ABA level is important for steady-state stomatal conductance, and OST1 is crucial for air humidity-induced stomatal closure.
Abstract
Guard cells shrink and close stomatal pores when air humidity decreases (i.e. when the difference between the vapor pressures of leaf and atmosphere [VPD] increases). The role of abscisic acid (ABA) in VPD-induced stomatal closure has been studied using ABA-related mutants that respond to VPD in some studies and not in others. The importance of ABA biosynthesis in guard cells versus vasculature for whole-plant stomatal regulation is unclear as well. Here, we show that Arabidopsis (Arabidopsis thaliana) lines carrying mutations in different steps of ABA biosynthesis as well as pea (Pisum sativum) wilty and tomato (Solanum lycopersicum) flacca ABA-deficient mutants had higher stomatal conductance compared with wild-type plants. To characterize the role of ABA production in different cells, we generated transgenic plants where ABA biosynthesis was rescued in guard cells or phloem companion cells of an ABA-deficient mutant. In both cases, the whole-plant stomatal conductance, stunted growth phenotype, and leaf ABA level were restored to wild-type values, pointing to the redundancy of ABA sources and to the effectiveness of leaf ABA transport. All ABA-deficient lines closed their stomata rapidly and extensively in response to high VPD, whereas plants with mutated protein kinase OST1 showed stunted VPD-induced responses. Another strongly ABA-insensitive mutant, defective in the six ABA PYR/RCAR receptors, responded to changes in VPD in both directions strongly and symmetrically, indicating that its VPD-induced closure could be passive hydraulic. We discuss that both the VPD-induced passive hydraulic stomatal closure and the stomatal VPD regulation of ABA-deficient mutants may be conditional on the initial pretreatment stomatal conductance.
Stomata close in response to reduced air humidity (i.e. large vapor pressure deficit [VPD], the difference between the vapor pressures of the leaf and atmosphere). The evolution of this mechanism was an important step in the colonization of land by plants, since it enabled plants to control water loss when they were exposed to a dry atmosphere. However, the mechanism of VPD-induced stomatal closure is still unclear, and the debate about the role of the plant stress hormone abscisic acid (ABA) in this process continues. The response to increased VPD is passive when the stomatal closure results solely from reduced leaf water content and turgor due to stronger evaporative demand in dry air. It has been shown that, in lycophytes, ferns, and conifers, VPD-induced stomatal closure is a passive response to reduced leaf turgor with no functional ABA synthesis detected in their leaves due to increased VPD (McAdam and Brodribb, 2015). Whether lycophytes, mosses, and ferns show functional ABA synthesis and ABA response is debatable at the moment (Brodribb and McAdam, 2011; Chater et al., 2011; Ruszala et al., 2011; Cai et al., 2017); however, in some fern species, stomatal response to VPD is probably more than just a passive process (Hõrak et al., 2017). Angiosperms, on the other hand, showed VPD-induced ABA synthesis associated with a rapid up-regulation of 9-cis-epoxycarotenoid dioxygenase (NCED) genes, indicating that an active, ABA-mediated component is involved (McAdam et al., 2016b). In woody crop species, a direct stomatal response to leaf turgor could explain the changes in stomatal conductance (gs) resulting from the variations in evaporative demand, plant hydraulic conductance, and soil water content (Rodriguez-Dominguez et al., 2016). The stomatal responses to changes in leaf turgor were mediated by ABA in angiosperms, as a subtle reduction of leaf turgor triggered rapid foliar ABA synthesis and stomatal closure in 10 to 20 min (McAdam and Brodribb, 2016; Sussmilch et al., 2017). Such a rapid, turgor-mediated de novo ABA synthesis highlights ABA as the metabolic signal for the hydroactive feedback response of guard cells to hydraulic disturbance in the leaves (Buckley, 2005, 2016).
The ABA signaling unit consists of cytosolic PYRABACTIN RESISTANCE1 (PYR1)/PYR1-LIKE (PYL)/ REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR) receptors that, in the presence of ABA sequester type 2 protein phosphatases, lead to the activation of protein kinase OST1, which, in turn, activates the slow-type anion channel SLAC1 and triggers stomatal closure (Geiger et al., 2009; Lee et al., 2009; Ma et al., 2009; Park et al., 2009). Stomatal responses to reduced air humidity often have been studied using mutants defective in ABA biosynthesis and/or signaling, with the assumption that the reduced VPD response of these plants points to the involvement of ABA. The results, however, are controversial. Initially, it was suggested that ABA is not obligatory in stomatal VPD response, since ABA-deficient and ABA-insensitive Arabidopsis (Arabidopsis thaliana) mutants responded to VPD like wild-type plants (Assmann et al., 2000). In contrast, in another study, ABA synthesis and signaling mutants showed reduced VPD response compared with the wild type (Xie et al., 2006). In the latter study, the final values of gs after reduced air humidity treatment, expressed as a percentage of the initial values, were 53%, 55%, and 32% in the wild type, ost1-4, and aba2-13, respectively, indicating that the closure might be only partially ABA dependent (Xie et al., 2006). Recent experiments demonstrated no VPD response in different ABA-deficient pea (Pisum sativum) and tomato (Solanum lycopersicum) mutants (McAdam et al., 2015, 2016b). Our previous experiments suggest that defects in ABA signaling rather than in its biosynthesis impair stomatal closure in response to reduced air humidity (Merilo et al., 2013). It is possible that plants with impaired ABA synthesis show increased sensitivity to its signaling, resulting in a strong stomatal responsiveness to minor changes in ABA concentration. Such a scenario is supported by experiments showing that changes in the ratios of different components of the ABA signaling unit can tune the ABA sensitivity (Szostkiewicz et al., 2010).
ABA-deficient mutants contain low levels of ABA and are able to synthesize it in response to water deficit (Fig. 1; Frey et al., 2012; González-Guzmán et al., 2002, 2004, 2012; Koornneef et al., 1982; Léon-Kloosterziel et al., 1996; Marx, 1976; Xiong et al., 2002; Yoshida et al., 2002). In these plants, ABA is produced via the residual activity of defective enzymes (Rock and Zeevaart, 1991) or via minor ABA biosynthetic pathways hypothesized to exist in higher plants (North et al., 2007). With regard to the location of ABA synthesis, shoots are the main source of ABA in the plant, as found in grafting studies (Holbrook et al., 2002; Christmann et al., 2007; McAdam et al., 2016a) and also supported by gene expression data (Ernst et al., 2010; Boursiac et al., 2013; Kuromori et al., 2014). The leaf-borne ABA is synthesized mainly in the phloem companion cells of the vasculature (Endo et al., 2008; Kuromori et al., 2014). Nonetheless, the guard cells also express all ABA biosynthetic genes and show increased expression of these under high VPD. Thus, the guard cells themselves can synthesize ABA that initiates the stomatal closure under high-VPD conditions (Bauer et al., 2013). The physiological relevance of ABA synthesis in guard cells versus phloem companion cells in different conditions and time scales is not clear. Furthermore, it is not known whether the minimum ABA threshold needed to activate stomatal ABA signaling can be adjusted to different conditions. However, the ABA threshold to initiate downstream processes is thought to be lower in the guard cells of ABA-deficient mutants (Bauer et al., 2013; Merilo et al., 2013, 2015a).
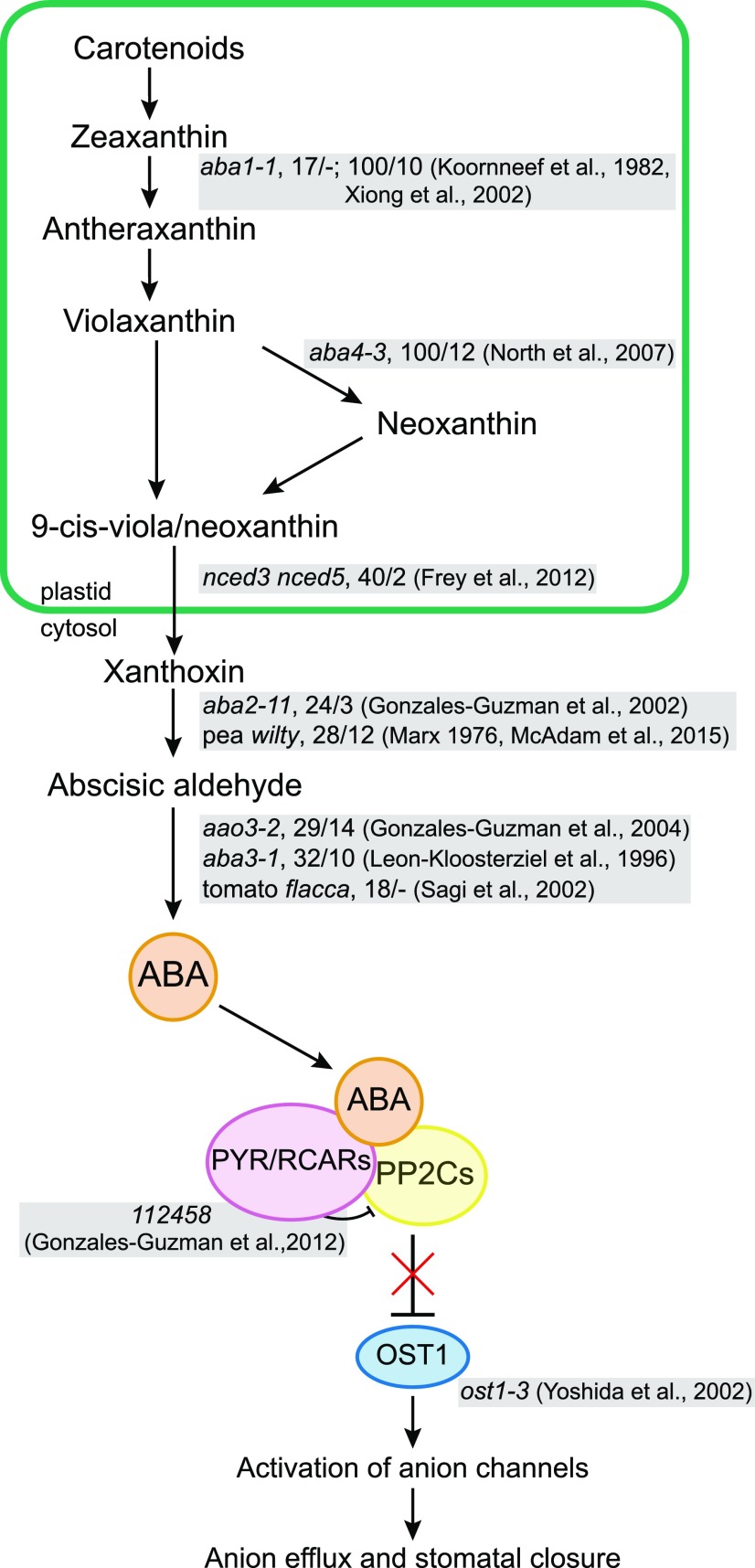
Figure 1.
