Tomato RIN-MC fusion plays a negative role in ripening and encodes a chimeric transcription factor that modulates the expression of many ripening genes, thereby contributing to the rin mutant phenotype.
Abstract
Fruit development and ripening is regulated by genetic and environmental factors and is of critical importance for seed dispersal, reproduction, and fruit quality. Tomato (Solanum lycopersicum) ripening inhibitor (rin) mutant fruit have a classic ripening-inhibited phenotype, which is attributed to a genomic DNA deletion resulting in the fusion of two truncated transcription factors, RIN and MC. In wild-type fruit, RIN, a MADS-box transcription factor, is a key regulator of the ripening gene expression network, with hundreds of gene targets controlling changes in color, flavor, texture, and taste during tomato fruit ripening; MC, on the other hand, has low expression in fruit, and the potential functions of the RIN-MC fusion gene in ripening remain unclear. Here, overexpression of RIN-MC in transgenic wild-type cv Ailsa Craig tomato fruits impaired several ripening processes, and down-regulating RIN-MC expression in the rin mutant was found to stimulate the normal yellow mutant fruit to produce a weak red color, suggesting a distinct negative role for RIN-MC in tomato fruit ripening. By comparative transcriptome analysis of rin and rin 35S::RIN-MC RNA interference fruits, a total of 1,168 and 1,234 genes were identified as potential targets of RIN-MC activation and inhibition. Furthermore, the RIN-MC fusion gene was shown to be translated into a chimeric transcription factor that was localized to the nucleus and was capable of protein interactions with other MADS-box factors. These results indicated that tomato RIN-MC fusion plays a negative role in ripening and encodes a chimeric transcription factor that modulates the expression of many ripening genes, thereby contributing to the rin mutant phenotype.
Fruit ripening is a physiological process involving the development of quality attributes such as color, texture, flavor, and aroma that facilitate seed dispersal and generate the nutritional and organoleptic properties valued by humans (Alba et al., 2005; Klee and Giovannoni, 2011). The dramatic changes occurring during this complex developmental process are genetically regulated and also influenced by environmental factors such as temperature and light (Matas et al., 2009) plus internal regulators (Seymour et al., 2008), including hormones, particularly ethylene (Barry and Giovannoni, 2007; Grierson, 2013), transcription factors (Qin et al., 2012), and epigenetic modifications (Zhong et al., 2013). Investigation of a series of ripening-inhibited tomato (Solanum lycopersicum) mutants has identified key transcription factors such as ripening inhibitor (rin; Vrebalov et al., 2002), colorless nonripening (cnr; Manning et al., 2006), and nonripening (nor; Giovannoni, 2004), whose disruption results in impaired ripening, and some ethylene response factors (ERFs), which control different facets of the response (Liu et al., 2016).
The characterization of the rin mutant led directly to the identification of the RIN MADS-box transcription factor, which plays a central regulating role in tomato fruit ripening (Vrebalov et al., 2002). Based on comparison between wild type cv Ailsa Craig (AC) and rin mutant plants, the mutation was shown to cause a severely inhibited ripening phenotype, including loss of the characteristic burst of ethylene production and respiratory climacteric normally associated with the onset of ripening and a severe reduction in pigment accumulation, flavor production, and softening (Vrebalov et al., 2002). The rin mutation alters the expression of at least 241 genes (Fujisawa et al., 2013) involved in many aspects of ripening-related pathways, such as ethylene synthesis (ACS2 and ACS4; Fujisawa et al., 2013, 2014), cell wall modification (PG, TBG4, and EXP1; Fujisawa et al., 2013), and volatile production (LoxC; Qin et al., 2012). Moreover, RIN is involved in epigenetic modification such as DNA methylation, and the global methylation level of rin fruit remained higher than in the wild type at the onset of ripening (Zhong et al., 2013). Comparisons of transcriptome, proteome, and metabolome between the rin mutant and wild-type fruits have confirmed that RIN is a global regulator of the tomato fruit-ripening process (Osorio et al., 2011).
The rin ripening mutation in tomato is caused by the deletion of a genomic DNA fragment on chromosome 5, resulting in the fusion of adjacent truncated RIN and MC genes (RIN-MC), and the ripening-inhibited phenotype is attributed to the lack of a functional RIN protein. It is known that fusions of distinct genetic loci also have the potential to generate novel functions, which can change the phenotype in plants (Long et al., 2003; Hagel and Facchini, 2017). In opium poppy (Papaver somniferum), for example, fusion of the DRS and DRR genes led directly to an abnormal benzylisoquinoline alkaloid biosynthesis pathway (Li et al., 2016; Hagel and Facchini, 2017). Furthermore, in Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa), approximately 110 and 45 genes, respectively, could be transcribed as fusion mRNAs resulting potentially in chimeric proteins, which might provide clues to explore intrinsic regulating mechanisms (Shahmuradov et al., 2010). However, although high expression of the RIN-MC fusion gene has been detected in rin mutant fruit at the ripe stage based on RNA sequencing technology (Zhong et al., 2013; Fujisawa et al., 2014), possible functions of the RIN-MC fusion gene in the rin mutant are unknown.
In this study, the functions of RIN-MC in tomato fruit ripening were identified both in mutant fruit, in which RIN-MC was silenced, and in overexpressing wild-type fruit, which had an altered phenotype. RIN-MC was shown to be a new transcriptional factor by nuclear localization of the RIN-MC fusion protein and the demonstration that it could interact with other transcription factors, and RIN-MC functions were confirmed by comparative transcriptome analysis of rin and rin 35S::RIN-MC RNA interference (RNAi) fruits as well as AC and AC 35S::RIN RNAi fruits.
RESULTS
Transcription and Translation Assay of RIN-MC in Tomato Fruit Ripening
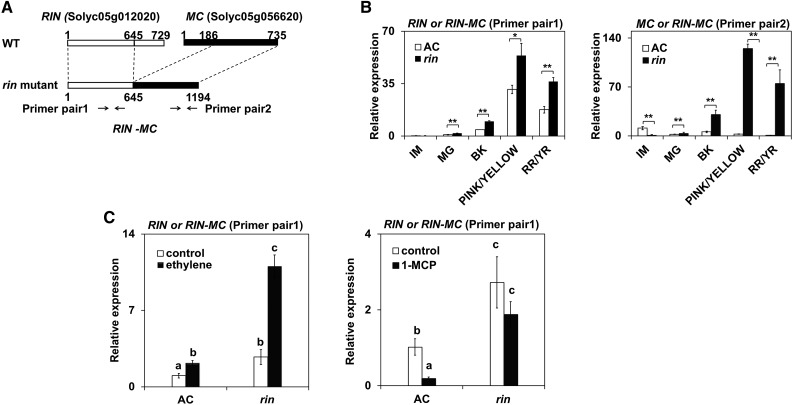
Two pairs of primers were designed to analyze RIN and RIN-MC transcripts in normal (AC) and rin mutant tomato fruits. Primer pair 1 mapped to the portion of the truncated RIN and primer pair 2 mapped to a specific region of the truncated MC present in the RIN-MC fusion (Fig. 1A). The reverse transcription-quantitative PCR (RT-qPCR) results showed that RIN-MC was expressed at high levels in rin fruits at MG (mature green), BK (breaker; the onset of color changes), yellow, and yellow ripe stages compared with normal RIN and MC genes in wild-type AC fruit (Fig. 1B). In contrast, only a very low level of transcripts from the normal MC gene was detected with primer pair 2 in the wild type (AC), in accordance with earlier findings (Vrebalov et al., 2002; Fig. 1B). The data obtained with both primer pairs suggested high abundance of RIN-MC transcripts in the rin mutant and were entirely consistent with the RIN-MC expression pattern reported in recent transcriptome assays of rin mutant fruit (Zhong et al., 2013; Fujisawa et al., 2014).
Figure 1.
Transcription assay of the RIN-MC gene in tomato fruit. A, The expression pattern of the RIN-MC gene was measured with different primer pairs, primer pair 1 and primer pair 2, mapped to partial RIN and MC, respectively, which detect RIN or RIN-MC (primer pair 1) and MC or RIN-MC (primer pair 2). WT, Wild type. B and C, Expression was analyzed at various ripening stages in wild-type and rin fruit (B) and after treatment with ethylene and 1-MCP at the MG stage (C). Relative transcript levels were determined by RT-qPCR, relative to the expression of the tomato internal control ACTIN gene, expressed as 2−ΔΔCt (Livak and Schmittgen, 2001). The stages of fruit ripening are as follows: immature (IM), MG, BK, pink/yellow, and red ripe (RR)/yellow ripe (YR). Relative values were based on comparisons of expression levels at different ripening stages with expression at the IM stage. Asterisks above the bars indicate values with significant differences, which were determined by Student’s t test (*, P < 0.05 and **, P < 0.01), and lowercase letters also indicate significant differences.
The effects of the ripening hormone ethylene and its competitive inhibitor 1-methylcyclopropene (1-MCP) on the accumulation of RIN mRNA in wild-type (AC) fruit and of RIN-MC transcripts in rin fruit at the MG stage were examined using primer pair 1 (Fig. 1C). Ethylene stimulated the accumulation of RIN-MC transcripts, and there was also a small stimulation of RIN mRNA. The significance of this is not clear, since both genes have the same promoter, but it may be related to differences in transcript processing, stability, or regulation by upstream factors. The addition of 1-MCP reduced RIN transcripts, suggesting that at least part of the increase is a genuine ethylene response, but the small reduction of RIN-MC RNA caused by 1-MCP was not statistically significant.
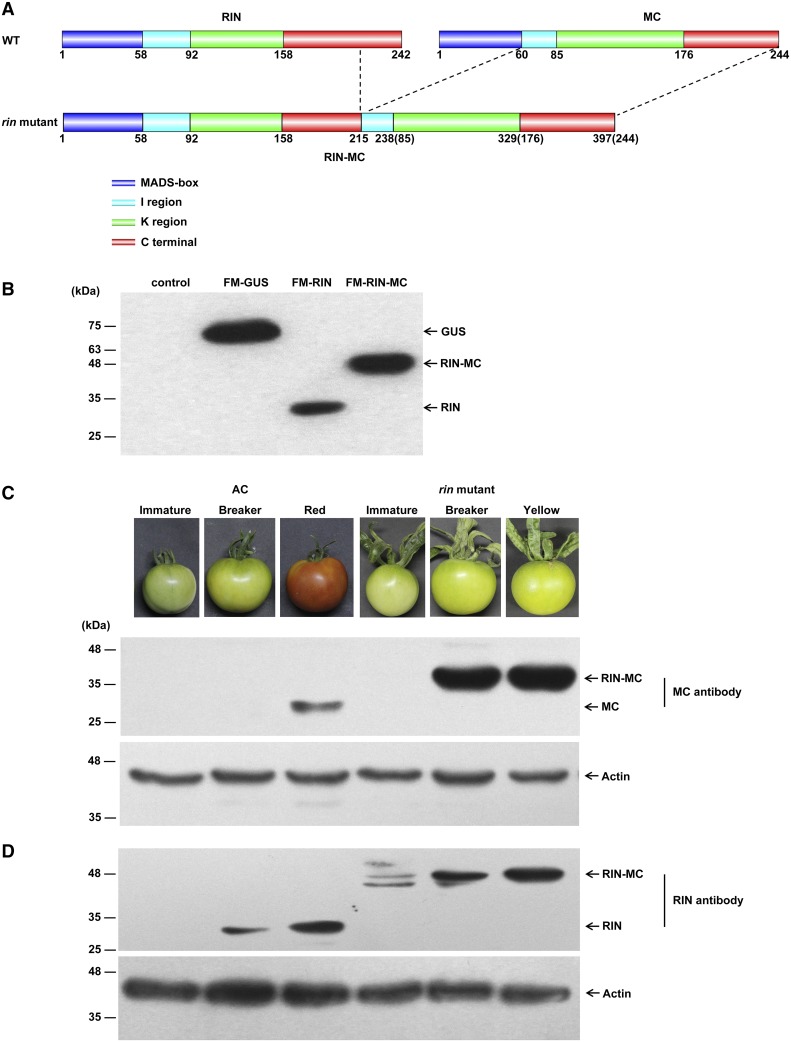
In wild-type (AC) fruit, RIN and MC encode the MADS-box transcription factors, RIN and MC, which belong to the MIKC class. According to the coding sequence (CDS) of RIN-MC (AF448523) in the National Center for Biotechnology Information database, the RIN-MC fusion gene could encode a MADS-box protein consisting of the MADS box (M), Intervening (I), keratin-like (K), and a major part of the C-terminal end (C) of RIN and nearly the complete I region, complete K region, and C-terminal end of MC (Fig. 2A). When the RIN-MC fusion protein with an N-terminal Flag-4myc (FM) tag was overexpressed in the Nicotiana benthamiana leaf transient expression system, the translation product from the RIN-MC gene was detected using anti-myc antibody (Fig. 2B). The potential endogenous RIN-MC chimeric protein also was assayed in rin mutant ripening fruits at different stages of development. Previously, it was reported that little or no signal could be detected for RIN-MC chimeric protein using antibody against partial RIN protein in rin mutant fruit (Ito et al., 2008; Martel et al., 2011; Qin et al., 2012); therefore, a specific antibody raised against the C-terminal end of RIN-MC (named MC antibody) was used in this study, in order to probe for MC polypeptide sequences in ripening wild-type (AC) fruit. The RIN-MC protein was detected in great abundance at the BK and yellow stages of rin fruits, whereas only a small amount of MC protein was found in AC (wild-type) tomato (Fig. 2C). A specific antibody was raised against the partial C-terminal end of RIN present in RIN-MC, named RIN-specific antibody, as a probe for the RIN-MC chimeric protein, which was detected at the BK and yellow stages of rin mutant tomato fruits (Fig. 2D) and was found with strong signals that indicated its high abundance compared with endogenous RIN protein in wild-type AC fruits. Taken together, this evidence showed that RIN-MC protein accumulates in rin mutant fruit and is present in greater abundance at the BK and yellow stages compared with either RIN or MC protein at the corresponding stages in wild-type AC fruits (Fig. 2, C and D); a similar conclusion was published by Ito et al. (2017).
