MPK17 and PMD1 are required with the actin cytoskeleton for peroxisome proliferation in response to salt treatment.
Abstract
Peroxisomes are small organelles that house many oxidative reactions. Peroxisome proliferation is induced under multiple stress conditions, including salt stress; however, factors regulating this process are not well defined. We have identified a role for Arabidopsis (Arabidopsis thaliana) MAP KINASE17 (MPK17) in affecting peroxisome division in a manner that requires the known peroxisome division factor PEROXISOME AND MITOCHONDRIAL DIVISION FACTOR1 (PMD1). MPK17 and PMD1 are involved in peroxisome proliferation in response to NaCl stress. Additionally, we found that PMD1 is an actin-binding protein and that a functioning actin cytoskeleton is required for NaCl-induced peroxisome division. Our data suggest roles for MPK17 and PMD1 in influencing the numbers and cellular distribution of peroxisomes through the cytoskeleton-peroxisome connection. These findings expand our understanding of peroxisome division and potentially identify factors connecting the actin cytoskeleton and peroxisome proliferation.
Peroxisomes are small conserved organelles that carry out a variety of critical functions. Universally, peroxisomes are responsible for fatty acid β-oxidation and metabolism of reactive oxygen species (ROS; for review, see Islinger et al., 2012). In plants, peroxisomes have acquired a number of specialized roles, including synthesis of biotin, branched chain amino acids, vitamins, and processing some proto-hormones into their active forms (for review, see Islinger et al., 2012).
Peroxisomes arise via two distinct pathways: through de novo biogenesis from the endoplasmic reticulum and by growth and division of mature peroxisomes (Agrawal and Subramani, 2016). Each of these pathways exist in all examined species, but the predominant method varies by organism. For example, yeast cells only undergo de novo biogenesis from the endoplasmic reticulum if no mature peroxisomes are available to undergo division (Motley and Hettema, 2007), whereas mammalian cells preferentially undergo de novo synthesis even when mature peroxisomes are present (Kim et al., 2006). Mature peroxisome division requires three distinct steps: growth and elongation, constriction, and fission (Schrader, 2006). The PEROXIN11 (PEX11) family of proteins may be master regulators of peroxisome division, working in concert at all three steps, in combination with additional factors. Peroxisome elongation in yeast, mammals, and plants is dependent on Pex11 proteins (Abe and Fujiki, 1998; Li and Gould, 2002; Lingard and Trelease, 2006). Yeast and mammalian Pex11 proteins are also involved in peroxisome fission through interaction with dynamin-like proteins, and also participate in constriction (Williams et al., 2015; Yoshida et al., 2015). Whether other, undiscovered protein factors also participate in constriction is currently unknown. In Arabidopsis (Arabidopsis thaliana), peroxisome elongation is dependent on Pex11 (Koch et al., 2010; Lingard and Trelease, 2006; Orth et al., 2007), and fission is dependent on members of the DYNAMIN RELATED PROTEIN (DRP) and FISSION1 protein families (Mano et al., 2004; Zhang and Hu, 2008). A role for constriction by Arabidopsis Pex11 family members has not yet been shown, nor are there any other constriction-related proteins known (for review, see Kaur and Hu, 2009). At least one plant-specific division factor, PEROXISOME AND MITOCHONDRIAL DIVISION FACTOR1 (PMD1), also participates in plant peroxisome division in a DRP/FIS-independent manner (Aung and Hu, 2011). The mechanism of PMD1 action on peroxisomes has not yet been elucidated. Clearly, our understanding of the machinery, timing, and roles of peroxisome division factors remains incomplete.
Peroxisome division occurs in response both to developmental cues and in response to certain stress conditions. A sudden increase in peroxisome number is called “peroxisome proliferation” (Kaur and Hu 2009). Often, proliferation is the result of various stresses to the plant. Peroxisomes in Arabidopsis proliferate in response to a variety of both biotic and abiotic stresses, including salt (Fahy et al., 2017; Mitsuya et al., 2010), pathogens (Koh et al., 2005), high light (Desai and Hu, 2008), cadmium (Rodríguez-Serrano et al., 2009, 2016), and general ROS stress (Lopez-Huertas et al., 2000). However, multiple lines of evidence suggest that stress induction of peroxisome proliferation is differentially triggered by each stress. PEX1 transcripts increase in response to light, pathogens, and salt stresses, but remains unchanged in response to osmotic stress (Charlton et al., 2005). In contrast, PEX10 transcripts increase in response to both salt stress and to osmotic stress (Charlton et al., 2005). Pathogen attack not only increases the number of peroxisomes but also reorients peroxisomes to the site of pathogen attack (Koh et al., 2005; Lipka et al., 2005). Under high light stress, plants proliferate peroxisomes and also extend peroxules from these peroxisomes, which associate with mitochondria (Jaipargas et al., 2016). Peroxules also form preferentially under cadmium stress (Rodríguez-Serrano et al., 2016), but have not been reported under high salt conditions or pathogen attacks. Together, these data suggest plants can distinguish among these stresses and trigger different peroxisome responses for each of them. An adaptive benefit from peroxisome proliferation remains elusive for most stresses, with the exception of pathogen attack, and artificially increasing peroxisome number by overexpressing peroxisome division factors fails to appreciably increase abiotic stress tolerance in Arabidopsis (Koh et al., 2005; Mitsuya et al., 2010). However, in response to pathogen attack, peroxisomes directly produce antifungal compounds (Lipka et al., 2005), and the rice (Oryza sativa) PEX5 peroxisome receptor is an active antifungal protein (Lee et al., 2007). Despite the many conditions that influence peroxisome numbers, gaps remain in our understanding of the regulation of plant peroxisome proliferation.
In this study, we describe a role for Arabidopsis MAP KINASE17 (MPK17) as a potential regulator of peroxisome division, and expand the known functions of the previously described peroxisome division factor PMD1. We found that a mutant defective in MPK17, mpk17-1, is hypersensitive to inactive long-chain auxins that are metabolized to active auxins in the peroxisome and that mpk17-1 displays increased numbers of peroxisomes compared to wild type. We further found that changes to peroxisome numbers and long-chain auxin sensitivity in mpk17-1 is dependent on the previously described PMD1 functioning normally; the mpk17-1 pmd1-1 double mutant displays restored sensitivity to long-chain auxins and wild-type peroxisome numbers. Consistent with a dual role for PMD1 regulating mitochondrial as well as peroxisome division (Aung and Hu, 2011), mpk17-1 also displays more mitochondria than wild type. These data implicate PMD1 misregulation in the increased peroxisome number and indole-3-butyric acid (IBA) sensitivity in the mpk17-1 mutant. Additionally, we found that PMD1 binds to the actin cytoskeleton and may act as a connector between peroxisomes and their highway through the cell. Together, these results help define the roles of the peroxisome division factor PMD1 and suggests MPK17 influences peroxisome division in Arabidopsis.
RESULTS
MAP KINASE17 Affects Peroxisome Number
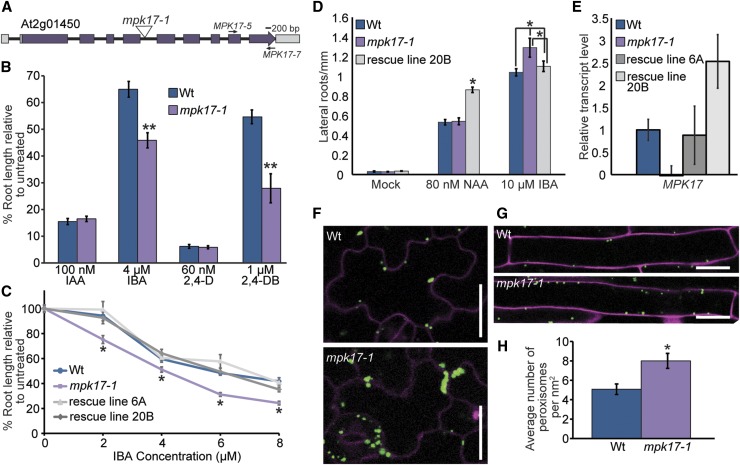
Although MAP kinases are known to influence auxin responses (Mockaitis and Howell 2000), the roles of MAP kinase signaling in regulating conversion of auxin precursors to active auxin is unexplored. We therefore examined the available map kinase (mpk) insertional alleles for altered responsiveness to the auxin precursor IBA. Converse to the mpk1 mutant displaying increased sensitivity to all tested auxins (Enders et al., 2017), we found that an insertional allele defective in MPK17 (At2g01450; Fig. 1A) displayed increased sensitivity to IBA in root elongation assays (Fig. 1, B and C) and lateral root induction assays (Fig. 1D) without affecting sensitivity to short-chain, active auxins. We named this allele mpk17-1 (SALK_020801). Expressing MPK17 behind its native upstream regulatory region in the mpk17-1 mutant rescued the IBA hypersensitivity phenotype (Fig. 1C), confirming that the lesion in MPK17 caused the observed IBA hypersensitivity. mpk17-1 carries a T-DNA insertion in between the fourth and fifth exons of the MPK17 gene and displays nearly undetectable MPK17 transcript accumulation (Fig. 1, A and E), suggesting that mpk17-1 is likely a null mutant.
