The microRNA396d connects OsBZR1 with OsGRFs to regulate the signal of brassinosteroid and gibberellin for controlling the key agriculture yield traits in rice.
Abstract
Genetic improvement of plant architecture is one of the strategies for increasing the yield potential of rice (Oryza sativa). Although great progress has been made in the understanding of plant architecture regulation, the precise mechanism is still an urgent need to be revealed. Here, we report that over-expression of OsMIR396d in rice results in semidwarf and increased leaf angle, a typical phenotype of brassinosteroid (BR) enhanced mutant. OsmiR396d is involved in the interaction network of BR and gibberellin (GA) signaling. In OsMIR396d over-expression plants, BR signaling was enhanced. In contrast, both the signaling and biosynthesis of GA were impaired. BRASSINAZOLE-RESISTANT1, a core transcription activator of BR signaling, directly promoted the accumulation of OsmiR396d, which controlled BR response and GA biosynthesis by regulating the expression of different target genes respectively. GROWTH REGULATING FACTOR 6, one of OsmiR396d targets, participated in GA biosynthesis and signal transduction but was not directly involved in BR signaling. This study provides a new insight into the understanding of interaction between BR and GA from multiple levels on controlling plant architecture.
One of the major goals for cereal crop breeding is to increase grain yields. An ideal plant architecture is a concept for high grain yield in rice (Oryza sativa) breeding (Khush, 2013). Moderate plant height and erect leaves are two of the main factors for ideal plant architecture in rice (Yuan, 2001). In the 1960s, the introduction of semidwarf gene sd1 greatly increased rice yields throughout Asia and brought about the “green revolution” as a result of increased lodging resistance and harvest index in the semidwarf sd1 mutant (Spielmeyer et al., 2002). Leaf angle has progressively decreased due to genetic improvement of japonica and indica rice cultivars in China in recent decades (Yang et al., 2006; Wu et al., 2007; Hao et al., 2010). Erect leaves can capture more sunlight and store more nitrogen, thus improving grain filling and increasing rice yield (Sinclair and Sheehy, 1999). For example, the erect leaf rice mutant, osdwarf4-1, which is partially deficient in biosynthesis of brassinosteroid (BR), can improve biomass production and grain yield under dense planting conditions without extra fertilizer (Sakamoto et al., 2006).
Gibberellin (GA) and BR are two predominant plant hormones that determine plant height and leaf angle by regulating cell growth (Tong et al., 2014; Zhang et al., 2014). GA is perceived and bound by the soluble protein GIBBERELLIN INSENSITIVE DWARF1 (GID1), which results in GID1 conformation change; thus, the interaction of GID1 and DELLA proteins (GA response repressors) is promoted (Ueguchi-Tanaka et al., 2007; Hedden and Sponsel, 2015). The GA-GID1-DELLA complex recruits an F-box protein (SLY1 in Arabidopsis [Arabidopsis thaliana] and GID2 in rice), and subsequently DELLA is ubiquitinated by SCF E3 ubiquitin ligase and then degraded through the proteasomal degradation pathway. Following this, GA signal transduction is released from repression by DELLA proteins and cell elongation is promoted (McGinnis et al., 2003; Sasaki et al., 2003; Hirano et al., 2010).
BR signal transduction pathway is mainly shared in Arabidopsis and rice (Zhang et al., 2014). BR is perceived by Leu-rich repeat receptor-like kinases BRASSINOSTEROID-INSENSITIVE 1 (BRI1) and its coreceptor BRI1-ASSOCIATED RECEPTOR KINASE 1 (Clouse et al., 1996; Yamamuro et al., 2000; Li et al., 2002; Wang, et al., 2008). Then, the signal is passed along to a key negative regulator, BRASSINOSTEROID-INSENSITIVE 2 (BIN2), which is dephosphorylated by BRI1 SUPPRESSOR 1 (Li et al., 2001; Mora-Garciá et al., 2004; Kim et al., 2011). In rice, the dephosphorylation of GSK3/SHAGGY-like Kinase (OsGSK2, a rice homolog of Arabidopsis BIN2) releases its suppression on BRASSINAZOLE-RESISTANT1 (OsBZR1), LEAF AND TILLER ANGLE INCREASED CONTROLLER (OsLIC), DWARF AND LOW-TILLERING (OsDLT), OVATE Family Protein 8, and REDUCED LEAF ANGLE1 to activate BR response and increase leaf angle (Bai et al., 2007; Wang, L et al., 2008; Tong et al., 2009, 2012; Zhang et al., 2012; Yang et al., 2016, Qiao et al., 2017). The kinase activity of OsGSK2 can also be directly inhibited by membrane protein GW5 (quantitative trait locus for grain width and weight on chromosome 5), which is a calmodulin-binding protein (Liu et al., 2017).
The crosstalk network between BR and GA is quite complicated. BR and GA interact not only at the biosynthesis regulation level, but also at the signaling level. And their crosstalk differs depending on hormone concentrations, development stages, tissues, and species (Tong and Chu, 2016; Unterholzner et al., 2016). BR signaling-defected mutants are impaired in GA biosynthesis in Arabidopsis and rice (Tong et al., 2014; Unterholzner et al., 2015). Expression of GA biosynthesis gene GA20ox1 under the promoter of BRI1 in bri1-301 mutant can rescue most of its defects (Unterholzner et al., 2015). Arabidopsis BES1 and rice OsBZR1 can bind the promoters of GA biosynthesis or inactivation genes to regulate GA biosynthesis (Tong et al., 2014; Unterholzner et al., 2015). On the other hand, GA regulates BR biosynthesis at the transcription level. OsSPY, a negative regulator of GA signaling, negatively regulates BR biosynthesis (Shimada et al., 2006). OsGSR1, a positive regulator of GA signaling, activates BR synthesis by directly interacting with BR biosynthesis enzyme, DWF1 (Wang et al., 2009). There is also a physical interaction between BR and GA at the signaling level. The GA signaling negative regulator, DELLA protein, interacts with BZR1/BES1 and inhibits their function. Thus, GA can release the inhibition of DELLA on BZR1/BES1 to induce downstream BR responses in Arabidopsis (Bai et al., 2012; Gallego-Bartolomé et al., 2012; Li et al., 2012). Although great progress has been made in this aspect, the knowledge we have now is far from enough to understand the interaction network of BR and GA. More related research work needs to be carried out.
MicroRNAs also participate in hormone regulation and plant development, especially in integrating distinct agricultural traits (Tang and Chu, 2017). miR396 represents one of the most deeply conserved miRNA families in plants (Liu et al., 2009). Many studies have been performed in Arabidopsis and rice focusing on miR396 and its targets, GROWTH REGULATING FACTORS (GRFs). In rice, OsmiR396 and its targets, OsGRFs, regulate leaf development, plant height, meristem function, flowering time, inflorescence architecture, and seed size (Luo et al., 2005; Kuijt et al., 2014; Liu et al., 2014; Che et al., 2015; Duan et al., 2015; Gao et al., 2015). OsGRF4 interacts with OsGIFs to control seed size through BR signaling, and its transcription activity can be directly regulated by OsGSK2 (Che et al., 2015; Duan et al., 2015). OsGRF6 is involved in floral organogenesis by regulating the expression of JMJD2 family jmjC gene 706 (OsJMJ706) and Crinkly4 receptor-like Kinase (OsCR4; Liu et al., 2014). OsGRF6 also modulates inflorescence architecture through the IAA regulating pathway (Gao et al., 2015).
It is well established that OsmiR396 targets OsGRFs to function in the regulation pathway of multiple hormones such as GA, BR, and auxin (van der Knaap et al., 2000; Che et al., 2015; Gao et al., 2015). However, little is known about how miR396 is regulated in plant development. Here, we show that OsMIR396d is involved in the BR signaling transduction pathway and is directly activated by the key transcription factor OsBZR1. OsMIR396d also participates in the regulation of GA signaling by modulating the expression of OsGRF6, which is independent of BR signaling. Our findings reveal that the OsmiR396d can combine the BR and GA regulation pathway to control rice plant architecture.
