Abstract
Advances over recent years underlines a growing interest in investigating endocytosis in plants.
The plasma membrane (PM) serves as the interface between the cell and its environment. Accordingly, cells have the capacity to modulate their complement of PM-associated receptors, transporters, channels, lipids, and other membrane components to facilitate numerous physiological functions, including synthesis of the extracellular matrix, intercellular communication, nutrient uptake, environmental sensing, and directional growth, among others. Exocytosis/secretion, which delivers newly synthesized and recycled cargo from the trans-Golgi network (TGN) and endosomes to the PM, and endocytosis, wherein PM cargo is internalized and sorted into early endosomes (EEs), are the two complimentary trafficking pathways chiefly responsible for the maintenance of PM composition. Much of our understanding of these processes derives from work in yeast and mammalian systems; indeed, only recently has endocytosis been conclusively demonstrated in plants (for review, see Robinson et al., 2008). This review summarizes recent progress that has enhanced our understanding of the mechanisms of internalization of proteins and lipids from the PM; other reviews of earlier work in the field, including the following, are highly recommended for a thorough investigation of the subject: Robinson et al. (2008), Chen et al. (2011), Baisa et al. (2013), Fan et al. (2015), and Paez Valencia et al. (2016).
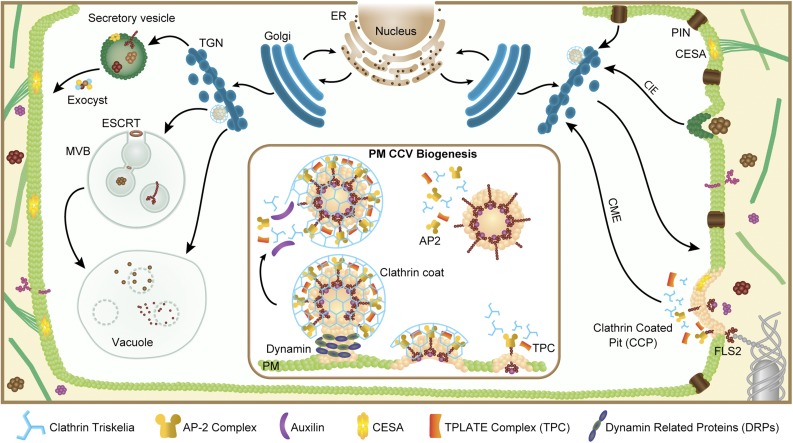
Cells employ a variety of pathways for internalization of membrane-associated and soluble cargo from the PM and extracellular environment (Fig. 1), of which clathrin-mediated endocytosis (CME) is the most studied. Integral PM proteins to be internalized via CME are marked for uptake by virtue of intrinsic signaling peptide (amino acid) sequences and posttranslational modifications (e.g. ubiquitin). In metazoans and yeast, the initial stages of clathrin-coated pit (CCP) formation involve adaptor complexes, such as the heterotetrameric adaptin protein-2 (AP-2) complex, that recognize these motifs and specific membrane lipids [e.g. phosphatidylinositol 4,5-bisphosphate, PI(4,5)P2] and recruit clathrin triskelia composed of clathrin heavy and light chain subunits (CHCs and CLCs). Oligomerization of clathrin, together with the recruitment of additional endocytic accessory proteins (EAPs), promotes increasing localized membrane curvature that resolves into a vesicle upon scission from the PM; in mammals (metazoans), this scission of budding clathrin-coated vesicles (CCVs) is facilitated by the dynamin GTPase. After uncoating via Hsc70 and auxilin-like proteins, the vesicle delivers its contents via fusion to the TGN/EE for subsequent sorting back to the PM (recycling) and/or delivery to the vacuole. Cargo that is destined to be degraded in the vacuole is internalized into intraluminal vesicles via the Endosomal Sorting Complexes Required for Transport (ESCRT) machinery to form multivesicular bodies (MVBs), which upon maturation eventually fuse with the vacuole to complete degradation. For recent perspective on endosomal trafficking, see Paez Valencia et al. (2016).
Figure 1.
Plant cells maintain several pathways for the distribution of membrane-bound material in both anterograde and retrograde fashions. Newly synthesized proteins traveling through the endoplasmic reticulum (ER) and Golgi apparatus are delivered via membrane transport from the TGN/EE, an organelle acting as a hub in secretory and endocytic trafficking pathways, on to the PM via exocyst-dependent docking and fusion. PM and extracellular material are internalized by multiple means, including CIE and CME. Initiation of CCPs (inset) employs various adaptor complexes, including AP-2 and TPC, which associate with the PM, recognize membrane-associated cargoes (including, for example, the flagellin-sensing FLS2 receptor and the CESA), and recruit the clathrin coat. Following release from the PM, endocytic CCVs undergo uncoating before fusing with the TGN/EE. Endocytosed proteins may undergo recycling to the PM via vesicular trafficking and/or sorting into the late endosome I MVB interior via ESCRT for subsequent vacuolar degradation.
Given the essential role of the PM in a large number of cellular processes, exquisite control of endocytosis must be exercised to maintain the appropriate PM protein and lipid composition. Consistent with this, the process of plant endocytosis seems to be cell-type dependent (Baral et al., 2015; Löfke et al., 2015) and is responsive to endogenous plant signaling molecules (e.g. auxin, salicylic acid; Yu et al., 2016) as well as other environmental stimuli including plant pathogens (Smith et al., 2014b; Mbengue et al., 2016; Li and Pan, 2017), nutrients (Takano et al., 2010; Barberon et al., 2014), and light (Wang et al., 2016; Zhang et al., 2017). Significant progress has been made in the last 5 years to identify and characterize the evolutionarily conserved and plant-specific endocytic machinery and its regulation.
EXPERIMENTAL TOOLS: CHEMICAL TREATMENTS AFFECTING ENDOCYTOSIS
Our understanding of the molecular machinery and dynamics of endocytosis in plants and other organisms has been greatly facilitated by the application of pharmacological agents and use of live cell imaging of fluorescent protein-tagged CME machinery and PM cargo proteins. The fungal metabolite Brefeldin A (BFA), a reversible vesicle trafficking inhibitor, has been widely used in Arabidopsis (Arabidopsis thaliana) to block the trafficking of material from the TGN/EE to probe the identity of newly synthesized and endocytosed proteins passing through this compartment. Specifically, BFA inhibition of a subset of Sec7 domain-containing ADP-ribosylation factor (ARF) guanine nucleotide exchange factors (ARF-GEF), including GNOM, results in the formation of characteristic intracellular endosomal and trans-Golgi compartment aggregates (BFA bodies) that contain newly synthesized and endocytosed proteins (Robinson et al., 2008; Paez Valencia et al., 2016). The appearance of fluorescently tagged PM proteins in BFA bodies in the presence of the protein synthesis inhibitor cycloheximide has been utilized to monitor endocytosis in wild-type and CME machinery defective mutants (Dhonukshe et al., 2007; Tanaka et al., 2009; Gadeyne et al., 2014), and to assess how CME is regulated in plants in response to environmental conditions (Geldner et al., 2001; Kleine-Vehn et al., 2010; Gadeyne et al., 2014; Wang et al., 2016; Yu et al., 2016). A major finding has been that the phytohormones auxin and salicylic acid rapidly inhibit the internalization of PM marker proteins into BFA bodies (Paciorek et al., 2005; Pan et al., 2009; Robert et al., 2010; Du et al., 2013; Wang et al., 2013, 2016). However, a recent study using photoconvertible fluorescent protein-tagged PIN-FORMED2 (PIN2; Jásik et al., 2016) concluded that auxins do not inhibit endocytosis, but rather affect BFA body formation and thereby inhibit delivery of newly synthesized PIN2 to the BFA bodies, raising questions regarding the validity of the use of the BFA body assay for assessing PM protein internalization. It will be important to reconcile these findings in light of previous studies showing that exogenous auxin treatment does not affect formation of BFA bodies (Paciorek et al., 2005) and disrupts clathrin association with the PM, thereby affecting the endocytosis of PIN auxin efflux transporters and other PM proteins (Robert et al., 2010; Wang et al., 2013, 2016).
