Abstract
Plant nuclear CBC consisted of two subunits (CBP20 and CBP80) is involved in both conserved processes related to RNA metabolism and simultaneously in extremely dynamic plant stress response.
The nuclear cap-binding complex (nCBC) in higher eukaryotes specifically binds to the monomethylated (7-methylguanosine) cap structure at the 5′ end of freshly transcribed mRNA. In addition to protecting mRNAs from degradation by exonucleases, the nCBC functions in transcription, polyadenylation, splicing, miRNA (micro RNA) biogenesis, and the nuclear export of capped RNAs. Growing evidence suggests that nCBC-guided RNA metabolism plays a central role in the plant’s response to phytohormones like abscisic acid (ABA) and ethylene and to abiotic stresses such as salt and drought. Mutations in the genes encoding the two nCBC subunits CBP20 and CBP80 have become an important tool for elucidating the role of the nCBC in plant species such as Arabidopsis (Arabidopsis thaliana), potato (Solanum tuberosum), and barley (Hordeum vulgare). This review focuses on the role of the nCBC in mRNA processing events in plants and on the cellular response to ABA and abiotic stress.
CAP-Binding Proteins from Humans to Plants
Capping occurs cotranscriptionally and is one of the earliest modifications of nascent RNA Polymerase II (RNAPII) transcripts. RNAPII pauses near the transcription start site after the synthesis of a pre-RNA of about 20 nucleotides to allow for the cotranscriptional addition of the 5′-cap. This step is a prerequisite for the productive elongation of transcription (Wen et al., 1998; Calero et al., 2002; Bentley, 2014; Ramanathan et al., 2016). The cap structure consists of a 7-methylguanosine residue linked to the first transcribed nucleotide [m7G(5′)ppp(5′)X] via a 5′–5′ triphosphate bridge (Filipowicz, 1978). Capping occurs in three enzymatic steps: (1) removal of the 5′-γ-phosphate group from the first transcribed nucleotide of premRNA by an RNA triphosphate (RT); (2) transfer of the GMP nucleotide to the RNA 5′-diphosphate end, which results in a guanosine cap (GpppN); and (3) methylation by guanine-N7-methyltransferase to produce a 7-methylguanosine cap (Bentley, 2014; Ramanathan et al., 2016). Capping enzymes are recruited to the 5′ end of nascent transcripts through interaction with the C-terminal domain of the largest subunit of RNAPII. The interaction is activated by the phosphorylation of Ser-5 in C-terminal domain by CYCLIN DEPENDENT KINASE 7 (Egloff and Murphy, 2008).
The cap can be recognized, cotranscriptionally bound, and protected by the nCBC. The nCBC was identified more than 20 years ago in HeLa cervical cancer cells (Henrietta Lacks cell line) on the basis of its affinity for the m7G structure (Izaurralde et al., 1994; Izaurralde and Mattaj 1995). In 2002, the first plant nCBC components were discovered in Arabidopsis (Kmieciak et al., 2002). The nCBC is a heterodimer consisting of two subunits, named CBP20 (Cap-Binding Protein 20) and CBP80 (Cap-Binding Protein 80) based on their Mrs (20 and 80 kD, respectively). The stability of CBP20 depends on the presence of CBP80; CBP20 is no longer detected in CBP80-depleted cell extracts (Fortes et al., 1999, Kierzkowski et al., 2009).
The residues at the interface between the two subunits were identified by protein crystallography, which also revealed the presence of an m7G-binding pocket within the CBP20 protein in mammals (Mazza et al., 2001; Calero et al., 2002; Worch et al., 2005). CBP20 is conserved in yeast, animals, and plants. Studies of the RNA-binding domain of Arabidopsis CBP20, which consists of one RNP2 and one RNP1 motif (Kmieciak et al., 2002; Supplemental Material S1), demonstrated that RNP2 functions in the initiation of the CBP20-CBP80 interaction, whereas RNP1 participates in the stabilization of the CBP20-CBP80 complex (Kierzkowski et al., 2009). Plant CBP20 has a long C-terminal tail that is rich in Arg, Gly, and Asp (Kmieciak et al., 2002; Daszkowska-Golec et al., 2017); this structure is absent in animal CBP20s. The presence of conserved motifs in the C-terminal tail of plant CBP20 suggests plant-specific role(s) of CBP20 (Daszkowska-Golec et al., 2017). Among the conserved motifs are two Nuclear Localization Signals (NLSs), HRKRQR and RKRR (Kmieciak et al., 2002; Pieczynski et al., 2013; Daszkowska-Golec et al., 2017). In Arabidopsis, these NLSs mediate the import of AtCBP20, either alone or as part of the entire nCBC, into the nucleus (Kierzkowski et al., 2009). Further studies revealed that the presence of one NLS is sufficient for translocation into the nucleus (Kierzkowski et al., 2009). In contrast to the situation in plants, a NLS motif is only present in the N terminus of the CBP80 subunit in animals and fungi (Izaurralde and Mattaj, 1995). In these organisms, CBP20 is thought to enter the nucleus independently or in a complex with CBP80. Although only CBP20 is in direct contact with the 5′-cap of the mRNA, actual binding requires the presence of the CBP80 subunit (Izaurralde et al., 1995; Kierzkowski et al., 2009). CBP80 is responsible for the conformational changes of CBP20 that enable cap binding. Moreover, CBP80 acts as a platform that recruits splicing factors during spliceosome assembly (Calero et al., 2002; Mazza et al., 2001; Pabis et al., 2013). CBP80 consists of three helical domains that are connected by two linkers. The central (Domain 2) and C-terminal (Domain 3) domains of CBP80 mediate the physical interaction with CBP20 (Mazza et al., 2001; Supplemental Material S2). The N-terminal helical domain (Domain 1) of CBP80, which is similar to the MIF4G (central domain of eukaryotic translation initiation factor 4G [eIF4G]) domain, is required for a cap-dependent pioneer round of translation (Calero et al., 2002). In contrast to CBP20, the CBP80 subunit of the nCBC is not highly conserved among eukaryotes (Supplemental Material S2). Arabidopsis CBP80 was first isolated in a genetic screen for mutants with hypersensitivity to ABA and thus was named CAP BINDING PROTEIN 80/ABA HYPERSENSITIVE1 (CBP80/ABH1) (Hugouvieux et al., 2001).
The Role of the NCBC in RNA-Related Processes
Studies in yeast and human cells have shown that both the cap structure and the cap-binding complex play important roles in many RNA-related processes (for review, see Gonatopoulos-Pournatzis and Cowling, 2014). The levels of mature RNAs in eukaryotic cells, including plant cells, are determined by transcription, cotranscriptional processing, and RNA degradation. nCBC is an important factor in each of these processes.