RESULTS
Over-expression of OsMIR396d Changed Rice Plant Architecture
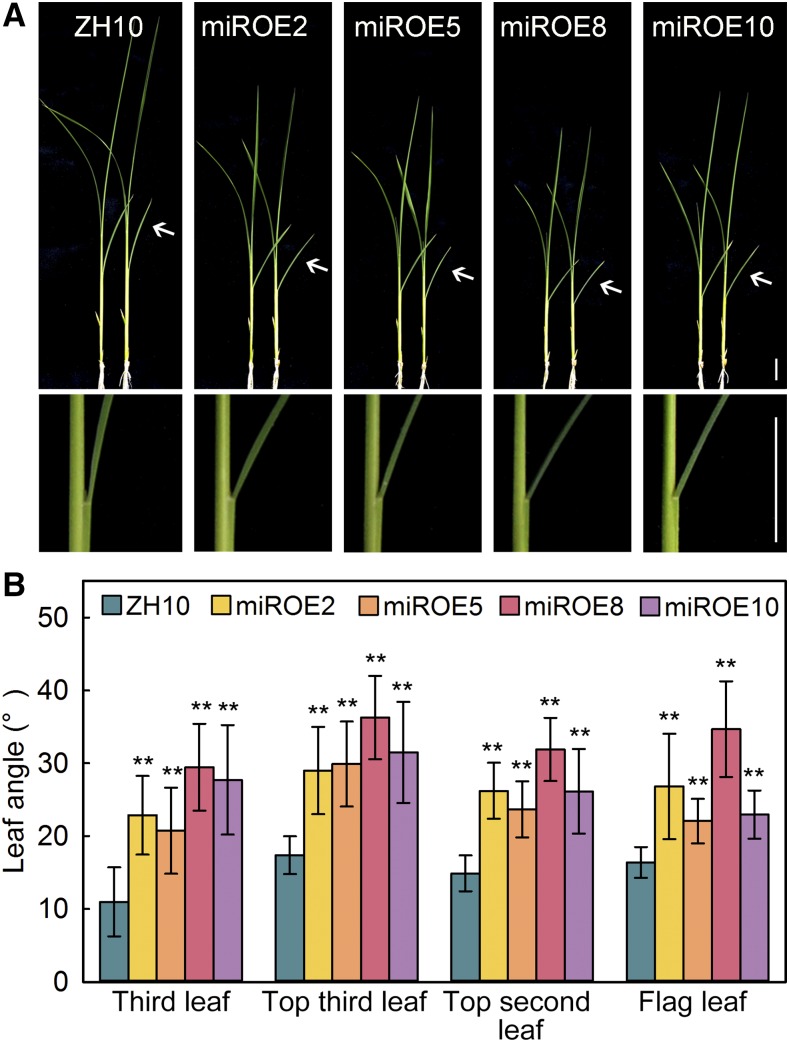
Our previous study showed that OsMIR396d over-expression transgenic plants (miROE) exhibited abnormal florets. OsmiR396d target genes, OsGRF6 and OsGRF10, were involved in regulating floral organ identity and husk opening (Liu et al., 2014). In miROE lines, the third leaf angles were increased to two times more than that of Zhonghua 10 (ZH10) at the seedling stage (Fig. 1, A and B). The increased leaf angle phenotype was also observed on the top three leaves of miROE lines at the seed filling stage (Fig. 1B). miROE8 had the highest expression level of OsmiR396d among the transgenic lines (Liu et al., 2014) and exhibited the largest leaf angle. The angles of the top third leaf of ZH10, miROE2, miROE5, miROE8, and miROE10 were 17°, 29°, 30°, 36°, and 32°, respectively (Fig. 1B).
Figure 1.
The leaf angle of OsMIR396d over-expression plants was increased. A, The third leaf angle of miROEs was specifically increased. Bar = 1 cm. B, Statistical analysis of leaf angles. The third leaf angle was measured at the seedling stage and the angles of the top three leaves were measured at the seed filling stage. Error bars indicate SD for at least 15 plants. Asterisks indicate P < 0.01 (**) compared with wild type in the Student’s t test analysis.
The height of miROE plants was also significantly decreased compared with ZH10 (Fig. 2A). The data showed that the plant height of miROE was only 70% to 80% of that of ZH10, and every internode of miROE was shortened compared with ZH10 (Fig. 2, B and C). The fifth internode of miROE exhibited severe growth retardation in particular (Fig. 2D). The fifth internode length decreased from 2.6 cm in ZH10 to about 1.0 cm in miROE lines. The relative lengths of each internode (percentage of each internode length to total culm length) had no obvious differences between miROE and ZH10, except that the fifth internode was reduced from 2.6% in ZH10 to about 1.5% in miROE (Fig. 2E).
Figure 2.
The plant height and length of internodes in OsMIR396d over-expression plants were reduced. A, The phenotype of miROEs and ZH10 at the seed filling stage. Bar = 5 cm. B, The panicle and internodes of miROEs and ZH10. Bar = 5 cm. C, Results of statistical analysis of plant height and internode length of miROE lines and ZH10. More than 15 mature plants were used in each analysis. D, Results of statistical analysis of fifth internode lengths of miROEs and ZH10. Error bars indicate SD for at least 15 plants. Asterisks indicate P < 0.01 (**) compared with ZH10 in the Student’s t test analysis. E, Relative internode length of miROEs and ZH10. The data shows each internode length relative to the total culm length. Error bars indicate SD for at least 15 plants. Asterisks indicate P < 0.05 (*) and P < 0.01 (**) compared with ZH10 in the Student’s t test analysis.
The Collar Adaxial Cell Size Was Enlarged in OsMIR396d Over-Expression Plants
The promotion of lamina inclination is one of the most typical BR responses in rice, and it is often caused by proliferation or expansion of collar adaxial cells (Wada et al., 1981; Cao and Chen, 1995; Sun et al., 2015). To determine the cell structure basis for increased leaf angle of miROE, the adaxial surface and longitudinal sections of miROE8 and ZH10 lamina joints were observed using scanning electron microscopy. The lamina joint adaxial side of miROE8 was obviously expanded compared to ZH10 (Fig. 3, A and D), and the cell size in the adaxial region of miROE8 was much larger than that of ZH10 (Fig. 3, B, C, E, and F). Especially, miROE8 cells were expanded nearly 1-fold larger than ZH10 along the proximal-distal axis (Fig. 3G). In contrast, the cell layers in the lamina joint adaxial side were not distinct between miROE8 and ZH10 at both proximal-distal axis (half frame indicated regions in Fig. 3, B and E) and adaxial-abaxial axis. Therefore, these results indicated that the increased leaf angle of miROE might mainly result from enlarged cell size at the adaxial side of the lamina joint.
Figure 3.
Scanning electron microscopic examination of the leaf lamina joint. A and B, Adaxial surface of the leaf lamina joint in ZH10 (A) and miROE8 (B). Arrows indicate the increased growth of cells. C and D, Longitudinal sections of the leaf lamina joint of ZH10 (C) and miROE8 (D). Half frame indicated regions were used to calculate cell layers. E and F, Close-up of regions denoted by rectangles in C and D respectively. Ad, adaxial; Ab, abaxial. Scale bars = 200 μm. G, Statistical analysis of cell length in C and F. Cell length along the adaxial-abaxial axis and proximal-distal axis were measured respectively. Error bars indicate SD for at least 100 cells. Asterisks indicate P < 0.01 (**) compared with ZH10 in the Student’s t test analysis.
Cell Elongation and Division Were Arrested in OsMIR396d Over-Expression Plants
To figure out the reason for the semidwarf phenotype of miROE plants, the cell sizes in the second leaf sheaths were analyzed. Compared with ZH10, the cell length of miROE8 was slightly decreased (Fig. 4, A and B). In addition, the cell cycle progression was analyzed by flow cytometry using root apical cells. The results showed that the DNA content of miROE8 was decreased compared to ZH10 (Fig. 4C). Thus, miROE possessed fewer cells in the G2/M phase. The mitotic index, a measure for the proliferation status of a cell population (Simpson et al., 1992), was about 9.4% in miROE8, which was significantly lower than that of ZH10 (16.4%; Fig. 4D). Therefore, the semidwarf phenotype of miROE plants was caused by both impaired cell elongation and reduced cell division.
