VND1, VND2, and VND3 are required for xylem vessel element formation in the secondary veins of Arabidopsis cotyledons grown under continuous dark conditions.
Abstract
Arabidopsis (Arabidopsis thaliana) VASCULAR-RELATED NAC-DOMAIN1 (VND1) to VND7 encode a group of NAC domain transcription factors that function as master regulators of xylem vessel element differentiation. These transcription factors activate the transcription of genes required for secondary cell wall formation and programmed cell death, key events in xylem vessel element differentiation. Because constitutive overexpression of VND6 and VND7 induces ectopic xylem vessel element differentiation, functional studies of VND proteins have largely focused on these two proteins. Here, we report the roles of VND1, VND2, and VND3 in xylem vessel formation in cotyledons. Using our newly established in vitro system in which excised Arabidopsis cotyledons are stimulated to undergo xylem cell differentiation by cytokinin, auxin, and brassinosteroid treatment, we found that ectopic xylem vessel element differentiation required VND1, VND2, and VND3 but not VND6 or VND7. The importance of VND1, VND2, and VND3 also was indicated in vivo; in the vnd1 vnd2 vnd3 seedlings, xylem vessel element differentiation of secondary veins in cotyledons was inhibited under dark conditions. Furthermore, the light responsiveness of VND gene expression was disturbed in the vnd1 vnd2 vnd3 mutant, and vnd1 vnd2 vnd3 failed to recover lateral root development in response to the change of light conditions. These findings suggest that VND1 to VND3 have specific molecular functions, possibly linking light conditions to xylem vessel formation, during seedling development.
The water-conducting activity of xylem is carried out by pipe-like xylem vessels consisting of xylem vessel elements. Recent advances in molecular genetics have greatly improved our understanding of xylem vessel element differentiation in Arabidopsis (Arabidopsis thaliana). In the current model, all vascular cells, including xylem vessel elements, are thought to differentiate from vascular precursor cells, namely procambium (Miyashima et al., 2013; Nieminen et al., 2015; De Rybel et al., 2016), in a process regulated by several key transcription factors, some of which have been deeply related to the auxin, cytokinin, and brassinosteroid signaling pathways (Ohashi-Ito and Fukuda, 2010; Růžička et al., 2015; De Rybel et al., 2016).
Arabidopsis VASCULAR-RELATED NAC-DOMAIN1 (VND1) to VND7, which encode a specific group of NAC (No Apical Meristem [NAM], Arabidopsis Transcription Activation Factor [ATAF1,2], and Cup-Shaped Cotyledon [CUC2]) domain transcription factors, were identified as master regulators of xylem vessel element differentiation in a study involving microarray analysis and an in vitro induction system of xylem vessel element differentiation based on Arabidopsis suspension cells, followed by an overexpression analysis (Kubo et al., 2005). Overexpression of VND6 and VND7 induces the formation of ectopic xylem vessel elements with metaxylem- and protoxylem-like secondary cell wall (SCW) patterning, respectively. Thus, VND6 and VND7 are key transcriptional switches of metaxylem and protoxylem vessel differentiation, respectively (Kubo et al., 2005). Functional studies of VND6 and VND7 revealed groups of downstream genes known to be required for xylem cell differentiation (Ohashi-Ito et al., 2010; Zhong et al., 2010; Yamaguchi et al., 2011), including genes involved in SCW biosynthesis (e.g. CELLULOSE SYNTHASE4/IRREGULAR XYLEM5 [CESA4/IRX5], CESA7/IRX3, and CESA8/IRX1 for cellulose biosynthesis [Brown et al., 2005] and IRX9 and IRX10 for xylan biosynthesis [Brown et al., 2009; Wu et al., 2009, 2010]) and programmed cell death (PCD; e.g. XYLEM CYSTEINE PEPTIDASE1 [XCP1; Funk et al., 2002] and METACASPASE9 [MC9; Bollhöner et al., 2013]) and genes encoding second-layer master transcription factors (such as MYB46 and MYB83 [Zhong et al., 2007a; McCarthy et al., 2009]). Recent comparative studies of VND homologs in a wide range of plant species suggested that the basic scheme of the VND-based transcriptional network is conserved among land plant species (Hussey et al., 2013; Xu et al., 2014; Nakano et al., 2015).
Although dominant repression of VND6 and VND7 inhibited xylem vessel element differentiation, no defects were detected in the vnd6 or vnd7 T-DNA insertion mutants (Kubo et al., 2005). In addition, promoter-reporter analysis of VND1 to VND7 revealed that all VND genes are expressed preferentially in developing vascular tissues (Kubo et al., 2005; Yamaguchi et al., 2008). These findings suggest high functional redundancy among the VND genes. However, several lines of evidence imply that the VND genes have differential functions as well; for example, detailed expression analysis showed that the VND genes exhibit diverse developmental expression patterns in xylem tissues (Kubo et al., 2005; Yamaguchi et al., 2008; Zhou et al., 2014). Within the plant, each organ has a specialized vascular system; thus, the cotyledon (seed leaf) has a different vascular pattern from the mature leaf or root. The precise roles of the different VND genes in xylem tissues of diverse organs are unknown; however, the subtle differences observed in VND1-7pro:GUS patterns suggest that these genes may have differential functions. In addition, protein-protein interaction analysis showed that VND proteins have different abilities to form homodimers or heterodimers (Yamaguchi et al., 2008). For example, VND7 forms heterodimers with VND1 to VND6 and also forms homodimers (Yamaguchi et al., 2008). Transient expression analysis of VND promoters in Arabidopsis leaves showed that VND1 to VND5 have the potential to induce VND7 expression (Endo et al., 2015). Based on these observations, we hypothesized that the VND genes have diversified roles in xylem vessel element differentiation. However, experimental evidence to support this hypothesis is lacking.
In this study, we demonstrated that VND1, VND2, and VND3 have specific roles in xylem vessel element differentiation in cotyledons. Using a new in vitro induction system of xylem vessel element differentiation in Arabidopsis cotyledons, we found that ectopic xylem vessel element differentiation was highly dependent on VND1, VND2, and VND3 but not on VND6 or VND7. Similarly, xylem vessel element differentiation of secondary veins in the cotyledons of seedlings grown under dark conditions was inhibited in the vnd1 vnd2 vnd3 triple mutant but not in the vnd6 vnd7 double mutant. Interestingly, the vnd1 vnd2 vnd3 phenotype was detected only under dark conditions, and light-mediated up-regulation of VND expression was disrupted in vnd1 vnd2 vnd3. Moreover, the lateral root formation in vnd1 vnd2 vnd3 seedlings was affected when the seedlings were transferred from weak to normal light conditions. Our findings suggest that VND1 to VND3 have the diversified roles that link environmental cues to xylem vessel formation during seedling development.
RESULTS
A Novel in Vitro Induction System of Xylem Vessel Element Differentiation (the KDB System) Using Arabidopsis Cotyledons
To investigate the functional diversity of VND proteins, we established a novel in vitro induction system. We used cotyledons in this system, as they have a well-characterized vascular system (Carland et al., 1999), can readily be treated with phytohormones in vitro, and can be obtained from mutant and reporter lines. Since cytokinin, auxin, and brassinosteroid are critical regulators of xylem cell differentiation (Fukuda, 2004; Ohashi-Ito and Fukuda, 2010), kinetin (K; as cytokinin), 2,4-dichlorophenoxyacetic acid (D; as auxin), and brassinolide (B; as brassinosteroid) were used in the in vitro induction system, which we named the KDB system.