The nCBC Functions in Constitutive and Alternative Splicing Processes
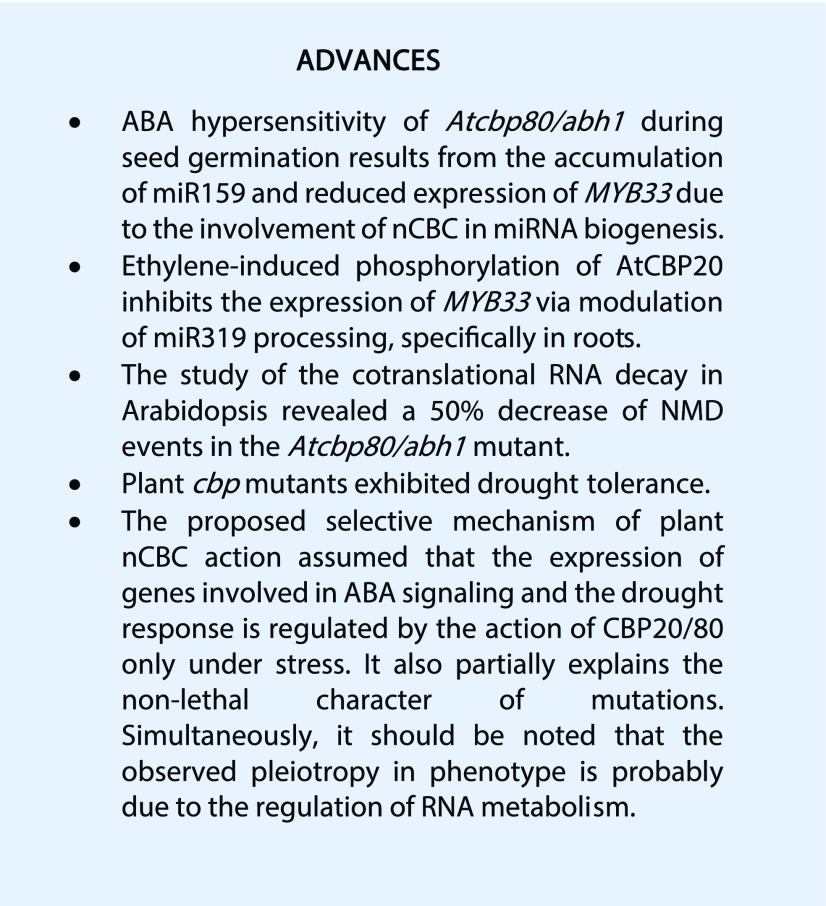
Constitutive and alternative splicing are mediated by the spliceosome, a large complex composed of five ribonucleoprotein subcomplexes. During early spliceosome assembly, the U1 small ribonucleoprotein interacts with the 5′ splice site of the intron. Since this interaction is stabilized by the nCBC (Lewis et al., 1996; Gonatopoulos-Pournatzis and Cowling, 2014), it shows that nCBC is crucial for the efficient excision of the cap proximal intron (Lewis et al., 1996; Lewis and Izaurralde, 1997; Gonatopoulos-Pournatzis and Cowling, 2014). In Arabidopsis, splicing of the first intron is regulated by the nCBC in cooperation with SERRATED (SE), a zinc finger protein that is mostly localized in nuclear Dicing-bodies (Laubinger et al., 2008; Raczynska et al., 2014; Fig. 1). The recently identified LUC7 (Lethal Unless CBC 7), which is essential for terminal intron splicing in plants, may also interact with the CBC-SE complex (Amorim et al., 2017). Both CBC-SE and LUC7 fine-tune gene expression via splicing under stress conditions (Laubinger et al., 2008; Kong et al., 2014; Raczynska et al., 2014; Amorim et al., 2017).
Figure 1.
Involvement of plant nCBC in crucial RNA-related processes. (A) nCBC and SE action during constitutive and alternative splicing. (B) The interplay between nCBC and histone modification factors such as COMPASS-like and EFS during RNA processing. (C) Interactions between the plant nCBC and its partners during the initial steps of miRNA processing. COMPASS-like, complex consisting of three subunits–ATX1 (ARABIDOPSIS TRITORAX 1), ASH2R, and WDR5a.
In Arabidopsis, one of the transcripts affected by nCBC-regulated splicing is FLC (FLOWERING LOCUS C). This gene encodes a transcription factor containing a MADS domain, which is a strong flowering repressor. FLC is positively regulated by FRIGIDA (FRI). Bezerra et al. (2004) described the early flowering phenotype of the Atcbp80/abh1 mutant. Additional studies linked the mutation in CBP80 to the suppression of the late flowering phenotype. The early flowering phenotype was shown to be caused by constitutively active FRI and its inability to increase the FLC level in the Atcbp80/abh1 mutant background. The same mutant also accumulated the splicing variant of FLC containing the first intron. The fact that FLC contains a large first intron may explain why FLC premRNA is more sensitive than other premRNAs to the loss of the nCBC. An alternative explanation raised by Bezerra et al. (2004) is that specific regulation of FLC mRNA levels involves components that interact with a CBC-bound FLC mRNA or premRNA. In Arabidopsis, nCBC and methyltransferases cooperate during the cotranscriptional splicing of FLC (Li et al., 2016). A similar effect, that is suppression of the late flowering phenotype and a low level of FLC with an increased proportion of unspliced FLC transcripts, was reported by Geraldo et al. (2009) in the Atcbp20 mutant. To elucidate the molecular mechanism underlying this process, Laubinger et al. (2008) analyzed intron accumulation in Atcbp80/abh1, Atcbp20, and a double mutant cbp80 cbp20 using a whole genome tiling array. A total of 518 and 931 intron retention events in the Atcbp80/abh1 and Atcbp20 mutants were identified, respectively. Of these, 298 were shared by both mutants. Generally, the genes of the mutants retained only a single intron, usually the first one. This suggests that in these mutants splicing efficiency diminishes toward the 5′ end bearing the cap structure. Splicing itself, however, was not abolished in the nCBC mutants, which is consistent with the fact that these genes are not essential for viability. Additionally, singular introns of most analyzed transcripts had reduced splicing efficiencies (Laubinger et al., 2008), which may be linked to the specific sensitivity of certain introns to nCBC loss. Since the nCBC affects splicing by facilitating the spliceosomal assembly in organisms like humans or yeasts, Laubinger et al. (2008) hypothesized that the introns affected by the loss of the nCBC undergo cotranscriptional splicing, whereas other introns might be spliced posttranscriptionally.
To resolve whether the nCBC also functions in alternative splicing in Arabidopsis, Raczynska et al. (2010) investigated the Atcbp20 and Atcbp80/abh1 mutants using an alternative splicing panel (a custom high-resolution RT-PCR panel) consisting of 435 alternative splicing events and 7 controls. The AS events were transcripts from gene-encoding transcription factors, splicing factors, stress-related proteins (ABA-related), stomatal ABA signaling, flowering time regulators, and other proteins. For controls, genes were used that were intronless, having constitutively spliced introns or U12-dependent introns. The study revealed the influence of CBC on the splicing of 101 Arabidopsis genes. Moreover, the splicing was preferentially affected in the first intron (50% of the alternatively spliced transcripts in the nCBC mutants) and particularly at its 5′ splice site. Furthermore, AtCBP80 was shown to play a more significant role in splicing than AtCBP20. Taken together, these results show that in both plants and animals, the nCBC affects the efficiency of the first intron excision.
An Analysis of the Plant nCBC Revealed a Link between Splicing and Histone Modification
Active histone marks, including acetylation, H2B monoubiquitination (H2Bub1), H3K4 trimethylation (H3K4me3), and H3K36me3, have been linked to cotranscriptional RNA splicing (Spies et al., 2009; Gunderson et al., 2011). It is unclear whether and how the CBC and the active histone modifiers function together to regulate mRNA production.
