Ca2+ across the plasma membrane, increasing cytosolic Ca2+ concentrations (Monshausen et al., 2011). This response occurs within 10 s and is accompanied by apoplastic alkalinization, which can be measured as a change in root surface pH. Pretreatment with the Ca2+ channel blocker La3+ inhibits both the Ca2+ and pH responses, suggesting that the pH changes are dependent on the increase in cytosolic Ca2+.
Such rapid changes in ion fluxes have been detected previously in guard cells and particularly in protoplast systems where their physiological significance has remained unclear (Rück et al., 1993; Blatt and Thiel, 1994). The high level of spatiotemporal resolution now possible in growing roots, coupled with the power of Arabidopsis genetics, has provided robust evidence linking these fluxes to growth. Both the rapid auxin-induced cytosolic Ca2+ elevation and the apoplastic alkalinization have been shown to depend on a cyclic nucleotide-gated channel, CNGC14 (Shih et al., 2015). Both responses are completely abolished in cngc14 loss-of-function mutants (Shih et al., 2015). Members of this family are known to transport Ca2+, and although this protein accumulates only to very low levels in root tips, it can be detected at the plasma membrane in at least some cell types (Shih et al., 2015). Crucially, auxin-induced root growth inhibition is significantly delayed in cngc14 roots (Shih et al., 2015). In wild-type roots, a significant reduction in elongation rate across the elongation zone can be detected within 1 min of auxin addition. In cngc14 mutants, it is not until 7 min after auxin application that the rate of root growth slows significantly. These data suggest that auxin stimulates the activity of CNGC14 triggering Ca2+ influx, which in turn results in cell wall alkalinization, inhibiting cell elongation.
This idea is supported by analysis of the root gravity response, bypassing the possibility of any artifacts associated with the use of applied auxin (Shih et al., 2015). The ability of roots to reorient their growth in response to changes in the gravity vector is dependent on the asymmetric redistribution of auxin at the root tip. Auxin transported toward the tip in the central stele is recirculated back up the root through the lateral root cap and root epidermis (Luschnig et al., 1998; Müller et al., 1998; Swarup et al., 2005). This pattern of auxin transport is mediated by polarly localized transporters of the PIN-FORMED (PIN) family (Blilou et al., 2005). In the columella root cap, auxin arriving through central tissues is transported laterally by PIN3, where it enters the peripheral shootward transport system. Tight control over the flow of auxin through these tissues is achieved by rapid removal from the apoplast mediated by the AUX/LAX family of auxin importers (Swarup et al., 2005). Upon gravistimulation detected in the columella root cap, PIN3 is polarized in the root cap cells, directing auxin disproportionately to the lower root surface, resulting in asymmetric distribution of auxin across the root and consequent asymmetric growth (Friml et al., 2002; Harrison and Masson, 2008).
Current methods for live imaging of auxin concentrations are not sufficiently sensitive to detect directly this auxin asymmetry rapidly after gravistimulation (Band et al., 2012). The best available method at present is based on the auxin-triggered degradation of a fluorescent reporter protein fused to a relatively long half-life Aux/IAA degron (Vernoux et al., 2011). A long half-life degron is required to allow sufficient accumulation of the fluorescent protein for detection, but comes at the expense of temporal resolution. Using a parameterized model for degradation of this protein in response to auxin, it has been estimated that asymmetry is established at 5 min after gravistimulation (Band et al., 2012). This is broadly consistent with the asymmetric distribution of Ca2+, which was detected within 3 to 6 min of gravistimulation, with higher levels on the lower surface of the root than the upper surface (Shih et al., 2015). Similarly, when auxin is applied locally at the root tip, a wave of elevated cytosolic Ca2+ is detected, moving shootward at a rate 150 to 600 μm/min, consistent with typical rates of polar auxin transport (Shih et al., 2015). That this wave is auxin-mediated is supported by the fact that it is dependent on the auxin influx carrier AUX1. In the case of gravistimulation, the pH at the upper surface reduces and at the lower surface increases with similar kinetics. These rapid changes in ion flux correlate with differential growth, which can be detected after 4 min. In the cgnc14 mutant, all three of these effects are delayed by approximately 6 min (Shih et al., 2015).
These data provide compelling evidence for a mode of auxin action too rapid for the transcriptional pathway. A 10-s response timeframe does not seem plausible for even the extremely rapid changes in gene expression elicited by auxin. Consistent with this idea, the ion flux changes are unaffected in a mutant lacking three of the TIR1/AFB auxin receptors (Shih et al., 2015), which is severely compromised in the transcriptional response system (Dharmasiri et al., 2005). In this context it is important to note that gravitropic root growth is only very mildly affected in cgnc14 mutant roots but is strongly compromised in mutants defective in the transcriptional response system. This suggests that, while the CNG14-dependent response contributes to the very rapid initiation of root reorientation, the transcriptional system is required for longer timeframe, sustained reorientation responses.
The nontranscriptional detection of auxin extends further the timeframes over which cells can directly sense changes in auxin concentrations from seconds to many hours. The importance of these diverse response times is beautifully illustrated at the root tip. Here, there is good evidence that a tip-high gradient of auxin patterns root development (Sabatini et al., 1999; Galinha et al., 2007; Mähönen et al., 2014), but also fine-tunes growth rates, as in the example of gravitropism (Luschnig et al., 1998; Müller et al., 1998; Swarup et al., 2005). A cluster of initial cells form a stem cell niche at the root tip, where the auxin concentration is high (Sabatini et al., 1999). These cells give rise to a rapidly dividing population of cells that make up the division zone. On exiting the cell cycle, the cells expand rapidly, forming an elongation zone, after which they mature and differentiate, for example, as root hairs in the differentiation zone. There is good evidence that the rate of progress of cells through these stages is dependent on the tip-high auxin concentration gradient mentioned above but interpreted indirectly through the auxin-regulated expression of a set of transcription factors of the PLETHORA family (Galinha et al., 2007; Mähönen et al., 2014). These proteins are expressed in response to the high auxin concentrations of the root tip. They are very stable, persisting through cell divisions. As a result, a stable gradient of PLETHORA forms, buffered against rapid changes in auxin concentration, for example, due to gravistimulated auxin redistribution at the root tip (Mähönen et al., 2014). Meanwhile, as described above, these transient fluctuations in auxin level can be interpreted over very short timescales by calcium spiking, and over intermediate timescales by rapid primary transcriptional changes, supporting dynamic responses in root growth rate and direction. Thus, multiple direct and indirect auxin-sensing systems operating over different timescales deliver robust multiscale growth coordination.