Figure 1.
mpk17-1 displays hypersensitivity to long-chain auxins and increased numbers of peroxisomes. A, mpk17-1 carries a T-DNA insertion between exons four and five of the MPK17 (At2g01450) gene. B, Normalized primary root lengths (n ≥ 12) of 8-d-old wild type (Col-0) and mpk17-1 grown under yellow-filtered light at 22°C on medium supplemented with 100 nm IAA, 4 μM IBA, 60 nm 2,4-D, or 1 μM 2,4-DB. Error bars represent se of the means. Statistically significant differences from wild type in ANOVA tests are indicated by an asterisk (* indicates P ≤ 0.05, ** indicates P ≤ 0.01). C, Expression of MPK17 driven by its native upstream regulatory region restores IBA sensitivity to mpk17-1. Normalized primary root lengths (n > 45) of 8-d-old wild type (Col-0), mpk17-1, and two independent mpk17-1 rescue lines carrying a genomic copy of MPK17 grown under yellow-filtered light at 22°C on medium supplemented with ethanol (0 μM IBA) or the indicated concentration of IBA. D, Emerged lateral roots of wild type (Col-0), mpk17-1, and two independent mpk17-1 rescue lines carrying a genomic copy of MPK17 were counted 4 d after transfer of 4-d-old seedlings to medium supplemented with ethanol (mock), 80 nm NAA, or 10 μM IBA (mean ± se, n ≥ 20; * indicates P ≤ 0.05 by ANOVA). E, Mean relative quantity of MPK17 transcript from 7-d-old wild type (Col-0), mpk17-1, and two independent mpk17-1 rescue lines carrying a genomic copy of MPK17 grown under continuous white light at 22°C, determined by qPCR. F, mpk17-1 displays increased peroxisome numbers compared to wild type. Confocal images of leaf epidermal cells from 4-d-old wild type (Col-0) and mpk17-1 carrying the 35S:GFP-PTS1 (Zolman and Bartel 2004) peroxisome marker. Seedlings were counterstained with propidium iodide (magenta). Scale bar = 20 μm. G, Confocal images of root epidermal cells from 4-d-old wild type and mpk17-1 carrying the 35S:GFP-PTS1 (Zolman and Bartel 2004) peroxisome marker. Seedlings were counterstained with propidium iodide (magenta). Scale bar = 20 μm. H, Mean number of peroxisomes per square nanometer from the maturation zone root cells of 4-d-old wild type and mpk17-1 carrying the 35S:GFP-PTS1 (Zolman and Bartel 2004) peroxisome marker. Wt, wild type.
Converse to its IBA hypersensitivity, mpk17-1 displays wild-type sensitivity to the short-chain synthetic auxin 2-4-dichlorophenoxyacetic acid (2,4-d) in root elongation assays (Fig. 1B) and the short-chain synthetic auxin NAA in lateral root induction assays (Fig. 1D). Because this pattern of differential sensitivity to short-chain versus long-chain auxins is characteristic of mutants with peroxisome defects (Hayashi et al., 1998, Zolman et al., 2000), we examined the peroxisomes in wild-type and mpk17-1 backgrounds using the 35S:GFP-PTS1 reporter (Zolman and Bartel 2004). We found that mpk17-1 displayed more peroxisomes than wild type (Fig. 1, F, G, and H). In addition, we observed that more peroxisomes in mpk17-1 were more likely to be clustered together than those from wild type (Fig. 1F). Because mpk17 displays hypersensitivity to the protoauxins IBA and 2,4-DB (Fig. 1B), which require functional peroxisomes for conversion to active auxins (Zolman et al., 2000), it seems likely that these additional peroxisomes are functional.
mpk17 Mutant Phenotypes Are Suppressed by Loss of PMD1
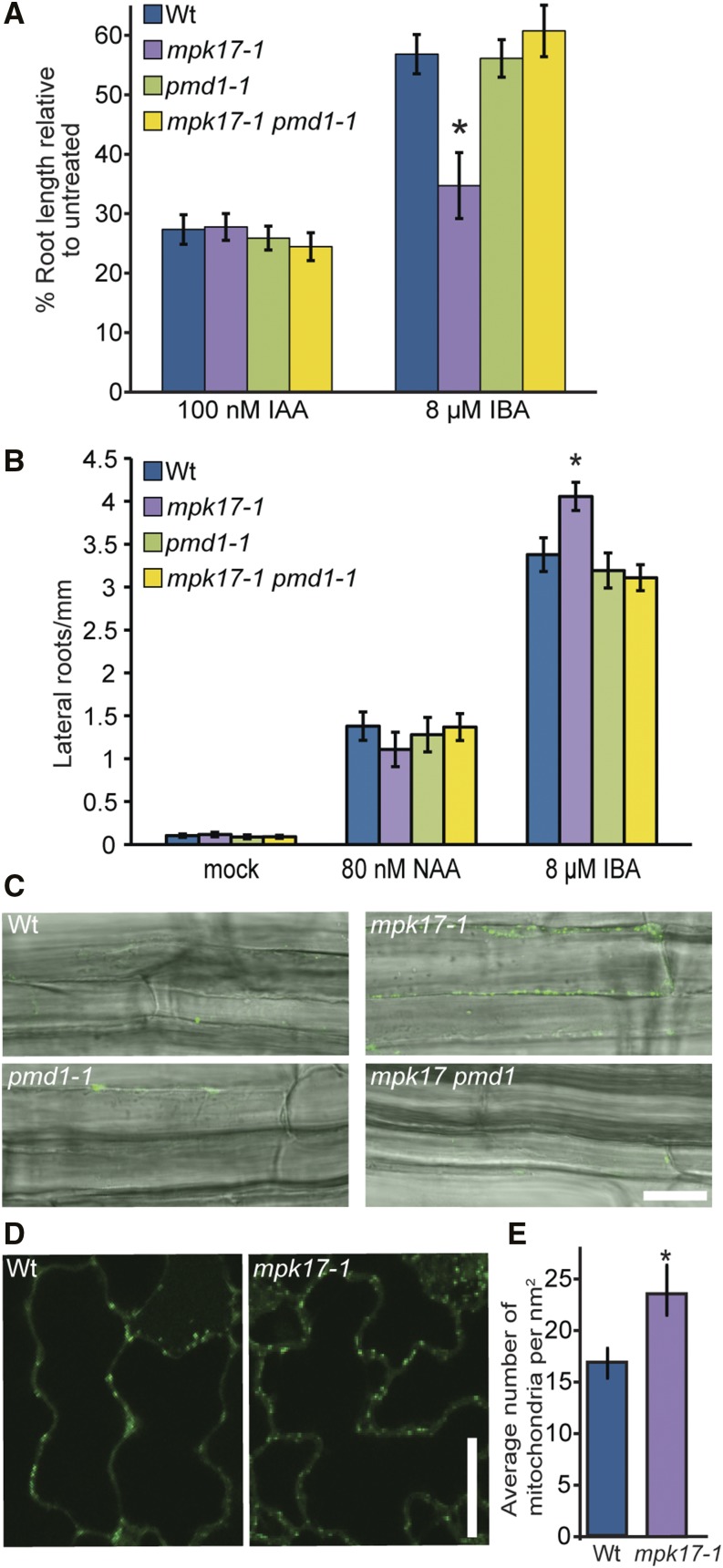
Overexpressing the peroxisome division factor PEROXISOME AND MITOCHONDRIAL DIVISION FACTOR1 (PMD1) results in increased peroxisome numbers, with many of these peroxisomes present in clusters (Aung and Hu, 2011). Because this peroxisome phenotype was similar to our observations of mpk17-1 (Fig. 1F), we examined whether MPK17 acted through this peroxisome division factor. Although pmd1-1 does not display resistance to the auxin precursor IBA (Fig. 2, A and B; Aung and Hu, 2011), we found that pmd1-1 suppressed the IBA hypersensitivity displayed by mpk17-1; the mpk17-1 pmd1-1 double mutant displayed wild-type sensitivity to the auxin precursor IBA in root elongation (Fig. 2A) and lateral root induction (Fig. 2B) assays. Additionally, pmd1-1 suppressed the increased peroxisome numbers found in mpk17-1 (Fig. 2C).
Figure 2.