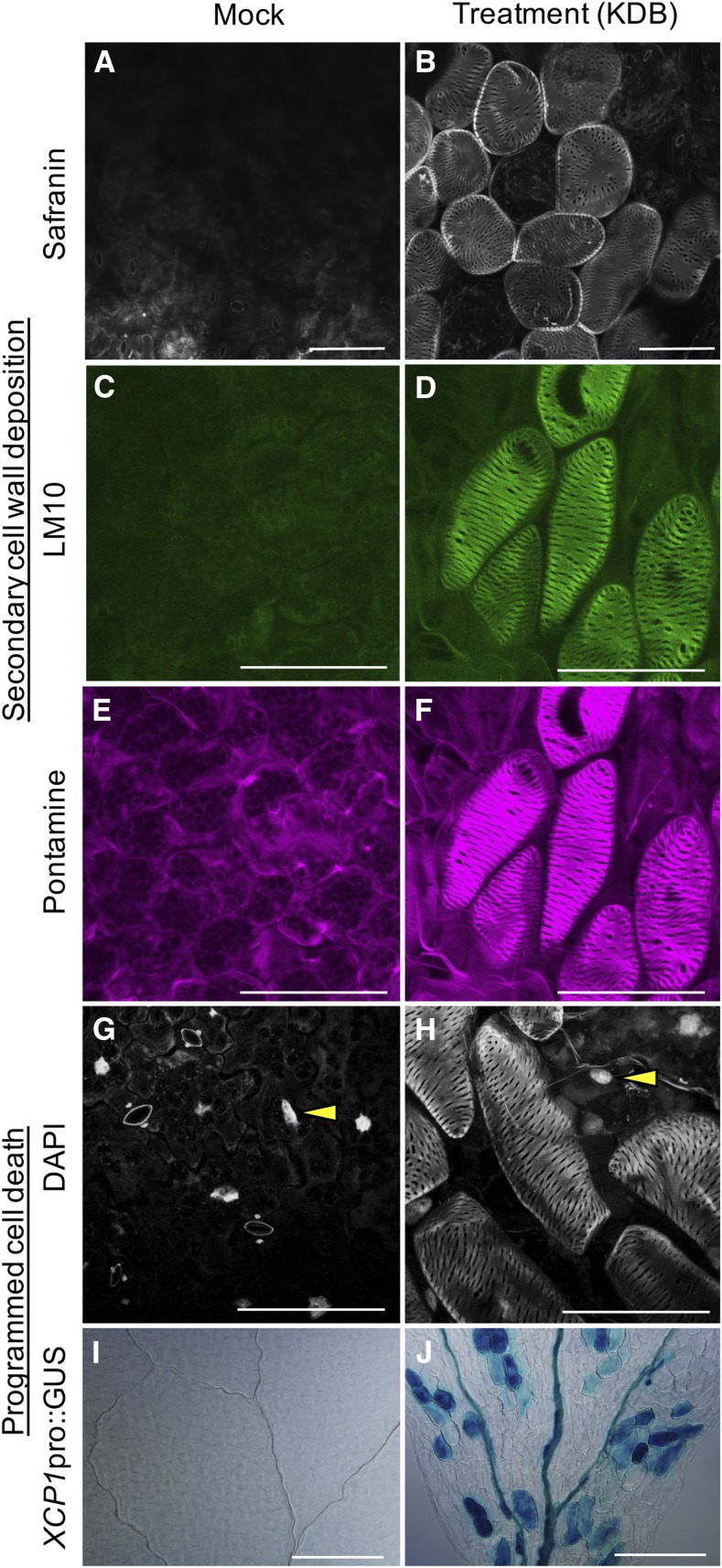
Using this system (Fig. 1A), we found that thickened secondary cell walls were induced effectively in the cotyledon cells of 6-d-old Arabidopsis seedlings under continuous light conditions (Fig. 1, B–G; Supplemental Table S1). Furthermore, these cells showed strong autofluorescence under UV illumination, similar to native xylem vessels in the cotyledon (Fig. 1, F and G), suggesting that the thickened walls contained lignin, the phenolic polymer present in the SCWs of xylem vessel elements. To confirm that lignin accumulated in the induced cell walls, we performed safranin-O staining (Fig. 2, A and B). Bright fluorescence in the induced cells demonstrated that the SCW indeed contained lignin (Fig. 2B). In addition, we tested for other SCW components, such as xylan, the predominant hemicellulose in the Arabidopsis SCW, and cellulose in the induced cells (Fig. 2, C–F). Double immunolabeling of xylan and cellulose with LM10 xylan antibody followed by pontamine staining showed that the induced cell walls contained xylan and were rich in cellulose (Fig. 2, C–F). Thus, the induced cells had a similar composition to xylem vessel elements, containing SCW polymers, cellulose, hemicellulose, and lignin. Careful observation via confocal laser scanning microscopy also suggested that the induced cells had the reticulate SCW patterns characteristic of metaxylem vessel elements (Fig. 2, B, D, and F).
Figure 1.
The Arabidopsis in vitro KDB induction system. A, Experimental setup for the KDB system. Arabidopsis seedlings and the cotyledons of 6-d-old Arabidopsis seedlings were excised and incubated in one-half-strength Murashige and Skoog (MS) liquid medium supplemented with phytohormones (KDB), as described in the text, at 22°C under continuous light. B to G, Induction of xylem cell differentiation in cotyledons by KDB treatment. The excised cotyledons of 6-d-old wild-type seedlings were treated without (B, D, and F) or with (C, E, and G) KDB for 5 d. Cotyledons were observed under differential interference contrast (B–E) or fluorescence (F and G) microscopy. White arrowheads indicate native xylem vessels, and white arrows indicate ectopic xylem vessel elements. Bars = 1 mm (B and C) and 200 µm (D–G). H, Time course of the frequency of ectopic xylem vessel element differentiation. The excised cotyledons of 6-d-old wild-type seedlings were treated with KDB for 7 d. n indicates the numbers of tested cotyledons.
Figure 2.
Characterization of induced ectopic xylem vessel elements. A to H, The excised cotyledons of 6-d-old wild-type seedlings were treated without (A, C, E, and G) or with (B, D, F, and H) KDB for 5 d. Cotyledons were observed using a confocal scanning laser microscope. The cotyledon cells were stained with safranin-O (A and B), probed with the LM10 xylan antibody (C and D), stained with pontamine (E and F), or stained with DAPI (G and H). Yellow arrowheads indicate the nucleus. Bars = 100 µm. I and J, Six-day-old XCP1pro:GUS seedlings were treated without (I) or with (J) KDB for 5 d. Bars = 500 µm.
In addition to having SCWs, xylem vessel elements undergo PCD, which converts them to hollow vessel elements capable of transporting water at maturity. To examine whether the induced cells underwent PCD, we stained the nuclei of induced Columbia cells with 4′,6-diamidino-2-phenylindole (DAPI). Confocal microscopy analysis showed that cells with thickened (and autofluorescent) SCWs lacked DAPI staining (Fig. 2, G and H). Moreover, high levels of GUS signal driven by the promoter of XCP1 (the PCD-related peptidase gene; Pyo et al., 2007; Yamaguchi et al., 2011) were observed in cells with thickened SCWs (Fig. 2, I and J), suggesting that the induced cells had undergone PCD. Taken together, these findings suggest that the cells induced by the cytokinin, auxin, and brassinosteroid (KDB) treatment were ectopic xylem vessel elements.
Ectopic xylem vessel elements were observed after 3 d of treatment and progressively increased in number over time (Fig. 1H). Interestingly, no ectopic xylem vessel elements differentiated under dark conditions (Supplemental Fig. S1).
Changes in Expression Patterns of Well-Known Marker Genes Related to Xylem Vessel Element Differentiation in the KDB System
To decipher the molecular processes of xylem vessel element formation in the KDB system, we performed quantitative reverse transcription-PCR (RT-qPCR) analysis of well-known marker genes related to xylem vessel element differentiation, such as procambial cell-related genes (TMO5, LHW, and MP; Hardtke and Berleth, 1998; Ohashi-Ito and Bergmann, 2007; Wenzel et al., 2007; Ohashi-Ito et al., 2010, 2013a, 2013b; De Rybel et al., 2013), HD-Zip III family genes (PHB, PHV, REV, ATHB8, and ATHB15; Baima et al., 2001; Carlsbecker et al., 2010; Ilegems et al., 2010), and genes functioning downstream of VND7 (MYB46, MYB83, CESA7, and LBD30; Yamaguchi et al., 2011; Fig. 3). The expression patterns of these genes seemed to roughly reflect their functions. The expression of LHW and MP, the procambial cell-related genes, peaked during the early stages of treatment (within 12 h of treatment; Fig. 3). The expression of PHB, PHV, ATHB8, and ATHB15, the HD-Zip III family genes involved in both procambial activity and the determination of xylem vessel element types (Carlsbecker et al., 2010; Miyashima et al., 2011), increased after 12 h of treatment and peaked at 3 d of treatment (Fig. 3). By contrast, genes downstream of VND7, which functions in xylem vessel element differentiation (Kubo et al., 2005; Yamaguchi et al., 2008, 2011), were down-regulated at 12 h of culture in the KDB system and then up-regulated, peaking at 3 d of treatment (Fig. 3). These data indicate that the KDB system is consistent with the known patterns of endogenous molecular processes for xylem vessel element differentiation (Ohashi-Ito and Fukuda, 2010; Růžička et al., 2015; De Rybel et al., 2016).
Figure 3.
Expression analysis of vascular formation-related genes in the KDB system. RT-qPCR results are shown for genes involved in xylem vessel element formation between 0 and 7 d of treatment with or without KDB. UBIQUITIN10 (UBQ10) was used as the internal control. Error bars indicate sd of three independent biological replicates. KDB, Treatment; M, mock control.
Global Transcript Profiling during Ectopic Xylem Vessel Element Differentiation in the KDB System
For a detailed view of the transcriptomic changes in the KDB system, we performed a time-course mRNA sequencing (mRNA-seq) analysis using the experimental setup outlined in Figure 1A. The resulting sequence reads reflected a high-quality transcriptomic data set (average mapping rate, 97% of the Arabidopsis genome; biological coefficient of variation, 0.2569; Supplemental Table S2), with most biological replicates consistently clustering together in a hierarchical analysis (Supplemental Fig. S2A). The day-5 samples of the control (c5d) and KDB treatment (k5d) were more variable than the other biological replicates, perhaps due to the prolonged time-course period or to batch or treatment effects. Overall, control samples (c0d, c1d, and c3d) clustered together with the early phytohormone treatment (k1d), while samples at days 3 and 5 of the phytohormone treatment (k3d and k5d) were distinct. Similarly, multidimensional scaling using edgeR showed the phytohormone treatment samples to be clearly separated from the control, except at the earliest time point (Supplemental Fig. S2B). We validated the mRNA-seq data using RT-qPCR analysis and found that there was good agreement between the two methods based on Pearson’s correlation coefficient analysis (r = 0.6–0.9; Supplemental Fig. S3).
For an overview of the molecular processes taking place in the KDB system, Gene Ontology (GO) and pathway enrichment analyses were performed (Supplemental Tables S3 and S4). The results suggested that there were dynamic changes in environmental response pathways, phytohormone signaling, and biosynthesis pathways in the KDB system. Notably, at 3 d of induction, cellulose biosynthesis seemed to be activated (in BP terms of Supplemental Table S3), and at 5 d of treatment, inactivation of photosynthesis and anthocyanin biosynthesis pathways became apparent (in BP terms and pathway of Supplemental Tables S3 and S4). These findings are consistent with previous observations of xylem vessel element differentiation (Bhargava et al., 2010; Yamaguchi et al., 2010).