Schematic overview of ABA biosynthesis and signaling. Mutants used in our experiments are shown together with their rosette ABA concentrations from previous studies (percentage of wild-type values) in normal/water-stressed conditions and the respective references.
To summarize, there is substantial evidence that, in angiosperms, ABA mediation is involved in the VPD-induced stomatal closure. However, it is not resolved whether the passive hydraulic stomatal closure also participates in VPD-induced stomatal regulation of angiosperms. Moreover, the roles of vasculature- and guard cell-borne ABA for VPD-induced stomatal regulation need clarification. Here, we analyzed VPD-induced rapid changes in whole-plant gs in Arabidopsis lines impaired in ABA signaling or in all steps of the ABA biosynthetic pathway starting from zeaxanthin (Fig. 1). The stomatal VPD responses of ABA-deficient pea wilty and tomato flacca lines also were studied. Additionally, we show that transgenic restoration of ABA biosynthesis either in guard cells or phloem companion cells of the strongly ABA-deficient aba2-11 Arabidopsis mutant was enough to increase the leaf ABA level and reduce the steady-state gs of the transgenic plants to the wild-type level.
RESULTS
Both ABA Biosynthesis and ABA Signaling Affect the Steady-State Stomatal Conductance
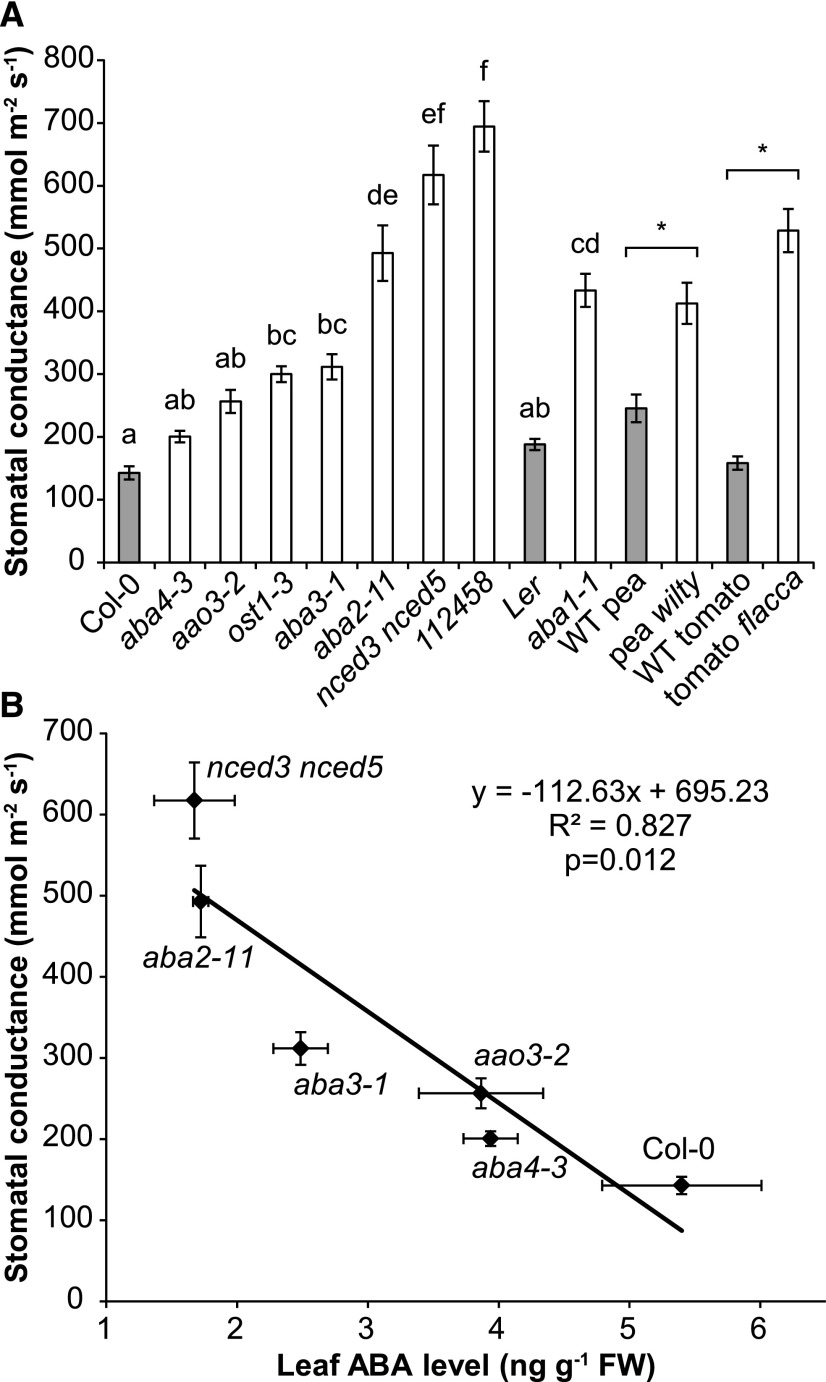
Steady-state gs was calculated from the values of whole-shoot transpiration of plants kept under standard conditions for 1 to 2 h, using a custom-made gas-exchange apparatus and software (Kollist et al., 2007). All ABA-deficient and insensitive lines (listed in Fig. 1) had higher steady-state gs compared with the wild type, although the increase was not significant in aba4-3 and aao3-2 (Fig. 2A). The gs value of aba3-1 was similar to that of ost1-3, whereas aba2-11 and nced3 nced5 had significantly higher gs. Plants of 112458, carrying mutations in six ABA PYR/RCAR receptors, showed the highest gs (Fig. 2A). The gs values of Arabidopsis Col-0-based mutants defective in various steps of ABA biosynthesis were correlated significantly with the leaf ABA level of these mutants (Fig. 2B): under well-watered conditions, ABA levels of aba4-3 and aao3-2 were reduced slightly, whereas those of aba2-11 and nced3 nced5 constituted only 31% of wild-type ABA. ABA-deficient pea wilty and tomato flacca showed significantly higher gs than the respective wild-type lines (Fig. 2A).
Figure 2.
Steady-state gs of all studied lines and its relationship with leaf ABA level in Columbia-0 (Col-0)-based ABA-deficient mutants. A, Stomatal conductance of plants carrying mutations in ABA biosynthesis and the signaling pathway. Significant differences between studied Arabidopsis lines are denoted with different lowercase letters (average ± se; n = 5–8) and with asterisks between a mutant and the respective wild type (WT) in pea and tomato (n = 6 or 7). B, Relationship between leaf ABA level and steady-state gs of Arabidopsis ABA-deficient mutants and the Col-0 wild type. FW, Fresh weight.
Recovering ABA Production Either in Guard Cells or in Phloem Companion Cells Restores Leaf ABA Level, Visual Phenotype, and Steady-State Stomatal Conductance
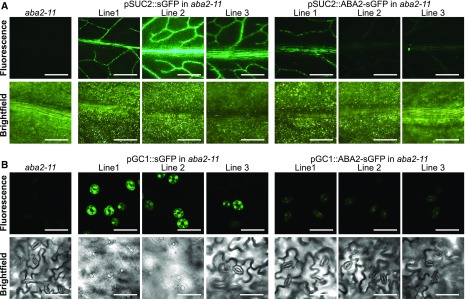
It has been demonstrated that ABA can be synthesized in guard cells (Bauer et al., 2013) in addition to vasculature. The relative importance of these two sources in the total foliar ABA pool and the regulation of physiological processes, however, is not clear. We used tissue-specific promoters to generate transgenic plants where ABA biosynthesis would be restored specifically in the guard or phloem companion cells. The aba2-11 mutant was chosen as the genetic background due to its high gs and low ABA level (Fig. 2B). The guard cell-specific GC1 promoter (Yang et al., 2008) and the phloem-specific SUC2 promoter (Truernit and Sauer, 1995) were employed to express ABA2 tagged with sGFP in guard cells and phloem companion cells, respectively. Control plants with GC1 or SUC2 promoters driving the expression of sGFP were generated as well. Plants of the aba2-11 mutant transformed with the pSUC2::ABA2-sGFP and pSUC2::sGFP constructs demonstrated sGFP signal in the vasculature of leaves and roots but not in the guard cells (Fig. 3; Supplemental Figs. S1 and S2). Transgenic aba2-11 lines expressing ABA2-sGFP or sGFP under the control of the GC1 promoter demonstrated targeted expression of the proteins in guard cells (Fig. 3; Supplemental Figs. S1 and S2). We also detected a faint activity of the GC1 promoter in roots of the pGC1::sGFP plants, although the fluorescence pattern was clearly different from that of plants with the pSUC2::ABA2-sGFP and pSUC2::sGFP insertions (Supplemental Fig. S2). Thus, in contrast to the SUC2 promoter, the GC1 promoter was not active in the phloem companion cells, where ABA is mainly synthesized (Endo et al., 2008; Kuromori et al., 2014). According to the sGFP signals, ABA2-sGFP expression was much weaker than the expression of sGFP only, indicating the possibility of a posttranslational regulation mechanism for ABA2 protein.