The mpk17 mutant phenotypes are suppressed by loss of PMD1. A, Normalized primary root lengths of 8-d-old wild type (Col-0), pmd1-1, mpk17-1, and mpk17-1 pmd1-1 grown on media supplemented with ethanol (mock) or 8 μM IBA (* = P ≤ 0.05, ANOVA). B, Emerged lateral roots of wild type (Col-0), pmd1-1, mpk17-1, and mpk17-1 pmd1-1 were counted 4 d after transfer of 4-d-old seedlings to medium supplemented with indicated hormones. Error bars indicate mean ± se. C, Confocal images of wild type, mpk17-1, pmd1-1, and mpk17-1 pmd1-1 seedling roots stained with BODIPY to visualize peroxisomes. Scale bar = 25 μm. D, Confocal images of wild type and mpk17-carrying the COX4-YFP (Aung and Hu 2011, Nelson et al., 2007) mitochondrial marker. Scale bar = 20 μm. E, Mean number ± se of mitochondria in wild type and mpk17-1 COX4-YFP lines. mpk17-1 displays significantly more mitochondria than wild type (* = P ≤ 0.05, two-tailed unpaired t test). Wt, wild type.
In addition to regulating peroxisome proliferation, PMD1 regulates mitochondria proliferation (Aung and Hu, 2011). Therefore, if MPK17 acts upstream of PMD1, we expect the mpk17-1 mutant to display increased mitochondria numbers, in addition to the observed increase in peroxisome numbers. We therefore crossed mpk17-1 to the mitochondria reporter line COX4-YFP (Aung and Hu, 2011; Nelson et al., 2007) and found that mpk17-1 displays increased mitochondrial numbers compared to wild type (Fig. 2, D and E). Because PMD1 and MPK17 appear to act in both peroxisome and mitochondria division and because pmd1-1 suppresses mpk17-1, it is possible that MPK17 and PMD1 act in the same pathway to regulate the proliferation of these organelles, with PMD1 acting downstream of MPK17. However, we do not yet know the phosphorylation targets of MPK17 and therefore cannot directly link MPK17 to PMD1; thus, it remains a possibility that these proteins act in independent pathways that affect these processes.
MPK17 and PMD1 Are Required for Salt-Induced Peroxisome Proliferation
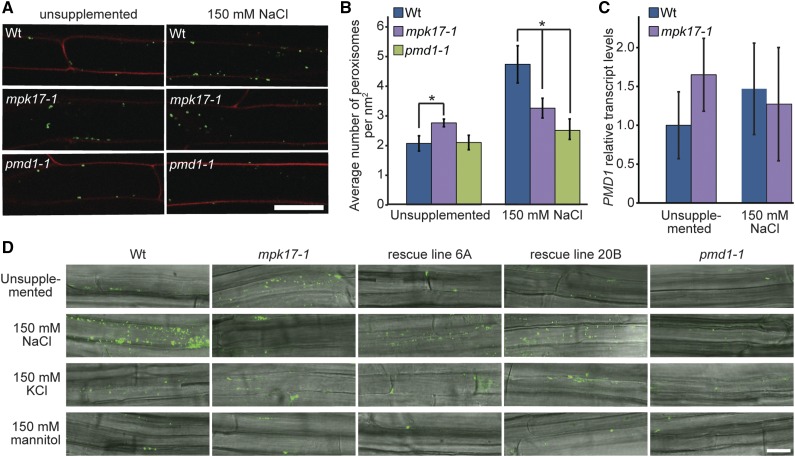
Because peroxisomes divide in response to a variety of stressful conditions (Charlton et al., 2005; Desai and Hu, 2008; Koh et al., 2005; Lipka et al., 2005; Mitsuya et al., 2010), and because both mpk17-1 (Fig. 1G) and pmd1-1 (Aung and Hu, 2011) display clearly aberrant peroxisome division, we examined stress-induced peroxisome proliferation in mpk17-1 and pmd1-1. If MPK17 and PMD1 are not involved in peroxisome division upon salt stress, we would expect the same increase in peroxisome number in the mutants as wild type when salt-stressed. If both MPK17 and PMD1 increase peroxisome division during salt stress, we would expect to see no difference in peroxisome number between the mutants grown in the presence or absence of salt. Neither mutant proliferates peroxisomes in response to NaCl stress (Fig. 3, A and D). This is consistent with both MPK17 and PMD1 acting in a salt-responsive peroxisome division pathway. Indeed, although mpk17-1 has significantly more peroxisomes than wild type when grown on unsupplemented media, mpk17-1 has fewer peroxisomes than wild type when grown on media supplemented with NaCl (Fig. 3B). Further, expression of wild-type MPK17 under its native promoter in the mpk17-1 background restores peroxisome proliferation on NaCl (Fig. 3D). In addition, PMD1 transcript trends toward a mild elevation in both the mpk17 mutant and in response to NaCl in wild type, although these differences are not statistically significant (Fig. 3C). The tested KCl and mannitol concentrations failed to stimulate peroxisome proliferation in wild type (Fig. 3D), despite affecting seedling physiology, suggesting that peroxisome proliferation in response to NaCl is not caused by osmotic changes and is specific to Na+ ions.
Figure 3.
mpk17-1 and pmd1-1 fail to proliferate peroxisomes in response to NaCl treatment. A, Seedlings carrying the GFP-PTS1 peroxisome marker (Zolman and Bartel, 2004) were grown on unsupplemented media for 3 d, then transferred to indicated treatments overnight before counterstaining with propidium iodide and imaging by confocal microscopy. GFP-PTS1 signal has been false-colored green and the propidium iodide signal has been false-colored red. B, Mean number (± se) of peroxisomes in wild type (Col-0), mpk17-1, and pmd1-1 when grown in the absence (unsupplemented) or presence of 150 mm NaCl. mpk17-1 displays significantly more peroxisomes (* indicates P ≤ 0.05, two-tailed unpaired t test) than wild type on unsupplemented media, but significantly fewer peroxisomes than wild type when treated with NaCl. pmd1-1 is statistically indistinguishable from wild type on unsupplemented media, and displays significantly fewer peroxisomes than wild type when treated with NaCl (P < 0.05, two-tailed unpaired t test). C, Mean relative PMD1 transcript accumulation in 7-d-old wild type and mpk17-1 grown under continuous white light at 22°C on unsupplemented media or media supplemented with 150 mm NaCl, determined by qPCR. D, Seedlings of wild type (Col-0), mpk17-1, pmd1-1, and two independent mpk17 rescue lines were grown for 3 d on unsupplemented media, then transferred to indicated treatments overnight. Seedlings were stained with BODIPY to visualize peroxisomes (Landrum et al., 2010) and imaged by confocal microscopy. Scale bar = 25 μm. Wt, wild type.
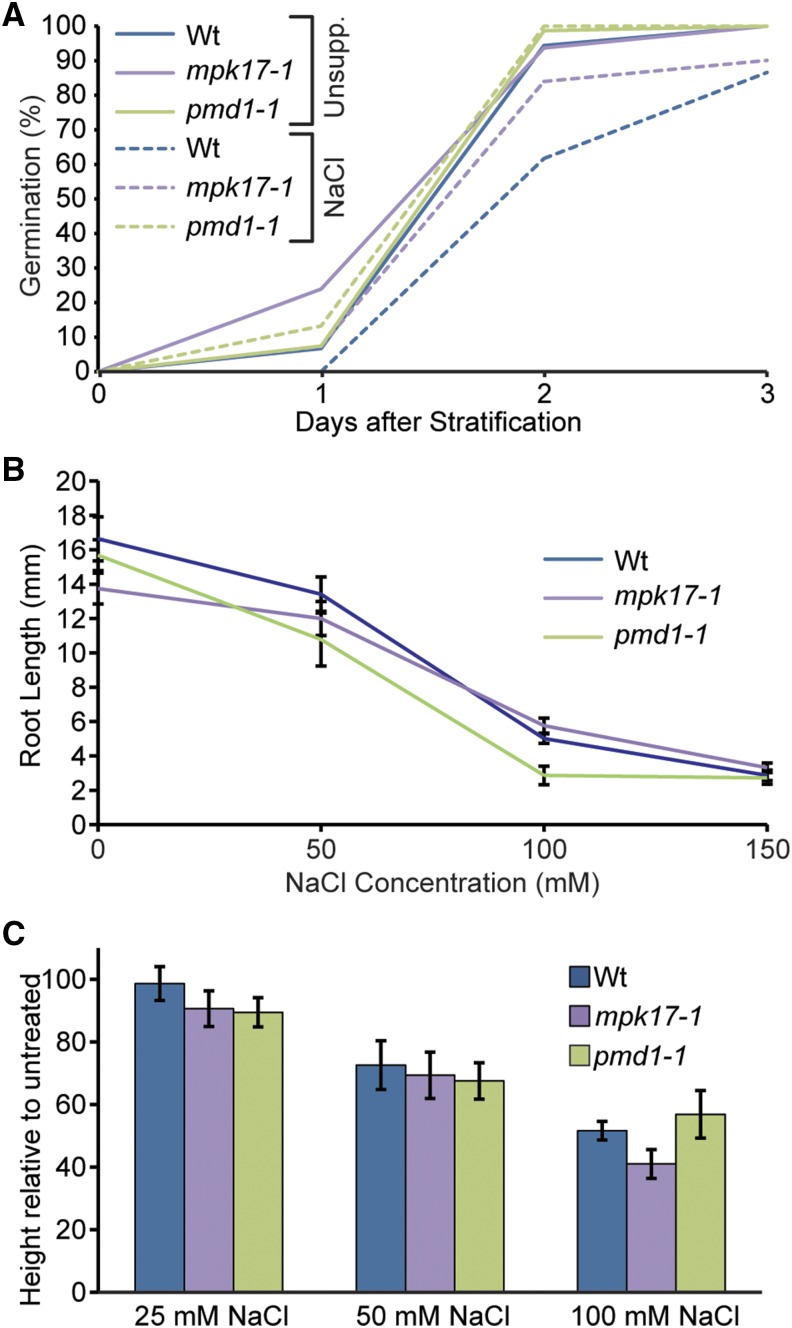
We then investigated whether the inability to proliferate peroxisomes in response to NaCl without MPK17 or PMD1 affects the whole plant tolerance to NaCl. We examined the sensitivity of mpk17-1 and pmd1-1 to NaCl using multiple salt tolerance assays, including examination of germination in the presence of salt (Fig. 4A; Supplemental Fig. S1), seedling root elongation inhibition by salt (Fig. 4B), and final adult plant height in response to salt watering (Fig. 4C). These assays cover a range of NaCl stress conditions, from a short-term acute stress in root elongation to low-level, persistent stress over the majority of the plant’s lifespan, through continuous watering with NaCl from 3 weeks old until senescence. We were unable to detect any consistent, significant differences between wild type and mpk1 or pmd1 mutants in any of these assays; however, it may be possible that peroxisome proliferation confers an advantage under salt stress under nonlab conditions.