A POLARIZING ISSUE
The existence of a nontranscriptional auxin signaling system is also supported by several theoretical considerations. Transcriptional regulation has only a limited ability to account for the apparent importance of auxin in polarity and polarization. There is a considerable body of evidence that auxin is intimately involved in the regulation of polarity. The mechanisms by which auxin contributes to cellular and tissue level polarity are poorly understood, and consequently they are currently a very active area of research. In some cases, it seems likely that auxin acts to allow proteins to respond to polarizing cues provided by other systems. For example, auxin, acting through its transcriptional pathway, influences polar PIN accumulation and activity (Hazak et al., 2010). However, there is also evidence that auxin can contribute to the polarizing cue itself.
Here again, in some cases, the canonical TIR1/AFB-Aux/IAA-ARF pathway can account for auxin-mediated polarization phenomena. For example, organ primordia at the shoot apical meristem are formed at sites of locally high auxin (Reinhardt et al., 2003; Heisler et al., 2005). Auxin accumulates at these maxima through the action of specific members of the PIN family of auxin efflux carrier. In the meristem epidermis, these PINs are polarized toward the neighboring cell inferred from auxin response reporters to have the highest auxin concentration, in a so-called “up the gradient” pattern (Reinhardt et al., 2003; Heisler et al., 2005; Jönsson et al., 2006; Smith et al., 2006). One mechanism that has been proposed to account for this polarization is based on the ability of cells to detect differences in physical forces across the cell wall (Heisler et al., 2010). If auxin promotes cell expansion in the meristem epidermis, then the increased expansion of cells with higher auxin levels than their neighbors will create tensile stresses in the cell wall adjacent to the expanding cell. If PIN proteins are delivered or retained in the plasma membrane in proportion to adjacent cell wall stresses, then this could drive up-the-gradient PIN polarization, as observed. In this way, nuclear auxin signaling, working via auxin-regulated gene expression, could regulate polarization of neighboring cells.
There is now strong evidence to suggest that this noncell-autonomous polarization depends on the TIR1/AFB-Aux/IAA-ARF system (Bhatia et al., 2016). The ARF MONOPTEROS (MP) is a central player in patterning organ emergence at the shoot apical meristem. Meristems deficient in MP are barren, producing no organs, nor can they be induced to produce organs by local auxin application (Reinhardt et al., 2000). When small clones of wild-type MP were induced in such an mp mutant background, PIN1 polarized toward the clones and organ-like outgrowths were initiated at these sites (Bhatia et al., 2016). This strongly suggests auxin signaling via the TIR1/AFB-Aux/IAA-ARF system can act as a polarizing cue for neighboring cells in the shoot apical meristem. This could act via the wall stress mechanism described above, but any directional signal from the MP-expressing cells could contribute to orienting PINs in neighboring cells.
However, there are other examples of auxin-regulated cell polarization that are more difficult to explain using only nuclear auxin signaling. Cellular polarization fundamentally requires symmetry breaking, and within a cell it is difficult to see how this could be accomplished by changes in transcription alone. During organ formation at the shoot apical meristem, the role of auxin is noncell autonomous, with asymmetry established in the polarizing cell by auxin signaling in its neighbors. There are situations where this type of explanation is more difficult to reconcile with the observed phenomena.
A good example is the positioning of Arabidopsis root hairs. As described above, various assays suggest that there is a tip-high gradient of auxin that patterns the Arabidopsis root, regulating the transition of cells between the division, elongation, and differentiation zones. This gradient also appears to play a role in positioning root hairs. Root epidermal cells elongate highly anisotropically in the elongation zone. In trichoblast cell files, upon exit from the elongation zone, root hairs develop as tip-growing projections from the epidermal cells (Nakamura et al., 2012). The hairs are positioned close to, but a little way back from, the rootward end of the trichoblast. The site of root hair development is predicted by accumulation of a patch of the type I Rho of Plants (ROP) GTPase protein on the plasma membrane (Molendijk et al., 2001; Jones et al., 2002). This patch acts as an organizing center for the cytoskeletal changes associated with root hair development.
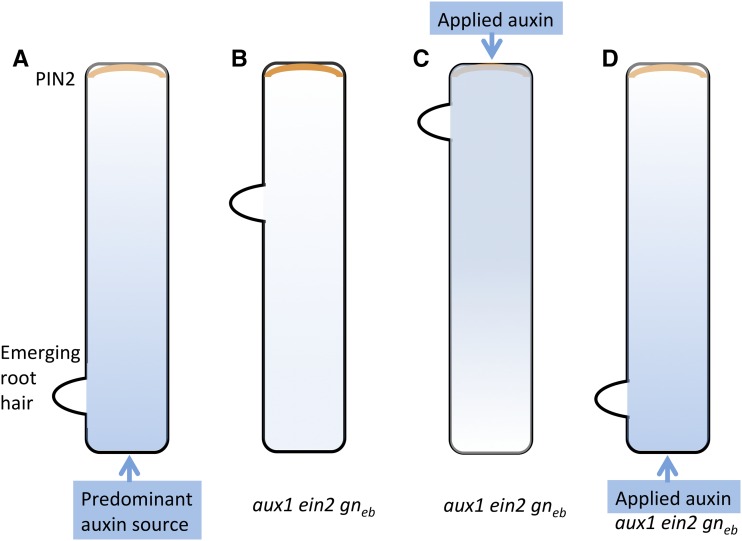
The positioning of the ROP patch and therefore the root hair along the trichoblast can be shifted by manipulation of the auxin gradient along the root (Fig. 2; Fischer et al., 2006; Ikeda et al., 2009). For example, when this gradient is flattened as in the aux1 ethylene-insensitive2 (ein2) gnomeb (gneb) triple mutant, root hairs emerge at variable positions along the trichoblast cells. Although the hairs can emerge at any position, there is a bias toward either the rootward or the shootward end of the cell. Correct, rootward positioning can be restored by application of auxin rootward of the differentiation zone (Fischer et al., 2006). Strikingly, root hair positioning can be biased to the shootward end by application of auxin shootward of the differentiating hair (Fig. 2). These data support the hypothesis that an auxin gradient contributes to the positioning of the root hair, and consistent with this idea, mutants with defects in root tip auxin biosynthesis have a shootward shift in root hair positioning (Ikeda et al., 2009).
Figure 2.
