Abstract
Stromules are plastid stroma-filled tubules that increase the surface area of the envelope and extend the reach of the plastid within the plant cell, affecting biosynthesis, metabolism, and signaling.
Stromules are narrow tubular structures, comprised of stroma surrounded by the envelope membrane, which emanate from all types of plastids found in vascular plants. The mechanism for formation of stromules is not understood, but investigating how they arise will be stimulated by the recent observation that they can form in vitro from chloroplasts isolated in particular conditions or in the presence of cellular protein extracts. Stromules allow the plastid compartment, with all its biosynthetic and metabolic capacity, to be placed near other subcellular locations in the cells, sometimes at considerable distance from the main plastid body. Proteins, and undoubtedly other molecules, but not DNA or ribosomes, flow through stromules, which have been implicated in retrograde signaling of pathogen invasion or light stress from chloroplast to nucleus. Stromules increase in frequency following exposures of cells to reactive oxygen species, sugar, hormones, and pathogen effector proteins, in chloroplast division mutants, and in transformed cells that overexpress plastid outer-envelope proteins. Through breakage or tip shedding, stromules may also be a source of plastid-derived vesicles that can recycle plastid content during nutrient stress but that also may have unknown roles in removal of toxic molecules or in intra- or intercellular communication.
Stromules emanate from plastids at varying frequencies, which differ between plant or algal species, cell type, and environmental conditions. Structures that can now be recognized as stromules have been described in the literature for over a hundred years (for review, see Gray et al., 2001; Kwok and Hanson, 2004a), but until the advent of GFP technology (Köhler et al., 1997; Hanson and Köhler, 2001), their existence was largely ignored by the scientific community. Previously, imaging them required special preparations and skilled technique (Wildman et al., 1962; Holzinger et al., 2008), and the number of cell types in which they could be visualized by standard microscopic methods was limited. Currently, labeling of plastid proteins with fluorescent proteins allows investigators to examine plastid and stromule morphology throughout the plant and in response to environmental or genetic changes. Recently, the fluorescent dye carboxyfluorescein acetate has been found to label plastids and stromules (Borucki et al., 2015) and will thus provide a convenient method to examine stromule morphology, frequency, and formation without the necessity for transformation of cells with genes encoding plastid-localized fluorescent proteins.
A series of early papers examining plastids labeled with GFP in our lab and others established stromules as a genuine feature of plant cells. Stromules were named in 2000 in order to distinguish the structures from other types of tubular structures in the cell (Köhler and Hanson, 2000). Since then, stromules have been observed through fluorescent labeling in a wide variety of plant species and cell types (for review, see Gray et al., 2001; Kwok and Hanson, 2004a; Natesan et al., 2005; Hanson and Sattarzadeh, 2008; Borucki et al., 2015). Stromules are implicated as structures that function in a large variety of cellular activities (Box 1). Now marking the 20th anniversary of the rediscovery of stromules (Köhler et al., 1997), this review will focus on the developments concerning stromules that have occurred since 2011, when our group last provided an update for Plant Physiology (Hanson and Sattarzadeh, 2011).
FORMATION OF STROMULES
Two main hypotheses, which are not mutually exclusive, are that stromules form due to forces from within or from without the main plastid body. When the plastid envelope is anchored to some structure such as the cytoskeleton and the structure itself moves away from the main plastid body or the plastid body itself moves away, perhaps “caught” by another cytoskeletal element and moved by myosin motors, a stromule might be produced. Alternatively, uneven outward pressure or membrane deformation within the plastid might cause one region to protrude, and if some structure constricts the membrane, a tubule would form. The constriction could occur either on the outside of the plastid membrane or at the base of an incipient stromule. Another hypothesis for the creation of internally generated tubules is that some feature of particular proteins within the envelope membrane can alter lipid/protein interactions and generate tubular projections. Mixing certain short peptides or dynamin with lipid bilayers in vitro resulted in remarkable production of tubular structures (Domanov and Kinnunen, 2006; Pucadyil and Schmid, 2008). Protein crowding has also been shown to produce membrane tabulation in vitro (Stachowiak et al., 2010). Either local changes in protein concentration within the chloroplast envelope or on its outer surface might induce stromule formation.