Figure 4.
Neither mpk17-1 nor pmd1-1 display detectable tolerance to NaCl. A, Percent seed germination of wild type (Col-0), mpk17-1, and pmd1-1 when grown on unsupplemented PNS media or PNS supplemented with 150 mm NaCl. B, Mean primary root lengths (± se; n ≥ 10) of wild type (Col-0), mpk17-1, and pmd1-1 seedlings when grown on PNS media supplemented with the indicated concentration of NaCl. C, Mean (± se; n ≥ 5) final relative adult plant height of wild type (Col-0), mpk17-1, and pmd1-1 when watered with the indicated concentration of NaCl during growth. Wt, wild type.
PMD1 Binds Actin
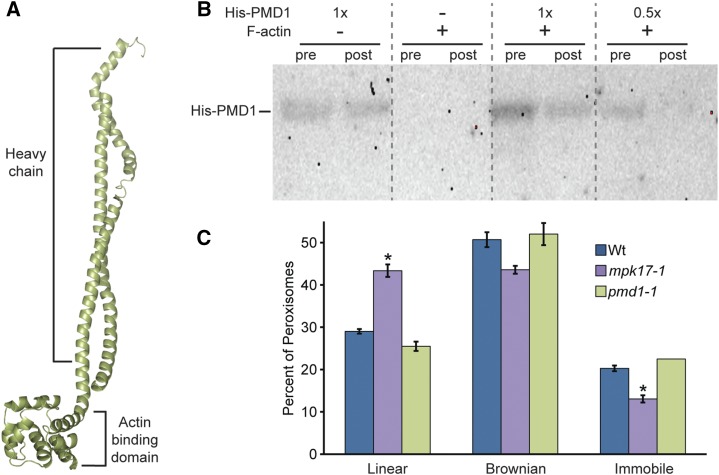
To further elucidate the function of the recently discovered PMD1 protein, we used the on-line protein structure prediction program Phyre2 to model secondary protein structure (Kelley et al., 2009). Phyre2 created a homology model of PMD1 (Fig. 5A) and predicted the PMD1 protein structure to have similarity to the heavy chains of dynein and myosin proteins. The PMD1 model suggests that, similar to dynein and myosin, the N-terminal portion of PMD1 (from residues 1 to 303) possess a H chain composed of the coil-coil protein structure (Fig. 5A). However, unlike dynein and myosin, PMD1 is not predicted to have a region conferring ATPase activity (Fig. 5A), and therefore is unlikely to facilitate movement in the absence of motor proteins. This Phyre2-generated model suggests the possibility that PMD1 roles in peroxisome biogenesis might rely on the plant cytoskeleton.
Figure 5.
PMD1 binds actin. A, Phyre2 (Kelley and Sternberg, 2009) predicted structure of PMD1 resembles dynein and myosin H chain proteins. The top portion has high similarity to the coil-coil portion of a H chain, and the bottom domain is highly similar to the actin binding domain, but without a catalytic domain. B, PMD1 binds to actin. Anti-His antibody was used to detect His-PMD11-303 protein in an actin cosedimentation assay. Assays were performed with and without F-actin and the indicated amount of His-PMD11-303 protein. Pre-spin (pre) and post-spin (post) supernatant were used for each lane, as indicated. C, Average percent of peroxisomes in wild type, mpk1-1, and pmd1-1 seedlings carrying the 35S:GFP-PTS1 (Zolman and Bartel, 2004) peroxisome marker grown in unsupplemented media moving in a linear fashion, Brownian fashion, or not moving. Error bars represent the se of the mean. Roots from 7-d-old seedlings were imaged in the maturation zone on the LSM510 (Leica) at a rate of 13 frames/s, for 29 s, then peroxisomes were tracked and the primary type of movement was logged for each peroxisome. At least five individuals of each genotype were imaged, with three images per individual, totaling more than 400 peroxisomes per genotype. mpk17-1 GFP-PTS1 displays a significantly higher percent of linear peroxisome movement than either Col GFP-PTS1 or pmd1-1 GFP-PTS1 (ANOVA, Tukey’s HSD P ≤ 0.05), and a significantly smaller percentage of immobile peroxisomes (ANOVA; P ≤ 0.05). Wt, wild type.
The cytoskeleton consists of microtubules and actin filaments. Peroxisomes travel along the cytoskeleton, but which cytoskeleton varies by kingdom. In animals, peroxisomes travel along the microtubule cytoskeleton (Rapp et al., 1996; Schrader et al., 1996; Wiemer et al., 1997). In plants and yeast, peroxisomes travel along the actin cytoskeleton (Hoepfner et al., 2001; Mathur et al., 2002); DRP peroxisome division factors interact with actin-associated proteins in yeast (Yu and Cai, 2004). However, direct interaction of a plant peroxisome division factor with actin filaments has not been reported.
The PMD1 homology model is consistent with the possibility that PMD1 interacts with either microtubules or actin. Because peroxisomes travel along the actin cytoskeleton in plants (Mathur et al., 2002) and PMD1 localizes to peroxisomes (Aung and Hu, 2011), we tested whether PMD1 could directly interact with the actin cytoskeleton using an actin-cosedimentation assay with heterologously expressed His-PMD11-303 protein and purified G-actin (Schafer et al., 1998). In this assay, proteins are incubated in the presence of G-actin, and polymerization of G-actin to F-actin is induced. Afterward, the reaction is subjected to high-speed centrifugation and proteins that bind F-actin are depleted from the supernatant as the polymerized F-actin pellets to the bottom of the tube. Proteins remaining in the supernatant are separated by SDS-PAGE, and immunoblot analysis is used to examine protein levels. Similar to the characterized actin-binding protein α-catenin (Rimm et al., 1995; Supplemental Fig. S2), His-PMD11-303 protein is depleted from the supernatant in this assay (Fig. 5B), indicating that PMD1 can directly bind actin in vitro.
Because PMD1 associates with actin (Fig. 5B), along which peroxisomes move (Mathur et al., 2002), we examined in planta peroxisome movement in the pmd1-1 and mpk17-1 mutants. In wild type, peroxisomes display various movement types when examined over a 30-s period. Approximately half the peroxisomes in wild type exhibit Brownian movement, approximately 20% are immobile, and approximately 30% display linear movement (Fig. 5C). Although peroxisomes in pmd1-1 display a slight decrease in linear movement, this difference is not statistically significant (P = 0.10; Fig. 5C). However, a significantly smaller percentage of peroxisomes in mpk17-1 were immobile compared to either wild type or pmd1-1 (Fig. 5C), and a significantly higher percentage of peroxisomes in mpk17-1 moved in a linear fashion (Fig. 5C). These results suggest increased peroxisome movement along actin filaments in mpk17-1, despite the increased clustering of peroxisomes observed in this mutant (Fig. 1). These data are consistent with the possibility that PMD1 directly tethers peroxisomes to actin; however, because PMD1 lacks a region conferring ATPase activity, it is unlikely to act as a motor to facilitate peroxisome movement along the actin filament.