For detailed molecular characterization of the KDB system, we performed soft-clustering analysis of the 526 genes found in literature data sets to contain known vascular-related genes (n = 314; Truernit and Sauer, 1995; Bonke et al., 2003; Mähönen et al., 2006; Ohashi-Ito and Bergmann, 2007; Gardiner et al., 2010; Ohashi-Ito et al., 2010, 2013a, 2013b, 2014; Schlereth et al., 2010; Yamaguchi et al., 2011; De Rybel et al., 2013, 2014; Miyashima et al., 2013; Furuta et al., 2014; Endo et al., 2015; Kondo et al., 2015) and epidermal and mesophyll cell-related genes (n = 212; Susek et al., 1993; Lu et al., 1996; Abe et al., 2003; Bruex et al., 2012; Supplemental Tables S6 and S7). In all, 77% of vascular-related genes clustered into cluster 2, 4, or 5, in which the genes were specifically up-regulated by the KDB treatment. By contrast, 72% of epidermal and mesophyll cell-related genes were clustered into cluster 1, 3, or 6, which contained genes that were not up-regulated by the KDB treatment (Fig. 4; Supplemental Tables S6 and S7). These exclusive distributions of genes indicated that the KDB system shares similar molecular processes with endogenous xylem vessel element formation. Interestingly, the seven VND genes were classified into different clusters: VND1 and VND2 were in cluster 5, VND3 and VND5 were in cluster 2, and VND4, VND6, and VND7 were in cluster 4 (Fig. 4; Supplemental Tables S6 and S7).
Figure 4.
Clustering analysis of vascular and epidermal and mesophyll cell-related genes. A, Clustering of 526 nonredundant vascular and epidermal and mesophyll cell-related genes using Mfuzz (Futschik and Carlisle, 2005). The gene clusters are listed in Supplemental Tables S6 and S7. For each sample, m or K indicates mock control or KBD treatment, respectively, and the number indicates the days of treatment (dai). B, Pie charts of vascular-related (n = 314) and epidermal and mesophyll cell-related (n = 212) genes. The numbers of genes in each cluster are shown in parentheses.
Ectopic Xylem Vessel Element Differentiation in the KDB System Is Dependent on VND1, VND2, and VND3
The differential clustering of the VND genes suggested that those genes might differentially contribute to ectopic xylem vessel element differentiation in the KDB system. To test this hypothesis, we explored the expression of the VND genes using the mRNA-seq data (Supplemental Fig. S4) and RT-qPCR (Fig. 5). VND1 and VND2 were greatly up-regulated after the KDB treatment, while the other VND genes were only moderately induced (Fig. 5; Supplemental Fig. S4), suggesting that VND1 and VND2 could have prominent roles in the KDB system. Previous phylogenetic analysis grouped VND1, VND2, and VND3 together (Kubo et al., 2005); thus, we used T-DNA insertion lines of vnd1, vnd2, and vnd3, in which the indicated VND gene was knocked down, for mutant analysis (Supplemental Figs. S5 and S6). We also analyzed the vnd6 vnd7 double mutant, in which both VND6 and VND7 were knocked down (Supplemental Figs. S5 and S6), in the KDB system, because VND6 and VND7 possess relatively strong activity for cell differentiation induction when overexpressed (Kubo et al., 2005). Excised wild-type and mutant cotyledons were subjected to the KDB treatment, and the numbers of differentiated ectopic xylem vessel elements were counted after 5 d of treatment. Differentiated ectopic xylem vessel elements were decreased significantly in the vnd2 and vnd3 single mutants compared with the wild type, with the vnd2 phenotype showing an especially strong reduction in ectopic xylem vessel element differentiation (Fig. 6B). The vnd1 vnd2 vnd3 triple mutant had the strongest phenotype of all mutants tested, with only a few ectopic xylem vessel elements observed (Fig. 6). The vnd6 vnd7 double mutant had fewer ectopic xylem vessel elements than the wild type, but some ectopic xylem vessel elements were still observed (Fig. 6). These data indicated that ectopic xylem vessel element differentiation in the KDB system is strongly dependent on the VND1, VND2, and VND3 transcription factors and that, while the VND6 and VND7 transcription factors play roles in the KDB system, they are secondary to those of VND1 to VND3.
Figure 5.

Expression analysis of VND genes in the KDB system. RT-qPCR results are shown for VND genes between 0 and 7 d of treatment with or without KDB. UBQ10 was used as the internal control. Error bars indicate sd of three independent biological replicates. KDB, Treatment (black squares with black lines); M, mock control (white diamonds with black lines).
Figure 6.
Analysis of the effects of KDB treatment in the vnd and nst backgrounds. A, Excised cotyledons of 6-d-old wild-type (WT), vnd6 vnd7, vnd1 vnd2 vnd3, and nst1 nst3 seedlings were treated with KDB for 5 d. White arrowheads indicate native xylem vessels, and white arrows indicate ectopic xylem vessel elements. Bars = 1 mm. B, Estimation of the number of ectopic xylem vessel elements. Excised cotyledons (n = 30) of 6-d-old wild type, vnd1, vnd2, vnd3, vnd1 vnd2, vnd1 vnd3, vnd2 vnd3, vnd1 vnd2 vnd3, vnd6 vnd7, and nst1 nst3 seedlings were treated with KDB for 5 d. The box plot shows median, interquartile range, and sample minimum and maximum. Significance for multiple comparisons was calculated using one-way ANOVA with a posthoc Tukey’s honestly significant difference (HSD) test (different letters indicate significantly different values at P < 0.05).
Our RT-qPCR analysis of vascular-related genes in cotyledons of vnd1 vnd2 vnd3 triple mutants demonstrated that the vnd1 vnd2 vnd3 mutations affected the expression of MYB46 and XCP1, an SCW regulator gene and a PCD-related enzyme gene, respectively (Fig. 7). However, the triple mutation did not alter the up-regulation of MP, TMO5, LHW, ATHB8, ATHB15, PHB, and PHV, which are involved in the formation of procambium and/or xylem precursor cells (Fig. 7). These data suggest that, in the KDB system, VND1, VND2, and VND3 act downstream of the determination of procambium cell fate and play major roles in the induction of xylem vessel element fate.
Figure 7.
Expression analysis of xylem vessel element formation-related genes in the vnd triple mutant in the KDB system. RT-qPCR values are shown for xylem vessel element formation related-genes in the wild type (WT) and vnd1 vnd2 vnd3 at 0, 1, 3, and 5 d of treatment with or without KDB. UBQ10 was used as the internal control. Error bars indicate sd of three independent biological replicates. KDB, Treatment (black squares with black lines); M, mock control (white diamonds with black lines).
The KDB System Is Dependent on MYB46 and MYB83, Second-Layer Master Regulators of Xylem Vessel Element Differentiation
We further examined the involvement of sister genes of the VND family, NAC SECONDARY WALL THICKENING PROMOTING FACTOR1 (NST1) and NST3, master regulators of the differentiation of fiber cells, which also develop SCWs (Zhong et al., 2006, 2007b; Mitsuda et al., 2007; Mitsuda and Ohme-Takagi, 2008). Interestingly, the nst1 nst3 mutants had more ectopic xylem vessel element differentiation than did the wild type (Fig. 6), suggesting that NST1 and NST3 negatively regulate xylem vessel element formation.
As MYB46 and MYB83 function as second-layer master transcription factors for SCW formation downstream of VND and NST (Zhong and Ye, 2012; Hussey et al., 2013; Nakano et al., 2015), we tested if MYB46 and MYB83 are required for xylem differentiation in the KDB system. Strikingly, ectopic xylem vessel element differentiation was abolished completely in the myb46 myb83 double mutant compared with the wild type (Supplemental Fig. S7), indicating that the ectopic vessels induced by KDB require MYB46 and MYB83 function. This again speaks to the similar molecular machinery operating in the in vitro and endogenous systems, as previous observations showed that xylem vessel formation is inhibited severely in myb46 myb83 mutants (McCarthy et al., 2009; Nakano et al., 2015).
Effects of vnd Mutations on Secondary Vein Formation in Cotyledons
The high dependency of xylem vessel element formation on VND1, VND2, and VND3 in the KDB system raised the question of what functions VND1, VND2, and VND3 play in endogenous xylem vessel formation in cotyledons. Therefore, we investigated the effects of vnd mutations on xylem formation in cotyledons. As the KDB system is highly dependent on light conditions (Supplemental Fig. S1), we performed these observations with close attention to the light conditions under which the cotyledons developed.
Wild-type 7-d-old cotyledons were analyzed based on the elements for categorization of vein formation defined by Verna et al. (2015; i.e. break points, touch points, end points, and exit points, to calculate cardinality, continuity, and connectivity of vein networks; Fig. 8). Qualitatively, no differences were obvious in the vein networks between those maintained under continuous light and continuous dark conditions in wild-type cotyledons (Fig. 8G), while more active vein development was observed when grown under light conditions (Fig. 8A) than under dark conditions (Fig. 8B). Under continuous light conditions, any vnd mutants showed no differences in vein networks as compared with the wild type (Fig. 8G). However, under continuous dark conditions, the secondary vein was not observed in the vnd1 vnd2 vnd3 triple mutants (Fig. 8F), and quantification of the features of the vasculature showed that 7-d dark-grown vnd mutants have defects in vein networks (Fig. 8G). The defect in cardinality was observed in vnd2, vnd1 vnd2, vnd2 vnd3, and vnd1 vnd2 vnd3 mutants grown under continuous dark conditions, but the defects in vnd2 single and vnd2 vnd3 double mutants were less severe than those of vnd1 vnd2 vnd3 triple mutants (Fig. 8G). In addition, the connectivity was affected significantly in vnd1 vnd2 vnd3 triple mutants (Fig. 8G). Therefore, VND1, VND2, and VND3 would contribute greatly to vein network formation under continuous dark conditions. Prolonged culture (for 14 d) of vnd1 vnd2 vnd3 triple mutant cotyledons under continuous dark conditions did not change the defects in cardinality and connectivity of vein networks (Supplemental Fig. S8), suggesting that the defects in secondary veins in vnd1 vnd2 vnd3 under dark conditions were not due to delayed differentiation. In addition, we examined the expression of the ATHB8pro:GUS reporter, which is expressed in procambial cells and xylem precursor cells (Scarpella et al., 2004; Donner et al., 2009), in the wild type and vnd1 vnd2 vnd3 (Supplemental Fig. S9). The GUS signal patterns were similar in the wild type and the vnd1 vnd2 vnd3 mutant; therefore, procambium cells could be established in the vnd1 vnd2 vnd3 cotyledon, even under dark conditions. These results indicated that VND1, VND2, and VND3 have crucial roles in endogenous xylem vessel element differentiation during secondary vein formation but not in procambium cell formation.