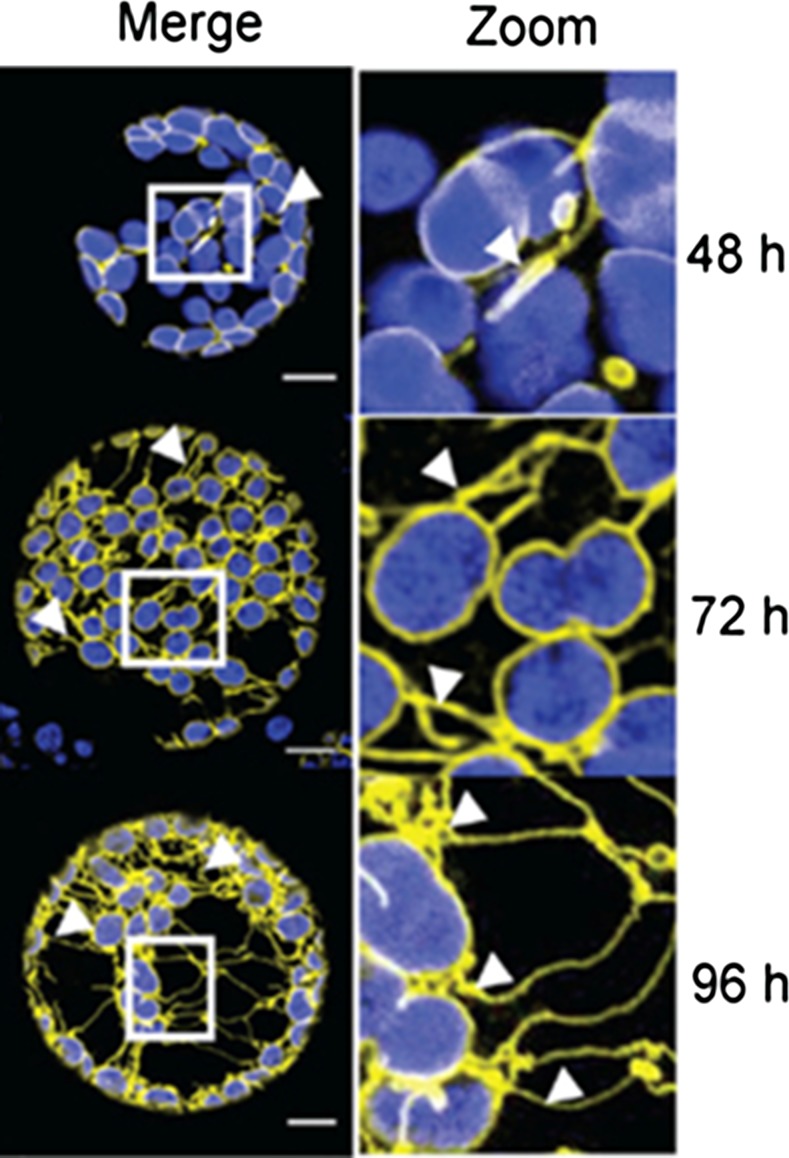
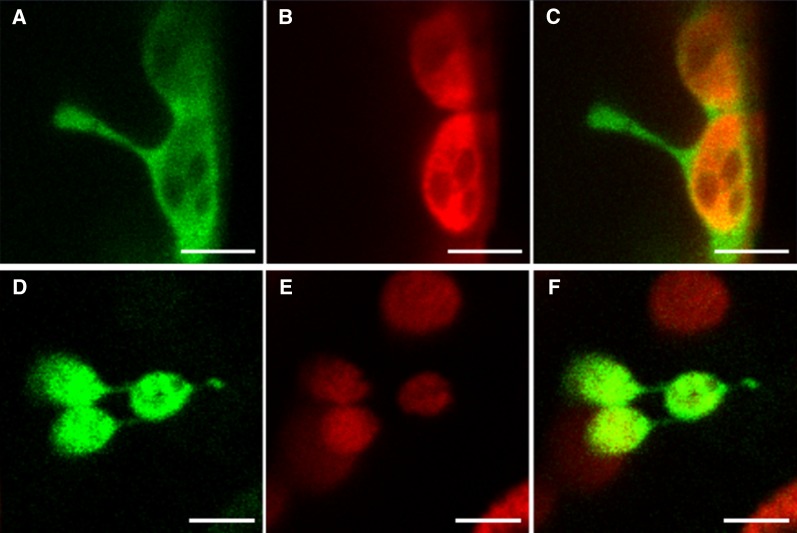
Investigations of the effects of overexpression of inner and outer envelope chloroplast membrane proteins have led to important insights that bear on the production of stromules. When either the outer envelope proteins AtLACS9 (Breuers et al., 2012) or OEP7 or the transmembrane portion of CHUP1 (Machettira et al., 2012) were overexpressed in Nicotiana benthamiana or Arabidopsis (Arabidopsis thaliana) leaf protoplasts, membrane protrusions similar to stromules were observed (Fig. 1). These apparent stromules were dependent on level of transgenic protein expression and were not seen when inner envelope membrane proteins alone were overexpressed. Simultaneous overexpression of both an inner and outer envelope protein was more effective in inducing long tubular structures in the Arabidopsis chloroplasts than expressing the outer envelope protein alone (Machettira et al., 2012). Electron microscopy revealed that extensions induced by AtLACS9 from N. benthamiana chloroplasts were comprised of both the outer and inner envelope membranes, thus suggesting that they were true stromules and that proliferation of the outer envelope was accompanied by increases in the inner envelope (Breuers et al., 2012). Machettira et al. (2012) advanced the novel hypothesis that another function of stromules could be storage of proteins imported into the outer envelope. Whether this might be a function in other types of tissues is not known.
Figure 1.
Induction of stromules by outer envelope membrane protein overexpression. Four days after Agrobacterium infiltration of AtLACS9-GFP into N. benthamiana protoplasts, there is extensive proliferation of stromules, which appear to connect chloroplasts to one another. Size of bars, 10 μm. From Breuers et al. (2012). Image supplied by Andreas Weber.
Whether proteins involved in chloroplast division are also directly involved in stromule formation or breakage is not known. Mutations in the ARC3 and ARC6 genes that reduce chloroplast division in Arabidopsis also result in increased stromule length (Holzinger et al., 2008). ARC3 mutants act through minE (Angel et al., 2013), and stromule frequency is increased in Arabidopsis minE1 mutants, which are affected in chloroplast division site placement (Fujiwara et al., 2015). FtsZ rings could theoretically provide constrictions that narrow incipient plastid projections into stromules, but experimental evidence for such a process is currently lacking.