Hypothetical intracellular auxin gradients and root hair position. A, Epidermal cells in trichoblast cell files of the Arabidopsis root produce a root hair toward the rootward end of the cell. This is patterned by a root-tip-high auxin gradient, which is predicted to feed a tip-high intracellular auxin gradient in trichoblasts (blue shading), reinforced by shootward localization of the PIN2 auxin exporter (orange line). B, Triple mutation in aux1 ein2 gneb flattens the tip-high auxin gradient and presumably also the intracellular auxin gradient. This is associated with shifts in root hair placement. C, Application of auxin to aux1 ein2 gneb mutants shootward of the root hair differentiation zone shifts the root hair position to the shootward end of the cell, presumably associated with an inverted intracellular auxin gradient. D, Application of auxin to aux1 ein2 gneb mutants rootward of the root hair differentiation zone restores a more wild-type root hair position, presumably associated with restoration of the tip-high intracellular auxin gradient.
Manipulation of ROP activity can also affect the position of root hair emergence. For example, overexpression of ROPs, or expression of a constitutively active form, can trigger the development of multiple root hairs per cell (Jones et al., 2002). Combined, this phenomenology, and particularly the wide range of root hair positions observed in response to auxin and ROP manipulation, is difficult to explain with a system in which polarization is driven noncell autonomously from neighboring cells.
An attractive hypothesis has been proposed to explain these data. At its core is the ability of a gradient to stabilize a self-organizing Turing pattern. Turing patterns emerge from a regulatory architecture involving a slow diffusing activator that promotes its own activity, combined with a faster diffusing inhibitor of this activity (Turing, 1952). Type I ROPs could act in a Turing system since upon activation they can be S-acylated and recruited into lipid rafts (Sorek et al., 2007). Thus, in their inactive form they may diffuse much more rapidly than in their active state. With the assumption of autocatalytic activation, this can create patches of active ROPs on the membrane (Payne and Grierson, 2009). However, to position the patch at a specific site along the cell, additional information is needed, and this is hypothesized to derive from the auxin gradient. If local auxin concentration tunes, for example, the rate of autocatalytic activation of ROPs, then the site(s) of ROP nucleation can depend on local auxin concentration (Payne and Grierson, 2009). Consistent with this idea, there is some evidence that auxin can rapidly activate ROPs in a dose-dependent manner (Tao et al., 2002; Xu et al., 2010). This can be detected using antibodies specific to the active form of ROPs. Active ROPs are known to regulate cytoskeletal organization, for example, via RIC1 and RIC4 (Yalovsky et al., 2008), which could coordinate the events necessary for root hair emergence.
This model is compelling because of its impressive ability to generate the patterns of root hair emergence observed as a result of various genetic and pharmacological manipulations by mechanistically plausible model parameter changes. The idea that symmetry breaking to generate a root hair relies on a self-organizing Turing-like patterning system for ROPs has an evidence base, particularly with respect to polarization events in slime mold and animal cells (Jilkine et al., 2007; Otsuji et al., 2007), as well as from more general considerations of the requirements for self-organizing polarity. The role of auxin is simply to bias this system, effectively providing a quantitative positional cue. In particular, the model involves sensing an intracellular, tip-high auxin gradient in each trichoblast. Such a gradient is predicted to be fed by the macroscopic tip-high auxin gradient but patterned substantially by the polar shootward localization of PIN2 efflux carriers in each trichoblast cell (Grieneisen et al., 2007). The active efflux of auxin from the shootward end of these cells is predicted to deplete the adjacent cytoplasm of auxin, establishing a diffusion-limited auxin gradient along the cell. Under this hypothesis, the aux1 ein2 gneb triple mutant could reduce the steepness of the intracellular gradient by reducing cellular auxin supply and uptake (Fig. 2). Unfortunately, it is not currently possible to measure intracellular gradients, so these ideas cannot be directly tested.
If root hair position is indeed determined by this mechanism, auxin’s role is effectively to convert an inherent apical-basal polarity axis, reflected in PIN2 polarity, into a new lateral growth axis for root hair emergence. However, intracellular auxin gradients have also been proposed as a mechanism to regulate polar PIN protein distribution itself, raising the possibility of feedback between PIN-generated auxin gradients, and PIN polarity. This idea is particularly interesting in the context of auxin transport canalization. Auxin transport canalization is a hypothesis proposed by Tsvi Sachs to account for a variety of observations associated with the regulation of vascular strand patterning by auxin (Sachs, 1968, 1969, 1981). Vascular strands develop between auxin sources, such as young expanding leaves, and auxin sinks, such as existing vascular strands, which include files of cells with highly polar rootward auxin transport. Vascular strand development is preceded by the emergence of files of cells expressing highly polar PIN transporters connecting the auxin source to the sink (Sauer et al., 2006; Scarpella et al., 2006; Sawchuk et al., 2013). This can be explained if an initial passive flux of auxin between the source and the sink is up-regulated and polarized in the direction of the sink by the accumulation of PIN proteins at the plasma membrane in proportion to net auxin flux across the membrane (Mitchison, 1980; Sachs, 1981; Rolland-Lagan and Prusinkiewicz, 2005). Although this idea can account for a wide range of observations, the mechanism by which PIN proteins can be allocated in proportion to flux (so-called “with the flux” polarization) is entirely obscure (Bennett et al., 2014). One mechanism that has been proposed to account for this is the allocation of PIN proteins to membranes dependent on intracellular auxin gradients (Kramer, 2009). Modeling has demonstrated that if cells with an intracellular auxin gradient greater than 1% allocate PINs to the plasma membrane adjacent to the lowest cytoplasmic auxin concentration, this can contribute to the positive feedback necessary to drive canalization of auxin transport between an auxin source and a sink. Integral to this feedback is the amplification of the intracellular gradient by PIN polarization.
Alternative or additional explanations for flux-correlated PIN allocation involve positive feedback between PIN accumulation and PIN activity, modified by extracellular auxin concentration (Cieslak et al., 2015). This requires an extracellular auxin sensor of some kind, as well as the ability of PIN proteins to act, in effect, as auxin sensors, for example, if PIN activity inhibits their removal from the plasma membrane. Interestingly, this kind of dual-sensing system can generate either with-the-flux type patterns of PIN accumulation or up-the-gradient type patterns depending on whether extracellular auxin decreases or increases local PIN accumulation (Cieslak et al., 2015).