Chromatin modifications play an important role in eukaryotic gene expression. H3K4me3, formed when Histone H3 Lys-4 (H3K4) is methylated by COMPASS or COMPASS-like H3K4 methyltransferase complexes that are enriched in the promoter region of actively expressed genes (Ruthenburg et al., 2007; Shilatifard, 2008), is a mark that is linked to active gene transcription (Ruthenburg et al., 2007; Chen et al., 2015; Hyun et al., 2017). In addition to H3K4me3, H3K36 di- and trimethylation (H3K36me3) are also associated with active gene expression (Wagner and Carpenter, 2012; Sui et al., 2012; Sui et al., 2013; Li et al., 2015; Hyun et al., 2017). H3K36me3 is deposited in the gene body mainly by an H3K36 methyltransferase EARLY FLOWERING IN SHORT DAYS (EFS)/SET DOMAIN GROUP 8 in Arabidopsis (Xu et al., 2008; Kim et al., 2005). Interestingly, H3K4me3 and H3K36me3 are also involved in cotranscriptional premRNA splicing, while histone acetylation and H2Bub1 have been shown to function in splicing in yeast and mammalian cells, respectively (Gunderson et al., 2011; Zhang et al., 2013).
Since FLC is the target locus of COMPASS-like, EFS, and the nCBC (Geraldo et al., 2009; Jiang et al., 2009), Li et al. (2016) investigated the interaction of these factors in the regulation of FLC expression. They found that the CBC forms multiprotein complexes through a direct interaction of the C terminus of AtCBP20 with conserved methyltransferases, the COMPASS-like H3K4 methyltransferase complex, and EFS H3K36 methyltransferase (Fig. 1B). These multiprotein complexes were shown to integrate active histone methylation with cotranscriptional mRNA processing and cap preservation, which further leads to a high level of mature mRNA production. It was also revealed that nCBC is required for the H3K4 and H3K36 trimethylation marks on FLC chromatin, since these marks were reduced in the cbp20 mutant. On the other hand, histone methyltransferases are required for CBC-mediated cap preservation and efficient splicing at their target loci, for example FLC. Together, these results suggest that COMPASS-like, EFS, and nCBC act interdependently in Arabidopsis (Li et al., 2016). Moreover, COMPASS-like and EFS were found to promote CBC-mediated mRNA cap protection and efficient cotranscriptional premRNA splicing of FLC (Li et al., 2016).
The Plant nCBC Functions in miRNA Biogenesis
Pri-miRNA produced by RNAPII carry m7G on their 5′ end, which is recognized and bound by the nCBC (Cai et al., 2004; Lee et al., 2004). AtCBP20 and AtCBP80 bind to pri-miRNA transcripts, thus increasing their processing efficiency mostly in a step that involves SERRATE, a zinc finger protein proposed to bridge the CBC and the spliceosome (Kim et al., 2008; Laubinger et al., 2008). Bielewicz et al. (2013) suggested that splicing and processing of pri‐miRNAs are coupled processes that might influence each other. The nCBC facilitates the loading of miRNA processing factors onto pri-miRNAs (Fig. 1). Prior to processing, the RNA-binding protein DAWDLE (DDL) presumably stabilizes pri-miRNAs and facilitates DICER-LIKE1 (DCL1) to access or recognize pri-miRNAs (Park et al., 2002; Yu et al., 2008). In addition, NOT2a and NOT2b (previously known as At-Negative on TATA less2 [NOT2] and VIRE2-INTERACTING PROTEIN2, respectively) proteins interact with RNAPII and other miRNA processing factors, including CBP80, CBP20, and SE, and facilitate efficient DCL1 recruitment in miRNA biogenesis (Wang et al., 2013). DCL1 sequentially processes pri-miRNAs into stem-loop precursors and eventually into miRNA/miRNA* (asterisk indicates passenger strand) duplexes (Kurihara and Watanabe, 2004). In this function, DCL1 interacts with other microprocessor components, including the dsRNA-binding domain protein HYL1, the zinc finger protein SE, and TOUGH (Ren et al., 2012). In plants, these steps of miRNA biogenesis occur in highly specialized nuclear foci called D-bodies (Liu et al., 2012). The miRNA/miRNA* duplex is further methylated by the small RNA methyltransferase HEN1 in the 2′-OH of the 3′ terminal nucleotide. Mature miRNA/miRNA* may be transported in an HST-dependent or -independent manner through the nuclear pore complex. Alternatively, the guide strand of mature miRNA/miRNA* may be selectively loaded into ARGONAUTE 1 - RNA-INDUCED SILENCING COMPLEX, thus transporting the miRNA-Induced Silencing Complex into the cytoplasm.
Compared to the wild type, the Atcbp80/abh1 and Atcbp20 mutants have reduced miRNA levels but increased levels of the corresponding pri-miRNAs. The accumulation of miRNA target transcripts in the Atcbp mutants is additional proof that the nCBC functions in miRNA processing (Kim et al., 2008). Interestingly, not all miRNAs are equally affected by the inactivation of AtCBP80 or AtCBP20 (Laubinger et al., 2008). Though mutations in AtCBP20 and AtCBP80 negatively affect plant morphology and physiology, they are not lethal. By contrast, mutations in DCL1 (Dicer like 1), another enzyme component of the miRNA biogenesis machinery, cause embryonic lethality (Schauer et al., 2002). This indicates that instead of being an essential regulator of the miRNA-processing machinery, the CBC plays a supporting role in this process. Laubinger et al. (2008) hypothesized that nCBC function is not required for pri-miRNAs that are particularly strongly expressed and that other proteins with cap-binding activity act redundantly with nCBC.
The Nuclear and Cytoplasmic Functions of the CBC Are Linked
Although most translation in eukaryotes depends on the eukaryotic translation initiation factor 4F (eIF4F) complex, the pioneer round of translation is driven by nCBC. This first round of translation in mammals occurs during the export of the newly spliced and nCBC-bound mRNA from the nucleus into the cytoplasm (Oh et al., 2007). The transition from the pioneer round to the standard mode of translation is regulated by importins. The dissociation of mRNA from nCBC is promoted by the interaction of importin-β with importin-α. Next, the eIF4F complex, which is conserved in higher eukaryotes, interacts with the cap structure, thus initiating translation (Browning and Bailey-Serres, 2015). The eIF4F complex consists of cap-binding eIF4E and the scaffolding protein eIF4G, which binds the dead-box helicase eIF4A. The mRNA and eIF4F factors are recruited to the 40S ribosome and associated eIFs to form the 48S initiation complex, which subsequently joins the 60S ribosome to form the completed 80S complex. The eIF4F complex is conserved in mammals, plants, and fungi. However, flowering plants have two distinct isoforms of the heterodimeric eIF4F complex, that is the evolutionarily conserved eIF4F and the plant-specific eIFiso4F complex (Patrick and Browning, 2012). The eIF4F and its plant-specific eIFiso4F isoform are both composed of two subunits, eIF4E and eIF4G or eIFiso4E and eIFiso4G, respectively (Browning and Bailey-Serres, 2015; Levins et al., 2016. There is structural and ancestral similarity between the subunits of the nuclear cap-binding complex and the cytoplasmic cap-binding complex (CBP20 and eIF4E/eIFiso4E and CBP80 and eIF4G/eIFiso4G, respectively; Marcotrigiano et al., 1997; Matsuo et al., 1997; Mazza et al., 2001, 2002; Calero et al., 2002; Marintchev and Wagner, 2005).