Actin Polymerization Is Required for Salt-Induced Peroxisome Proliferation
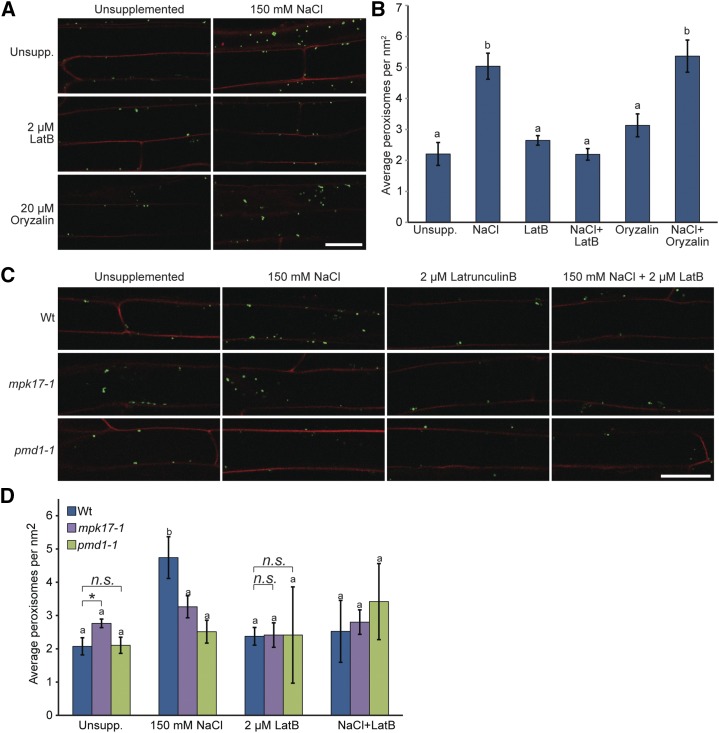
Binding of actin by PMD1 (Fig. 5B), combined with the alterations in peroxisome movement in mpk17-1 (Fig. 5C), raises the possibility that at least some of the phenotypes observed in these mutants result from altered interactions between peroxisomes and the actin cytoskeleton. To test whether peroxisome proliferation in response to salt stress relies on the actin cytoskeleton, we examined the effects of the latrunculin B (which prevents F-actin assembly) and oryzalin (which depolymerizes microtubules) on salt-induced peroxisome proliferation. We treated 4-d-old wild-type seedlings carrying the GFP-PTS1 reporter (Zolman and Bartel 2004) with NaCl, with and without latrunculin B or oryzalin supplements, for 16 h before imaging peroxisomes from upper root cells that were fully differentiated before treatment (Fig. 6A). Disruption of salt-induced peroxisome proliferation by oryzalin would indicate that intact microtubules are necessary for peroxisome proliferation, whereas disruption of salt-induced peroxisome proliferation by latrunculin B treatment would indicate that actin polymerization is necessary for peroxisome division. We quantified peroxisome numbers from seedling treated with various combinations of NaCl, latrunculin B, and oryzalin (Fig. 6B), and found that the increased peroxisome numbers in seedlings treated with 150 mm NaCl were not reduced in seedlings treated with 150 mm NaCl and oryzalin (Fig. 6, A and B), suggesting that disrupting the microtubule cytoskeleton does not affect salt-induced peroxisome proliferation. Conversely, peroxisome numbers from seedlings treated with NaCl and latrunculin B were statistically indistinguishable from untreated seedlings and seedlings treated with latrunculin B alone (Fig. 6B), suggesting that disrupting the actin cytoskeleton prevents NaCl-induced peroxisome proliferation in Arabidopsis.
Figure 6.
Salt-induced peroxisome proliferation depends on actin polymerization. A, Latrunculin B blocks salt-induced peroxisome division. Here, 3-d-old Col-0 GFP-PTS1 seedlings grown on unsupplemented media at 22°C under continuous light were transferred to the indicated treatments for 16 h. Seedlings were then counterstained with propidium iodide before imaging by confocal microscopy. Signal from GFP-PTS1 was false-colored green and signal from propidium iodide was false-colored red. Scale bar = 25 μm. B, Mean number of peroxisomes (± se) from 3-d-old Col-0 GFP-PTS1 seedlings treated as indicated. Peroxisome numbers in seedlings treated with oryzalin + NaCl are statistically indistinguishable from seedlings treated with NaCl alone (using ANOVA), whereas peroxisome numbers on latrunculin + NaCl are statistically indistinguishable from peroxisome numbers in seedlings grown on unsupplemented media and latrunculin alone (using ANOVA). C, Confocal images of 3-d-old wild type (Col-0), mpk17-1, and pmd1-1 carrying the 35S:GFP-PTS1 reporter (Zolman and Bartel, 2004) treated for 16 h as indicated. Scale bar = 25 μm. Seedlings were counterstained with propidium iodide before imaging; signal from GFP-PTS1 was false-colored green and signal from propidium iodide was false-colored red. D, Mean number of peroxisomes (μ se) from 3-d-old wild type (Col-0), mpk17-1, and pmd1-1 carrying the 35S:GFP-PTS1 reporter (Zolman and Bartel, 2004) treated for 16 h as indicated. Peroxisome numbers in mpk17-1 and pmd1-1 do not significantly change with the addition of NaCl or with latrunculin + NaCl. Data analyzed using ANOVA and Tukey’s HSD (letters) and by a two-tailed unpaired t test (* = P ≤ 0.05). Wt, wild type.
Because latrunculin B suppressed salt-induced peroxisome proliferation (Fig. 6B), we examined the effects of latrunculin B on salt-induced peroxisome proliferation in the mpk17-1 and pmd1-1 mutants (Fig. 6C). Similar to our previous findings (Fig. 3, A and B), we found that both mpk17-1 and pmd1-1 were less responsive to salt-induced peroxisome proliferation than wild type (Fig. 6, C and D). In addition, although latrunculin B restored salt-treated peroxisome numbers to match those of untreated seedlings in wild type, latrunculin B did not strongly alter peroxisome numbers in salt-treated mpk17-1 or pmd1-1 (Fig. 6D). Interestingly, we found that latrunculin B treatment of mpk17-1 resulted in peroxisome numbers similar to those of wild type (Fig. 6, C and D), suggesting that the increased peroxisome numbers observed in mpk17-1 rely on formation of F-actin.
DISCUSSION
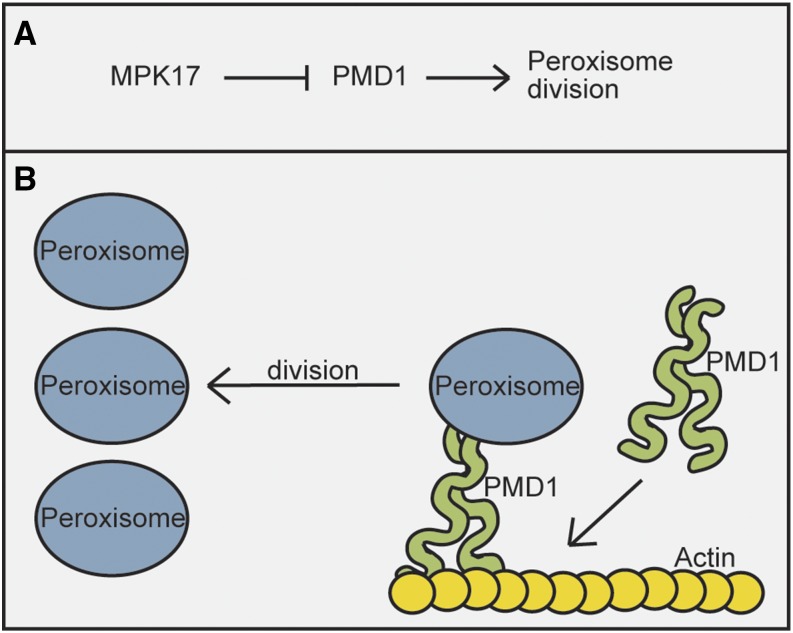
Based on the phenotype of mpk17-1 as IBA hypersensitivity with increased peroxisome numbers, and the known role of PMD1 increasing peroxisome division (Aung and Hu 2011), we suggest a genetic model in which MPK17 inhibits PMD1, a factor promoting peroxisome division (Fig. 7). Because pmd1-1 suppresses the mpk17-1 increased peroxisome number phenotype, PMD1 acts downstream of MPK17 (Fig. 7). We do not yet know the phosphorylation targets of MPK17. We were unable to detect an interaction between MPK17 and PMD1 using a yeast two-hybrid assay, consistent with the possibility that PMD1 is not a direct target of putative MPK17 kinase activity. Therefore, there are likely additional components missing from this model. Additionally, we cannot exclude the possibility that MPK17 inhibits peroxisome biogenesis generally, not just division through one specific factor. Further work will need to be done to determine precisely how broad or narrow an effect MPK17 has on peroxisome division.
Figure 7.