Figure 2.
Translation assay of the RIN-MC gene in tomato fruit. A, Predicted structure of RIN-MC protein based on its published CDS (AF448523; Vrebalov et al., 2002), visualized in DOG2.0.1 (http://dog.biocuckoo.org/; Ren et al., 2009). WT, Wild type. B, Assay of RIN and RIN-MC protein following transient expression in N. benthamiana leaves of FM-GUS, FM-RIN-MC, and FM-RIN. The western blot shows control (expressing empty vector) and experimental constructs using anti-myc antibody. C and D, Endogenous RIN-MC protein in the wild type (AC) and the rin mutant at different ripening stages using MC antibody (C) and RIN antibody (D). Actin was used as an internal protein control.
Overexpression of the RIN-MC Fusion Gene Inhibits Tomato Fruit Ripening
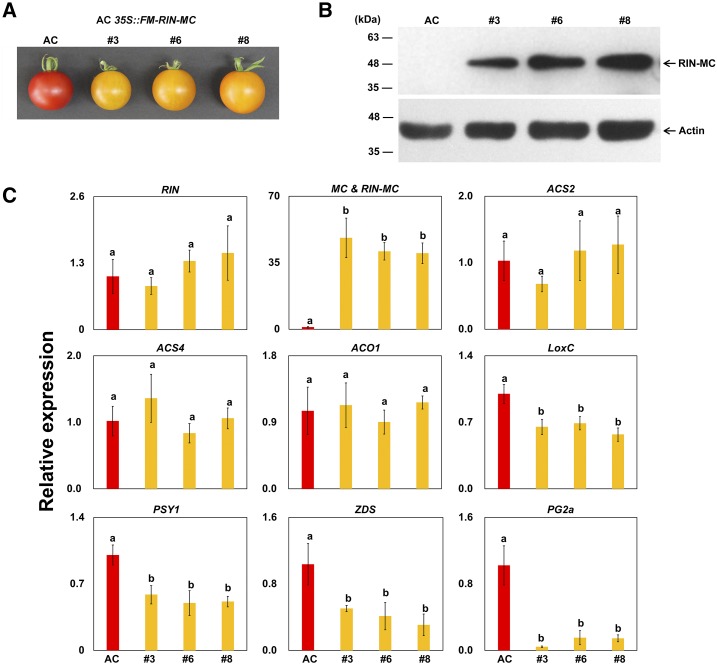
The function of the RIN-MC fusion gene was analyzed by overexpressing it in transgenic wild-type (AC) fruits under the control of the cauliflower mosaic virus (CaMV) 35S promoter (Fig. 3). Overexpression of RIN-MC in wild-type fruits resulted in a yellow-colored phenotype at the BK+10 stage (10 d after the start of color change; Fig. 3A). There was no statistically significant difference in RIN expression between the wild-type control and transgenic fruits (Fig. 3C), indicating a distinct inhibitory effect of the RIN-MC gene or protein on tomato fruit ripening that did not operate by silencing the normal RIN gene. Expression of the RIN-MC protein was confirmed in AC::35S-FM-RIN-MC fruits by western-blot assay using anti-myc antibody (Fig. 3B), consistent with that observed expressing FM-RIN-MC in the leaf transient expression system (Fig. 2B). Several ripening-related genes were down-regulated in the transgenic AC::35S-FM-RIN-MC fruits, such as lipoxygenase (LoxC), phytoene synthase (PSY1), ζ-carotene desaturase (ZDS), and polygalacturonase (PG2a). Genes related to the ethylene biosynthesis pathway, on the other hand, including ACC synthase (ACS2 and ACS4) and ACC oxidase (ACO1), displayed no statistically significant difference between AC and AC::35S-FM-RIN-MC fruits (Fig. 3C), indicating that the altered ripening was unlikely to be due to an effect on ethylene synthesis.
Figure 3.
Effects on tomato ripening of the overexpression of RIN-MC in wild-type (AC) fruits. Results from three transgenic lines (3, 6, and 8) are compared with the wild-type control. A, Phenotypes. B, Levels of RIN-MC protein. C, Accumulation of specific transcripts in transgenic tomato (AC 35S::FM-RIN-MC) fruits at B+10. The western blots of fruits extracts from wild-type (AC) and RIN-MC-overexpressing plants in B used anti-myc antibody (top) with actin (bottom) as a control. In C, relative transcript levels were determined by RT-qPCR, relative to the expression of the tomato internal control ACTIN gene, expressed as 2−ΔΔCt (Livak and Schmittgen, 2001). Lowercase letters indicate values with significant differences. At least three biological replicates were included for each assay.
Silencing RIN-MC Accelerated Yellow Ripening in rin Mutant Tomato Fruit
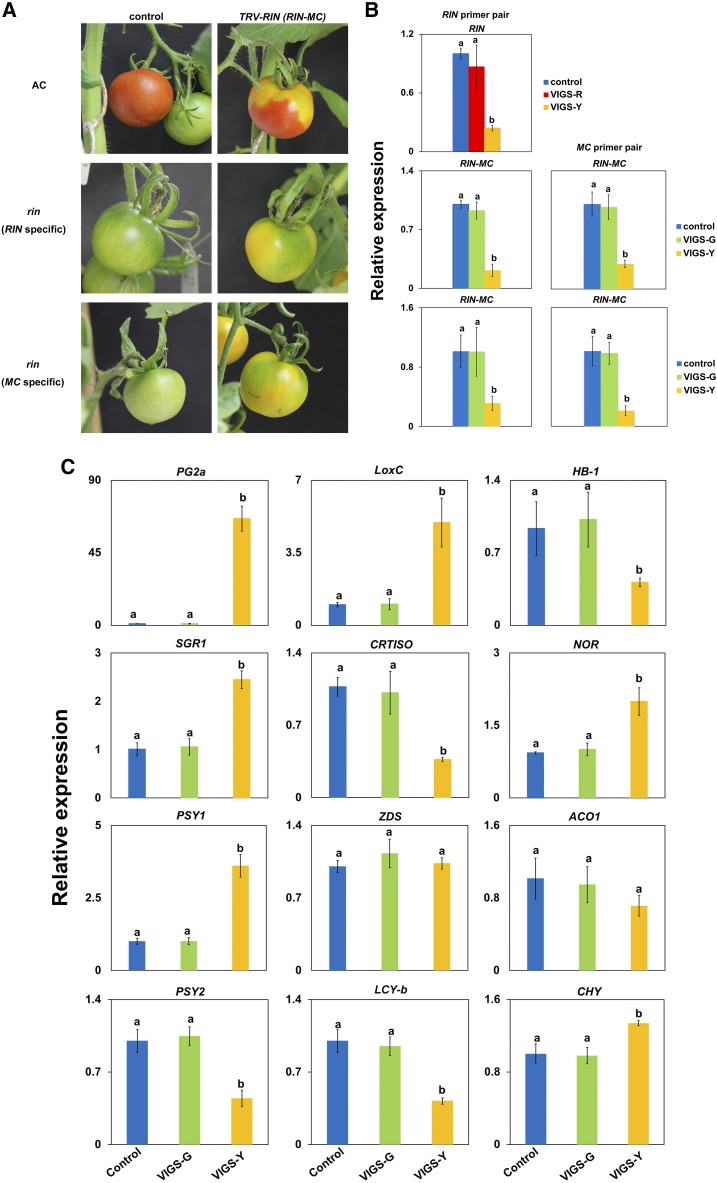
As high expression of the RIN-MC fusion gene was detected in rin mutant fruits at and after ripening onset (Fig. 1B), the question arises whether it functions in the tomato ripening process. TRV-mediated virus-induced gene silencing (VIGS) was employed as a fast and efficient method to down-regulate the high expression of RIN-MC fusion genes in rin mutant fruit, in order to address this question. Two fragments specific to different regions of the RIN-MC fusion gene were selected for VIGS plasmid vector construction and used to generate pTRV2-RIN and pTRV2-MC (Supplemental Fig. S1, B and C). The pTRV2-RIN construct was used to silence the RIN gene in the wild type (AC) as the positive control (Supplemental Fig. S1A). Approximately 10 d after infiltration of the fruit stalk with mixed Agrobacterium tumefaciens strain GV3101 containing pTRV1 and pTRV2 constructs, fruits of the infiltrated plants developed an uneven coloring, and the RIN-silenced wild-type fruit (AC; positive control) displayed a partial yellow coloring phenotype symptomatic of ripening inhibition (Ito et al., 2015). Interestingly, silencing of RIN-MC genes, whether with pTRV2-RIN or pTRV2-MC vector, resulted in significantly patchy coloring of the fruits, with areas exhibiting different sections of yellow or green (Fig. 4A). The mRNA levels of the RIN-MC fusion gene in the yellow colored areas were reduced by more than 70% (Fig. 4B) compared with those in the green sectors, and the presence of the virus in the yellow areas was confirmed (Supplemental Fig. S1), indicating that silencing of RIN-MC accelerated the rate of ripening of rin fruit from green to yellow.
Figure 4.
Effects of silencing RIN and RIN-MC on gene expression and affected ripening in wild-type (AC) and rin fruit. A, Phenotypes of silencing RIN and RIN-MC in wild-type and rin fruit (A). B and C, Gene expression assay of silenced RIN and RIN-MC genes (B) and ripening-related genes (C) analyzed using TRV-mediated VIGS in tomato fruits. The fruit stalks were injected with A. tumefaciens transformed with TRV alone or with pTRV2 carrying a fragment of the target gene (RIN in AC and RIN-MC in the rin mutant). Silencing RIN in wild-type (AC) fruit led to patchy red and yellowing, and silencing RIN-MC in the rin mutant led to uneven green and yellow coloring (A). RNA was extracted from the control and the different coloring areas of the gene-silenced tomato fruits. After reverse transcription, relative transcript levels were determined by RT-qPCR in the two parts of the fruits. Silencing of target genes led to significant decreases in gene expression (B). VIGS-R and VIGS-Y indicate the red and yellow parts of RIN-silenced fruits in the wild type, and VIGS-G and VIGS-Y indicate the green and yellow parts of RIN-MC-silenced fruits in the rin mutant (B and C). Relative transcript levels of some ripening-related genes were analyzed in RIN-MC-silenced fruits in the rin mutant (C).