The eIF4A subunit, a member of the DEAD box helicase family, is loosely associated with other components of the cytoplasmic cap-binding complex and is easily removed during purification (Browning and Bailey-Serres, 2015). However, eIF4A is important for plant growth and development in Brachypodium and Arabidopsis (Vain et al., 2011; Bush et al., 2015). Apparently, eIF4A associates with cap complexes such as eIF4F, nCBC, and the plant-specific eIFiso4F in proliferating cells (Bush et al., 2016). eIF4E binds to the scaffold protein eIF4G to form a two-subunit eIF4F complex (Hinnebusch and Lorsch, 2012; Grüner et al., 2016; Miras et al., 2017). Protein crystallography revealed a universal binding mode of eIF4E to eIF4G in both higher plants and mammals (Grüner et al., 2016; Miras et al., 2017). eIF4E and eIFiso4E share 50% amino acid sequence similarity and form a specific complex with an eIF4G and eIFiso4G binding partner, respectively (Levins et al., 2016). Similar to CBP20 in the nCBC, eIF4E recognizes and directly binds to the cap structure. Although eIF4E and CBP20 differ substantially, the cap-binding pockets of these proteins are similar, consisting of two aromatic amino acids surrounded by basic and acidic areas that accommodate a negatively charged 5′-5′ triphosphate bridge and positively charged π-ring system of m7G, respectively. In both mammals and plants, the purine ring of m7G is sandwiched between the Trp and Tyr residues of eIF4E and CBP20 (Marcotrigiano et al., 1997; Matsuo et al., 1997; Mazza et al., 2001, 2002; Calero et al., 2002; Miras et al., 2017).
Arabidopsis contains a single gene encoding eIFiso4E and three encoding eIF4E proteins. Two of the eIF4E proteins, eIF4E1b and eIF4E1c, appear to be gene duplication products specific to the Brassicaceae family. These proteins do not belong to the canonical translation apparatus and are thought to have specialized functions (Patrick et al., 2014). The large subunits of the plant eIF4F or eIFiso4F complexes, eIF4G and eIFiso4G, are responsible for ribosome attachment and enhancing the efficiency of translation. In mammals and yeast, eIF4G interacts directly with nCBC. McKendrick et al. (2001) showed that in addition to the cytoplasmic localization of eIF4G, there is also a significant nuclear pool of this protein. It is possible that eIF4G accompanies nCBC-bound mRNA during nucleus-cytoplasm export and then facilitates the substitution of nCBC for the eIF4F complex (Fortes et al., 1999; McKendrick et al., 2001). The timing and location of the exchange of the nuclear with the cytoplasmic complex components that bind the mRNA cap remain to be determined (Browning and Bailey-Serres, 2015). The large subunits eIF4G and eIFiso4G share similarity in the C-terminal half containing the eIF4E binding site and two HEAT (Huntington elongation factor 3, protein phosphatase 2A and the yeast TOR1 kinase) domains. The difference between these two subunits is the lack of the N-terminal region in the eIFiso4G subunit. Studies on animal eIF4G and CBP80 have revealed a common origin and domain structure (Marintchev and Wagner, 2005). The high degree of similarity between CBP80 and eIF4G allowed the interdomain orientation of eIF4G to be predicted.
The eIF4G has three MIF4G (middle portion of eIF4G) domains, which are similar to the HEAT domains and composed of helical hairpins. The high degree of similarity between eIF4G and CBP80 amino acid sequences was shown mainly within HEAT domains. The orientation of the three HEAT domains of eIF4G may be similar to the one present in CBP80 when in complex with CBP20 (Marintchev and Wagner, 2005; Miras et al., 2017). However, the most intriguing aspect is the presence of two eIF4F complexes in plants. The plant-specific heterodimeric cap-binding eIFiso4F complex was found to be more abundant in wheat germ extracts (approximately 5- to 10-fold higher) compared to eIF4F. This suggested that eIFiso4F is the general cytoplasmic cap-binding complex in plant cells (Browning et al., 1990). The functional deletion of the genes encoding eIFiso4G in Arabidopsis results in impaired growth, poor seed quality, and altered stress response (Lellis et al., 2010). These observations indicated that eIFiso4G plays an important role in the plant response to environmental stress (Mayberry et al., 2011). It was also shown that eIFiso4G expression is required for violaxanthin de-epoxidase expression and thus for supporting photosynthetic activity (Chen et al., 2014).
The Plant nCBC Plays an Important Role in Nonsense-Mediated mRNA Decay
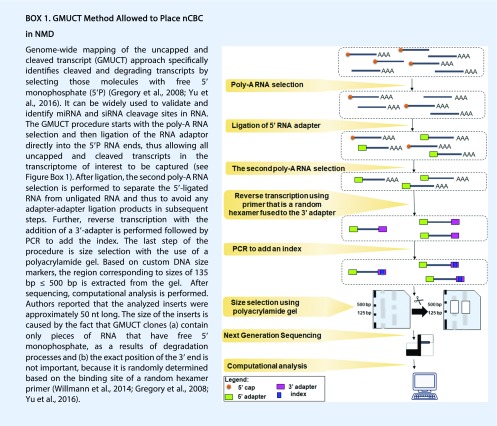
Nonsense-mediated mRNA decay (NMD) is a pathway of RNA surveillance that ensures that transcripts with premature termination codons are recognized and further degraded. Control of mRNA stability is an essential component of the regulation of gene expression. The model of the NMD pathway in animals assumes that this process is performed during the initial round of translation when mRNA is still bound by nCBC at the 5′ end (Ishigaki et al., 2001; Chiu et al., 2004). Yu et al. (2016) explored the function of the nCBC in plant cotranslational RNA decay using next-generation sequencing methods (Box 1). This work revealed that not only cotranslational RNA degradation occurs in plants, but also that CBP80 plays a significant role in that process. Genome-wide mapping of the uncapped and cleaved transcript was performed on two allelic Atcbp80/abh1 insertional mutants. Cotranslational RNA decay was decreased to approximately 50% in the Atcbp80/abh1 mutants compared with the wild type, indicating that AtCBP80 affects only specific cotranslational RNA decay target transcripts. Interestingly, the ontology analysis of the transcripts affected by the loss of AtCBP80 revealed their engagement in the response to stress, cold, temperature stimulus, and abiotic stress (Yu et al., 2016). The authors proposed that the functions of AtCBP80 in cotranslational RNA decay include: (1) determining which transcripts are going to be degraded by cotranslational or general RNA decay; (2) directing the transcripts into the cotranslational RNA decay pathway during the initiation round of translation; and (3) affecting alternative splicing leading to greater degradation via the cotranslational RNA decay pathway.
cbp Plant Mutants under ABA Treatment and Abiotic Stresses
Studies of the genes encoding CBPs using plant mutants revealed that both CBP20 and CBP80 function in RNA metabolism and in the ABA-related response to abiotic stresses. Although the first plant mutants of CBC were identified and described over 15 years ago, the mechanism of CBP20 and CBP80 action in the presence of ABA or abiotic stress remains elusive (Hugouvieux et al., 2001; Papp et al., 2004).
Analysis of cbp Mutants Links miRNA to the Phytohormonal Response
An Arabidopsis mutant harboring a defect in the CBP80 gene, Atcbp80/abh1, was identified as being hypersensitive to ABA during a germination screen performed in the presence of ABA. Atcbp80/abh1 was also slightly insensitive to gibberellin during a hypocotyl elongation assay, but no other hormonal cross talk was identified during the early stages of development (Hugouvieux et al., 2001). Similarly, the Atcbp20 mutant was hypersensitive to ABA during seed germination (Papp et al., 2004). In addition, Atcbp80/abh1 was sensitive to salt and osmotic stresses during seed germination (Daszkowska-Golec et al., 2013).