An additional mechanism has been reported that can generate either up-the-gradient or with-the-flux type patterns of PIN polarization. This involves an auxin-biased spontaneous polarization mechanism similar to that described above for ROPs but operating at the whole-cell level to create a single axis of polarity across the cell rather than a small patch (Abley et al., 2013). If this axis is oriented by extracellular auxin, even a very shallow auxin gradient can create coordinated cell polarity across a tissue oriented from high extracellular auxin, as might be expected at an auxin source, to low extracellular auxin, as might be expected at an auxin sink. Since polarization depends on extracellular auxin, orientation of PINs toward cells with high intracellular auxin, as in up-the-gradient polarization patterns, can be achieved if these cells express high levels of auxin importers and therefore have low extracellular auxin. This is consistent with the observation that up-the-gradient PIN polarization is correlated with, and in some cases dependent on, expression of auxin importers of the Aux/LAX family (Abley et al., 2016). Unfortunately, as for intracellular auxin gradients, there is currently no way to measure extracellular auxin concentration at the level of resolution necessary to assess correlations with PIN accumulation.
Together, these considerations suggest that additional auxin-sensing mechanisms beyond transcription are necessary to explain the full range of auxin activities. These could include an intracellular sensor that can detect intracellular gradients and/or an extracellular auxin sensor. In both cases, these could act to bias self-organizing polarization systems, such as ROP partitioning. There is some evidence that auxin can regulate ROP activity and thus ROP partitioning (Tao et al., 2002; Xu et al., 2010). The detection systems involved are poorly understood but a small family of plasma membrane-localized receptor kinases have been implicated (Xu et al., 2014). Similarly, the relationship between these hypothetical auxin detection systems and the rapid auxin-induced calcium transients described above is also unclear.
OTHER AUXIN RECEPTORS
Given the evidence for auxin signaling beyond the TIR1/AFB-Aux/IAA-ARF system, including nontranscriptional effects, there should be additional auxin receptors/sensors. Indeed, several have been proposed, with varying degrees of support and varying levels of evidence for their functional significance. Prominent among them is Auxin Binding Protein1 (ABP1), which has been a constant and controversial feature on the auxin signaling landscape throughout the full 20 years I have been writing reviews about auxin. As its name suggests, ABP1 was originally identified biochemically through its ability to bind auxin (Löbler and Klämbt, 1985a, 1985b). A substantial body of data on the biochemistry of ABP1 has accumulated since, including its crystal structure (Woo et al., 2002). ABP1 binds the natural auxin IAA with a Kd of 5 to 10 μm (Napier, 1995). It has a much higher affinity, in the region of 100 nm, for the synthetic auxin NAA. Binding of NAA to ABP1 is highly pH sensitive, with an optimum of 5.0 to 5.5, with very little binding at pH 7. The cell biology of ABP1 has also been studied quite intensively because the majority of the protein is retained in the endoplasmic reticulum (ER), where the pH is such that auxin binding is predicted to be weak (Napier, 1997; Klode et al., 2011). Although there is considerable speculation about the role of ER-localized auxin in nuclear and cytoplasmic auxin homeostasis (Friml and Jones, 2010), there is currently no evidence that auxin signals from the ER. However, some ABP1 appears to escape ER retention and is secreted, where the pH matches better the auxin binding maximum (Jones and Herman, 1993; Napier, 1997).
The analysis of protoplast auxin responses provides some evidence that ABP1 might function as an extracellular auxin receptor (Rück et al., 1993; Steffens et al., 2001; Badescu and Napier, 2006). For example, protoplasts derived from pea epicotyl epidermal cells swell slightly in response to IAA addition. Measured 90 min after auxin treatment, this response is biphasic with maxima at around 1 and 10 μm. The dose response curve for NAA has only a single maximum at around 1 μm. When these assays are performed in the presence of antibodies against ABP1, the response to NAA is completely abolished, and the response to IAA is simplified, with a single peak retained at 1 μm (Yamagami et al., 2004; Badescu and Napier, 2006). One interpretation of these results is that auxin can induce protoplast swelling by two different mechanisms, one of which is ABP1 dependent.
However, while these responses are intriguing, their physiological significance is unclear. Robust in planta analysis requires genetic resources, and it is here that the ABP1 story becomes most problematic. Various methods have been used to assess the effects of modulating ABP1 activity in planta. These include conventional antisense expression, as well as overexpression of both the native protein and a mutant version lacking the ER retention sequence (Jones et al., 1998; Braun et al., 2008; Tromas et al., 2009; Robert et al., 2010). In addition, lines overexpressing the anti-ABP1 antibody have also been widely used, again either ER retained or secreted (Venis et al., 1992; Leblanc et al., 1999; Braun et al., 2008; Tromas et al., 2009; Robert et al., 2010). These approaches come with caveats because, for example, antibodies might have off-target effects, and overexpression is prone to neomorphism artifacts. The use of these lines has been a mainstay of ABP1 research because of initial suggestions that its stable loss of function resulted in early embryonic lethality, precluding the use of clean null mutants for analyzing the postembryonic roles of ABP1 (Chen et al., 2001). To circumvent this problem, TILLING was used to isolate weaker alleles, including the abp1-5 allele, which carries a point mutation in the auxin binding pocket (Robert et al., 2010; Xu et al., 2010). This was predicted to prevent auxin binding without markedly altering other ABP1 properties.
A number of auxin response defects have been reported for these ABP1-perturbed lines. Most have focused on cellular-level responses, such as inhibition of endocytosis (Robert et al., 2010; Chen et al., 2012) and microtubule reorientation (Chen et al., 2014). These phenotypes have been linked to ROP activity, which as described above may be auxin regulated. ROPs, auxin, and ABP1 have also all been implicated in pavement cell morphogenesis in the leaf (Xu et al., 2010).
However, the interpretation of these results has been called into question by the identification of new null mutant alleles in the ABP1 gene (Gao et al., 2015). These mutations have no obvious phenotypic differences compared with wild-type plants. Meanwhile, the lethality attributed to loss of ABP1 in the originally analyzed line results from mutation in a closely linked gene (Michalko et al., 2015). Thus, rather than being an essential gene, ABP1 is apparently completely dispensable for normal plant growth and development under lab conditions. Compounding the problem, an isolate of the abp1-5 allele was found to include thousands of polymorphisms compared with the parental genetic background, as well as a chromosomal segment derived from a different background, consistent with errors in the backcrossing regime that followed the isolation of this allele (Enders et al., 2015). As a result, the phenotypic differences between this line and its wild-type previously attributed to the abp1-5 allele may in fact result from other mutations in the background. Together, these results require a major reexamination of the evidence supporting a functionally significant role for ABP1, including in endocytosis, cytoskeletal arrangement, and ROP modulation. Clean null mutants are now available, and assessment of these auxin responses in the new mutant background will establish whether they require ABP1.