Figure 3.
Expression of ABA2-sGFP and sGFP transgenes in Arabidopsis. A, The fluorescence of ABA2-sGFP and sGFP in the vascular system (pSUC2::ABA2-sGFP and pSUC2::sGFP, respectively) was detected using a stereomicroscope. A 15-s exposure was used to collect the fluorescent light. The corresponding bright-field images of studied leaf samples also are shown. Bars = 1 mm. B, The green fluorescence in guard cells of the lines carrying pGC1::ABA2-sGFP and pGC1::sGFP insertions was detected using confocal microscopy. The corresponding bright-field images of studied leaf samples also are shown. Bars = 50 µm.
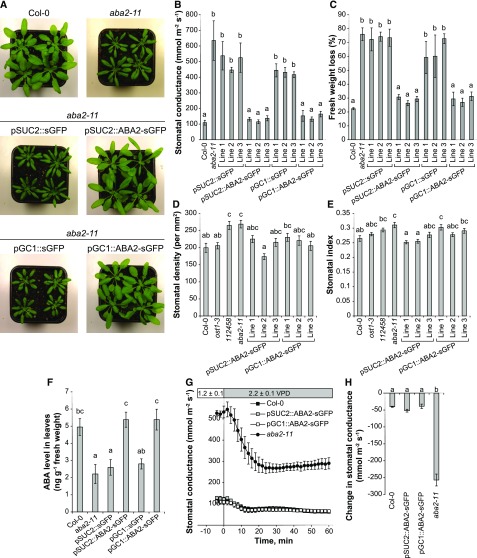
Restoring ABA biosynthesis in the guard cells or phloem companion cells rescued the visual phenotype of the aba2-11 mutant. While aba2-11 leaves were narrow and overall plant size was greatly reduced, plants expressing pGC1::ABA2-sGFP or pSUC2::ABA2-sGFP were similar to Col-0 wild-type plants (Fig. 4A). Stomatal function in these lines was restored, too. Several-fold higher steady-state gs observed in aba2-11 was reduced to the wild-type level by the expression of ABA2-sGFP under the control of either guard cell-specific GC1 or phloem-specific SUC1 promoters (Fig. 4B). Furthermore, pSUC2::ABA2-sGFP and pGC1::ABA2-sGFP lines demonstrated reduced fresh weight loss from detached leaves compared with the parental aba2-11 mutant (Fig. 4C). At the same time, the expression of sGFP did not influence the phenotype of transgenic plants. The ABA level also affects stomatal development, as ABA-deficient mutants show increased stomatal density (Tanaka et al., 2013; Chater et al., 2015). We found higher stomatal density and stomatal index of aba2-11 compared with Col-0. Similarly, the 112458 mutant showed higher stomatal density than the wild type (Fig. 4, D and E), indicating that not only ABA level, but also ABA perception by PYR/RCAR proteins, is important for stomatal development. The ost1-3 plants demonstrated unaffected stomatal density and stomatal index compared with Col-0. In transgenic lines, stomatal density and index were reduced to values not significantly different from wild-type ones, except one pGC1::ABA2-sGFP line (Fig. 4, D and E). Altogether, our results indicate that the guard and phloem companion cells are functionally redundant in ABA production; restored ABA biosynthesis in either site significantly affected plant development and the regulation of steady-state gs. As expected from the visual phenotype and gs results, the restored ABA production in guard cells or phloem companion cells was enough to increase the leaf ABA level to wild-type value in well-watered conditions (Fig. 4F). While the leaf ABA level of aba2-11 was more than 2-fold lower compared with Col-0 plants in well-watered conditions, the expression of ABA2-sGFP in aba2-11 under the control of GC1 or SUC2 promoters resulted in rescued leaf ABA levels (Fig. 4F).
Figure 4.
Expression of ABA2-sGFP either in aba2-11 phloem companion cells (pSUC2::ABA2-sGFP) or in guard cells (pGC1::ABA2-sGFP) recovered the aba2-11 mutant phenotypes, according to the rescued plant growth, recovered stomatal traits, and ABA level. Expression of sGFP under the control of the same promoters (pSUC2::sGFP and pGC1::sGFP) did not influence the phenotype of aba2-11. A, Representative photographs of each genotype at the age of 4 weeks. B and C, Steady-state gs (B) and fresh weight loss (C) presented as averages ± se (n = 3 or 4). D and E, Stomatal density (D) and stomatal index (E) were determined in 23 to 28 leaves per line, and measurements were performed in three biological repeats. F, ABA levels were measured in the leaves of 4-week-old plants. The results were pooled for each genotype; the values are averages ± se (n = 12 for pSUC2::sGFP and pSUC2::ABA2-sGFP, n = 11 for pGC1::ABA2-sGFP, n = 9 for pGC1::sGFP, and n = 7 for Col-0 and aba2-11). Different letters denote significant differences (Tukey’s HSD, P < 0.05). G and H, High-VPD-induced stomatal closure of aba2-11 plants transformed with ABA2 in the vascular system (pSUC2::ABA2-sGFP) or in the guard cells (pGC1::ABA2-sGFP; G) and high-VPD-induced 40-min stomatal closures for these lines (H).
OST1 Is Required for VPD-Induced Stomatal Responses, and ABA-Defective Mutants Show Strong Stomatal Closure in Response to Reduced Air Humidity
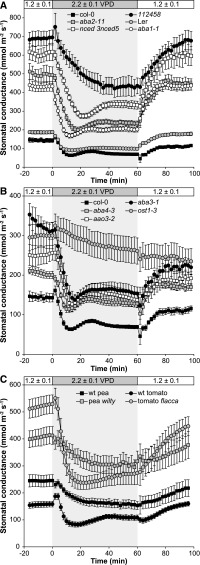
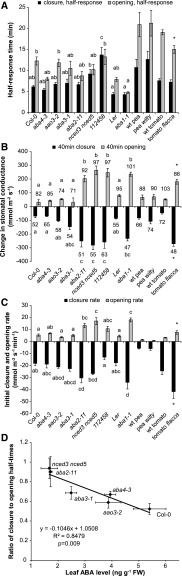
Reduced air humidity (i.e. increase in VPD) around the leaves results in increased transpiration and rapid stomatal closure to prevent desiccation. To further clarify the role of ABA biosynthesis and signaling in VPD-induced stomatal closure, we used respective mutants and monitored their stomatal closure in response to a rapid increase in VPD from 1.2 ± 0.1 to 2.2 ± 0.1 kPa, corresponding to a change in relative air humidity from 65 to 70% to 30 to 35% (Fig. 5). In order to provide quantitative values for measured responses, we calculated three characteristics: (1) the stomatal response half-times, which represent the time when 50% of the entire response was completed; (2) 40-min closures, which represent the change in gs after 40 min in high VPD compared with the pretreatment value; and (3) initial rate of stomatal closure, which was calculated by fitting a second-order polynomial on the time series of gs values after the change in VPD. These characteristics describe different aspects of the response: initial rate is a characteristic of fast response to a change in VPD, half-response time is a combination of the magnitude and the rate of the response, and 40-min closure describes the magnitude of the response. The strongest impairment in VPD-induced stomatal response was observed in ost1-3 (Fig. 5B). The gs of ost1-3 did not decrease significantly in response to VPD change when pretreatment gs values were compared with those of plants kept in high VPD for 60 min (repeated-measures ANOVA with Tukey’s posthoc test). Therefore, the closure-describing characteristics were not calculated for ost1-3. However, when the high VPD-induced initial transient stomatal opening due to the mechanical advantage of epidermal cells over guard cells is considered as well, stomatal closure of ost1-3, although very different from the fast and extensive responses of other lines, is noticeable. VPD-induced closure of ost1-3 follows linear regression (R2 = 0.93, P = 0.00) and is most probably a passive hydraulic response. The sextuple mutant of ABA receptors, 112458, showed significantly longer half-response time, but its initial closure rate was similar to that of Col-0 and its 40-min closure was significantly larger than in Col-0 (Fig. 6, A–C). Interestingly, all ABA-deficient mutants showed half-response times comparable to Col-0 or Landsberg erecta; furthermore, the initial closure rates and/or 40-min closures of aba2-11, nced3 nced5, and aba1-1 were significantly higher than those observed in the respective wild-type plants (Fig. 6, A–C). In tomato, the stomata of the wild type and flacca closed with similar half-response times and larger initial closure rate and 40-min closure of flacca. In pea, wilty and wild-type genotypes showed no significant differences in any of the closure-describing characteristics (Figs. 5C and 6, A–C).