The molecular mechanism of AtCBP20 and AtCBP80 action during seed germination in the presence of ABA was partially discerned based on the involvement of AtCBP80 and AtCBP20 in miRNA biogenesis. A defect in either AtCBP80 or AtCBP20 resulted in decreased levels of mature miRNAs, accompanied by the apparent stabilization of pri-miRNAs. Laubinger et al. (2008) performed whole-genome tiling array analyses and showed that Atcbp80/abh1 and Atcbp20 mutants share similar splicing defects. They identified the dual role of cap-binding proteins in regulating gene expression through miRNA biogenesis and splicing. At the same time, Kim et al. (2008) experimentally showed that pri-miRNAs were bound by CBP20 at the 5′ end. miR159 was one of many miRNAs that exhibited a changed level in the Atcbp80/abh1 and Atcbp20 mutants. Reyes and Chua (2007) identified miR159 as being up-regulated by ABA and drought. Furthermore, they showed that miR159 negatively regulates the expression of the positive regulators of ABA, the MYB33 and MYB101 transcription factors (Reyes and Chua, 2007). Kim et al. (2008) observed that the regulation of miR159 level may be dependent on developmental stage, since the greatest reduction in the expression level was observed in the seeds and young seedlings of the Atcbp80/abh1 and Atcbp20 mutants. ABA-mediated induction of miR159 levels was largely suppressed in the Atcbp80/abh1 mutant (Kim et al., 2008). Under the same conditions, higher MYB33 transcript levels were observed, which could account, in part, for the ABA hypersensitivity of the Atcbp80/abh1 mutant compared with the wild type. Possibly, CBP20 and CBP80 function as negative regulators of ABA through promoting miR159 accumulation at certain developmental stages, such as germination. MIR159 expression is regulated by ABI3, and partially by ABI5, in the presence of ABA (Reyes and Chua 2007). The action of miR159 during seed germination connected CBP80 and CBP20 with ABA signaling components such as ABI3 and ABI5 in Arabidopsis. ABI3 activates ABI4, which has been shown to bind the ABI5 promoter (Nakamura et al., 2001; Brady et al., 2003; Bossi et al., 2009). Interestingly, in an Atcbp80/abh1 suppressor screen, a mutation in ABI4 was shown to suppress the hypersensitive phenotype of Atcbp80/abh1 during seed germination (Daszkowska-Golec et al., 2013). It was hypothesized that ABI4 might regulate MIR159 expression either in a direct manner by interacting with the ABRE or S-box elements within the MIR159 promoter or indirectly by interacting with ABI5 and/or ABI3 (Daszkowska-Golec et al., 2013). Taken together, these results show that the nCBC contributes to the regulation of germination as part of the core ABA signaling pathway.
Zhang et al. (2016) demonstrated that CBP20 is phosphorylated in response to ethylene at Ser-245 and that the phosphorylation is required for root development (Box 2). Genome-wide analysis of the Atcbp20 mutant and its corresponding wild type showed that the miR319b level was upregulated in the roots, but not in the shoots, after ethylene treatment. Moreover, MYB33 expression was inversely correlated with the expression of miR319b, specifically in the roots in the presence of ethylene. These results together indicate that the ethylene-induced phosphorylation of CBP20 inhibits MYB33 expression in roots. In addition, evidence was provided that miR319b, but not miR159, has an impact on MYB33 expression in the presence of ethylene in roots (Box 2). These findings broaden our current understanding of the involvement of nCBC in the plant physiology, which for a long time was limited to its contributions to abiotic stress and ABA responses.
cbp Mutants under Abiotic Stress Conditions: Phenotype and Molecular Basis
In response to drought stress, the Atcbp80/abh1 mutant showed enhanced stomatal closure and better photosynthesis performance when compared to the wild type (Hugouvieux et al., 2002; Daszkowska-Golec et al., 2013). Atcbp80/abh1 was able to store more water than the wild type after 3 weeks of drought stress and lost water much more slowly than the wild type (Daszkowska-Golec et al., 2013). A possible physiological mechanism contributing to the reduced water loss and wilting involves stomatal action in response to drought stress. It was also experimentally confirmed that the Atcbp80/abh1 mutation increases cytoplasmic calcium fluctuations in guard cells, enhances slow anion channel activity, and simultaneously decreases inward-rectifying potassium channel currents in response to ABA (Hugouvieux et al., 2001, 2002). Further research on the Atcbp80/abh1 mutant revealed phenotypic changes such as increased number of trichomes, increased stomatal density on the abaxial leaf surface, and decreased stomatal density on the adaxial surface (Pieczynski et al., 2013; Daszkowska-Golec et al., 2013)
An Arabidopsis mutant harboring defects in CBP20 performed better than its corresponding wild type under water deficit (Papp et al., 2004; Jäger et al., 2011). The phenotypic characterization of the Atcbp20 mutant under drought stress was similar to the one presented by Hugouvieux et al. (2001) for Atcbp80/abh1. It also displayed an increased tolerance to water deprivation via the rapid closure of stomata (Papp et al., 2004). Additionally, the Atcbp20 mutant displayed an altered pattern of cellular organization of the epidermis. Relative to its corresponding wild type, the mutant was characterized by a greater number of epidermal cells that were smaller in size, an increased stomatal density, and a thicker cuticle. Moreover, the Atcbp20 leaf surface was coated with significantly more trichomes (Jäger at al., 2011). An increased number of trichomes was already reported to enhance drought tolerance, since it protects the plant from evaporation under drought stress (Huttunen et al., 2010; Hauser, 2014). The morphological features described above may help to explain the water-saving characteristics of Atcbp20.
The barley mutant hvcbp20.ab has been characterized in terms of its water deficit response (Daszkowska-Golec et al., 2017). Detailed phenotypic analyses demonstrated that hvcbp20.ab had a reduced growth rate under optimal water conditions and a less rapid inhibition of growth rate under stress, rapid stomatal closure at the onset of drought stress, and an altered epidermal pattern. Moreover, the barley mutant exhibited earlier inward leaf rolling. It was shown that the mutant performed an earlier shift in basic metabolism, which was manifested by better adaptation of the photosynthetic apparatus and a more efficient activation of the protective processes such as protein folding and ROS-scavenging under water deficit conditions, which was also supported by a transcriptomic analysis. All of the above-mentioned traits allowed hvcbp20.ab to optimize growth in response to a water shortage. The network hubs in the hvcbp20.ab mutant that are involved in the adjustment to drought conditions were proposed based on the integration of transcriptomic and physiological data (Daszkowska-Golec et al., 2017).
Interestingly, the connection between nCBC and miR159, together with the MYB33 and MYB101 transcription factors, was also studied regarding drought stress in both Arabidopsis and potato (Solanum tuberosum; Pieczynski et al., 2013). Pieczynski et al. (2013) investigated the response of transgenic potato plants to a down-regulated CBP80 gene using an artificial microRNA. The drought-tolerant phenotype of the mutant was manifested at the physiological and morphological level by its greater water maintenance, higher density of trichomes, and altered epidermal pattern. Moreover, it was observed that the level of miR159 was decreased and, simultaneously, the levels of the miR159 target mRNAs, MYB33 and MYB101, increased in the transgenic plants under drought.
In contrast to the drought-tolerant phenotype of the cbp mutants, Kong et al. (2014) reported a salt-sensitive response of both the Atcbp80/abh1 and Atcbp20 mutants. Using a proteomic approach, Kong et al. (2014) identified 77 differentially expressed proteins in the mutants compared with the wild type. Their further analysis showed that CBP20 and CBP80 regulate the splicing of genes involved in Pro and sugar metabolism. It was also demonstrated that salt stress induced massive events related to ubiquitination/sumoylation in the wild type, but not in the mutants. The authors postulated that CBP20/CBP80-dependent protein ubiquitination/sumoylation are linked to the salt-stress response and the salt-sensitive phenotype of the mutants (Kong et al., 2014).
