Dear Editor,
Our recent work discovered a role of the Arabidopsis E3 ubiquitin ligase SP1 in peroxisome matrix protein import (Pan et al., 2016). Recently, a Letter to the Editor published in Plant Physiology raised criticism against the localization and function of SP1 in peroxisomes (Ling et al., 2017). Here, we respond to this criticism and present additional evidence to reinforce our conclusion for the role of SP1 in peroxisomes.
Ling et al. stated that “there was nothing in the original datasets to suggest functions elsewhere (other than chloroplasts) in the cell,” and that “it was difficult to understand how a key regulator of chloroplast protein import” can “additionally operate in a second organelle with a very different protein import system” (Ling et al., 2017). We respectfully disagree with this statement based on information from the literature and our own observations. SP1’s closest homolog in human, MAPL/MULAN/MUL1/GIDE/HADES, which shares a similar domain structure and some levels of similarity in protein sequence with SP1, as was cited in both of our previous reports (Ling et al., 2012; Pan et al., 2016), localizes to the outer membrane of mitochondria and can be transported to peroxisomes via mitochondrion-derived vesicles (Neuspiel et al., 2008; Braschi et al., 2010). It is not uncommon for an E3 ubiquitin ligase to be multifunctional and have different substrates. For example, MAPL itself has several known substrate proteins in different physiological processes, including the mitochondrial and peroxisomal fission factor dynamin-related protein DRP1 (Braschi et al., 2009), E3 ubiquitin ligase TRAF2 in mitochondrial hyperfusion (Zemirli et al., 2014), ULK1 in mitophagy (Li et al., 2015), mitofusin in mitochondrial integrity maintenance (Yun et al., 2014), RIG-I in mitochondrial antiviral response (Jenkins et al., 2013), the Ser/Thr kinase Akt in cell proliferation and viability (Bae et al., 2012), and p53 and p73 when they translocate to mitochondria under cell stress (Jung et al., 2011; Min et al., 2015). When we performed a phylogenetic analysis using SP1, its two homologs from Arabidopsis SPL1 and SPL2, and homologous sequences from other eukaryotic species, MAPL was grouped together with SP1 and SPL1 in the same subclade conserved in plants and animals, whereas SPL2 belonged to a plant-specific subclade, suggesting a strong evolutionary relationship between SP1 and MAPL (Pan et al., 2016). In fact, we found SP1 to target to three types of organelles in Arabidopsis: chloroplasts, peroxisomes, and mitochondria (see below).
For subcellular localization studies, Ling et al. (2017) were unable to obtain transgenic lines that express 35Spro:SP1-YFP or SP1pro:SP1-YFP with detectable fluorescence signals, which they attributed to the ability of E3 ligases to target and destabilize themselves. However, several possible reasons could have led to this negative result. In addition, although E3s can destabilize themselves, a fraction of the protein may remain and the remaining fluorescent molecules can be detected by confocal microscopy. In our hands, we indeed obtained multiple transgenic lines that express 35Spro:SP1-YFP or SP1pro:SP1-YFP and exhibit detectable SP1-YFP signals.