Global differences in ripening-related gene changes in the yellow parts of the RIN-MC-silenced rin fruit compared with the nonsilenced parts were explored by RNA sequencing, and 1,321 differentially expressed genes (DEGs) using the threshold of fragments per kilobase of exon model per million fragments mapped (FPKM) > 10, log2 fold change > 1, and q < 0.01 (Supplemental Data Set S1) were identified. Many DEGs known to be involved in the ripening process were influenced significantly by silencing the expression of the RIN-MC fusion gene, including some involved in cell wall metabolism and ethylene and carotenoid biosynthesis (Supplemental Fig. S2). The expression of a Laccase1a (Solyc10g085090) gene, which encodes a laccase involved in flavonoid and anthocyanin metabolism (DellaPenna et al., 1989), also was up-regulated in RIN-MC-silenced rin fruit and showed the largest fold change among all of the DEGs (up to 126-fold; Supplemental Data Set S1). Genes such as GGPPS, LCYB1, and PSY1 in the MEP-carotenoid pathway (Enfissi et al., 2017) also were affected by RIN-MC silencing, suggesting that RIN-MC played a role in the regulation of carotenoid biosynthesis. The expression of LoxC, a lipoxygenase gene involved in flavor and volatile formation (Chen et al., 2004; Karlova et al., 2011), also was up-regulated in RIN-MC-silenced regions of rin fruit. In addition, some genes encoding fruit-specific transcriptional factors such as NOR and HB-1 were influenced by RIN-MC silencing, as were several genes participating in the ethylene biosynthesis and signaling pathway, such as SAM3, ETR6, and EIN3 (Supplemental Fig. S2). Changes in the expression of several important ripening genes identified by transcriptome analysis were studied by RT-qPCR assay (Fig. 4C). The results confirmed that silencing the expression of the RIN-MC fusion gene up-regulated the expression of SGR1, LoxC, PG2a, NOR, and PSY1, direct target genes of RIN, and the indirect target gene CHY, which showed coordinated regulation during tomato fruit ripening (Fujisawa et al., 2013; Zhong et al., 2013; Sakuraba et al., 2015). Silencing of RIN-MC also led to the inhibition of expression of HB-1, an HD-zip transcription factor; CRTISO, which encodes an enzyme involved in carotenoid biosynthesis; PSY2, which is required for carotenoid biosynthesis; and LCY-b, involved in the MEP-carotenoid pathway (Enfissi et al., 2017). No significant changes were found in the expression of CNR, ACO1, ETR4, and ZDS in RT-qPCR analysis, however, which was consistent with the transcriptome analysis (Supplemental Fig. S2; Supplemental Data Set S1). These results indicated that the silencing of RIN-MC affected genes in various ripening-related pathways, implying a potential direct regulatory role of RIN-MC in tomato fruit ripening.
Difference in Phenotype between RIN-MC-Silenced and rin Fruit
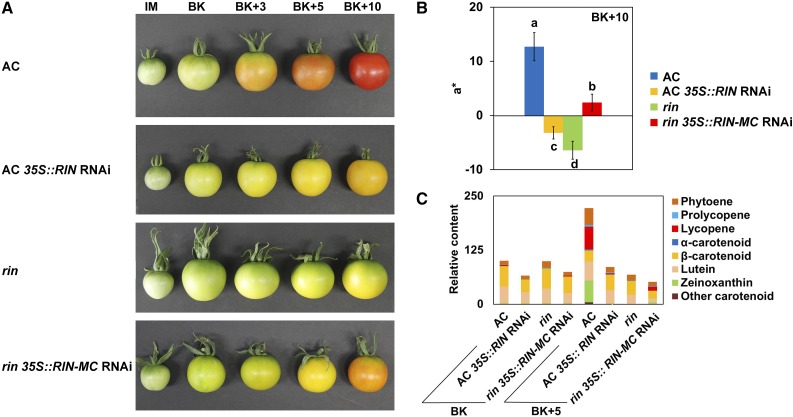
The role of the RIN-MC fusion in the rin mutant was tested directly by expressing a 35S-RIN-MC RNAi construct in transgenic rin fruit (rin 35S::RIN-MC RNAi plants). This led to a more rapid fruit color change to yellow, compared with the rin control (Fig. 5A; Supplemental Fig. S3C), and the yellow segments began to turn orange, with slight red color appearing at the BK+10 stage (Fig. 5A; Supplemental Fig. S3D). These results were very similar to those obtained by VIGS of RIN-MC (Fig. 4) and also were similar to the phenotype of AC::CRISPR-Cas9-RIN tomato fruit in a recent study (Ito et al., 2015). In our experiments, the fruit colors of AC 35S::RIN RNAi and rin plants were easily distinguishable from BK+3 to BK+10 stages, with the most obvious difference being the late generation of an orange coloration in RIN-MC RNAi plants (Fig. 5; Supplemental Fig. S3).
Figure 5.
Effects of RIN and RIN-MC on fruit color and the accumulation of carotenoids. A, Phenotypes of four different lines: the wild type (AC), the wild type expressing a RIN-silencing construct, the rin mutant, and the rin mutant expressing a RIN-MC-silencing construct. B and C, Color (a*; B) and relative contents of carotenoids (C). For A, fruits were picked at IM, BK, BK+3, BK+5, and BK+10 stages. A hand-held colorimeter with the CIE L*a*b color system was used for pericarp color assay, and a* represents red to green (Komatsu et al., 2016). At least nine replicates were included for each assay. The contents of different carotenoids in each type of fruit are shown in C. At least three biological replicates were included for each assay.
The tomato fruit pericarp colors were measured with a colorimeter using the CIE L*a*b color system (Komatsu et al., 2016). The a* value refers to the degree of red to green, determined by the degradation of chlorophyll and the accumulation of carotenoids, such as β-carotene and lycopene, producing the characteristic yellow and red coloration (Luo et al., 2013). Wild-type (AC) fruits turned red gradually, but significantly different phenotypes were manifested by the three other kinds of fruits, with transgenic AC 35S::RIN RNAi appearing orange-red and the rin mutant remaining yellow (Fig. 5; Supplemental Figs. S3 and S5A).
The color differences were confirmed by determining the concentrations of individual constituents of the carotenoid pathway in wild-type AC, AC 35S::RIN RNAi, rin, and rin 35S::RIN-MC RNAi fruits at BK and BK+5 stages. Wild-type (AC) accumulated obvious lycopene at BK+5, and a small amount of lycopene was detected in tomato fruits of transgenic rin 35S::RIN-MC RNAi, while very little was detected in fruits of transgenic AC 35S::RIN RNAi, and almost no lycopene was detected rin mutant fruits (Fig. 5C).
Differences in the expression of carotenoid biosynthesis genes were measured by RNA sequencing and visualized using the MapMan database (Usadel et al., 2009; Jaiswal and Usadel, 2016; Supplemental Fig. S4B). RIN RNAi inhibited the expression of all carotenoid biosynthesis genes tested, apart from PSY2, which is involved in carotenoid production in green fruit, prior to ripening (Fray and Grierson, 1993), and the greatest differences were found for PSY1, ZDS, CrtISO, LCY-b, and CHY. Key differences were found by comparing mRNAs in rin and RIN RNAi fruit, and these were confirmed by RT-qPCR (Supplemental Fig. S4C), which showed differences in transcripts of LYC-b and CHY. Furthermore, RIN RNAi failed to down-regulate PSY2 compared with AC fruit, whereas this was reduced in RIN-MC RNAi fruit compared with rin fruit. Although small compared with RIN-MC RNAi fruit, there were significantly higher levels of PSY1 mRNA, which is required for lycopene accumulation during ripening (Fray and Grierson, 1993).
Two other aspects of ripening, pericarp firmness and ethylene production, also were investigated in the wild type (AC), AC 35S::RIN RNAi, rin, and rin 35S::RIN-MC RNAi at different stages of ripening. Only wild-type fruits showed a burst of ethylene synthesis and underwent softening, and there were no obvious differences in the pericarp firmness or ethylene production between the fruits of the rin mutant and the transgenic rin 35S::RIN-MC RNAi plants (Supplemental Fig. S5, B and C). Silencing of RIN gene expression in wild-type fruit significantly reduced the transcripts of genes involved in ethylene biosynthesis, such as ACO1, ACS2, and ACS4, and ethylene receptors, such as ETR3 (Supplemental Fig. S5D). However, silencing of the RIN-MC fusion gene up-regulated the expression of genes involved in ethylene perception and response, such as ERF6, ETR3, and EBF1 (Supplemental Fig. S5D), indicating that the normal function of RIN is as an activator of ethylene synthesis and perception; the down-regulation of RIN-MC in the rin mutant, however, was unable to restore ethylene production.
Comprehensive Analysis of RIN-MC-Regulated Genes
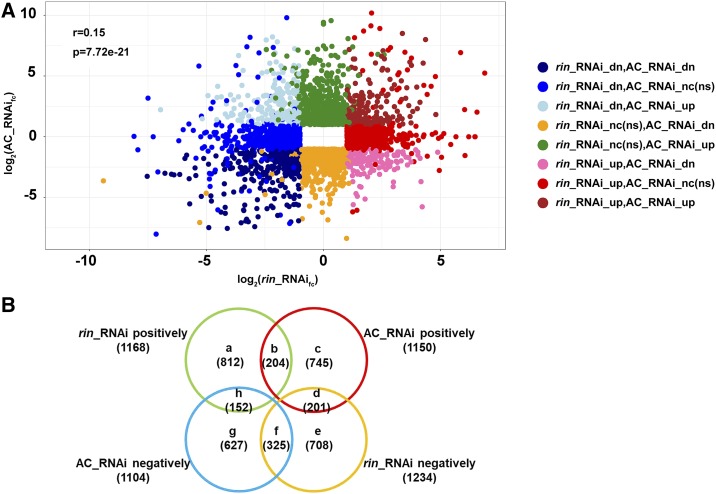
Expression of the RIN-MC fusion gene significantly affected coloring (Fig. 5; Supplemental Fig. S4) and other physiological traits (Supplemental Fig. S5) in ripening tomato fruit, and transcriptome analysis by RNA sequencing was performed in order to identify RIN-MC functions, using the threshold for DEGs of log2 fold change ≥ 1, q < 0.05, and FPKM > 10 for either sample, which discarded many genes with very low expression. Significant difference existed in comparison of wild-type (AC) with AC 35S::RIN RNAi (AC/AC 35S::RIN RNAi), comparison of rin mutant with rin 35S::RIN-MC RNAi (rin/rin 35S::RIN-MC RNAi), and comparison of AC with rin (AC/rin; Supplemental Fig. S6). Analysis of rin/rin 35S::RIN-MC RNAi identified 1,168 positively and 1,234 negatively DEGs (summarized in Fig. 6B; Supplemental Data Set S3). Most DEGs regulated by RIN-MC showed low identity (r = 0.15, P = 7.72e-21; Fig. 6A) compared with those regulated by RIN (AC/AC 35S::RIN RNAi; Fig. 6A), and genes in each subset in the Venn diagram (Fig. 6B) also are listed in Supplemental Data Sets S4 to S11.
Figure 6.
Comparison between DEGs in AC/AC 35S::RIN RNAi and rin/rin 35S::RIN-MC RNAi. Scatterplot (A) and Venn diagram (B) of DEGs show comparisons between AC/AC 35S::RIN RNAi and rin/rin 35S::RIN-MC RNAi. AC_RNAi, AC/AC 35S::RIN RNAi; rin_RNAi, rin/rin 35S::RIN-MC RNAi; dn, down-regulated; fc, fold change; nc, no change; ns, no significant difference; up, up-regulated. Letters a to h are different regions in the Venn diagram. DEGs were selected with a threshold of log2 fold change ≥ 1, q < 0.05, and FPKM > 10 in each sample. RNA sequencing and DEG assay were for four genotypes of tomato fruits at the BK+5 stage, as mentioned in Supplemental Figure S4A and Figure 5. Genes in different regions in the Venn diagram are listed in Supplemental Data Sets S4 to S11.
Genes regulated by RIN-MC participate in various aspects of ripening, including Ethylene Receptor3 (ETR3/NR); cell wall metabolism, such as PG2a, polygalacturonase (PGcat), Pectinesterase1 (PME1.9), and PME2.1; carotenoid formation, such as PSY1, PSY2, and geranylgeranyl pyrophosphate synthase (GGPPS2); and transcription factors, such as TDR4 (Tables I and III; Supplemental Data Set S3). DEGs were categorized by Gene Ontology (GO) enrichment, and in a comparison between rin mutant and transgenic rin 35S::RIN-MC RNAi (rin/rin 35S::RIN-MC RNAi) (Supplementary Data Set 12), negatively regulated DEGs were enriched in three categories and positively regulated DEGs were enriched in only one category (biological process; Supplemental Fig. S7A). Groups such as thylakoid, response to abiotic stimulus, and signal transduction were highly enriched (Supplemental Fig. S7A). Many DEGs involved in ripening were negatively regulated by RIN-MC, indicating that the RIN-MC fusion gene might play an inhibitory role, which was consistent with the results of both VIGS (Fig. 4) and its inhibition of red coloring (Fig. 5) in tomato fruit ripening. In contrast, DEGs positively regulated by RIN were associated with known ripening pathways (Supplementary Data Set 13), consistent with the widely recognized role of RIN as a ripening activator. DEGs regulated by RIN-MC were highly enriched in metabolic pathway and biosynthesis of secondary metabolites by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses, and DEGs regulated by RIN were highly enriched in biosynthesis of secondary metabolites (Supplemental Fig. S7B; Supplementary Data Set 14 and 15). These results indicate significant regulatory differences between RIN-MC and MADS-RIN, although their MADS-box domains are homologous.