Figure 4.
Cell length and cell division analysis. A, The second leaf sheath cells of 2-week-old seedlings of miROE8 and ZH10. Bars = 50 μm. B, The statistical analysis of cell length in the second leaf sheath. Error bars indicate SD for more than 400 cells. Asterisks indicate P < 0.01 (**) compared with ZH10 in student’s t test analysis. C, The DNA content of miROE8 and ZH10. After the seeds germinated for 3 d at 28°C, the root tips were harvested. Cell nuclei (10,000) were stained with 1 μg mL−1 DAPI and analyzed by flow cytometry. 2C and 4C represent the DAPI signals that correspond to nuclei with different DNA contents. D, Cell mitotic index in root apical meristem in miROE8 and ZH10. The results were from three independent replications. Error bars indicate SD. Asterisks indicate P < 0.01 (**) compared with ZH10 in the Student’s t test analysis.
OsmiR396d Is Involved in the BR Signaling Pathway
The increased leaf angle and semidwarf phenotypes of miROE plants are similar to the phenotypes of BR signal-enhanced rice plants, such as OsLIC antisense lines (Zhang et al., 2012) and ili1-d mutant (Zhang et al., 2009). Therefore, it is possible that OsmiR396d is involved in positive regulation of BR signaling. To investigate this possibility, the effects of OsmiR396d on BR signaling were examined by leaf angle and root length growth of miROE8 and ZH10 in the presence of 24-epibrassinolide (24-eBL). The leaf angle of miROE8 was increased more efficiently than that of ZH10 when exogenous 24-eBL was applied (Fig. 5A). The root growth of miROE8 was also more sensitive to 24-eBL compared to ZH10 (Fig. 5B). These 24-eBL treatment results implied that OsmiR396d positively regulated BR signaling in rice. To figure out the points of BR signaling impacted with OsMIR396d over-expression, the expression levels of several BR regulation related genes were compared between miROEs and ZH10. The qRT-PCR result showed that the positive regulatory genes of BR signaling, OsBRI1 and OsDLT1, were up-regulated in miROEs, while the negative regulatory gene of BR signaling OsLIC was down-regulated (Supplemental Fig. S1). This result indicated that OsmiR396d might regulate multiple steps of the BR signaling pathway.
Figure 5.
OsmiR396d is involved in BR signaling. A, Lamina joint inclination assay of miROE8 and ZH10. The second lamina joints of 1-week-old seedlings grown under dark condition were cut off and treated with different concentrations of 24-eBL for 3 days. Error bars indicate SD for at least 15 plants. Asterisks indicate P < 0.05 (*) and P < 0.01 (**) compared with ZH10 in the Student’s t test analysis. B, The seminal root growth of miROE8 and ZH10 under treatment with different concentrations of 24-eBL. The seminal root length was measured after seedlings were grown in 24-eBL containing 1/2 Murashige and Skoog medium for 1 week. Error bars indicate SD for at least 15 plants. Asterisks indicate P < 0.05 (*) and P < 0.01 (**) compared with ZH10 in the Student’s t test analysis. C, The expression pattern of OsMIR396d under treatment with 24-eBL. Two-week-old ZH10 seedlings were treated with 1 μm 24-eBL. Whole plants were sampled 1, 3, 6, 12, and 24 h after treatment. The plants sampled from the normal growth condition were used as control. UBQUITIN1 was used as a reference gene. Error bars indicate SD for three replicates. Asterisks indicate P < 0.05 (*) and P < 0.01 (**) compared with control in the Student’s t test analysis. D, The expression of LIC-AS, BZR1R, and BZR1-OE plants. LIC-AS and BZR1R were under the background of ZH10 and BZR1-OE was under the background of NIP. UBQUITIN1 was used as reference gene. Error bars indicate SD for three replicates. Asterisks indicate P < 0.05 (*) and P < 0.01 (**) compared with wild-type ZH10 or NIP, respectively, in the Student’s t test analysis.
How BR influences OsMIR396d expression was also investigated. The response of OsMIR396d to 24-eBL was detected by quantitative reverse transcription-PCR (qRT-PCR). Compared with the control, the expression level of OsMIR396d was up-regulated under 24-eBL treatment and reached the highest level after treatment for 3 h (Fig. 5C). Consistently, the transcript level of several OsGRFs decreased after BR treatment for 3 h (Supplemental Fig. S2). Furthermore, the expression level of OsMIR396d in BR signaling enhanced or impaired rice plants was checked. The qRT-PCR results showed that the expression of OsMIR396d was increased in the BR-signal enhanced OsLIC antisense line (LIC-AS; Zhang et al., 2012) and the OsBZR1 over-expression line (BZR1-OE), but decreased in the BR-signal reduced OsBZR1 RNAi line (BZR1R; Bai et al., 2007; Fig. 5D). These results indicated that OsMIR396d transcription was induced by both exogenous BR treatment and endogenous BR signal.
OsMIR396d Is a Direct Target of OsBZR1
The expression of OsMIR396d was up-regulated in BZR1-OE plants and down-regulated in BZR1R lines (Fig. 5D). It was reported that transcription factor BZR1 binds to CGTGT/CG element (He et al., 2005; Zhang et al., 2012). The 1.5-kb sequence of the OsMIR396d promoter was screened, and three putative BZR1 binding sites were found at −479 (S1), −667 (S2), and −1,069 (S3) bp upstream of the premiR396d transcription start site (Fig. 6A). These results implied that OsMIR396d is potentially targeted by the transcription factor OsBZR1.
Figure 6.
OsBZR1 directly activates the expression of OsMIR396d. A, Localization of three putative OsBZR1 binding sites S1, S2, and S3 in the OsMIR396d promoter. B, Yeast one-hybrid analysis of OsBZR1 binding the promoter of OsMIR396d. Empty pHIS vector was used as negative control. C, EMSA analysis of OsBZR1 binding the promoter of OsMIR396d. S1-containing DNA fragment was labeled with biotin. The arrow indicates the complex of OsBZR1-His and the DNA probe. D, ChIP-qPCR analysis of OsBZR1 binding the promoter of OsMIR396d using OsBZR1 antibody to enrich DNA. The UBIQUITIN DNA fragment was used as an internal control. Error bars indicate SD for three replicates. Asterisks indicate P < 0.01 (**) compared with ZH10 in the Student’s t test analysis. E, Transactivation assay of OsBZR1 with the luciferase reporter system. Error bars indicate SD for three replicates. Asterisks indicate P < 0.01 (**) compared with control in the Student’s t test analysis. F, The phenotype of OsBZR1 RNA interference transgenic plants (miROE8/BZR1R) under the background of miROE8. Bar = 10 cm.
To test the direct regulation of OsBZR1 to OsMIR396d, yeast one-hybrid assay was carried out. The data showed that OsBZR1 could bind the S1 site but not the S2 or S3 site. The mutation of S1 sites led to disappeared binding ability (Fig. 6B). The EMSA assay further confirmed this possibility. The His-tagged OsBZR1 protein could physically bind the S1 site containing DNA fragment in vitro, and the excess unlabeled competitive probe could effectively inhibit the biotin probe bound by OsBZR1 (Fig. 6C). Furthermore, OsBZR1 bound the promoter of OsMIR396d in vivo was analyzed through chromatin immunoprecipitation (ChIP)-qPCR via the antibody against OsBZR1. The S1-containing DNA fragment was significantly decreased in BZR1R plants compared with ZH10, while the amount of S2- and S3-containing DNA fragments was not significantly different between BZR1R and ZH10 (Fig. 6D). In addition, activity of luciferase was used as a reporter to detect the effect of OsBZR1 on OsMIR396d expression in Arabidopsis protoplasts. The data showed that the luciferase activity of OsMIR396d::LUC transfected protoplasts was increased when OsBZR1-GFP was coexpressed (Fig. 6E). This result suggested that OsBZR1 positively regulates the expression of OsMIR396d.