Figure 8.
Effects of vnd mutation on vein formation in cotyledons. A to F, Representative images of vein formation patterns in cotyledons of 7-d-old light-grown (A, C, and E) or dark-grown (B, D, and F) seedlings: the wild type (WT; A and B), vnd6 vnd7 (C and D), and vnd1 vnd2 vnd3 (E and F). Bars = 1 mm (A, C, and E) and 250 µm (B, D, and F). G, Vein formation patterns in the cotyledons of 7-d-old light- and dark-grown seedlings: the wild type, single mutants vnd1, vnd2, and vnd3, double mutants vnd1 vnd2, vnd1 vnd3, vnd2 vnd3, and vnd6 vnd7, and the vnd1 vnd2 vnd3 triple mutant. Based on the elements defined by Verna et al. (2015; i.e. break points, touch points, end points, and exit points), the cardinality, continuity, and connectivity of vein networks were calculated. For each genotype, 30 cotyledons were examined. Significance for multiple comparisons was calculated using one-way ANOVA with a posthoc Tukey’s HSD test (different letters indicate significantly different values at P < 0.05).
Effects of Light Conditions and vnd1 vnd2 vnd3 Mutations on VND Expression in Cotyledons
We examined the expression of VND genes in the cotyledon using promoter:GUS reporter assays (Supplemental Fig. S10; Yamaguchi et al., 2008) and RT-qPCR analysis (Fig. 9). The reporter gene XCP1pro:GUS, which is strongly expressed during PCD in developing xylem vessel elements (Yamaguchi et al., 2011), was used as a marker of xylem vessel formation. Seedlings grown under continuous light or dark conditions were harvested at 81, 93, and 105 h after imbibition of seeds, fixed, and treated with the GUS substrate, and the GUS signals were observed in cotyledons (Supplemental Fig. S10). The light conditions did not affect the spatiotemporal expression patterns of VNDpro:GUS or XCP1pro:GUS (Supplemental Fig. S10). The promoter activities of VND2, VND3, and VND6 were detected in both primary and secondary veins, while the VND7 and XCP1 promoters seemed to be active only in primary veins. In addition, VND1, VND2, and VND6 promoter activities were detected in the distal margin of the primary vein in cotyledons (Supplemental Fig. S10). No promoter:GUS activity was detected for VND4 and VND5 in the tested young seedlings, regardless of the light conditions, and VND1pro:GUS activity was found mainly in the mesophyll cells of cotyledons (Supplemental Fig. S10).
Figure 9.

Effects of light conditions on VND transcript abundance in cotyledons. The copy numbers are shown for VND transcripts obtained from cotyledons of wild-type (WT) and vnd1 vnd2 vnd3 seedlings imbibed and germinated under continuous light or dark conditions at 240 h after imbibition using RT-qPCR. Error bars indicate sd of three independent biological replicates. Significance for multiple comparisons was calculated using one-way ANOVA with a posthoc Tukey’s HSD test (different letters indicate significantly different values at P < 0.05); n.d., not detected.
Next, we quantified the transcript abundance of VND family genes by RT-qPCR using cotyledon samples prepared from wild-type and vnd1 vnd2 vnd3 seedlings grown under continuous light or dark conditions (Fig. 9). In the wild type, the transcript abundance of VND1, VND2, and VND6 was decreased under dark conditions compared with light conditions (Fig. 9), implying that light stimuli could up-regulate the expression of these VND genes, probably without drastic changes in spatial expression patterns (Supplemental Fig. S10). By contrast, in the vnd1 vnd2 vnd3 triple mutant cotyledons, in which no expression of VND2 and reduction of VND1 and VND3 were detected, the abundance of VND transcripts was not significantly different between light and dark conditions; regardless of the light conditions, the expression levels of VND1 and VND4 to VND7 were similar to those of the wild type under dark conditions (Fig. 9). These results suggest that light changes the expression levels of specific VND genes. Furthermore, given that the cotyledons of the light-grown vnd1 vnd2 vnd3 mutant formed normal secondary veins (Fig. 8, F and H), xylem vessel element differentiation in cotyledons under dark conditions appears to require a certain level of expression of specific VND genes (i.e. VND1, VND2, and VND3; Fig. 9).
Effects of vnd1 vnd2 vnd3 Mutations on Light Condition-Dependent Root Development
Finally, the effects of vnd1 vnd2 vnd3 mutations on seedling growth were examined. Under normal light conditions of our laboratory for Arabidopsis (continuous light and 45–85 µmol m−2 s−1), the vnd1 vnd2 vnd3 seedlings showed no severe morphological and growth defects, while the primary root length of vnd1 vnd2 vnd3 was slightly longer than that of the wild type (Table I). When the light intensity was decreased to 5 to 12 µmol m−2 s−1 (weak light condition), both the wild-type and vnd1 vnd2 vnd3 seedlings showed retarded growth, resulting in decreased length of primary roots and lack of visible lateral roots (Table I). To monitor responses to the change of light conditions, the wild-type and vnd1 vnd2 vnd3 seedlings were grown under the weak light condition for 3 d and then transferred to the normal light condition. While the wild-type seedlings recovered lateral root development by transfer to the normal light condition, the vnd1 vnd2 vnd3 seedlings failed to restore lateral root numbers (Table I). It is known that light intensity and quality are critical factors to regulate lateral root development (Thomas et al., 2014). Our observations imply physiological roles of VND1, VND2, and VND3 in the regulation of seedling development (i.e. cotyledon venation and lateral root formation) in response to light conditions.
Table I. Effects of the vnd1 vnd2 vnd3 mutation on light condition-dependent root development.
For each genotype and condition, 30 seedlings were examined. Different letters indicate statistically significant differences among light conditions (P < 0.05; Tukey’s multiple test for primary root length and Student’s t test for number and density of lateral roots).
| Plant | Parameter | Light Condition |
||
|---|---|---|---|---|
| Normal | Weak | Weak→Normal | ||
| Wild type | Primary root length (mm) | 37.0 ± 4.4 a | 22.5 ± 2.6 b | 33.3 ± 2.9 c |
| No. of lateral roots (per plant) | 3.2 ± 1.5 | 0 | 2.9 ± 1.3 | |
| Density of lateral roots (No. per cm) | 0.85 ± 0.41 | – | 0.85 ± 0.32 | |
| vnd1 vnd2 vnd3 | Primary root length (mm) | 43.0 ± 7.3 a | 21.0 ± 3.5 b | 33.5 ± 2.4 c |
| No. of lateral roots (per plant) | 6.5 ± 2.5 a | 0 | 2.3 ± 1.2 b | |
| Density of lateral roots (No. per cm) | 1.54 ± 0.62 a | – | 0.68 ± 0.37 b | |
DISCUSSION
Specific Contributions of VND1, VND2, and VND3 to the Phytohormone KDB System and Cotyledon Vein Formation
In this work, we successfully elucidated the specific functions of VND1, VND2, and VND3 in xylem vessel element differentiation of cotyledon cells. The KDB system, an innovative in vitro induction system for xylem vessel element differentiation using Arabidopsis cotyledons, showed clear dependency on VND1, VND2, and VND3 but not on VND6 or VND7 (Figs. 1A and 6). Currently, an evolutionarily conserved NAC-MYB-based transcriptional regulatory scheme has been suggested to regulate SCW-related cell differentiation (Hussey et al., 2013; Nakano et al., 2015). Considering our results using the myb46 myb83 double mutant (Supplemental Fig. S7) and expression analysis of MYB46 genes in the vnd1 vnd2 vnd3 mutant (Fig. 7), xylem vessel element differentiation in the KDB system appears to be driven by a regulatory system consisting of VND1, VND2, and VND3 as the NAC proteins and MYB46 and MYB83 as the MYB proteins. The importance of VND1, VND2, and VND3 for xylem vessel element differentiation also was observed in the cotyledons of seedlings: in the vnd1 vnd2 vnd3 mutant seedlings, xylem formation in secondary vein formation in the cotyledon was affected under dark conditions (Figs. 8G and 10). Notably, the single vnd2 mutant showed a significant decrease in ectopic xylem vessel element differentiation in the KDB system (Fig. 6) and in the cardinality of vein networks in cotyledons under dark conditions (Fig. 8G). Thus, it is possible that VND2 makes relatively strong contributions to the integrity of the vascular network of cotyledons through xylem vessel element differentiation in response to extracellular stimuli.
Figure 10.
Proposed schematic model of secondary vein formation in cotyledons. A and B, Formation of secondary veins in light-grown (A) and dark-grown (B) wild-type cotyledons. C and D, Formation of secondary veins in the cotyledons of light-grown (C) and dark-grown (D) vnd1 vnd2 vnd3 triple mutant.
The mRNA-seq and RT-qPCR data indicated higher transcript levels of VND1 and VND2 after KDB treatment when compared with the other VND genes (Fig. 5; Supplemental Fig. S4), suggesting that the dependency of the KDB system on VND1 and VND2 can be largely explained by the transcriptional regulation of VND genes in the cotyledons in response to phytohormones. A recently developed in vitro induction system for vascular cell differentiation uses excised Arabidopsis rosette leaf samples (Kondo et al., 2014, 2015, 2016). In that system, leaf disc samples or excised leaves are cultured in the presence of auxin, cytokinin, and bikinin, which strongly activates brassinosteroid signaling via the inhibition of BRASSINOSTEROID-INSENSITIVE2, a negative regulator of brassinosteroid signaling in Arabidopsis. As a result, not only xylem vessel elements but also phloem cells can be ectopically induced in this system (Kondo et al., 2016). In the system developed by Kondo et al. (2016), the ectopic xylem vessel element differentiation is largely dependent on VND6, and the expression of all VND genes seems to be equally up-regulated (Kondo et al., 2015, 2016). Therefore, our observation of the dependency of the KDB system on VND1 and VND2 likely reflects cotyledon-specific aspects of vascular development.