After ABA and drought treatment, the transcript level of neither CBP was affected in plants. However, in response to ABA (but not salt), the level of the CBP80 and CBP20 protein increased (Hugouvieux et al., 2001; Papp et al., 2004; Kim et al., 2008). A longer exposure to ABA led to a higher level of the CBP80 protein. Kim et al. (2008) determined the time course of both the CBP20 and CBP80 proteins. CBP80 showed a half-life of 6 h, which was prolonged to >12 h in the presence of ABA. CBP20 showed a half-life of 12 h, which was also prolonged in the presence of ABA. These results together show that both CBPs are stabilized by ABA treatment in a posttranslational manner. The authors hypothesized a link between the increased levels of stress-responsive miRNA and the regulatory role of CBPs in miRNA processing. It was postulated that increased levels of CBP20 and CBP80 may, in turn, facilitate miRNA accumulation in the presence of ABA or stress (Kim et al., 2008). A recent study of SUC NON FERMENTING KINASES 2 (SnRK2s), which are activated in response to ABA and environmental stress and reprogram gene expression, showed that these kinases are required for proper miRNA accumulation via the regulation of miRNA processing factors (Yan et al., 2017). Potential substrates for posttranslational modification performed by SnRK2s are SE and HYL1. The protein level of HYL1, a core component of microprocessor, was reduced in the snrk2.2/3/6 triple mutant. The phosphorylation of both HYL1 and SE has been shown only in vitro. Further studies should determine whether the SnRK2s phosphorylate HYL1 and SE in vivo too. It was suggested that phosphorylation of SE by SnRK2s might affect SE localization or its interaction with other factors, rather than its abundance, since SE protein abundance was not affected substantially in the snrk2 mutant. Taking into account the strong interaction between SE and nCBC, SnRK2-mediated phosphorylation might also influence nCBC activity.
CONCLUSIONS AND FURTHER PERSPECTIVES
It has been well documented that nCBC coordinates many cellular processes in plants, such as miRNA biogenesis, alternative and constitutive splicing regulation, histone modifications, and nonsense-mediated mRNA decay. Moreover, linking the physiological analyses of the cbp mutants under hormonal or stress treatment using cutting-edge molecular biology techniques is likely to elucidate the regulatory role of the CBC. Although growing evidence has suggested the huge potential of nCBC as a regulatory master of the abiotic stress and phytohormone responses in plants, a more detailed analysis of the function of CBP20 and CBP80 in plants is needed to fully understand the molecular mechanism underlying the regulatory role of nCBC (see Outstanding Questions).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AtCBP20 (AT5G44200), AtCBP80 (AT2G13540), HvCBP20 (FJ548567).
Supplemental Data
The following supplemental materials are available.
Supplemental Material S1. Sequence alignment of human and plant CBP20 proteins.
Supplemental Material S2. Sequence alignment of human and plant CBP80 proteins.
Footnotes
Articles can be viewed without a subscription.
References
- Amorim MF, Wiling EM, Droste-Borel I, Macek B, Schneeberger K, Laubinger S (2017) Arabidopsis U1 snRNP subunit LUC7 functions in alternative splicing and 3 preferential removal of terminal introns. bioRxiv 10.1101/150805 [DOI] [Google Scholar]
- Bentley DL. (2014) Coupling mRNA processing with transcription in time and space. Nat Rev Genet 15: 163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra IC, Michaels SD, Schomburg FM, Amasino RM (2004) Lesions in the mRNA cap-binding gene ABA HYPERSENSITIVE 1 suppress FRIGIDA-mediated delayed flowering in Arabidopsis. Plant J 40: 112–119 [DOI] [PubMed] [Google Scholar]
- Bielewicz D, Kalak M, Kalyna M, Windels D, Barta A, Vazquez F, Szweykowska-Kulinska Z, Jarmolowski A (2013) Introns of plant pri-miRNAs enhance miRNA biogenesis. EMBO Rep 14: 622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi F, Cordoba E, Dupré P, Mendoza MS, Román CS, León P (2009) The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59: 359–374 [DOI] [PubMed] [Google Scholar]
- Brady SM, Sarkar SF, Bonetta D, McCourt P (2003) The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34: 67–75 [DOI] [PubMed] [Google Scholar]
- Browning KS, Bailey-Serres J (2015) Mechanism of cytoplasmic mRNA translation. Arabidopsis Book 13: e0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KS, Humphreys J, Hobbs W, Smith GB, Ravel JM (1990) Determination of the amounts of the protein synthesis initiation and elongation factors in wheat germ. J Biol Chem 265: 17967–17973 [PubMed] [Google Scholar]
- Bush MS, Crowe N, Zheng T, Doonan JH (2015) The RNA helicase, eIF4A-1, is required for ovule development and cell size homeostasis in Arabidopsis. Plant J 84: 989–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush MS, Pierrat O, Nibau C, Mikitova V, Zheng T, Corke FM, Vlachonasios K, Mayberry LK, Browning KS, Doonan JH (2016) eIF4A RNA helicase associates with cyclin-dependent protein kinase A in proliferating cells and is modulated by phosphorylation. Plant Physiol 172: 128–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Hagedorn CH, Cullen BR (2004) Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 10: 1957–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero G, Wilson KF, Ly T, Rios-Steiner JL, Clardy JC, Cerione RA (2002) Structural basis of m7GpppG binding to the nuclear cap-binding protein complex. Nat Struct Biol 9: 912–917 [DOI] [PubMed] [Google Scholar]
- Chen X, Liu X, Zhao Y, Zhou DX (2015) Histone H3K4me3 and H3K27me3 regulatory genes control stable transmission of an epimutation in rice. Sci Rep 5: 13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jolley B, Caldwell C, Gallie DR (2014) Eukaryotic translation initiation factor eIFiso4G is required to regulate violaxanthin De-epoxidase expression in Arabidopsis. J Biol Chem 289: 13926–13936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SY, Lejeune F, Ranganathan AC, Maquat LE (2004) The pioneer translation initiation complex is functionally distinct from but structurally overlaps with the steady-state translation initiation complex. Genes Dev 18: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszkowska-Golec A, Skubacz A, Marzec M, Słota M, Kurowska M, Gajecka M, Gajewska P, Płociniczak T, Sitko K, Pacak A, et al. (2017) Mutation in HvCBP20 (Cap Binding Protein 20) adapts barley to drought stress at phenotypic and transcriptomic levels. Front Plant Sci 8: 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszkowska-Golec A, Wojnar W, Rosikiewicz M, Szarejko I, Maluszynski M, Szweykowska-Kulinska Z, Jarmolowski A (2013) Arabidopsis suppressor mutant of abh1 shows a new face of the already known players: ABH1 (CBP80) and ABI4-in response to ABA and abiotic stresses during seed germination. Plant Mol Biol 81: 189–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff S, Murphy S (2008) Cracking the RNA polymerase II CTD code. Trends Genet 24: 280–288 [DOI] [PubMed] [Google Scholar]
- Filipowicz W. (1978) Functions of the 5,-terminal m7G cap in eukaryotic mRNA. FEBS Lett 96: 1–11 [DOI] [PubMed] [Google Scholar]