To explore the genetic relationship of OsMIR396d and OsBZR1, the OsBZR1 RNAi vector was transformed into miROE8 to get double transgenic plants (BZR1R/miROE8; Supplemental Fig. S3). The leaf angle of BZR1R/miROE8 was increased compared with BZR1R or ZH10 (Fig. 6F; Supplemental Fig. S4, A and B). This result suggested that the function of OsBZR1 might be partially dependent on OsmiR396d. Additionally, miROE8 was crossed with BR receptor OsBRI1 mutant d61 (Nakamura et al., 2006). The leaf angles were significantly increased in the progenies of miROE8/d61 compared with the parents (Supplemental Fig. S4). This result further confirmed that OsMIR396d was involved in the BR signal regulated leaf angle development process. These biochemical and genetic evidences indicate that OsMIR396d was one direct target gene of OsBZR1.
OsmiR396d Is Involved in the GA Regulation Pathway
The OsMIR396d over-expression plants displayed decreased internode length, especially the shortened fifth internode (Fig. 2). This characteristic was similar to GA deficient mutants such as d18, d35, and sd1 (Itoh et al., 2004). The cross progenies miROE8/d61 showed not only increased leaf angle but also reduced plant height compared with their parents (Supplemental Fig. S4). These phenotypes implied that OsmiR396d was possible to control plant height through GA signaling in addition to the BR-dependent pathway.
To examine if OsmiR396d controls plant height through GA signaling, the GA sensitivity of miROEs was analyzed by measuring the second leaf sheath length under GA treatment. The results showed that the second leaf sheath length of both miROE8 and ZH10 were distinctly elongated under GA treatment (Fig. 7A). However, by comparison, the leaf sheath of miROE8 was less sensitive to GA3 than that of ZH10. The expression levels of GA signaling and biosynthesis related genes were also checked in miROEs and ZH10. Results from qRT-PCR showed that the transcription levels of GA signaling pathway gene OsGID2 and several GA biosynthesis pathway genes (including OsCPS1, OsKO2, OsGA20ox1, and OsGA20ox3) were apparently reduced in miROEs compared with ZH10 (Fig. 7B). The expression pattern of OsMIR396d after treatment with paclobutrazol (PAC, GA biosynthesis inhibitor) or GA3 was also analyzed in ZH10 seedlings. The result showed the expression of OsMIR396d was typically repressed by continuous PAC treatment, but was up-regulated after treatment with a high concentration of GA3 (Supplemental Fig. S5). These findings suggested that OsmiR396d participates in the GA signaling pathway and regulates internal GA biosynthesis as well.
Figure 7.
GA signaling and biosynthesis were impaired in OsMIR396d over-expression plants. A, The phenotype of miROE8 and ZH10 seedlings growth under different concentrations of GA3 treatment. The seeds were treated with 10 μm PAC for 2 d before cultivation in nutrient solution containing the indicated concentration of GA3. After 10 d of growth, the seedlings were photographed. The measurements of the second leaf sheath are shown. Error bars indicate SD for at least 15 plants. Bar = 5 cm. B, The expression of GA pathway related genes in miROE and ZH10. UBQUITIN1 was used as reference gene. Error bars indicate SD for three replicates.
OsmiR396d Targeted OsGRF6 to Regulate the GA Pathway
GRFs are predicted to be the targets of miR396 (Jones-Rhoades and Bartel, 2004; Wu et al., 2009). OsGRF6, one target of OsmiR396d, was involved in floral organogenesis under the regulation of OsmiR396d (Liu et al., 2014). We were also interested in determining whether OsGRF6 participates in BR and GA signaling in a similar manner as OsMIR396d. Our previous data showed that the plant height of osgrf6 mutant was decreased compared with wild-type Dongjin (DJ; Liu et al., 2014). OsGRF6 antisense transgenic plants (GRF6as) were also moderately reduced compared with wild-type (ZH10) plants, while the OsmiR396d-resistant form of OsGRF6 transgenic plants (rGRF6OE) had a slightly increased plant height (Fig. 8A). Microscopy data further showed that the cell length of the osgrf6 second leaf sheath was significantly shortened compared with DJ (Fig. 8B). In contrast, the leaf angle of osgrf6 was very similar to DJ (Fig. 8C). These results suggested that OsmiR396d might target OsGRF6 to regulate plant height but nearly not affect leaf angle.
Figure 8.
BR signaling was not affected in osgrf6 mutant. A, The phenotype of GRF6as and the rGRF6OE. Bar = 5 cm. B, The second leaf sheath cells of 2-week-old seedlings of osgrf6 and DJ. The cell length was measured. Error bars indicate SD for more than 400 cells. Asterisks indicate P < 0.01 (**) compared with DJ in the Student’s t test analysis. Bar = 50 μm. C, The leaf angle of osgrf6 was not changed. The third leaf angle was measured. Error bars indicate SD for at least 15 plants. D, Lamina joint inclination assay of osgrf6 and DJ. Error bars indicate SD for at least 15 plants. E, The seminal root growth of miROE8 and ZH10 under treatment with different concentrations of 24-eBL. Error bars indicate SD for at least 15 plants.
In lamina inclination assay, the result showed that the sensitivity of osgrf6 to 24-eBL was not apparently different from that of DJ (Fig. 8D). In seminal root growth experiment, the root length of osgrf6 mutant was much shorter than that of DJ under normal growth condition, so we used relative root length to do the analysis following the method used by Tong et al. (2014), and the root length under normal growth condition was used as reference. The relative root length of osgrf6 was 0.56 and that of DJ was 0.61 at 10−7 M 24-eBL (Fig. 8E). This result also showed that there was no apparent distinction in BR sensitivity between osgrf6 and DJ. Above results indicated that OsGRF6 might not be directly involved in the BR signaling mediated lamina joint development and root growth processes.
To determine if OsGRF6 participates in GA regulation, osgrf6 and DJ seeds were pretreated with 10−5 M PAC (to inhibit endogenous GA biosynthesis) or water (control) for 2 d. Then the germinated seeds were planted in nutrient solution containing 10−5 M PAC and/or 10−5 M GA3 as indicated in the Figure 9A. The second leaf sheath of osgrf6 was significantly shorter than DJ after the treatment for 10 d. When treated only with PAC, there was no difference in second leaf sheath length between osgrf6 and DJ. However, this difference appeared again when GA3 was added into the nutrient solution (Fig. 9, A and B). This result implied that GA signaling or biosynthesis was likely impaired in osgrf6. The reduced sensitivity of osgrf6 to GA3 was further confirmed by treating osgrf6 and DJ with different concentration of exogenous GA3 (Supplemental Fig. S6). The genes that were down-regulated in miROEs were also analyzed in osgrf6. Results from qRT-PCR showed that the expression levels of OsCPS1, OsGA20ox1, OsGA20ox3, and OsGA3ox2 were also down-regulated in osgrf6 compared with DJ (Fig. 9C).
Figure 9.
GA signaling and biosynthesis are impaired in the osgrf6 mutant. A, osgrf6 and DJ seedlings treated with PAC or GA3. The seeds were treated with water (control) or 10−5 M PAC for 2 d and then germinated seeds were planted in PAC and/or GA3 containing culture solution for 10 d. Bar = 5 cm. B, The statistical result of the second leaf sheath length of osgrf6 and DJ grown under PAC and/or GA3 treatment. Error bars indicate SD for at least 15 plants. The relative leaf sheath length between DJ and osrf6 was calculated and shown. Asterisks indicate P < 0.01 (**) compared with DJ in Student’s t test analysis. C, The expression of GA pathway related genes in osgrf6 and DJ. UBQUITIN1 was used as reference gene. Error bars indicate SD for three replicates. D, Quantification of endogenous GAs in osgrf6 and DJ. Two-week-old seedlings were sampled. Error bars indicate SD for three replicates. Asterisks indicate P < 0.01 (**) compared with DJ in the Student’s t test analysis.