In the secondary veins of cotyledons in seedlings, however, the vnd1 vnd2 vnd3 phenotype did not appear to be correlated with the transcript level of these VND genes. Although the expression levels of all other VND genes were comparable in the vnd1 vnd2 vnd3 cotyledons grown under continuous light and dark conditions, secondary vein formation was inhibited only in vnd1 vnd2 vnd3 cotyledons under dark conditions (Figs. 8G, 9, and 10), suggesting that the expression of VND4 to VND7 is sufficient for xylem vessel element differentiation under light conditions but not for xylem vessel element differentiation under dark conditions. This can be explained by some posttranscriptional regulation of VND functions specific for dark conditions. Phylogenetic analysis with amino acid sequences of NAC domains showed that VND1, VND2, and VND3 are subgrouped within the VND family and that VND1 and VND2 are closely related (Kubo et al., 2005; Zhou et al., 2014; Nakano et al., 2015). It is possible that the VND1-, VND2-, and VND3-specific molecular features of protein structure, such as NAC domain structure, could be related to the phenotype of vnd1 vnd2 vnd3 for xylem formation in secondary veins. Further detailed study of the DNA-binding properties of VNDs could provide clues to the molecular basis of VND1-, VND2-, and VND3-specific functions.
VND Genes at the Junction between Light Conditions and Vein Formation
Xylem vessel development is regulated in response to environmental signals. VND genes were shown to be involved in xylem vessel element formation in response to Verticillium spp. fungal infection (Reusche et al., 2012) and salt stress (Taylor-Teeples et al., 2015). Here, we revealed that light signals change the expression of VND genes in the cotyledons during the early stages of seedling development (Fig. 9); however, our promotor:GUS expression analysis showed that the light conditions did not change the spatial patterns of VND expression (Supplemental Fig. S10). These data suggest that light promotes VND expression in cotyledons. Vein development in leaves is more active under light conditions than under dark conditions (e.g. compare the cardinality of the wild type between light and dark conditions in Fig. 8G). The activation of VND transcription could be one of the reasons underlying such advanced development of vein formation in the light.
The AtGenExpress Light Series project showed that the irradiation of 4-d-old dark-grown seedlings with different types of light (blue, red, far-red, and white light) for 4 h did not significantly change the expression level of VND (AtGenExpress light series in Arabidopsis eFP browser; Winter et al., 2007). Thus, the up-regulation of VND expression by light signals shown here would require several steps mediated by transcriptional regulators after light signal perception by a suite of photoreceptors, such as the red/far-red light receptors (phytochrome [PHYs]) and blue light receptors (cryptochromes [CRYs] and phototropins [PHOTs]; de Lucas and Prat, 2014; Kong and Okajima, 2016). Moreover, vein formation in leaves is altered in Arabidopsis photoreceptor mutants such as cry1, cry2, phot1, phot1 phot2, phya phyb phyd, phya phyb phye, phyb phyd phye, and phya phyb phyd phye (Sherr, 2012). The branching of veins is increased in these mutants, implying that UV-A/blue and red/far-red light signaling are negative regulators of vein network formation (Sherr, 2012). The PHY proteins interact directly with phytochrome-interacting factors (PIFs), a subset of bHLH transcription factors that function as key regulators of light-regulated plant development (Chen and Chory, 2011; Leivar and Quail, 2011; de Lucas and Prat, 2014). The PIFs accumulate in darkness or shade but undergo phosphorylation and subsequent degradation upon interaction with light-activated PHYs (Ni et al., 2013). A recent study showed that PIF4 interacts with the BR-signaling transcription factors BRASSINAZOLE RESISTANT1 (BZR1) and BZR2/BES1 (Oh et al., 2012). The latter, BZR2/BES1, is involved in the differentiation of cambial cells into xylem cells under the regulation of the Tracheary Element Differentiation Inhibitory Factor (TDIF)-TDIF Receptor (TDR) signaling pathway (Kondo et al., 2014, 2015; Kondo and Fukuda, 2015). Thus, it is possible that the interaction between VNDs and light signaling-related transcription factors, such as PIFs and/or BZRs, could be involved in xylem vessel element differentiation downstream of photoreceptor-mediated light perception and signaling.
Interestingly, the light responsiveness of VND gene expression was not observed in the cotyledons of vnd1 vnd2 vnd3 mutant seedlings (Fig. 9). This result implies the involvement of VND1, VND2, and VND3 in the light-dependent up-regulation of VND genes. Endo et al. (2015) reported that VND1 to VND6 activate the transcription of VND7 and hypothesized that VND1 to VND6 boost VND7 expression to sufficient levels to induce xylem vessel element differentiation effectively. Our data suggest that some VND proteins regulate the expression of other VND genes in response to light signals. The VND genes may have functionally diversified to modulate the activity of other VND family proteins as part of a mechanism that ensures the development of environmentally appropriate xylem vessels. Indeed, the vnd1 vnd2 vnd3 seedlings showed the abnormality of responsiveness to changes in light conditions (Table I), suggesting the importance of VND1 to VND3 for light condition-dependent xylem differentiation in seedlings.
In conclusion, we revealed that VND1 to VND3, but not VND6 or VND7, are the main contributors of xylem vessel element differentiation in the secondary veins of cotyledons in darkness (Fig. 10). These data strongly suggest that not only VND6 and VND7, but all the VND proteins, promote xylem vessel formation throughout the plant body under various environmental conditions (e.g. continuous light, as shown here); as such, the VND proteins appear to have important roles in the adaptation and survival of plants on dry land.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana) ecotype Columbia was used as the wild type and to generate transgenic plants. VND1 to VND7pro:GUS and XCP1pro:GUS plants were as described by Yamaguchi et al. (2008, 2011). The vnd mutant lines, vnd1 (SALK_022534), vnd2 (SALK_022124), vnd3 (SAIL_58_D12), vnd6 (GABI_567F08), and vnd7 (SALK_115812), were crossed to generate multiple vnd mutants, including vnd1 vnd2, vnd1 vnd3, vnd2 vnd3, vnd1 vnd2 vnd3, and vnd6 vnd7. The nst1 nst3 double mutant was kindly provided by Dr. Nobutaka Mitsuda (Mitsuda et al., 2007, Mitsuda and Ohme-Takagi, 2008). The myb46 myb83 double mutant was as described by Nakano et al. (2015). The wild type and vnd1 vnd2 vnd3 triple mutants harboring ATHB8pro:GUS, in which the 2-kb region upstream of the start codon of ATHB8 drives the expression of GUS, were generated by the direct introduction of the ATHB8pro:GUS construct into wild-type or vnd1 vnd2 vnd3 plants.
Plasmid Construction and Plant Transformation
For the promoter analysis, promoter fragments of ATHB8 (Supplemental Table S5) were subcloned into the pENTR/D-TOPO vector and then integrated into the Gateway destination vector, pBGGUS (Kubo et al., 2005). For transformation, the generated plasmids were electroporated into Agrobacterium tumefaciens strain GV3101::pMP90, and 4- to 5-week-old Arabidopsis plants were transformed by the floral dip method (Clough and Bent, 1998).
Arabidopsis in Vitro Xylem Vessel Element Differentiation System (KDB System)
Arabidopsis seeds were sterilized with 70% (v/v) ethanol followed by Plant Preservation Mixture (PPM) and then sown on MS agar medium containing MS plant salt mixture (Wako), 0.5% (w/v) Suc (Wako), 0.05% (w/v) MES adjusted to pH 5.8 with potassium hydroxide (Dojindo), 500× vitamin mix (Wako), and 0.6% (w/v) gellan gum (Wako). The plates with seeds were incubated for 2 d at 4°C and then grown at 22°C under continuous light for 6 d. The cotyledons of 6-d-old seedlings were excised and incubated in one-half-strength MS liquid medium supplemented with phytohormones (KDB): cytokinin (50 ng mL−1 kinetin [K]), auxin (500 ng mL−1 2,4-dichlorophenoxyacetic acid [D]), and brassinosteroid (1 μm brassinolide [B]) at 22°C under continuous light. For the mock treatment, the excised cotyledons were incubated in one-half-strength MS liquid medium without any phytohormones.
Growth Conditions for Seedlings under Continuous Light or Dark Conditions
Arabidopsis seeds were sterilized and sown on MS agar medium as described for the KDB system above. Plates were incubated for 72 h in darkness at 4°C and then transferred to 22°C incubators with continuous lighting (45–85 µmol m−2 s−1). For the continuous dark condition, the plates were wrapped in aluminum foil.
For the investigation of primary root growth, the wild-type and vnd1 vnd2 vnd3 seeds sown on MS agar medium were incubated for 72 h in darkness at 4°C and then transferred to 22°C incubators with continuous lighting (45–85 µmol m−2 s−1 as the normal light condition and 5–12 µmol m−2 s−1 as the weak light condition) for 7 d. To shift the light condition, the seedlings grown under the weak light condition for 3 d were transferred to the normal light condition and grown for an additional 4 d. Primary root length were measured based on the images of plates containing 7-d-old seedlings by ImageJ software (https://imagej.nih.gov/ij/).
Observation of Autofluorescence Signals of Lignin
Excised cotyledons, either mock or KDB treated for 5 d, were fixed in 90% (v/v) acetone at –30°C for 24 h. After three washes with distilled water, the samples were mounted in clearing solution (a mixture of 8 g of chloral hydrate, 1 mL of glycerol, and 2 mL of water). Lignin was visualized using mercury lamp excitation with a WU filter (dichroic mirror DM400, excitation filter BP330-385, and barrier filter BA420) under microscopy (BX51; Olympus) and photographed with a digital camera (DP70; Olympus).