ABP1 is highly conserved across the plant kingdom, although interestingly it is apparently missing from the M. polymorpha genome (Kato et al., 2015). Nonetheless, this suggests that it confers a significant selective advantage, despite the fact that it is not required for the auxin-regulated processes so far examined, and to the extent to which they have been examined in abp1 null mutants. It may be that either fully parallel systems and/or different auxin response systems regulate the same processes, resulting in functional redundancy. This is the case for auxin-regulated root growth described above. Here, the very earliest stages of gravitropic bending depend on auxin-stimulated Ca2+ influx, mediated independently of the TIR1/AFB-Aux/IAA-ARF system. However, the slightly later effects of the TIR1/AFB-Aux/IAA-ARF system on gravitropism all but mask the loss of the nontranscriptional pathway at a macroscopic level. For this response, however, auxin-stimulated Ca2+ influx has been assessed in an abp1 null mutant background and found to be normal (Shih et al., 2015).
It is noteworthy that for many of the cell biological responses reported as being ABP1-dependent, associated whole-plant level phenotypes have not been examined in much detail, and where they have, relatively modest effects are typically reported, despite strong effects reported for the cellular level (Robert et al., 2010; Chen et al., 2014; Baskin, 2015). This is consistent with the idea that these cell biological responses are either not very important at the organismal level, or there is sufficient redundancy in their regulation to mask any morphological effects of ABP1 manipulation. There are, however, some examples of strong morphological effects from perturbed ABP1 levels, including standard ABP1 antisense expression (Braun et al., 2008; Tromas et al., 2009). These experiments involve inducible antisense expression, raising the possibility that sudden changes in ABP1 levels have more significant effects than stable loss of function. This could be consistent with one or more redundant compensating systems requiring time to reequilibrate in response to ABP1 loss.
Whatever the role of ABP1, it is clear that it is not sufficient to explain the known and/or strongly suspected nontranscriptional effects of auxin, for example, on root Ca2+ spiking. There must therefore be other auxin perception systems. There are certainly additional biochemically identified auxin binding proteins, although there is very limited evidence that they play any role in auxin responses (Napier and Venis, 1995). Perhaps the best-supported auxin receptor mediating an at least partially nontranscriptional auxin response is the Arabidopsis S-Phase Kinase-Associated Protein2A (SKP2A). SKP2A is an F-box protein with structural similarities to the TIR1 family (Jurado et al., 2008, 2010). It regulates cell cycle progression by promoting degradation of cell cycle transcriptional regulators including E2FC and DPB (del Pozo et al., 2006). Auxin has been shown to trigger these degradative events as well as trigger degradation of SKP2A itself (Jurado et al., 2010). Structural modeling against TIR1 suggested that auxin could bind directly to SKP2A, and this was experimentally validated. Furthermore, mutation of the predicted auxin binding site compromised auxin binding, as well as auxin-induced destabilization of SKP2A and interaction with DPB. These data directly link auxin to cell cycle progression, and the functional significance of this link is supported by the auxin-resistant root growth phenotype of plants in overexpressing SPK2A that is unable to bind auxin.
SUMMARY AND PERSPECTIVES
Auxin can be considered as a general coordinator of growth and development, used and reused throughout the life cycle of plants to mediate communication between cells and tissues at short and long ranges. How this information is decoded at the receiving tissues is unsurprisingly complex. Relevant information is present in the absolute as well as spatially and temporally relative levels of auxin. The appropriate response to all this information depends on myriad other factors, requiring an extensive and highly tunable information-processing system in every cell. The TIR1/AFB-Aux/IAA-ARF system provides impressive power to deliver the necessary response properties, but there is mounting evidence that it cannot and does not account for all auxin responses. Additional auxin binding activities, for example, mediated by ETTIN and SKP2A, have been linked to specific downstream responses, and specific auxin responses unlikely to be mediated by known auxin reception systems have been identified, such as root Ca2+ transients and root hair positioning. Evolutionary approaches are a promising route for the characterization of these auxin responses, and integration of computational modeling continues to make important contributions. However, limitations in currently available in vivo auxin detection systems are a major constraint on progress.
Footnotes
O.L.’s research is funded by the Gatsby Charitable Foundation, the European Research Council, and the UK Biotechnology and Biological Sciences Research Council.
Articles can be viewed without a subscription.
References
- Abel S, Oeller PW, Theologis A (1994) Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci USA 91: 326–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel S, Theologis A (1996) Early genes and auxin action. Plant Physiol 111: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abley K, De Reuille PB, Strutt D, Bangham A, Prusinkiewicz P, Marée AF, Grieneisen VA, Coen E (2013) An intracellular partitioning-based framework for tissue cell polarity in plants and animals. Development 140: 2061–2074 [DOI] [PubMed] [Google Scholar]
- Abley K, Sauret-Güeto S, Marée AFM, Coen E (2016) Formation of polarity convergences underlying shoot outgrowths. eLife 5: e18165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badescu GO, Napier RM (2006) Receptors for auxin: Will it all end in TIRs? Trends Plant Sci 11: 217–223 [DOI] [PubMed] [Google Scholar]
- Band LR, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Péret B, et al. (2012) Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci USA 109: 4668–4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann BO, Vanneste S, Krouk G, Nawy T, Efroni I, Shani E, Choe G, Friml J, Bergmann DC, Estelle M, et al. (2013) A map of cell type-specific auxin responses. Mol Syst Biol 9: 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. (2015) Auxin inhibits expansion rate independently of cortical microtubules. Trends Plant Sci 20: 471–472 [DOI] [PubMed] [Google Scholar]
- Bennett T, Hines G, Leyser O (2014) Canalization: what the flux? Trends Genet 30: 41–48 [DOI] [PubMed] [Google Scholar]
- Bennett T, Leyser O (2014) The auxin question: a philosophical overview. In Zažímalová E, Petrasek J, Benkova E, eds, Auxin and Its Role in Plant Development. Springer, Berlin, pp 3–20 [Google Scholar]
- Bhatia N, Bozorg B, Larsson A, Ohno C, Jönsson H, Heisler MG (2016) Auxin acts through MONOPTEROS to regulate plant cell polarity and pattern phyllotaxis. Curr Biol 26: 3202–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Thiel G (1994) K+ channels of stomatal guard cells: bimodal control of the K+ inward-rectifier evoked by auxin. Plant J 5: 55–68 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Boer DR, Freire-Rios A, van den Berg WA, Saaki T, Manfield IW, Kepinski S, López-Vidrieo I, Franco-Zorrilla JM, de Vries SC, Solano R, et al. (2014) Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156: 577–589 [DOI] [PubMed] [Google Scholar]