To confirm that GA biosynthesis was impaired in osgrf6, endogenous GAs were quantified. The results showed that GA1, GA19, and GA53 levels were significantly decreased in osgrf6 (Fig. 9D). These results demonstrated that OsGFR6 was involved in the regulation of both GA signaling and biosynthesis.
DISCUSSION
BR and GA are vital hormones in plant that influence various developmental processes, such as cell division, cell elongation, flowering, leaf senescence, and seed germination (Greenboim-Wainberg et al., 2005; Vriet et al., 2013; Fariduddin et al., 2014; Sakata et al., 2014; Hedden and Sponsel, 2015). BR and GA interact with each other at both biosynthesis and signaling levels to form a potential complex network (Wang et al., 2009; Zhang et al., 2012). Here, we report that OsmiR396d participates in this interaction network of BR and GA. OsBZR1, as a node of BR signaling pathway, can directly activate the expression of OsMIR396d to repress the expression of OsGRF6 for regulating GA signaling and biosynthesis (Fig. 10).
Figure 10.
A proposed model for OsmiR396d controlling rice plant architecture. OsMIR396d is activated by OsBZR1, which is the key regulator transcription factor of BR signaling. On the one hand, OsmiR396d regulates leaf angle by repressing the expression of OsGRF4. On the other hand, OsmiR396d is involved in GA signaling and biosynthesis by regulating the expression of OsGRF6 to control rice plant height.
OsBZR1 Targets OsMIR396d to Control Leaf Angle through OsGRF4-Mediated BR Response
In this study, we propose that OsmiR396d is involved in the BR signaling pathway based on data with four lines. Firstly, over-expression of OsMIR396d in rice resulted in increased leaf angle and semidwarf phenotype (Figs. 1 and 2), which was similar to the phenotype of rice plants with accumulated BR or with enhanced BR signal (Tanaka et al., 2009; Tong et al., 2009, 2012; Zhang et al., 2009, 2012). Secondly, we demonstrated that the CGTGT/CG element in the OsMIR396d promoter was directly bound by OsBZR1 using yeast one-hybrid experiment and EMSA assay in vitro and also ChIP-qPCR detection in vivo. The results of the luciferase reporter assay indicated that OsBZR1 could activate OsMIR396d expression (Fig. 6). Coincidently, the OsMIR396d transcript level was increased in OsBZR1 over-expression plants, but was decreased in OsBZR1 RNAi plants (Fig. 5D). Thirdly, in the double transgenic plants BZR1R/miROE8, the leaf angle was increased compared with BZR1R (Fig. 6F; Supplemental Fig. S4), which further confirmed that OsMIR396d was possibly epistatic to OsBZR1. Finally, the miROE8 seedlings with a decreased OsGRF4 transcript level (Liu et al., 2014) were sensitive to BR (Fig. 5). In addition, near-isogenic line carrying GL2 plants, which possessed enhanced OsGRF4 expression, showed impaired sensitivity to BR in lamina joints (Che et al., 2015). Therefore, OsmiR396d, under the regulation of OsBZR1, may control rice leaf angle by regulating the expression of OsGRF4.
Our data support OsMIR396d is directly controlled by OsBZR1 on the transcription level, but we also noticed that over-expression of OsMIR396d in OsBZR1 RNAi background only partially rescues the phenotype of OsBZR1i plant. There are three potential reasons for this phenomenon. Firstly, OsBZR1 is not only involved in BR signaling but also participates in some other biological processes. There are thousands of target genes of OsBZR1, such as OsLIC, DLT, IBH1, ILI1, and so on (Tong and Chu, 2009; Zhang et al., 2009, 2012; Sun et al., 2010). OsMIR396d is just one of its targets, so OsMIR396d alone is not enough to explain all phenotypes of OsBZR1i plant, even the phenotypes specific to BR response. Secondly, OsMIR396d is not only controlled by OsBZR1 but is also controlled by other transcription factors, such as unknown GA response factors mentioned below. Thirdly, there are 12 putative targets (OsGRFs) of OsmiR396d, and they are involved in different development progress. Some of them are not involved in OsBZR1-regulated signaling pathway. For example, OsGRF3 and OsGRF10 regulate meristem function through repressing the expression of KNOX gene OsKN2 (Kuijt et al., 2014), OsGRF6 is involved in IAA regulation to control panicle size (Gao et al., 2015), and OsGRF6 and OsGRF10 also influence flower organ development through binding to the promoters of OsJMJ706 and OsCR4 (Liu et al., 2014). So OsBZR1 and OsmiR396d are not in a single signaling pathway, but in a complex network system; their functions just partially overlay with each other. In this study, we only reveal one kind of connections between OsBZR1 and OsmiR396d through biochemical and genetic approaches.
OsmiR396d Regulates OsGRF6 to Control Rice Plant Height through GA Pathway
The plant heights of BZR1R/miROE8 and miROE8/d61 were shorter than both parent plants (Fig. 6F; Supplemental Fig. S4). The additive genetic effect between OsMIR396d and these BR signaling pathway genes may result from the fact that OsmiR396d and its target genes are not only involved in BR signaling but also in other hormone responses. Our experiments showed that over-expression of OsMIR396d caused enhanced BR signal and reduced GA signal in rice seedlings (Figs. 5 and 7). In the osgrf6 mutant, the BR sensitivity was similar to wild type (Fig. 8); however, both GA signaling and biosynthesis were impaired (Fig. 9). Furthermore, the expression of both OsMIR396d and OsGRF6 were regulated by GA. OsMIR396d expression level was down-regulated by treatment with GA biosynthesis inhibitor PAC, but was up-regulated by treatment with high concentrations of GA. However, the OsGRF6 transcript level was changed in an opposite way to OsMIR396d (Supplemental Fig. S5). These results indicate that OsmiR396d controls rice height by directly regulating OsGRF6 expression and GA signaling.
OsmiR396d Integrates Different Hormone Regulation Pathway
Our results showed that OsMIR396d transcription was induced by both exogenous BR and GA, and OsMIR396d can be directly activated by OsBZR1. Based on a previous report that the protein level of OsBZR1 decreased under 10−5 M GA3 treatment (Tong et al., 2014), the transcription level of OsBZR1’s target gene OsMIR396d should decrease under the same GA3 concentration. However, qRT-PCR results showed that the transcripts of OsMIR396d were increased (Supplemental Fig. S5). These conflicting results implied that there might be another GA response factor, but not OsBZR1, which also controlled the transcription of OsMIR396d in GA signal. As one of the OsmiR396d targets, OsGRF4 functions in the BR response but not in GA signaling to control seed size and leaf angle (Che et al., 2015; Duan et al., 2015). Here, we reported another target of OsmiR396d, OsGRF6, was involved in GA biosynthesis regulation and signal transduction to regulate plant height, but did not directly participate in the BR response to control leaf angle. In addition, OsmiR396-OsGRF6 was also involved in auxin signaling to determine panicle architecture (Gao et al., 2015). Therefore, OsMIR396d could be regulated by BR, GA, and auxin respectively, and controls various yield traits through different downstream targets.
OsmiR396d Positively Regulates BR Signaling but Negatively Regulates GA Function
Although BR and GA coordinately regulate plant development, they are distinct from each other in the specific biological processes. GA powerfully promotes cell elongation in a great range of concentrations, while BR only moderately promotes cell elongation under specific physiological concentrations (Tong et al., 2014). BR is a master controller of leaf angle, while GA has only limited effect on this aspect (Tong and Chu, 2016). Here, we report that OsmiR396d positively regulates BR singling mainly focused on leaf angle, and OsmiR396d negatively controls GA biosynthesis and signaling mainly focused on plant height. Furthermore, we found that OsMIR396d expression is also differentially regulated by varying concentrations of GA. The expression of OsMIR396d is down-regulated by low GA conditions while up-regulated by high GA conditions (Supplemental Fig. S5). It indicated that the repression of GA biosynthesis by OsmiR396d is attenuated when GA signaling is weak and is enhanced when GA signaling is strong. It hints OsmiR396d possibly participates in the feedback regulation of GA biosynthesis. In addition, OsMIR396d expression is up-regulated under exogenous 1 μm BR treatment (Fig. 5C). It suggested that the repression of GA biosynthesis by OsmiR396d will become stronger when BR signaling is greatly enhanced.