Figure 5.
Stomatal responses of ABA-deficient and -insensitive plants to changes in air humidity. Time courses are shown for gs in response to VPD in Arabidopsis (average ± se; n = 5–8; A and B) and pea wilty and tomato flacca (n = 6 or 7) mutants and the respective wild-type plants (C). At time 0, VPD was increased from 1.2 ± 0.1 to 2.2 ± 0.1 kPa, and at 62 min, VPD was returned to its previous level.
Figure 6.
Characteristics describing high-VPD-induced stomatal closure and low-VPD-induced stomatal opening. A, Stomatal closure and opening half-response times indicate the times when half of the measured VPD response was completed. B, High-VPD-induced stomatal closures and low-VPD-induced openings within the initial 40 min of VPD change (gs40 − gs0). Percentages show the values of gs after 40 min under the respective VPD treatment, expressed as a ratio of the initial pretreatment value (in percentage). C, Initial closure and opening rates calculated by fitting a second-order polynomial on the time series of gs values after the change in VPD. Significant differences are denoted with different letters in Arabidopsis and with asterisks in tomato (ANOVA and Tukey’s posthoc test). In pea, no significant differences between wilty and the wild type (wt) were detected. D, Relationship between leaf ABA level and the ratio of closure to opening half-response times in Arabidopsis ABA-deficient mutants and Col-0.
In contrast to the significantly larger initial closure rate and 40-min closure observed in aba2-11, transgenic plants with restored ABA production in guard cells or in phloem companion cells had wild-type-like VPD-induced stomatal closure patterns and 40-min stomatal closures (Fig. 4, G and H) and initial closure rates. This indicates that ABA sourced from guard cells or vasculature is equally effective in restoring the wild-type-like stomatal VPD response.
Stomatal Opening after Returning to High Air Humidity
After 60 min of treatment with low air humidity, we increased the air humidity back to the initial level. Such treatment induced stomatal opening in all lines, except ost1-3, further illustrating the exceptionally important role of OST1 protein kinase in the VPD-induced stomatal movements (Fig. 5B). Interestingly, even the slight hydropassive opening trend, analogous to the closure one, was not evident in ost1-3 when humidity was restored. In Arabidopsis, no significant differences were found in opening half-times, whereas the initial opening rates and 40-min openings of aba2-11, nced3 nced5, and aba1-1 were higher than in the respective wild types (Fig. 6, A–C). In tomato, the opening half-time, initial opening rate, and 40-min opening of flacca were faster and higher, respectively, compared with the wild type (Fig. 6, A–C). No differences between the stomatal opening characteristics of pea wilty and the wild type were detected, and of all the studied plants, their reopening in low VPD was the most modest. The characteristics describing stomatal opening (half-response times, initial opening rates, and 40-min openings) of the wild types were considerably longer/smaller than the respective closure-describing characteristics (Fig. 6, A–C). Thus, the recovery of gs displayed considerable hysteresis that could be caused by high-VPD-induced ABA biosynthesis in these plants, which suppresses the restoration of gs to the previous level, as also discussed by McAdam and Brodribb (2015). Interestingly, the hysteresis in the recovery of gs was not evident in the sextuple ABA receptor mutant 112458, which showed almost identical closure/opening half-response times, initial rates, and 40-min closure and opening values (Fig. 6, A–C). This resulted in almost full recovery (97% of the pretreatment value) of gs in 112458 within 40 min after turning the humidity back to normal (70%). In Arabidopsis ABA-deficient lines, the recovery of gs depended on the leaf ABA level and was faster in more ABA-deficient lines (aba2-11 and nced3 nced5; Fig. 6D). However, as leaf ABA level and steady-state gs were closely correlated as well (Fig. 2B), this contributes to the symmetry of the VPD response through greater dilution of ABA by the water flux in lines with higher gs. Thus, in ABA-deficient lines, the higher gs is associated with faster water flow and recovery of their basal ABA level when humidity is restored to initial levels, thereby reducing hysteresis.
Stomatal Responses to Exogenous ABA
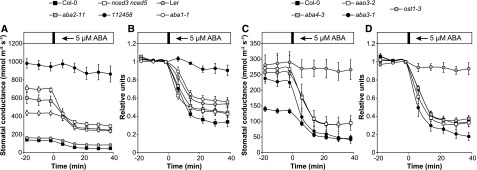
Here, we addressed how the ABA-insensitive mutants ost1-3 and 112458 respond to ABA spraying, as it is possible that some of the PYR/RCAR ABA receptors are still functional in the sextuple receptor mutant and this was the reason for its considerable VPD response. Both ost1-3 and 112458 showed strong insensitivity to exogenous ABA: their gs did not decrease significantly in response to ABA spraying (repeated-measures ANOVA with Tukey’s posthoc test; Fig. 7). Thus, the activity of the remaining PYR/RCAR proteins in 112458 did not mediate the ABA response, although this conclusion may not hold if higher ABA concentration or VPD stress is applied. ABA-deficient mutants responded strongly, even hypersensitively, to exogenous ABA (Fig. 7).
Figure 7.
Stomatal responses of different Arabidopsis lines to foliar spraying with 5 µm ABA. Time courses of gs in response to ABA spraying in ABA-insensitive and ABA-deficient mutants (average ± se; n = 4–7) are shown in absolute units (A and C) and relative units (B and D).
DISCUSSION
Among the atmospheric factors that control stomatal behavior, air humidity (i.e. VPD) is one of the most important: the stomatal closure in response to high VPD serves to decrease water loss and prevent desiccation under high evaporative demand. As an example, changes in VPD during the day result in transient stomatal closure and, accordingly, in reduced net photosynthetic rate around midday, when the VPD values are largest (Raschke and Resemann, 1986; Brodribb and Holbrook, 2004). Furthermore, the combination of increased temperature and larger variability of water stress predicted for the future results in a VPD-modulated increase in transpiration and higher mortality rate during terminal drought in some species/areas (Will et al., 2013). This underlines the need to understand stomatal VPD regulation. Here, we aimed to elucidate the role of ABA in steady-state gs to water vapor and in stomatal responsiveness to VPD. We found that ABA level is of great importance for steady-state gs and that OST1 is crucial for the air humidity-induced regulation of gs. We also show that ABA biosynthesis in guard and phloem companion cells is functionally redundant, as both sources are equivalent in restoring the visual phenotype, whole-plant gs, and leaf ABA level of aba2-11 plants.
Whole-Plant Stomatal Conductance Is Controlled More by ABA Concentration and PYR/RCAR Receptors Than by Signaling through OST1
The several times higher steady-state gs of strongly ABA-deficient and -insensitive plants indicates that ABA plays a major role in controlling basal water flux through stomata (Fig. 2A). Interestingly, plants carrying mutation in OST1, a central kinase in stomatal regulation in response to ABA, but also to environmental factors such as CO2, ozone, and darkness (Vahisalu et al., 2010; Xue et al., 2011; Merilo et al., 2013, 2015a), had considerably lower steady-state gs than aba2-11, nced3 nced5, and 112458. This indicates that ABA, independently of OST1, is involved in the regulation of some other processes related to leaf hydraulics or stomatal signaling that affect gs. Obvious candidates could be the guard cell plasma membrane H+-ATPases that initiate stomatal opening (Shimazaki et al., 2007) or the K+in channels that are required for K+ accumulation during stomatal opening. ABA inhibits phosphorylation and the activation of H+-ATPase, either directly or via the secondary messengers hydrogen peroxide or nitric oxide (Zhang et al., 2004, 2007). Furthermore, a phospholipid second messenger of ABA signaling, phosphatidic acid, has been shown to inhibit H+-ATPase activity (Takemiya and Shimazaki, 2010) and K+in channels (Jacob et al., 1999). The higher gs values of strongly ABA-deficient and -insensitive plants compared with those lacking functional OST1 also could be explained by the role of ABA in reducing leaf hydraulic conductance independently of OST1 (Shatil-Cohen et al., 2011; Pantin et al., 2013a). Alternatively, as OST1 is expressed in the vascular tissues of other plant organs and has unknown functions besides guard cell signaling (Belin et al., 2006; Yoshida et al., 2006), it is possible that the lack of OST1 affects plant hydraulic conductance and counteracts the increase in gs independently of ABA.