Proposed model of NaCl-regulated peroxisome division mediated through PMD1. A, Our genetic analysis suggests a model in which PMD1 acts downstream of MPK17 in peroxisome division. B, PMD1 interacts both with peroxisomes and with the actin cytoskeleton to aid in peroxisome division.
In this work, we have demonstrated a novel function for Arabidopsis MPK17 that affects peroxisome and mitochondrial division. These effects by MPK17 depend on PMD1, a peroxisome and mitochondrial division factor. Although Arabidopsis MPK17 is essentially uncharacterized compared to its well-understood relatives MPK3 and MPK6 (for review, see Mishra et al., 2006), studies on MPK17 homologs from other plants suggest it plays roles in stress response. For example, cotton GhMPK17 salinity stress tolerance and ABA treatment, and overexpression of GhMPK17 in Arabidopsis, led to increased tolerance of both salinity and ABA (Zhang et al., 2014). Similarly, in Setaria italica, SiMPK17 transcript is upregulated in response to dehydration stress (Lata et al., 2010). Transcript of the maize (Zea mays) homolog, ZmMPK17, increases upon cold, ROS, or osmotic stresses and during treatments with abscisic acid, salicylic acid, jasmonic acid, and ethylene (Pan et al., 2012). Further, the two closest rice MPK17 homologs, OsMPK13 and OsMPK14, are induced upon inoculation with a rice fungal pathogen (Reyna and Yang, 2006). Clearly, MPK17 and its homologs respond to stress transcriptionally and, at least in some cases, mediates tolerance to various stress conditions.
Disruption of either MPK17 or PMD1 results in decreased salt-induced peroxisome proliferation, thus both MPK17 and PMD1 are necessary for this dynamic salt response. Because the peroxisome numbers in non-salt-stressed mpk17-1 are not as high as wild type grown on NaCl, division through the actions of MPK17 and PMD1 cannot be the sole salt-responsive peroxisome division pathway(s). The ability to divide peroxisomes in response to NaCl does not substantially impact survival or growth of these mutants under high-NaCl conditions (Fig. 4), which suggests that peroxisome proliferation may not enhance the fitness of NaCl- stressed plants. This result contrasts with recent results by Fahy et al. (2017), who observed that the salt-hypersensitive mutants fry1 and sos1 did not proliferate peroxisomes in response to NaCl, and have very poor survival on high NaCl. Both mpk17 and pmd1 display the same nonproliferation molecular phenotype, but no whole plant NaCl phenotype. It remains unclear whether peroxisome proliferation in response to NaCl may provide salt tolerance to the plant under specific conditions, or if peroxisome proliferation is a side effect caused by regulation of a different pathway.
In this study, we also discovered a novel function for PMD1 as an actin-binding protein (Fig. 5B). PMD1 may act as a mechanical input to the peroxisome (and mitochondrial) division process, an idea that is supported by the peroxisome clustering phenotype seen in PMD1 overexpression lines (Aung and Hu, 2011). The increased fraction of mpk17-1 peroxisomes moving in a linear versus Brownian pattern is also consistent with the hypothesis that connections between PMD1 and the actin cytoskeleton contribute to peroxisome distribution in planta, as mpk17-1 contains more PMD1, and mpk17-1 peroxisomes show an increased ability to move around the cell than in wild type or pmd1-1. Peroxisomes in cells treated with latrunculin B still undergo Brownian movement (Mathur et al., 2002), further supporting the hypothesis by Aung and Hu (2011) that PMD1 might act in peroxisome distribution within the plant cell. Recently, the distribution, not just the number, of peroxisomes was shown to be vital for proper cell division in mice skin cells (Asare et al., 2017). Knocking down Pex11b retained peroxisome attachment to the microtubule cytoskeleton, but peroxisomes were mislocalized. This mislocalization led to improper positioning of the peroxisomes during cell division and mitotic delay, as well as aberrant angles of the mitotic plane of division (Asare et al., 2017). Other findings suggest the ability of plants to traffic actin-dependent contents is important for ordinary growth and development, not just organelle distribution during stress. The speed of myosins was shown to directly affect plant size, with expression of a faster myosin leading to larger plant size, and slower myosin causing smaller plant size (Tominaga et al., 2013). These data further support a role for localization, not just number, in peroxisome function.
These findings illuminate the importance of the actin cytoskeleton in peroxisome division. Future research to determine whether additional peroxisome or mitochondrial division factors associate with actin will be of interest and may provide molecular insight into how actin affects peroxisome division. Many questions remain about how, why, and when plants regulate peroxisome numbers and what adaptive function peroxisome proliferation may provide to plants.
MATERIALS AND METHODS
Growth Conditions and Phenotypic Assays
Arabidopsis (Arabidopsis thaliana) mutants were all in the Columbia-0 (Col-0) background, which was used as the wild type in all assays. Seeds were surface sterilized (Last and Fink, 1988) and stratified overnight at 4°C before plating on plant nutrient medium (Haughn and Somerville, 1986) supplemented with 0.5% w/v Suc and solidified with 0.6% agar, unless otherwise noted. Seedlings were grown at 22°C under continuous illumination.
To examine auxin-responsive root elongation, seeds were plated on media supplemented with ethanol (mock), or the indicated concentrations of indole-3-acetic acid (Sigma-Aldrich), indole-3-butyric acid (Sigma-Aldrich), 2,4-dichlorophenoxyacetic acid (Sigma-Aldrich), or 2,4-dichlorophenoxybutyric acid (Sigma-Aldrich). All hormone stocks were dissolved in 100% ethanol. Plates were incubated horizontally at 22°C under yellow-filtered light to prevent indole backbone degradation (Stasinopoulos and Hangarter, 1990). After 8 d of growth, seedlings were removed from the agar and root length measured.
To examine auxin-responsive lateral root formation, seeds were plated on unsupplemented media and grown under continuous white light for 4 d before transfer to media supplemented with ethanol (mock) or the indicated auxin. Seedlings were grown horizontally for an additional 4 d under continuous yellow light. Emerged lateral roots were counted under a dissecting scope and root length measured to determine the number of lateral roots formed per mm root length.
To examine inhibition of root elongation on salt, seeds were plated on unsupplemented media and grown horizontally under continuous white light for 4 d before transfer to plates supplemented with the indicated concentration of NaCl, KCl, or mannitol and solidified with 0.7% agar. Upon transferring, root tips were aligned and marked. Plates were then placed vertically under continuous white light and grown for an additional 4 d before imaging and measurement of post-transfer growth using the software ImageJ (National Institutes of Health).
To examine seedling bleaching caused by salt, seeds were plated on sterile filter paper (Whatman) set on top of plant nutrient media and grown under continuous white light for 4 d. Filter papers with seedlings were then transferred to plates supplemented with the indicated concentration of NaCl and grown under continuous white light. After both 7 d and 14 d of additional growth on salt or control conditions, the number of green seedlings (seedlings with at least one green cotyledon) and bleached (colorless) were counted.
To examine adult plant growth response to salt watering, seedlings were grown for one week on unsupplemented media, then transferred to individual 9 × 9 cm pots containing soil. Ten individuals of each genotype were used for each treatment. Plants were grown at 21°C, 50% humidity under continuous light in a growth chamber and were top-watered uniformly for one week with distilled water before daily top-watering with 25 mL of distilled water supplemented with the indicated amount of NaCl. Plant mortality was logged daily. Plant height was measured after 2 weeks of watering treatment, when plants were beginning to senesce.
Vector Construction and Transformation
To create mpk17-1 rescue lines, the 2 kB region upstream of MPK17 and the full-length MPK17 gene were amplified using Pfx Platinum (Life Technologies) polymerase with MPK17-16 (5´-CTAACGATTCGGAAAGCTAAAG-3´) and MPK17-withstop (5´-CTATGACACTGCAGAGGAGACAC-3´). This region was subcloned into pENTR-DTOPO (Life Technologies), then cloned into pMDC123 (Curtis and Grossniklaus, 2003) using LR Clonase II (Life Technologies) to create MPK17promoter:MPK17. MPK17promoter:MPK17 was then transformed into GV3101 Agrobacterium tumefaciens strain, which was used for floral dip transformation (Clough and Bent, 1998) of mpk17-1 plants.
To create the construct for PMD1 protein expression, the portion of PMD1 cDNA (U83915) encoding PMD1 from amino acids 1 to 303 and thus lacking the predicted PMD1 transmembrane domain was PCR-amplified using PMD1-NdeI (5´-CATATGGCGGATGTTGAAGATC-3´) and PMD1-XhoI 5´-(CTCGAGCTAAGCAGCTCCAACTGATCC-3´) and the resultant product was cloned into pCR4 (Life Technologies). PMD11-303 was then released using restriction enzymes NdeI and XhoI and subcloned into the protein expression vector pET28A (Novagen) to create pET28-PMD11-303.