Improving Agronomic Traits through Manipulating miR396-GRF-Regulated Pathways
Rice miR396 family and their targets control various yield traits via multiple phytohormone signals. OsmiR396-OsGRF6 controls plant height and panicle size (Gao et al., 2015), while OsmiR396-OsGRF4 controls leaf angle and seed size (Che et al., 2015; Duan et al., 2015). So it is possible to improve these traits at the same time through regulating the expression of multiple OsmiR396 family members or their targets. Enhancing the expression of OsGRF4 and OsGRF6 directly may cause more spikelets, increased seed size, erect leaves, and slightly higher plant height, which may be close to the concept of ideal plant architecture for high-grain-yield breeding. It is also possible to create OsmiR396d-resistant OsGRF4 and OsGRF6 alleles through CRISPR–Cas9 nickase-cytidine deaminase fusion system (Zong et al., 2017) to convert C to T in OsmiR396 targeted DNA regions. An additional approach may also be achieved through combining different members of OsmiR396 deleted “nontransgenic” mutants by CRISPR-CAS9 technology (Feng et al., 2013) to improve rice yield.
In this study, an important component of the BR signaling, OsBZR1, was identified as the first direct upstream regulator of OsMIR396d. OsmiR396d can integrate the BR and GA signal interaction network to control plant architecture by repressing the expression of multiple target genes, such as OsGRF4 and OsGRF6, which participate in BR and GA regulation, respectively (Fig. 10). Effective regulation of the expression of OsMIR396 and its target genes may not only increase spikelet number (Gao et al., 2015) and seed size (Che et al., 2015; Duan et al., 2015), but also improve plant architecture.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Rice (Oryza sativa) japonica cultivars ZH10, Nipponbare (NIP), and DJ were used in this study. Transgenic plants were produced in our laboratory, including: miROE, GRF6as, rGRF6OE, LIC-AS, BZR1-OE, and BZR1R (Bai et al., 2007; Zhang et al., 2012; Liu et al., 2014). The T3 generation of double transgenic plants BZR1R/miROE8 and the F3 generation of cross progenies miROE8/d61 were used in this study. The osgrf6 mutant from the DJ background was obtained from the Rice Functional Genomics Express Database of Korea (Jeong et al., 2002). Mutant d61 was kindly provided by Makoto Matsuoka (Yamamuro et al., 2000). Rice plants used in this study were grown in a greenhouse at 30°C/25°C (day/night) or in the field located in Beijing under natural conditions.
Hormone Treatment
For BR treatment, rice seeds were sterilized with 5% NaClO3 and grown in 1/2 Murashige and Skoog medium (M519, Phytotech) with different concentrations of 24-eBL (CE5051, Coolaber) for 1 week, and the seminal root length was measured. The lamina inclination experiment was carried out according to Zhang et al. (2012).
To detect GA sensitivity, rice seeds were first soaked in 10−5 M PAC (CP8121, Coolaber) for 2 d to inhibit endogenous GA biosynthesis, and then germinated seeds were planted in Yoshida’s culture solution (Yoshida et al., 1976) with various concentrations of GA3 (G8040, Solarbio). After 10 d of growth, the length of the second leaf sheath was measured, and the seedlings were used to detect the transcription levels of OsMIR396d and OsGRF6.
Microscopy Observation
For scanning electron microscopy analysis, the lamina joints of OsMIR396d over-expression plants and wild-type ZH10 at heading stage were excised to image the adaxial surface according to Wang et al. (2008). To determine the cell length of second leaf sheaths, surface cells in the same position of the second leaf sheaths of miROE, osgrf6, and wild-type ZH10 and DJ 2-week-old seedlings were torn off and observed under Differential Interference Contrast microscopy according to Li et al. (2011).
Flow Cytometric Analysis of Cell Cycle Progression
For flow cytometry, wild-type and miROE seeds were germinated for 7 d in petri dishes (diameter, 20 cm) on filter paper with sterilized water in the dark at 28°C. Root apical tips (1 mm) were excised and immediately collected in chilled chopping buffer and chopped with a single-edged razor blade in a glass petri dish (diameter, 5 cm) on ice. Chopping buffer (45 mm MgCl2, 30 mm sodium citrate, 20 mm 4-morpholinepropane sulfonate, and 1 mg mL−1 Triton X-100, pH 7.0) was used to release cells from the chopped tissues. The DNA content of individual cells was determined by flow cytometry. Cell nuclei were prepared for FACSAria by staining with 2 μg mL−1 4',6-diamidino-2-phenylindole (DAPI). Each root apical tip sample was prepared three times and subjected to FACS Caliber cytometry (BD Bioscience) three times. Ten thousand nuclei were measured per analysis as previously described (Ma et al., 2009).
RNA Extraction and qRT-PCR
Total RNA was extracted from 2-week-old rice seedlings using the Trizol RNA extraction kit according to the user manual (15596, Invitrogen). First-strand cDNA was then synthesized from 1 to 2 μg total RNA using the FastQuant RT Kit (with gDNase; KR106, Tiangen) according to the manufacturer’s instructions. qRT-PCR analyses were carried out using the SYBR Green Master Mix (QPK-201, TOYOBO) on the Mx3000P real-time PCR System (Stratagene) according to the manufacturer’s instructions. Rice UBIQUITIN was used as internal reference, and gene expression levels were normalized to the expression level of UBIQUITIN. The expression level of OsMIR396d was analyzed as described by Liu et al. (2014). All primers are described in Supplemental Table S1.
ChIP-qPCR
Two grams of leaves from 2-week-old rice seedlings of wild-type (ZH10) and OsBZR1 RNAi plants (BZR1R) were used for ChIP assay; the experiment was performed as described in He et al. (2005). The OsBZR1 antibody was used for immunoblot and ChIP analysis. The immunoprecipitated DNA content was determined by qPCR. Three replications were performed independently. The primers used in ChIP assays are listed in Supplemental Table S1.
Yeast One-Hybrid Assays
Yeast one-hybrid assays were used to check binding of OsBZR1 to the OsMIR396d promoter according to a previous study (Chen et al., 2013). The wild-type putative OsBZR1 binding sites, S1, S2, S3, and mutated site S1 (mS1) of the OsMIR396d promoter tandem repeat sequences (S1, CGTCGTGTGGAA; S2, GTTCGTGCGTCT; S3, CGGCGTGTGTGT; and mS1, CGTAAAAAAGAA) were placed upstream of the minimal promoter in pHISi-1 vector. The full-length coding region of OsBZR1 was fused to the pGAD424 vector. pGAD424-OsBZR1 was then transformed into the yeast strain YM4271, which carries the reporter gene HIS3 under the control of wild-type S1/S2/S3 or mS1. The transformed yeast cells were selected on SD/–His/–Leu medium containing 0, 15, 30, 45, and 60 mm 3-AT (a competitive inhibitor of the HIS3 gene product) by standard protocols (Clontech), and 15 mm 3-AT was used as the final concentration for screening.
EMSA
The full-length cDNA of OsBZR1 was fused into the KpnI and EcoRI sites of vector pColdTM TF DNA. The fusion protein was purified with Ni Sepharose High Performance purification column (17-5268, Amersham) according to the product manual. Biotin end-labeled DNA fragment of the OsMIR396d promoter was prepared by PCR amplification using 5′-biotin labeled primers 5′-CGGGATCGTGCAATTCTCA-3′ and 5′-TAAATAGCGGGAGGAGATAACC-3′. EMSA assay was performed using the LightShift Chemiluminescent EMSA Kit (20148, Thermo) according to the manufacturer’s instructions. Briefly, the reaction mixtures (20 μL) for EMSA contained 2 μg purified OsBZR1, 20 fmol biotin end-labeled target DNA, 2 μL 10× binding buffer, 1 μL 1 µg μL−1 Poly (dI•dC), and double-distilled water. The binding reactions were incubated at room temperature for 20 min and electrophoresed on a 10% native polyacrylamide gel, then transferred to a nylon membrane (S4056, Millipore) in 0.5× TBE buffer at 380 mA for 60 min. Biotin-labeled DNA was detected by chemiluminescence.
