Abstract
Reanalysis of published experimental data shows that in dehydrating leaves ABA accumulation is linked with reduction of cell volume rather than turgor, providing clues toward signaling mechanisms.
Identifying the mechanisms for cell responses as plants dehydrate is crucial for analyzing and predicting crop and ecosystem responses to climate change (Blum, 1996, 2017; Bartlett et al., 2016), for isolating the proteins and the genes underlying the responses (Christmann et al., 2013), and for the design of model plants and crops with increased water use efficiency and/or drought tolerance (e.g. Nemhauser and Torii, 2016; Yang et al., 2016). The dehydration-sensing mechanisms involved in driving the accumulation of the hormone abscisic acid (ABA; see symbols in Table I) are of special importance as it is implicated in stomatal closure during drought (Rodriguez-Dominguez et al., 2016) or increasing vapor pressure deficit (McAdam et al., 2016), and may contribute to the decline of leaf hydraulic conductance (Shatil-Cohen et al., 2011; Pantin et al., 2013). Cellular ABA accumulation during dehydration may occur due to modulation of transport from cellular or apoplastic stores, de novo synthesis, and/or turnover (Finkelstein, 2013). However, disentangling the factors that leaf cells sense during dehydration is difficult as many changes typically occur in tandem: turgor is lost, solute concentrations increase, relative water content (RWC) decreases, and cell membranes shrink, altering interactions with the cytoskeleton and cell wall (Haswell and Verslues, 2015). Two recent articles (McAdam and Brodribb, 2016; Sussmilch et al., 2017) have argued based on applying external pressure to leaves that turgor loss provides the endogenous signal triggering ABA accumulation and that species differ greatly in the turgor loss threshold that triggers ABA accumulation. We derived new equations from plant water relations theory enabling the calculation of turgor, solute potential, and RWC for the experimental leaves in those studies. These calculations establish that the accumulation of ABA in these artificially dehydrated leaves was not due to decline of turgor pressure but instead was associated with the decline of RWC. These analyses further show that the RWC loss associated with ABA accumulation varied by approximately 10% across the diverse angiosperm species, indicating functional convergence in cellular drought sensing and providing clues for identification of the components of the signaling pathway.
Table I. Symbols used in the text.
| Symbol | Term | Unit |
|---|---|---|
| ABA | Abscisic acid | n/a |
| Ψleaf | Bulk leaf water potential | MPa |
| ΨS | Osmotic potential, a.k.a. solute potential | MPa |
| ΨP | Pressure potential, a.k.a. turgor pressure | MPa |
| ΨS,o | Solute potential at full turgor | MPa |
| ΨS,tlp | Solute potential at turgor loss point | MPa |
| af | Apoplastic water fraction at full hydration | % |
| Ψx | Apoplast (and xylem) water potential | MPa |
| Px | Apoplast (and xylem) pressure potential | MPa |
| Total RWC | Total relative water content of apoplast and symplast | MPa |
| Symplastic RWC | Relative water content in the symplast (cellular compartment) | % |
| RWCtlp | Relative water content at turgor loss point | % |
| c | Solute concentration | mol L−1 |
The debate on the precise determinants of ABA accumulation began decades ago. In a dehydrating leaf, cell volume, turgor, osmotic potential, and leaf water potential decline together, and making a distinction among these may seem at first semantic. However, it is critical to distinguish exactly which of these or related physical properties is ultimately sensed and leads to ABA accumulation. For example, changes in cell volume independently of turgor may affect sensors of cytoskeletal properties, ion concentrations or ion transport rates, or cell membrane interactions with the cell wall, whereas sensing of membrane tension might be affected by volume and/or turgor. The idea that turgor loss was the driver for ABA accumulation arose from early experiments showing the hormone levels increased in drying leaves as leaf water potential (Ψleaf) declined (e.g. Zabadal, 1974; Beardsell and Cohen, 1975; Wright, 1977), and was later further supported circumstantially by the finding that in many species, stomatal closure, known to be driven by ABA levels, apparently coincides roughly, on average, with turgor loss point (global data recently synthesized in Bartlett et al., 2016). Subsequent experiments took the necessary next step by dehydrating leaves of several species on the bench top and measuring ABA accumulation, and used pressure volume curves to estimate solute and pressure potentials from leaf water potentials (Pierce and Raschke, 1980). These calculations showed that increases in ABA accumulation correlated more closely with the decline of turgor pressure (ΨP) than with the declines of either osmotic potential (ΨS) or Ψleaf. Yet, those studies did not consider the decline of RWC as a potential driver.