Stromules from Isolated Plastids
An important development has been the finding that stromules can form on isolated plastids from N. benthamiana, reported by two different groups (Brunkard et al., 2015, 2016; Ho and Theg, 2016). Because the cytoskeleton is disrupted upon cell breakage, this finding favors the hypothesis that some force from within the plastid body or envelope membrane can cause stromules to form. Nevertheless, after fractionation of cells into plastids, proteins from the cytoplasm may remain on the outer surface of the plastid and could produce the constriction needed to produce a stromule. One group used a relatively simple chloroplast isolation procedure to produce plastids that were able extend stromules without the addition of any other factors (Brunkard et al., 2016), while the chloroplast preparations made by a second group required addition of a cell protein extract to chloroplasts to induce stromules (Ho and Theg, 2016). The reason for the difference in requirement for a protein extract is unknown, perhaps due to variation in the composition of extraction buffers between the two studies. It is possible that some cytoplasmic factors remained bound in the preparations not requiring exogenous protein. For example, chloroplasts are known to exhibit actin-mediated movement, and perhaps cytoskeletal elements might remain bound to chloroplasts following extraction under certain conditions. Short actin microfilaments are known to decorate the outside of chloroplasts (Kong et al., 2013). Treatment of isolated chloroplasts with actin inhibitors could clarify whether external microfilaments are required for stromule formation. However, the finding that the loss of CHUP1, an outer envelope protein that interacts with these short microfilaments, induces stromules rather than decreases their formation (Caplan et al., 2015), suggests that short remnants of microfilaments are not a factor allowing formation of stromules from cell-free plastids.
Only those proteins surviving a 100-kD filtration step were able to generate stromules on preparations made by Ho and Theg (2016), and the stromule-inducing activity of the extract was heat sensitive. Further fractionation of this extract could provide important insights into stromule formation. Because molecules such as drugs, dyes, and proteins can easily be added to a liquid suspension, extension of stromules by isolated plastids in vitro is an excellent system in which to investigate how stromules extend and retract and how they interact with other isolated organelles and each other.
PLASTID INTERCONNECTIONS THROUGH STROMULES
Our initial rediscovery of stromules in 1997 (Köhler et al., 1997) raised anew the question whether plastids frequently communicated with one another through direct connection. Earlier extraordinary movies by Sam Wildman and his colleagues visualized dynamic tubular structures that sometimes appeared to connect multiple chloroplasts (Wildman et al., 1962). However, at the time that Wildman’s images were produced, technology did not exist that could determine whether the “projections” could actually allow movement of molecules from one plastid to another. By 2000, photobleaching experiments had established that plastids are not usually part of a network, as only a few plastids at any one time were attached to each other in cultured cells, which display an unusually large number of stromules (Köhler and Hanson, 2000). When the GFP molecules in a portion of the stromules and plastids within a cultured cell were photobleached, recovery of fluorescence within the photobleached area did not occur, indicating that GFP could not flow from unbleached stromules and plastids into the bleached plastids. Furthermore, long-term photobleaching of one region of the cells did not result in loss of GFP fluorescence in the entire cell (Köhler and Hanson, 2000). In contrast, GFP-labeled mammalian endoplasmic reticulum within a cell becomes entirely nonfluorescent when only a region of the cell is irradiated, indicating that the endoplasmic reticulum (ER) is a network (Cole et al., 1996). The absence of a plastid network was reconfirmed twelve years later with the use of a photoconvertible GFP (Schattat et al., 2012a).
Although at any one time, only a few plastids are connected by stromules, plastids separated over considerable distance do exchange proteins. Semantic arguments have been used to claim that two plastid bodies connected by a stromule are the same plastid (Schattat et al., 2012b); however, even if two plastids are separated by 10–50 μ are products of the same parental plastid, they are clearly no longer the same plastid, but instead are two different plastids that are joined by a stromule. Time-lapse movies demonstrating recovery of GFP by a plastid after photobleaching through flow of GFP from a distant plastid are available as supplemental data in several publications (Hanson and Sattarzadeh, 2008, 2011, 2013). Thus, movements of proteins from one plastid to another have been rigorously demonstrated, even though most plastids are not connected to other plastids under usual conditions.
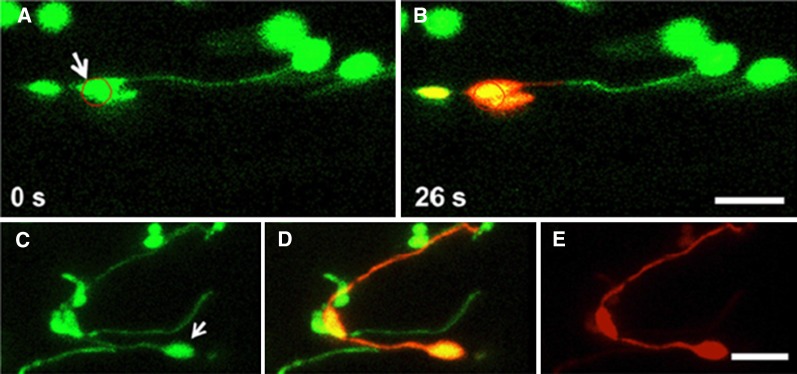
In addition to photobleaching, protein movement within a cell can be monitored by photoconversion of fluorescent protein from green to red fluorescence (Mathur et al., 2010; Bross et al., 2017). A striking image in which one green plastid is extending a green stromule toward another stromule that is red, which projects from a plastid containing red photoconverted mEosFP (Schattat et al., 2012a), has begun to be miscited as evidence that proteins do not flow from one plastid to another. Instead, the image is likely the result of an artifact caused by strong irradiation of the plastid in order to achieve photoconversion, which has resulted either in coagulation of the photoconverted protein that restricts its flow or breakage of a stromule that initially connected the two plastids. This artifact could be reproduced by our group by photoconverting mEosFP with high laser power (Fig. 2; Hanson and Sattarzadeh, 2013). However, when we used low laser power for photoconversion, the red protein readily flowed from the photoconverted plastid through a stromule to a second plastid more than 5μ away and subsequently to the tip of a stromule more than 30 μ away from the original site of photoconversion (Fig. 2).