Transient Assays for Activation Activity in Vivo
The transcriptional activity assay was carried out in the transient-transformed protoplast prepared from 4-week-old Arabidopsis seedlings of the Col-0 line grown in short-day conditions as described previously (Lin et al., 2007). For the specific binding and activating activity of OsBZR1 to the OsMIR396d promoter assay, full-length cDNA of OsBZR1 were fused into the pBI221-GFP vector driven by the CaMV 35S promoter to generate pBI221-OsBZR1-GFP. The pBI221-GFP vector was used as the negative control. The OsMIR396d promoter was amplified to generate the OsMIR396d::LUC reporter gene. The plasmid carrying the GUS gene under the control of the CaMV 35S promoter was used as a normalization control. Values represent means ± sd of three technical replicates.
GA Quantification
For GA quantification of DJ and osgrf6 plants, 3 g of 2-week-old seedlings were harvested and frozen in liquid nitrogen. Quantification of endogenous GAs was performed as described previously (Li et al., 2011).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers OsBZR1 (Os07g0580500), OsGRF4 (Os02g0701300), OsGRF6 (Os03g0729500), LIC (Os06g0704300), CPS1 (Os02g0278700), KS (Os04g0611800), KO2 (Os06g0569500), GA20ox1 (Os03g0856700), GA20ox2 (Os01g0883800), GA20ox3 (Os07g0169700), GA2ox1 (Os05g0158600), GA2ox3 (Os01g0757200), GA3ox2 (Os01g0177400), SLR1 (Os03g0707600), GID1 (Os05g0407500), GID2 (Os02g0580300), SPY (Os08g0559300).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The expression level of several BR-related genes in miROEs.
Supplemental Figure S2. The expression pattern of OsGRFs under 24-eBL treatment.
Supplemental Figure S3. The expression of OsBZR1 in miROE8 and BZR1R/miROE double transgenic lines.
Supplemental Figure S4. Genetic relationship of OsMIR396d and OsBZR1 or D61.
Supplemental Figure S5. The expression pattern of OsMIR396d and OsGRF6 under PAC or GA3 treatment.
Supplemental Figure S6. The sensitivity of osgrf6 to GA was decreased compared with DJ.
Supplemental Table S1. List of primers used in this study.
Acknowledgments
We thank Dr. J. H. Snyder for examining the English usage in the manuscript, Wuhan Greensword Creation Technology Co., Ltd. for GA measurements, and Yuda Niu for rice transformation.
Footnotes
This work was supported by the National Natural Science Foundation of China (91335101) and a grant from the Chinese Academy of Sciences (XDA08010205).
Articles can be viewed without a subscription.
References
- Bai MY, Shang JX, Oh E, Fan M, Bai Y, Zentella R, Sun TP, Wang ZY (2012) Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat Cell Biol 14: 810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai MY, Zhang LY, Gampala SS, Zhu SW, Song WY, Chong K, Wang ZY (2007) Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Natl Acad Sci USA 104: 13839–13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao HP, Chen SK (1995) Brassinosteroid-induced rice lamina joint inclination and its relation to indole-3-acetic acid and ethylene. Plant Growth Regul 16: 189–196 [Google Scholar]
- Che R, Tong H, Shi B, Liu Y, Fang S, Liu D, Xiao Y, Hu B, Liu L, Wang H, et al. (2015) Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat Plants 2: 15195. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li F, Ma Y, Chong K, Xu Y (2013) Overexpression of OrbHLH001, a putative helix-loop-helix transcription factor, causes increased expression of AKT1 and maintains ionic balance under salt stress in rice. J Plant Physiol 170: 93–100 [DOI] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC (1996) A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111: 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan P, Ni S, Wang J, Zhang B, Xu R, Wang Y, Chen H, Zhu X, Li Y (2015) Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat Plants 2: 15203. [DOI] [PubMed] [Google Scholar]
- Fariduddin Q, Yusuf M, Ahmad I, Ahmad A (2014) Brassinosteroids and their role in response of plants to abiotic stresses. Biol Plant 58: 9–17 [Google Scholar]
- Feng Z, Zhang B, Ding W, Liu X, Yang DL, Wei P, Cao F, Zhu S, Zhang F, Mao Y, et al. (2013) Efficient genome editing in plants using a CRISPR/Cas system. Cell Res 23: 1229–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Bartolomé J, Minguet EG, Grau-Enguix F, Abbas M, Locascio A, Thomas SG, Alabadí D, Blázquez MA (2012) Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proc Natl Acad Sci USA 109: 13446–13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Wang K, Liu Y, Chen Y, Chen P, Shi Z, Luo J, Jiang D, Fan F, Zhu Y, et al. (2015) Blocking miR396 increases rice yield by shaping inflorescence architecture. Nat Plants 2: 15196. [DOI] [PubMed] [Google Scholar]
- Greenboim-Wainberg Y, Maymon I, Borochov R, Alvarez J, Olszewski N, Ori N, Eshed Y, Weiss D (2005) Cross talk between gibberellin and cytokinin: the Arabidopsis GA response inhibitor SPINDLY plays a positive role in cytokinin signaling. Plant Cell 17: 92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Gui-Lu TAN, Ya-Guang XUE, Zhi-Qin W, Li-Jun LIU, Jian-Chang Y (2010) Changes in grain yield and morphological and physiological characteristics during 60-year evolution of japonica rice Cultivars in Jiangsu Province, China. Acta Agron Sin 36: 133–140 [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SSL, Gendron N, Sun CQ, Wang ZY (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Sponsel V (2015) A century of gibberellin research. J Plant Growth Regul 34: 740–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Asano K, Tsuji H, Kawamura M, Mori H, Kitano H, Ueguchi-Tanaka M, Matsuoka M (2010) Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice. Plant Cell 22: 2680–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, Kitano H, Ashikari M, Ichihara S, Matsuoka M (2004) A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol Biol 54: 533–547 [DOI] [PubMed] [Google Scholar]
- Jeong DH, An S, Kang HG, Moon S, Han JJ, Park S, Lee HS, An K, An G (2002) T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol 130: 1636–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14: 787–799 [DOI] [PubMed] [Google Scholar]
- Khush GS. (2013) Strategies for increasing the yield potential of cereals: case of rice as an example. Plant Breed 132: 433–436 [Google Scholar]
- Kim TW, Guan S, Burlingame AL, Wang ZY (2011) The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol Cell 43: 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijt SJ, Greco R, Agalou A, Shao J, ’t Hoen CC, Overnäs E, Osnato M, Curiale S, Meynard D, van Gulik R, et al. (2014) Interaction between the GROWTH-REGULATING FACTOR and KNOTTED1-LIKE HOMEOBOX families of transcription factors. Plant Physiol 164: 1952–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Jiang J, Qian Q, Xu Y, Zhang C, Xiao J, Du C, Luo W, Zou G, Chen M, et al. (2011) Mutation of rice BC12/GDD1, which encodes a kinesin-like protein that binds to a GA biosynthesis gene promoter, leads to dwarfism with impaired cell elongation. Plant Cell 23: 628–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222 [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH, Vafeados D, Chory J (2001) BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol 127: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QF, Wang C, Jiang L, Li S, Sun SSM, He JX (2012) An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 5: ra72. [DOI] [PubMed] [Google Scholar]
- Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H (2007) Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Song Y, Chen Z, Yu D (2009) Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plant 136: 223–236 [DOI] [PubMed] [Google Scholar]
- Liu H, Guo S, Xu Y, Li C, Zhang Z, Zhang D, Xu S, Zhang C, Chong K (2014) OsmiR396d-regulated OsGRFs function in floral organogenesis in rice through binding to their targets OsJMJ706 and OsCR4. Plant Physiol 165: 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen J, Zheng X, Wu F, Lin Q, Heng Y, Tian P, Cheng Z, Yu X, Zhou K, et al. (2017) GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat Plants 3: 17043. [DOI] [PubMed] [Google Scholar]