Safranin-O Staining for the Visualization of Lignin
Excised cotyledons mock or KDB treated for 5 d were fixed in 90% (v/v) acetone at –30°C for 24 h. After three rounds of washing with distilled water, the samples were stained with 0.1% (v/v) safranin-O (Kitin et al., 2000) for 5 min at room temperature. Thereafter, the samples were dehydrated using an ethanol series (10%, 30%, 50%, 70%, 90%, and 100% [v/v] ethanol, 40-min incubation for each concentration) and then rehydrated with a second ethanol series (90%, 70%, 50%, 30%, and 10% [v/v] ethanol, 40-min incubation for each concentration) on a rotator. Finally, samples were mounted in distilled water. Observation was performed with UV excitation and a DAPI filter (wavelength, 410–585 nm) using a confocal laser scanning microscope system (Zeiss LSM 710).
Double Staining of Xylan and Cellulose
Double staining of xylan and cellulose was performed. Xylan was detected with the LM10 antibody, which specifically recognizes xylan (McCartney et al., 2005), and cellulose was visualized with Pontamine Fast Scarlet 4B (Sigma-Aldrich), as described previously (Schuetz et al., 2014). Briefly, excised cotyledons mock or KDB treated for 5 d were fixed in fixation solution (4% [w/v] paraformaldehyde, 50 mm PIPES, 5 mm MgSO4, and 5 mm EGTA) for 2 h at room temperature. After two washes with TBST buffer (TBST; 10 mm Tris-HCl, pH 7, 0.25 m NaCl, and 0.1% [v/v] Tween 20) for 10 min, the samples were incubated in 5% (w/v) bovine serum albumin (Sigma-Aldrich) in TBST for 1 h at room temperature and subsequently incubated in a 1:36 dilution of LM10 primary antibody in TBST buffer overnight at 4°C. The samples were then rinsed three times with TBST for 5 min and incubated in a 1:100 dilution of secondary anti-rat Alexa 488 antibody (Invitrogen) overnight at 4°C. After washing twice with TBST for 10 min, the samples were incubated in one-half-strength MS medium containing 10 mg mL−1 Pontamine Fast Scarlet 4B (Sigma-Aldrich) for 30 min at room temperature. After two washes with TBST, the samples were mounted in one-half-strength MS liquid medium. Xylan and cellulose were observed using Alexa Fluor 488 (wavelength, 493–556 nm) and Alexa Fluor 594 (wavelength, 585–734 nm), respectively, using a confocal laser scanning microscope system (Zeiss LSM 710).
DAPI Staining for Nucleus Visualization
Excised cotyledons, either mock or KDB treated for 5 d, were fixed in fixation solution (1.5% [v/v] formaldehyde, 0.5% [v/v] glutaraldehyde in PEMT buffer containing 50 mm PIPES, 2 mm EGTA, 2 mm MgSO4, and 0.05% [v/v] Triton X-100, pH 7.2; Sugimoto et al., 2000) for 40 min at room temperature. The samples were rinsed with PEMT buffer three times for 10 min each, followed by rinsing with phosphate-buffered saline (PBS) buffer at room temperature three times for 10 min each. As described by Ishida et al. (2009), the samples were further stained with 1 mm DAPI solution for 10 min at room temperature and then washed with PBS buffer three times for 10 min each. Finally, the samples were mounted in PBS buffer prior to imaging with UV excitation and a DAPI filter (wavelength, 410–585 nm) using a confocal laser scanning microscope system (Zeiss LSM 710).
GUS Staining
Samples were fixed in 90% (v/v) acetone overnight at –30°C and subjected to GUS staining according to the method of Pyo et al. (2007). The fixed samples were washed three times with 100 mm sodium phosphate buffer (pH 7) and then incubated in the substrate solution (1 mm 5-bromo-4-chloro-3-indolyl glucuronide, 0.5 mm potassium ferricyanide, and 0.5 mm potassium ferrocyanide) in 100 mm sodium phosphate (pH 7) at 37°C for 24 h, except for KDB-treated XCP1pro:GUS samples, which were incubated for 1 h. After washing with 100 mm sodium phosphate buffer (pH 7), the samples were mounted in the clearing solution. Samples were observed with a microscope equipped with Nomarski optics (BX51; Olympus) and photographed with a digital camera (DP70; Olympus).
Vein Pattern Observation
Seven-day-old seedlings grown under continuous light or dark conditions were fixed in 90% (v/v) acetone for 24 h at –30°C. After three rounds of washing with distilled water, the samples were mounted in the clearing solution. Samples were observed with a microscope equipped with Nomarski optics (BX51; Olympus) and photographed with a digital camera (DP70; Olympus). The vein patterns were categorized according to the method described by Verna et al. (2015).
Quantitative and Semiquantitative RT-PCR Analysis
Total RNA was isolated using Trizol reagent (Invitrogen) with DNase I (Qiagen). First-strand cDNA was synthesized using oligo(dT) primers and Transcriptor Reverse Transcriptase (Roche) from 1 µg of total RNA samples.
RT-qPCR analysis was performed using the first-strand cDNA as a template with the Light Cycler 480 SYBR Green 1 Master (Roche) according to the manufacturer’s instructions. The reactions were incubated at 95°C for 5 min, followed by 45 repeated cycles of 95°C for 10 s, 59°C for 10 s, and 72°C for 10 s. The final cycle was followed by the generation of a melting curve by incubation at 95°C for 5 s and 65°C for 1 min and cooling with incubation at 50°C for 30 s. Each gene expression level was determined by the second derivative maximum method (Roche). UBQ10 was used as the internal control, and the relative abundance of transcripts to UBQ10 was calculated for the tested genes. Three biological replicates were performed.
For semiquantitative RT-PCR analysis to evaluate the expression of the VND genes, 1 µL of first-strand cDNA product was used as template, with 1.4 or 2 μL of 25 mm MgCl2, 4 μL of 5× PCR buffer, 0.6 μL of 10 mm deoxyribonucleotide triphosphate mix, 1 μL of each gene-specific forward and reverse primer, 0.1 μL of Kapa Taq Extra DNA Polymerase (Kapa Biosystems), and nuclease-free water to make a 10-μL reaction volume for each PCR. The PCR products were separated on 2% (w/v) agarose gels and stained with ethidium bromide. UBQ10 was used as the internal control.
The information for primers used here is shown in Supplemental Table S5.
mRNA-seq Library Preparation and Sequencing
Total RNA extraction using Trizol reagent (Invitrogen) was performed according to the manufacturer’s instructions. The purity and concentration of the RNA were measured using the NanoDrop 1000 Spectrophotometer (Thermo Scientific), and samples with an A260/A280 ratio of 1.8 to 2 were used for further analysis. The quality of RNA samples was assessed by agarose gel electrophoresis and on an Agilent 2100 Bioanalyzer (Agilent Technologies), and samples with a high RNA integrity number (7 or greater) were used for mRNA library sample preparation. Poly(A+) mRNA was purified from 20 μg of total RNA using FastTrack MAG mRNA Isolation Kits (Ambion) and then used to construct cDNA libraries with the NEBNext mRNA Library Prep Reagent Set for Illumina (New England Biolabs), according to the manufacturer’s instructions. Briefly, 100 ng of purified mRNA was fragmented (to ∼200-nucleotide fragments) at 94°C for 5 min. Double-stranded cDNA was synthesized from the fractionated RNA pool, and then the cDNA fragments were end repaired. After A-tailed and indexed adapters were ligated, the products were purified using Agencourt AMPure XP Beads (Beckman Coulter) and amplified by PCR to create the final cDNA libraries. The libraries were further quantitated using a KAPA Library Quantification Kit (Kapa Biosystems), and a total of 21 cDNA libraries (seven experimental conditions [i.e. samples for 1, 3, and 5 d of mock and KDB treatment in addition to 0 d of treatment] × three biological replicates) were pooled in equal amounts (2 nm) for multiplexing. These libraries were sequenced on seven lanes per run (two runs) of Illumina GAIIx at the Plant Global Unit, Nara Institute of Science and Technology, Japan. The data generated by the Illumina Genome Analyzer II were processed using open-source Firecrest (image analysis), Bustard (base calling), and CASAVA version 1.8.2 (convert base calling file into demultiplexed Fastq file) applications (Illumina) to produce digital-quality data (33 nucleotides).
mRNA Library Data Analysis
Each 33-nucleotide end read (raw data set) for each library was mapped independently using Bowtie in TopHat version 2.0.10 (http://tophat.cbcb.umd.edu/; Trapnell et al., 2009) against the Arabidopsis genome (TAIR10 [ftp://ftp.arabidopsis.org/home/tair/Genes/TAIR10_genome_release/TAIR10_gff3/TAIR10_GFF3_genes.gff]; Lamesch et al., 2012). Genome Analyzer II libraries were aligned with the following options: —segment-length 16, —max-multihits 20, —report-secondary-alignments, and GTF TAIR10_GFF3_genes.gff. The other parameters were set as default. Transcript expression and differentially expressed genes (DEGs) between all possible pairwise combinations of conditions were defined with the Cuffdiff2 program (Trapnell et al., 2009), a part of the Cufflinks package (http://cufflinks.cbcb.umd.edu). Cuffdiff2 was run with default settings except —min-alignment-count 1. The relative expression level was calculated in paired-end fragments per kilobase of exon model per million mapped reads, and genes with q < 0.01 were regarded as DEGs.