- Braun N, Wyrzykowska J, Muller P, David K, Couch D, Perrot-Rechenmann C, Fleming AJ (2008) Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell 20: 2746–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge LJ, Mirams GR, Kieffer ML, King JR, Kepinski S (2012) Distinguishing possible mechanisms for auxin-mediated developmental control in Arabidopsis: models with two Aux/IAA and ARF proteins, and two target gene-sets. Math Biosci 235: 32–44 [DOI] [PubMed] [Google Scholar]
- Calderón Villalobos LI, Lee S, De Oliveira C, Ivetac A, Brandt W, Armitage L, Sheard LB, Tan X, Parry G, Mao H, et al. (2012) A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat Chem Biol 8: 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Isaacs CG, Reeves PH, Maloney GS, Muday GK, Nagpal P, Reed JW (2012) Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J 71: 684–697 [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M (2009) Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet 43: 265–285 [DOI] [PubMed] [Google Scholar]
- Chen JG, Ullah H, Young JC, Sussman MR, Jones AM (2001) ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev 15: 902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Grandont L, Li H, Hauschild R, Paque S, Abuzeineh A, Rakusová H, Benková E, Perrot-Rechenmann C, Friml J (2014) Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature 516: 90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Naramoto S, Robert S, Tejos R, Löfke C, Lin D, Yang Z, Friml J (2012) ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr Biol 22: 1326–1332 [DOI] [PubMed] [Google Scholar]
- Chuang HW, Zhang W, Gray WM (2004) Arabidopsis ETA2, an apparent ortholog of the human cullin-interacting protein CAND1, is required for auxin responses mediated by the SCFTIR1 ubiquitin ligase. Plant Cell 16: 1883–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslak M, Runions A, Prusinkiewicz P (2015) Auxin-driven patterning with unidirectional fluxes. J Exp Bot 66: 5083–5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Dharmasiri S, Hellmann H, Walker L, Gray WM, Estelle M (2002) AXR1-ECR1-dependent conjugation of RUB1 to the Arabidopsis Cullin AtCUL1 is required for auxin response. Plant Cell 14: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C (2006) The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 18: 2224–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9: 109–119 [DOI] [PubMed] [Google Scholar]
- Dinesh DC, Kovermann M, Gopalswamy M, Hellmuth A, Calderón Villalobos LI, Lilie H, Balbach J, Abel S (2015) Solution structure of the PsIAA4 oligomerization domain reveals interaction modes for transcription factors in early auxin response. Proc Natl Acad Sci USA 112: 6230–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher KA, Brown J, Saw RE, Callis J (2006) The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 18: 699–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10: 1135–1142 [DOI] [PubMed] [Google Scholar]
- Enders TA, Oh S, Yang Z, Montgomery BL, Strader LC (2015) Genome sequencing of Arabidopsis abp1-5 reveals second-site mutations that may affect phenotypes. Plant Cell 27: 1820–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle M, Weijers D, Ljung K, Leyser O, editors (2011) Auxin Signaling: From Synthesis to Systems Biology. Cold Spring Harbor Press, Cold Spring Harbor, NY [Google Scholar]
- Fendrych M, Leung J, Friml J (2016) TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife 5: e19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Ikeda Y, Ljung K, Serralbo O, Singh M, Heidstra R, Palme K, Scheres B, Grebe M (2006) Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr Biol 16: 2143–2149 [DOI] [PubMed] [Google Scholar]
- Flores-Sandoval E, Eklund DM, Bowman JL (2015) A simple auxin transcriptional response system regulates multiple morphogenetic processes in the liverwort Marchantia polymorpha. PLoS Genet 11: e1005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Jones AR (2010) Endoplasmic reticulum: the rising compartment in auxin biology. Plant Physiol 154: 458–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG (1999) Binding of 14-3-3 protein to the plasma membrane H(+)-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J Biol Chem 274: 36774–36780 [DOI] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y (2015) Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci USA 112: 2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Gray WM, Muskett PR, Chuang HW, Parker JE (2003) Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell 15: 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Marée AF, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449: 1008–1013 [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ. (2015) The PB1 domain in auxin response factor and Aux/IAA proteins: a versatile protein interaction module in the auxin response. Plant Cell 27: 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseman JM, Hellmuth A, Lanctot A, Feldman TP, Moss BL, Klavins E, Calderón Villalobos LI, Nemhauser JL (2015) Auxin-induced degradation dynamics set the pace for lateral root development. Development 142: 905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A. (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116: 483–505 [DOI] [PubMed] [Google Scholar]
- Harrison BR, Masson PH (2008) ARL2, ARG1 and PIN3 define a gravity signal transduction pathway in root statocytes. Plant J 53: 380–392 [DOI] [PubMed] [Google Scholar]
- Havens KA, Guseman JM, Jang SS, Pierre-Jerome E, Bolten N, Klavins E, Nemhauser JL (2012) A synthetic approach reveals extensive tunability of auxin signaling. Plant Physiol 160: 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazak O, Bloch D, Poraty L, Sternberg H, Zhang J, Friml J, Yalovsky S (2010) A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol 8: e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler MG, Hamant O, Krupinski P, Uyttewaal M, Ohno C, Jönsson H, Traas J, Meyerowitz EM (2010) Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol 8: e1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15: 1899–1911 [DOI] [PubMed] [Google Scholar]
- Hellmann H, Hobbie L, Chapman A, Dharmasiri S, Dharmasiri N, del Pozo C, Reinhardt D, Estelle M (2003) Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J 22: 3314–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Men S, Fischer U, Stepanova AN, Alonso JM, Ljung K, Grebe M (2009) Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat Cell Biol 11: 731–738 [DOI] [PubMed] [Google Scholar]
- Jilkine A, Marée AF, Edelstein-Keshet L (2007) Mathematical model for spatial segregation of the Rho-family GTPases based on inhibitory crosstalk. Bull Math Biol 69: 1943–1978 [DOI] [PubMed] [Google Scholar]
- Jones AM, Herman EM (1993) KDEL-containing auxin-binding protein is secreted to the plasma membrane and cell wall. Plant Physiol 101: 595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Im KH, Savka MA, Wu MJ, DeWitt NG, Shillito R, Binns AN (1998) Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 282: 1114–1117 [DOI] [PubMed] [Google Scholar]