In addition to BFA and other previously described trafficking inhibitors, hundreds of small molecules affecting endomembrane trafficking in plants have been discovered through recent chemical genetic screens (Robert et al., 2008; Drakakaki et al., 2011; Chuprov-Netochin et al., 2016). These compounds offer unique benefits, including transient and tunable phenotypes, the potential to overcome potential genetic redundancy of essential membrane trafficking factors, and utility as a means of biochemically identifying their targets (Norambuena and Tejos, 2017). Several members of one group of compounds, known as endosidins, based on their identification as compounds that affect endocytosis and endosomal function, have been characterized recently. A subset of endosidin compounds including Endosidin1 (ES1), ES2, and ES16 has been shown to affect post-Golgi trafficking, including exocytosis and endosome function (Robert et al., 2008; Tóth et al., 2012; Zhang et al., 2016; Li et al., 2017). ES9 was shown to inhibit endocytosis of the known CME cargo, transferrin receptor, in animal cells (Dejonghe et al., 2016) and of the amphiphilic styryl dye N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide (FM4-64) in plants. As an integral PM dye, FM4-64 is likely taken up by multiple endocytic pathways, though the observation that FM4-64 internalization is highly sensitive to clathrin HUB overexpression indicates the majority of the dye is taken up via CME (Dhonukshe et al., 2007). Consistent with its inhibition of CME, ES9 caused a dramatic increase in the lifetime of CCPs in root epidermal cells as observed with fluorescent protein-tagged CLC, CHC, TPLATE complex (TPC), and AP-2 complex subunits. PI(4,5)P2 but not phosphatidylinositol 4-phosphate levels at the PM were also affected in root cells treated with ES9. However, it appears these effects are not due to specific targeting of CME machinery, but instead manifest primarily through the mitochondrial uncoupling and protonophoric activity of ES9, thereby causing cellular depletion of ATP and cytoplasmic acidification, respectively (Dejonghe et al., 2016). This mode of action appears to be shared with the phosphotyrosine analog tyrphostin A23 (TyrA23), which has been utilized as a pharmacological tool to interfere with CME in animal and plant cells. In animal cells, the effects of TyrA23 on endocytosis were postulated to occur by virtue of disrupting the interaction of the AP-2 complex with YXXΦ (Φ represents any bulky hydrophobic residue) motifs found in many CME cargoes (Banbury et al., 2003). In plants, TyrA23 disrupts the PM association of AP2α1, AP2μ, AP2σ, and the TPC complex subunit TPLATE (Van Damme et al., 2011; Di Rubbo et al., 2013; Fan et al., 2013; Kim et al., 2013; Yamaoka et al., 2013; Wang et al., 2016), and inhibits the internalization of a number of PM-resident proteins involved in hormone signaling (Robert et al., 2010; Irani et al., 2012), nutrient uptake (Barberon et al., 2011), and pathogen defense or stress response (Beck et al., 2012; Hao et al., 2014; Smith et al., 2014a, 2014b). Intriguingly, at environmental pHs greater than 6.5, the inhibition of FM4-64 uptake when exposed to TyrA23 and the protonophore chlorophenyl hydrazine is diminished, whereas the inhibitory effect of ES9 application is preserved, suggesting ES9 may yet have CME inhibiting effects beyond those resulting from cytoplasmic acidification (Dejonghe et al., 2016). Moreover, elevating TyrA23 concentration beyond those typically used to inhibit endocytosis (30–50 µm; Dejonghe et al., 2016) has demonstrated additional effects, including dissociation of the TPC from the PM (75 µm; Van Damme et al., 2011) and inhibition of flg22-elicted reactive oxygen species formation (100 µm; Smith et al., 2014a, 2014b). The careful characterization of the effects of ES9 and TyrA23 serves as a reminder that it is critical that the mechanism of action of other compounds used for the characterization of membrane trafficking be well defined, and the impact of potential off-target effects must be considered. In light of their varied effects on endocytosis apparently separate to those resulting from cytoplasmic acidification, ES9 and TyrA23 may yet serve as powerful tools for the dissection of endocytic pathways when accompanied by critical examination of off-target effects.
EXPERIMENTAL TOOLS: QUANTITATIVE IMAGING OF PLANT ENDOCYTOSIS
Live cell imaging of fluorescent protein-tagged cargo and vesicle trafficking proteins has proven invaluable for understanding the CME and clathrin-independent endocytosis (CIE) molecular machinery and cargo. In an effort study the dynamics of receptor-mediated endocytosis, several bioactive fluorescent cargo analogs have been developed recently. The fluorescent brassinosteroid ligand analog Alexa fluor 674 castasterone enables quantitative measurement of brassinosteroid receptor (BRI1) endocytosis (Irani et al., 2012, 2014), and has been utilized to characterize the ES9 mode of action (Dejonghe et al., 2016) and the function of the AP2α subunit in CME (Di Rubbo et al., 2013). Additionally, the fluorescently labeled endogenous elicitor peptide TAMRA-pep1 has been used to examine trafficking of its cognate receptor, PEP RECEPTOR1 (PEPR1; Ortiz-Morea et al., 2016).
The advent of live cell imaging techniques applicable to plant cells has greatly expanded our ability to probe dynamic events at the cell cortex. Expanding on the powerful signal-to-noise ratios offered by traditional laser scanning confocal microscopy, the improved scanning rates of spinning disk confocal microscopy and subsequently increased temporal resolution have enabled in-depth study of dynamic events in vivo. At the leading edge of efforts to improve spatial resolution, several techniques have been developed recently that, while lacking high temporal resolution, may have utility in probing the spatial distribution of CME and CIE components, including coat proteins and EAPs (Komis et al., 2015). Adaptation of total internal reflection microscopy (TIRFM) and variable angle epifluorescence microscopy (VAEM) for use in plant systems combines extremely high spatiotemporal resolution with novel illumination techniques to capture dynamic events at the cell surface. The unique physics of TIRFM/VAEM allow for the selective excitation of a limited portion of the sample adjacent to the glass slide, ideal for imaging events at the PM without background fluorescence from the sample interior (Kaksonen et al., 2005; Schneckenburger, 2005; Konopka and Bednarek, 2008b; Wan et al., 2011). Imaging of fluorescently tagged clathrin and/or EAPs at the PM using spinning disk confocal microscopy or TIRFM/VAEM reveals dynamic foci of rising and falling intensity reflecting the formation of CCPs and the internalization of budding CCVs away from the plane of focus, respectively. Measurements of subdiffraction size CCP lifetimes is an important metric of endocytic efficiency under varying conditions (Dejonghe et al., 2016), though quantitative analysis of these data manually is labor intensive and is susceptible to human subjectivity. To address these issues, commercial and open source automated particle tracking software tools have been developed (Debeir et al., 2005; Murray et al., 2006; Jaqaman et al., 2008; Tinevez et al., 2017). Recently, the use of Trackmate, a plug-in for the open source ImageJ/FIJI software platform (Schneider et al., 2012) that offers automated and manually segmentation and tracking (Tinevez et al., 2017), and the cmeAnalysis package (Aguet et al., 2013) for the proprietary/commercial MATLAB computing platform were validated for tracking and lifetime analysis of CME dynamics in plant cells. In agreement with the values obtained through manual annotation (Konopka et al., 2008; Bashline et al., 2013; Gadeyne et al., 2014), automated tracking of VAEM/TIRFM data of fluorescent protein-tagged clathrin and CME accessory proteins in root cells using Trackmate revealed CCP lifetimes of 24.2 ± 11.6 s (Tinevez et al., 2017). Similarly, using TIRFM imaging and cmeAnalysis software, others have determined the lifetimes of several fluorescent protein-tagged, PM CCP-resident proteins, including CLC (10.4–11.7 s), AP2α (12.5 s), AP2μ (15.9 s), and the plant dynamin homolog dynamin related protein 1C (DRP1C; 16.0-20.4 s; Johnson and Vert, 2017).