Restoring Guard Cell or Phloem ABA Synthesis in the ABA-Deficient Genotype Recovers Whole-Plant Stomatal Conductance, Growth Phenotype, and Leaf ABA Level
For a mechanistic insight into the regulation of whole-plant water balance, it is important to understand to what extent the water flux through the stomata is controlled by guard cell-autonomous ABA synthesis (Bauer et al., 2013) versus ABA transport from the leaf vasculature (McAdam and Brodribb, 2015). Bauer et al. (2013) created transgenic plants in the aba3-1 background that had ABA synthesis restored only in guard cells and found that these plants did not wilt in dry air like the aba3-1 mutant did. Stomatal conductance was not measured by these authors, and even though the leaf wilting/nonwilting under low air humidity conditions was impressively captured, it is not necessarily an indication of ABA involvement in stomatal VPD responses. Despite their fast and extensive closure, ABA-deficient mutants lose more water compared with the wild type in both humid and dry air, because their stomata are more open initially and remain more open under high VPD (Fig. 5), explaining the higher water loss and potential wilting. Considering the close correlation between leaf ABA level and gs in humid air (Fig. 2B), ABA level might determine the final, stable value of gs under low-humidity conditions as well, whereas the closure process itself might be partially ABA independent or be determined by a high VPD-induced pulse in ABA synthesis that occurs in ABA-deficient mutants too. In another study, Kuromori et al. (2014) created transgenic plants with enhanced ABA synthesis in the phloem companion cells that had higher leaf temperature and reduced water loss from detached leaves. This result points to more closed stomata and indicates ABA transport to the guard cells; however, again, the gs was not measured directly. We chose aba2-11 as the background to generate transgenic plants with ABA synthesis restored in the guard cells or in the phloem, because aba2-11 plants were strongly ABA deficient and displayed remarkably high gs (Fig. 2B). These transgenic lines had wild-type-like steady-state gs, indicating that not only guard cell- but also phloem-borne ABA is enough to reduce the high gs of aba2-11 plants. Moreover, both transgenic lines exhibited growth phenotypes, stomatal densities, and leaf ABA levels that were not different from the wild type (Fig. 4). These results strengthen the perception of ABA as the plant’s brake system put up by Chater et al. (2014): ABA affects physiology and development, transmitting its message via short- and long-distance communication.
The above-described results indicate plasticity to mobilize ABA as a signaling molecule and highlight the importance of ABA transport systems within the leaf. Similarly, restoration of molybdenum hydroxylase activity in roots alone was sufficient to augment leaf ABA concentration of the tomato flacca mutant (Sagi et al., 2002), and directing ABA biosynthesis to the immature epidermis and stomatal lineage cells of the nced3 nced5 mutant restored the plant ABA level as compared with the ABA-deficient parental line (Chater et al., 2015). We previously showed that plants carrying defects in proteins involved in ABA transport have no significant differences in stomatal behavior relative to the wild type, suggesting that ABA transport is well covered with redundant proteins involved (Merilo et al., 2015a). Crossing plants with ABA synthesis restored in the phloem with plants defective in the different ABA transporters would serve to identify the transporters that are physiologically important in the route from vasculature to guard cells. Our above results show that ABA can be transported from the vasculature to guard cells and from guard cells to the rest of the leaf, indicating that ABA synthetic and transport systems are duplicated and plastic in leaves, as also discussed by Merilo et al. (2015b).
OST1 Kinase Rather Than ABA Concentration per se Controls Humidity-Induced Stomatal Responses
ABA-insensitive mutants lacking OST1 or six ABA receptors (ost1-3 and 112458) showed no significant reduction in gs after spraying with 5 µm ABA solution. The VPD-induced stomatal closure of ost1-3 plants was small, slow, and linear (Fig. 5B), whereas the gs of 112458 decreased prominently under high VPD. Furthermore, in 112458, no hysteresis in gs recovery after returning to low VPD was detected (Fig. 6, A–C), a result different from wild-type Col-0 and similar to the passive hydraulic behavior in ferns (McAdam and Brodribb, 2015). These results allow for two interpretations. First, it is possible that substantial passive hydraulic closure takes place in 112458 but not that much in ost1-3. As the soil water content was not limiting in our experiment and ost1-3 showed only modestly increased pretreatment gs (Fig. 2A), it is possible that, even under high VPD, the hydraulic conductance of ost1-3 was large enough to support the water flow with no reductions in leaf turgor and, consequently, relatively small passive stomatal closure. 112458, on the other hand, had 2-fold larger gs compared with ost1-3, potentially resulting in reduced leaf water content and turgor and, in turn, passive hydraulic stomatal closure was larger. Symmetrical stomatal responses to VPD fit with the hypothesis of passive hydraulic closure in 112458 according to McAdam and Brodribb (2015). As the stomata of ost1-3 closed relatively little under high VPD, the functionality of passive hydraulic regulation seems to depend on the steady-state gs: only very high gs results in hydropassive VPD-induced stomatal closure of Arabidopsis. Alternatively, as the signaling through OST1 is of utmost importance for rapid stomatal closure in response to high VPD and there are obvious differences in the stomatal VPD responses between ost1-3 and 112458, an ABA-independent activation of OST1 during the humidity response may take place. Previously, both ABA-dependent and -independent pathways were shown to activate OST1 through different regions of the C-terminal domain under low humidity; together, these pathways may ensure complete stomatal closure (Yoshida et al., 2006). Similarly, different phosphorylation mechanisms and pathways participated in the activation of OST1 in response to osmotic stress and ABA (Boudsocq et al., 2007; Vlad et al., 2010).
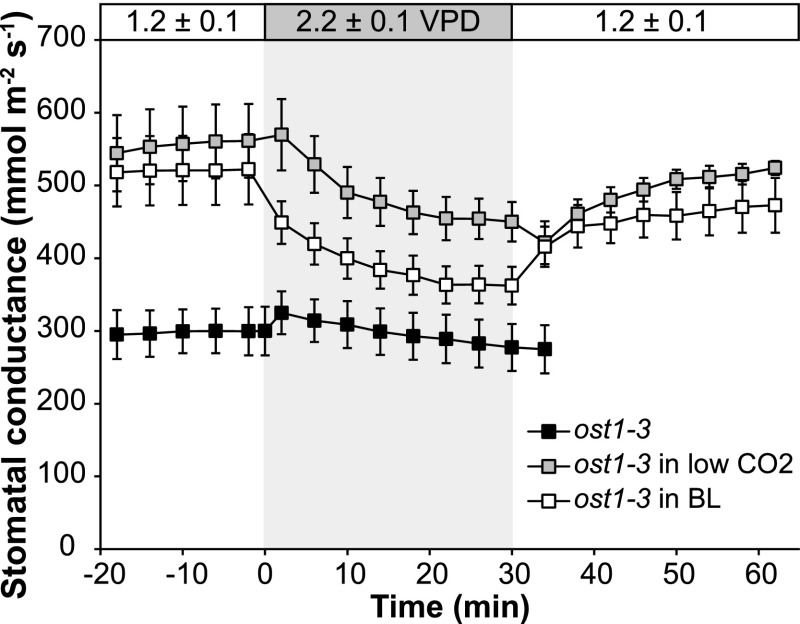
We addressed which of the above arguments, the conditionality of passive hydraulic closure on gs or the ABA-independent activation of OST1, could explain the differences in the VPD response of ost1-3 and 112458 with an additional experiment. Namely, we argued that, if the hydropassive VPD-induced closure depends on initial gs, then ost1-3 plants with more open stomata might start to close under high VPD. Plants of ost1-3 are rather insensitive to CO2 enrichment and darkness (Xue et al., 2011; Merilo et al., 2013), but they respond somewhat to CO2 withdrawal and blue light. When ost1-3 plants were kept in low CO2 (50 µL L−1) or white + blue light conditions, their gs increased approximately 1.7 to 1.9 times compared with the values in Figure 2A. Importantly, high-VPD-induced stomatal closure was detected in these plants, followed by low-VPD-induced stomatal opening (Fig. 8). This result supports the hypothesis that the passive hydraulic stomatal closure is conditional on initial gs. Nevertheless, the possibility of ABA-independent activation of OST1 under some stresses is still not excluded.
Figure 8.
Stomatal VPD response of ost1-3 plants with increased gs. ost1-3 plants were kept in low CO2 (50 µL L−1) or increased light (white + blue light, photosynthetic photon flux density [PPFD] of ∼500 μmol m−2 s−1) for at least 1.5 h to induce stomatal opening, and when gs had stabilized, VPD was increased for 30 min, then turned back. For comparison, a segment of ost1-3 data from Figure 5 also is presented. n = 5 to 7.
Strong VPD Response of ABA-Deficient Mutants
We found that all ABA-deficient mutants had larger initial gs but closed under high VPD like, or even more intensely than, the wild type (Figs. 5 and 6, A–C; Merilo et al., 2013, 2015a). This contrasts with the studies where ABA-deficient tomato and pea mutants had steady-state gs similar to that of the respective wild types while their VPD-induced closures were reduced substantially (McAdam et al., 2015, 2016b). It has been shown that stomatal responsiveness may be conditional on growth conditions (e.g. plants grown under very high humidity show reduced stomatal responsiveness to ABA; Pospíšilová, 1996; Pantin et al., 2013b; Arve et al., 2014). However, different growth conditions seem to be insufficient to explain the above-mentioned differences between our results and those of McAdam et al. (2015, 2016b), even though different humidity regimes were used in our experiments. Instead, we propose three interpretations to explain these contradictions. First, the stomatal responses of ABA-deficient mutants could be conditional on the pretreatment gs: when the gs of an ABA-deficient mutant is significantly higher than in the wild type, the plants are highly responsive to environmental factors, including high VPD. This could result from the higher local drop of humidity in the substomatal cavity of the lines with higher gs or their lower leaf water potential and, consequently, an ABA signaling system that is more sensitized to ABA. Even a slight nanomolar increase in ABA concentration could be sufficient to activate ABA signaling (Szostkiewicz et al., 2010), and ABA-deficient mutants are all able to synthesize ABA to some degree (Figs. 1, refs., and 2B). Furthermore, reactive oxygen species (ROS) can nonenzymatically convert ABA precursors to ABA according to a recent report (McAdam et al., 2017), offering another way to explain the increased stress-induced ABA levels in mutants defective in ABA biosynthesis, as stress is often accompanied by a ROS burst. The time-resolved course of ABA synthesis in ABA-deficient lines under low-humidity treatment would clarify how fast and how much ABA is synthesized and whether leaf ABA level correlates with stabilized gs values under low VPD. Second, it is possible that the VPD-induced passive hydraulic stomatal closure is present in those ABA-deficient mutants that showed the highest gs values, similar to 112458. Furthermore, conflicting results with ABA-deficient plants could be explained by the use of different experimental setups as well. In McAdam et al. (2015; 2016b), the leaf chamber encloses only part of the leaf in the measurement cuvette, whereas we monitored VPD responses of the whole rosette/shoot. Hydraulic conditions, particularly the availability of water from the rest of the plant and the rate of turgor loss, may differ in these setups. Simultaneous measurements of changes in ABA, ROS, and water content/turgor are needed to elucidate the temporal hierarchy of components involved in stomatal responses to changes in evaporative demand.