Figure 2.
Photoconversion of mEosFP at high laser power prevents flow through a stromule, while protein flow and conversion occurs at low laser power. A and B, 100% laser power with 20 iterations was used to photoconvert mEosFP from green to red within the circle. C to E, Photoconversion of the plastid (arrow) with 1% laser power resulted in conversion to red protein that flowed through stromules. Details of the experiment and imaging can be found in Hanson and Sattarzadeh (2013).
How stromule connections arise between two plastids is not known. Because interconnected plastids are seen only rarely in wild-type plants in favorable growth conditions, no one has been able to observe a new connection being made in vivo and then carry out photobleaching or photoconversion in one of the connected plastids to determine whether the stromule is merely touching the other plastid’s envelope or whether membrane fusion is allowing transfer of molecules between them. As a result, the hypothesis has been advanced that plastids that are functionally connected by stromules are merely daughter plastids that have not entirely separated despite having moved considerable distance from one another (Schattat et al., 2012b, 2015), but as yet there is no evidence for or against this hypothesis. A way to meet the technical challenge of finding new stromule/plastid interactions might be through exploitation of the envelope membrane protein overexpression (Machettira et al., 2012; Breuers et al., 2012), which results in new extensions of stromules that appear to contact multiple chloroplasts that are clearly not daughter plastids (Fig. 1). By overexpressing the membrane proteins in plants with fluorescent-protein-labeled stroma, photobleaching or photoconversion could reveal whether the newly induced apparent stromules directly connect one chloroplast to another. Alternatively, stromules could be induced to proliferate by inoculation of pathogens, protein overexpression, chemical treatments, or photosynthetic stress (Breuers et al., 2012; Gray et al., 2012; Brunkard et al., 2015; Caplan et al., 2015) and then observed to find whether new connections have been established.
The fact that multiple separate plastids are rarely seen to be connected by stromules (in cells that are not subjected to various stresses) should not obscure the fact that proteins (and thus likely other types of molecules such as RNA and small molecules) flow long distance within stromules connected to a single plastid. Several different genuine plastid proteins, such as Rubisco, Asp aminotransferase (Kwok and Hanson, 2004b), cpHSP70 (Krenz et al., 2010a), NRIP1 (Caplan et al., 2015), arogenate dehydratase (Bross et al., 2017), and carbonic anhydrase (Fig. 3) have been labeled with fluorescent proteins and found to be present within stromules. In contrast, DNA and ribosomes do not usually move into stromules from the main plastid body. In a clever experiment, Newell et al. (2012) imaged plastid DNA with a GFP-labeled DNA binding protein (lacI) and found that nucleoids did not enter stromules. Likewise, GFP-labeled ribosomes usually remained within the main plastid body, apparently associated with plastid nucleoids (Newell et al., 2012). Early genetic experiments involving somatic fusion of protoplasts carrying different plastid genomes had previously shown that DNA from separate plastids, in contrast to mitochondrial genomes (Rothenberg and Hanson, 1988), recombines very rarely (Medgyesy et al., 1985; Clark et al., 1986). The genetic findings are consistent with the absence of DNA in stromules and the rarity of interconnected plastids.
Figure 3.
Two β-carbonic anhydrases of N. tabacum are found in stromules. βCA1 and βCA5 cDNAs were fused to a GFP coding region at the 3′ end and transiently expressed by Agroinfiltration into N. tabacum leaves. A, βCA1-GFP. B, Chlorophyll autofluorescence. C, Merge of A and B. D, βCA5-GFP. E, Chlorophyll autofluorescence. F, Merge of D and E. Green represents GFP; red represents chlorophyll Bars = 5 μm.
Given that plastids descended from engulfed cyanobacteria, stromules may be merely one example of the many types of narrow tubular structures that have been observed to connect cells of various types. For example, mammalian immune cells are well known to connect to each other through so-called nanotubes (Onfelt et al., 2004, 2005). Similar structures known as cytonemes signal between animal cells during development (Kornberg, 2017).
STROMULES—A SOURCE OF PLASTID-DERIVED VESICLES?
Vesicles have been observed to break off from stromules, a process Gunning termed “tip-shedding” (Wildman et al., 1962; Gunning, 2005, 2009). In other systems, it is known that vesicles can be created through the action of dynamin; plant cytoplasmic dynamin-type proteins might not only be involved in stromule formation but also in stromule breakage. A role of the dynamin-related GTPase ARC5 in production of stromule-derived vesicles merits further investigation. The possible involvement of FtsZ in “stromule fission” has been proposed (Fujiwara et al., 2015). The ultimate fate of the broken stromule “tips” is unknown—are they taken up by plastids, or do they enter other organelles within the cells?
Studies of chloroplast degradation suggest that at least some plastid-derived vesicles can end up in the vacuole in both Arabidopsis and rice (Oryza sativa). Small bodies containing stroma-localized fluorescent protein can be visualized in the vacuoles, provided their lytic activity is inhibited. The accumulation of these bodies in the vacuole occurs during nutrient stress, and is dependent on the autophagy system, as the stroma-containing bodies are not seen in mutants lacking ATG7 (Ishida et al., 2008; Izumi et al., 2010, 2015). Whether stromules are the actual source of these bodies through tip-shedding is not known.