Table I. Comparison of RIN and RIN-MC regulation in the expression of ripening genes.
Ripening-related genes were regulated by both RIN and RIN-MC, with the fold change given for each gene in AC/AC 35S::RIN RNAi (AC_RNAi) and in rin/rin 35S::RIN-MC RNAi (rin_RNAi).
| Function | Description | Identifier | Gene | Differentially Expressed |
ChIP_seqa | ChIP_chipb | |
|---|---|---|---|---|---|---|---|
| AC_RNAi | rin_RNAi | ||||||
| Transcriptional factors | AP2-EREBP | Solyc03g044300 | Solyc03g044300 | 1.53 | 0.29 | Yes | Yes |
| AP2-EREBP | Solyc09g075420 | ERF2 | 6.84 | 0.27 | Yes | No | |
| GRAS | Solyc06g036170 | GRAS9 | 1.79 | 0.57 | Yes | Yes | |
| MYB | Solyc06g076770 | Solyc06g076770 | 2.59 | 0.21 | No | Yes | |
| MADS | Solyc06g069430 | TDR4 | 3.39 | 4.80 | Yes | No | |
| GRAS | Solyc02g085600 | Solyc02g085600 | 0.28 | 0.45 | Yes | Yes | |
| C2H2 | Solyc04g077980 | Solyc04g077980 | 2.31 | 3.40 | Yes | No | |
| bZIP | Solyc08g006110 | Opaque 2 | 0.19 | 0.37 | No | No | |
| HSF | Solyc12g007070 | Solyc12g007070 | 0.53 | 0.32 | Yes | No | |
| MADS | Solyc05g012020 | RIN (RIN-MC) | 2.84 | 3.51 | Yes | Yes | |
| NAC | Solyc10g006880 | NAC-NOR | 0.49 | 0.52 | Yes | Yes | |
| Auxin-responsive protein | Solyc06g053840 | Solyc06g053840 | 2.47 | 2.92 | No | Yes | |
| Histone-Lys N-methyltransferase NSD3 | Solyc01g103250 | Solyc01g103250 | 0.12 | 1.83 | Yes | No | |
| Ethylene | Synthesis | Solyc01g095080 | ACS2 | 2.51 | 0.48 | Yes | Yes |
| Synthesis | Solyc07g026650 | ACO5 | 99.11 | 5.59 | No | No | |
| Receptors | Solyc09g075440 | ETR3/NR | 2.16 | 0.43 | Yes | No | |
| Receptors | Solyc09g089610 | ETR6 | 3.90 | 0.25 | No | No | |
| Response | Solyc01g009170 | EIN3 | 0.19 | 0.51 | Yes | No | |
| Response | Solyc12g009560 | EBF1 | 1.85 | 0.28 | Yes | No | |
| MEP-carotenoid pathway | Phytoene synthases | Solyc03g031860 | PSY1 | 6.87 | 0.32 | Yes | No |
| 9,15,9′-tri-cis-ζ-Carotene isomerase | Solyc12g098710 | ZISO | 2.65 | 0.19 | Yes | Yes | |
| Nonheme hydroxylases | Solyc03g007960 | CHY | 2.47 | 0.17 | Yes | No | |
| Geranylgeranyl pyrophosphate synthases | Solyc04g079960 | GGPPS2 | 0.66 | 0.25 | No | No | |
| Lycopene β-cyclases | Solyc04g040190 | LCY-b | 2.18 | 3.33 | No | Yes | |
| Flavonoid/anthocyanin | 4-Coumarate-CoA ligase | Solyc03g117870 | 4CL | 1.96 | 0.38 | Yes | Yes |
| Phe ammonia lyase | Solyc05g056170 | PAL | 0.18 | 0.48 | Yes | No | |
| Cell wall | Endo-1,4-β-glucanase | Solyc01g102580 | Cel3 | 1.83 | 0.30 | Yes | Yes |
| Endo-1,4-β-glucanase | Solyc05g005080 | Cel6 | 0.45 | 2.28 | No | No | |
| Expansin-like protein precursor (EXLA1) | Solyc01g112000 | LeEXLA1 | 3.93 | 0.10 | Yes | No | |
| LeXYL1 | Solyc10g047030 | LeXYL1 | 3.11 | 0.23 | Yes | No | |
| Xyloglucan endotransglucosylase hydrolase XTH3 | Solyc03g093120 | SlXTH3 | 4.04 | 0.10 | No | No | |
| Extensin (class II) | Solyc11g005150 | tegII | 2.80 | 0.03 | Yes | No | |
| Arabinosidase ARA-1 | Solyc10g081120 | ARA-1 | 0.20 | 1.64 | No | No | |
| Expansin precursor (EXPA5) | Solyc02g088100 | EXPA5 | 0.16 | 0.48 | No | No | |
| Fruit ripening-regulated expansin | Solyc06g051800 | LeEXP1 | 8.39 | 10.13 | Yes | Yes | |
| β-Fructofuranosidase, cell wall invertase | Solyc09g010080 | lin5 | 0.18 | 0.14 | No | No | |
| Dehiscence polygalacturonase | Solyc04g015530 | PS-2 | 0.01 | 0.38 | No | No | |
| Arabinogalactan | Solyc02g092790 | AGP-1c | 0.52 | 0.05 | Yes | Yes | |
| Expansin (EXPA6) | Solyc10g086520 | EXPA6 | 0.34 | 0.04 | Yes | No | |
| Polygalacturonase | Solyc08g060970 | PGcat | 7.51 | 6.49 | No | No | |
| Pectinesterase2.1 | Solyc07g064180 | PME2.1 | 0.43 | 0.10 | No | No | |
| Endoxyloglucan transferase | Solyc04g008210 | SlXTH8 | 0.35 | 0.13 | No | No | |
| β-Galactosidase precursor | Solyc12g044880 | TBG1 | 2.03 | 2.19 | No | No | |
| β-Galactosidase | Solyc03g019890 | TBG7 | 9.89 | 3.18 | No | No | |
Chromatin immunoprecipitation sequencing data from Zhong et al. (2013). bChIP-chip data from Fujisawa et al. (2012).
Table III. Comparison of RIN and RIN-MC regulation in the expression of ripening genes.
Ripening-related genes were regulated by RIN but not RIN-MC, with the fold change for each gene in AC/AC 35S::RIN RNAi. AC_RNAi, AC/AC 35S::RIN RNAi; rin_RNAi, rin/rin 35S::RIN-MC RNAi.
| Function | Description | Identifier | Gene | Differentially Expressed |
ChIP_seqa | ChIP_chipb | |
|---|---|---|---|---|---|---|---|
| AC_RNAi | rin_RNAi | ||||||
| Transcriptional factors | bZIP | Solyc01g100460 | abz1 | 2.67 | No | Yes | Yes |
| C2C2-CO-like | Solyc12g096500 | Solyc12g096500 | 0.16 | No | Yes | Yes | |
| SBP | Solyc02g077920 | LeSPL-CNR | 1.66 | No | Yes | Yes | |
| ULT | Solyc07g054450 | Solyc07g054450 | 1.52 | No | Yes | Yes | |
| WRKY | Solyc02g021680 | Solyc02g021680 | 4.04 | No | Yes | No | |
| GRAS | Solyc01g008910 | Solyc01g008910 | 2.75 | No | Yes | Yes | |
| GRAS | Solyc11g012510 | GRAS1 | 0.27 | No | Yes | No | |
| Transcriptional regulator | Auxin-responsive protein | Solyc03g120390 | Solyc03g120390 | 0.29 | No | Yes | Yes |
| Transcription elongation factor A protein2 | Solyc10g080930 | Solyc10g080930 | 2.19 | No | No | No | |
| DNA-repair protein | Solyc06g050510 | Solyc06g050510 | 0.54 | No | No | No | |
| Ethylene | Synthesis | Solyc07g049550 | ACO3 | 16.02 | No | Yes | No |
| Synthesis | Solyc05g050010 | ACS4 | 4.93 | No | Yes | Yes | |
| Synthesis | Solyc07g049530 | ACO1 | 4.70 | No | Yes | No | |
| Receptors | Solyc06g053710 | ETR4 | 2.54 | No | Yes | No | |
| Response | Solyc06g073730 | EIL4 | 2.22 | No | No | No | |
| Response | Solyc08g060810 | EBF2 | 0.50 | No | Yes | Yes | |
| Response | Solyc03g111720 | E4 | 3.39 | No | No | No | |
| MEP-carotenoid pathway | Isopentenyl diphosphate isomerases | Solyc05g055760 | IPP2 | 0.22 | No | No | No |
| ζ-Carotene desaturase | Solyc01g097810 | ZDS | 2.57 | No | No | No | |
| 7,9,7′,9′-tetra-cis-Lycopene isomerase | Solyc10g081650 | CrtISO | 3.15 | No | Yes | Yes | |
| Alternative oxidase | Solyc11g011990 | PTOX | 2.55 | No | Yes | No | |
| P450 hydroxylases | Solyc04g051190 | CYP97A3 | 0.44 | No | No | No | |
| Cell wall | Endo-1,4-β-glucanase | Solyc09g010210 | Cel2 | 270.30 | No | Yes | Yes |
ChIP-seq data from Zhong et al. (2013). bChIP-chip data from Fujisawa et al. (2012).
RIN-MC Protein Has Transcription Factor Activity
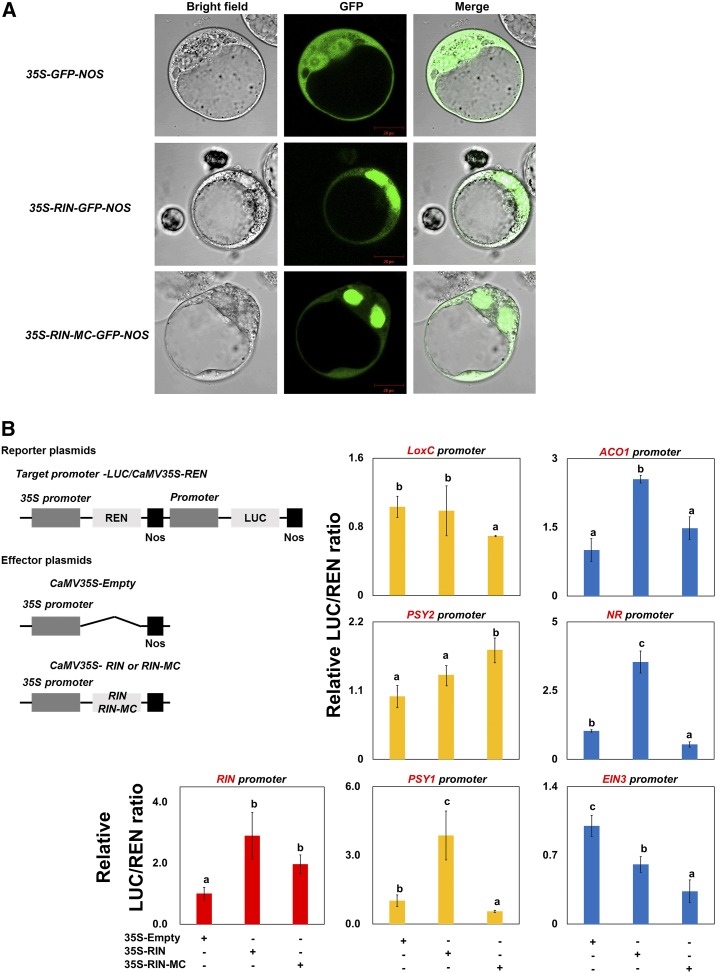
The predicted domain structures of the RIN-MC protein (Fig. 2A) are likely to be sufficiently similar to the regular structure of MADS transcription factors to be able to participate in target promoter binding and possibly protein dimerization (Kaufmann et al., 2005). Accordingly, the subcellular localization and trans-activation activities of RIN-MC protein were analyzed in vivo, and RIN-MC fused to GFP protein was shown to be localized in nuclei, as found for RIN (Fig. 7A).
Figure 7.
Transcription factor activity of RIN-MC protein. Subcellular localization (A) and trans-activation assay (B) of RIN-MC for RIN-MC protein are shown. In the subcellular localization assay (A), BY2 cell protoplasts were transiently transformed with RIN-GFP or RIN-MC-GFP constructs or GFP vector using the polyethylene glycol method. GFP fluorescence was observed with a fluorescence microscope. Trans-activation activities of tomato RIN and RIN-MC (B) were analyzed in the N. benthamiana transient expression system, using the double-reporter plasmid containing the promoters of ripening genes fused to LUC luciferase and REN luciferase driven by CaMV 35S. The effectors were RIN or RIN-MC driven by CaMV 35S. The effector and reporter plasmids were transfected into A. tumefaciens separately and coinfected into N. benthamiana leaves. The luciferase was measured 2 to 5 d after infiltration. At least nine biological replicates were included for each assay. The lowercase indicates significant difference.