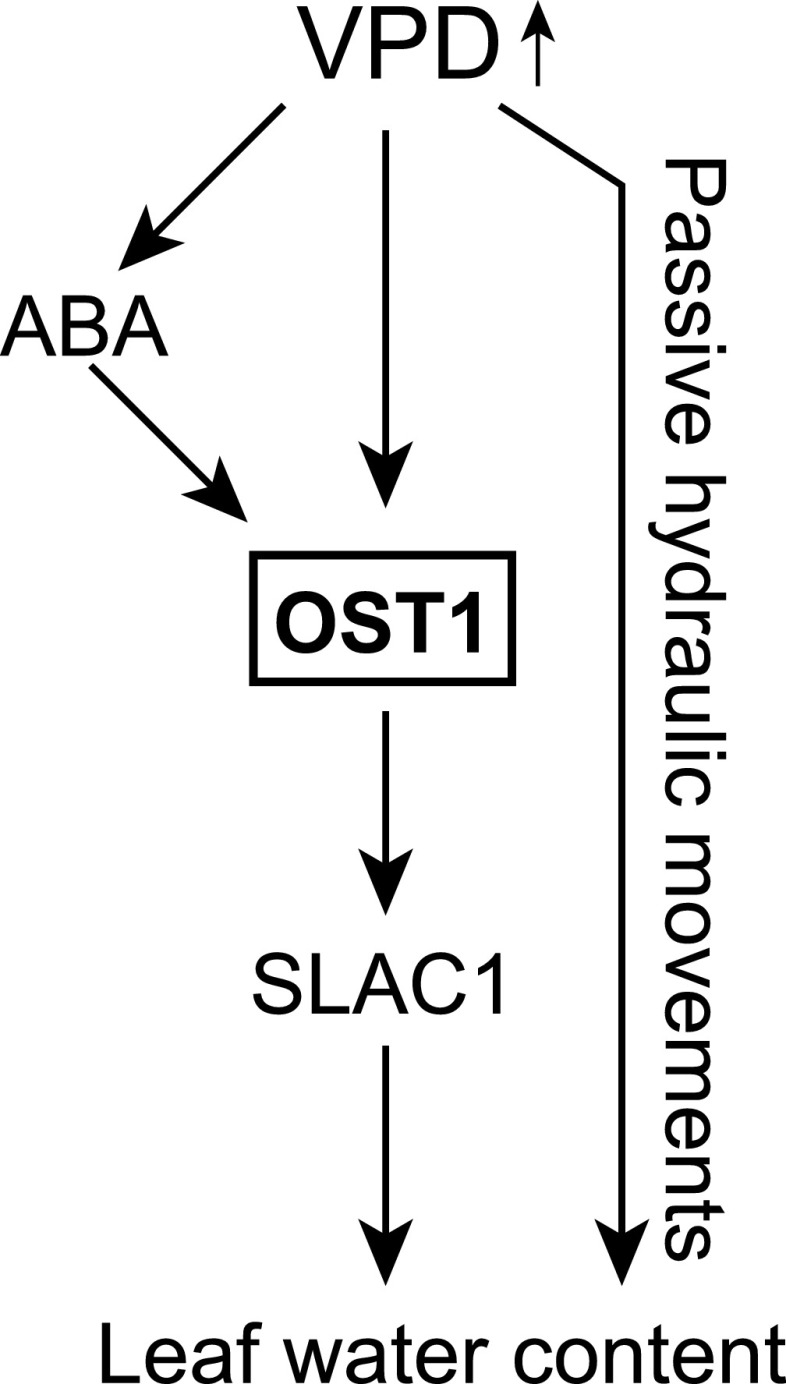
In conclusion, ABA appears to be a highly mobile molecule, since restoring ABA synthesis either in the vasculature or in the guard cells was enough to recover plant growth- and stomata-related phenotypes of ABA-deficient plants. The impaired VPD response of ost1-3 highlights that signaling through OST1, which may be partially ABA independent, is of utmost importance for VPD-induced stomatal closure. A strong VPD response, together with the nonsignificant ABA response of the sextuple mutant of ABA receptors, 112458, could be explained by a passive hydraulic stomatal closure due to high gs. All ABA-deficient mutants showed some ABA synthesis (31%–73% of the wild-type level in well-watered conditions) and responded to high VPD with stomatal closure. In more severe ABA-deficient mutants, part of this VPD-induced closure may be passive hydraulic. To sum up, we propose a hypothetical model of processes participating in VPD-induced stomatal closure as discussed here and in the literature (Fig. 9).
Figure 9.
Hypothetical model of VPD-induced stomatal closure. Results of this and previous studies indicate that the protein kinase OST1 is required for VPD-induced stomatal closure; its activation could be either ABA dependent or independent. In addition, a passive hydraulic response to reduced leaf turgor is evident under high VPD.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds were planted in soil containing 4:3 (v/v) peat:vermiculite and grown through a hole in a glass plate covering the pot as described by Kollist et al. (2007). The full list of mutants used is given in Figure 1. Soil moisture was kept at 80% of maximum water capacity. Plants were grown in growth chambers (AR-66LX and AR-22L [Percival Scientific] and MCA1600 [Snijders Scientific]) in a 12/12-h photoperiod, 23°C/18°C temperature, 150 µmol m−2 s−1 light, and 70% relative humidity. For gas-exchange experiments, we used plants with total leaf area between 5 and 15 cm2. This corresponds to 21- to 30-d-old Arabidopsis plants. Plants used for ABA measurements were grown in growth chambers under similar conditions and harvested at the age of 28 d. To study the expression of sGFP and ABA2-sGFP in roots, transgenic plants were grown in petri dishes containing 0.5× Murashige and Skoog medium, 1% Suc, 10 mm MES (pH 5.7), and 0.8% agar.
The seeds of pea (Pisum sativum) wild type (cv Torsdag; Hobart Line 107) and wilty, which is defective in the xanthoxin dehydrogenase step of ABA biosynthesis (McAdam et al., 2015), were sown into 1-L pots containing the soil mixture described above. Growth conditions in the plant chambers were as described above. For gas-exchange experiments, we used pea seedlings at the age of 10 to 18 d. We also studied ABA-deficient tomato (Solanum lycopersicum) flacca and its wild type cv Ailsa Craig (Sagi et al., 2002); the tomato plants were grown under similar conditions and measured at the age of 20 to 23 d. All experimental Arabidopsis, pea, and tomato plants were in a vegetative growth stage.
Stomatal Conductance Measurements
The eight-chamber whole-plant rapid-response gas-exchange measurement device has been described previously (Kollist et al., 2007). This device enables the measurement of eight plants within a 16-min interval. Currently, we used only one plant at a time to get gs data every 2 min. One Arabidopsis plant was inserted into the device, and the high-VPD treatment started when gs had stabilized (i.e. at least 1 h later). Standard conditions during the stabilization were as follows: ambient CO2, ∼400 µL L−1; light, 150 µmol m−2 s−1; relative air humidity, ∼70%. Photographs of plants were taken after the experiment, and leaf area was calculated using ImageJ 1.37v (National Institutes of Health). gs was calculated with a custom-written program as described by Kollist et al. (2007).
Pea and tomato plants were measured with a custom-made flow-through four-chamber device, which is suitable for larger and taller plants. The main body of the system consists of four thermostated gas-exchange cuvettes formed by two glass cylinders (i.d., 10.6 cm; height, 15.6 cm) and a thermostated water jacket between them. The cuvettes are placed on a stand composed of two well-fitted glass plates that form a bottom of the gas-exchange cuvette. One of these plates contains perforations for the plant stem and is removable. The other glass plate contains gas input and output ports, a temperature sensor, and a fan to guarantee high turbulence and uniform gas mixing. The fan ensured a high boundary layer conductance of leaves (4,800 mmol m−2 s−1) and a high heat-exchange coefficient between the leaves and chamber air (36 cal m−2 s−1 °C−1), minimizing the effect of transpiration on leaf temperature. Modeling gum was used to ensure an air-tight separation of plant shoots within the cuvette from roots in the soil. Chambers are hermetically sealed and operate under slight overpressure of a few millibars to avoid uncontrolled intake of ambient air. Air flow rate through the chamber was 2.5 L min−1. Ambient air passing through a large buffer volume of 25 L was used. The air temperature inside the chambers was measured continuously with thermistors (model -001; RTI Electronics) and was between 24°C and 25°C. Leaf temperature was calculated from the energy balance of leaves based on absorbed light and transpiration (Kollist et al., 2007). All tubing and connections were made of Teflon and stainless steel. For illumination, four 50-W halogen lamps (Kanlux MR16C; Philips) provided PPFD of 500 μmol m−2 s−1 into each cuvette. Concentrations of CO2 and water vapor in the reference channel (i.e. air entering the measuring cuvette) and measurement channel (air coming out from the cuvette) were measured with an infrared gas analyzer (Li-7000; Li-Cor), and gs was calculated with a custom-written program as described by Kollist et al. (2007). Standard conditions during the stabilization period in the gas-exchange cuvettes were as follows: ambient CO2 concentration, ∼400 µL L−1; PPFD, 500 µmol m−2 s−1; and relative air humidity, ∼70%.