Many types of cells, ranging from those in bacteria to mammals, utilize vesicle release to communicate with other cells. These extracellular vesicles often contain DNA and regulatory RNA and proteins that can influence the function of recipient cells (Yáñez-Mó et al., 2015; Bitto et al., 2017; Pérez-Bermúdez et al., 2017). The abundant cyanobacterium Prochlorococcus as well as other photosynthetic bacteria have been observed to release vesicles into the ocean (Biller et al., 2014, 2017). Among the speculated purposes for these vesicles are provision of nutrients to beneficial heterotrophic bacteria, transfer of RNA and DNA to other bacteria, or defense against bacteriophage that would mistakenly bind to the vesicles instead of their intended bacterial targets (Biller et al., 2014). Plastids are endosymbionts, essentially cells within a cell. Could stromule-derived vesicles be remnants of ancient communication, nutrient supply, or defense systems? Are they still playing some of these roles? And could such vesicles have been co-opted by pathogens for transmission within the cell or between cells?
STROMULES AND SIGNAL TRANSDUCTION
Infection and Plant Defense
Chloroplasts may be a refuge for viruses from the RNA silencing defenses mounted by plant cells outside the organelle. A number of plant viruses have been found to associate with plant envelope membranes or to enter the chloroplast (Bhattacharyya and Chakraborty, 2017). For example, outer envelope protein CHUP1 interacts with cauliflower mosaic virus (Angel et al., 2013), and cpHSP70 associates with movement protein of the geminivirus Abutilon mosaic virus (Krenz et al., 2010b). A subsequent study revealed that Abutilon mosaic virus induces long stromules that extend between plastids and from the cell periphery to the nucleus (Krenz et al., 2012). The authors suggest that stromules might serve as conduits for viruses from the plastids to the nucleus or even to other cells. Perhaps stromule-derived vesicles could also be transmitting viruses or viral effector proteins to the nucleus.
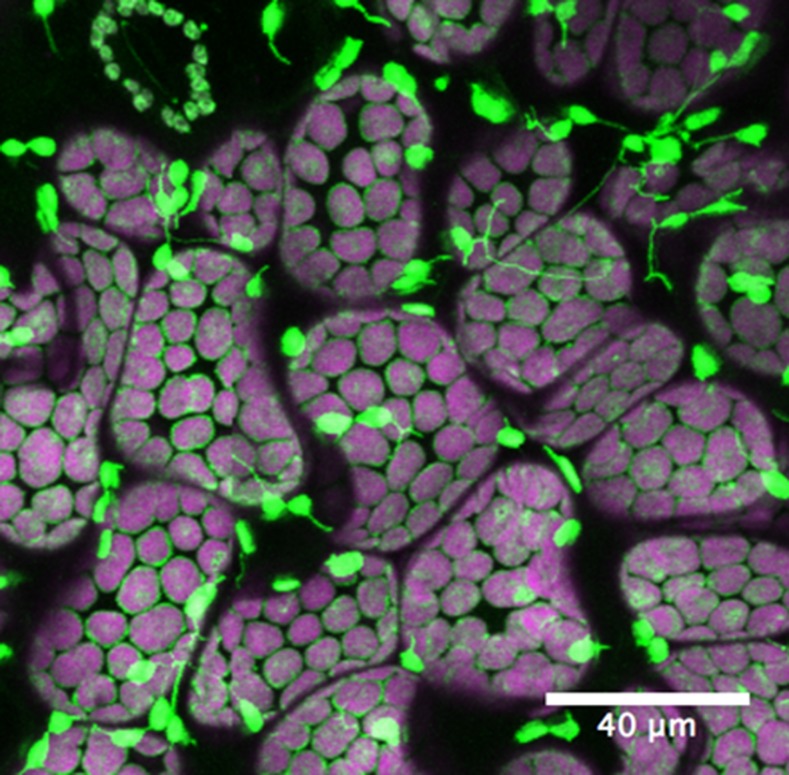
The strongest evidence for a role of stromules in plant defense comes from a study in which pathogen effector proteins were expressed transiently in N. benthamiana (Caplan et al., 2015; Gu and Dong, 2015; Fig. 4). Tobacco mosaic virus (TMV) effector protein p50, as well as a mixture of AvrBS2 and its receptor BS2, induced extensive proliferation of stromules following Agrobacterium-mediated transient expression. Likewise, infection of Arabidopsis with Pseudomonas syringae resulted in stromule induction, but strains lacking the ability to trigger effector-induced immunity had no such effect. The stromules that were induced exhibited close associations with nuclei. Evidence that the association is functional comes from the observation that that stromules accumulated a fluorescently labeled chloroplast TMV defense protein (NRIP1) that could be found in nuclei following effector p50 expression. By examining plants encoding a protein sensor of H2O2 in either the chloroplast or the nucleus, Caplan et al. (2015) were able to observe H2O2 bursts in chloroplasts following p50 expression and H2O2 accumulation in nuclei from surrounding chloroplasts.
Figure 4.
Stromule induction during plant immune response. Transgenic N. benthamiana plant expressing N NLR immune receptor and chloroplast localized NRIP1 fused to cerulean was infiltrated with Agrobacterium containing TMV effector p50. Significant induction of stromules was observed 24 h post-p50 infiltration. Green, NRIP1-Cerulean; magenta, chloroplast autofluorescence. (Image from Eunsook Park, Jeffrey Caplan, and Dinesh-Kumar.)