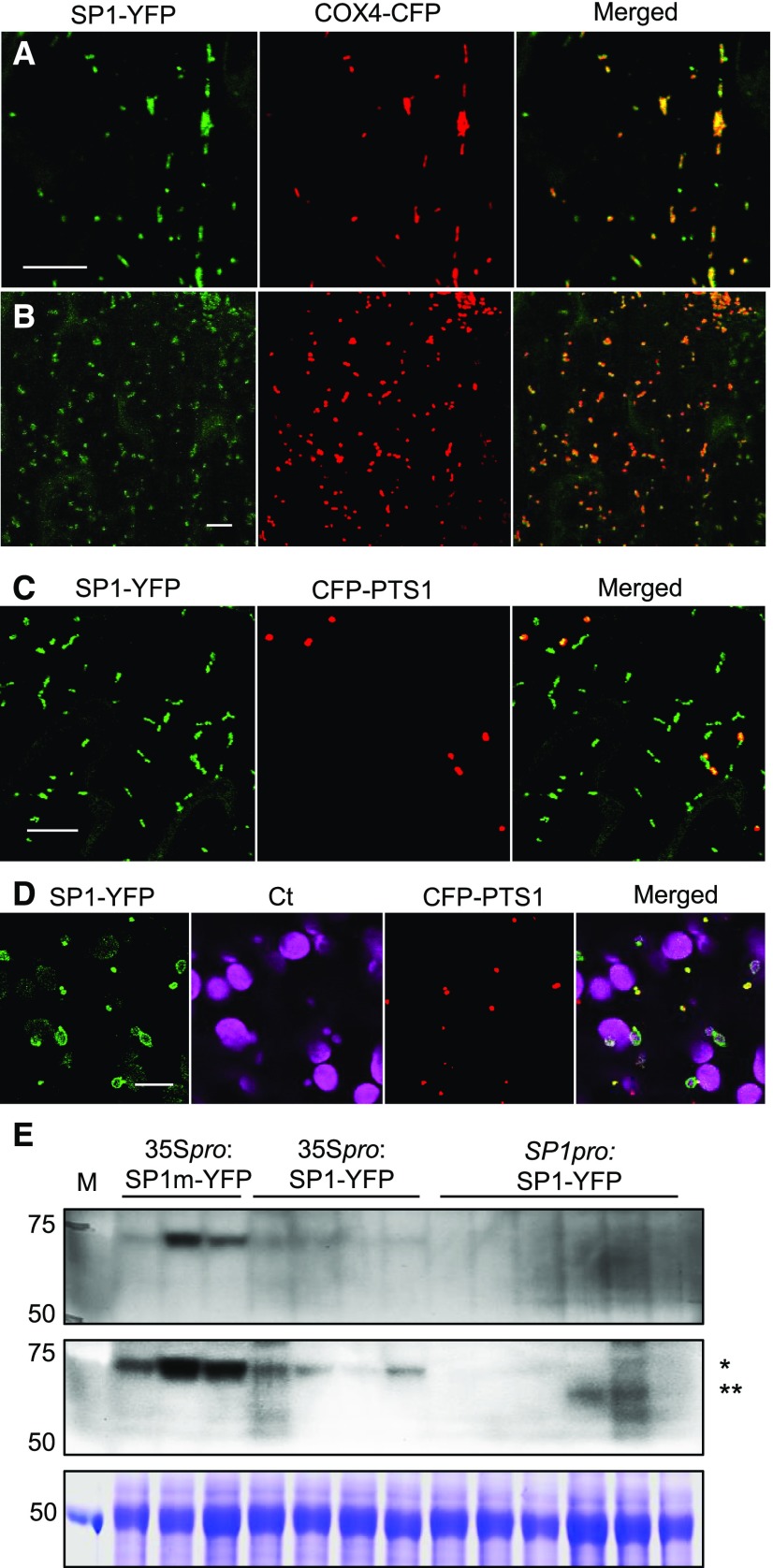
In our previous report, we generated multiple Arabidopsis lines that stably coexpress 35Spro:SP1-YFP and the peroxisome marker CFP-PTS1, and found the protein to be associated with peroxisomes and chloroplasts (Pan et al., 2016). Simultaneously, we also generated transgenic lines that stably coexpress 35Spro:SP1-YFP and the mitochondrial marker COX4-CFP and found SP1-YFP to also target to mitochondria (Fig. 1A), which we did not report at that time because peroxisomes were the focus of that study. To further check into the multiple locations of SP1 in plant cells, we later generated Arabidopsis plants coexpressing SP1-YFP under the 2-kb native promoter of SP1 (Pan et al., 2016) and CFP-PTS1 or COX4-CFP. Similar to what we had observed in plants overexpressing SP1-YFP, the subcellular distribution of SP1-YFP in SP1pro:SP1-YFP lines appeared to vary to some extent during development. For example, during the first 4 d of seedling establishment, SP1-YFP showed strong mitochondrial targeting (Fig. 1B), very weak peroxisome signals, and targeting to the surface of all chloroplasts. After this early stage, SP1-YFP exhibited strong targeting to mitochondria and peroxisomes (Fig. 1C). Interestingly, SP1-YFP signals were much stronger on some smaller chloroplasts in mesophyll cells (Fig. 1D), which is consistent with what we had reported previously for 35Spro:SP1-YFP lines (Pan et al., 2016).