- Fortes P, Kufel J, Fornerod M, Polycarpou-Schwarz M, Lafontaine D, Tollervey D, Mattaj IW (1999) Genetic and physical interactions involving the yeast nuclear cap-binding complex. Mol Cell Biol 19: 6543–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo N, Bäurle I, Kidou S, Hu X, Dean C (2009) FRIGIDA delays flowering in Arabidopsis via a cotranscriptional mechanism involving direct interaction with the nuclear cap-binding complex. Plant Physiol 150: 1611–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatopoulos-Pournatzis T, Cowling VH (2014) Cap-binding complex (CBC). Biochem J 457: 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüner S, Peter D, Weber R, Wohlbold L, Chung M-Y, Weichenrieder O, Valkov E, Igreja C, Izaurralde E (2016) The Structures of eIF4E-eIF4G Complexes Reveal an Extended Interface to Regulate Translation Initiation. Mol Cell 64: 467–479 [DOI] [PubMed] [Google Scholar]
- Gunderson FQ, Merkhofer EC, Johnson TL (2011) Dynamic histone acetylation is critical for cotranscriptional spliceosome assembly and spliceosomal rearrangements. Proc Natl Acad Sci USA 108: 2004–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MT. (2014) Molecular basis of natural variation and environmental control of trichome patterning. Front Plant Sci 5: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG, Lorsch JR (2012) The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb Perspect Biol 4: a011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda N, Kim YK, Lejeune F, Maquat LE (2005) CBP80 promotes interaction of Upf1 with Upf2 during nonsense-mediated mRNA decay in mammalian cells. Nat Struct Mol Biol 12: 893–901 [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Kwak JM, Schroeder JI (2001) An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106: 477–487 [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Murata Y, Young JJ, Kwak JM, Mackesy DZ, Schroeder JI (2002) Localization, ion channel regulation, and genetic interactions during abscisic acid signaling of the nuclear mRNA cap-binding protein, ABH1. Plant Physiol 130: 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen P, Karkkainen K, Loe G, Rautio P, Agren J (2010) Leaf trichome production and responses to defoliation and drought in Arabidopsis lyrata (Brassicaceae). Ann Bot Fenn 47: 199–207 [Google Scholar]
- Hyun K, Jeon J, Park K, Kim J (2017) Writing, erasing and reading histone lysine methylations. Exp Mol Med 49: e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki Y, Li X, Serin G, Maquat LE (2001) Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 106: 607–617 [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Lewis J, McGuigan C, Jankowska M, Darzynkiewicz E, Mattaj IW (1994) A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell 78: 657–668 [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Mattaj IW (1995) RNA export. Cell 81: 153–159 [DOI] [PubMed] [Google Scholar]
- Izaurralde E, McGuigan C, Mattaj IW (1995) Nuclear localization of a cap-binding protein complex. Cold Spring Harb Symp Quant Biol 60: 669–675 [DOI] [PubMed] [Google Scholar]
- Jäger K, Fábián A, Tompa G, Deák C, Höhn M, Olmedilla A, Barnabás B, Papp I (2011) New phenotypes of the drought-tolerant cbp20 Arabidopsis thaliana mutant have changed epidermal morphology. Plant Biol (Stuttg) 13: 78–84 [DOI] [PubMed] [Google Scholar]
- Jiang D, Gu X, He Y (2009) Establishment of the winter-annual growth habit via FRIGIDA-mediated histone methylation at FLOWERING LOCUS C in Arabidopsis. Plant Cell 21: 1733–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierzkowski D, Kmieciak M, Piontek P, Wojtaszek P, Szweykowska-Kulinska Z, Jarmolowski A (2009) The Arabidopsis CBP20 targets the cap-binding complex to the nucleus, and is stabilized by CBP80. Plant J 59: 814–825 [DOI] [PubMed] [Google Scholar]
- Kim S, Yang JY, Xu J, Jang IC, Prigge MJ, Chua NH (2008) Two cap-binding proteins CBP20 and CBP80 are involved in processing primary MicroRNAs. Plant Cell Physiol 49: 1634–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, He Y, Jacob Y, Noh YS, Michaels S, Amasino R (2005) Establishment of the vernalization-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. Plant Cell 17: 3301–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmieciak M, Simpson CG, Lewandowska D, Brown JWS, Jarmolowski A (2002) Cloning and characterization of two subunits of Arabidopsis thaliana nuclear cap-binding complex. Gene 283: 171–183 [DOI] [PubMed] [Google Scholar]
- Kong X, Ma L, Yang L, Chen Q, Xiang N, Yang Y, Hu X (2014) Quantitative proteomics analysis reveals that the nuclear cap-binding complex proteins arabidopsis CBP20 and CBP80 modulate the salt stress response. J Proteome Res 13: 2495–2510 [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Watanabe Y (2004) Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc Natl Acad Sci USA 101: 12753–12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubinger S, Sachsenberg T, Zeller G, Busch W, Lohmann JU, Rätsch G, Weigel D (2008) Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc Natl Acad Sci USA 105: 8795–8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN (2004) MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23: 4051–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lellis AD, Allen ML, Aertker AW, Tran JK, Hillis DM, Harbin CR, Caldwell C, Gallie DR, Browning KS (2010) Deletion of the eIFiso4G subunit of the Arabidopsis eIFiso4F translation initiation complex impairs health and viability. Plant Mol Biol 74: 249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins E, Tseng CY, Patrick RM, Mayberry LK, Cole N, Browning KS (2016) Fusion proteins of Arabidopsis cap-binding proteins: cautionary “tails” of woe. Translation (Austin) 4: e1257408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Görlich D, Mattaj IW (1996) A yeast cap binding protein complex (yCBC) acts at an early step in pre-mRNA splicing. Nucleic Acids Res 24: 3332–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Izaurralde E (1997) The role of the cap structure in RNA processing and nuclear export. Eur J Biochem 247: 461–469 [DOI] [PubMed] [Google Scholar]
- Li Y, Mukherjee I, Thum KE, Tanurdzic M, Katari MS, Obertello M, Edwards MB, McCombie WR, Martienssen RA, Coruzzi GM (2015) The histone methyltransferase SDG8 mediates the epigenetic modification of light and carbon responsive genes in plants. Genome Biol 16: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jiang D, Fu X, Luo X, Liu R, He Y (2016) Coupling of histone methylation and RNA processing by the nuclear mRNA cap-binding complex. Nat Plants 2: 16015. [DOI] [PubMed] [Google Scholar]
- Liu Q, Shi L, Fang Y (2012) Dicing bodies. Plant Physiol 158: 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK (1997) Cocrystal structure of the messenger RNA 5′ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell 89: 951–961 [DOI] [PubMed] [Google Scholar]
- Marintchev A, Wagner G (2005) eIF4G and CBP80 share a common origin and similar domain organization: implications for the structure and function of eIF4G. Biochemistry 44: 12265–12272 [DOI] [PubMed] [Google Scholar]
- Matsuo H, Li H, McGuire AM, Fletcher CM, Gingras AC, Sonenberg N, Wagner G (1997) Structure of translation factor eIF4E bound to m7GDP and interaction with 4E-binding protein. Nat Struct Biol 4: 717–724 [DOI] [PubMed] [Google Scholar]