NRIP1 is one of two chloroplast proteins that have been shown to move to the nucleus, likely to mediate pathogen responses. A clever strategy was used to verify that NRIP1 translocated from within the chloroplast into the nucleus, as an alternative possibility would be that cytoplasmically synthesized NRIP1 directly entered the nucleus. A nuclear export signal was put onto the N terminus of fluorescently labeled NRIP1, thus preventing any accumulation of NRIP1 in the nucleus unless the protein first enters the chloroplast where the N-terminal transit sequence is cleaved along with the nuclear export signal (Caplan et al., 2015). Another novel strategy to verify movement of a protein from within the chloroplast was utilized by Isemer et al. (2012), who expressed an HA-tagged Whirly1 from the chloroplast genome. Proteins can be synthesized in abundance from the chloroplast genome, and the tagged Whirly1 could be observed in both chloroplast and nuclear preparations. The plants containing nuclear-localized chloroplast-derived Whirly1 exhibited greatly increased RNA levels of two pathogen-response genes (Isemer et al., 2012). Thus, although there is no evidence that plastid envelope membranes fuse with the nucleus, close association of chloroplast and stromules with nuclei may facilitate movement of these two proteins, as well as other proteins that signal pathogen attack or environmental distress, to the nucleus.
Sensing of Light and Oxidative Stress
Biotic stress from pathogens is not the only challenge that results in stromule formation. As well as varying in different cell and tissue types (Köhler and Hanson, 2000), stromule frequency changes in response to abiotic stress. Brunkard et al. (2015) demonstrated that stromule frequency varies in N. benthamiana seedling during the diurnal cycle. They also explored the effect of inhibiting photosynthesis chemically and observed increase in reactive oxygen species as well as increase in stromules. When a key protein in regulation of chloroplast redox status, NADPH-dependent thioredoxin reductase (NbNTRC), was down-regulated by virus-induced gene silencing, stromules also increased. Careful controls were used to determine that the effect was specific to chloroplast redox status rather than to other types of disruptions of chloroplast or mitochondrial function (Brunkard et al., 2015; Hanson, 2015). These results are consistent with Caplan et al.’s (2015) finding of stromule proliferation following exogenous application of H2O2. Furthermore, through expression of a fluorescent H2O2 sensor, high light stress has been shown to result in accumulation of H2O2 in chloroplasts and nuclei. Reduction of H2O2 in the chloroplast was accompanied with a reduction in its level in nuclei (Exposito-Rodriguez et al., 2017).
Induction by Plant Hormones
Stromules were induced in both Arabidopsis and N. tabacum by treatment with strigolactones, hormones that are secreted by roots and signal parasitic plants that possible hosts are present, as well as promote branching of arbuscular mycorrhizal fungi (Ruyter-Spira et al., 2013). Low phosphate is known to induce both stromules and strigolactone production, so the effect of exogenous strigolactone production was investigated (Vismans et al., 2016). Indeed, hypocotyl cells of both species exhibited increased stromule frequency upon strigolactone treatment, while either chemical or genetic inhibition of strigolactone production decreased stromule frequency (Vismans et al., 2016). The authors suggest that the previous finding that ABA induces stromule formation in wheat by Gray et al. (2012) might actually be due to an effect on strigolactone synthesis. Evidence for this conclusion is that ABA could not induce stromules in an Arabidopsis mutant unable to synthesize strigolactones (Vismans et al., 2016)
Associations of Stromules with Other Organelles and Subcellular Structures
Close associations of stromules and plastids with other organelles have frequently been reported. Some of the associations captured in images may be coincidental, due to crowding of cytoplasmic contents. A number of images of mitochondria, peroxisomes, and chloroplasts in close proximity are presented in textbooks in discussions of photorespiration, which requires substrate passage between those three organelles and the cytoplasm (Bauwe et al., 2010; Eisenhut et al., 2015). Some apparent associations of different organelles may result from their attachment to the same actin microfilament. There also may be unknown mechanisms that bring plastids and stromules into close contact with other parts of the cells.
Contact of stromules and plastids with the nucleus may be needed for retrograde signaling by proteins and other molecules emanating from plastids (Bobik and Burch-Smith, 2015). Erickson et al. (2017) undertook a study to determine whether the apparent association of stromules with nuclei in epidermal cells was simply coincidental, the result of an increased number of stromules in some cells, or whether the presence of the nucleus affected the frequency of stromules. Indeed, a zone within 8 μm of the nucleus was identified where most stromules either face or appear to touch the nucleus. Stromules initiated more often within the zone than elsewhere in the cell. Outside the vicinity of the nucleus, the stromules that formed did not appear to “aim” toward the nucleus and are also less likely to form. The authors present the plausible hypothesis that the actin cytoskeleton that surrounds the nucleus may be interacting with stromules and plastids, thus pulling on the plastid envelope to produce new stromules as the nucleus itself moves within the cell (Erickson et al., 2017). An association of stromules with the actin cytoskeleton was previously demonstrated by direct visualization of both microfilaments and stromules, and through treatment of tissue with actin inhibitors, resulting in reduced stromule length and looping of stromules back onto the main plastid body (Kwok and Hanson, 2003, 2004c).