Genetic Analyses
Col-0 carrying the GFP-PTS1 reporter (Zolman and Bartel 2004) was crossed to mpk17-1 and pmd1-1 and the resultant F2 seedlings genotyped to obtain mpk17-1 GFP-PTS1 and pmd1-1 GFP-PTS1. Col-0 and pmd1-1 carrying the COX4-YFP mitochondrial reporter (Aung and Hu, 2011; Nelson et al., 2007) were crossed to mpk17-1 and the resultant F2 seedlings genotyped to obtain mpk17-1 COX4-YFP. Fluorescent reporter lines were genotyped with a combination of PCR and by the presence of the fluorescent reporter.
To obtain mpk17 rescue lines, mpk17-1 mutants were transformed using the floral dip method (Clough and Bent, 1998). mpk17-1 seedlings carrying the MPK17promoter:MPK17 transgene were selected for Basta (phosphinothricin; Gold Biotechnology) resistance in the T1 generation and lines homozygous for the transgene were selected in subsequent generations. Nonsegregating T3 lines were genotyped for both the mpk17-1 TDNA insertion and presence of wild-type MPK17 from the transgene. Later generations were genotyped by both PCR and Basta plating.
Imaging and Peroxisome Quantification
To determine the effects of salt treatment and cytoskeleton inhibitors on peroxisome numbers, 3-d-old seedlings were transferred to plant growth media supplemented with 150 mm NaCl, 2 μM latrunculin with and without 150 mm NaCl, and 20 μM oryzalin with and without 150 mm NaCl. Plants were grown on salt and cytoskeleton inhibitors overnight at 22°C under continuous white light, then incubated in propidium iodide (Invitrogen) and confocally imaged, using identical settings for each image. Peroxisomes were quantified in ImageJ by selecting the root area, then using “Find Maxima” with appropriate parameters (thresholding between 6 and 10, dark background mode).
To determine peroxisome numbers and movement, 7-d-old seedlings were mounted on microscope slides in liquid plant media containing 0.1% agar; slides had a Tough-Tag (Sigma-Aldrich) positioned on either end of the coverslip to provide a cushion between the slide and coverslip. Seedlings were imaged at a rate of 13 frames/s for 29 s on a model no. DM6 B upright fluorescence scope (Leica) equipped with a model no. DFC 3000 G camera (Leica). For each genotype, at least nine different individuals were imaged, with three images/root in the maturation zone taken. Videos were analyzed using ImageJ. The movement type for each peroxisome was categorized as Linear (indicates rapid, mostly unidirectional movement through the cell), Brownian (refers to back-and-forth movement of a peroxisome), or “Immobile” (the peroxisome exhibited no movement during the 29 s recording).
To image and quantify mitochondria, four-d-old seedlings carrying the COX4-YFP were counterstained with propidium iodide and imaged by confocal with a model no. LSM510 (Zeiss) using identical settings for each image. Images were analyzed in ImageJ using the “Find Maxima” tool as described for peroxisomes above.
Protein Expression and Purification
PMD11-303 was expressed in Escherichia coli (DE3) Rosetta cells (Invitrogen) as an N-terminal His-tagged protein. Bacterial cultures were grown at 37°C to an A600nm = ∼0.5. Protein expression was induced with a final concentration of 1 mm isopropyl β-d-1-thiogalactopyranoside, then grown for an additional 18 h at 18°C. Bacterial cells were pelleted and resuspended in lysis buffer [50 mm Tris pH 8.0, 20 mm imidazole, 500 mm NaCl, 10% (vol/vol) glycerol, 1% Tween 20]. Resuspended cells were lysed by sonication, and cell debris was pelleted by centrifugation. The soluble cell lysate was passed over a Ni2+-nitrilotriacetic acid chromatography column. The column was washed with wash buffer [50 mm Tris pH 8.0, 20 mm imidazole, 500 mm NaCl, 10% (vol/vol) glycerol] and bound protein eluted with elution buffer [50 mm Tris pH 8.0, 250 mm imidazole, 500 mm NaCl, 10% (vol/vol) glycerol].
Actin Cosedimentation Assay
The actin cosedimentation assay was performed with purified His-PMD11-303 and G-actin, which was a kind gift from the lab of John Cooper (Washington University in St. Louis). Before the assay, the 10 μM G-actin and His-PMD11-303 were independently centrifuged at 200,000g at 4°C for 20 min to remove any protein complexes. One X and half X concentrations of His-PMD11-303 were incubated with G-actin, 20× KMEI polymerization buffer (0.2 m imidazole pH 7.0, 1 m KCl, 20 mm MgCl2, 20 mm EGTA, 1 mm NaN3) and G-actin buffer with ATP (2 mm Tris-Cl pH 8.0, 0.1 mm CaCl2, 1 mm NaN3, 0.2 mm Na-ATP, 0.5 mm DTT). Samples were rocked at room temperature for 1 h. A 50-μL aliquot was removed from each sample before centrifugation to create the pre-spin sample. The remaining sample was then moved to a tube containing 50 μL of 20% Suc in G-actin buffer (2.0 mm Tris-Cl pH 8.0, 0.1 mm CaCl2, 1 mm NaN3) and centrifuged at 100,000g at 4°C for 30 min. After centrifugation, a 50-μL aliquot from the post-spin supernatant was removed from each sample to new tubes to create the post-spin samples. Each sample was mixed with an equal volume of Nu-PAGE sample buffer (141 mm Tris, 2% LDS, 0.51 mm EDTA, 10% glycerol, 0.175 mm phenol red, 0.22 mm Coomassie Blue) and boiled for 5 min before separation by electrophoresis through a 10% Bis-Tris Plus gel (Invitrogen). After separation, proteins were transferred to a nitrocellulose membrane. The membrane was blocked in 8% milk diluted in 1× TBS-T (20 mm Tris, pH 7.5, 150 mm NaCl, 1% Tween) for 1 h, then incubated with a 1:200 dilution of α-His (Santa Cruz Biotechnology) in blocking buffer at 4°C overnight. The membrane was washed three times with 1× TBS-T for 5 min per wash before a 4-h incubation with 1:5000 goat α-rabbit HRP-conjugated antibody dilution (Santa Cruz Biotechnology). After incubation with secondary antibody, the membrane was washed 3 times with 1× TBS-T for 5 min per wash. The membrane was immersed in westernBright ECL HRP (Bioexpress) substrate and imaged using a ChemiDoc (Bio-Rad).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession no. At2g014500 (MPK17) and At3g58840 (PMD1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. mpk17-1 resembles wild type in multiple germination assay trials.
Supplemental Figure S2. Validation of actin cosedimentation assay.
Acknowledgments
We thank the ABRC for providing mpk17-1 insertional allele and MPK17 cDNA, Jianping Hu for providing pmd1-1, Col COX4-YFP, and pmd1-1 COX4-YFP; Bonnie Bartel for providing Col GFP-PTS1; the lab of John Cooper for providing actin for cosedimentation assays and technical advice on performing these assays; Joseph Jez and Hani Zaher and their labs for use of their equipment; and Bonnie Bartel, Hongwei Jing, Samantha Powers, and Katie Schreiber for critical comments on the manuscript.
Footnotes
This research was supported by the National Science Foundation (NSF, under IOS-1453750 to L.C.S.), the United States Department of Agriculture-National Institute of Food and Agriculture Fellowship Program (under 2016-67011-25096 to E.M.F.), and the National Institutes of Health (NIH, under R01 GM112898 to L.C.S.).
Articles can be viewed without a subscription.