- Mayberry LK, Allen ML, Nitka KR, Campbell L, Murphy PA, Browning KS (2011) Plant cap-binding complexes eukaryotic initiation factors eIF4F and eIFISO4F: molecular specificity of subunit binding. J Biol Chem 286: 42566–42574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza C, Ohno M, Segref A, Mattaj IW, Cusack S (2001) Crystal structure of the human nuclear cap binding complex. Mol Cell 8: 383–396 [DOI] [PubMed] [Google Scholar]
- Mazza C, Segref A, Mattaj IW, Cusack S (2002) Large-scale induced fit recognition of an m(7)GpppG cap analogue by the human nuclear cap-binding complex. EMBO J 21: 5548–5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendrick L, Morley SJ, Pain VM, Jagus R, Joshi B (2001) Phosphorylation of eukaryotic initiation factor 4E (eIF4E) at Ser209 is not required for protein synthesis in vitro and in vivo. Eur J Biochem 268: 5375–5385 [DOI] [PubMed] [Google Scholar]
- Miras M, Truniger V, Silva C, Verdaguer N, Aranda MA, Querol-Audí J (2017) Structure of eIF4E in complex with an eIF4G peptide supports a universal bipartite binding mode for protein translation. Plant Physiol 174: 1476–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Lynch TJ, Finkelstein RR (2001) Physical interactions between ABA response loci of Arabidopsis. Plant J 26: 627–635 [DOI] [PubMed] [Google Scholar]
- Oh N, Kim KM, Choe J, Kim YK (2007) Pioneer round of translation mediated by nuclear cap-binding proteins CBP80/20 occurs during prolonged hypoxia. FEBS Lett 581: 5158–5164 [DOI] [PubMed] [Google Scholar]
- Pabis M, Neufeld N, Steiner MC, Bojic T, Shav-Tal Y, Neugebauer KM (2013) The nuclear cap-binding complex interacts with the U4/U6·U5 tri-snRNP and promotes spliceosome assembly in mammalian cells. RNA 19: 1054–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp I, Mur LA, Dalmadi A, Dulai S, Koncz C (2004) A mutation in the Cap Binding Protein 20 gene confers drought tolerance to Arabidopsis. Plant Mol Biol 55: 679–686 [DOI] [PubMed] [Google Scholar]
- Park W, Li J, Song R, Messing J, Chen X (2002) CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12: 1484–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick RM, Browning KS (2012) The eIF4F and eIFiso4F complexes of plants: an evolutionary perspective. Comp Funct Genomics 2012: 287814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick RM, Mayberry LK, Choy G, Woodard LE, Liu JS, White A, Mullen RA, Tanavin TM, Latz CA, Browning KS (2014) Two Arabidopsis thaliana loci encode novel eIF4E isoforms that are functionally distinct from the conserved plant eIF4E. Plant Physiol 164: 1820–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieczynski M, Marczewski W, Hennig J, Dolata J, Bielewicz D, Piontek P, Wyrzykowska A, Krusiewicz D, Strzelczyk-Zyta D, Konopka-Postupolska D, et al. (2013) Down-regulation of CBP80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol J 11: 459–469 [DOI] [PubMed] [Google Scholar]
- Raczynska KD, Simpson CG, Ciesiolka A, Szewc L, Lewandowska D, McNicol J, Szweykowska-Kulinska Z, Brown JW, Jarmolowski A (2010) Involvement of the nuclear cap-binding protein complex in alternative splicing in Arabidopsis thaliana. Nucleic Acids Res 38: 265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan A, Robb GB, Chan SH (2016) mRNA capping: biological functions and applications. Nucleic Acids Res 44: 7511–7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Xie M, Dou Y, Zhang S, Zhang C, Yu B (2012) Regulation of miRNA abundance by RNA binding protein TOUGH in Arabidopsis. Proc Natl Acad Sci USA 109: 12817–12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JL, Chua NH (2007) ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J 49: 592–606 [DOI] [PubMed] [Google Scholar]
- Ruthenburg AJ, Allis CD, Wysocka J (2007) Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell 25: 15–30 [DOI] [PubMed] [Google Scholar]
- Sabin LR, Zhou R, Gruber JJ, Lukinova N, Bambina S, Berman A, Lau C-K, Thompson CB, Cherry S (2009) Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell 138: 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer SE, Jacobsen SE, Meinke DW, Ray A (2002) DICER-LIKE1: blind men and elephants in Arabidopsis development. Trends Plant Sci 7: 487–491 [DOI] [PubMed] [Google Scholar]
- Shilatifard A. (2008) Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr Opin Cell Biol 20: 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies N, Nielsen CB, Padgett RA, Burge CB (2009) Biased chromatin signatures around polyadenylation sites and exons. Mol Cell 36: 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui P, Jin J, Ye S, Mu C, Gao J, Feng H, Shen WH, Yu Y, Dong A (2012) H3K36 methylation is critical for brassinosteroid-regulated plant growth and development in rice. Plant J 70: 340–347 [DOI] [PubMed] [Google Scholar]
- Sui P, Shi J, Gao X, Shen WH, Dong A (2013) H3K36 methylation is involved in promoting rice flowering. Mol Plant 6: 975–977 [DOI] [PubMed] [Google Scholar]
- Vain P, Thole V, Worland B, Opanowicz M, Bush MS, Doonan JH (2011) A T-DNA mutation in the RNA helicase eIF4A confers a dose-dependent dwarfing phenotype in Brachypodium distachyon. Plant J 66: 929–940 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Carpenter PB (2012) Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol 13: 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Song X, Gu L, Li X, Cao S, Chu C, Cui X, Chen X, Cao X (2013) NOT2 proteins promote polymerase II-dependent transcription and interact with multiple MicroRNA biogenesis factors in Arabidopsis. Plant Cell 25: 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Yue Z, Shatkin AJ (1998) Mammalian capping enzyme binds RNA and uses protein tyrosine phosphatase mechanism. Proc Natl Acad Sci USA 95: 12226–12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worch R, Niedzwiecka A, Stepinski J, Mazza C, Jankowska-Anyszka M, Darzynkiewicz E, Cusack S, Stolarski R (2005) Specificity of recognition of mRNA 5′ cap by human nuclear cap-binding complex. RNA 11: 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhao Z, Dong A, Soubigou-Taconnat L, Renou JP, Steinmetz A, Shen WH (2008) Di- and tri- but not monomethylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in Arabidopsis thaliana. Mol Cell Biol 28: 1348–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Bi L, Zheng B, Ji L, Chevalier D, Agarwal M, Ramachandran V, Li W, Lagrange T, Walker JC, Chen X (2008) The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proc Natl Acad Sci USA 105: 10073–10078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Wang P, Wang B, Hsu CC, Tang K, Zhang H, Hou YJ, Zhao Y, Wang Q, Zhao C, Zhu X, Tao WA, Li J, Zhu JK (2017) The SnRK2 kinases modulate miRNA accumulation in Arabidopsis. PLoS Genet 13: e1006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Willmann MR, Anderson SJ, Gregory BD (2016) Genome-wide mapping of uncapped and cleaved transcripts reveals a role for the nuclear mRNA cap-binding complex in cotranslational RNA decay in Arabidopsis. Plant Cell 28: 2385–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang L, Lim JY, Kim T, Pyo Y, Sung S, Shin C, Qiao H (2016) Phosphorylation of CBP20 links MicroRNA to root growth in the ethylene response. PLoS Genet 12: e1006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jones A, Joo HY, Zhou D, Cao Y, Chen S, Erdjument-Bromage H, Renfrow M, He H, Tempst P, et al. (2013) USP49 deubiquitinates histone H2B and regulates cotranscriptional pre-mRNA splicing. Genes Dev 27: 1581–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]