Figure 1.
Analysis of the subcellular localization of SP1. A, Confocal images taken in leaf epidermal cells of 4-week-old Arabidopsis stably expressing 35Spro:SP1-YFP and mitochondrial marker COX4-CFP. Scale bar = 10 µm. B, Confocal images taken in cotyledon epidermal cells of 3-d-old Arabidopsis expressing SP1pro:SP1-YFP and the mitochondrial marker COX4-CFP. Scale bar = 10 µm. C and D, Confocal images taken in cotyledon epidermal (C) and mesophyll (D) cells of 5-d-old Arabidopsis expressing SP1pro:SP1-YFP and the peroxisomal marker CFP-PTS1. Ct, Chloroplasts shown by chlorophyll autofluorescence. Scale bars = 10 µm. E, Immunoblot analysis of proteins from 7-d-old Arabidopsis plants expressing 35Spro:SP1m-YFP, 35Spro:SP1-YFP, or SP1pro:SP1-YFP using α-GFP antibody. Top, One-hour incubation with α-GFP. Middle, Overnight incubation with α-GFP. Bottom, Coomassie Blue staining as the loading control. 35Spro:SP1m-YFP and 35Spro:SP1-YFP proteins (single asterisk) are larger than SP1pro:SP1-YFP (double asterisks) because the 35S-driven constructs are in the pEarley101 gateway vector, which has longer linker sequences and an HA tag at the C terminus of YFP. M, Marker lane. Molecular masses in kD are indicated on the left.
Ling et al. speculated that their inability to detect YFP signals suggested that the cells in our previous study may have accumulated SP1-YFP to unusually high levels (Ling et al., 2017). In addition to 35Spro:SP1-YFP lines, we have obtained multiple SP1pro:SP1-YFP lines with detectable YFP signals; the chance that SP1-YFP is highly accumulated in every line would be rare. To compare the level of SP1-YFP proteins in different transgenic lines, we performed immunoblot analysis on proteins extracted from plants expressing 35Spro:SP1-YFP, 35Spro:SP1m-YFP, or SP1pro:SP1-YFP. SP1m is a dominant negative form of SP1, which contains point mutations in the conserved residues in the catalytic (RING) domain and partially suppressed the phenotypes of pex14-2 when overproduced (Pan et al., 2016). As expected, SP1pro:SP1-YFP lines expressed lower levels of the SP1-YFP protein compared with the overexpressors (Fig. 1E). We were able to visualize SP1-YFP signals in all the six SP1pro:SP1-YFP lines by confocal microscopy, confirming that the fluorescent proteins do not need to be overaccumulated to be detected by microscopy. Consistent with its level of expression, we did find SP1-YFP signals to be weaker in most 35Spro:SP1-YFP lines than in 35Spro:SP1m-YFP plants and very weak in all SP1pro:SP1-YFP lines (data not shown).
Ling et al. (2017) could only detect SP1-YFP signals by transient expression of the protein through protoplast transfection, where they found consistent chloroplast localization but little peroxisome association of the fluorescent protein. Protoplasts derived from mesophyll cells contain many more chloroplasts than most other cell types, and chloroplasts, mitochondria, and peroxisomes are often clumped together. Therefore, it may be difficult to distinguish SP1-YFP signals on peroxisomes from those on the surface of chloroplasts because of the tight physical association between these organelles, especially when peroxisomal SP1 signals are weaker than those on chloroplasts. Finally, organelle association of SP1 may be affected by physiological conditions or signals, which differ between the protoplast system and cells in intact plants.