Clustering Analysis
Clustering of 21 mRNA libraries was performed using Pvclust (Suzuki and Shimodaira, 2006) with bootstrap replication of 1,000 for hierarchical clustering and edgeR (Robinson et al., 2010) for plotting multidimensional scaling. Soft clustering using Mfuzz (Futschik and Carlisle, 2005) was performed on gene sets reported in the literature to contain the vascular-related genes (Supplemental Table S6; Truernit and Sauer, 1995; Bonke et al., 2003; Mähönen et al., 2006; Ohashi-Ito and Bergmann, 2007; Gardiner et al., 2010; Ohashi-Ito et al., 2010, 2013a, 2013b, 2014; Schlereth et al., 2010; Yamaguchi et al., 2011; De Rybel et al., 2013, 2014; Miyashima et al., 2013; Furuta et al., 2014; Endo et al., 2015; Kondo et al., 2015) and epidermal and mesophyll cell-related genes (Supplemental Table S7; Susek et al., 1993; Lu et al., 1996; Abe et al., 2003; Bruex et al., 2012) based on the fragments per kilobase of exon model per million mapped reads values of each gene in the 21 mRNA libraries. All analyses were performed with default parameter settings.
GO and Pathway Enrichment Analysis
For GO analyses, DEG data sets between mock- and KDB-treated samples at 1, 3, and 5 d of treatment with more than a 5-fold change were separated into up- or down-regulated gene sets and then independently subjected to the agriGO engine (http://bioinfo.cau.edu.cn/agriGO/; Du et al., 2010). For pathway enrichment analysis, the same gene lists were subjected to Arabidopsis Reactome Skypainter (http://www.arabidopsisreactome.org/cgi-bin/skypainter2; Tsesmetzis et al., 2008). The GO terms and pathways with a Benjamini and Hochberg false discovery rate of less than 0.05 were considered to be statistically overrepresented.
Large Data Sets
The mRNA-seq data presented in this study were submitted to the DNA Data Bank of Japan Sequence Read Archive (http://trace.ddbj.nig.ac.jp/dra/index_e.html) and can be retrieved via accession number DRA006129.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: AT2G18060 (VND1), AT4G36160 (VND2), AT5G66300 (VND3), AT1G12260 (VND4), AT1G62700 (VND5), AT5G62380 (VND6), AT1G71930 (VND7), AT1G19850 (MP), AT3G25710 (TMO5), AT2G27230 (LHW), AT4G32880 (ATHB8), AT1G52150 (ATHB15), AT2G34710 (PHB), AT1G30490 (PHV), AT5G60690 (REV), AT5G12870 (MYB46), AT3G08500 (MYB83), AT5G17420 (CESA7), AT4G00220 (LBD30), AT4G35350 (XCP1), and AT4G05320 (UBQ10).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. KDB treatment of excised cotyledons of Arabidopsis under light and dark conditions.
Supplemental Figure S2. Reproducibility of transcriptome analysis, shown by clustering of 21 mRNA libraries.
Supplemental Figure S3. Pearson correlation analysis of KDB treatment with mRNA-seq versus RT-qPCR.
Supplemental Figure S4. Expression of VND genes in the KDB system based on mRNA-seq.
Supplemental Figure S5. T-DNA insertion mutant lines of VND genes used in this study.
Supplemental Figure S6. Semiquantitative RT-PCR of VND gene expression in vnd mutants.
Supplemental Figure S7. The myb46 myb83 mutants are unresponsive to induction by KDB.
Supplemental Figure S8. The vnd1 vnd2 vnd3 mutant cotyledon vein formation phenotype persists over development (14 d).
Supplemental Figure S9. Promoter-GUS analysis shows that ATHB8 expression is similar in wild-type and vnd1 vnd2 vnd3 seedlings during imbibition and germination under light and dark conditions.
Supplemental Figure S10. Spatial and temporal expression patterns of VNDs and XCP1 during imbibition and germination under continuous light and dark conditions.
Supplemental Table S1. Summary of doses of phytohormones used.
Supplemental Table S2. Summary of mapping of reads from 21 mRNA libraries to the Arabidopsis genome (TAIR10).
Supplemental Table S3. List of overrepresented plant slim-GO terms for significantly up- or down-regulated genes at each time point (control versus treatment) in excised cotyledons of Arabidopsis.
Supplemental Table S4. List of overrepresented pathways for significantly up- or down-regulated genes at each time point (control versus treatment) in excised cotyledons of Arabidopsis.
Supplemental Table S5. List of primers used in this study.
Supplemental Table S6. Redundant list of all vascular-related genes in the literature data set.
Supplemental Table S7. Redundant list of all epidermal and mesophyll cell-related genes in the literature data set.
Acknowledgments
We thank the Arabidopsis Biological Research Center for providing Arabidopsis seeds. We also thank Dr. Nobutaka Mitsuda and Dr. Yoshimi Nakano (National Institute of Advanced Industrial Science and Technology, Japan) for providing plant materials, Shizuka Nishida and Eriko Tanaka (Nara Institute of Science and Technology, Japan) for technical support, and Dr. Lacey A. Samuels (University of British Columbia) for critical reading of the article.
Footnotes
This work was supported in part by the Naito Science & Engineering Foundation (to M.O.), the Japan Society for the Promotion of Science (KAKENHI Grant Number 25291062 to T.D.), the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant-in-Aid for Scientific Research on Innovative Areas “The Plant Cell Wall as Information Processing System” Grant Number 25114520 and 15H01235 to M.O., 24114002 to T.D., and Grants-in-Aid from the NC-CARP project to T.D.), and the Exploratory Research for Advanced Technology (ERATO) from Japan Science and Technology Agency (Grant Number JPMJER1602 to M.O.) and Japan Advanced Plant Science Network.
Articles can be viewed without a subscription.
References
- Abe M, Katsumata H, Komeda Y, Takahashi T (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643 [DOI] [PubMed] [Google Scholar]
- Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, Ruberti I, Morelli G (2001) The Arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol 126: 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A, Mansfield SD, Hall HC, Douglas CJ, Ellis BE (2010) MYB75 functions in regulation of secondary cell wall formation in the Arabidopsis inflorescence stem. Plant Physiol 154: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollhöner B, Zhang B, Stael S, Denancé N, Overmyer K, Goffner D, Van Breusegem F, Tuominen H (2013) Post mortem function of AtMC9 in xylem vessel elements. New Phytol 200: 498–510 [DOI] [PubMed] [Google Scholar]
- Bonke M, Thitamadee S, Mähönen AP, Hauser MT, Helariutta Y (2003) APL regulates vascular tissue identity in Arabidopsis. Nature 426: 181–186 [DOI] [PubMed] [Google Scholar]
- Brown DM, Zeef LAH, Ellis J, Goodacre R, Turner SR (2005) Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17: 2281–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Zhang Z, Stephens E, Dupree P, Turner SR (2009) Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. Plant J 57: 732–746 [DOI] [PubMed] [Google Scholar]
- Bruex A, Kainkaryam RM, Wieckowski Y, Kang YH, Bernhardt C, Xia Y, Zheng X, Wang JY, Lee MM, Benfey P, et al. (2012) A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genet 8: e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, Berg BL, FitzGerald JN, Jinamornphongs S, Nelson T, Keith B (1999) Genetic regulation of vascular tissue patterning in Arabidopsis. Plant Cell 11: 2123–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vatén A, Thitamadee S, et al. (2010) Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chory J (2011) Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol 21: 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- de Lucas M, Prat S (2014) PIFs get BRright: PHYTOCHROME INTERACTING FACTORs as integrators of light and hormonal signals. New Phytol 202: 1126–1141 [DOI] [PubMed] [Google Scholar]
- De Rybel B, Adibi M, Breda AS, Wendrich JR, Smit ME, Novák O, Yamaguchi N, Yoshida S, Van Isterdael G, Palovaara J, et al. (2014) Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 345: 1255215. [DOI] [PubMed] [Google Scholar]
- De Rybel B, Mähönen AP, Helariutta Y, Weijers D (2016) Plant vascular development: from early specification to differentiation. Nat Rev Mol Cell Biol 17: 30–40 [DOI] [PubMed] [Google Scholar]
- De Rybel B, Möller B, Yoshida S, Grabowicz I, Barbier de Reuille P, Boeren S, Smith RS, Borst JW, Weijers D (2013) A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in Arabidopsis. Dev Cell 24: 426–437 [DOI] [PubMed] [Google Scholar]
- Donner TJ, Sherr I, Scarpella E (2009) Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 136: 3235–3246 [DOI] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38: W64–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Yamaguchi M, Tamura T, Nakano Y, Nishikubo N, Yoneda A, Kato K, Kubo M, Kajita S, Katayama Y, et al. (2015) Multiple classes of transcription factors regulate the expression of VASCULAR-RELATED NAC-DOMAIN7, a master switch of xylem vessel differentiation. Plant Cell Physiol 56: 242–254 [DOI] [PubMed] [Google Scholar]
- Fukuda H. (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5: 379–391 [DOI] [PubMed] [Google Scholar]
- Funk V, Kositsup B, Zhao C, Beers EP (2002) The Arabidopsis xylem peptidase XCP1 is a tracheary element vacuolar protein that may be a papain ortholog. Plant Physiol 128: 84–94 [PMC free article] [PubMed] [Google Scholar]
- Furuta KM, Yadav SR, Lehesranta S, Belevich I, Miyashima S, Heo JO, Vatén A, Lindgren O, De Rybel B, Van Isterdael G, et al. (2014) Arabidopsis NAC45/86 direct sieve element morphogenesis culminating in enucleation. Science 345: 933–937 [DOI] [PubMed] [Google Scholar]
- Futschik ME, Carlisle B (2005) Noise-robust soft clustering of gene expression time-course data. J Bioinform Comput Biol 3: 965–988 [DOI] [PubMed] [Google Scholar]
- Gardiner J, Sherr I, Scarpella E (2010) Expression of DOF genes identifies early stages of vascular development in Arabidopsis leaves. Int J Dev Biol 54: 1389–1396 [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey SG, Mizrachi E, Creux NM, Myburg AA (2013) Navigating the transcriptional roadmap regulating plant secondary cell wall deposition. Front Plant Sci 4: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilegems M, Douet V, Meylan-Bettex M, Uyttewaal M, Brand L, Bowman JL, Stieger PA (2010) Interplay of auxin, KANADI and class III HD-ZIP transcription factors in vascular tissue formation. Development 137: 975–984 [DOI] [PubMed] [Google Scholar]