- Luo AD, Liu L, Tang ZS, Bai XQ, Cao SY, Chu CC (2005) Down-regulation of OsGRF1 gene in rice rhd1 mutant results in reduced heading date. J Integr Plant Biol 47: 745–752 [Google Scholar]
- Ma Q, Dai X, Xu Y, Guo J, Liu Y, Chen N, Xiao J, Zhang D, Xu Z, Zhang X, et al. (2009) Enhanced tolerance to chilling stress in OsMYB3R-2 transgenic rice is mediated by alteration in cell cycle and ectopic expression of stress genes. Plant Physiol 150: 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15: 1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-García S, Vert G, Yin Y, Caño-Delgado A, Cheong H, Chory J (2004) Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev 18: 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Fujioka S, Sunohara H, Kamiya N, Hong Z, Inukai Y, Miura K, Takatsuto S, Yoshida S, Ueguchi-Tanaka M, et al. (2006) The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol 140: 580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao S, Sun S, Wang L, Wu Z, Li C, Li X, Wang T, Leng L, Tian W, Lu T, Wang X (2017) The RLA1/SMOS1 transcription factor functions with OsBZR1 to regulate brassinosteroid signaling and rice architecture. Plant Cell 29: 292–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Morinaka Y, Ohnishi T, Sunohara H, Fujioka S, Ueguchi-Tanaka M, Mizutani M, Sakata K, Takatsuto S, Yoshida S, et al. (2006) Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol 24: 105–109 [DOI] [PubMed] [Google Scholar]
- Sakata T, Oda S, Tsunaga Y, Shomura H, Kawagishi-Kobayashi M, Aya K, Saeki K, Endo T, Nagano K, Kojima M, et al. (2014) Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiol 164: 2011–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Kitano H, Ashikari M, Matsuoka M (2003) Characterization of rice dwarf mutant, GA insensitive dwarf 2 (gid2). Plant Cell Physiol 44: S186 [Google Scholar]
- Shimada A, Ueguchi-Tanaka M, Sakamoto T, Fujioka S, Takatsuto S, Yoshida S, Sazuka T, Ashikari M, Matsuoka M (2006) The rice SPINDLY gene functions as a negative regulator of gibberellin signaling by controlling the suppressive function of the DELLA protein, SLR1, and modulating brassinosteroid synthesis. Plant J 48: 390–402 [DOI] [PubMed] [Google Scholar]
- Simpson JF, Dutt PL, Page DL (1992) Expression of mitoses per thousand cells and cell density in breast carcinomas: a proposal. Hum Pathol 23: 608–611 [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Sheehy JE (1999) Erect leaves and photosynthesis in rice. Science 283: 1455 [Google Scholar]
- Spielmeyer W, Ellis MH, Chandler PM (2002) Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci USA 99: 9043–9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Chen D, Li X, Qiao S, Shi C, Li C, Shen H, Wang X (2015) Brassinosteroid signaling regulates leaf erectness in Oryza sativa via the control of a specific U-type cyclin and cell proliferation. Dev Cell 34: 220–228 [DOI] [PubMed] [Google Scholar]
- Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E, et al. (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19: 765–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Nakagawa H, Tomita C, Shimatani Z, Ohtake M, Nomura T, Jiang C-J, Dubouzet JG, Kikuchi S, Sekimoto H, et al. (2009) BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol 151: 669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Chu C (2017) MicroRNAs in crop improvement: fine-tuners for complex traits. Nat Plants 3: 17077. [DOI] [PubMed] [Google Scholar]
- Tong H, Chu C (2009) Roles of DLT in fine modulation on brassinosteroid response in rice. Plant Signal Behav 4: 438–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Chu C (2016) Reply: brassinosteroid regulates gibberellin synthesis to promote cell elongation in rice: critical comments on ross and quittenden’s letter. Plant Cell 28: 833–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Jin Y, Liu W, Li F, Fang J, Yin Y, Qian Q, Zhu L, Chu C (2009) DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J 58: 803–816 [DOI] [PubMed] [Google Scholar]
- Tong H, Liu L, Jin Y, Du L, Yin Y, Qian Q, Zhu L, Chu C (2012) DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24: 2562–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Xiao Y, Liu D, Gao S, Liu L, Yin Y, Jin Y, Qian Q, Chu C (2014) Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 26: 4376–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I, et al. (2007) Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 19: 2140–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner SJ, Rozhon W, Papacek M, Ciomas J, Lange T, Kugler KG, Mayer KF, Sieberer T, Poppenberger B (2015) Brassinosteroids are master regulators of gibberellin biosynthesis in Arabidopsis. Plant Cell 27: 2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner SJ, Rozhon W, Poppenberger B (2016) Reply: interaction between brassinosteroids and gibberellins: synthesis or signaling? In Arabidopsis, both! Plant Cell 28: 836–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Kim JH, Kende H (2000) A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth. Plant Physiol 122: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriet C, Russinova E, Reuzeau C (2013) From squalene to brassinolide: the steroid metabolic and signaling pathways across the plant kingdom. Mol Plant 6: 1738–1757 [DOI] [PubMed] [Google Scholar]
- Wada K, Marumo S, Ikekawa N, Morisaki M, Mori K (1981) Brassinolide and homobrassinolide promotion of lamina inclination of rice seedlings. Plant Cell Physiol 22: 323–325 [Google Scholar]
- Wang L, Wang Z, Xu Y, Joo S-H, Kim S-K, Xue Z, Xu Z, Wang Z, Chong K (2009). OsGSR1 is involved in crosstalk between gibberellins and brassinosteroids in rice. Plant Journal 57: 498–510 [DOI] [PubMed] [Google Scholar]
- Wang L, Xu Y, Zhang C, Ma Q, Joo SH, Kim SK, Xu Z, Chong K (2008) OsLIC, a novel CCCH-type zinc finger protein with transcription activation, mediates rice architecture via brassinosteroids signaling. PLoS One 3: e3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhang Q, Zhou H, Ni F, Wu X, Qi Y (2009) Rice MicroRNA effector complexes and targets. Plant Cell 21: 3421–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Xu K, Zhao Y, He X, Wang X, Ling F (2007) Changes of some agronomic traits in japonica Rice varieties during forty-seven years of genetic improvement in Jilin Province, China. Zhongguo Shuidao Kexue 21: 507–512 [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12: 1591–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shen W, He Y, Tian Z, Li J (2016) OVATE Family Protein 8 positively mediates brassinosteroid signaling through interacting with the GSK3-like Kinase in rice. PLoS Genet 12: e1006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wang P, Liu L, Wang Z, Zhu Q (2006) Evolution characteristics of grain yield and plant type for mid-season indica rice cultivars. Acta Agron Sin 32: 949–955 [Google Scholar]
- Yoshida S, Forno DA, Cock JH, Gomez KA. (1976). Laboratory manual for physiological studies of rice. 3rd edn Manila, Philippines: International Rice Research Institute; pp. 61–64 [Google Scholar]
- Yuan L. 2001. Breeding of super hybrid rice. In Peng S, Hardy B, eds, Rice Research for Food Security and Poverty Alleviation. International Rice Research Institute, Los Baños, Philippines, pp 143–149. [Google Scholar]
- Zhang C, Bai MY, Chong K (2014) Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep 33: 683–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Xu Y, Guo S, Zhu J, Huan Q, Liu H, Wang L, Luo G, Wang X, Chong K (2012) Dynamics of brassinosteroid response modulated by negative regulator LIC in rice. PLoS Genet 8: e1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LY, Bai MY, Wu J, Zhu JY, Wang H, Zhang Z, Wang W, Sun Y, Zhao J, Sun X, et al. (2009) Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21: 3767–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Wang Y, Li C, Zhang R, Chen K, Ran Y, Qiu JL, Wang D, Gao C (2017) Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat Biotechnol 35: 438–440 [DOI] [PubMed] [Google Scholar]