A plant was inserted into one of the four measurement cuvettes and kept under standard conditions for about 1 to 2 h to allow the stabilization of gs. After stabilization, VPD was increased sharply from 1.2 ± 0.1 to 2.2 ± 0.1 (i.e. air humidity was decreased from ∼70% to ∼35%) in all experiments. After 1 h in low-humidity conditions, VPD was decreased to the previous level; thereafter, measurements continued for 40 min. In order to compare the VPD responses of different lines, we calculated the following. (1) The initial rates of stomatal closure/opening, found by fitting a second-order polynomial on the time series of gs values after changes in humidity. The slope of that polynomial at the 4-min time point represents the initial closure/opening rate. (2) The closure/opening half-times obtained by scaling the whole 60-min (closure due to increased VPD) or 40-min (opening due to reduced VPD) stomatal responses to a range from 0% to 100% and by calculating the time when 50% of the stomatal response was achieved. And (3) changes in gs after stimulus application, calculated as the difference gs40 − gs0, where gs40 is the value of gs after 40 min under high/low VPD and gs0 is the average of the two last gs values under low VPD/high VPD. These characteristics were not calculated for ost1-3. To induce stomatal opening of ost1-3 plants in Figure 8, we first kept them in low CO2 (∼50 µL L−1) or high light (white + blue lights, PPFD of ∼500 µmol m−2 s−1) and applied VPD change after 1.5 to 2 h, when stomata had stabilized. VPD was increased as described above for 30 min and then turned back for another 30 min.
In ABA-spraying experiments, plants were inserted into measurement chambers and, after gs had stabilized, intact plants were sprayed with 5 μm ABA solution (distilled water, 0.012% Silwet L-77 [Duchefa], and 0.05% ethanol). We also performed control experiments, where plants of different genotypes were sprayed with mock solution containing no ABA but 0.012% Silwet L-77 and 0.05% ethanol in distilled water. Supplemental Figure S3 presents the results for Col-0. The volume of solution (ABA or mock) sprayed on one plant was ∼20 µL cm−2 leaf area. After spraying, gs was measured for 40 min as described previously (Merilo et al., 2015a).
Generation of Transgenic Plants
Genomic fragments containing SUC2 (Truernit and Sauer, 1995) and GC1 promoters (Yang et al., 2008) specific to phloem and guard cells, respectively, were amplified and cloned into pART7 plasmid using appropriate restriction sites to replace the 35S promoter. All primers, which were used in this work for cloning, are shown in Supplemental Table S1. The coding region of GFP (sGFP; Chiu et al., 1996) was amplified and cloned downstream of the SUC2 and GC1 promoter regions in BamHI and XbaI restriction sites. The amplified ABA2 coding region was fused with sGFP using XhoI and BamHI restriction sites. All cloned inserts were sequenced to avoid unwanted mutations. The expression cassettes containing pSUC2::ABA2-sGFP, pSUC2::sGFP, pGC1::ABA2-sGFP, and pGC1:sGFP were cut out using NotI restriction sites and were recloned into the NotI site of the pMLBart plasmid. The final constructs were introduced into Agrobacterium tumefaciens GV3101 strain. Agrotransformation of the ABA-deficient mutant aba2-11 was performed using the floral dip method (Bent, 2006). Transgenic plants were selected by spraying with Basta (glufosinate ammonium) as well as by detection of sGFP signal in phloem and guard cells. The presence of the insertions as well as the aba2-11 genetic background of the transgenic lines were confirmed by PCR with the primers indicated in Supplemental Table S1. Selected transgenic lines contained only one T-DNA insertion according to plant segregation after spraying with Basta.
Detection of sGFP and ABA2-sGFP Fluorescence in Transgenic Plants
Guard cells of transgenic plants were examined with the LSM 710 META Laser Scanning Microscope (Carl Zeiss) using a 488-nm laser beam for excitation of sGFP and a 510- to 550-nm channel for emission of light. The fluorescent proteins in vascular tissue were detected using the SteREO Discovery V20 stereomicroscope with attached AxioCamMR5 digital camera (Carl Zeiss). sGFP and ABA2-sGFP fluorescence were excited using a 470/40 emission filter and detected with a 525/50 emission filter. The light was passed though the FT495 beam splitter.
Measurements of Leaf ABA Concentration
ABA was extracted from leaf samples according to McAdam and Brodribb (2014) and McAdam (2015), with slight modifications. Plant samples (70–100 mg of leaf tissue) were collected from plants at the age of 4 weeks. The plant tissue was ground with glass beads and incubated in 12 mL of 80% (v/v) methanol in water at −20°C for 24 h. To each sample, 3 ng of [2H6]ABA was added as an internal standard. ABA was extracted from plant cells during an additional incubation at +4°C for 24 h. An aliquot of 5 mL was taken and dried under vacuum; samples were then resuspended in 500 µL of 2% (v/v) acetic acid in water and partitioned three times against 300-µL aliquots of diethyl ether. Samples in diethyl ether were then dried and taken up in 150 µL of 5% (v/v) methanol, 94% (v/v) water, and 1% (v/v) acetic acid. A 20-µL aliquot was taken for HPLC (Agilent 1200 series with Phenomenex Kinetex 2.6 µm EVO C18 100×4.6 mm column)-mass spectrometry (Sciex Q-Trap 4500) analysis according to Pan et al. (2010). The retention time and identity of ABA were confirmed with commercial ABA samples. The selected reaction monitoring transitions were 263/153 for ABA and 269/159 for [2H6]ABA, and collision potential was −15 V.
Measurements of Fresh Weight Loss from Detached Leaves
The fresh weight loss of detached leaves after 2 h of drying was determined. Leaves of 4-week-old plants were cut and weighed, then they were left in open petri dishes on a laboratory table. The leaves were weighed after 2 h, and the relative fresh weight loss was calculated.
Measurement of Stomatal Density
To measure stomatal density (SD) and stomatal index (SI), leaves of 4-week-old plants, one leaf per plant, were excised, and the abaxial side was covered with dental resin (Xantropen VL Plus; Heraeus Kulzer). The resin impressions were covered with transparent nail varnish after leaves were removed. The hardened nail varnish imprints were attached to a microscope glass slide with transparent tape. Images were taken with the Zeiss SteREO Discovery V20 stereomicroscope. For quantification, an image with an area of ∼0.12 mm2 was taken from the middle of the leaf, close to the middle vein. SD and SI were calculated as follows:
Statistical Analysis
ANOVA (General Linear Model [GLM] procedure) was used to assess the effect of line on steady-state gs and characteristics calculated to describe VPD-induced stomatal closure and opening. Comparisons between individual means were performed using Tukey’s HSD test. The significance of linear regressions was tested with GLM simple regression analysis. Data were ln transformed when necessary. All effects were considered significant at P < 0.05. Statistical analyses were performed with Statistica, version 7.0 (StatSoft).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Images showing that the SUC2 promoter is not active in guard cells.
Supplemental Figure S2. Images showing the high tissue specificity of the SUC2 and GC1 promoters.
Supplemental Figure S3. Stomatal response of Arabidopsis Col-0 plants to foliar spraying with control solution or 5 μm ABA.
Supplemental Table S1. Primers used in this study.
Acknowledgments
We thank Julie Gray, Ian Dodd, Timothy Brodribb, and Pedro Rodriguez for sending us seeds. We are grateful to Scott McAdam for scientific discussions and technical advice regarding ABA measurements. We also thank Moshe Sagi for providing pART7 and pMLBart plasmids. Finally, we thank the editorial board and reviewers for their thorough and elaborate comments and discussion.
Footnotes
This work was supported by the Estonian Research Council (PUT1133 [E.M.], PUT331 [D.Y.], and IUT2-21 [H.K.]) and by the European Regional Development Fund (Center of Excellence in Molecular Cell Engineering and Center of Excellence for Genomics and Translational Medicine).
Articles can be viewed without a subscription.