- Ishida T, Fujiwara S, Miura K, Stacey N, Yoshimura M, Schneider K, Adachi S, Minamisawa K, Umeda M, Sugimoto K (2009) SUMO E3 ligase HIGH PLOIDY2 regulates endocycle onset and meristem maintenance in Arabidopsis. Plant Cell 21: 2284–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitin P, Funada R, Sano Y, Ohtani J (2000) Analysis by confocal microscopy of the structure of cambium in the hardwood Kalopanax pictus. Ann Bot (Lond) 86: 109–117 [Google Scholar]
- Kondo Y, Fujita T, Sugiyama M, Fukuda H (2015) A novel system for xylem cell differentiation in Arabidopsis thaliana. Mol Plant 8: 612–621 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Fukuda H (2015) The TDIF signaling network. Curr Opin Plant Biol 28: 106–110 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Ito T, Nakagami H, Hirakawa Y, Saito M, Tamaki T, Shirasu K, Fukuda H (2014) Plant GSK3 proteins regulate xylem cell differentiation downstream of TDIF-TDR signalling. Nat Commun 5: 3504. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Nurani AM, Saito C, Ichihashi Y, Saito M, Yamazaki K, Mitsuda N, Ohme-Takagi M, Fukuda H (2016) Vascular cell induction culture system using Arabidopsis leaves (VISUAL) reveals the sequential differentiation of sieve element-like cells. Plant Cell 28: 1250–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SG, Okajima K (2016) Diverse photoreceptors and light responses in plants. J Plant Res 129: 111–114 [DOI] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19: 1855–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, et al. (2012) The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res 40: D1202–D1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Porat R, Nadeau JA, O’Neill SD (1996) Identification of a meristem L1 layer-specific gene in Arabidopsis that is expressed during embryonic pattern formation and defines a new class of homeobox genes. Plant Cell 8: 2155–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Törmäkangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y (2006) Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 311: 94–98 [DOI] [PubMed] [Google Scholar]
- McCarthy RL, Zhong R, Ye ZH (2009) MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol 50: 1950–1964 [DOI] [PubMed] [Google Scholar]
- McCartney L, Marcus SE, Knox JP (2005) Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J Histochem Cytochem 53: 543–546 [DOI] [PubMed] [Google Scholar]
- Mitsuda N, Iwase A, Yamamoto H, Yoshida M, Seki M, Shinozaki K, Ohme-Takagi M (2007) NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 19: 270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda N, Ohme-Takagi M (2008) NAC transcription factors NST1 and NST3 regulate pod shattering in a partially redundant manner by promoting secondary wall formation after the establishment of tissue identity. Plant J 56: 768–778 [DOI] [PubMed] [Google Scholar]
- Miyashima S, Koi S, Hashimoto T, Nakajima K (2011) Non-cell-autonomous microRNA165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development 138: 2303–2313 [DOI] [PubMed] [Google Scholar]
- Miyashima S, Sebastian J, Lee JY, Helariutta Y (2013) Stem cell function during plant vascular development. EMBO J 32: 178–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Yamaguchi M, Endo H, Rejab NA, Ohtani M (2015) NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front Plant Sci 6: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Xu SL, Chalkley RJ, Pham TN, Guan S, Maltby DA, Burlingame AL, Wang ZY, Quail PH (2013) Multisite light-induced phosphorylation of the transcription factor PIF3 is necessary for both its rapid degradation and concomitant negative feedback modulation of photoreceptor phyB levels in Arabidopsis. Plant Cell 25: 2679–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen K, Blomster T, Helariutta Y, Mähönen AP (2015) Vascular cambium development. The Arabidopsis Book 13: e0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Wang ZY (2012) Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 14: 802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC (2007) Regulation of the Arabidopsis root vascular initial population by LONESOME HIGHWAY. Development 134: 2959–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Fukuda H (2010) Transcriptional regulation of vascular cell fates. Curr Opin Plant Biol 13: 670–676 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Matsukawa M, Fukuda H (2013a) An atypical bHLH transcription factor regulates early xylem development downstream of auxin. Plant Cell Physiol 54: 398–405 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Oda Y, Fukuda H (2010) Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation. Plant Cell 22: 3461–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Oguchi M, Kojima M, Sakakibara H, Fukuda H (2013b) Auxin-associated initiation of vascular cell differentiation by LONESOME HIGHWAY. Development 140: 765–769 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Saegusa M, Iwamoto K, Oda Y, Katayama H, Kojima M, Sakakibara H, Fukuda H (2014) A bHLH complex activates vascular cell division via cytokinin action in root apical meristem. Curr Biol 24: 2053–2058 [DOI] [PubMed] [Google Scholar]
- Pyo H, Demura T, Fukuda H (2007) TERE: a novel cis-element responsible for a coordinated expression of genes related to programmed cell death and secondary wall formation during differentiation of tracheary elements. Plant J 51: 955–965 [DOI] [PubMed] [Google Scholar]
- Reusche M, Thole K, Janz D, Truskina J, Rindfleisch S, Drübert C, Polle A, Lipka V, Teichmann T (2012) Verticillium infection triggers VASCULAR-RELATED NAC DOMAIN7-dependent de novo xylem formation and enhances drought tolerance in Arabidopsis. Plant Cell 24: 3823–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K, Ursache R, Hejátko J, Helariutta Y (2015) Xylem development: from the cradle to the grave. New Phytol 207: 519–535 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Francis P, Berleth T (2004) Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development 131: 3445–3455 [DOI] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D (2010) MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916 [DOI] [PubMed] [Google Scholar]
- Schuetz M, Benske A, Smith RA, Watanabe Y, Tobimatsu Y, Ralph J, Demura T, Ellis B, Samuels AL (2014) Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiol 166: 798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr I. (2012) Regulation of auxin transport in Arabidopsis leaf vascular development. Master’s thesis. University of Alberta, Edmonton, Canada [Google Scholar]
- Sugimoto K, Williamson RE, Wasteneys GO (2000) New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis. Plant Physiol 124: 1493–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek RE, Ausubel FM, Chory J (1993) Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74: 787–799 [DOI] [PubMed] [Google Scholar]
- Suzuki R, Shimodaira H (2006) Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22: 1540–1542 [DOI] [PubMed] [Google Scholar]
- Taylor-Teeples M, Lin L, de Lucas M, Turco G, Toal TW, Gaudinier A, Young NF, Trabucco GM, Veling MT, Lamothe R, et al. (2015) An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 517: 571–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Sreelakshmi Y, Sharma R (2014) Light modulates the root tip excision induced lateral root formation in tomato. Plant Signal Behav 9: e970098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Sauer N (1995) The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta 196: 564–570 [DOI] [PubMed] [Google Scholar]
- Tsesmetzis N, Couchman M, Higgins J, Smith A, Doonan JH, Seifert GJ, Schmidt EE, Vastrik I, Birney E, Wu G, et al. (2008) Arabidopsis reactome: a foundation knowledgebase for plant systems biology. Plant Cell 20: 1426–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna C, Sawchuk MG, Linh NM, Scarpella E (2015) Control of vein network topology by auxin transport. BMC Biol 13: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel CL, Schuetz M, Yu Q, Mattsson J (2007) Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J 49: 387–398 [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AM, Hörnblad E, Voxeur A, Gerber L, Rihouey C, Lerouge P, Marchant A (2010) Analysis of the Arabidopsis IRX9/IRX9-L and IRX14/IRX14-L pairs of glycosyltransferase genes reveals critical contributions to biosynthesis of the hemicellulose glucuronoxylan. Plant Physiol 153: 542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AM, Rihouey C, Seveno M, Hörnblad E, Singh SK, Matsunaga T, Ishii T, Lerouge P, Marchant A (2009) The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for glucuronoxylan biosynthesis during secondary cell wall formation. Plant J 57: 718–731 [DOI] [PubMed] [Google Scholar]
- Xu B, Ohtani M, Yamaguchi M, Toyooka K, Wakazaki M, Sato M, Kubo M, Nakano Y, Sano R, Hiwatashi Y, et al. (2014) Contribution of NAC transcription factors to plant adaptation to land. Science 343: 1505–1508 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Goué N, Igarashi H, Ohtani M, Nakano Y, Mortimer JC, Nishikubo N, Kubo M, Katayama Y, Kakegawa K, et al. (2010) VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol 153: 906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) Vascular-related NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55: 652–664 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Mitsuda N, Ohtani M, Ohme-Takagi M, Kato K, Demura T (2011) VASCULAR-RELATED NAC-DOMAIN7 directly regulates the expression of a broad range of genes for xylem vessel formation. Plant J 66: 579–590 [DOI] [PubMed] [Google Scholar]
- Zhong R, Demura T, Ye ZH (2006) SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell 18: 3158–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Lee C, Ye ZH (2010) Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol Plant 3: 1087–1103 [DOI] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye ZH (2007a) The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 19: 2776–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye ZH (2007b) Two NAC domain transcription factors, SND1 and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis. Planta 225: 1603–1611 [DOI] [PubMed] [Google Scholar]
- Zhong R, Ye ZH (2012) MYB46 and MYB83 bind to the SMRE sites and directly activate a suite of transcription factors and secondary wall biosynthetic genes. Plant Cell Physiol 53: 368–380 [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhong R, Ye ZH (2014) Arabidopsis NAC domain proteins, VND1 to VND5, are transcriptional regulators of secondary wall biosynthesis in vessels. PLoS ONE 9: e105726. [DOI] [PMC free article] [PubMed] [Google Scholar]