Subsequent experiments confirmed that ΨS did not drive ABA accumulation: leaf sections of spinach (Spinacia oleracea) and maize (Zea mays) accumulated ABA if incubated in mannitol or polyethylene glycol, which dehydrated the leaf, but not when incubated with ethylene glycol, which penetrates the cell membrane and thus decreases ΨS with only transient changes in ΨP or RWC (Creelman and Zeevaart, 1985; Jia et al., 2001). Additionally, osmotic adjustment (i.e. the decrease of ΨS) generally enhances or sustains gas exchange during drought, whereas if decreased ΨS per se enhanced ABA accumulation, one would expect the opposite response (Turner et al., 1978). In subsequent years, with the increasing recognition of the importance of xylem negative pressure (tension) in driving cavitation and the importance of water potential and xylem pressure gradients as driving forces for water movement in the soil-plant-atmosphere continuum (Kramer, 1988; Tyree and Zimmermann, 2002), Ψleaf and leaf ΨP have eclipsed changes in cell volume or RWC as indicator variables for predicting plant function during drought.
Subsequent studies, however, suggested that ΨP decline is not in fact the primary determinant of ABA production. When cotton (Gossypium hirsutum) or maize leaves were dehydrated under sustained pressure in a pressure chamber, such that the leaves lost water by extrusion through the petiole, ABA accumulated (Ackerson and Radin, 1983; Jia et al., 2001). The authors argued that pressurizing the leaves during dehydration maintained cell turgor, and thus that ABA accumulation was driven instead by cellular volume shrinkage or relaxation of the cell wall, i.e. corresponding to a decline in RWC or volume, independently of ΨP. An important control showed that pressurizing leaves entirely enclosed within the pressure chamber—without the petiole protruding and thus without leaf water loss—only led to minimal stimulation of ABA accumulation, indicating that increases in ΨP alone were not the stimulus (Ackerson and Radin, 1983). This same approach was revived in recent articles (McAdam and Brodribb, 2016; Sussmilch et al., 2017), though these authors argued that the application of external pressure would reduce leaf turgor and that this reduction of turgor triggered ABA accumulation. In these experiments, leaves were treated in a pressure chamber with petiole protruding, and subjected to a range of pressures (in 0.5 MPa intervals from 0 to 3.5 MPa) for 20 or 60 min, after which they were immediately snap-frozen and analyzed for ABA concentration (McAdam and Brodribb, 2016). In a subsequent study, this approach was applied to Arabidopsis (Arabidopsis thaliana) leaves pressurized at 1.5 MPa for 1, 5, 10, or 20 min (Sussmilch et al., 2017). Leaves of angiosperm species subjected to sufficient pressures for a long enough time showed increased ABA accumulation, whereas the three conifer species, two fern species, and one lycophyte species tested did not, and the authors concluded that turgor reduction was responsible for triggering ABA accumulation in angiosperms. This conclusion was based on the assumption that turgor pressure declined in the treated leaves from its value in fully hydrated leaves (determined from pressure volume curves) by an amount equal to the applied external pressure, and plots of [ABA] increase against reduction of turgor calculated in this way apparently showed threshold responses, which varied strongly across the four angiosperm species (supplemental figure S1 of McAdam and Brodribb, 2016). A major advance of these experiments is that they used modern analytical methods to show ABA accumulation occurred at lesser levels and durations of dehydration than previously thought based on earlier work. The authors argued that subtle decreases of turgor would drive ABA production in angiosperms, thus triggering stomatal closure. They argued further that the turgor threshold for ABA production varied strongly across species and was closely related to their turgor loss points (see differences in thresholds required in Table II).
Table II. New analyses of cell water relation parameters during external pressurization experiments that stimulated ABA accumulation (McAdam and Brodribb, 2016; Sussmilch et al., 2017).