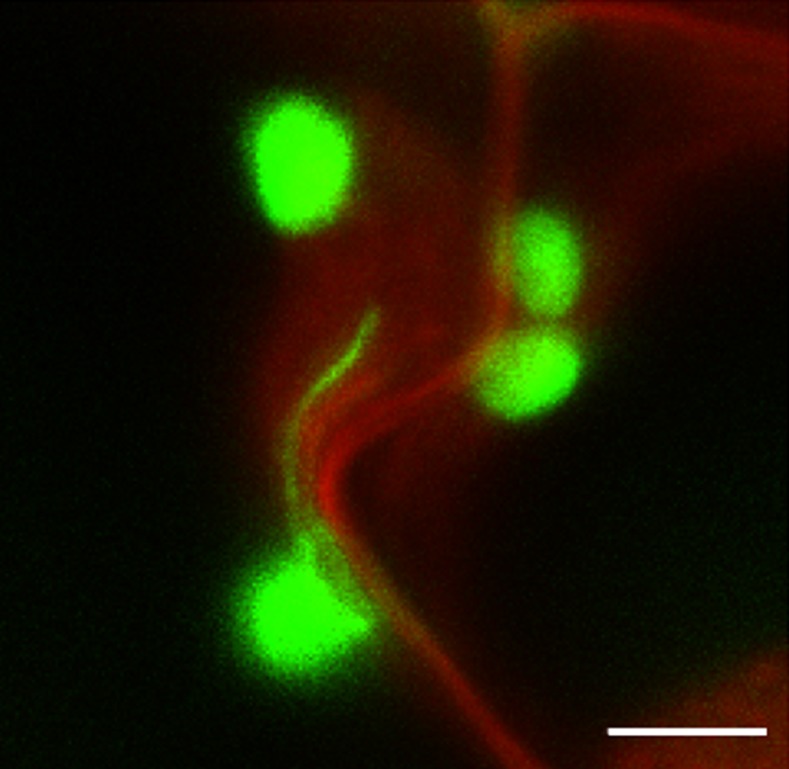
As well as the nucleus, stromules have been visualized in close proximity to other subcellular organelles or locations such as mitochondria, peroxisomes, and plasma membrane (Köhler and Hanson, 2000; Kwok and Hanson, 2004a; Barton et al., 2017; Fig. 5). Even though there may be no specific mechanism that enhances interaction of mitochondria, peroxisomes, and stromules (Barton et al., 2017), their proximity could still have functional significance in exchange of metabolites. Close association of stromules with the plasma membrane is particularly striking in cultured cells (Köhler and Hanson, 2000) and in cotyledons, in which images of stromules near each other on opposite sides of a cell wall suggest they could be involved in intercellular communication (Kwok and Hanson, 2004d). A plastid protein known as HTF1, found in both the stroma and outer envelope, was observed to interact with a plasma membrane G-protein and to be involved in sugar sensing, which could be facilitated by stromule proximity to the cell periphery (Huang et al., 2006). Stromules have been reported to be induced by exogenous Suc and Glc in Arabidopsis leaf epidermis (Schattat and Klösgen, 2011).
Figure 5.
Association of chloroplasts and a stromule with the plasma membrane/cell wall region. Cotyledons of N. tabacum line MR220 expressing GFP from the chloroplast genome (Reed et al., 2001) were stained in 1 mg/mL propidium iodide (PI) for 20 min before being imaged. GFP excited at 488 nm, PI at 561 nm. Bar = 5 μm.
Stromules also have been observed to be associated with the ER. Using fluorescent proteins that labeled the ER and plastids and stromules, Schattat et al. (2011) observed stromules extending and retracting along with the movement of nearby ER tubules. This coordinated movement might result from attachments at membrane contact sites, which were previously discovered to occur between chloroplasts and ER through the use of optical tweezers. In ruptured Arabidopsis protoplasts, chloroplasts remained attached to ER, which could be stretched out by applying a force onto the chloroplast (Andersson et al., 2007). A possible function of these membrane contacts could be hemifusion of membranes to allow passage of nonpolar metabolites, as indicated by the ability of enzymes relocated from the chloroplast to the ER to complement the metabolic deficiency caused by loss of the chloroplast enzymes (Mehrshahi et al., 2013; Mehrshahi et al., 2014).
CONCLUSION
Considerable progress in characterizing stromules has been made since fluorescent protein technology made it possible to label multiple components of the cells and visualize them in vivo. Many different vascular and nonvascular plants have been observed to produce stromules, the frequency of which varies between cell types and under different environmental stresses. Proteins and other molecules move from the main plastid body into stromules, which sometimes, albeit rarely under normal growth conditions, exhibit direct connections to other plastid bodies that allow transmission of proteins between separate plastids. Stromules are also often found in intimate association with the nucleus and may be an important part of the retrograde signaling pathway that occurs between plastids and nuclei, particularly during pathogen attack or light stress. Stromules can fragments into vesicles whose function merits further exploration. Initiation of stromules experimentally in vitro will allow future unraveling of the mechanism(s) behind their formation.
Footnotes
The authors’ prior work on stromules was supported by grants from the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, US Department of Energy to M.R.H. (especially grant De-FG02-09ER16070). Unpublished data in this article was funded by NSF MCB 1642386 (Bilateral NSF/BIO-BBSRC). Acquisition of a Zeiss LSM 710 confocal microscope by the Cornell BioResource Center was made possible by NIH grant S10RR025502.
Articles can be viewed without a subscription.