- Jones MA, Shen J-J, Fu Y, Li H, Yang Z, Grierson CS (2002) The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E (2006) An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci USA 103: 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S, Abraham Z, Manzano C, López-Torrejón G, Pacios LF, Del Pozo JC (2010) The Arabidopsis cell cycle F-box protein SKP2A binds to auxin. Plant Cell 22: 3891–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S, Díaz-Triviño S, Abraham Z, Manzano C, Gutierrez C, del Pozo C (2008) SKP2A, an F-box protein that regulates cell division, is degraded via the ubiquitin pathway. Plant J 53: 828–841 [DOI] [PubMed] [Google Scholar]
- Kanczewska J, Marco S, Vandermeeren C, Maudoux O, Rigaud JL, Boutry M (2005) Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimer into a hexamer. Proc Natl Acad Sci USA 102: 11675–11680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Ishizaki K, Kouno M, Shirakawa M, Bowman JL, Nishihama R, Kohchi T (2015) Auxin-mediated transcriptional system with a minimal set of components is critical for morphogenesis through the life cycle in Marchantia polymorpha. PLoS Genet 11: e1005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klode M, Dahlke RI, Sauter M, Steffens B (2011) Expression and subcellular localization of Arabidopsis thaliana auxin-binding protein 1 (ABP1). J Plant Growth Regul 30: 416–424 [Google Scholar]
- Knox K, Grierson CS, Leyser O (2003) AXR3 and SHY2 interact to regulate root hair development. Development 130: 5769–5777 [DOI] [PubMed] [Google Scholar]
- Korasick DA, Westfall CS, Lee SG, Nanao MH, Dumas R, Hagen G, Guilfoyle TJ, Jez JM, Strader LC (2014) Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc Natl Acad Sci USA 111: 5427–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM. (2009) Auxin-regulated cell polarity: an inside job? Trends Plant Sci 14: 242–247 [DOI] [PubMed] [Google Scholar]
- Kutschera U. (1994) The current status of the acid-growth hypothesis. New Phytol 126: 549–569 [Google Scholar]
- Lavy M, Prigge MJ, Tao S, Shain S, Kuo A, Kirchsteiger K, Estelle M (2016) Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. eLife 5: e13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N, David K, Grosclaude J, Pradier JM, Barbier-Brygoo H, Labiau S, Perrot-Rechenmann C (1999) A novel immunological approach establishes that the auxin-binding protein, Nt-abp1, is an element involved in auxin signaling at the plasma membrane. J Biol Chem 274: 28314–28320 [DOI] [PubMed] [Google Scholar]
- Leyser HMO. (1997) Auxin: lessons from a mutant weed. Physiol Plant 100: 407–414 [Google Scholar]
- Leyser O. (2005) Auxin distribution and plant pattern formation: How many angels can dance on the point of PIN? Cell 121: 819–822 [DOI] [PubMed] [Google Scholar]
- Leyser O. (2016) Angiosperm multicellularity: the whole, the parts and the sum. In KJ Niklas, SA Newman, eds, The Evolution and Consequences of Multicellularity. Vienna Series of Theoretical Biology. MIT Press, Cambridge, MA, pp 87–102 [Google Scholar]
- Löbler M, Klämbt D (1985a) Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). I. Purification by immunological methods and characterization. J Biol Chem 260: 9848–9853 [PubMed] [Google Scholar]
- Löbler M, Klämbt D (1985b) Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). II. Localization of a putative auxin receptor. J Biol Chem 260: 9854–9859 [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mähönen AP, Ten Tusscher K, Siligato R, Smetana O, Díaz-Triviño S, Salojärvi J, Wachsman G, Prasad K, Heidstra R, Scheres B (2014) PLETHORA gradient formation mechanism separates auxin responses. Nature 515: 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraschin FdosS, Memelink J, Offringa R (2009) Auxin-induced, SCF(TIR1)-mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J 59: 100–109 [DOI] [PubMed] [Google Scholar]
- McClure BA, Hagen G, Brown CS, Gee MA, Guilfoyle TJ (1989) Transcription, organization, and sequence of an auxin-regulated gene cluster in soybean. Plant Cell 1: 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalko J, Dravecká M, Bollenbach T, Friml J (2015) Embryo-lethal phenotypes in early abp1 mutants are due to disruption of the neighboring BSM gene. F1000 Res 4: 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironova VV, Omelyanchuk NA, Wiebe DS, Levitsky VG (2014) Computational analysis of auxin responsive elements in the Arabidopsis thaliana L. genome. BMC Genomics (Suppl 12) 15: S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison GJ. (1980) A model for vein formation in higher plants. Proc R Soc Lond B Biol Sci 207: 79–109 [Google Scholar]
- Molendijk AJ, Bischoff F, Rajendrakumar CSV, Friml J, Braun M, Gilroy S, Palme K (2001) Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J 20: 2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy S (2011) Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J 65: 309–318 [DOI] [PubMed] [Google Scholar]
- Moss BL, Mao H, Guseman JM, Hinds TR, Hellmuth A, Kovenock M, Noorassa A, Lanctot A, Villalobos LI, Zheng N, et al. (2015) Rate motifs tune auxin/indole-3-acetic acid degradation dynamics. Plant Physiol 169: 803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Kiefer CS, Grebe M (2012) Planar polarity, tissue polarity and planar morphogenesis in plants. Curr Opin Plant Biol 15: 593–600 [DOI] [PubMed] [Google Scholar]
- Nanao MH, Vinos-Poyo T, Brunoud G, Thévenon E, Mazzoleni M, Mast D, Lainé S, Wang S, Hagen G, Li H, et al. (2014) Structural basis for oligomerization of auxin transcriptional regulators. Nat Commun 5: 3617. [DOI] [PubMed] [Google Scholar]
- Napier R. (1995) Towards an understanding of ABP1. J Exp Bot 46: 1787–1795 [Google Scholar]
- Napier RM. (1997) Trafficking of the auxin-binding protein. Trends Plant Sci 2: 251–255 [Google Scholar]
- Napier RM, Venis MA (1995) Auxin action and auxin-binding proteins. New Phytol 129: 167–201 [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Feldman LJ, Zambryski PC (2000) Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127: 3877–3888 [DOI] [PubMed] [Google Scholar]
- Otsuji M, Ishihara S, Co C, Kaibuchi K, Mochizuki A, Kuroda S (2007) A mass conserved reaction-diffusion system captures properties of cell polarity. PLOS Comput Biol 3: e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paponov IA, Paponov M, Teale W, Menges M, Chakrabortee S, Murray JA, Palme K (2008) Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol Plant 1: 321–337 [DOI] [PubMed] [Google Scholar]
- Payne RJH, Grierson CS (2009) A theoretical model for ROP localisation by auxin in Arabidopsis root hair cells. PLoS One 4: e8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Jerome E, Moss BL, Lanctot A, Hageman A, Nemhauser JL (2016) Functional analysis of molecular interactions in synthetic auxin response circuits. Proc Natl Acad Sci USA 113: 11354–11359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Greenham K, Zhang Y, Santner A, Castillejo C, Mutka AM, O’Malley RC, Ecker JR, Kunkel BN, Estelle M (2016) The Arabidopsis auxin receptor F-box proteins AFB4 and AFB5 are required for response to the synthetic auxin picloram. G3 (Bethesda) 6: 1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Lavy M, Ashton NW, Estelle M (2010) Physcomitrella patens auxin-resistant mutants affect conserved elements of an auxin-signaling pathway. Curr Biol 20: 1907–1912 [DOI] [PubMed] [Google Scholar]