AP-2
As the canonical CME adaptor complex, the evolutionarily conserved heterotetrameric AP-2 complex, comprised of α/A, β/B, μ/M, and σ/S subunits, has been well characterized in many systems, including plants (Fig. 1; Bashline et al., 2013; Di Rubbo et al., 2013; Fan et al., 2013; Kim et al., 2013; Yamaoka et al., 2013; Wang et al., 2016; Johnson and Vert, 2017). Imaging of early events in metazoan CME has indicated that the AP-2 complex nucleates CCPs by virtue of interactions with PM signaling phospholipids such as PI(4,5)P2 and phosphatidylinositol 3-phosphate as well as various EAPs, including the AP180 N-terminal homology, epsin N-terminal homology, and FCHo (F-BAR) proteins, which together serve to generate membrane curvature (Cocucci et al., 2012; Mayers et al., 2013). Consistent with its function as an interaction hub in other systems, plant AP-2 interacts with clathrin and CME cargoes, and functions in plant CME (Fan et al., 2013). PI(4,5)P2 is involved in plant endocytosis, as demonstrated by the sensitivity of CHC PM association to PI(4,5)P2 production (Zhao et al., 2010) and the reduction in PIN internalization and perturbed CCP abundance and dynamics observed in PI(4)P 5-kinase pip5k1 pip5k2 double mutants (Yamaoka et al., 2013). However, a place for PI(4,5)P2 in the AP-2 interaction network in plants, either via other EAPs or through AP-2 subunits directly, remains to be determined.
In line with its nature as a major interaction hub in CME, the AP-2 complex is essential for embryonic development and morphogenesis in mammals (Mitsunari et al., 2005). However, HeLa cell lines remain viable despite loss of the AP2μ subunit and the concomitant inhibition of CME of some cargoes (Motley et al., 2003; Aguet et al., 2013). Similarly, Arabidopsis mutants defective for individual AP-2 subunits remain viable (Bashline et al., 2013; Fan et al., 2013; Kim et al., 2013). An advantage of the viability of AP-2 subunit knockout alleles is the ability to examine the impact of such mutations on CME dynamics and cargo selection in vivo; analysis of individual AP-2 subunit knockout mutants has revealed an AP-2 interaction with the cellulose synthase complex (Bashline et al., 2013), floral and pollen tube defects consistent with aberrant PIN polarity (Kim et al., 2013), and reduced BRI1 internalization (Di Rubbo et al., 2013), suggesting AP-2 has a central role in CME. The diverse functions of AP-2 may be partly facilitated by the independent function(s) of its constituent subunits as demonstrated by subunit-specific phenotypes and high temporal resolution TIRFM imaging with single particle tracking revealing that the arrival of AP2μ precedes the concomitant recruitment of AP2α1 and clathrin at CCPs (Johnson and Vert, 2017). Moreover, in Arabidopsis individuals deficient in a given AP-2 subunit, the remaining subunits can assemble into “subcomplexes” that associate with the PM (Wang et al., 2016). Loss of AP2μ strongly impairs the recruitment of the remaining subunits to the PM relative to ap2σ mutants (Wang et al., 2016), reflecting the former’s earlier arrival at the PM (Johnson and Vert, 2017). Together, these findings suggest that the relatively mild developmental phenotypes observed in Arabidopsis ap-2 subunit mutants (Bashline et al., 2013; Di Rubbo et al., 2013; Fan et al., 2013; Kim et al., 2013; Yamaoka et al., 2013) may be due to formation of partially active AP-2 complexes that functionally overlap with other adaptor complexes.
TPC
In addition to the AP-2 complex, the recently discovered heterooctameric TPC functions as an important CME adaptor in plants (Van Damme et al., 2011; Gadeyne et al., 2014). Indicative of the critical function of the TPC, Arabidopsis TPC subunit loss-of-function mutants exhibit pollen lethality (Van Damme et al., 2011; Gadeyne et al., 2014). Although yeast and metazoans lack clearly definable TPC subunit homologs, a complex composed of six TPC subunit homologs, named TSET, was identified in Dictyostelium (Hirst et al., 2014; Zhang et al., 2015). Phylogenetic analysis of TPC/TSET subunits suggests that these complexes are evolutionarily ancient, arising between the advent of COPI and the adaptins, two of the primary coat/adapting complexes in eukaryotes (Zhang et al., 2015). Over such an extended evolutionary period, it follows that extant organisms may have adapted the subunits of the ancient TPC complex to fit unique requirements. Indeed, TPC subunits TML, AtEH1, and AtEH2 are structurally similar to metazoan FCHo, Eps15, and Intersectin, components of a complex that stabilizes AP-2 at the PM (McMahon and Boucrot, 2011; Mayers et al., 2013). TPC subunits biochemically interact with clathrin, AP-2, and DRPs, and are required for AP2α subunit and clathrin recruitment to the PM (Gadeyne et al., 2014; Wang et al., 2016). Moreover, TPC subunits appear at CCPs prior to AP2α (Gadeyne et al., 2014) but concomitantly with AP2μ (Bashline et al., 2015), suggesting that the TPC participates in concert with the AP-2 complex in the initiation of endocytic events (Fig. 1). Consistent with a putative role in CME, analysis of inducible knockdown mutants or nonlethal alleles that affect TPC subunit expression reveals defective internalization of FM4-64 and various PM proteins, including cellulose synthase complexes (CESA), BRI1, PIN1, and PIN2 (Van Damme et al., 2011; Gadeyne et al., 2014; Bashline et al. 2015), a phenotype reminiscent of AP-2 subunit knockouts. However, genetic and pharmacological studies indicate that TPC and AP-2 likely have independent roles in CME as well (Gadeyne et al., 2014; Bashline et al., 2015; Wang et al., 2016). Moreover, salicylic acid application reduces the membrane association of clathrin and AP-2, but not that of the TPC (Wang et al., 2016). Further investigation thus is required to tease apart the individual and concerted functions of the plant-specific TPC and the evolutionarily conserved AP-2 in plant CME.
CARGO SELECTION
Fine control of PM protein abundance is predicated on the ability of CME machinery to recognize relevant cargo via intrinsic signals (i.e. peptide sequences) and posttranslational modifications. In metazoans, AP-2 α and μ subunits recognize various sorting motifs, including the di-Leu [DE]xxxL[LI] and Tyr-based YXXΦ peptide sequences, respectively (Kelly and Owen, 2011). A role in CME for the Tyr-based motif may be conserved in plants given that mutation of a YXXΦ Tyr in the cytoplasmic region of the Arabidopsis fungal pathogen sensor LeEix2 abrogates its function and prevents internalization from the PM (Bar and Avni, 2009). Moreover, YXXΦ-associated CME has been coopted by the bacterial pathogen Agrobacterium tumefaciens to internalize the motif-containing virulence factor VirE2. Mutation of both YXXΦ motifs in the VirE2 C-terminal domain abolished interaction with AP2μ and, similar to the effects of overexpression of the dominant-negative CME inhibitor clathrin HUB (Dhonukshe et al., 2007), reduced uptake of the protein from the PM (Li and Pan, 2017). Additionally, the Tyr residues in three YXXΦ motifs in the cytoplasmic region of PIN1 have been demonstrated to interact with AP2μ, though single YXXΦ mutation did not alter PIN1 localization (Sancho-Andrés et al., 2016). However, other studies have shown disruption of multiple YXXΦ motifs may be necessary to overcome functional redundancy (Li and Pan, 2017). Conversely, mutational analysis of three YXXΦ motifs in the cytoplasmic region of the Arabidopsis boron transporter BOR1 revealed that they are not required for CME but instead for endosomal recycling to maintain the proper polar distribution of the protein in the inner PM domain of root cells (Takano et al., 2010), suggesting the motif may be involved in several post-Golgi pathways.
In addition to intrinsic peptide signals in cargo proteins, post-translational protein modifications, including phosphorylation and ubiquitylation of cytoplasmic domains of transmembrane proteins, are critical for endocytic cargo trafficking and proper PM proteomic distribution. The phosphorylation states of auxin efflux carriers (PINs) govern the polar distribution of these proteins to apical and basal PM domains (Luschnig and Vert, 2014); however, it is unclear whether the endocytosis of PINs is modulated directly by phosphorylation. In contrast, phosphorylation of Thr residues in the NODULIN 26-LIKE INTRINSIC PROTEIN5;1 PM boric acid importer N-terminal Thr-Pro-Gly repeat motif enhances AP-2-dependent CME and is critical to the maintenance of channel polarity and endocytosis (Wang et al., 2017).