The trans-activation capability of the RIN-MC chimeric protein was tested by transient dual luciferase (LUC) reporter assays in N. benthamiana leaves. Promoters of potential target genes were fused to the LUC reporter separately, with a Renilla (REN) reporter under the control of the 35S promoter as an internal control, and tested against either the RIN or RIN-MC protein. The RIN-MC protein activated the LUC reporter gene when driven by RIN promoter segments of 2, 1.5, 1, and 0.75 kb compared with the empty vector negative control (Supplemental Fig. S8), but there was no activity with a 0.5-kb RIN promoter fragment, as also found for the RIN protein, indicating a requirement for sufficient length of promoter to contain important regulatory motifs (Fujisawa et al., 2013). RIN-MC activated the expression of PSY2, which is required for carotenoid biosynthesis; genes such as LoxC, PSY1, NR, and EIN3 were repressed by RIN-MC, whereas it had no significant trans-activation on the ACO1 promoter (Fig. 7B). These responses were different from those observed with RIN, particularly for target genes such as LoxC, ACO1, PSY2, NR (ETR3), and PSY1. Both RIN-MC and RIN, on the other hand, inhibited expression from the EIN3 promoter (Fig. 7B).
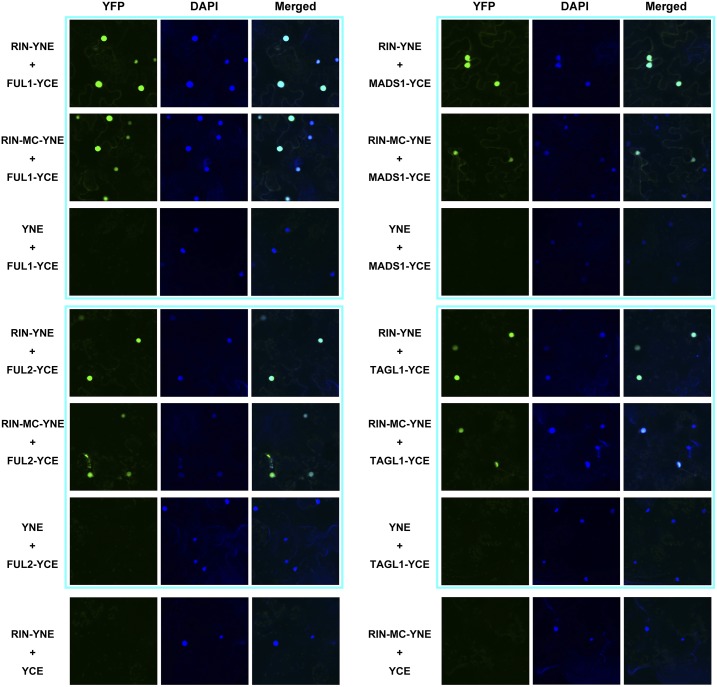
MADS-box transcription factors usually form protein complexes in vivo, and the predicted RIN-MC protein contains the complete MADS-box domain from RIN, which could interact physically with other important ripening regulators such as FUL1 and FUL2 (Shima et al., 2013; Fujisawa et al., 2014), MADS1 (Dong et al., 2013), and TAGL1 (Itkin et al., 2009; Fujisawa et al., 2014). Accordingly, these four MADS-box factors were selected as potential targets to analyze protein-protein interactions with the RIN-MC fusion protein. Initially, the interactions between RIN-MC and FUL1, FUL2, MADS1, and TAGL1 were performed by colocalization assays using bimolecular fluorescence complementation (BiFC) technology. The RIN and RIN-MC genes were constructed with an N-terminal YFP coding sequence to generate a chimeric protein, and the four MADS-box genes were constructed with C-terminal YFP sequences. RIN-MC interacted with FUL1, FUL2, MADS1, and TAGL1 (Fig. 8), and the BiFC assays also showed that RIN colocalized and interacted with these MADS-box factors in cell nuclei. These results were very similar to those demonstrated for RIN in previous studies (Martel et al., 2011; Dong et al., 2013; Shima et al., 2013; Fujisawa et al., 2014).
Figure 8.
Interactions between RIN and RIN-MC with MADS-box factors measured by BiFC assay. RIN or RIN-MC was fused with the N-terminal end of YFP (YNE), and FUL1, FUL2, TAGL1, and MADS1 were fused individually with the C-terminal end of YFP (YCE). The expression of RIN/RIN-MC or FUL1/FUL2/TAGL1/MADS1 alone was used as a negative control. 4′,6-Diamino-phenylindole (DAPI) staining was included as a control for nuclear localization.
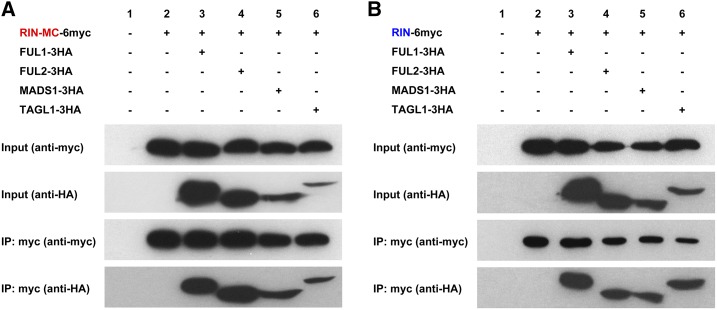
To confirm the interaction between RIN-MC and FUL1, FUL2, MADS1, and TAGL1 observed in the BiFC assay, coimmunoprecipitation (CoIP) assays were performed. Full-length RIN or RIN-MC (with a deleted stop codon) plus a C-terminal 6myc tag (RIN-6myc and RIN-MC-6myc) and MADS-box factors (FUL1-3HA, FUL6HA, MADS1-3HA, and TAGL1-3HA) with C-terminal 3HA tags were coexpressed in N. benthamiana leaves. The results showed that FUL1-3HA, FUL2-3HA, MADS1-3HA, and TAGL1-3HA coimmunoprecipitated with RIN-MC-6myc (Fig. 9A). Similar results also were observed when these MADS-box factors with 3HA tags also were coimmunoprecipitated with RIN-6myc (Fig. 9B).
Figure 9.
Interactions between RIN-MC and RIN and MADS-box factors measured by CoIP. Total protein extracts (Input) and protein complexes immunoprecipitated with anti-myc agarose (IP) were separated on gels and blotted. RIN-MC and MADS-box factors interacted (A), similar to RIN (B) interactions measured by CoIP. Anti-HA and anti-myc antibodies were used in western blotting.
DISCUSSION
In this study, we investigated the possible functions of the RIN-MC fusion gene in fruit of the rin tomato mutant. Our results showed that the RIN-MC fusion protein accumulated to a high level in rin fruit. Overexpression of RIN-MC in wild-type fruit impaired several ripening changes, and silencing of RIN-MC expression led to these fruit turning weakly red. GFP localization, BiFC, and CoIP assays suggested that RIN-MC could associate physically with FUL1, FUL2, MADS2, and TAGL1 and form potential heteroprotein complexes in planta, and trans-activation studies indicated that RIN-MC had transcription factor activity.
Gene Fusions Can Play an Important Role in Developmental Biology
Important biological effects of gene fusion can result from chromosomal rearrangements, DNA shuffling, abnormal transcription, and alternative splicing, leading to the formation of chimeric proteins that can have significance in human cancer (Mitelman et al., 2007; Annala et al., 2013). The altered chimeric protein can constitutively activate downstream target genes or destroy a critical cellular function (Annala et al., 2013), such as the chimeric BCR-ABL1 in leukemia (McWhirter et al., 1993), FGFR3-TACC3 fusions in glioblastoma (Singh et al., 2012), and the ALK fusions in anaplastic large cell lymphoma (Chiarle et al., 2008). The reports of gene fusion relevant to plant metabolism are limited, but at least two gene fusion events have been confirmed as relevant to benzylisoquinoline alkaloid metabolism (Li et al., 2016; Hagel and Facchini, 2017). This disparity probably reflects a knowledge gap concerning plant gene fusions rather than a reduced occurrence of functional importance compared with studies related to human disease. It also has been reported that a considerable number of fusion mRNAs might produce chimeric proteins in Arabidopsis and rice (Shahmuradov et al., 2010). Moreover, regardless of the specific mechanism involved in gene fusion, the final multidomain enzymes, particularly those sharing domain-level homology with different enzyme types, might function in metabolic pathways (Boycheva et al., 2014; Nützmann and Osbourn, 2014), as in the enormous diversity of terpene synthases found in plants (Zi et al., 2014).
However, in our experiments, we investigated the potential role of the fusion of adjacent transcription factor genes resulting from a genomic DNA fragment deletion (Vrebalov et al., 2002) and showed that the fusion protein was expressed abundantly in ripening fruit (Fig. 2), accumulated in the nucleus (Fig. 7), interacted with other transcription factors (Figs. 8 and 9), and altered the transcriptome and ripening phenotype (Fig. 6; Supplemental Fig. S3), which would help promote studies related to gene fusion in plants.
RIN-MC Transcripts and Protein Are Present in Abundance in rin Fruit
The RIN transcription factor is widely recognized as one of the hub regulators controlling ripening, and the rin mutation results in a severely ripening-inhibited phenotype (Vrebalov et al., 2002). At the transcriptional level, the expression of numerous genes involved in almost all branches of the ripening-related pathways is affected (Qin et al., 2012; Fujisawa et al., 2013, 2014). Our understanding of the role of RIN has been based on the characterization of rin mutant fruit, in which the fruit-specific factor RIN is impaired by the formation of the RIN-MC fusion, whereas the normal MC was believed not to be a fruit regulator (Vrebalov et al., 2002).
In most previous studies, the expression of RIN-MC was found to be low (Vrebalov et al., 2002; Martel et al., 2011; Fujisawa et al., 2012, 2013), but recently, it was shown, based on RNA sequencing technology, that rin mutant fruit accumulate high levels of RIN-MC transcripts at 42 DPA and at the pink ripening stage (Zhong et al., 2013; Fujisawa et al., 2014). In previous studies, the specific RIN-MC fusion gene fragments detected mapped mainly to part of the 3′ region of the normal RIN gene, which is absent from the rin mutant, explaining why almost no transcript signals were detected. For example, probes used for northern blotting were homologous to the 3′ untranslated region of RIN (Vrebalov et al., 2002), probe sets selected for microarrays mapped mostly to the missing region of the RIN gene (Fujisawa et al., 2012), and primer pairs designed for RT-qPCR amplification were targeted to fragments that do not exist in their entirety in the RIN-MC fusion gene (Martel et al., 2011; Fujisawa et al., 2013). Recently, the high expression level of RIN-MC was determined from transcriptome data derived by high-throughput sequencing technology (Zhong et al., 2013; Fujisawa et al., 2014), and this was confirmed at both the RNA and protein levels in this study (Figs. 1 and 2). A polyclonal antibody against RIN partial protein also was used to detect RIN-MC protein successfully in the rin mutant in this study, unlike previous reports (Ito et al., 2008; Martel et al., 2011; Qin et al., 2012). The MC-specific antibody is much more suitable for distinguishing the RIN-MC fusion protein in rin fruit, because minor signals from translational products of the MC gene exist in ripening fruits of wild-type AC (Vrebalov et al., 2002).
The Role of RIN-MC in Gene Expression in rin Mutant Fruit
The RIN transcription factor is a central regulator in tomato fruit ripening (Vrebalov et al., 2002), and RIN is expressed in a fruit ripening-specific pattern, which has almost no abundance of transcripts in other tissues such as bud, leaf, root, and immature green (IG) fruits (Tomato Genome Consortium, 2012). As a result, the regulation of RIN and RIN-MC in tomato fruit ripening was much more focused. Genes regulated by RIN-MC participate in various aspects of ripening, including ETR3/NR; cell wall metabolism, such as PG2a, PGcat, PME1.9, and PME2.1; carotenoid formation, such as PSY1, PSY2, and GGPPS2; and transcription factors, such as TDR4 (Tables I and III; Supplemental Data Set S3). Moreover, nuclear localization and interaction studies with other transcription factors showed that RIN-MC protein had the expected characteristics of a transcription factor. Although RIN-MC protein could, like RIN, form MADS-box complexes with FUL1, FUL2, MADS1, and TAGL1 (Figs. 8 and 9), the regulatory effects on target genes were not so similar to those found for RIN (Tables I and III; Supplemental Data Set S2). For genes such as TDR4 and LCY-b, RIN-MC silencing reduced their expression, similar to the effect induced by RIN silencing (Table I). The comparison of AC/AC 35S::RIN RNAi and rin/rin 35S::RIN-MC RNAi, however, (Table I), showed opposite trends for genes such as ERF2, GRAS9, ACS2, NR, EBF1, PSY1, ZISO, 4CL, and Cel3. More interestingly, the expression of genes such as ERF2b, ARF8, LoxC, LeMAN4a, PG2a, and TBG4 was not affected by RIN silencing; these genes were expressed significantly in comparison with rin/rin 35S::RIN-MC RNAi (Table II).