References
- Arve LE, Carvalho DR, Olsen JE, Torre S (2014) ABA induces H2O2 production in guard cells, but does not close the stomata on Vicia faba leaves developed at high air humidity. Plant Signal Behav 9: e29192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Snyder JA, Lee YRJ (2000) ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ 23: 387–395 [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KAS, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Belin C, de Franco PO, Bourbousse C, Chaignepain S, Schmitter JM, Vavasseur A, Giraudat J, Barbier-Brygoo H, Thomine S (2006) Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiol 141: 1316–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent A. (2006) Arabidopsis thaliana floral dip transformation method. Methods Mol Biol 343: 87–103 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Droillard MJ, Barbier-Brygoo H, Laurière C (2007) Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol Biol 63: 491–503 [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Léran S, Corratgé-Faillie C, Gojon A, Krouk G, Lacombe B (2013) ABA transport and transporters. Trends Plant Sci 18: 325–333 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM (2004) Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant Cell Environ 27: 820–827 [Google Scholar]
- Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Buckley TN. (2005) The control of stomata by water balance. New Phytol 168: 275–292 [DOI] [PubMed] [Google Scholar]
- Buckley TN. (2016) Stomatal responses to humidity: has the ‘black box’ finally been opened? Plant Cell Environ 39: 482–484 [DOI] [PubMed] [Google Scholar]
- Cai S, Chen G, Wang Y, Huang Y, Marchant DB, Wang Y, Yang Q, Dai F, Hills A, Franks PJ, et al. (2017) Evolutionary conservation of ABA signaling for stomatal closure. Plant Physiol 174: 732–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, Fleming A, Cuming AC, Gray JE, Beerling DJ (2011) Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Curr Biol 21: 1025–1029 [DOI] [PubMed] [Google Scholar]
- Chater C, Peng K, Movahedi M, Dunn JA, Walker HJ, Liang YK, McLachlan DH, Casson S, Isner JC, Wilson I, et al. (2015) Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr Biol 25: 2709–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater CC, Oliver J, Casson S, Gray JE (2014) Putting the brakes on: abscisic acid as a central environmental regulator of stomatal development. New Phytol 202: 376–391 [DOI] [PubMed] [Google Scholar]
- Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6: 325–330 [DOI] [PubMed] [Google Scholar]
- Christmann A, Weiler EW, Steudle E, Grill E (2007) A hydraulic signal in root-to-shoot signalling of water shortage. Plant J 52: 167–174 [DOI] [PubMed] [Google Scholar]
- Endo A, Sawada Y, Takahashi H, Okamoto M, Ikegami K, Koiwai H, Seo M, Toyomasu T, Mitsuhashi W, Shinozaki K, et al. (2008) Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol 147: 1984–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst L, Goodger JQD, Alvarez S, Marsh EL, Berla B, Lockhart E, Jung J, Li P, Bohnert HJ, Schachtman DP (2010) Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J Exp Bot 61: 3395–3405 [DOI] [PubMed] [Google Scholar]
- Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, Sechet J, To A, North HM, Marion-Poll A (2012) Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J 70: 501–512 [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guzmán M, Abia D, Salinas J, Serrano R, Rodríguez PL (2004) Two new alleles of the abscisic aldehyde oxidase 3 gene reveal its role in abscisic acid biosynthesis in seeds. Plant Physiol 135: 325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guzmán M, Apostolova N, Bellés JM, Barrero JM, Piqueras P, Ponce MR, Micol JL, Serrano R, Rodríguez PL (2002) The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 14: 1833–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernández MA, Holdsworth MJ, Perez-Amador MA, Kollist H, et al. (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24: 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook NM, Shashidhar VR, James RA, Munns R (2002) Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J Exp Bot 53: 1503–1514 [PubMed] [Google Scholar]
- Hõrak H, Kollist H, Merilo E (2017) Fern stomatal responses to ABA and CO2 depend on species and growth conditions. Plant Physiol 174: 672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T, Ritchie S, Assmann SM, Gilroy S (1999) Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA 96: 12192–12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, Oja V, Rämma H, Hüve K, Jaspers P, Kangasjärvi J, Kollist H (2007) A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol Plant 129: 796–803 [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 61: 385–393 [DOI] [PubMed] [Google Scholar]
- Kuromori T, Sugimoto E, Shinozaki K (2014) Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiol 164: 1587–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JAD, Koornneef M (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Marx GA. (1976) “Wilty”: a new gene of Pisum. Pisum Newsl 8: 40–41 [Google Scholar]
- McAdam EL, Brodribb TJ, McAdam SAM (2017) Does ozone increase ABA levels by non-enzymatic synthesis causing stomata to close? Plant Cell Environ 40: 741–747 [DOI] [PubMed] [Google Scholar]
- McAdam SAM. (2015) Physicochemical quantification of abscisic acid levels in plant tissues with an added internal standard by ultra-performance liquid chromatography. Bio-protocol 5: e1599 [Google Scholar]
- McAdam SAM, Brodribb TJ (2014) Separating active and passive influences on stomatal control of transpiration. Plant Physiol 164: 1578–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2015) The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiol 167: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2016) Linking turgor with ABA biosynthesis: implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiol 171: 2008–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ, Ross JJ (2016a) Shoot-derived abscisic acid promotes root growth. Plant Cell Environ 39: 652–659 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC, Brodribb TJ (2016b) Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ 39: 485–491 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC, Brodribb TJ, Ross JJ (2015) Molecular characterization of a mutation affecting abscisic acid biosynthesis and consequently stomatal responses to humidity in an agriculturally important species. AoB Plants 7: plv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Jalakas P, Kollist H, Brosché M (2015a) The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Mol Plant 8: 657–659 [DOI] [PubMed] [Google Scholar]
- Merilo E, Jalakas P, Laanemets K, Mohammadi O, Hõrak H, Kollist H, Brosché M (2015b) Abscisic acid transport and homeostasis in the context of stomatal regulation. Mol Plant 8: 1321–1333 [DOI] [PubMed] [Google Scholar]
- Merilo E, Laanemets K, Hu H, Xue S, Jakobson L, Tulva I, Gonzalez-Guzman M, Rodriguez PL, Schroeder JI, Broschè M, et al. (2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol 162: 1652–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North HM, De Almeida A, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50: 810–824 [DOI] [PubMed] [Google Scholar]
- Pan X, Welti R, Wang X (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5: 986–992 [DOI] [PubMed] [Google Scholar]
- Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B (2013a) The dual effect of abscisic acid on stomata. New Phytol 197: 65–72 [DOI] [PubMed] [Google Scholar]
- Pantin F, Renaud J, Barbier F, Vavasseur A, Le Thiec D, Rose C, Bariac T, Casson S, McLachlan DH, Hetherington AM, et al. (2013b) Developmental priming of stomatal sensitivity to abscisic acid by leaf microclimate. Curr Biol 23: 1805–1811 [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospíšilová J. (1996) Effect of air humidity on the development of functional stomatal apparatus. Biol Plant 38: 197 [Google Scholar]
- Raschke K, Resemann A (1986) The midday depression of CO2 assimilation in leaves of Arbutus unedo L.: diurnal changes in photosynthetic capacity related to changes in temperature and humidity. Planta 168: 546–558 [DOI] [PubMed] [Google Scholar]
- Rock CD, Zeevaart JAD (1991) The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci USA 88: 7496–7499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Dominguez CM, Buckley TN, Egea G, de Cires A, Hernandez-Santana V, Martorell S, Diaz-Espejo A (2016) Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant Cell Environ 39: 2014–2026 [DOI] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011) Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 21: 1030–1035 [DOI] [PubMed] [Google Scholar]
- Sagi M, Scazzocchio C, Fluhr R (2002) The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. Plant J 31: 305–317 [DOI] [PubMed] [Google Scholar]
- Shatil-Cohen A, Attia Z, Moshelion M (2011) Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: a target of xylem-borne ABA? Plant J 67: 72–80 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Sussmilch FC, Brodribb TJ, McAdam SAM (2017) Up-regulation of NCED3 and ABA biosynthesis occur within minutes of a decrease in leaf turgor but AHK1 is not required. J Exp Bot 68: 2913–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostkiewicz I, Richter K, Kepka M, Demmel S, Ma Y, Korte A, Assaad FF, Christmann A, Grill E (2010) Closely related receptor complexes differ in their ABA selectivity and sensitivity. Plant J 61: 25–35 [DOI] [PubMed] [Google Scholar]
- Takemiya A, Shimazaki K (2010) Phosphatidic acid inhibits blue light-induced stomatal opening via inhibition of protein phosphatase 1 [corrected]. Plant Physiol 153: 1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Nose T, Jikumaru Y, Kamiya Y (2013) ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J 74: 448–457 [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N (1995) The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of beta-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta 196: 564–570 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Puzõrjova I, Brosché M, Valk E, Lepiku M, Moldau H, Pechter P, Wang YS, Lindgren O, Salojärvi J, et al. (2010) Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J 62: 442–453 [DOI] [PubMed] [Google Scholar]
- Vlad F, Droillard MJ, Valot B, Khafif M, Rodrigues A, Brault M, Zivy M, Rodriguez PL, Merlot S, Laurière C (2010) Phospho-site mapping, genetic and in planta activation studies reveal key aspects of the different phosphorylation mechanisms involved in activation of SnRK2s. Plant J 63: 778–790 [DOI] [PubMed] [Google Scholar]
- Will RE, Wilson SM, Zou CB, Hennessey TC (2013) Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest-grassland ecotone. New Phytol 200: 366–374 [DOI] [PubMed] [Google Scholar]
- Xie X, Wang Y, Williamson L, Holroyd GH, Tagliavia C, Murchie E, Theobald J, Knight MR, Davies WJ, Leyser HMO, et al. (2006) The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr Biol 16: 882–887 [DOI] [PubMed] [Google Scholar]
- Xiong L, Lee H, Ishitani M, Zhu JK (2002) Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277: 8588–8596 [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI (2011) Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J 30: 1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Zhang X, Takemiya A, Kinoshita T, Shimazaki K (2007) Nitric oxide inhibits blue light-specific stomatal opening via abscisic acid signaling pathways in Vicia guard cells. Plant Cell Physiol 48: 715–723 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang H, Takemiya A, Song CP, Kinoshita T, Shimazaki K (2004) Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol 136: 4150–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]