Both mono- and polyubiquitylation serve as molecular recognition signals for the internalization of PM proteins. In mammalian and yeast species, the major endocytosis determinant is Lys-63 (K-63)-linked polyubiquitylation (Haglund and Dikic, 2012), and this modification also is used as an endocytic signal in plants. After sensing the flg22 peptide, the plant immune receptor kinase FLAGELLIN-SENSING2 (FLS2) binds its coreceptor, the kinase BAK1, which phosphorylates the PUB12/13 E3 ubiquitin ligases that subsequently K-63 polyubiquitylate FLS2 prior to internalization and degradation (Fig. 1; Lu et al., 2011). K-63 polyubiquitylation also is critical for polar localization of the PM proteins BRI1 (Martins et al., 2015) and PIN2 (Leitner et al., 2012).
Endocytic signaling via monoubiquitylation has also been implicated in the regulation of nutrient uptake by marking the iron transport protein IRON-REGULATED TRANSPORTER1 for constitutive endocytosis and turnover (Barberon et al., 2011). Conversely, mono- and diubiquitylation is not required for constitutive CME of the BOR1 transporter, but is necessary for targeting to the MVB for degradation in response to high environmental boron (Kasai et al., 2011). Ubiquitylated CME cargo proteins are recognized in metazoans via the EAPs Eps15 and intersectin, which collaborate with the ESCRT to sort such cargoes at the PM (Mayers et al., 2013; Schuh and Audhya, 2014). It remains to be determined whether plant EAPs, including homologs of Eps15, intersectin, and epsin, function in recognition of mono- and/or polyubiquitylated CME cargo. Other ubiquitin-binding families in Arabidopsis, including the TARGET OF MYB1-LIKE proteins, have been shown to localize to the PM, bind ubiquitin, and are required for degradation of ubiquitylated PIN2 (Korbei et al., 2013). While a significant number of ubiquitylated PM proteins and the enzymes that attach and recognize these tags have been discovered, the extent to which these systems mediate PM cargo endocytosis, recycling, and/or degradation remains unknown (Isono and Kalinowska, 2017).
DRPS
As CCPs mature, increasing curvature of the membrane results in the emerging vesicle being connected to the donor membrane by a comparatively narrow neck (Fig. 1). Overcoming the thermodynamic energy barrier necessary for scission of the vesicle from the PM requires the coordinated activity of a number of EAPs, including dynamin, amphiphysin, and endophilins (Antonny et al., 2016). In plants, CME is dependent on the function of two DRP families: the DRP2 family that is the most similar to metazoan dynamin as well as the plant-specific DRP1 family (Bednarek and Backues, 2010; Fujimoto and Tsutsumi, 2014).
DRP2B has been implicated in pathogen response signaling. In Arabidopsis, DRP2B is phosphorylated in response to elicitation with the flg22 peptide (Benschop et al., 2007), and drp2b mutants display higher susceptibility to Pseudomonas syringae (Smith et al., 2014a; Leslie et al., 2016). Furthermore, flg22 ligand-induced endocytosis of FLS2 was found to be dependent on DRP2B but not DRP2A (Smith et al., 2014a). Similar results demonstrating reduced FLS2 internalization resulting from DRP2 silencing and impaired flg22-induced reactive oxygen species response upon overexpression of the DRP2B homolog NtDRP2-1 in Nicotiana benthamiana (Chaparro-Garcia et al., 2015) indicate a conserved DRP2 function in plant immunity across plant species. Moreover, DRP2A and DRP2B interact with each other (Huang et al., 2015), and localize in PM CCPs, at the TGN/EE, and at the leading edge of the cell plate (Fujimoto et al., 2008, 2010). Indicative of their essential function, drp2a/drp2b double mutants arrest prior to the first mitotic division in male and female gametogenesis, whereas single mutant plants do not display obvious morphological phenotypes, suggesting that DRP2A and DRP2B have overlapping and/or functionally redundant roles in development (Backues et al., 2010).
Like the DRP2s, the DRP1s are essential; in Arabidopsis, DRP1A, DRP1C, and DRP1E are required for various stages of plant development, including pollen maturation, embryogenesis, seedling growth, and fertilization, and mutants display cell plate and PM biogenesis defects (Kang et al., 2003; Konopka and Bednarek, 2008a; Fujimoto and Tsutsumi, 2014). Consistent with their function in endocytosis, DRP1s colocalize with clathrin in dynamic PM CCPs (Konopka et al., 2008; Fujimoto et al., 2010), and drp1 mutants display abnormal CME (Collings et al., 2008). BOR1-GFP, which is polarly localized on the inner/stele-side PM of root cells, colocalizes with DRP1A, and inducible expression of the dominant negative DRP1A K47A mutant inhibits BOR1-GFP endocytosis, boron-induced degradation, and polar localization (Yoshinari et al., 2016).
In recent years, an increasing amount of evidence has accumulated suggesting clathrin-mediated trafficking is important in membrane recycling from the growing cell plate and in mediating cell plate protein and lipid distribution (McMichael and Bednarek, 2013). Indeed, key players in CME have been localized to various regions of the cell plate, including clathrin (Konopka et al., 2008; Miart et al., 2014) and the TPC (Van Damme et al., 2011). During cytokinesis, DRP1s colocalize with DRP2s at the leading edge of the cell plate (Kang et al., 2003; Collings et al., 2008; Konopka et al., 2008; Mravec et al., 2011; Ito et al., 2012; Song et al., 2012; Frescatada-Rosa et al., 2014). Clathrin is predominantly associated with the maturing central regions of the cell plate, suggesting that DRP1s and DRP2s function in a clathrin-independent manner at the periphery of the cell plate. However, reminiscent of the dynamin-recruiting activity of the SRC homology 3 (SH3) domain- and BAR (Bin/Amphiphysin/Rvs) domain-containing mammalian EAPs amphiphysin and endophilin (Antonny et al., 2016), the SH3 domain-containing protein 2 (SH3P2) was recently shown to interact and facilitate the association of DRP1 with the cell plate (Ahn et al., 2017). Similar to amphiphysin and endophilin, the SH3P2 protein contains a membrane curvature-inducing BAR domain, suggesting that the association of SH3P2 and DRP1 in the absence of clathrin promotes tubulation of cell plate membrane (Ahn et al., 2017). In addition to its function at the cell plate, SH3P2 localizes to the PM and cofractionates with isolated CCVs (Nagel et al., 2017), potentially indicating the SH3P2/DRP1 interaction extends to CME.
Given the concomitant arrival and departure of DRP2s and DRP1s to/from CCPs (Fujimoto et al., 2010), it is likely that these distinct DRP families function coordinately in CME through their ability to self-associate and thus potentially copolymerize (Fig. 1; Fujimoto et al., 2008), as well as through indirect association with EAPs, including the SH3 domain-containing TASH3 subunit of the TPC (Gadeyne et al., 2014) and lipids. Their coordinate function may extend to the cell plate, where DRP1A, DRP2B, and CLC2 have been shown to colocalize and interact with sterols in a regulatory cross talk mechanism governing lipid composition in the organelle (Frescatada-Rosa et al., 2014). However, CCP-associated DRP1A dynamics are disrupted by TyrA23 application (Konopka et al., 2008), whereas DRP2B were not (Fujimoto et al., 2010), indicating that despite their coordinated function in CME and clathrin-independent roles in cell plate biogenesis, DRP1 and DRP2 proteins may be regulated in distinct ways. Further study is needed to understand the functions of DRP1/2 in CME and cytokinesis, and whether the various DRPs serve different roles between these processes, how they are regulated, and whether they function in an analogous manner as mammalian dynamin in the process of CCV budding and scission in plants. Understanding all aspects of DRP function throughout the cell will be essential for our understanding of how plants utilize DRPs in CME, specifically.