References
- Abe I, Fujiki Y (1998) cDNA cloning and characterization of a constitutively expressed isoform of the human peroxin Pex11p. Biochem Biophys Res Commun 252: 529–533 [DOI] [PubMed] [Google Scholar]
- Agrawal G, Subramani S (2016) De novo peroxisome biogenesis: evolving concepts and conundrums. Biochim Biophys Acta 1863: 892–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare A, Levorse J, Fuchs E (2017) Coupling organelle inheritance with mitosis to balance growth and differentiation. Science 355: 6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung K, Hu J (2011) The Arabidopsis tail-anchored protein PEROXISOMAL AND MITOCHONDRIAL DIVISION FACTOR1 is involved in the morphogenesis and proliferation of peroxisomes and mitochondria. Plant Cell 23: 4446–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton WL, Matsui K, Johnson B, Graham IA, Ohme-Takagi M, Baker A (2005) Salt-induced expression of peroxisome-associated genes requires components of the ethylene, jasmonate and abscisic acid signaling pathways. Plant Cell Environ 28: 513–524 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Hu J (2008) Light induces peroxisome proliferation in Arabidopsis seedlings through the photoreceptor phytochrome A, the transcription factor HY5 HOMOLOG, and the peroxisomal protein PEROXIN11b. Plant Physiol 146: 1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders TA, Frick EM, Strader LC (2017) An Arabidopsis kinase cascade influences auxin-responsive cell expansion. Plant J 92: 68–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy D, Sanad MN, Duscha K, Lyons M, Liu F, Bozhkov P, Kunz HH, Hu J, Neuhaus HE, Steel PG, Smertenko A (2017) Impact of salt stress, cell death, and autophagy on peroxisomes: quantitative and morphological analyses using small fluorescent probe N-BODIPY. Sci Rep 7: 39069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn GW, Somerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204: 430–434 [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M (1998) 2,4-dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10: 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D, van den Berg M, Philippsen P, Tabak HF, Hettema EH (2001) A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J Cell Biol 155: 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islinger M, Grille S, Fahimi HD, Schrader M (2012) The peroxisome: an update on mysteries. Histochem Cell Biol 137: 547–574 [DOI] [PubMed] [Google Scholar]
- Jaipargas EA, Mathur N, Bou Daher F, Wasteneys GO, Mathur J (2016) High light intensity leads to increased peroxule-mitochondria interactions in plants. Front Cell Dev Biol 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N, Hu J (2009) Dynamics of peroxisome abundance: a tale of division and proliferation. Curr Opin Plant Biol 12: 781–788 [DOI] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4: 363–371 [DOI] [PubMed] [Google Scholar]
- Kim PK, Mullen RT, Schumann U, Lippincott-Schwartz J (2006) The origin and maintenance of mammalian peroxisomes involves a de novo PEX16-dependent pathway from the ER. J Cell Biol 173: 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J, Pranjic K, Huber A, Ellinger A, Hartig A, Kragler F, Brocard C (2010) PEX11 family members are membrane elongation factors that coordinate peroxisome proliferation and maintenance. J Cell Sci 123: 3389–3400 [DOI] [PubMed] [Google Scholar]
- Koh S, André A, Edwards H, Ehrhardt D, Somerville S (2005) Arabidopsis thaliana subcellular responses to compatible Erysiphe cichoracearum infections. Plant J 44: 516–529 [DOI] [PubMed] [Google Scholar]
- Landrum M, Smertenko A, Edwards R, Hussey PJ, Steel PG (2010) BODIPY probes to study peroxisome dynamics in vivo. Plant J 62: 529–538 [DOI] [PubMed] [Google Scholar]
- Last RL, Fink GR (1988) Tryptophan-requiring mutants of the plant Arabidopsis thaliana. Science 240: 305–310 [DOI] [PubMed] [Google Scholar]
- Lata C, Sahu PP, Prasad M (2010) Comparative transcriptome analysis of differentially expressed genes in foxtail millet (Setaria italica L.) during dehydration stress. Biochem Biophys Res Commun 393: 720–727 [DOI] [PubMed] [Google Scholar]
- Lee JR, Park SC, Kim MH, Jung JH, Shin MR, Lee DH, Cheon MG, Park Y, Hahm KS, Lee SY (2007) Antifungal activity of rice Pex5p, a receptor for peroxisomal matrix proteins. Biochem Biophys Res Commun 359: 941–946 [DOI] [PubMed] [Google Scholar]
- Li X, Gould SJ (2002) PEX11 promotes peroxisome division independently of peroxisome metabolism. J Cell Biol 156: 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingard MJ, Trelease RN (2006) Five Arabidopsis peroxin 11 homologs individually promote peroxisome elongation, duplication or aggregation. J Cell Sci 119: 1961–1972 [DOI] [PubMed] [Google Scholar]
- Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, Llorente F, Molina A, et al. (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310: 1180–1183 [DOI] [PubMed] [Google Scholar]
- Lopez-Huertas E, Charlton WL, Johnson B, Graham IA, Baker A (2000) Stress induces peroxisome biogenesis genes. EMBO J 19: 6770–6777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano S, Nakamori C, Kondo M, Hayashi M, Nishimura M (2004) An Arabidopsis dynamin-related protein, DRP3A, controls both peroxisomal and mitochondrial division. Plant J 38: 487–498 [DOI] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Hülskamp M (2002) Simultaneous visualization of peroxisomes and cytoskeletal elements reveals actin and not microtubule-based peroxisome motility in plants. Plant Physiol 128: 1031–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra NS, Tuteja R, Tuteja N (2006) Signaling through MAP kinase networks in plants. Arch Biochem Biophys 452: 55–68 [DOI] [PubMed] [Google Scholar]
- Mitsuya S, El-Shami M, Sparkes IA, Charlton WL, de Marcos Lousa C, Johnson B, Baker A (2010) Salt stress causes peroxisome proliferation, but inducing peroxisome proliferation does not improve NaCl tolerance in Arabidopsis thaliana. PLoS One 5: e9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockaitis K, Howell SH (2000) Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J 24: 785–796 [DOI] [PubMed] [Google Scholar]
- Motley AM, Hettema EH (2007) Yeast peroxisomes multiply by growth and division. J Cell Biol 178: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Orth T, Reumann S, Zhang X, Fan J, Wenzel D, Quan S, Hu J (2007) The PEROXIN11 protein family controls peroxisome proliferation in Arabidopsis. Plant Cell 19: 333–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Zhang M, Kong X, Xing X, Liu Y, Zhou Y, Liu Y, Sun L, Li D (2012) ZmMPK17, a novel maize group D MAP kinase gene, is involved in multiple stress responses. Planta 235: 661–676 [DOI] [PubMed] [Google Scholar]
- Rapp S, Saffrich R, Anton M, Jäkle U, Ansorge W, Gorgas K, Just WW (1996) Microtubule-based peroxisome movement. J Cell Sci 109: 837–849 [DOI] [PubMed] [Google Scholar]
- Reyna NS, Yang Y (2006) Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Mol Plant Microbe Interact 19: 530–540 [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS (1995) Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA 92: 8813–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Sanz-Fernández M, Hu J, Sandalio LM (2016) Peroxisomes extend peroxules in a fast response to stress via a reactive oxygen species-mediated induction of the peroxin PEX11a. Plant Physiol 171: 1665–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Sparkes I, Hawes C, del Río LA, Sandalio LM (2009) Peroxisome dynamics in Arabidopsis plants under oxidative stress induced by cadmium. Free Radic Biol Med 47: 1632–1639 [DOI] [PubMed] [Google Scholar]
- Schafer DA, Jennings PB, Cooper JA (1998) Rapid and efficient purification of actin from nonmuscle sources. Cell Motil Cytoskeleton 39: 166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M. (2006) Shared components of mitochondrial and peroxisomal division. Biochim Biophys Acta 1763: 531–541 [DOI] [PubMed] [Google Scholar]
- Schrader M, Burkhardt JK, Baumgart E, Lüers G, Spring H, Völkl A, Fahimi HD (1996) Interaction of microtubules with peroxisomes. Tubular and spherical peroxisomes in HepG2 cells and their alterations induced by microtubule-active drugs. Eur J Cell Biol 69: 24–35 [PubMed] [Google Scholar]
- Stasinopoulos TC, Hangarter RP (1990) Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues. Plant Physiol 93: 1365–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Kimura A, Yokota E, Haraguchi T, Shimmen T, Yamamoto K, Nakano A, Ito K (2013) Cytoplasmic streaming velocity as a plant size determinant. Dev Cell 27: 345–352 [DOI] [PubMed] [Google Scholar]
- Wiemer EA, Wenzel T, Deerinck TJ, Ellisman MH, Subramani S (1997) Visualization of the peroxisomal compartment in living mammalian cells: dynamic behavior and association with microtubules. J Cell Biol 136: 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Opalinski L, Landgraf C, Costello J, Schrader M, Krikken AM, Knoops K, Kram AM, Volkmer R, van der Klei IJ (2015) The membrane remodeling protein Pex11p activates the GTPase Dnm1p during peroxisomal fission. Proc Natl Acad Sci USA 112: 6377–6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Niwa H, Honsho M, Itoyama A, Fujiki Y (2015) Pex11mediates peroxisomal proliferation by promoting deformation of the lipid membrane. Biol Open 4: 710–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Cai M (2004) The yeast dynamin-related GTPase Vps1p functions in the organization of the actin cytoskeleton via interaction with Sla1p. J Cell Sci 117: 3839–3853 [DOI] [PubMed] [Google Scholar]
- Zhang J, Zou D, Li Y, Sun X, Wang NN, Gong SY, Zheng Y, Li XB (2014) GhMPK17, a cotton mitogen-activated protein kinase, is involved in plant response to high salinity and osmotic stresses and ABA signaling. PLoS One 9: e95642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Hu JP (2008) FISSION1A and FISSION1B proteins mediate the fission of peroxisomes and mitochondria in Arabidopsis. Mol Plant 1: 1036–1047 [DOI] [PubMed] [Google Scholar]
- Zolman BK, Bartel B (2004) An Arabidopsis indole-3-butyric acid-response mutant defective in PEROXIN6, an apparent ATPase implicated in peroxisomal function. Proc Natl Acad Sci USA 101: 1786–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Yoder A, Bartel B (2000) Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156: 1323–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]