Pressure volume curve parameters are provided (based on methods of Sack et al., 2010): osmotic potential at full turgor (ΨS,o) and at turgor loss point (ΨS,tlp), the relative water content (RWC) at turgor loss point (RWCtlp), and the apoplastic fraction (af); see Appendix and Supplemental Data S1. The external pressure threshold for ABA production and the minimum tested time necessary for the pressure treatment to induce ABA production are provided, as well as calculated values for the water relations of the leaves using the equations provided in the Appendix: total and symplastic RWC, osmotic and turgor potentials (ΨS and ΨP, respectively), and the % increase in ΨP, the decrease in ΨS, the % increase in solute concentration c (estimated given that ΨS = −RTc, where R is the ideal gas constant and T is temperature), and the declines in total and symplastic RWC relative to turgid leaves. While the four first rows are based on data from McAdam and Brodribb (2016), the last row, marked with an asterisk, is from Sussmilch et al. (2017), and the external pressure was not a threshold for ABA accumulation but chosen to exceed turgor loss point.
| Species | ΨS,o | ΨS,tlp | RWCtlp | af | During External Pressure Treatment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| External Pressure Threshold | Shortest Measured Time Req't | Total RWC | Symplastic RWC | ΨP | ΨS | ΨP Increase | ΨS Decrease | c Increase | Total RWC Decline | Symplastic RWC Decline | |||||
| MPa | MPa | % | % | MPa | min | % | MPa | % | % | % | |||||
| Arabidopsis thaliana | −0.684 | −0.807 | 87.7 | 19.4 | 0.5 | 20 | 0.923 | 0.905 | 0.76 | −0.76 | 10.5 | −0.072 | 10.5 | 7.66 | 9.51 |
| Pisum sativum | −0.927 | −1.29 | 80.2 | 29.6 | 1 | 60 | 0.843 | 0.777 | 1.19 | −1.19 | 28.7 | −0.266 | 28.7 | 15.7 | 22.3 |
| Nothofagus cunninghamii | −0.955 | −1.24 | 96.0 | 82.8 | 1 | 20 | 0.967 | 0.810 | 1.18 | −1.18 | 23.4 | −0.223 | 23.4 | 3.25 | 18.9 |
| Olea oleaster | −1.91 | −2.34 | 91.6 | 53.6 | 1.5 | 60 | 0.945 | 0.882 | 2.17 | −2.17 | 13.4 | −0.256 | 13.4 | 5.48 | 11.8 |
| Arabidopsis thaliana* | −0.684 | −0.807 | 87.7 | 19.4 | 1.5* | 5 | 0.562 | 0.456 | 1.50 | −1.50 | 119 | −0.816 | 119 | 43.8 | 54.4 |
We show here that applying external pressure to the leaf increases cell turgor throughout the leaf, and thus that decrease in ΨP is not itself the stimulus for ABA accumulation. Indeed, the external pressure dehydration treatment (Ackerson and Radin, 1983; Jia et al., 2001; McAdam and Brodribb, 2016; Sussmilch et al., 2017) enables the independent resolution of ΨP decline from RWC decline, whereas in vivo these typically occur together. The method thus provides a powerful tool to distinguish these as drivers of physiological responses. We derived new theory from the pressure chamber equations to quantitatively determine their changes within the treated leaves in the recent high-resolution studies (McAdam and Brodribb, 2016; Sussmilch et al., 2017).
For a leaf being dehydrated at a given applied pressure in the pressure chamber, once water is extruded from the petiole and has stopped flowing at that pressure, and the leaf equilibrates at the new balance pressure, the xylem is at equilibrium with the atmosphere and thus
where Px is the pressure in the xylem and 0 is gauge pressure (relative to atmospheric pressure). Assuming the solute potential of the xylem and apoplast is negligible (i.e. less negative than −0.05 MPa; Scoffoni et al., 2012), then
In a leaf held at balance pressure until water ceases to exude from the petiole and equilibrates among cells and tissues, the bulk cell water potential will equal that of the xylem and apoplast surrounding the cells, so it follows that
because 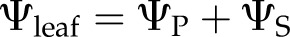 , at balance pressure,
, at balance pressure,
During these experiments, in which the leaf is subjected to pressure in the pressure chamber, given that water has been squeezed out of cells, ΨS has become more negative relative to its value in a leaf at full turgor. Therefore, ΨP will have increased, not decreased as assumed by McAdam and Brodribb (2016) and Sussmilch et al. (2017).
Our new analysis of the pressure volume equations (see Appendix) enables quantification of the RWC, ΨP and ΨS for the treated leaves in those authors’ experiments, and shows that the increase of ΨP was substantial: at the threshold pressures that corresponded to ABA accumulation, ΨP had increased by 11% to 119% (Table II). Our analysis also establishes that declining Ψleaf itself was not the driver, because it was equal across treatments and held at  0 MPa. Assuming that ABA accumulation was not driven by the increase of ΨP (Ackerson and Radin, 1983), nor by the reduction of ΨS associated with the increase in cellular solute concentrations (Creelman and Zeevaart, 1985), the decline of cell volume and its associated processes would be responsible (Table III). We note that a strong but very brief reduction of ΨP would have been triggered when the external pressure was released and the leaf removed from the chamber before snap freezing for ABA analysis. However, ABA accumulation was closely related to the duration of the pressure chamber treatment (figures 1 and 2 of McAdam and Brodribb, 2016), which indicates that it was not this brief exposure to a low ΨP that drove ABA accumulation.
0 MPa. Assuming that ABA accumulation was not driven by the increase of ΨP (Ackerson and Radin, 1983), nor by the reduction of ΨS associated with the increase in cellular solute concentrations (Creelman and Zeevaart, 1985), the decline of cell volume and its associated processes would be responsible (Table III). We note that a strong but very brief reduction of ΨP would have been triggered when the external pressure was released and the leaf removed from the chamber before snap freezing for ABA analysis. However, ABA accumulation was closely related to the duration of the pressure chamber treatment (figures 1 and 2 of McAdam and Brodribb, 2016), which indicates that it was not this brief exposure to a low ΨP that drove ABA accumulation.
Table III. Summary of evidence for and against the declines in leaf water potential (Ψleaf), turgor potential (ΨP), solute potential (ΨS), or RWC or cell volume as drivers of ABA accumulation in previous studies, and whether the putative driver is supported as important for ABA accumulation in studies using externally applied pressure, as analyzed in Table II.
| Cell Behavior during Dehydration Potentially Driving ABA Accumulation | Correlative Evidence for a Role in Driving ABA Accumulation | Evidence against a Role in Driving ABA Accumulation | Supported by Observed Effect of External Pressure on ABA Accumulation? |
|---|---|---|---|
| 1. Leaf water potential decline | Correlation with ABA production in dehydrating leavesa | Weak relation with ABA production in dehydrating leavesa | No: Ψleaf is 0 for the treated leaves |
| 2. Turgor pressure decline | Correlation with ABA production in dehydrating leavesa | – | No: ΨP increased in the treatmentb,c; this itself should not cause ABA accumulationc |
| 3. Solute potential decline | – | Weak relation with ABA production in dehydrating leavesa | Potentially: ΨS declined in the treatmentb |
| No relation to ABA production in leaf samples floated on solute solutionsd | |||
| 4. Relative water content or cell volume decline | Correlation with ABA production in dehydrating leavesa | – | Yes: Increase of ABA coincided with RWC decline in four studiesb,c |
Pierce and Raschke (1980) and references therein.
Our analysis of data of McAdam and Brodribb (2016); Sussmilch et al. (2017; Table I).
The decline of RWC during dehydration would correspond to reduction of cell volumes within the leaf. While a substantial portion of the leaf water is apoplastic (i.e. within cell walls or xylem), this apoplastic water would be “bound” by surface tension in the cell wall pores or xylem conduits, until very strong tissue dehydration would trigger embolism and drain xylem conduits. Thus, under mild dehydration above turgor loss point, whether naturally or using the pressure chamber, the loss of leaf water would be virtually all cellular, and would necessitate volume shrinkage of cells, such that declining RWC would correspond to declining cell volumes.
The idea that turgor loss drove ABA accumulation led to the conclusion that angiosperm species showed striking differences in their water status thresholds for rapid increases in ABA levels, coinciding with their strong differences in turgor loss point (Table II; McAdam and Brodribb, 2016). However, our finding of the importance of cell shrinkage instead emphasizes potential convergence, not diversity, in these thresholds across the tested species. The decline of RWC associated with ABA accumulation was 3% to 16% across the four species tested (McAdam and Brodribb, 2016), corresponding to symplastic RWC declines of 10% to 22% (Table II), although we note that calculating symplastic RWC entails estimating apoplastic water fraction by extrapolating pressure-volume curves, which contributes a level of uncertainty (Andersen et al., 1991; Wardlaw, 2005). Further, the intervals of external pressure applied in the experiments to determine the thresholds for ABA accumulation were rather wide, and higher resolution studies may show the range of RWC decline associated with ABA accumulation across diverse angiosperms to be yet narrower.
Given the association of ABA accumulation with RWC decline in these experiments, our findings lend support to a role for RWC decline in ABA accumulation during rapid changes in vapor pressure deficit. Further, the finding that volume loss rather than turgor loss is associated with ABA accumulation is consistent with additional experiments, e.g. showing that ABA accumulated in leaf disks floated on saline solutions (McAdam and Brodribb, 2016), as the cells would have reduced RWC as well as ΨP.
Why sense cell volume rather than turgor? Declining cell volume may trigger signals via sensors within the cytoskeleton, or the cell membrane (i.e. sensors of membrane tension, of membrane protein distances, of the increase of specific ions or metabolites), or at the interface of cell membrane and cell wall (Christmann et al., 2013; Kumar et al., 2013; Haswell and Verslues, 2015; Pandey, 2017). These proteins may directly sense negative effects on processes or structures threatened by cell volume shrinkage, such as the cytoskeleton, membrane-cell wall contacts, or ion transport, even before detrimental biochemical effects arise (Oliver, 1996; Zhang et al., 2001; Pandey 2017). By contrast, a direct detrimental impact of declining turgor on cell processes, independent of cell volume, has not been demonstrated to our knowledge in mesophyll cells, though key functions of specialized, often semi-isolated tissues do depend on the maintenance of critical positive pressures—e.g. growth, phloem translocation, guard cell opening, and plant movements.
Identification of dehydration sensors thus depends on knowing whether the decline of ΨP or RWC is important, as this will inform screens of ecotypes and mutants, and application of genetic association studies to find key genes (Wohlbach et al., 2008; Haswell and Verslues, 2015; Gupta et al., 2016). Notably, in vivo, dehydration may initiate different processes throughout the mesophyll or surrounding tissues (i.e. epidermis, bundle sheath, vasculature) that may differ from the bulk leaf in RWC, ΨP, ΨS, and/or Ψleaf (Bennett et al., 1987). It is thus both a strength and a weakness that the external pressure experiment (Ackerson and Radin, 1983; Jia et al., 2001; McAdam and Brodribb, 2016; Sussmilch et al., 2017) imposes an equal and simultaneous decline in water status in all leaf cells: this ensures the sensing cells’ status is reflected in bulk leaf variables while precluding the determination of those cells’ identity. New tools are needed to measure cell volumes and turgor, osmolyte movement, ion flux, and organelle and cell membrane tension cells in different tissues in dehydrating leaves. Such work will enable resolution of the most important thresholds for declines in function and triggers for active processes such as ABA accumulation, osmotic adjustment, and stomatal closure (Haswell and Verslues, 2015).
Supplemental Data
The following supplemental materials are available.
Supplemental Data S1. Spreadsheet tool.
Supplemental Data S2. Supplemental information.
Footnotes
Articles can be viewed without a subscription.
This work was supported by the U.S. National Science Foundation (award nos. 1457279 and 1557906), the Australian Research Council (DP150103863 and LP130101183), and International Wheat Yield Partnership/Grains Research and Development Corporation US00082.
References
- Ackerson RC, Radin JW (1983) Abscisic acid accumulation in cotton leaves in response to dehydration at high pressure. Plant Physiol 71: 432–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MN, Jensen CR, Losch R (1991) Derivation of pressure volume curves by a nonlinear regression procedure and determination of apoplastic water. J Exp Bot 42: 159–165 [Google Scholar]
- Bartlett MK, Klein T, Jansen S, Choat B, Sack L (2016) The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc Natl Acad Sci USA 113: 13098–13103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L (2012) The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol Lett 15: 393–405 [DOI] [PubMed] [Google Scholar]
- Beardsell MF, Cohen D (1975) Relationships between leaf water status, abscisic acid levels, and stomatal resistance in maize and sorghum. Plant Physiol 56: 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JM, Sinclair TR, Muchow RC, Costello SR (1987) Dependence of stomatal conductance on leaf water potential, turgor potential and relative water content in field-grown soybean and maize. Crop Sci 27: 984–990 [Google Scholar]
- Blum A. (1996) Crop responses to drought and the interpretation of adaptation. Plant Growth Regul 20: 135–148 [Google Scholar]
- Blum A. (2017) Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ 40: 4–10 [DOI] [PubMed] [Google Scholar]
- Christmann A, Grill E, Huang J (2013) Hydraulic signals in long-distance signaling. Curr Opin Plant Biol 16: 293–300 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Zeevaart JAD (1985) Abscisic acid accumulation in spinach leaf slices in the presence of penetrating and nonpenetrating solutes. Plant Physiol 77: 25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. (2013) Abscisic acid synthesis and response. Arabidopsis Book 11: e0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta DB, Rai Y, Gayali S, Chakraborty S, Chakraborty N (2016) Plant organellar proteomics in response to dehydration: turning protein repertoire into insights. Front Plant Sci 7: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell ES, Verslues PE (2015) The ongoing search for the molecular basis of plant osmosensing. J Gen Physiol 145: 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W, Zhang J, Liang J (2001) Initiation and regulation of water deficit-induced abscisic acid accumulation in maize leaves and roots: cellular volume and water relations. J Exp Bot 52: 295–300 [PubMed] [Google Scholar]