VESICLE UNCOATING
Following scission from the PM, clathrin triskelia are liberated and EAPs are shed to expose the regulatory proteins critical for vesicle transport, targeting, tethering, and eventually fusion. In animal systems, this process is facilitated through the action of auxilin and the heat shock cognate 70 (HSC70) ATPase (Ungewickell et al., 1995; Kampinga and Craig, 2010). In Arabidopsis, the auxilin-related protein-1 (AUXI1) interacts with clathrin and SH3P1, and stimulates vesicle uncoating in vitro in the presence of HSC70 (Fig. 1; Lam et al., 2001). Similarly, the rice (Oryza sativa) ortholog of AUXI1, XB21, similarly increases clathrin dissociation in the presence of animal HSC70 in vitro (Park et al., 2017), suggesting the auxilin/HSC70 uncoating mechanism is conserved in plants. In vivo, overexpression of another auxilin-related protein, auxilin-like protein 2 (AX2), abrogates internalization of FM4-64 and the fluorescently labeled endogenous danger-associated peptide (TAMRA-pep1) and its receptor PEPR1 (Ortiz-Morea et al., 2016), suggesting that high levels of AX2 interfere with clathrin membrane recruitment and CCP formation. Further investigation is needed to determine if AUXI1, AX2, or other proteins accomplish vesicle uncoating in vivo.
CIE
In addition to the significant amount of research that has focused on the process of CME, there also has been increasing recognition of the importance of a nonclathrin-dependent mechanism(s) for the internalization of PM and extracellular material in plants. In metazoans, several CIE pathways exist that can be largely divided into dynamin-dependent (including caveolin and RhoA-mediated pathways) and dynamin-independent (possibly mediated by ARF6, CDC42, and flotillins) groups (Mayor and Pagano, 2007). The latter pathway has been implicated with membrane microdomains insofar as flotillin is associated with detergent-resistant membrane fractions enriched for sterols, sphingolipids, and some specific proteins, putatively derived from PM microdomains (Borner et al., 2005; Raffaele et al., 2009). Homologs of the flotillin family (e.g. flotillin 1 [flot1] in Arabidopsis) have been identified in many plant species (Haney and Long, 2010; Li et al., 2012) and are similarly associated with detergent-resistant membrane fractions (Borner et al., 2005). While the mechanics of flotillin endocytosis have yet to be clearly elucidated, the dynamics of fluorescently tagged flot1 foci at the PM are distinct from those of CCPs (Li et al., 2012). Flotillin-associated CIE partly mediates the internalization of membrane-anchored protein cargo, including GPI-anchored proteins in metazoans (Sabharanjak et al., 2002); likewise, GPI-anchored cargo internalization in Arabidopsis is partially insensitive to CME inhibition (Baral et al., 2015). However, this sensitivity appears to be cell type specific, implying cargo distribution among CME and CIE pathways is tissue dependent (Baral et al., 2015). Moreover, environmental conditions such as salt stress appear to influence cargo distribution between CME and CIE pathways (Baral et al., 2015). In addition to CIE of PM-associated proteins, extracellular solutes may be internalized by means of fluid-phase endocytic pathways, a phenomenon that permits cells to take up critical solutes within membrane-enclosed vesicles, thus avoiding disruption of cytoplasmic concentrations (Baroja-Fernandez et al., 2006). Studies of fluid-phase endocytosis in plants have primarily relied on the use of ikarugamycin (IKA) to distinguish between CME and CIE uptake of extracellular markers. IKA is a natural product that has been utilized as a CME inhibitor in plants and animals, although its mode of action remains to be determined (Elkin et al., 2016). Accumulation of fluorescent glucose in the endomembrane system of BY-2 protoplasts (Bandmann and Homann, 2012), as well as the internalization of charged nanogold particles targeted to the degradative vacuole into tobacco pollen tubes, is insensitive to IKA (Moscatelli et al., 2007), suggesting that the uptake of these reporters is mediated through the CIE mechanism(s). Similarly, IKA application impedes internalization of FM4-64 and, to a lesser extent, fluorescent nanobeads of approximately 20 nm, suggesting the latter might be internalized by CME and CIE (Bandmann et al., 2012).
HORMONAL REGULATION OF ENDOCYTOSIS
Polarized auxin transport, which facilitates asymmetric distribution of auxin, is critical for organ patterning and differential growth during tropic responses (Abbas et al., 2013; Rakusová et al., 2015). Several recent studies in Arabidopsis have demonstrated that gravity- and light-induced changes in directional auxin flow are partially mediated through membrane trafficking-dependent intracellular relocalization and/or degradation of the PM-resident PIN auxin efflux transporters (Abas et al., 2006; Kleine-Vehn et al., 2010; Ding et al., 2011; Zhang et al., 2017). PIN2 and PIN3 are essential for root gravitropism and hypocotyl phototropism, respectively, and genetic interference with clathrin function abrogates the polarity of PIN2 and PIN3, resulting in uniform PM distribution (Kitakura et al., 2011; Wang et al., 2013; Zhang et al., 2017). Consistent with this, loss-of-function chc2 and clc2/clc3 mutants and lines expressing clathrin HUB display altered auxin transport and distribution, as well as various auxin-dependent developmental phenotypes, including agravitropism, indicating that CME is important for proper spatial distribution of auxin necessary for plant growth and morphogenesis (Yu et al., 2016). Recent studies have found that gravity or blue light differentially regulate clathrin recruitment to the PM in root epidermal cells or hypocotyl endodermal cells at both sides of the gravity-stimulated root or light-illuminated hypocotyl (Wang et al., 2016; Yu et al., 2016; Zhang et al., 2017).
Not only is auxin distribution dependent on CME, but CME itself appears to be modulated by auxin, although as discussed above this has been questioned recently by the findings of Jasik and colleagues (Jásik et al., 2016). Indeed, the application of another hormone, salicylic acid, has been shown to inhibit CME cargo uptake and disrupt clathrin and AP-2 association with the PM (Du et al., 2013; Wang et al., 2016). Inhibition of CME by treatment with natural or synthetic auxin as well as modulation of auxin levels in vivo results in inhibition of bona fide CME cargo uptake and the rapid disappearance of CLCs, but not CHCs, from the PM. Moreover, CLCs are essential for auxin inhibition of CME (Robert et al., 2010; Wang et al., 2013). The mechanism(s) by which auxin differentially affects CLC and CHC membrane association has yet to be determined. However, not all components of the CME machinery are affected; recruitment of the adaptor complexes TPC and AP-2, which are required for clathrin membrane association, to the PM is not sensitive to auxin application (Wang et al., 2016). In light of the critical contribution of CLCs to clathrin triskelia assembly and stability, it may be that auxin inhibits CME by affecting the stability and/or structure of the clathrin coat. However, the biochemical mechanism by which auxin might affect CLCs remains to be discovered, and the disparity between these data and those of Jasik and colleagues has yet to be reconciled.
Acknowledgments
We thank Laura Vanderploeg for graphic design assistance and apologize to those colleagues whose work we were unable to cover due space restrictions.
Footnotes
This work was supported by the National Science Foundation (G.D.R. and S.Y.B.; award nos. 1121998 and 1614915) and the National Natural Science Foundation of China (C.W. and J.P.; award nos. 31670283, 31370313, and 91317304).
Articles can be viewed without a subscription.
References
- Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256; erratum Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Nat Cell Biol 8: 424 [DOI] [PubMed] [Google Scholar]
- Abbas M, Alabadí D, Blázquez MA (2013) Differential growth at the apical hook: all roads lead to auxin. Front Plant Sci 4: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguet F, Antonescu CN, Mettlen M, Schmid SL, Danuser G (2013) Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev Cell 26: 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn G, Kim H, Kim DH, Hanh NH, Yoon Y, Singaram I, Wijesinghe KJ, Johnson KA, Zhuang XH, Liang Z, et al. (2017) SH3P2 plays a crucial role at the step of membrane tubulation during cell plate formation in plants. Plant Cell http://dx.doi.org/10.1105/tpc.17.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Burd C, De Camilli P, Chen E, Daumke O, Faelber K, Ford M, Frolov VA, Frost A, Hinshaw JE, et al. (2016) Membrane fission by dynamin: what we know and what we need to know. EMBO J 35: 2270–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backues SK, Korasick DA, Heese A, Bednarek SY (2010) The Arabidopsis dynamin-related protein2 family is essential for gametophyte development. Plant Cell 22: 3218–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisa GA, Mayers JR, Bednarek SY (2013) Budding and braking news about clathrin-mediated endocytosis. Curr Opin Plant Biol 16: 718–725 [DOI] [PubMed] [Google Scholar]
- Banbury DN, Oakley JD, Sessions RB, Banting G (2003) Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J Biol Chem 278: 12022–12028 [DOI] [PubMed] [Google Scholar]
- Bandmann V, Homann U (2012) Clathrin-independent endocytosis contributes to uptake of glucose into BY-2 protoplasts. Plant J 70: 578–584 [DOI] [PubMed] [Google Scholar]
- Bandmann V, Müller JD, Köhler T, Homann U (2012) Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett 586: 3626–3632 [DOI] [PubMed] [Google Scholar]
- Bar M, Avni A (2009) EHD2 inhibits ligand-induced endocytosis and signaling of the leucine-rich repeat receptor-like protein LeEix2. Plant J 59: 600–611 [DOI] [PubMed] [Google Scholar]
- Baral A, Irani NG, Fujimoto M, Nakano A, Mayor S, Mathew MK (2015) Salt-induced remodeling of spatially restricted clathrin-independent endocytic pathways in Arabidopsis root. Plant Cell 27: 1297–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Dubeaux G, Kolb C, Isono E, Zelazny E, Vert G (2014) Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc Natl Acad Sci USA 111: 8293–8298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Zelazny E, Robert S, Conéjéro G, Curie C, Friml J, Vert G (2011) Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA 108: E450–E458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroja-Fernandez E, Etxeberria E, Muñoz FJ, Morán-Zorzano MT, Alonso-Casajús N, Gonzalez P, Pozueta-Romero J (2006) An important pool of sucrose linked to starch biosynthesis is taken up by endocytosis in heterotrophic cells. Plant Cell Physiol 47: 447–456 [DOI] [PubMed] [Google Scholar]
- Bashline L, Li S, Zhu X, Gu Y (2015) The TWD40-2 protein and the AP2 complex cooperate in the clathrin-mediated endocytosis of cellulose synthase to regulate cellulose biosynthesis. Proc Natl Acad Sci USA 112: 12870–12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashline L, Li S, Anderson CT, Lei L, Gu Y (2013) The endocytosis of cellulose synthase in Arabidopsis is dependent on μ2, a clathrin-mediated endocytosis adaptin. Plant Physiol 163: 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Heard W, Mbengue M, Robatzek S (2012) The INs and OUTs of pattern recognition receptors at the cell surface. Curr Opin Plant Biol 15: 367–374 [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Backues SK (2010) Plant dynamin-related protein families DRP1 and DRP2 in plant development. Biochem Soc Trans 38: 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop JJ, Mohammed S, O’Flaherty M, Heck AJ, Slijper M, Menke FL (2007) Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics 6: 1198–1214 [DOI] [PubMed] [Google Scholar]
- Borner GH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, Macaskill A, Napier JA, Beale MH, Lilley KS, Dupree P (2005) Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol 137: 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro-Garcia A, Schwizer S, Sklenar J, Yoshida K, Petre B, Bos JI, Schornack S, Jones AM, Bozkurt TO, Kamoun S (2015) Phytophthora infestans RXLR-WY effector AVR3a associates with Dynamin-Related Protein 2 required for endocytosis of the plant pattern recognition receptor FLS2. PLoS One 10: e0137071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Irani NG, Friml J (2011) Clathrin-mediated endocytosis: the gateway into plant cells. Curr Opin Plant Biol 14: 674–682 [DOI] [PubMed] [Google Scholar]
- Chuprov-Netochin R, Neskorodov Y, Marusich E, Mishutkina Y, Volynchuk P, Leonov S, Skryabin K, Ivashenko A, Palme K, Touraev A (2016) Novel small molecule modulators of plant growth and development identified by high-content screening with plant pollen. BMC Plant Biol 16: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Aguet F, Boulant S, Kirchhausen T (2012) The first five seconds in the life of a clathrin-coated pit. Cell 150: 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, Gebbie LK, Howles PA, Hurley UA, Birch RJ, Cork AH, Hocart CH, Arioli T, Williamson RE (2008) Arabidopsis dynamin-like protein DRP1A: a null mutant with widespread defects in endocytosis, cellulose synthesis, cytokinesis, and cell expansion. J Exp Bot 59: 361–376 [DOI] [PubMed] [Google Scholar]
- Debeir O, Van Ham P, Kiss R, Decaestecker C (2005) Tracking of migrating cells under phase-contrast video microscopy with combined mean-shift processes. IEEE Trans Med Imaging 24: 697–711 [DOI] [PubMed] [Google Scholar]
- Dejonghe W, Kuenen S, Mylle E, Vasileva M, Keech O, Viotti C, Swerts J, Fendrych M, Ortiz-Morea FA, Mishev K, et al. (2016) Mitochondrial uncouplers inhibit clathrin-mediated endocytosis largely through cytoplasmic acidification. Nat Commun 7: 11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Di Rubbo S, Irani NG, Kim SY, Xu ZY, Gadeyne A, Dejonghe W, Vanhoutte I, Persiau G, Eeckhout D, Simon S, et al. (2013) The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell 25: 2986–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski Ł, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, et al. (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Drakakaki G, Robert S, Szatmari AM, Brown MQ, Nagawa S, Van Damme D, Leonard M, Yang Z, Girke T, Schmid SL, et al. (2011) Clusters of bioactive compounds target dynamic endomembrane networks in vivo. Proc Natl Acad Sci USA 108: 17850–17855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Tejos R, Beck M, Himschoot E, Li H, Robatzek S, Vanneste S, Friml J (2013) Salicylic acid interferes with clathrin-mediated endocytic protein trafficking. Proc Natl Acad Sci USA 110: 7946–7951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin SR, Oswald NW, Reed DK, Mettlen M, MacMillan JB, Schmid SL (2016) Ikarugamycin: a natural product inhibitor of clathrin-mediated endocytosis. Traffic 17: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Hao H, Xue Y, Zhang L, Song K, Ding Z, Botella MA, Wang H, Lin J (2013) Dynamic analysis of Arabidopsis AP2 σ subunit reveals a key role in clathrin-mediated endocytosis and plant development. Development 140: 3826–3837 [DOI] [PubMed] [Google Scholar]
- Fan L, Li R, Pan J, Ding Z, Lin J (2015) Endocytosis and its regulation in plants. Trends Plant Sci 20: 388–397 [DOI] [PubMed] [Google Scholar]
- Frescatada-Rosa M, Stanislas T, Backues SK, Reichardt I, Men S, Boutté Y, Jürgens G, Moritz T, Bednarek SY, Grebe M (2014) High lipid order of Arabidopsis cell-plate membranes mediated by sterol and DYNAMIN-RELATED PROTEIN1A function. Plant J 80: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Arimura S, Nakazono M, Tsutsumi N (2008) Arabidopsis dynamin-related protein DRP2B is co-localized with DRP1A on the leading edge of the forming cell plate. Plant Cell Rep 27: 1581–1586 [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Arimura S, Ueda T, Takanashi H, Hayashi Y, Nakano A, Tsutsumi N (2010) Arabidopsis dynamin-related proteins DRP2B and DRP1A participate together in clathrin-coated vesicle formation during endocytosis. Proc Natl Acad Sci USA 107: 6094–6099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Tsutsumi N (2014) Dynamin-related proteins in plant post-Golgi traffic. Front Plant Sci 5: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeyne A, Sánchez-Rodríguez C, Vanneste S, Di Rubbo S, Zauber H, Vanneste K, Van Leene J, De Winne N, Eeckhout D, Persiau G, et al. (2014) The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell 156: 691–704 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Haglund K, Dikic I (2012) The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci 125: 265–275 [DOI] [PubMed] [Google Scholar]
- Haney CH, Long SR (2010) Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc Natl Acad Sci USA 107: 478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Fan L, Chen T, Li R, Li X, He Q, Botella MA, Lin J (2014) Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 26: 1729–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Schlacht A, Norcott JP, Traynor D, Bloomfield G, Antrobus R, Kay RR, Dacks JB, Robinson MS (2014) Characterization of TSET, an ancient and widespread membrane trafficking complex. eLife 3: e02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Fujimoto M, Fujiwara M, Fukao Y, Arimura S, Tsutsumi N (2015) Arabidopsis dynamin-related proteins, DRP2A and DRP2B, function coordinately in post-Golgi trafficking. Biochem Biophys Res Commun 456: 238–244 [DOI] [PubMed] [Google Scholar]
- Irani NG, Di Rubbo S, Mylle E, Van den Begin J, Schneider-Pizoń J, Hniliková J, Šíša M, Buyst D, Vilarrasa-Blasi J, Szatmári AM, et al. (2012) Fluorescent castasterone reveals BRI1 signaling from the plasma membrane. Nat Chem Biol 8: 583–589 [DOI] [PubMed] [Google Scholar]
- Irani NG, Di Rubbo S, Russinova E (2014) In vivo imaging of brassinosteroid endocytosis in Arabidopsis. Methods Mol Biol 1209: 107–117 [DOI] [PubMed] [Google Scholar]
- Isono E, Kalinowska K (2017) ESCRT-dependent degradation of ubiquitylated plasma membrane proteins in plants. Curr Opin Plant Biol 40: 49–55 [DOI] [PubMed] [Google Scholar]
- Ito E, Fujimoto M, Ebine K, Uemura T, Ueda T, Nakano A (2012) Dynamic behavior of clathrin in Arabidopsis thaliana unveiled by live imaging. Plant J 69: 204–216 [DOI] [PubMed] [Google Scholar]
- Jaqaman K, Loerke D, Mettlen M, Kuwata H, Grinstein S, Schmid SL, Danuser G (2008) Robust single-particle tracking in live-cell time-lapse sequences. Nat Methods 5: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jásik J, Bokor B, Stuchlík S, Mičieta K, Turňa J, Schmelzer E (2016) Effects of auxins on PIN-FORMED2 (PIN2) dynamics are not mediated by inhibiting PIN2 endocytosis. Plant Physiol 172: 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Vert G (2017) Single event resolution of plant plasma membrane protein endocytosis by TIRF microscopy. Front Plant Sci 8: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG (2005) A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123: 305–320 [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11: 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Rancour DM, Bednarek SY (2003) The dynamin-like protein ADL1C is essential for plasma membrane maintenance during pollen maturation. Plant J 35: 1–15 [DOI] [PubMed] [Google Scholar]
- Kasai K, Takano J, Miwa K, Toyoda A, Fujiwara T (2011) High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem 286: 6175–6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BT, Owen DJ (2011) Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol 23: 404–412 [DOI] [PubMed] [Google Scholar]
- Kim SY, Xu ZY, Song K, Kim DH, Kang H, Reichardt I, Sohn EJ, Friml J, Juergens G, Hwang I (2013) Adaptor protein complex 2-mediated endocytosis is crucial for male reproductive organ development in Arabidopsis. Plant Cell 25: 2970–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakura S, Vanneste S, Robert S, Löfke C, Teichmann T, Tanaka H, Friml J (2011) Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 23: 1920–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Ding Z, Jones AR, Tasaka M, Morita MT, Friml J (2010) Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc Natl Acad Sci USA 107: 22344–22349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Šamajová O, Ovečka M, Šamaj J (2015) Super-resolution microscopy in plant cell imaging. Trends Plant Sci 20: 834–843 [DOI] [PubMed] [Google Scholar]
- Konopka CA, Backues SK, Bednarek SY (2008) Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell 20: 1363–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka CA, Bednarek SY (2008a) Comparison of the dynamics and functional redundancy of the Arabidopsis dynamin-related isoforms DRP1A and DRP1C during plant development. Plant Physiol 147: 1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka CA, Bednarek SY (2008b) Variable-angle epifluorescence microscopy: a new way to look at protein dynamics in the plant cell cortex. Plant J 53: 186–196 [DOI] [PubMed] [Google Scholar]
- Korbei B, Moulinier-Anzola J, De-Araujo L, Lucyshyn D, Retzer K, Khan MA, Luschnig C (2013) Arabidopsis TOL proteins act as gatekeepers for vacuolar sorting of PIN2 plasma membrane protein. Curr Biol 23: 2500–2505 [DOI] [PubMed] [Google Scholar]
- Lam BC, Sage TL, Bianchi F, Blumwald E (2001) Role of SH3 domain-containing proteins in clathrin-mediated vesicle trafficking in Arabidopsis. Plant Cell 13: 2499–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J, Retzer K, Korbei B, Luschnig C (2012) Dynamics in PIN2 auxin carrier ubiquitylation in gravity-responding Arabidopsis roots. Plant Signal Behav 7: 1271–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie ME, Rogers SW, Heese A (2016) Increased callose deposition in plants lacking DYNAMIN-RELATED PROTEIN 2B is dependent upon POWDERY MILDEW RESISTANT 4. Plant Signal Behav 11: e1244594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liu P, Wan Y, Chen T, Wang Q, Mettbach U, Baluska F, Samaj J, Fang X, Lucas WJ, et al. (2012) A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell 24: 2105–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Rodriguez-Furlan C, Wang J, van de Ven W, Gao T, Raikhel NV, Hicks GR (2017) Different endomembrane trafficking pathways establish apical and basal polarities. Plant Cell 29: 90–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pan SQ (2017) Agrobacterium delivers VirE2 protein into host cells via clathrin-mediated endocytosis. Sci Adv 3: e1601528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfke C, Scheuring D, Dünser K, Schöller M, Luschnig C, Kleine-Vehn J (2015) Tricho- and atrichoblast cell files show distinct PIN2 auxin efflux carrier exploitations and are jointly required for defined auxin-dependent root organ growth. J Exp Bot 66: 5103–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Lin W, Gao X, Wu S, Cheng C, Avila J, Heese A, Devarenne TP, He P, Shan L (2011) Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332: 1439–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Vert G (2014) The dynamics of plant plasma membrane proteins: PINs and beyond. Development 141: 2924–2938 [DOI] [PubMed] [Google Scholar]
- Martins S, Dohmann EM, Cayrel A, Johnson A, Fischer W, Pojer F, Satiat-Jeunemaître B, Jaillais Y, Chory J, Geldner N, et al. (2015) Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination. Nat Commun 6: 6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers JR, Wang L, Pramanik J, Johnson A, Sarkeshik A, Wang Y, Saengsawang W, Yates JR III, Audhya A (2013) Regulation of ubiquitin-dependent cargo sorting by multiple endocytic adaptors at the plasma membrane. Proc Natl Acad Sci USA 110: 11857–11862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano RE (2007) Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8: 603–612 [DOI] [PubMed] [Google Scholar]
- Mbengue M, Bourdais G, Gervasi F, Beck M, Zhou J, Spallek T, Bartels S, Boller T, Ueda T, Kuhn H, et al. (2016) Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc Natl Acad Sci USA 113: 11034–11039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533 [DOI] [PubMed] [Google Scholar]
- McMichael CM, Bednarek SY (2013) Cytoskeletal and membrane dynamics during higher plant cytokinesis. New Phytol 197: 1039–1057 [DOI] [PubMed] [Google Scholar]
- Miart F, Desprez T, Biot E, Morin H, Belcram K, Höfte H, Gonneau M, Vernhettes S (2014) Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant J 77: 71–84 [DOI] [PubMed] [Google Scholar]
- Mitsunari T, Nakatsu F, Shioda N, Love PE, Grinberg A, Bonifacino JS, Ohno H (2005) Clathrin adaptor AP-2 is essential for early embryonal development. Mol Cell Biol 25: 9318–9323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli A, Ciampolini F, Rodighiero S, Onelli E, Cresti M, Santo N, Idilli A (2007) Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J Cell Sci 120: 3804–3819 [DOI] [PubMed] [Google Scholar]
- Motley A, Bright NA, Seaman MN, Robinson MS (2003) Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol 162: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J, Petrášek J, Li N, Boeren S, Karlova R, Kitakura S, Pařezová M, Naramoto S, Nodzyński T, Dhonukshe P, et al. (2011) Cell plate restricted association of DRP1A and PIN proteins is required for cell polarity establishment in Arabidopsis. Curr Biol 21: 1055–1060 [DOI] [PubMed] [Google Scholar]
- Murray JI, Bao Z, Boyle TJ, Waterston RH (2006) The lineaging of fluorescently-labeled Caenorhabditis elegans embryos with StarryNite and AceTree. Nat Protoc 1: 1468–1476 [DOI] [PubMed] [Google Scholar]
- Nagel MK, Kalinowska K, Vogel K, Reynolds GD, Wu Z, Anzenberger F, Ichikawa M, Tsutsumi C, Sato MH, Kuster B, et al. (2017). Arabidopsis SH3P2 is an ubiquitin-binding protein that functions together with ESCRT-I and the deubiquitylating enzyme AMSH3. Proc Natl Acad Sci USA 114: E7197–E7204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norambuena L, Tejos R (2017) Chemical genetic dissection of membrane trafficking. Annu Rev Plant Biol 68: 197–224 [DOI] [PubMed] [Google Scholar]
- Ortiz-Morea FA, Savatin DV, Dejonghe W, Kumar R, Luo Y, Adamowski M, Van den Begin J, Dressano K, Pereira de Oliveira G, Zhao X, et al. (2016) Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc Natl Acad Sci USA 113: 11028–11033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Zazímalová E, Ruthardt N, Petrásek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, et al. (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Paez Valencia J, Goodman K, Otegui MS (2016) Endocytosis and endosomal trafficking in plants. Annu Rev Plant Biol 67: 309–335 [DOI] [PubMed] [Google Scholar]
- Pan J, Fujioka S, Peng J, Chen J, Li G, Chen R (2009) The E3 ubiquitin ligase SCFTIR1/AFB and membrane sterols play key roles in auxin regulation of endocytosis, recycling, and plasma membrane accumulation of the auxin efflux transporter PIN2 in Arabidopsis thaliana. Plant Cell 21: 568–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CJ, Wei T, Sharma R, Ronald PC (2017) Overexpression of rice auxilin-like protein, XB21, induces necrotic lesions, up-regulates endocytosis-related genes, and confers enhanced resistance to Xanthomonas oryzae pv. oryzae. Rice (N Y) 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S, Bayer E, Lafarge D, Cluzet S, German Retana S, Boubekeur T, Leborgne-Castel N, Carde JP, Lherminier J, Noirot E, et al. (2009) Remorin, a Solanaceae protein resident in membrane rafts and plasmodesmata, impairs Potato virus X movement. Plant Cell 21: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusová H, Fendrych M, Friml J (2015) Intracellular trafficking and PIN-mediated cell polarity during tropic responses in plants. Curr Opin Plant Biol 23: 116–123 [DOI] [PubMed] [Google Scholar]
- Robert S, Chary SN, Drakakaki G, Li S, Yang Z, Raikhel NV, Hicks GR (2008) Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc Natl Acad Sci USA 105: 8464–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, Vanneste S, Zhang J, Simon S, Čovanová M, et al. (2010) ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143: 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Jiang L, Schumacher K (2008) The endosomal system of plants: charting new and familiar territories. Plant Physiol 147: 1482–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharanjak S, Sharma P, Parton RG, Mayor S (2002) GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell 2: 411–423 [DOI] [PubMed] [Google Scholar]
- Sancho-Andrés G, Soriano-Ortega E, Gao C, Bernabé-Orts JM, Narasimhan M, Müller AO, Tejos R, Jiang L, Friml J, Aniento F, et al. (2016) Sorting motifs involved in the trafficking and localization of the PIN1 auxin efflux carrier. Plant Physiol 171: 1965–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneckenburger H. (2005) Total internal reflection fluorescence microscopy: technical innovations and novel applications. Curr Opin Biotechnol 16: 13–18 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh AL, Audhya A (2014) The ESCRT machinery: from the plasma membrane to endosomes and back again. Crit Rev Biochem Mol Biol 49: 242–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Leslie ME, Robinson SJ, Korasick DA, Zhang T, Backues SK, Cornish PV, Koo AJ, Bednarek SY, Heese A (2014a) Loss of Arabidopsis thaliana Dynamin-Related Protein 2B reveals separation of innate immune signaling pathways. PLoS Pathog 10: e1004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Salamango DJ, Leslie ME, Collins CA, Heese A (2014b) Sensitivity to Flg22 is modulated by ligand-induced degradation and de novo synthesis of the endogenous flagellin-receptor FLAGELLIN-SENSING2. Plant Physiol 164: 440–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Jang M, Kim SY, Lee G, Lee GJ, Kim DH, Lee Y, Cho W, Hwang I (2012) An A/ENTH domain-containing protein functions as an adaptor for clathrin-coated vesicles on the growing cell plate in Arabidopsis root cells. Plant Physiol 159: 1013–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T (2010) Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA 107: 5220–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Kitakura S, De Rycke R, De Groodt R, Friml J (2009) Fluorescence imaging-based screen identifies ARF GEF component of early endosomal trafficking. Curr Biol 19: 391–397 [DOI] [PubMed] [Google Scholar]
- Tinevez JY, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E, Bednarek SY, Shorte SL, Eliceiri KW (2017) TrackMate: an open and extensible platform for single-particle tracking. Methods 115: 80–90 [DOI] [PubMed] [Google Scholar]
- Tóth R, Gerding-Reimers C, Deeks MJ, Menninger S, Gallegos RM, Tonaco IA, Hübel K, Hussey PJ, Waldmann H, Coupland G (2012) Prieurianin/endosidin 1 is an actin-stabilizing small molecule identified from a chemical genetic screen for circadian clock effectors in Arabidopsis thaliana. Plant J 71: 338–352 [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E (1995) Role of auxilin in uncoating clathrin-coated vesicles. Nature 378: 632–635 [DOI] [PubMed] [Google Scholar]
- Van Damme D, Gadeyne A, Vanstraelen M, Inzé D, Van Montagu MC, De Jaeger G, Russinova E, Geelen D (2011) Adaptin-like protein TPLATE and clathrin recruitment during plant somatic cytokinesis occurs via two distinct pathways. Proc Natl Acad Sci USA 108: 615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Ash WM III, Fan L, Hao H, Kim MK, Lin J (2011) Variable-angle total internal reflection fluorescence microscopy of intact cells of Arabidopsis thaliana. Plant Methods 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Hu T, Yan X, Meng T, Wang Y, Wang Q, Zhang X, Gu Y, Sánchez-Rodríguez C, Gadeyne A, et al. (2016) Differential regulation of clathrin and its adaptor proteins during membrane recruitment for endocytosis. Plant Physiol 171: 215–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yan X, Chen Q, Jiang N, Fu W, Ma B, Liu J, Li C, Bednarek SY, Pan J (2013) Clathrin light chains regulate clathrin-mediated trafficking, auxin signaling, and development in Arabidopsis. Plant Cell 25: 499–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yoshinari A, Shimada T, Hara-Nishimura I, Mitani-Ueno N, Feng Ma J, Naito S, Takano J (2017) Polar localization of the NIP5;1 boric acid channel is maintained by endocytosis and facilitates boron transport in Arabidopsis roots. Plant Cell 29: 824–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka S, Shimono Y, Shirakawa M, Fukao Y, Kawase T, Hatsugai N, Tamura K, Shimada T, Hara-Nishimura I (2013) Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell 25: 2958–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari A, Fujimoto M, Ueda T, Inada N, Naito S, Takano J (2016) DRP1-dependent endocytosis is essential for polar localization and boron-induced degradation of the borate transporter BOR1 in Arabidopsis thaliana. Plant Cell Physiol 57: 1985–2000 [DOI] [PubMed] [Google Scholar]
- Yu Q, Zhang Y, Wang J, Yan X, Wang C, Xu J, Pan J (2016) Clathrin-mediated auxin efflux and maxima regulate hypocotyl hook formation and light-stimulated hook opening in Arabidopsis. Mol Plant 9: 101–112 [DOI] [PubMed] [Google Scholar]
- Zhang C, Brown MQ, van de Ven W, Zhang ZM, Wu B, Young MC, Synek L, Borchardt D, Harrison R, Pan S, et al. (2016) Endosidin2 targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc Natl Acad Sci USA 113: E41–E50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Persson S, Hirst J, Robinson MS, van Damme D, Sánchez-Rodríguez C (2015) Change your TPLATE, change your fate: plant CME and beyond. Trends Plant Sci 20: 41–48 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu Q, Jiang N, Yan X, Wang C, Wang Q, Liu J, Zhu M, Bednarek SY, Xu J, Pan J (2017) Clathrin regulates blue light-triggered lateral auxin distribution and hypocotyl phototropism in Arabidopsis. Plant Cell Environ 40: 165–176 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yan A, Feijó JA, Furutani M, Takenawa T, Hwang I, Fu Y, Yang Z (2010) Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell 22: 4031–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]