- Rademacher EH, Lokerse AS, Schlereth A, Llavata-Peris CI, Bayer M, Kientz M, Freire Rios A, Borst JW, Lukowitz W, Jürgens G, et al. (2012) Different auxin response machineries control distinct cell fates in the early plant embryo. Dev Cell 22: 211–222 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, Vanneste S, Zhang J, Simon S, Čovanová M, et al. (2010) ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143: 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland-Lagan AG, Prusinkiewicz P (2005) Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis. Plant J 44: 854–865 [DOI] [PubMed] [Google Scholar]
- Rück A, Palme K, Venis MA, Napier RM, Felle HH (1993) Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J 4: 41–46 [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Sachs T. (1968) On the determination of the pattern of vascular tissue in peas. Ann Bot (Lond) 32: 781–790 [Google Scholar]
- Sachs T. (1969) Polarity and the induction of organized vascular tissues. Ann Bot (Lond) 33: 263–275 [Google Scholar]
- Sachs T. (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9: 151–162 [Google Scholar]
- Salehin M, Bagchi R, Estelle M (2015) SCFTIR1/AFB-based auxin perception: mechanism and role in plant growth and development. Plant Cell 27: 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wisniewska J, Reinöhl V, Friml J, Benková E (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20: 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchuk MG, Edgar A, Scarpella E (2013) Patterning of leaf vein networks by convergent auxin transport pathways. PLoS Genet 9: e1003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Nemhauser JL, McColl A, Roe JL, Feldmann KA, Zambryski PC (1997) ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124: 4481–4491 [DOI] [PubMed] [Google Scholar]
- Shih HW, DePew CL, Miller ND, Monshausen GB (2015) The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr Biol 25: 3119–3125 [DOI] [PubMed] [Google Scholar]
- Simonini S, Deb J, Moubayidin L, Stephenson P, Valluru M, Freire-Rios A, Sorefan K, Weijers D, Friml J, Østergaard L (2016) A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev 30: 2286–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 55: 555–590 [DOI] [PubMed] [Google Scholar]
- Smith RS, Guyomarc’h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P (2006) A plausible model of phyllotaxis. Proc Natl Acad Sci USA 103: 1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek N, Poraty L, Sternberg H, Bar E, Lewinsohn E, Yalovsky S (2007) Activation status-coupled transient S acylation determines membrane partitioning of a plant Rho-related GTPase. Mol Cell Biol 27: 2144–2154; retraction [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sorek N, Poraty L Sternberg H Bar E Lewinsohn E Yalovsky S(2017) Mol Cell Biol 37: e00321–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Lee SH, Wenger JP, Gonzalez N, Itoh H, Inzé D, Peer WA, Murphy AS, Overvoorde PJ, Gray WM (2012) The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J 70: 978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Lor VS, Ren H, Olszewski NE, Miller ND, Wu G, Spalding EP, Gray WM (2017) Constitutive expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in tomato confers auxin-independent hypocotyl elongation. Plant Physiol 173: 1453–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM (2014) SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26: 2129–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Feckler C, Palme K, Christian M, Böttger M, Lüthen H (2001) The auxin signal for protoplast swelling is perceived by extracellular ABP1. Plant J 27: 591–599 [DOI] [PubMed] [Google Scholar]
- Stewart JL, Nemhauser JL (2010) Do trees grow on money? Auxin as the currency of the cellular economy. Cold Spring Harb Perspect Biol 2: a001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Liu J-P, Ward S, Kendall SL, Leyser O (2012) Mutation of the cytosolic ribosomal protein-encoding RPS10B gene affects shoot meristematic function in Arabidopsis. BMC Plant Biol 12: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Wang J, Gao Z, Dong J, He H, Terzaghi W, Wei N, Deng XW, Chen H (2016) Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc Natl Acad Sci USA 113: 6071–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HM, Haseloff J, Beemster GT, Bhalerao R, Bennett MJ (2005) Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol 7: 1057–1065 [DOI] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hayashi K, Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol 159: 632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446: 640–645 [DOI] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Wu HM (2002) Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14: 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander M, Olsson T, Ronne H (2005) Effect of the energy supply on filamentous growth and development in Physcomitrella patens. J Exp Bot 56: 653–662 [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16: 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromas A, Braun N, Muller P, Khodus T, Paponov IA, Palme K, Ljung K, Lee J-Y, Benfey P, Murray JAH, et al. (2009) The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS One 4: e6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM. (1952) The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biol Sci 237: 37–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1999) Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci USA 96: 5844–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venis MA, Napier RM, Barbier-Brygoo H, Maurel C, Perrot-Rechenmann C, Guern J (1992) Antibodies to a peptide from the maize auxin-binding protein have auxin agonist activity. Proc Natl Acad Sci USA 89: 7208–7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, et al. (2011) The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol 7: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Walcher CL, Chory J, Nemhauser JL (2008) Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc Natl Acad Sci USA 105: 9829–9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers JDB, Paterson NW (2001) Plant hormones and the control of physiological processes. New Phytol 152: 375–407 [DOI] [PubMed] [Google Scholar]
- Woo E-J, Marshall J, Bauly J, Chen J-G, Venis M, Napier RM, Pickersgill RW (2002) Crystal structure of auxin-binding protein 1 in complex with auxin. EMBO J 21: 2877–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M-F, Yamaguchi N, Xiao J, Bargmann B, Estelle M, Sang Y, Wagner D (2015) Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. eLife 4: e09269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusová H, et al. (2014) Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343: 1025–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z (2010) Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143: 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Bloch D, Sorek N, Kost B (2008) Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol 147: 1527–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami M, Haga K, Napier RM, Iino M (2004) Two distinct signaling pathways participate in auxin-induced swelling of pea epidermal protoplasts. Plant Physiol 134: 735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser N, Ellsmore A, Leasure C, Callis J (2001) Auxin modulates the degradation rate of Aux/IAA proteins. Proc Natl Acad Sci USA 98: 11795–11800 [DOI] [PMC free article] [PubMed] [Google Scholar]