References
- Andersson MX, Goksör M, Sandelius AS (2007) Optical manipulation reveals strong attracting forces at membrane contact sites between endoplasmic reticulum and chloroplasts. J Biol Chem 282: 1170–1174 [DOI] [PubMed] [Google Scholar]
- Angel CA, Lutz L, Yang X, Rodriguez A, Adair A, Zhang Y, Leisner SM, Nelson RS, Schoelz JE (2013) The P6 protein of Cauliflower mosaic virus interacts with CHUP1, a plant protein which moves chloroplasts on actin microfilaments. Virology 443: 363–374 [DOI] [PubMed] [Google Scholar]
- Barton KA, Wozny MR, Mathur N, Jaipargas EA, Mathur J (2017) Chloroplast behaviour and interactions with other organelles in Arabidopsis thaliana pavement cells. J Cell Sci jcs.202275 (in press) [DOI] [PubMed] [Google Scholar]
- Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: Players, partners and origin. Trends Plant Sci 15: 330–336 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya D, Chakraborty S (2017) Chloroplast: The Trojan horse in plant-virus interaction. Mol Plant Pathol. Published online January 5, 2017. 10.1111/mpp.12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller SJ, McDaniel LD, Breitbart M, Rogers E, Paul JH, Chisholm SW (2017) Membrane vesicles in sea water: heterogeneous DNA content and implications for viral abundance estimates. ISME J 11: 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW (2014) Bacterial vesicles in marine ecosystems. Science 343: 183–186 [DOI] [PubMed] [Google Scholar]
- Bitto NJ, Chapman R, Pidot S, Costin A, Lo C, Choi J, D’Cruze T, Reynolds EC, Dashper SG, Turnbull L, et al. (2017) Bacterial membrane vesicles transport their DNA cargo into host cells. Sci Rep 7: 7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik K, Burch-Smith TM (2015) Chloroplast signaling within, between and beyond cells. Front Plant Sci 6: 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borucki W, Bederska M, Sujkowska-Rybkowska M (2015) Visualisation of plastid outgrowths in potato (Solanum tuberosum L.) tubers by carboxyfluorescein diacetate staining. Plant Cell Rep 34: 853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuers FK, Bräutigam A, Geimer S, Welzel UY, Stefano G, Renna L, Brandizzi F, Weber AP (2012) Dynamic remodeling of the plastid envelope membranes - a tool for chloroplast envelope in vivo localizations. Front Plant Sci 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross CD, Howes TR, Abolhassani Rad S, Kljakic O, Kohalmi SE (2017) Subcellular localization of Arabidopsis arogenate dehydratases suggests novel and non-enzymatic roles. J Exp Bot 68: 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkard JO, Runkel AM, Zambryski PC (2015) Chloroplasts extend stromules independently and in response to internal redox signals. Proc Natl Acad Sci USA 112: 10044–10049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkard JO, Runkel AM, Zambryski P (2016) Visualizing stromule frequency with fluorescence microscopy. J Vis Exp 23: 10.3791/54692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JL, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh-Kumar SP (2015) Chloroplast stromules function during innate immunity. Dev Cell 34: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E, Schnabelrauch L, Hanson MR, Sink KC (1986) Differential fate of plastid and mitochondrial genomes in Petunia somatic hybrids. Theor Appl Genet 72: 748–755 [DOI] [PubMed] [Google Scholar]
- Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J (1996) Diffusional mobility of Golgi proteins in membranes of living cells. Science 273: 797–801 [DOI] [PubMed] [Google Scholar]
- Domanov YA, Kinnunen PK (2006) Antimicrobial peptides temporins B and L induce formation of tubular lipid protrusions from supported phospholipid bilayers. Biophys J 91: 4427–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M, Hocken N, Weber AP (2015) Plastidial metabolite transporters integrate photorespiration with carbon, nitrogen, and sulfur metabolism. Cell Calcium 58: 98–104 [DOI] [PubMed] [Google Scholar]
- Erickson JL, Kantek M, Schattat MH (2017) Plastid-nucleus distance alters the behavior of stromules. Front Plant Sci 8: 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM (2017) Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat Commun 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara MT, Kojo KH, Kazama Y, Sasaki S, Abe T, Itoh RD (2015) The Arabidopsis minE mutation causes new plastid and FtsZ1 localization phenotypes in the leaf epidermis. Front Plant Sci 6: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, Hansen MR, Shaw DJ, Graham K, Dale R, Smallman P, Natesan SK, Newell CA (2012) Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J 69: 387–398 [DOI] [PubMed] [Google Scholar]
- Gray JC, Sullivan JA, Hibberd JM, Hansen MR (2001) Stromules: mobile protrusions and interconnections between plastids. Plant Biol (Stuttg) 3: 223–233 [Google Scholar]
- Gu Y, Dong X (2015) Stromules: Signal conduits for plant Immunity. Dev Cell 34: 3–4 [DOI] [PubMed] [Google Scholar]
- Gunning BE. (2005) Plastid stromules: Video microscopy of their outgrowth, retraction, tensioning, anchoring, branching, bridging, and tip-shedding. Protoplasma 225: 33–42 [DOI] [PubMed] [Google Scholar]
- Gunning BES. (2009) Plant Cell Biology on DVD. Springer-Verlag, Heidelberg, Germany [Google Scholar]
- Hanson MR. (2015) Reactive oxygen species signal chloroplasts to extend themselves. Proc Natl Acad Sci USA 112: 9799–9800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Köhler RH (2001) GFP imaging: Methodology and application to investigate cellular compartmentation in plants. J Exp Bot 52: 529–539 [PubMed] [Google Scholar]
- Hanson MR, Sattarzadeh A (2008) Dynamic morphology of plastids and stromules in angiosperm plants. Plant Cell Environ 31: 646–657 [DOI] [PubMed] [Google Scholar]
- Hanson MR, Sattarzadeh A (2011) Stromules: Recent insights into a long neglected feature of plastid morphology and function. Plant Physiol 155: 1486–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Sattarzadeh A (2013) Trafficking of proteins through plastid stromules. Plant Cell 25: 2774–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, Theg SM (2016) The formation of stromules in vitro from chloroplasts isolated from Nicotiana benthamiana. PLoS One 11: e0146489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A, Kwok EY, Hanson MR (2008) Effects of arc3, arc5 and arc6 mutations on plastid morphology and stromule formation in green and nongreen tissues of Arabidopsis thaliana. Photochem Photobiol 84: 1324–1335 [DOI] [PubMed] [Google Scholar]
- Huang J, Taylor JP, Chen JG, Uhrig JF, Schnell DJ, Nakagawa T, Korth KL, Jones AM (2006) The plastid protein THYLAKOID FORMATION1 and the plasma membrane G-protein GPA1 interact in a novel sugar-signaling mechanism in Arabidopsis. Plant Cell 18: 1226–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isemer R, Mulisch M, Schäfer A, