Abstract
Advanced bioimaging uncovers insights into subcellular structures of plants.
After the establishment of advanced fluorescence microscopy methods and the development of numerous fluorescent proteins, it is possible to follow the organization and dynamics of most organelles and subcellular compartments in cells of living plants. Nowadays, it is possible to address subcellular architecture at the nanoscale through the implementation of superresolution microscopy methods such as structured illumination microscopy, photoactivation localization microscopy, or stochastic optical reconstruction and stimulated emission depletion microscopy. In a developmental context, the dynamic cellular and subcellular changes can be monitored long term in whole plant organs by light-sheet fluorescence microscopy. This is a mesoscopic method offering high-speed imaging, very low phototoxicity, and bioimaging of vertically oriented plants. This Update aims to provide the principles, the current application range, and the expected potential of superresolution and light-sheet fluorescence microscopy methods as well as a brief description of the improvements of standard wide-field epifluorescence and confocal systems.
METHODS OF SUPERRESOLUTION MICROSCOPY
Superresolution microscopy is a term that collectively refers to various techniques that bend or overcome diffraction limitations that strictly define the resolution limit of classical far-field optical microscopy systems. Resolution in conventional far-field systems is proportional to the wavelength of excitation light and inversely proportional to the numerical aperture of the light-collecting lens.
One major category of superresolution microscopy includes techniques that illuminate the sample by means of patterned light, such as structured illumination microscopy (SIM; linear and nonlinear; Gustafsson, 2000; Rego et al., 2012) and stimulated emission depletion microscopy (STED; Dyba et al., 2003).
The second major category of superresolution techniques includes single-molecule localization strategies that interrogate the positioning of individual fluorophores with subdiffraction accuracy. Such techniques mainly include photoactivation localization microscopy (PALM; Betzig et al., 2006), stochastic optical reconstruction microscopy (STORM; Rust et al., 2006), and some related variants such as superresolution optical fluctuation microscopy (SOFI; Dertinger et al., 2010a, 2010b), Bayesian analysis of blinking and bleaching (3B), and superresolution radial fluctuations (Cox et al., 2011; Small and Parthasarathy, 2014; Gustafsson et al., 2016). These so-called pointillistic superresolution techniques exploit properties of specific fluorophores to undergo repeated transitions between on and off states under certain conditions (Dempsey et al., 2011; Vaughan and Zhuang, 2011).
SIM
SIM imposes a spatially modulated light pattern (generated by means of a grating with an effective spacing of λexc/2; Gustafsson, 2000) that combines with diffraction orders of the emitting sample to Moiré patterns. Such Moiré patterns are generated by rotations (either three or five rotations at 36° or 60° increments, respectively) and phase shifts (three or five at 2π/3 increments) of the grating. Individual images from each position of the grating are then combined, deconvolved, and used to reconstruct an image, which by definition is twice resolved compared with the Abbe limit (Gustafsson, 2000). Since linear SIM can rescue up to two diffraction orders, it is still diffraction limited. However, by means of linear SIM combined with high numerical aperture objectives (Komis et al., 2014), microtubules that were simply visualized using GFP-labeled tags (GFP-MBD [Marc et al., 1998] and GFP-TUA6 [Shaw et al., 2003]) could be practically resolved to the theoretical 100-nm threshold of linear SIM (Komis et al., 2014, 2015). This is twice as good as the theoretical optimum of 200 nm predicted by Abbe’s equation. The Z-resolution can be significantly better compared with confocal laser scanning microscopy (CLSM; Sahl et al., 2017, and refs. therein). The final spatial resolution achieved by SIM is dependent on the specifications of the platform used for image acquisition, since different commercial platforms employ different means to generate patterned light as well as different numbers of rotations and phase shifts (discussed by Komis et al. [2015]). The temporal resolution of SIM is limited by the mechanical rotations and phase shifts of the light pattern. Thus, reconstructed time series of highly dynamic structures can suffer from motion artifacts (Förster et al., 2016). However, the acquisition frame rate of any commercial SIM platform is sufficient to record moderately dynamic events such as end-wise length fluctuations of microtubules and tracking minor growth/shrinkage events with a length below 200 nm. On the basis of differences in the signal intensity, SIM allows one to discriminate the dynamics of very proximal or bundled microtubules even if these cannot be resolved optically (Komis et al., 2014). Although not as effective as STED (see below) or PALM/STORM in superresolving intracellular structures, linear SIM offers several advantages for cell imaging. It is fluorophore unlimited, moderately fast (with imaging speeds ranging from 100 ms for a single image with Deltavision OMX to 600 ms with Nikon NSIM or 1.2 s with Zeiss Elyra S.1), and well represented in the market (Komis et al., 2015).
Nonlinear SIM, whereby emission of the fluorophore is not linearly proportional to excitation illumination, is able to truly superresolve intracellular structures such as microtubules in a diffraction-unlimited manner (Rego et al., 2012). However, appropriate platforms are not yet widely accessible to the microscopy community.
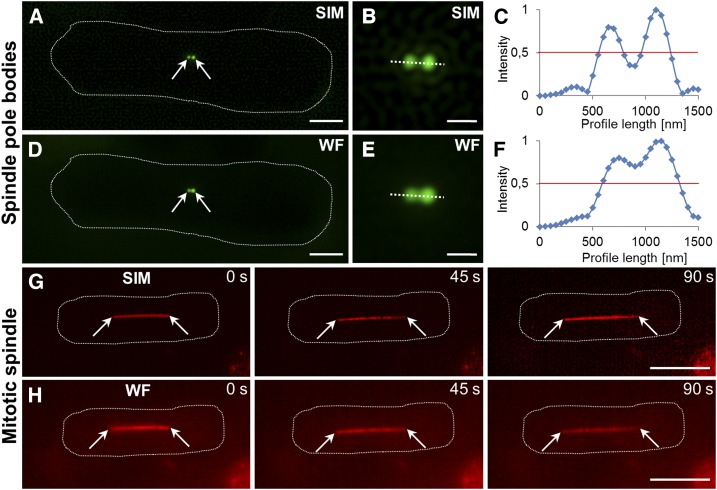
SIM can be used to image living cells of variable sizes. Thus, live SIM studies have been conducted in bacteria (Bottomley et al., 2017), showing the subcellular distribution of RNA polymerase (Stracy et al., 2015) and the time-resolved assembly of the FtsZ ring during bacterial division (Strauss et al., 2012). 2D and 3D SIM can temporally resolve subcellular structures of small eukaryotic cells, such as the spindle pole body (Fig. 1, A–F) and mitotic spindle (Fig. 1, G and H), or the reorganization of the actin cytoskeleton during cell fusion in fission yeast (Dudin et al., 2015). In custom-built systems, 2D and 3D SIM has been reportedly used at video frame rates and documented the organization and dynamics of microtubules (Kner et al., 2009; Schvartz et al., 2017) and mitochondria (Shao et al., 2011) in mammalian cells.
Figure 1.
Comparative structured illumination and wide-field epifluorescence microscopy imaging of Schizosaccharomyces pombe cytoskeletal structures. A to C, Newly separated spindle pole bodies in a premitotic cell (A and B) and their clear discrimination based on normalized fluorescence intensity profiling (C) by means of SIM in S. pombe cells expressing the GFP-pcp1 spindle pole marker. D to F, Spindle bodies as visualized by wide-field epifluorescence (D and E) with less prominent resolution (F) compared with SIM. G and H, Mitotic spindle elongation visualized by SIM (G) and wide-field epifluorescence (H) in S. pombe cells expressing an mCherry-atb2 microtubule marker. Arrows indicate spindle pole bodies (A and D) and mitotic spindle poles (G and H). Bars = 2 µm (A and D), 1 µm (B and E), and 5 µm (G and H).
Depending on the optics and, especially, the objective numerical aperture, intracellular structures can be dynamically visualized at significantly better resolution than 100 nm (Li et al., 2015). In cases where the optical path can be split between two or more cameras, there is a possibility for dual or multiple-channel dynamic imaging of two or more subcellular compartments at the same time (for review, see Hirano et al., 2015; Li et al., 2015). Time-lapse 3D SIM imaging has been markedly improved by combining SIM and light-sheet fluorescence microscopy (LSFM) into a lattice light sheet with exceptional frame rates of acquisition (Chen et al., 2014).
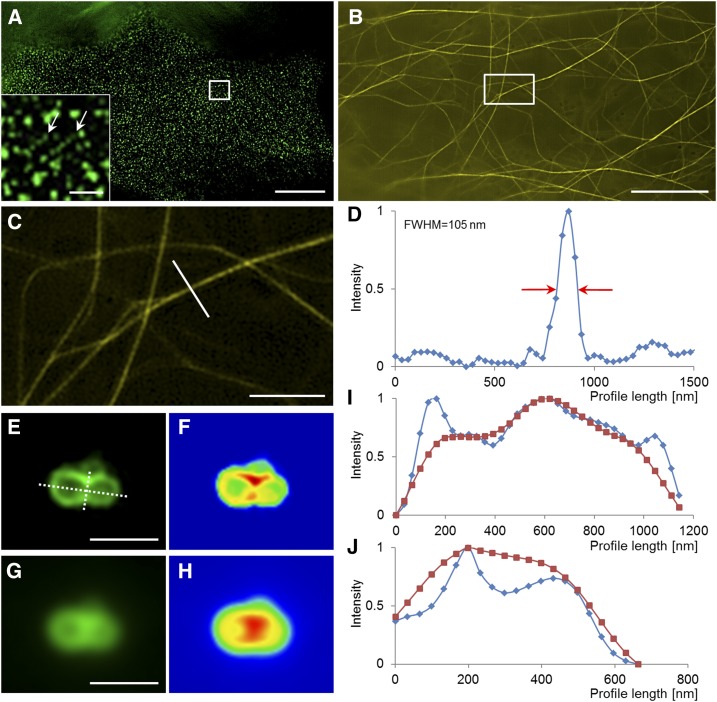
In plants, 2D SIM can visualize various superresolved subcellular structures. These structures may include plasma membrane microdomains containing FLOTILIN1 (Flot1; Fig. 2A), actin filaments (Fig. 2, B and C), microtubules (Komis et al., 2014, 2015), and late endosomes (Fig. 2, E–H), providing quantitative information on the resolution improvement (Fig. 2D). Rare events such as vesicle fusion, which cannot be resolved by wide-field epifluorescence methods, can be captured by SIM (Fig. 2, I and J).
Figure 2.
The superresolution potential of structured illumination microscopy applied to subcellular live imaging of plants. A, Imaging of Flot1 clusters at the plasma membrane of an Arabidopsis cotyledon pavement cell expressing a GFP-Flot1 marker. The inset shows two linear clusters of Flot1 particles (arrows). B to D, Imaging of the actin cytoskeleton in a hypocotyl epidermal cell expressing a YFP-FABD2-YFP actin marker (B; the boxed area is magnified in C) with the capacity to resolve fine actin bundles at approximately 100 nm at full width at half-maximum (FWHM) of the intensity profile (D) shown in C. E to J, Visualization of late endosome fusion with SIM imaging in root epidermal cells or Arabidopsis expressing a GFP-FYVE late endosomal reporter (E and F) as compared with wide-field epifluorescence (G and H). The graphic depiction of the longitudinal (I) and the transverse (J) profiles, drawn as dotted lines in E, highlights the potential of SIM to identify the area of fusion as an area of decreased fluorescence intensity. Bars = 10 μm (A and B), 2 μm (C), and 1 μm (E, G, and inset in A).
2D and 3D SIM also can elucidate the structures of organelles such as plastids (Fig. 3, A and B), mitochondria (Fig. 3, C and D), nuclei (Fig. 3, E–H), and the endoplasmic reticulum (Fig. 3, I and J). Moreover, 3D SIM was used successfully to decipher the architecture of plasmodesmata (Fitzgibbon et al., 2010; Knox et al., 2015), address the structure of virus replication complexes (Linnik et al., 2013), and localize the positions of viral movement proteins in plasmodesmata (Tilsner et al., 2013).
Figure 3.
Structured illumination microscopy can deliver superresolved images of various plant organelles as compared with wide-field (WF) epifluorescence imaging. A and B, Plastids labeled by the marker ADP-sugar pyrophosphatase fused with GFP. C and D, Mitochondria labeled by the mitochondria-targeting sequence of the γ-subunit of the F1-ATPase fused with GFP. E and F, Nuclear structures of root epidermal cells expressing a nuclear localization signal-GFP marker. G and H, Nuclear structures of root epidermal cells expressing an HTB-mRFP marker. I and J, Structures of endoplasmic reticulum in root hairs stably expressing a GFP-HDEL marker. Bars = 2 μm (A–D, I, and J) and 10 μm (E–H).
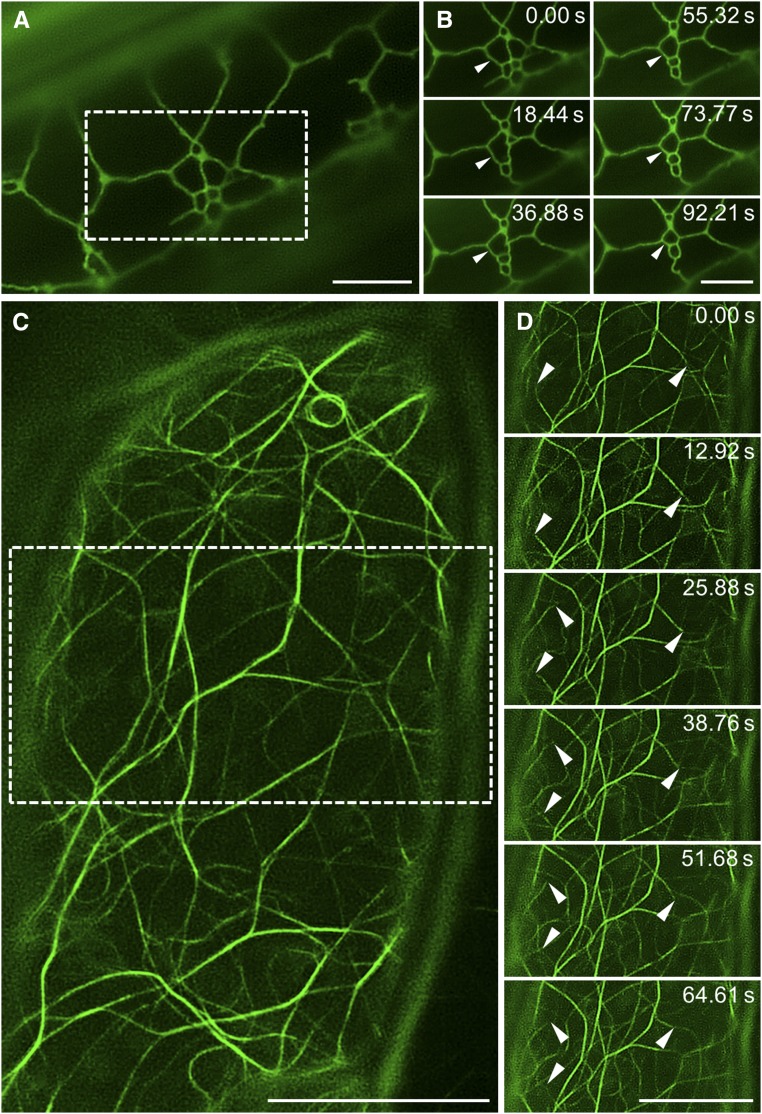
Nevertheless, time-lapse imaging using either 2D or 3D SIM is still very scarce in plant science. In the 2D mode, time-lapse SIM revealed superresolved cortical microtubule dynamics (Komis et al., 2014) and the dynamics of Flot1 under resting conditions or after flg22 treatment (Yu et al., 2017). In both cases, the subject of interest was confined at the cell periphery, being positioned close to the coverslip and, thus, facilitating image acquisition. In the same manner, other cortical structures, including the endoplasmic reticulum (Fig. 4, A and B) and the actin cytoskeleton (Fig. 4, C and D), can be tracked over time for relatively long periods.
Figure 4.
The potential for time-resolved structured illumination microscopy imaging. A and B, Overview (A) and time-lapse imaging (B) of cortical endoplasmic reticulum in a hypocotyl epidermal cell expressing a GFP-HDEL marker. The dynamic reorganization of the endoplasmic reticulum tubular structure is depicted by arrowheads (B). C and D, Overview (C) and time-lapse imaging (D) of actin filaments in a hypocotyl epidermal cell expressing a GFP-FABD2 marker. The dynamic reorganization of actin filaments is depicted by arrowheads (D). Bars = 5 μm (A and B) and 10 μm (C and D).
SIM also can be used for more advanced quantitative studies, which may include the colocalization of different subcellular structures. Colocalization studies using SIM combined with single-particle averaging were used to map the positions of individual components of complex subcellular organelles such as the spindle pole body of fission yeast (Burns et al., 2015; Bestul et al., 2017). Finally, it was shown that fluorescence recovery after photobleaching (FRAP) analysis can be adopted by SIM to provide quantitative information on protein kinetics (Schneider et al., 2013).
SIM is prone to spherical aberrations due to refractive index mismatches. This may hamper the visualization of the subcellular architecture of plant samples not adequately coated by the observation medium. It can be avoided by mounting plants in an oxygen-saturated perfluorocarbon (Littlejohn et al., 2010, 2014; Littlejohn and Love, 2012; Chi and Ambrose, 2016). SIM also is prone to optical artifacts that may be caused by inaccurate grid movements (Langhorst et al., 2009), and resolving capacity depends on the labeling quality and the signal-to-noise ratio (SNR) of the sample. If the object in focus is dimly labeled and obscured by out-of-focus light or background fluorescence, the contrast of the grating pattern will be deteriorated and will not allow the calculation of the final image at the expected high resolution (Langhorst et al., 2009). Moreover, SIM is heavily dependent on postacquisition image reconstruction, while settings of the deconvolution algorithms may lead to artifacts in image reconstruction (for review, see Komis et al., 2015; Demmerle et al., 2017).
The principle of structured illumination can be combined with other microscopy methods, such as spinning disk to deliver superresolution images or wide-field epifluorescence to reduce out-of-focus readouts and provide optical sectioning of the sample. In the first case, the speed of imaging using the combination of SIM and spinning disk can achieve temporal resolutions between 30 and 100 frames per second (Hayashi and Okada, 2015). To our knowledge, a hybrid system of SIM-spinning disk is offered as an add-on for wide-field epifluorescence microscopes provided by Andor (DSD system; http://www.andor.com/learning-academy/revolution-dsd-principles-of-operation). The Zeiss Apotome system is a structured illumination add-on for wide-field microscopes that markedly improves image quality and allows for optical sectioning minimizing out-of-focus blur (https://applications.zeiss.com/C125792900358A3F/0/05BA0ED63B5E4CA2C1257E85004AB36A/$FILE/EN_41_011_081_ApoTome2.pdf).
Single-Molecule Localization Microscopy
Single-molecule localization microscopy (SMLM) includes all techniques that improve the precision of localization of single fluorophores based on their blinking (transition between emitting and dark states) under redox conditions (STORM; Rust et al., 2006; Dempsey et al., 2011) or on the localization of photoactivatable, photoswitchable, or photoconvertible fluorescent proteins (PALM; Nienhaus and Nienhaus, 2016). The precision of localization itself depends on the number of photons collected from a single molecule, the pixel size of the detector, the background signal, and the sd of the point-spread function. Therefore, it is important to acquire images at high SNR using cameras with low noise. Thus, PALM/STORM acquisitions are preferably performed using a total internal reflection microscopy (TIRF) illumination mode and electron microscopy CCD cameras.
The resolution of SMLM techniques can be optimally 10 times better than Abbe’s limit (i.e. approximately 20 nm), but these techniques suffer from extreme limitations related to sample stability and fluorophore performance during acquisition. Since they investigate fluorophore localization at tens of nanometer precision, it is essential that the sample should be very firmly immobilized, and the examined structures must exhibit negligible dynamics during the thousands of time frames needed for image acquisition (Halpern et al., 2015). The two most important fluorophore properties affecting the performance of SMLM are the number of photons detected for every switching event (transition between on/off states) and the duty cycle of the fluorophore, which represents the fraction of time that the fluorophore spends in the on state. For optimal SMLM, a high photon yield and a low duty cycle of the fluorophore are desirable (Dempsey et al., 2011). Therefore, SMLM imaging is fluorophore limited, especially regarding genetically encoded fluorescent proteins. Although plant codon-optimized photoactivatable/photoswitchable proteins (Lummer et al., 2011, 2013) and photoconvertible proteins (Hosy et al., 2015) are available, these have been used very rarely for SMLM imaging of plant samples so far. One such study employed PALM to investigate the perinuclear organization of the actin cytoskeleton in living tobacco (Nicotiana tabacum) BY-2 suspension cells labeled with the tetrameric variant of photoswitchable EosFP (IrisFP) and achieved resolution of approximately 50 nm (Durst et al., 2014). Another study utilized fusions of the monomeric photoconvertible protein mEos3.2 with plasma membrane or tonoplast proteins to decipher the kinetics of their motility by means of single-particle tracking PALM (Hosy et al., 2015). Our provided example of PALM imaging shows the nuclear distribution of DRONPA-tagged plant-specific END BINDING1c (EB1c), a member of the microtubule plus end-binding protein family of Arabidopsis (Arabidopsis thaliana), in comparison with the diffuse nuclear signal observed by wide-field microscopy (Fig. 5, A–C). Moreover, PALM localization is more effective then SIM in deciphering EB1c nuclear distribution (Fig. 5, D and E).
Figure 5.
Photoactivation localization microscopy of EB1c protein in root epidermal cells of Arabidopsis expressing an EB1c-DRONPA marker and STED of actin filaments in Arabidopsis cotyledon epidermal cells expressing a GFP-FABD2 actin marker. A to C, Wide-field (WF) epifluorescence (A) and PALM (B) imaging and quantitative assessment (C) of the precision of localization of B at approximately 40 nm. D and E, Comparison of SIM (D) and PALM (E) localization of EB1c. F and G, Comparison of CLSM (F) and STED (G) imaging of actin filaments in Arabidopsis cotyledon epidermal cells. H and I, Volumetric CLSM (H) and STED (I) imaging of actin microfilament organization in Arabidopsis cotyledon epidermal cells. Bars = 2 μm (A, B, D, and E) and 10 μm (F–I).
STORM is heavily dependent on the photochemical properties of the fluorophores. This method is used preferentially for fixed and immunolabeled probes. Moreover, the blinking of fluorophores suitable for STORM requires special buffer conditions, but with the appropriate fluorophore-buffer combination, the resolution can reach 5 nm (Dempsey et al., 2011). Efficient blinking of commonly used STORM fluorophores can be achieved in commercial mounting media, especially in Vectashield (Olivier et al., 2013). The introduction of Escherichia coli dihydrofolate reductase (eDHFR) as a protein tag of intracellular structures allows the labeling of eDHFR fusion proteins with fluorophore-conjugated trimethoprim primers used for live STORM applications (Wombacher et al., 2010).
STORM studies are very limited in plants and restricted to fixed probes. Such studies include the mapping of RNA polymerase II in interphase nuclei of Arabidopsis in fixed samples with Cy and AlexaFluor fluorophores (Schubert and Weisshart, 2015) and the visualization of cortical microtubules in fixed root cells immunolabeled with antibodies conjugated to the photoswitchable fluorophore AlexaFluor 647 at a resolution of approximately 50 nm (Dong et al., 2015).
STED
STED is a conceptually simple technique that projects two laser beams on the sample. The central Gaussian beam excites the sample while being surrounded by a doughnut-shaped eraser pulsed laser beam (Dyba and Hell, 2003). Superresolution in STED is achieved by the return in the ground state of nearly all excited fluorophores, except for those in the center of the excitation focus. Current STED beams include lasers at 592 nm, 660 nm (which corresponds to the second excitation maximum of chlorophyll), and 775 nm (https://www.leica-microsystems.com/fileadmin/downloads/Leica%20TCS%20SP8%20STED%203X/Brochures/Leica%20TCS%20SP8%20STED%203X-Brochure_EN.pdf). If compared with all other superresolution techniques, STED is based on scanning the sample with the combination of laser beams; in this way, it bears resemblance to CLSM. Since its development (Dyba and Hell, 2003; Dyba et al., 2003), STED has not found fertile ground in plant cell biology. There is only one study available (Kleine-Vehn et al., 2011). This might relate to the extreme output of the laser lines used, rendering STED quite phototoxic and unsuitable for mid- to long-term observations of living plant samples (for review, see Turkowyd et al., 2016). The original commercial STED designs used continuous-wave (CW) lasers (CW-STED), increasing the photon load and phototoxicity. An additional problem with CW lasers is that, for a short time, excited fluorophores outside the zero intensity center of the STED beam still emit photons before emission is suppressed. Such residual photons contribute to blurring of the resulting image when they are detected. This problem can be managed by so-called gated STED, detecting photons with a certain delay, in order to exclude the possibility that photons arising outside the center of the STED beam contribute to the image (Moffitt et al., 2011; Neupane et al., 2015; Castello et al., 2016). Another approach to improve the resolution of STED is the use of pulsed lasers. In this case, both lasers can be delivered as synchronous or asynchronous picosecond pulses, as in the original development of the method (Dyba and Hell, 2003). Moreover, image acquisition can be improved by resonant scanning, allowing very fast acquisition rates and high-rate volumetric imaging of actin filaments in living cotyledon epidermal cells at better resolution compared with CLSM (Fig. 5, F–I). A positive feature of STED is that, being a confocal microscopy-based technology, it can be used for superresolution in combination with quantitative imaging methods such as fluorescence correlation spectroscopy (FCS) either at the cell surface (Andrade et al., 2015) or in deeper parts of the cell (Lanzanò et al., 2017). Unlike SIM and PALM/STORM, which require postacquisition image processing to deliver the superresolved result, STED provides superresolution during acquisition.
Software-Based Superresolution for Live Cell Imaging
Software-based superresolution relies on optical fluctuations of fluorophores occurring under standard imaging conditions and the ability to record these fluctuations via single-photon-sensitive cameras. Such methods are described briefly here because they require a relatively cheap microscopic setup and represent an attractive option for the plant cell biologist.
One such example includes the acquisition of oversampled Z-stacks (i.e. smaller sample section thickness than what is defined by the Nyquist sampling rate; see https://svi.nl/NyquistRate) using a spinning disk microscope and subsequent 3D deconvolution based on commercially available software (Okamoto et al., 2012; Nakano, 2013). This approach was used in the study of cellulose synthase trafficking (Fujimoto et al., 2015).
Another software-based approach that can deliver superresolution results from any basic wide-field epifluorescence setup is called 3B (Cox et al., 2011). This method can be implemented as an ImageJ (https://imagej.nih.gov/ij/) plugin or a stand-alone platform (Cox et al., 2011; Rosten et al., 2013; both available from http://www.coxphysics.com/3b/). It is useful for time-lapse data sets of samples labeled with conventional fluorescent proteins. A big drawback of 3B is that it is extremely time consuming, which makes the processing of large data sets troublesome. However, cloud computing at affordable prices can overcome this drawback (Hu et al., 2013). A recently developed method called superresolution radial fluctuations (SRRF; Gustafsson et al., 2016; https://bitbucket.org/rhenriqueslab/nanoj-srrf/wiki/Home) is implemented as a plugin for Fiji image-analysis software (Schindelin et al., 2012; https://fiji.sc/) and can deliver superresolution information from both wide-field and confocal sources at a good temporal frame rate (1 s). In this respect, Andor has commercialized a much accelerated SRRF stream live superresolution workflow (http://www.andor.com/srrf-stream).
SOFI is another postacquisition software-processing procedure (Dertinger et al., 2009). Like 3B and SRRF, SOFI processes data sets acquired by standard wide-field epifluorescence platforms with no specification on the fluorophore but with the requirement that the fluorophore must exhibit at least two detectable fluorescent states (a dark one and a bright one; Dertinger et al., 2009). SOFI can deliver superresolution images at a lower sample SNR compared with default PALM/STORM approaches (Geissbuehler et al., 2011). Importantly, SOFI postacquisition processing can be combined with spinning disk imaging (Hosny et al., 2013).
LSFM
LSFM is a fast mesoscopic imaging method combining advantages of wide-field fluorescence microscopy and 4D volumetric imaging. It is based on sample illumination by a thin sheet of light (with controllable thickness in the range of micrometers). The light sheet can be generated in either a unilateral or, ideally, a bilateral fashion. Such an adjustable sheet of light enables optical sectioning of the sample and excitation of the fluorophores only within the thin illuminated volume. This illuminated sample volume is well adjusted to the focal plane of the detection objective, thus eliminating out-of-focus fluorophore excitation and light emission. Thus, LSFM prevents the photobleaching of fluorophores outside of the light sheet volume. Because the detection path is oriented orthogonally to the illumination, the system is capable of spherical aberration-free acquisition (Weber and Huisken, 2011). To allow optical sectioning, it is possible to either move the light sheet through the sample (Kumar et al., 2014) or, alternatively, to move the sample with respect to a stationary light sheet (Huisken et al., 2004; Chen et al., 2014; Reynaud et al., 2015; Kumar et al., 2016). With the high speed of sample movement through the light sheet, the fluorophore photobleaching as well as phototoxicity in the sample are reduced considerably. The above setup is supported by the currently existing LSFM platforms, as it allows very fast optical sectioning and rapid image acquisition. Therefore, the total effective excitation energy absorbed by the sample in LSFM can be several orders of magnitude lower in comparison with the epifluorescence wide-field or confocal laser scanning systems (Ichikawa et al., 2014; Stelzer, 2015). The sample mounted on a rotor can be rotated during multiangular acquisition, allowing precise postacquisition rendering for 4D (x, y, z, and t) image reconstruction. Although LSFM is a mesoscopic imaging method not even reaching resolution close to the diffraction limit, it became the most advanced approach for long-term developmental live cell imaging at the subcellular, cellular, tissue, organ, and whole-organism levels.
A great potential of LSFM in developmental studies is provided by its ability to maintain biological samples under almost natural conditions. The standard approach of animal sample preparation is full embedding and immobilization of the sample in transparent gel-like agarose (Kaufmann et al., 2012). Immobilization of plants in agarose ensures their stabilization in the vertical orientation. However, embedding of plant aerial parts is not compatible with proper physiological conditions, which are necessary for long-term imaging. Recently developed open systems for plant preparation and maintenance during LSFM long-term imaging (Ovečka et al., 2015; von Wangenheim et al., 2017) ensure that plants are maintained in the microscope vertically oriented (according to the gravity vector). Simultaneously, continuous root growth is supported by appropriate culture medium, and the aerial part grows freely in the air. Controllable illumination devices maintain desirable photoperiod protocols; thus, imaging experiments can be performed over a period of several days. Altogether, LSFM is a forefront method for long-term developmental imaging of plants in a natural vertical orientation maintaining their undisturbed growth in near-environmental conditions.
The most common type of LSFM devices is based on a classical selective-plane illumination microscopy (SPIM) principle with a horizontal setup for both illumination and detection objectives, where the sample is introduced to the field of view vertically from above. Microscopy modalities based on this hardware arrangement are either commercially available (Lightsheet Z.1 from Carl Zeiss) or can be constructed in place directly according to the OpenSPIM concept (http://openspim.org). The OpenSPIM concept was developed to adapt modular SPIM systems to the particular requirements of end users (Gualda et al., 2013; Pitrone et al., 2013). The concept is well supported by the community and provides a broad range of biological applications (Girstmair et al., 2016). By means of the vertical placement of the sample, both commercially available and custom-made LSFM/SPIM systems are suitable and already have been used successfully for long-term developmental imaging of plants. An alternative LSFM system based on the inclined optical arrangement, like dual-view inverted SPIM, might be designed for the imaging of animal living samples at subcellular resolution (Kumar et al., 2014, 2016). Such a system is optimal for the horizontal placement of rather thin and transparent samples mounted in a standard glass slide format (Kumar et al., 2014); therefore, it has no practical application on living plants so far.
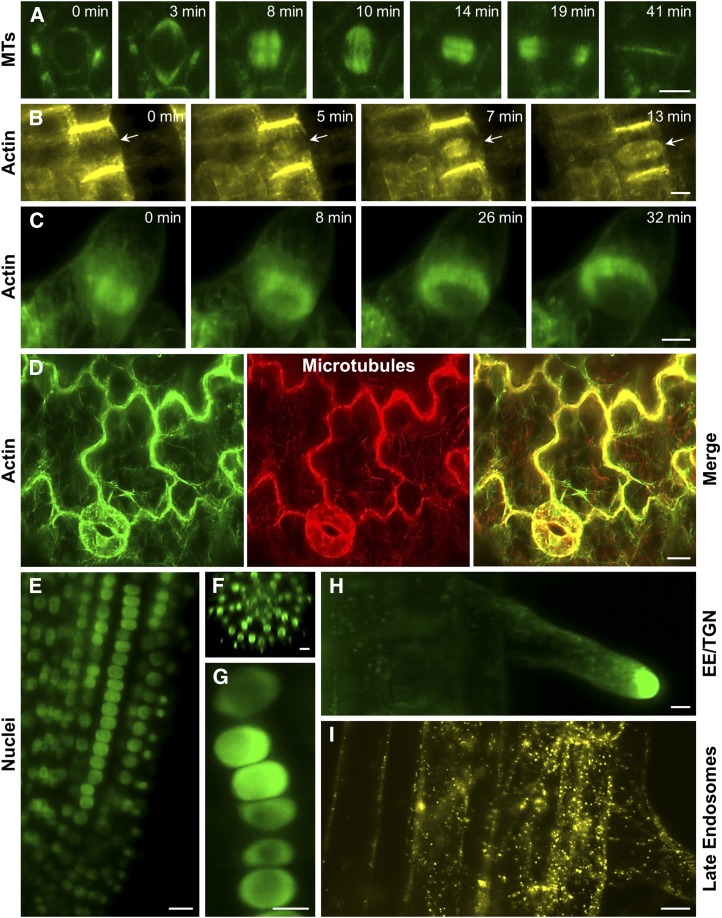
Applications of LFSM in plant biology are dynamically evolving, while studies on growing Arabidopsis roots provided long-term observations of cell division progression in cells carrying the microtubule markers GFP-MBD (Maizel et al., 2011) and GFP-TUA5 (Ovečka et al., 2015; Fig. 6A). However, the spatial and temporal resolutions of LSFM also allow characterizing the redistributions of actin filaments in the phragmoplast during cytokinesis of Arabidopsis root cells carrying the actin marker YFP-FABD2-YFP (Fig. 6B) or tobacco BY-2 suspension cells carrying another actin marker, GFP-Lifeact (Fig. 6C). Owing to the high speed of imaging, LSFM can track the dynamics of more than one subcellular structure (Fig. 6D). Thus, Ovečka et al. (2015) showed live coimaging of actin filaments and microtubules in the cotyledon epidermal cells, while von Wangenheim et al. (2016) tracked cell fate by simultaneous imaging of plasma membrane and nuclear markers during lateral root formation. LSFM, unlike other live cell-imaging techniques, was able to successfully localize signaling protein such as mitogen-activated protein kinase 6 (MPK6) to the preprophase band of Arabidopsis root meristematic cells (Smékalová et al., 2014). One possible target of MPK6 is plant-specific EB1c. This protein is localized in the nucleus of nondividing cells. Long-term developmental imaging by LSFM revealed the correlation between nuclear size and EB1c content in diverse root tissues (epidermis, cortex, and endodermis) and in different developmental zones (meristematic, transition, and elongation zones) of the Arabidopsis root (Fig. 6, E–G; Novák et al., 2016). LSFM also allowed the precise characterization of cell division patterns during the formation and growth of Arabidopsis lateral roots (von Wangenheim et al., 2016). Another important advantage of LSFM for dynamic developmental studies of plants is deep imaging of nuclei (Novák et al., 2016) and endosomes (Berson et al., 2014) in inner root tissues and the undisturbed development of root hairs (Fig. 6, E–I). The good spatial and excellent temporal resolutions of LSFM also facilitate the simultaneous monitoring of mitotic microtubules in several tissue layers of the root meristem of the crop species Medicago sativa that is significantly thicker in comparison with Arabidopsis (Vyplelová et al., 2017). Recently, it was demonstrated that developmental imaging can be scaled up in existing commercial platforms by customized sample imaging chambers, allowing high-throughput imaging of developmental processes such as root growth (de Luis Balaguer et al., 2016).
Figure 6.
Advances of LSFM imaging of dynamic cellular and developmental processes in plants. A, Imaging of microtubules (MTs) in a time-course recording of a dividing Arabidopsis root epidermal cell as visualized using a GFP-TUA5 marker. B, Time-course imaging of actin in phragmoplast of a dividing Arabidopsis root epidermal cell (arrows) as visualized using a YFP-FABD2-YFP marker. C, Time-course imaging of actin in phragmoplast of a dividing BY-2 suspension cell as visualized using a GFP-Lifeact marker. D, Covisualization of actin using GFP-FABD2 and microtubules using mCherry-TUA5 in cotyledon stomata and pavement cells of Arabidopsis. E to G, Localization of EB1c in nuclei of Arabidopsis root as visualized using an EB1c-GFP marker. The high potential of LSFM for deep tissue imaging provides insight into EB1c-GFP nuclear localization in cells of different developmental zones (E), different root cell layers (F), as well as in individual cell files (G). H, Localization of the small Rab GTPase RabA1d in early endosomal-trans-Golgi network (EE-TGN) compartments accumulating at the tip of growing root hairs as visualized using a GFP-RabA1d marker. I, Imaging of late endosomes in Arabidopsis cortical and epidermal root cells as visualized using a YFP-RabF2a marker. Bars = 5 µm (A, B, and G–I) and 10 µm (C–F).
LSFM also can track physiological processes such as calcium fluxes by using ratiometric calcium indicators in the intact and freely moving nematode Caenorhabditis elegans (Ardiel et al., 2017) or during the neuronal activity of zebrafish larvae (Barykina et al., 2017). In the first case, fast exposure (2.5–4.5 ms) was translated to a 5-Hz volumetric acquisition frame rate, allowing imaging of the entire animal. Along with advancing volumetric image acquisition by paralleled illumination with more than one light sheet (Dean et al., 2017), such results exemplify the potential of LSFM to monitor fast physiological events with subcellular resolution in whole organisms, organs, or tissues.
The growth of sensitive cells such as root hairs may be compromised under standard conditions of sample preparation and imaging with conventional microscopes (Ovečka et al., 2005). By using LSFM, it is possible to monitor the growth of root hairs from their initiation to full differentiation but also to track fast-moving organelles such as endosomes and correlate their dynamics to the pattern of root hair elongation (Fig. 6, H and I; Berson et al., 2014). Moreover, LSFM was combined recently with Förster resonance energy transfer (FRET) to address the respiration rates of root hairs using a FRET sensor for MgATP production (De Col et al., 2017). A similar FRET-based sensor was used to follow cytosolic calcium oscillations during root (Costa et al., 2013) and root hair (Candeo et al., 2017) growth.
Of considerable importance for the implementation of LSFM in any laboratory is the fact that LSFM data sets are very big and routinely exceed terabyte sizes. This makes the postacquisition storage, processing (e.g. multiview registration and fusion), and analysis of such data sets quite demanding. At present, there are software solutions that efficiently address the problem of data access and postacquisition processing, including BigDataViewer (Pietzsch et al., 2015) and TeraFly (Bria et al., 2016).
Obviously, LSFM is not able to compete with superresolution methods providing nanotracking of structures and organelles at the subcellular level. However, LSFM significantly advanced the developmental imaging of plants, providing near-environmental conditions. In the near future, the functional combination of LSFM and superresolution concepts (Chen et al., 2014) might provide a new platform for very powerful spatiotemporal imaging of living plants.
SHORT UPDATE ON WIDE-FIELD AND CONFOCAL MICROSCOPY SYSTEMS
Research-grade wide-field epifluorescence microscopes and single-photon CLSM devices are in the standard setup of any cell biology research unit, and there have been numerous studies employing such microscopes. Especially CLSM has been in the forefront of qualitative and quantitative studies, such as the assessment of protein-protein interactions through FRET, recording of protein dynamics with fluorescence lifetime imaging (FLIM), and interactions with FLIM/FRET (Hoffmann et al., 2014; Garagounis et al., 2017; Lv et al., 2017), or the recording and the quantification of diffusion/mobility of single molecular species through FCS (Li et al., 2016). Especially in CLSM, speed, sensitivity, and the spectral range of image acquisition have been improved by resonant scanning approaches and the implementation of gallium arsenide phosphide alloy-based multispectral or hybrid detectors using sensitive avalanche photodiodes combined with the high dynamic range of PMTs (for review, see Jonkman and Brown, 2015).
For more specialized applications, platforms are expanding to include TIRF, particularly suitable for in vitro microscopic assays, variable-angle emission microscopy, with increased depth of excitation compared with TIRF, and finally, spinning disk microscopy, which is ideal for fast 3D time-lapse imaging of demanding events such as mitosis (Komis et al., 2017) but with reduced resolution capacity compared with CLSM (Wang et al., 2005). Detailed descriptions of these modalities are beyond the scope of this Update, and the reader is referred to relevant reviews (Henty-Ridilla et al., 2013; Shaw and Ehrhardt, 2013; Li et al., 2015).
Airyscan CLSM
A recent advancement of CLSM with improved resolution is Airyscan CLSM. The Airyscan detector has 32 subAiry (0.25 AU each) gallium arsenide phosphide alloy-based elements that are geometrically fitted to acquire the entire Airy pattern of emitted light and assign it to the correct position by software reconstruction (Weisshart, 2014; Huff et al., 2015). The averaged image from the detector elements can have improved xy resolution by a factor of 1.7 (resulting in a theoretical value of 140 nm). This corresponds to a CLSM device with the pinhole adjusted to approximately 0.2 AU but with much improved SNR (Weisshart, 2014). At the fast imaging mode, Airyscan frame rates approximate resonant scanning, being able to reach 27 frames per second but with compromise in resolution. Airyscan CLSM is a very recent advancement of microscopy technologies, and its applications have been limited. By taking advantage of high-speed acquisition at improved resolution, it was possible to capture microtubule-buckling events during the contraction of cardiomyocytes at high resolution (Robison et al., 2016). Very recently, Airyscan CLSM was used to capture mitotic spindle and phragmoplast dynamics in Arabidopsis ktn1-2 mutants devoid of the KATANIN1 protein (Komis et al., 2017).
CONCLUSION AND OUTLOOK
A survey of the available literature can show the importance of microscopy in plant live imaging. All the existing fluorescence-based microscopy methods have been applied to visualize different organelles and subcellular compartments. For quantitative approaches such as FRAP, FRET, FLIM, and FCS, the CLSM apparatus remains the workhorse, although other methods may be used instead (i.e. SIM can be combined with FRAP [Conduit et al., 2015] and STED can be used for FCS [Lanzanò et al., 2017]). With no doubt, recently developed advanced superresolution methods (such as SIM, PALM, STORM, and STED, software-based superresolution approaches, and Airyscan CLSM) and methods for long-term developmental imaging (such as LSFM and SPIM) have provided new dimensions and perspectives for better imaging of plant cells, especially in terms of significantly improved spatial and temporal resolution.
The field of microscopy is vigorously advancing toward hybrid methods, including Bessel or Airy beam illumination and lattice light-sheet microscopy. These were developed to compensate for speed, spatial resolution, and physiological imaging in just one microscope (Fahrbach and Rohrbach, 2012; Chen et al., 2014; Vettenburg et al., 2014). In the near future, they will possibly be applicable to the cumbersome plant specimens (Meinert et al., 2016). Such platforms will allow subcellular tracking of complex structures like the mitotic spindle in plant cells with unprecedented spatiotemporal resolution and with developmental connotations, similar to already published lattice light-sheet imaging of animal cells (Yamashita et al., 2015).
Integration of the precision of localization-based superresolution methods in plant cell imaging (including PALM and STORM) is expected to expand and help us uncover details of the molecular architecture and intermolecular associations within complex subcellular structures in the near future.