Table II. Comparison of RIN and RIN-MC regulation in the expression of ripening genes.
Ripening related genes regulated by RIN-MC but not RIN, with the fold change for each gene in rin/rin 35S::RIN-MC RNAi. AC_RNAi, AC/AC 35S::RIN RNAi; rin_RNAi, rin/rin 35S::RIN-MC RNAi; ChIP-seq data are from Zhong et al., 2013; the ChIP-chip data from Fujisawa et al., 2012.
| Function | Description | Identifier | Gene | Differentially Expressed |
ChIP_seqa | ChIP_chipb | |
|---|---|---|---|---|---|---|---|
| AC_RNAi | rin_RNAi | ||||||
| Transcriptional factors | AP2-EREBP | Solyc01g065980 | ERF2b | No | 0.46 | Yes | No |
| AP2-EREBP | Solyc03g093560 | ERF5 | No | 1.82 | No | No | |
| AP2-EREBP | Solyc03g093540 | ERF1a | No | 3.55 | No | No | |
| AP2-EREBP | Solyc12g009240 | ERF4 | No | 7.83 | No | No | |
| AP2-EREBP | Solyc02g077370 | Pti5 | No | 3.19 | No | No | |
| ARF | Solyc02g037530 | ARF8 | No | 2.68 | Yes | No | |
| bZIP | Solyc01g079480 | bZIP-1 | No | 0.41 | No | No | |
| bZIP | Solyc04g011670 | Solyc04g011670 | No | 3.08 | No | No | |
| C2C2-CO-like | Solyc08g006530 | Solyc08g006530 | No | 6.53 | No | No | |
| C2H2 | Solyc08g063040 | Solyc08g063040 | No | 0.31 | Yes | Yes | |
| CCAAT | Solyc01g006930 | Solyc01g006930 | No | 2.51 | No | No | |
| GRAS | Solyc07g052960 | Solyc07g052960 | No | 0.24 | Yes | Yes | |
| HB | Solyc05g006980 | Solyc05g006980 | No | 2.10 | No | No | |
| HB | Solyc02g063520 | Solyc02g063520 | No | 4.41 | Yes | No | |
| LIM | Solyc04g077780 | Solyc04g077780 | No | 3.87 | No | No | |
| MYB | Solyc03g113620 | Solyc03g113620 | No | 1.90 | No | No | |
| WRKY | Solyc09g014990 | Solyc09g014990 | No | 4.66 | No | No | |
| GRAS | Solyc11g013150 | Solyc11g013150 | No | 2.94 | No | No | |
| Transcriptional regulator | Auxin-responsive protein | Solyc03g120500 | Solyc03g120500 | No | 1.93 | Yes | Yes |
| MEP-carotenoid pathway | Phytoene synthases | Solyc02g081330 | PSY2 | No | 2.99 | Yes | No |
| Zeaxanthin epoxidase | Solyc02g090890 | ZEP | No | 3.44 | Yes | No | |
| Flavonoid/anthocyanin | 4-Coumarate-CoA ligase | Solyc11g069050 | 4CL | No | 2.92 | Yes | No |
| Lipoxygenase | Solyc01g006540 | LoxC | No | 0.20 | Yes | Yes | |
| Cell wall | Endo-1,4-β-mannanase | Solyc01g008710 | LeMAN4a | No | 0.03 | Yes | No |
| LeXYL2 | Solyc01g104950 | LEXYL2 | No | 0.26 | Yes | No | |
| Polygalacturonase2a | Solyc10g080210 | PG2a | No | 0.01 | Yes | No | |
| Pectinesterase1 | Solyc07g064170 | PME1.9 | No | 0.05 | No | No | |
| β-Galactosidase | Solyc12g008840 | TBG4 | No | 0.39 | Yes | No | |
| S-Galactosidase | Solyc03g121540 | teg1A/TBG3 | No | 2.81 | Yes | No | |
ChIP-seq data from Zhong et al. (2013). bChIP-chip data from Fujisawa et al. (2012).
Genes regulated by RIN-MC participated not only in fruit-ripening processes but also in many other aspects, such as ascorbate biosynthesis and recycling, cell wall structure, resistance to stress, protein modification, and some kinase, which indicated that RIN-MC regulation spreads in various important biological aspects (for details, see Supplemental Data Sets S2 and S3). The amounts of genes regulated by RIN-MC had different regulation patterns by RIN in ascorbate biosynthesis and recycling. Genes such as dehydroascorbate reductase and GDP-l-Gal phosphorylase (GGP2), which were key regulators of fruit ascorbic acid concentrations (Mellidou et al., 2012), were significantly regulated by RIN-MC but not RIN. The cytochrome P450 monooxygenase (P450) superfamily is involved in the biosynthesis of various primary and secondary metabolites (Yu et al., 2017). Genes encoding cytochrome P450, such as CYP71D208 and CYP90B3, were obviously up-regulated by RIN-MC but could not be induced by RIN. Genes regulated by RIN-MC but not by RIN also were involved in resistance to stress and ubiquitination, such as multiantimicrobial extrusion protein (Solyc05g013460), hairpin-induced protein (Solyc03g121620), ubiquitin-like (Solyc05g056060), and ubiquitin-conjugating enzyme (Solyc10g012240). Meanwhile, many DEGs showed different regulation patterns by RIN and RIN-MC, such as glycoside hydrolase (Solyc01g067660), UDP-glucosyltransferase (Solyc02g070020), lipoxygenase (Solyc01g006540), and lipase (Solyc03g005020), involved in carbohydrate and lipid metabolism. All these genes were targets of RIN, except GGP2 and CYP71D208. Comparison between RIN and RIN-MC regulation in fruit ripening and other processes offered valuable clues for further identification of new functions of RIN-MC in future studies.
Furthermore, differences in trans-activating activities were distinguished between RIN and RIN-MC. Taking the results for LoxC and PSY2 as examples, RIN had no significant trans-activating activity, but RIN-MC regulated their expression (Fig. 7), which indicated that RIN-MC has its own independent transcriptional activity in planta. For RIN target genes, such as CNR, ACO3, and ACS4, expression was influenced by RIN silencing but not by RIN-MC silencing, which also indicates different mechanisms of action of RIN-MC protein and RIN on the same target genes (Table III). Meanwhile, RIN-MC could activate the LUC reporter gene when driven by the RIN promoter (Fig. 7; Supplemental Fig. S8), which is consistent with the up-regulation of RIN-MC expression in tomato fruit (Fig. 1B), indicating a similar positive regulatory feedback to RIN. These findings are in disagreement with the conclusion from a previous study, in which the RIN-MC fusion gene expressed in yeast cells was transcriptionally inactive (Ito et al., 2008). This discrepancy may be related to differences in transcriptional activation by RIN-MC protein in tobacco (Nicotiana tabacum) leaves and yeast cells, differences in regulating target genes, or the effects of differences in the ability to form protein complexes between the RIN-MC protein and other proteins recruited in vivo in yeast and tobacco. Bioinformatics analysis showed that an ERF-associated amphiphilic repression motif (LDLNL; Ohta et al., 2001) is present in the C terminus of the RIN-MC protein (Supplemental Fig. S9), which might explain the repression effect of RIN-MC on some target genes (Fig. 7). These results show that the RIN-MC fusion protein has a separate and distinct function from the normal and mutated RIN. Silencing RIN-MC in the rin mutant (rin 35S::RIN-MC RNAi fruits), where the mRNA was reduced to 4.2% (Supplemental Fig. S4A), could not restore the normal ripening process and only resulted in weak red coloration without an ethylene burst (Fig. 5; Supplemental Figs. S3–S5). Overexpressing the RIN protein in rin::CRISPR/Cas9-RIN-MC fruit would be expected to restore normal fruit ripening.
In conclusion, the possibility that RIN-MC plays a role in controlling the expression of ripening genes in the rin mutant has not received much credence previously because the evidence indicated that its transcripts were absent from the mutant fruit (Martel et al., 2011; Fujisawa et al., 2012, 2013). We have shown that rin fruit in fact contain abundant RIN-MC mRNA, which is translated. The encoded protein is located in the nucleus, interacts with other MADS-box transcription factors, and is capable of altering ripening gene expression when overexpressed in wild-type fruit. Further work will be necessary to identify all the gene targets of RIN-MC and to determine the transcriptional interactions between RIN-MC, other transcription factors, and target gene promoters.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type tomato (Solanum lycopersicum AC) and rin mutant (AC background) seedlings were grown in a greenhouse under long-day conditions (16 h of light and 8 h of dark) at a temperature of 26°C. For gene expression analysis, organs were collected, frozen in liquid nitrogen, and stored at −80°C until RNA extraction. Three independent samplings were performed.
Primers
All the primers designed and used in this study are listed in Supplemental Tables S1 and S2.
Tomato Genetic Transformation
The recombined pCAMBIA1300-35S-FM-RIN-MC vector was selected for protein overexpression in transformed tomato. A hairpin-inducing vector harboring two gene-specific fragments (targeting either RIN or RIN-MC) was chosen for gene silencing based on an RNAi strategy. The Agrobacterium tumefaciens-mediated transfer of T-DNA was used for stable transformation of tomato (Sun et al., 2006; Kimura and Sinha, 2008). At least three lines were obtained for each assay.
RNA Isolation and RT-qPCR
RNA was isolated from pericarp of tomato fruits at different ripening stages as described (Zhu et al., 2015). Total RNA extraction from tomato fruit pericarp was carried out using DeTRNa reagent (EarthOx) according to the manufacturer’s protocol, and RNA integrity was verified by 1.5% (v/v) agar gel electrophoresis. Genomic DNA was removed from RNA preparations by digestion with DNase I (TaKaRa), and RNA quality and quantity were confirmed by spectrophotometry (Thermo Scientific; NanoDrop 1000). RNA was reverse transcribed into cDNA using M-MLV Reverse Transcriptase (Promega) according to the manufacturer’s instructions. RT-qPCR was conducted using TransStart Top Green qPCR SuperMix (Transgen) with the CFX96 Real-Time PCR System (Bio-Rad). Relative gene expression values were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001). The tomato ACTIN gene (Solyc03g078400) was used as an internal reference gene. At least three biological replicates were included for each point, and each replicate was from independent sampling.
Ethylene and 1-MCP Treatment
Tomato fruits at the MG stage were placed into an air-tight 1-L plastic container with 100 μL ethylene L−1 air or 10 µL L−1 1-MCP generated by dissolving 48 mg of 1-MCP-releasing powder in 50 μL of water (Fujisawa et al., 2013). The treatment was conducted for 24 h in an incubator under 16 h of light and 8 h of dark at 25°C. After the treatment, the fruits were sliced, and seeds were removed, frozen in liquid nitrogen, and used for RNA isolation followed by RT-qPCR. At least three biological replicates were included for each treatment, and each replicate was from independent sampling.
TRV-Mediated VIGS in Tomato Fruit
The pTRV1 and pTRV2 VIGS vectors have been described previously (Liu et al., 2002). A. tumefaciens strain GV3101 containing pTRV1 or pTRV2 and its derivatives were used for the VIGS experiments. GV3101 containing the TRV-VIGS vectors was grown at 28°C in Luria-Bertani medium containing 10 mm MES (pH 5.6) and 20 mm acetosyringone with appropriate antibiotics (gentamicin and rifampicin for GV3101 and kanamycin for pTRV1 or pTRV2). After culturing overnight (28°C and 200 rpm), A. tumefaciens cells were harvested and resuspended in infiltration buffer (10 mm MgCl2, 10 mm MES, pH 5.6, and 150 mm acetosyringone) to a final OD600 of 2 (for both pTRV1 or pTRV2 and its derivatives). A. tumefaciens containing pTRV1 and pTRV2 or the recombinant vectors were mixed in a 1:1 ratio and left for 11 h at room temperature before infiltration, as described previously (Fu et al., 2005). The tomato inflorescence peduncles attached to the fruit were injected with cultures of A. tumefaciens harboring the vectors using a 1-mL syringe. To detect the accumulation of virus and the silencing efficiency of specific genes in tomato fruit, RT-PCR and RT-qPCR were performed separately.