- Koide RT, Robichaux RH, Morse SR, Smith CM (2000) Plant water status, hydraulic resistance and capacitance. In Pearcy RW, Ehleringer JR, Mooney HA, Rundel PW, eds, Plant Physiological Ecology: Field Methods and Instrumentation. Kluwer, Dordrecht, The Netherlands, pp 161–183 [Google Scholar]
- Kramer PJ. (1988) Changing concepts regarding plant water relations: opinion. Plant Cell Environ 11: 565–568 [Google Scholar]
- Kumar MN, Jane W-N, Verslues PE (2013) Role of the putative osmosensor Arabidopsis histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol 161: 942–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2016) Linking turgor with ABA biosynthesis: implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiol 171: 2008–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC, Brodribb TJ (2016) Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ 39: 485–491 [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Torii KU (2016) Plant synthetic biology for molecular engineering of signalling and development. Nat Plants 2: 16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver MJ. (1996) Desiccation tolerance in vegetative plant cells. Physiol Plant 97: 779–787 [Google Scholar]
- Pandey GK. (2017). Mechanism of Plant Hormone Signaling Under Stress, Vol 2 Wiley, Hoboken, NJ [Google Scholar]
- Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B (2013) The dual effect of abscisic acid on stomata. New Phytol 197: 65–72 [DOI] [PubMed] [Google Scholar]
- Pierce M, Raschke K (1980) Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 148: 174–182 [DOI] [PubMed] [Google Scholar]
- Robichaux RH. (1984) Variation in the tissue water relations of two sympatric Hawaiian Dubautia species and their natural hybrid. Oecologia 65: 75–81 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Dominguez CM, Buckley TN, Egea G, de Cires A, Hernandez-Santana V, Martorell S, Diaz-Espejo A (2016) Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant Cell Environ 39: 2014–2026 [DOI] [PubMed] [Google Scholar]
- Sack L, Pasquet-Kok J, PrometheusWiki Contributors (2010) Leaf pressure-volume curve parameters. http://prometheuswiki.org/tiki-index.php?page=Leaf%20pressure-volume%20curve%20parameters
- Scoffoni C, McKown AD, Rawls M, Sack L (2012) Dynamics of leaf hydraulic conductance with water status: quantification and analysis of species differences under steady state. J Exp Bot 63: 643–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatil-Cohen A, Attia Z, Moshelion M (2011) Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: a target of xylem-borne ABA? Plant J 67: 72–80 [DOI] [PubMed] [Google Scholar]
- Sussmilch FC, Brodribb TJ, McAdam SAM (2017) Up-regulation of NCED3 and ABA biosynthesis occur within minutes of a decrease in leaf turgor but AHK1 is not required. J Exp Bot 68: 2913–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner NC, Begg JE, Tonnet ML (1978) Osmotic adjustment of sorghum and sunflower crops in response to water deficits and its influence on the water potential at which stomata close. Aust J Plant Physiol 5: 597–608 [Google Scholar]
- Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap. Springer, Berlin [Google Scholar]
- Wardlaw IF. (2005) Consideration of apoplastic water in plant organs: a reminder. Funct Plant Biol 32: 561–569 [DOI] [PubMed] [Google Scholar]
- Wohlbach DJ, Quirino BF, Sussman MR (2008) Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell 20: 1101–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright STC. (1977) The relationship between leaf water potential ψleaf and the levels of abscisic acid and ethylene in excised wheat leaves. Planta 134: 183–189 [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu J, Tischer SV, Christmann A, Windisch W, Schnyder H, Grill E (2016) Leveraging abscisic acid receptors for efficient water use in Arabidopsis. Proc Natl Acad Sci USA 113: 6791–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabadal TJ. (1974) A water potential threshold for the increase of abscisic acid in leaves. Plant Physiol 53: 125–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, He F, Jia W (2001) Cell biological mechanism for triggering of ABA accumulation under water stress in Vicia faba leaves. Sci China C Life Sci 44: 421–428 [DOI] [PubMed] [Google Scholar]