Acknowledgments
We thank Jinxing Lin (Key Laboratory of Photosynthesis and Molecular Environmental Physiology, Institute of Botany, Chinese Academy of Sciences) for Arabidopsis seeds carrying the proFlot1::FLOT1:GFP marker, Dr. Juraj Gregan (Department of Genetics, Faculty of Natural Sciences, Comenius University) for Schizosaccharomyces pombe strains carrying GFP-pcp1 and mCherry-atb2 spindle pole body and microtubule markers, Dr. Yoshihisa Oda (Department of Biological Sciences, Graduate School of Science, University of Tokyo) for the HTB-mRFP chromatin marker, Dr. Javier Pozueta (Instituto de Agrobiotecnología, Universidad Pública de Navarra) for Arabidopsis seeds carrying ADP-sugar pyrophosphatase fused with GFP, Dr. Yasuo Niwa (Laboratory of Plant Cell Technology, Graduate School of Nutritional and Environmental Sciences, University of Shizuoka) for Arabidopsis seeds carrying GFP-tagged mitochondria-targeting presequence of the N terminus of the γ-subunit of F1-ATPase, Dr. Jim Haseloff (Division of Cell Biology, Medical Research Council Laboratory of Molecular Biology, United Kingdom) for Arabidopsis seeds carrying GFP fused to the HDEL endoplasmic reticulum retention sequence mGFP5, Dr. Jiří Macas (Institute of Plant Molecular Biology, Czech Republic) for Arabidopsis seeds carrying GFP fused to a nuclear localization signal and to the complete coding region of GUS, Dr. Arun Sampathkumar (Max Planck Institute of Molecular Plant Physiology) for Arabidopsis seeds carrying the double markers GFP-FABD2 and mCherry-TUA5, and Dr. Andrei Smertenko (Institute of Biological Chemistry, Washington State University) for BY-2 cultures expressing GFP-Lifeact.
Footnotes
This work was supported by the Czech Science Foundation (GAČR), projects 15-19284 and 16-24313S.
Articles can be viewed without a subscription.
References
- Andrade DM, Clausen MP, Keller J, Mueller V, Wu C, Bear JE, Hell SW, Lagerholm BC, Eggeling C (2015) Cortical actin networks induce spatio-temporal confinement of phospholipids in the plasma membrane: a minimally invasive investigation by STED-FCS. Sci Rep 5: 11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiel EL, Kumar A, Marbach J, Christensen R, Gupta R, Duncan W, Daniels JS, Stuurman N, Colón-Ramos D, Shroff H (2017) Visualizing calcium flux in freely moving nematode embryos. Biophys J 112: 1975–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barykina NV, Subach OM, Piatkevich KD, Jung EE, Malyshev AY, Smirnov IV, Bogorodskiy AO, Borshchevskiy VI, Varizhuk AM, Pozmogova GE, et al. (2017) Green fluorescent genetically encoded calcium indicator based on calmodulin/M13-peptide from fungi. PLoS ONE 12: e0183757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson T, von Wangenheim D, Takáč T, Šamajová O, Rosero A, Ovečka M, Komis G, Stelzer EH, Šamaj J (2014) Trans-Golgi network localized small GTPase RabA1d is involved in cell plate formation and oscillatory root hair growth. BMC Plant Biol 14: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestul AJ, Yu Z, Unruh JR, Jaspersen SL (2017) Molecular model of fission yeast centrosome assembly determined by superresolution imaging. J Cell Biol 216: 2409–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF (2006) Imaging intracellular fluorescent proteins at nanometer resolution. Science 313: 1642–1645 [DOI] [PubMed] [Google Scholar]
- Bottomley AL, Turnbull L, Whitchurch CB, Harry EJ (2017) Immobilization techniques of bacteria for live super-resolution imaging using structured illumination microscopy. Methods Mol Biol 1535: 197–209 [DOI] [PubMed] [Google Scholar]
- Bria A, Iannello G, Onofri L, Peng H (2016) TeraFly: real-time three-dimensional visualization and annotation of terabytes of multidimensional volumetric images. Nat Methods 13: 192–194 [DOI] [PubMed] [Google Scholar]
- Burns S, Avena JS, Unruh JR, Yu Z, Smith SE, Slaughter BD, Winey M, Jaspersen SL (2015) Structured illumination with particle averaging reveals novel roles for yeast centrosome components during duplication. eLife 4: 08586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candeo A, Doccula FG, Valentini G, Bassi A, Costa A (2017) Light sheet fluorescence microscopy quantifies calcium oscillations in root hairs of Arabidopsis thaliana. Plant Cell Physiol 58: 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello M, Tortarolo G, Hernández IC, Bianchini P, Buttafava M, Boso G, Tosi A, Diaspro A, Vicidomini G (2016) Gated-STED microscopy with subnanosecond pulsed fiber laser for reducing photobleaching. Microsc Res Tech 79: 785–791 [DOI] [PubMed] [Google Scholar]
- Chen BC, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, Janetopoulos C, Wu XS, Hammer JA III, Liu Z, et al. (2014) Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346: 1257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z, Ambrose C (2016) Microtubule encounter-based catastrophe in Arabidopsis cortical microtubule arrays. BMC Plant Biol 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conduit PT, Wainman A, Novak ZA, Weil TT, Raff JW (2015) Re-examining the role of Drosophila Sas-4 in centrosome assembly using two-colour-3D-SIM FRAP. eLife 4: 08483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Candeo A, Fieramonti L, Valentini G, Bassi A (2013) Calcium dynamics in root cells of Arabidopsis thaliana visualized with selective plane illumination microscopy. PLoS ONE 8: e75646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S, Rosten E, Monypenny J, Jovanovic-Talisman T, Burnette DT, Lippincott-Schwartz J, Jones GE, Heintzmann R (2011) Bayesian localization microscopy reveals nanoscale podosome dynamics. Nat Methods 9: 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean KM, Roudot P, Welf ES, Pohlkamp T, Garrelts G, Herz J, Fiolka R (2017) Imaging subcellular dynamics with fast and light-efficient volumetrically parallelized microscopy. Optica 4: 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Col V, Fuchs P, Nietzel T, Elsässer M, Voon CP, Candeo A, Seeliger I, Fricker MD, Grefen C, Møller IM, et al. (2017) ATP sensing in living plant cells reveals tissue gradients and stress dynamics of energy physiology. eLife 6: 26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luis Balaguer MA, Ramos-Pezzotti M, Rahhal MB, Melvin CE, Johannes E, Horn TJ, Sozzani R (2016) Multi-sample Arabidopsis Growth and Imaging Chamber (MAGIC) for long term imaging in the ZEISS Lightsheet Z.1. Dev Biol 419: 19–25 [DOI] [PubMed] [Google Scholar]
- Demmerle J, Innocent C, North AJ, Ball G, Müller M, Miron E, Matsuda A, Dobbie IM, Markaki Y, Schermelleh L (2017) Strategic and practical guidelines for successful structured illumination microscopy. Nat Protoc 12: 988–1010 [DOI] [PubMed] [Google Scholar]
- Dempsey GT, Vaughan JC, Chen KH, Bates M, Zhuang X (2011) Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat Methods 8: 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertinger T, Colyer R, Iyer G, Weiss S, Enderlein J (2009) Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI). Proc Natl Acad Sci USA 106: 22287–22292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertinger T, Colyer R, Vogel R, Enderlein J, Weiss S (2010a) Achieving increased resolution and more pixels with superresolution optical fluctuation imaging (SOFI). Opt Express 18: 18875–18885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertinger T, Heilemann M, Vogel R, Sauer M, Weiss S (2010b) Superresolution optical fluctuation imaging with organic dyes. Angew Chem Int Ed Engl 49: 9441–9443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Yang X, Zhu S, Bassham DC, Fang N (2015) Stochastic optical reconstruction microscopy imaging of microtubule arrays in intact Arabidopsis thaliana seedling roots. Sci Rep 5: 15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudin O, Bendezú FO, Groux R, Laroche T, Seitz A, Martin SG (2015) A formin-nucleated actin aster concentrates cell wall hydrolases for cell fusion in fission yeast. J Cell Biol 208: 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst S, Hedde PN, Brochhausen L, Nick P, Nienhaus GU, Maisch J (2014) Organization of perinuclear actin in live tobacco cells observed by PALM with optical sectioning. J Plant Physiol 171: 97–108 [DOI] [PubMed] [Google Scholar]
- Dyba M, Hell SW (2003) Photostability of a fluorescent marker under pulsed excited-state depletion through stimulated emission. Appl Opt 42: 5123–5129 [DOI] [PubMed] [Google Scholar]
- Dyba M, Jakobs S, Hell SW (2003) Immunofluorescence stimulated emission depletion microscopy. Nat Biotechnol 21: 1303–1304 [DOI] [PubMed] [Google Scholar]
- Fahrbach FO, Rohrbach A (2012) Propagation stability of self-reconstructing Bessel beams enables contrast-enhanced imaging in thick media. Nat Commun 3: 632. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon J, Bell K, King E, Oparka K (2010) Super-resolution imaging of plasmodesmata using three-dimensional structured illumination microscopy. Plant Physiol 153: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R, Wicker K, Müller W, Jost A, Heintzmann R (2016) Motion artefact detection in structured illumination microscopy for live cell imaging. Opt Express 24: 22121–22134 [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Suda Y, Vernhettes S, Nakano A, Ueda T (2015) Phosphatidylinositol 3-kinase and 4-kinase have distinct roles in intracellular trafficking of cellulose synthase complexes in Arabidopsis thaliana. Plant Cell Physiol 56: 287–298 [DOI] [PubMed] [Google Scholar]
- Garagounis C, Kostaki KI, Hawkins TJ, Cummins I, Fricker MD, Hussey PJ, Hetherington AM, Sweetlove LJ (2017) Microcompartmentation of cytosolic aldolase by interaction with the actin cytoskeleton in Arabidopsis. J Exp Bot 68: 885–898 [DOI] [PubMed] [Google Scholar]
- Geissbuehler S, Dellagiacoma C, Lasser T (2011) Comparison between SOFI and STORM. Biomed Opt Express 2: 408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girstmair J, Zakrzewski A, Lapraz F, Handberg-Thorsager M, Tomancak P, Pitrone PG, Simpson F, Telford MJ (2016) Light-sheet microscopy for everyone? Experience of building an OpenSPIM to study flatworm development. BMC Dev Biol 16: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualda EJ, Vale T, Almada P, Feijó JA, Martins GG, Moreno N (2013) OpenSpinMicroscopy: an open-source integrated microscopy platform. Nat Methods 10: 599–600 [DOI] [PubMed] [Google Scholar]
- Gustafsson MG. (2000) Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J Microsc 198: 82–87 [DOI] [PubMed] [Google Scholar]
- Gustafsson N, Culley S, Ashdown G, Owen DM, Pereira PM, Henriques R (2016) Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nat Commun 7: 12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern AR, Howard MD, Vaughan JC (2015) Point by point: an introductory guide to sample preparation for single-molecule, super-resolution fluorescence microscopy. Curr Protoc Chem Biol 7: 103–120 [DOI] [PubMed] [Google Scholar]
- Hayashi S, Okada Y (2015) Ultrafast superresolution fluorescence imaging with spinning disk confocal microscope optics. Mol Biol Cell 26: 1743–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henty-Ridilla JL, Li J, Blanchoin L, Staiger CJ (2013) Actin dynamics in the cortical array of plant cells. Curr Opin Plant Biol 16: 678–687 [DOI] [PubMed] [Google Scholar]
- Hirano Y, Matsuda A, Hiraoka Y (2015) Recent advancements in structured-illumination microscopy toward live-cell imaging. Microscopy (Oxf) 64: 237–249 [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Moes D, Dieterle M, Neumann K, Moreau F, Tavares Furtado A, Dumas D, Steinmetz A, Thomas C (2014) Live cell imaging reveals actin-cytoskeleton-induced self-association of the actin-bundling protein WLIM1. J Cell Sci 127: 583–598 [DOI] [PubMed] [Google Scholar]
- Hosny NA, Song M, Connelly JT, Ameer-Beg S, Knight MM, Wheeler AP (2013) Super-resolution imaging strategies for cell biologists using a spinning disk microscope. PLoS ONE 8: e74604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Martinière A, Choquet D, Maurel C, Luu DT (2015) Super-resolved and dynamic imaging of membrane proteins in plant cells reveal contrasting kinetic profiles and multiple confinement mechanisms. Mol Plant 8: 339–342 [DOI] [PubMed] [Google Scholar]
- Hu YS, Nan X, Sengupta P, Lippincott-Schwartz J, Cang H (2013) Accelerating 3B single-molecule super-resolution microscopy with cloud computing. Nat Methods 10: 96–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J, Bathe W, Netz R, Anhut T, Weisshart K (2015) The Airyscan detector from ZEISS: confocal imaging with improved signal-to-noise ratio and super-resolution. Nat Methods 12: 10.1038/nmeth.f.388 [DOI] [Google Scholar]
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EHK (2004) Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305: 1007–1009 [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Nakazato K, Keller PJ, Kajiura-Kobayashi H, Stelzer EHK, Mochizuki A, Nonaka S (2014) Live imaging and quantitative analysis of gastrulation in mouse embryos using light-sheet microscopy and 3D tracking tools. Nat Protoc 9: 575–585 [DOI] [PubMed] [Google Scholar]
- Jonkman J, Brown CM (2015) Any way you slice it: a comparison of confocal microscopy techniques. J Biomol Tech 26: 54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Mickoleit M, Weber M, Huisken J (2012) Multilayer mounting enables long-term imaging of zebrafish development in a light sheet microscope. Development 139: 3242–3247 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Wabnik K, Martinière A, Łangowski Ł, Willig K, Naramoto S, Leitner J, Tanaka H, Jakobs S, Robert S, et al. (2011) Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol Syst Biol 7: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kner P, Chhun BB, Griffis ER, Winoto L, Gustafsson MG (2009) Super-resolution video microscopy of live cells by structured illumination. Nat Methods 6: 339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K, Wang P, Kriechbaumer V, Tilsner J, Frigerio L, Sparkes I, Hawes C, Oparka K (2015) Putting the squeeze on plasmodesmata: a role for reticulons in primary plasmodesmata formation. Plant Physiol 168: 1563–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Luptovčiak I, Ovečka M, Samakovli D, Šamajová O, Šamaj J (2017) Katanin effects on dynamics of cortical microtubules and mitotic arrays in Arabidopsis thaliana revealed by advanced live-cell imaging. Front Plant Sci 8: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Mistrik M, Šamajová O, Doskočilová A, Ovečka M, Illés P, Bartek J, Šamaj J (2014) Dynamics and organization of cortical microtubules as revealed by superresolution structured illumination microscopy. Plant Physiol 165: 129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Mistrik M, Šamajová O, Ovečka M, Bartek J, Šamaj J (2015) Superresolution live imaging of plant cells using structured illumination microscopy. Nat Protoc 10: 1248–1263 [DOI] [PubMed] [Google Scholar]
- Kumar A, Christensen R, Guo M, Chandris P, Duncan W, Wu Y, Santella A, Moyle M, Winter PW, Colón-Ramos D, et al. (2016) Using stage- and slit-scanning to improve contrast and optical sectioning in dual-view inverted light sheet microscopy (diSPIM). Biol Bull 231: 26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Wu Y, Christensen R, Chandris P, Gandler W, McCreedy E, Bokinsky A, Colón-Ramos DA, Bao Z, McAuliffe M, et al. (2014) Dual-view plane illumination microscopy for rapid and spatially isotropic imaging. Nat Protoc 9: 2555–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorst MF, Schaffer J, Goetze B (2009) Structure brings clarity: structured illumination microscopy in cell biology. Biotechnol J 4: 858–865 [DOI] [PubMed] [Google Scholar]
- Lanzanò L, Scipioni L, Di Bona M, Bianchini P, Bizzarri R, Cardarelli F, Diaspro A, Vicidomini G (2017) Measurement of nanoscale three-dimensional diffusion in the interior of living cells by STED-FCS. Nat Commun 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shao L, Chen BC, Zhang X, Zhang M, Moses B, Milkie DE, Beach JR, Hammer JA III, Pasham M, et al. (2015) Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science 349: aab3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xing J, Qiu Z, He Q, Lin J (2016) Quantification of membrane protein dynamics and interactions in plant cells by fluorescence correlation spectroscopy. Mol Plant 9: 1229–1239 [DOI] [PubMed] [Google Scholar]
- Linnik O, Liesche J, Tilsner J, Oparka KJ (2013) Unraveling the structure of viral replication complexes at super-resolution. Front Plant Sci 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn GR, Gouveia JD, Edner C, Smirnoff N, Love J (2010) Perfluorodecalin enhances in vivo confocal microscopy resolution of Arabidopsis thaliana mesophyll. New Phytol 186: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Littlejohn GR, Love J (2012) A simple method for imaging Arabidopsis leaves using perfluorodecalin as an infiltrative imaging medium. J Vis Exp 59: 3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn GR, Mansfield JC, Christmas JT, Witterick E, Fricker MD, Grant MR, Smirnoff N, Everson RM, Moger J, Love J (2014) An update: improvements in imaging perfluorocarbon-mounted plant leaves with implications for studies of plant pathology, physiology, development and cell biology. Front Plant Sci 5: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummer M, Humpert F, Steuwe C, Caesar K, Schüttpelz M, Sauer M, Staiger D (2011) Reversible photoswitchable DRONPA-s monitors nucleocytoplasmic transport of an RNA-binding protein in transgenic plants. Traffic 12: 693–702 [DOI] [PubMed] [Google Scholar]
- Lummer M, Humpert F, Wiedenlübbert M, Sauer M, Schüttpelz M, Staiger D (2013) A new set of reversibly photoswitchable fluorescent proteins for use in transgenic plants. Mol Plant 6: 1518–1530 [DOI] [PubMed] [Google Scholar]
- Lv X, Jing Y, Xiao J, Zhang Y, Zhu Y, Julian R, Lin J (2017) Membrane microdomains and the cytoskeleton constrain AtHIR1 dynamics and facilitate the formation of an AtHIR1-associated immune complex. Plant J 90: 3–16 [DOI] [PubMed] [Google Scholar]
- Maizel A, von Wangenheim D, Federici F, Haseloff J, Stelzer EH (2011) High-resolution live imaging of plant growth in near physiological bright conditions using light sheet fluorescence microscopy. Plant J 68: 377–385 [DOI] [PubMed] [Google Scholar]
- Marc J, Granger CL, Brincat J, Fisher DD, Kao T, McCubbin AG, Cyr RJ (1998) A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell 10: 1927–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert T, Tietz O, Palme KJ, Rohrbach A (2016) Separation of ballistic and diffusive fluorescence photons in confocal light-sheet microscopy of Arabidopsis roots. Sci Rep 6: 30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt JR, Osseforth C, Michaelis J (2011) Time-gating improves the spatial resolution of STED microscopy. Opt Express 19: 4242–4254 [DOI] [PubMed] [Google Scholar]
- Nakano A. (2013) Super-resolution confocal live imaging microscopy (SCLIM): cutting-edge technology in cell biology. Conf Proc IEEE Eng Med Biol Soc 2013: 133–135 [DOI] [PubMed] [Google Scholar]
- Neupane B, Jin T, Mellor LF, Loboa EG, Ligler FS, Wang G (2015) Continuous-wave stimulated emission depletion microscope for imaging actin cytoskeleton in fixed and live cells. Sensors (Basel) 15: 24178–24190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus K, Nienhaus GU (2016) Chromophore photophysics and dynamics in fluorescent proteins of the GFP family. J Phys Condens Matter 28: 443001. [DOI] [PubMed] [Google Scholar]
- Novák D, Kuchařová A, Ovečka M, Komis G, Šamaj J (2016) Developmental nuclear localization and quantification of GFP-tagged EB1c in Arabidopsis root using light-sheet microscopy. Front Plant Sci 6: 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Kurokawa K, Matsuura-Tokita K, Saito C, Hirata R, Nakano A (2012) High-curvature domains of the ER are important for the organization of ER exit sites in Saccharomyces cerevisiae. J Cell Sci 125: 3412–3420 [DOI] [PubMed] [Google Scholar]
- Olivier N, Keller D, Rajan VS, Gönczy P, Manley S (2013) Simple buffers for 3D STORM microscopy. Biomed Opt Express 4: 885–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovečka M, Lang I, Baluška F, Ismail A, Illeš P, Lichtscheidl IK (2005) Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 226: 39–54 [DOI] [PubMed] [Google Scholar]
- Ovečka M, Vaškebová L, Komis G, Luptovčiak I, Smertenko A, Šamaj J (2015) Preparation of plants for developmental and cellular imaging by light-sheet microscopy. Nat Protoc 10: 1234–1247 [DOI] [PubMed] [Google Scholar]
- Pietzsch T, Saalfeld S, Preibisch S, Tomancak P (2015) BigDataViewer: visualization and processing for large image data sets. Nat Methods 12: 481–483 [DOI] [PubMed] [Google Scholar]
- Pitrone PG, Schindelin J, Stuyvenberg L, Preibisch S, Weber M, Eliceiri KW, Huisken J, Tomancak P (2013) OpenSPIM: an open-access light-sheet microscopy platform. Nat Methods 10: 598–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego EH, Shao L, Macklin JJ, Winoto L, Johansson GA, Kamps-Hughes N, Davidson MW, Gustafsson MG (2012) Nonlinear structured-illumination microscopy with a photoswitchable protein reveals cellular structures at 50-nm resolution. Proc Natl Acad Sci USA 109: E135–E143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud EG, Peychl J, Huisken J, Tomancak P (2015) Guide to light-sheet microscopy for adventurous biologists. Nat Methods 12: 30–34 [DOI] [PubMed] [Google Scholar]
- Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB, Prosser BL (2016) Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 352: 0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosten E, Jones GE, Cox S (2013) ImageJ plug-in for Bayesian analysis of blinking and bleaching. Nat Methods 10: 97–98 [DOI] [PubMed] [Google Scholar]
- Rust MJ, Bates M, Zhuang X (2006) Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods 3: 793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl SJ, Hell SW, Jakobs S (2017) Fluorescence nanoscopy in cell biology. Nat Rev Mol Cell Biol 18: 685–701 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Fuchs C, Dobay A, Rottach A, Qin W, Wolf P, Álvarez-Castro JM, Nalaskowski MM, Kremmer E, Schmid V, et al. (2013) Dissection of cell cycle-dependent dynamics of Dnmt1 by FRAP and diffusion-coupled modeling. Nucleic Acids Res 41: 4860–4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert V, Weisshart K (2015) Abundance and distribution of RNA polymerase II in Arabidopsis interphase nuclei. J Exp Bot 66: 1687–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz T, Aloush N, Goliand I, Segal I, Nachmias D, Arbely E, Elia N (2017) Direct fluorescent-dye labeling of α-tubulin in mammalian cells for live cell and superresolution imaging. Mol Biol Cell 28: 2747–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Kner P, Rego EH, Gustafsson MG (2011) Super-resolution 3D microscopy of live whole cells using structured illumination. Nat Methods 8: 1044–1046 [DOI] [PubMed] [Google Scholar]
- Shaw SL, Ehrhardt DW (2013) Smaller, faster, brighter: advances in optical imaging of living plant cells. Annu Rev Plant Biol 64: 351–375 [DOI] [PubMed] [Google Scholar]
- Shaw SL, Kamyar R, Ehrhardt DW (2003) Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 300: 1715–1718 [DOI] [PubMed] [Google Scholar]
- Small AR, Parthasarathy R (2014) Superresolution localization methods. Annu Rev Phys Chem 65: 107–125 [DOI] [PubMed] [Google Scholar]
- Smékalová V, Luptovčiak I, Komis G, Šamajová O, Ovečka M, Doskočilová A, Takáč T, Vadovič P, Novák O, Pechan T, et al. (2014) Involvement of YODA and mitogen activated protein kinase 6 in Arabidopsis post-embryogenic root development through auxin up-regulation and cell division plane orientation. New Phytol 203: 1175–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer EHK. (2015) Light-sheet fluorescence microscopy for quantitative biology. Nat Methods 12: 23–26 [DOI] [PubMed] [Google Scholar]
- Stracy M, Lesterlin C, Garza de Leon F, Uphoff S, Zawadzki P, Kapanidis AN (2015) Live-cell superresolution microscopy reveals the organization of RNA polymerase in the bacterial nucleoid. Proc Natl Acad Sci USA 112: E4390–E4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss MP, Liew AT, Turnbull L, Whitchurch CB, Monahan LG, Harry EJ (2012) 3D-SIM super resolution microscopy reveals a bead-like arrangement for FtsZ and the division machinery: implications for triggering cytokinesis. PLoS Biol 10: e1001389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilsner J, Linnik O, Louveaux M, Roberts IM, Chapman SN, Oparka KJ (2013) Replication and trafficking of a plant virus are coupled at the entrances of plasmodesmata. J Cell Biol 201: 981–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkowyd B, Virant D, Endesfelder U (2016) From single molecules to life: microscopy at the nanoscale. Anal Bioanal Chem 408: 6885–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan JC, Zhuang X (2011) New fluorescent probes for super-resolution imaging. Nat Biotechnol 29: 880–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettenburg T, Dalgarno HI, Nylk J, Coll-Lladó C, Ferrier DE, Čižmár T, Gunn-Moore FJ, Dholakia K (2014) Light-sheet microscopy using an Airy beam. Nat Methods 11: 541–544 [DOI] [PubMed] [Google Scholar]
- von Wangenheim D, Fangerau J, Schmitz A, Smith RS, Leitte H, Stelzer EH, Maizel A (2016) Rules and self-organizing properties of post-embryonic plant organ cell division patterns. Curr Biol 26: 439–449 [DOI] [PubMed] [Google Scholar]
- von Wangenheim D, Hauschild R, Friml J (2017) Light sheet fluorescence microscopy of plant roots growing on the surface of a gel. J Vis Exp 119: 55044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyplelová P, Ovečka M, Šamaj J (2017) Alfalfa root growth rate correlates with progression of microtubules during mitosis and cytokinesis as revealed by environmental light-sheet microscopy. Front Plant Sci 8: 1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Babbey CM, Dunn KW (2005) Performance comparison between the high-speed Yokogawa spinning disc confocal system and single-point scanning confocal systems. J Microsc 218: 148–159 [DOI] [PubMed] [Google Scholar]
- Weber M, Huisken J (2011) Light sheet microscopy for real-time developmental biology. Curr Opin Genet Dev 21: 566–572 [DOI] [PubMed] [Google Scholar]
- Weisshart K. (2014) The basic principle of Airyscanning. Technology note. Carl Zeiss Microscopy GmbH, 07745 Jena, Germany [Google Scholar]
- Wombacher R, Heidbreder M, van de Linde S, Sheetz MP, Heilemann M, Cornish VW, Sauer M (2010) Live-cell super-resolution imaging with trimethoprim conjugates. Nat Methods 7: 717–719 [DOI] [PubMed] [Google Scholar]
- Yamashita N, Morita M, Legant WR, Chen BC, Betzig E, Yokota H, Mimori-Kiyosue Y (2015) Three-dimensional tracking of plus-tips by lattice light-sheet microscopy permits the quantification of microtubule growth trajectories within the mitotic apparatus. J Biomed Opt 20: 101206. [DOI] [PubMed] [Google Scholar]
- Yu M, Liu H, Dong Z, Xiao J, Su B, Fan L, Komis G, Šamaj J, Lin J, Li R (2017) The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. J Plant Physiol 215: 73–84 [DOI] [PubMed] [Google Scholar]