Color and Firmness Measurement
A hand-held colorimeter (CR-10 Plus) with the CIE L*a*b color system was chosen for pericarp color assay (Komatsu et al., 2016). The firmness of the pericarp was assayed using a hand-held penetrometer (model FT327 made in Italy) according to the manufacturer’s instructions. At least nine biological replicates were used for each assay, and each replicate was from independent sampling.
Carotenoid Content and Pathway Gene Expression Assay
Carotenoid extractions were performed as described previously (Fantini et al., 2013). Briefly, lyophilized tomato fruit powder was extracted with chloroform and methanol (2:1, v/v). Subsequently, 1 volume of 50 mm Tris buffer (pH 7.5, containing 1 m NaCl) was added, and the samples were kept for 20 min on ice. After centrifugation (15,000g for 10 min at 4°C), the organic phase was collected and reextracted. The combined organic phases for each sample were then dried by nitrogen blowing and resuspended in 100 μL of ethyl acetate. For each genotype, at least three independent extractions were performed. To identity and quantify the carotenoids, the Accurate-Mass HPLC1200/MS-QTOF 6520A (Agilent Technol) system packed with a reverse-phase column, 4.6 × 150 mm, 3 μm (YMC), was used, and the carotenoid was washed out by mobile phase A (81% methanol + 15% MTBE (methyl tert-butyl ether) + 4% water) and B (8% methanol + 90% MTBE + 2% water) at a flow rate 0.4 mL min−1. Settings were as follows: DAD (diode array detection), 260 to 550 nm; mass range, 200 to 800; APCI (atmospheric pressure chemical ionization) ion source drying gas of N2 at a pressure of 40 p.s.i., 350°C, 8 L min−1; VCAP (voltage of capillary), 3,500 V; fragmentor, 160 V; skimmer, 65 V; OCT RF Vpp (OctPole radio frequency value of peak peak), 750 V; with the negative mass spectrometry scan mode 2GHzExt Dyn (3200). HPLC peak areas at 260 to 550 nm were integrated and calibrated by external standards (e.g. α-carotene, β-carotene, and lycopene; purchased from Sigma-Aldrich) mixed to generate multiple-diluted external calibration curves for quantification of the pigments. Carotenoids were identified based on typical retention times and specific published absorption spectra (Mialoundama et al., 2010; Régnier et al., 2015). DEGs in the carotenoid pathway were mapped using the MapMan database and visualized with colors representing the log2 fold change (Usadel et al., 2009; Jaiswal and Usadel, 2016).
Ethylene Production Measurement
For the measurement of ethylene production by fruit, each fruit was placed in a gas-tight 300-mL container at 25°C for 1 h, and a 1-mL headspace gas sample was analyzed using a gas chromatograph equipped with a flame ionization detector (Ma et al., 2016).
Statistical Analysis
Microsoft Excel 2010 and SPSS (SPSS Statistics, version 22) were used for statistical analyses. Data were subjected to ANOVA, and a comparison was carried out by Student’s t test (*, P < 0.05 and **, P < 0.01). Duncan’s multiple range test was used (P < 0.05).
Protein Extraction
Fruit proteins were isolated using the protocol described (Wang et al., 2006). Briefly, samples were ground into powder under liquid nitrogen, transferred to 2-mL tubes, which were filled with 10% TCA/acetone, mixed well, and centrifuged at 4°C, followed by removal of the supernatant, and the tubes were then topped up with 80% methanol and 0.1 m ammonium acetate. After centrifugation, the supernatant was discarded, the tubes were filled with 80% acetone, mixed well, and centrifuged, and the supernatant was again discarded and the samples air dried. Phenol (pH 8):SDS solution (30% Suc, 2% SDS, 0.2 m Tris, pH 8, and 5% β-mercaptoethanol [1:1, v/v]) was added to extract proteins, which were precipitated with 80% methanol and 0.1 m ammonium acetate, then the pellets were washed with 100% methanol and 80% acetone and finally dissolved in 2% SDS buffer (0.5 m Tris, pH 7, and 1.4% SDS).
Western Blotting
Protein extracts were separated on 10% SDS-PAGE gels and transferred to a polyvinylidene fluoride membrane. The membrane was blocked in 5% nonfat milk for 2 h at room temperature. Rabbit polyclonal or mouse monoclonal antibody was added at a ratio of 1:1,000 and incubated for 2 h at room temperature. Antibodies for protein tags (anti-myc and anti-HA) were from sigma (C3956 and H9658). Membranes were washed with Tris-buffered saline plus Tween 20 three times, 10 min each time. The anti-rabbit/anti-mouse horseradish peroxidase secondary antibody was added at a ratio of 1:10,000 and incubated for 2 h at room temperature. After three washes with Tris-buffered saline plus Tween 20, the membranes were visualized using a horseradish peroxidase-enhanced chemiluminescence system.
A. tumefaciens-Mediated Transient Expression in Planta
A. tumefaciens strain GV3101 (for BiFC and CoIP) or EHA105 (for trans-activation activity) containing recombinant vectors was used for transient expression assays. A. tumefaciens cells were cultured and harvested as described for the VIGS assay, and cells were resuspended to a final OD600 of 1 each. For BiFC, CoIP, and trans-activity assays, suspensions of the corresponding recombinant vectors were mixed in a 1:1 ratio, and the mixed suspension was infiltrated into Nicotiana benthamiana leaves using a 1-mL syringe without a needle. The fluorescence and luciferase activities were observed and measured at 2 to 5 d after infiltration.
Subcellular Localization
The CDS of target genes without the stop codon was amplified by PCR and subcloned into the pBI221-GFP vector, in frame with the GFP sequence. These fusion constructs and the control GFP vector were transformed into tobacco (Nicotiana tabacum) BY2 suspension culture cell protoplasts using the polyethylene glycol method (Shan et al., 2012; Ba et al., 2014). GFP fluorescence was observed with a fluorescence microscope (Zeiss7 10-3 channel). All transient expression assays were repeated at least three times.
Trans-Activation Assay
A dual luciferase trans-activity assay was performed as described by Ba et al. (2014) and Shan et al. (2014). The CDS of RIN or RIN-MC without stop codon was cloned into the pEAQ vector as effector (Sainsbury et al., 2009). Promoters of ripening-related genes were amplified by PCR, and the products were inserted into the pGreenII 0800-LUC double reporter vector fused to the LUC reporter gene; a REN luciferase under the control of the 35S promoter in the same vector was used as an internal control (Hellens et al., 2005). The constructed effector and reporter plasmids were transfected into A. tumefaciens strain EHA105 (pGreenII series holding psoup plasmid) separately and coinfected into N. benthamiana leaves. LUC/REN activities were measured using the dual luciferase assay kits (Promega) 2 to 5 d after infiltration. At least nine assay measurements were included for each assay.
BiFC Assay
Plasmids used were as described (Walter et al., 2004). The CDS of RIN or RIN-MC without stop codon was cloned into pSPYNE vector, and FUL1, FUL2, MADS1, and TAGL1 were cloned into pSPYCE vector and transfected into A. tumefaciens strain GV3101 separately, using protocols for A. tumefaciens-mediated transient expression in infiltrated leaves (Piotrzkowski et al., 2012). The fluorescence was observed at 2 to 5 d after infiltration.
CoIP Assay
The CDS of RIN or RIN-MC without stop codon was fused with 6myc tags and cloned into pCAMBIA1300-221 vector, and FUL1, FUL2, MADS1, and TAGL1 were fused with 3HA tags. Recombinant vectors were transfected into A. tumefaciens strain GV3101 separately, using protocols for A. tumefaciens-mediated transient expression in infiltrated leaves. The assay procedures were as described (Kim et al., 2009; Deng et al., 2014). N. benthamiana leaves were ground in liquid nitrogen, and proteins were extracted with extraction buffer (20 mm HEPES-KOH, pH 7.5, 40 mm KCl, 1 mm EDTA, 0.5% Triton X-100, and 1× protease inhibitors; Roche) at a ratio of 3 mL g−1 tissue powder. After 10 min of centrifugation at 20,000g, the supernatant was incubated with anti-myc agarose (Sigma-Aldrich) for 1 h. The retained proteins on beads were then washed four times with wash buffer (20 mm HEPES-KOH, pH 7.5, 40 mm KCl, and 0.01% Triton X-100), eluted by boiling in 2× SDS sample buffer for 10 min, and analyzed on protein blots after gel electrophoresis using anti-myc or anti-HA antibody (Sigma-Aldrich).
RNA Sequencing and Bioinformatics Assay
Total RNA samples were prepared from tomato fruits of the wild type (AC), rin mutants, and transgenic AC 35S::RIN RNAi and rin 35S::RIN-MC RNAi at BK+5 (three replicates per sample). The extraction and quality control of total RNA and DNA digestion were as described above. The pair-end sequencing was performed on an Illumina HiSeq2500 PE150 by Novogene, generating at least 6 G of raw data. Raw data were pretreated using FASTX-Toolkit (version 0.0.13.2; http://hannonlab.cshl.edu/fastx_toolkit/download.html), and the resulting clean reads were checked for quality using the Q < 20 threshold and aligned with the tomato reference genome using TopHat (version 2.0.8; http://ccb.jhu.edu/software/tophat/index.shtml). Reads with less than two mismatches were used to construct transcripts using Cufflinks (version 2.0.2; http://cole-trapnell-lab.github.io/cufflinks/). All clean reads were deposited in the National Center for Biotechnology Information Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra/) under the accession numbers SRP106816 (data in VIGS assay) and SRP106775 (data in RIN and RIN-MC-suppressed assay). Genes were considered as DEGs under the threshold of log2 fold change ≥ 1, q < 0.05, and FPKM > 10 in either sample. A heat map was plotted to visualize gene expression using the pheatmap package (Dailey, 2017).
GO Enrichment Analysis
GO enrichment analysis was performed using BiNGO (Maere et al., 2005) based on DEGs, using threshold values of P < 0.05 and FPKM > 10 for each sample. Genes were classified into three classes: cellular component, biological process, and molecular function.
KEGG Pathway Enrichment Analysis
Fasta format files containing DEG protein sequences were obtained using in-house Perl scripts, and KEGG enrichment analysis was then performed in KOBAS (version 2.0; http://obas.cbi.pku.edu.cn/download.do), based on native BLAST tools and organism annotation libraries. KEGG pathways with P < 0.05 and FPKM > 10 for each sample were analyzed and visualized with R Project (R version 3.4.0; https://www.r-project.org/).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers SRP106816 (data in VIGS assay) and SRP106775 (data in RIN and RIN-MC-suppressed assay).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Primer design and virus detection of VIGS in tomato fruits.
Supplemental Figure S2. Transcriptome assay of ripening genes in RIN-MC-silenced tomato fruits.
Supplemental Figure S3. Photographs of four kinds of tomato fruits at different ripening stages.
Supplemental Figure S4. Expression of genes involved in coloring during ripening of four kinds of tomato fruits.
Supplemental Figure S5. Physiological traits of four kinds of tomato fruits at different ripening stages.
Supplemental Figure S6. Comparison of DEGs in two kinds of tomato fruits.
Supplemental Figure S7. Comparison of DEGs in AC/AC 35S::RIN RNAi and rin/rin 35S::RIN-MC RNAi.
Supplemental Figure S8. Trans-activities of tomato RIN and RIN-MC using different lengths of RIN promoters in a transient in planta expression system.
Supplemental Figure S9. Trans-activation activities of tomato RIN and RIN-MC with different lengths of RIN promoters in a transient in planta expression system.
Supplemental Tables S1 and S2. Sequences of all the primers designed.
Supplemental Data Sets S1 to S3. RNA sequencing data.
Supplemental Data Sets S4 to S11. List of DEGs regulated by RIN or RIN-MC that exist in different regions in the Venn diagram.
Supplemental Data Sets S12 and S13. List of DEGs regulated by RIN or RIN-MC that are enriched in the GO enrichment assay (Supplemental Fig. S7A).
Supplemental Data Sets S14 and S15. List of DEGs regulated by RIN or RIN-MC that are enriched in the KEGG assay.
Acknowledgments
We thank Jianye Chen and Dr. Jianfei Kuang (South China Agricultural University) for the generous gift of tobacco BY2 suspension cells and pGreenII 0800 series vectors; Xian Li (Zhejiang University) for help with experimental technique; Zhipeng Deng (Zhejiang Academy of Agricultural Science) for help with article revision; and the Institute of Plant Physiology and Ecology, Shanghai institutes for biological sciences (SIBS) Chinese Academy of Sciences, for carotenoid content detection.
Footnotes
This work was supported by the National Natural Science Foundation of China (NSFC 31571894 and 31271959) and the Education Foundation of Da Bei Nong Group (1061-2415002).
References
- Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ (2005) Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17: 2954–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annala MJ, Parker BC, Zhang W, Nykter M (2013) Fusion genes and their discovery using high throughput sequencing. Cancer Lett 340: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba L, Shan W, Kuang J, Feng B, Xiao Y, Lu W, Chen J (2014) The banana MaLBD (LATERAL ORGAN BOUNDARIES DOMAIN) transcription factors regulate EXPANSIN expression and are involved in fruit ripening. Plant Mol Biol Rep 32: 1103–1113 [Google Scholar]
- Barry CS, Giovannoni JJ (2007) Ethylene and fruit ripening. J Plant Growth Regul 26: 143–159 [Google Scholar]
- Boycheva S, Daviet L, Wolfender JL, Fitzpatrick TB (2014) The rise of operon-like gene clusters in plants. Trends Plant Sci 19: 447–459 [DOI] [PubMed] [Google Scholar]
- Chen G, Hackett R, Walker D, Taylor A, Lin Z, Grierson D (2004) Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol 136: 2641–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G (2008) The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 8: 11–23 [DOI] [PubMed] [Google Scholar]
- DellaPenna D, Lincoln JE, Fischer RL, Bennett AB (1989) Transcriptional analysis of polygalacturonase and other ripening associated genes in Rutgers, rin, nor, and Nr tomato fruit. Plant Physiol 90: 1372–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Oses-Prieto JA, Kutschera U, Tseng TS, Hao L, Burlingame AL, Wang ZY, Briggs WR (2014) Blue light-induced proteomic changes in etiolated Arabidopsis seedlings. J Proteome Res 13: 2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T, Hu Z, Deng L, Wang Y, Zhu M, Zhang J, Chen G (2013) A tomato MADS-box transcription factor, SlMADS1, acts as a negative regulator of fruit ripening. Plant Physiol 163: 1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enfissi EMA, Nogueira M, Bramley PM, Fraser PD (2017) The regulation of carotenoid formation in tomato fruit. Plant J 89: 774–788 [DOI] [PubMed] [Google Scholar]
- Fantini E, Falcone G, Frusciante S, Giliberto L, Giuliano G (2013) Dissection of tomato lycopene biosynthesis through virus-induced gene silencing. Plant Physiol 163: 986–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray RG, Grierson D (1993) Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol Biol 22: 589–602 [DOI] [PubMed] [Google Scholar]
- Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB (2005) Virus-induced gene silencing in tomato fruit. Plant J 43: 299–308 [DOI] [PubMed] [Google Scholar]
- Fujisawa M, Nakano T, Shima Y, Ito Y (2013) A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25: 371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Shima Y, Higuchi N, Nakano T, Koyama Y, Kasumi T, Ito Y (2012) Direct targets of the tomato-ripening regulator RIN identified by transcriptome and chromatin immunoprecipitation analyses. Planta 235: 1107–1122 [DOI] [PubMed] [Google Scholar]
- Fujisawa M, Shima Y, Nakagawa H, Kitagawa M, Kimbara J, Nakano T, Kasumi T, Ito Y (2014) Transcriptional regulation of fruit ripening by tomato FRUITFULL homologs and associated MADS box proteins. Plant Cell 26: 89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. (2004) Genetic regulation of fruit development and ripening. Plant Cell (Suppl) 16: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson D. (2013) Ethylene and the control of fruit ripening. In Seymour GB, Poole M, Giovannoni JJ, Tucker GA, eds, The Molecular Biology and Biochemistry of Fruit Ripening. Blackwell Publishing, Oxford, doi/10.1002/9781118593714.ch3 [Google Scholar]
- Hagel JM, Facchini PJ (2017) Tying the knot: occurrence and possible significance of gene fusions in plant metabolism and beyond. J Exp Bot 68: 4029–4043 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkin M, Seybold H, Breitel D, Rogachev I, Meir S, Aharoni A (2009) TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J 60: 1081–1095 [DOI] [PubMed] [Google Scholar]
- Ito Y, Kitagawa M, Ihashi N, Yabe K, Kimbara J, Yasuda J, Ito H, Inakuma T, Hiroi S, Kasumi T (2008) DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. Plant J 55: 212–223 [DOI] [PubMed] [Google Scholar]
- Ito Y, Nishizawa-Yokoi A, Endo M, Mikami M, Shima Y, Nakamura N, Kotake-Nara E, Kawasaki S, Toki S (2017) Re-evaluation of the rin mutation and the role of RIN in the induction of tomato ripening. Nat Plants 3: 866–874 [DOI] [PubMed] [Google Scholar]
- Ito Y, Nishizawa-Yokoi A, Endo M, Mikami M, Toki S (2015) CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem Biophys Res Commun 467: 76–82 [DOI] [PubMed] [Google Scholar]
- Jaiswal P, Usadel B (2016) Plant pathway databases. Methods Mol Biol 1374: 71–87 [DOI] [PubMed] [Google Scholar]
- Karlova R, Rosin FM, Busscher-Lange J, Parapunova V, Do PT, Fernie AR, Fraser PD, Baxter C, Angenent GC, de Maagd RA (2011) Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell 23: 923–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Melzer R, Theissen G (2005) MIKC-type MADS-domain proteins: structural modularity, protein interactions and network evolution in land plants. Gene 347: 183–198 [DOI] [PubMed] [Google Scholar]
- Kim TW, Guan S, Sun Y, Deng Z, Tang W, Shang JX, Sun Y, Burlingame AL, Wang ZY (2009) Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol 11: 1254–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Sinha N (2008) Tomato transformation. CSH Protoc 2008: t5084. [DOI] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Komatsu T, Mohammadi S, Busa LS, Maeki M, Ishida A, Tani H, Tokeshi M (2016) Image analysis for a microfluidic paper-based analytical device using the CIE L*a*b* color system. Analyst (Lond) 141: 6507–6509 [DOI] [PubMed] [Google Scholar]
- Li J, Lee EJ, Chang L, Facchini PJ (2016) Genes encoding norcoclaurine synthase occur as tandem fusions in the Papaveraceae. Sci Rep 6: 39256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Gomes BL, Mila I, Purgatto E, Peres LEP, Frasse P, Maza E, Zouine M, Roustan JP, Bouzayen M, et al. (2016) Comprehensive profiling of ethylene response factor expression identifies ripening-associated ERF genes and their link to key regulators of fruit ripening in tomato. Plant Physiol 170: 1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Long M, Betrán E, Thornton K, Wang W (2003) The origin of new genes: glimpses from the young and old. Nat Rev Genet 4: 865–875 [DOI] [PubMed] [Google Scholar]
- Luo Z, Zhang J, Li J, Yang C, Wang T, Ouyang B, Li H, Giovannoni J, Ye Z (2013) A STAY-GREEN protein SlSGR1 regulates lycopene and β-carotene accumulation by interacting directly with SlPSY1 during ripening processes in tomato. New Phytol 198: 442–452 [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhou L, Wang Z, Chen J, Qu G (2016) Oligogalacturonic acids promote tomato fruit ripening through the regulation of 1-aminocyclopropane-1-carboxylic acid synthesis at the transcriptional and post-translational levels. BMC Plant Biol 16: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maere S, Heymans K, Kuiper M (2005) BiNGO: a Cytoscape plugin to assess overrepresentation of Gene Ontology categories in biological networks. Bioinformatics 21: 3448–3449 [DOI] [PubMed] [Google Scholar]
- Manning K, Tör M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38: 948–952 [DOI] [PubMed] [Google Scholar]
- Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ (2011) The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol 157: 1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas AJ, Gapper NE, Chung MY, Giovannoni JJ, Rose JK (2009) Biology and genetic engineering of fruit maturation for enhanced quality and shelf-life. Curr Opin Biotechnol 20: 197–203 [DOI] [PubMed] [Google Scholar]
- McWhirter JR, Galasso DL, Wang JY (1993) A coiled-coil oligomerization domain of Bcr is essential for the transforming function of Bcr-Abl oncoproteins. Mol Cell Biol 13: 7587–7595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellidou I, Chagné D, Laing WA, Keulemans J, Davey MW (2012) Allelic variation in paralogs of GDP-L-galactose phosphorylase is a major determinant of vitamin C concentrations in apple fruit. Plant Physiol 160: 1613–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mialoundama AS, Heintz D, Jadid N, Nkeng P, Rahier A, Deli J, Camara B, Bouvier F (2010) Characterization of plant carotenoid cyclases as members of the flavoprotein family functioning with no net redox change. Plant Physiol 153: 970–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman F, Johansson B, Mertens F (2007) The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer 7: 233–245 [DOI] [PubMed] [Google Scholar]
- Nützmann HW, Osbourn A (2014) Gene clustering in plant specialized metabolism. Curr Opin Biotechnol 26: 91–99 [DOI] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S, Alba R, Damasceno CM, Lopez-Casado G, Lohse M, Zanor MI, Tohge T, Usadel B, Rose JK, Fei Z, Giovannoni JJ, Fernie AR (2011) Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol 157: 405–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrzkowski N, Schillberg S, Rasche S (2012) Tackling heterogeneity: a leaf disc-based assay for the high-throughput screening of transient gene expression in tobacco. PLoS One 7: e45803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Wang Y, Cao B, Wang W, Tian S (2012) Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. Plant J 70: 243–255 [DOI] [PubMed] [Google Scholar]
- Régnier P, Bastias J, Rodriguez-Ruiz V, Caballero-Casero N, Caballo C, Sicilia D, Fuentes A, Maire M, Crepin M, Letourneur D, et al. (2015) Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar Drugs 13: 2857–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X (2009) DOG 1.0: illustrator of protein domain structures. Cell Res 19: 271–273 [DOI] [PubMed] [Google Scholar]
- Sainsbury F, Thuenemann EC, Lomonossoff GP (2009) pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J 7: 682–693 [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Park SY, Paek NC (2015) The divergent roles of STAYGREEN (SGR) homologs in chlorophyll degradation. Mol Cells 38: 390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour G, Poole M, Manning K, King GJ (2008) Genetics and epigenetics of fruit development and ripening. Curr Opin Plant Biol 11: 58–63 [DOI] [PubMed] [Google Scholar]
- Shahmuradov IA, Abdulazimova AU, Solovyev VV, Qamar R, Cholan SN, Aliyev JA (2010) Mono- and bi-cistronic chimeric mRNAs in Arabidopsis and rice genomes. Appl Comput Math 9: 66–81 [Google Scholar]
- Shan W, Kuang JF, Chen L, Xie H, Peng HH, Xiao YY, Li XP, Chen WX, He QG, Chen JY, et al. (2012) Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J Exp Bot 63: 5171–5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Kuang JF, Lu WJ, Chen JY (2014) Banana fruit NAC transcription factor MaNAC1 is a direct target of MaICE1 and involved in cold stress through interacting with MaCBF1. Plant Cell Environ 37: 2116–2127 [DOI] [PubMed] [Google Scholar]
- Shima Y, Kitagawa M, Fujisawa M, Nakano T, Kato H, Kimbara J, Kasumi T, Ito Y (2013) Tomato FRUITFULL homologues act in fruit ripening via forming MADS-box transcription factor complexes with RIN. Plant Mol Biol 82: 427–438 [DOI] [PubMed] [Google Scholar]
- Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P, Pellegatta S, et al. (2012) Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337: 1231–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HJ, Uchii S, Watanabe S, Ezura H (2006) A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol 47: 426–431 [DOI] [PubMed] [Google Scholar]
- Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Poree F, Nagel A, Lohse M, Czedik-Eysenberg A, Stitt M (2009) A guide to using MapMan to visualize and compare omics data in plants: a case study in the crop species, maize. Plant Cell Environ 32: 1211–1229 [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296: 343–346 [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang W, Vignani R, Scali M, Cresti M (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27: 2782–2786 [DOI] [PubMed] [Google Scholar]
- Yu J, Tehrim S, Wang L, Dossa K, Zhang X, Ke T, Liao B (2017) Evolutionary history and functional divergence of the cytochrome P450 gene superfamily between Arabidopsis thaliana and Brassica species uncover effects of whole genome and tandem duplications. BMC Genomics 18: 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Fei Z, Chen YR, Zheng Y, Huang M, Vrebalov J, McQuinn R, Gapper N, Liu B, Xiang J, et al. (2013) Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat Biotechnol 31: 154–159 [DOI] [PubMed] [Google Scholar]
- Zhu B, Yang Y, Li R, Fu D, Wen L, Luo Y, Zhu H (2015) RNA sequencing and functional analysis implicate the regulatory role of long non-coding RNAs in tomato fruit ripening. J Exp Bot 66: 4483–4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi J, Mafu S, Peters RJ (2014) To gibberellins and beyond! Surveying the evolution of (di)terpenoid metabolism. Annu Rev Plant Biol 65: 259–286 [DOI] [PMC free article] [PubMed] [Google Scholar]