Taken together, we stand by our conclusion that SP1 localizes to peroxisomes. This triple location pattern is exciting and in line with the fact that MAPL, the human homolog of SP1, is known to target to mitochondria and peroxisomes (Neuspiel et al., 2008; Braschi et al., 2010).
Ling et al. (2017) also criticized our physiological assays as unspecific to peroxisomes, and mentioned that chloroplast import mutants also have short hypocotyls and roots. The inefficient deetiolation phenotype of sp1 mutants reported by Ling et al. (2012) is not a specific phenotype for chloroplast protein import, as numerous factors such as plant hormones and the light perception network impact deetiolation/photomorphogenesis (Nemhauser, 2008). On the other hand, our measurements were done in the presence of 2,4-dichlorophenoxybutryic acid (2,4-DB), an analog of proauxin that is converted to the auxin analog 2,4-D by β-oxidation, a pathway occurring exclusively in the peroxisome in plants. The 2,4-DB assay is not a general assessment of plant growth, but instead is specifically used to evaluate peroxisome function and has been widely used by the plant peroxisome research community and our own lab (Cassin-Ross and Hu, 2014; Pan et al., 2016). Without 2,4-DB, the root or hypocotyl lengths of sp1 mutants are actually similar to that of the wild type. The sp1 loss-of-function mutants are more sensitive whereas SP1 overexpressors are more resistant to 2,4-DB, indicating that SP1 has a negative effect on peroxisome function (Pan et al., 2016).
Ling et al. (2017) were unable to detect apparent changes in the level of endogenous PEX13 or PEX14 protein in sp1 null and SP1 overexpressing plants. In sp1, we found no apparent changes in the level of PEX14 and subtle changes for PEX13 (Pan et al., 2016). Such subtle changes in protein level may be missed when using different batches of the PEX13 antibody and different overexpression lines. Moreover, SP1 is a regulatory protein whose function may be affected by feedback mechanisms that depend on physiological and environmental conditions. As we discussed in our previous report (Pan et al., 2016), a lack of obvious changes in PEX14 levels in transgenic Arabidopsis lines cannot prove that PEX14 is not a target by SP1 for destabilization. To that end, we chose to use the tobacco protein expression system, which transiently and abundantly expresses both enzymes and substrates to temporarily overcome the consequences of feedback regulation, so that we can demonstrate obvious effects in protein destabilization/stabilization. In this system, we could demonstrate that SP1 overexpression destabilizes PEX13 and PEX14, but not PEX5, PEX7, PEX4, PEX22, YFP-PTS, or actin. We not only tagged our proteins with YFP, which was pointed out by Ling et al. (2017) as a large tag, but also used the small tag FLAG and found that PEX13-FLAG could be more efficiently destabilized by SP1 than PEX13-YFP, which we attributed to more efficient peroxisome targeting of PEX13-FLAG (Pan et al., 2016). Nevertheless, both PEX13-FLAG and PEX13-YFP can be strongly destabilized by SP1 overexpression in repeated experiments (Pan et al., 2016). The tobacco system is for transient protein expression, and so is the protoplast transient expression system that has been employed in studies such as Ling et al. (2012) and (2017). Although tobacco is a heterologous system, it is an intact plant under natural physiological conditions and has been widely used in plant research as a powerful alternative to transgenic lines. In this case, we observed the same results after repeated experiments in tobacco, which are consistent with our microscopic, genetic, and physiological data from Arabidopsis.
In summary, we have multiple lines of evidence to support our conclusion that SP1 targets to peroxisomes and functions in peroxisome protein import. Similar to its mammalian homolog MAPL, SP1 also localizes to mitochondria, an interesting phenomenon that is currently under investigation. In plants, chloroplasts, mitochondria, and peroxisomes are functionally linked and coordinately participate in a number of metabolic pathways in energy metabolism (Hu et al., 2012). SP1, together with its homologs SPL1 and SPL2 in Arabidopsis, may constitute a small E3 family that regulates the biogenesis and dynamics of multiple energy organelles in plants, and this mechanism may be conserved to some degrees across diverse eukaryotic species.
ACKNOWLEDGMENTS
We thank Drs. Christoph Benning and John Froehlich for comments on this manuscript.
References
- Bae S, Kim SY, Jung JH, Yoon Y, Cha HJ, Lee H, Kim K, Kim J, An IS, Kim J, et al. (2012) Akt is negatively regulated by the MULAN E3 ligase. Cell Res 22: 873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E, Goyon V, Zunino R, Mohanty A, Xu L, McBride HM (2010) Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol 20: 1310–1315 [DOI] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM (2009) MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep 10: 748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassin-Ross G, Hu J (2014) Systematic phenotypic screen of Arabidopsis peroxisomal mutants identifies proteins involved in β-oxidation. Plant Physiol 166: 1546–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Baker A, Bartel B, Linka N, Mullen RT, Reumann S, Zolman BK (2012) Plant peroxisomes: biogenesis and function. Plant Cell 24: 2279–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins K, Khoo JJ, Sadler A, Piganis R, Wang D, Borg NA, Hjerrild K, Gould J, Thomas BJ, Nagley P, et al. (2013) Mitochondrially localised MUL1 is a novel modulator of antiviral signaling. Immunol Cell Biol 91: 321–330 [DOI] [PubMed] [Google Scholar]
- Jung JH, Bae S, Lee JY, Woo SR, Cha HJ, Yoon Y, Suh KS, Lee SJ, Park IC, Jin YW, et al. (2011) E3 ubiquitin ligase Hades negatively regulates the exonuclear function of p53. Cell Death Differ 18: 1865–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qi W, Chen G, Feng D, Liu J, Ma B, Zhou C, Mu C, Zhang W, Chen Q, et al. (2015) Mitochondrial outer-membrane E3 ligase MUL1 ubiquitinates ULK1 and regulates selenite-induced mitophagy. Autophagy 11: 1216–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Q, Huang W, Baldwin A, Jarvis P (2012) Chloroplast biogenesis is regulated by direct action of the ubiquitin-proteasome system. Science 338: 655–659 [DOI] [PubMed] [Google Scholar]
- Ling Q, Li N, Jarvis P (2017) Chloroplast ubiquitin E3 ligase SP1: Does it really function in peroxisomes? Plant Physiol 175: 586–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Ryu J, Chi SW, Yi GS (2015) Ubiquitination-dependent degradation of p73 by the mitochondrial E3 ubiquitin ligase Hades. Biochem Biophys Res Commun 467: 316–321 [DOI] [PubMed] [Google Scholar]
- Nemhauser JL. (2008) Dawning of a new era: photomorphogenesis as an integrated molecular network. Curr Opin Plant Biol 11: 4–8 [DOI] [PubMed] [Google Scholar]
- Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM (2008) Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol 18: 102–108 [DOI] [PubMed] [Google Scholar]
- Pan R, Satkovich J, Hu J (2016) E3 ubiquitin ligase SP1 regulates peroxisome biogenesis in Arabidopsis. Proc Natl Acad Sci USA 113: E7307–E7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Puri R, Yang H, Lizzio MA, Wu C, Sheng ZH, Guo M (2014) MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. eLife 3: e01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemirli N, Pourcelot M, Ambroise G, Hatchi E, Vazquez A, Arnoult D (2014) Mitochondrial hyperfusion promotes NF-κB activation via the mitochondrial E3 ligase MULAN. FEBS J 281: 3095–3112 [DOI] [PubMed] [Google Scholar]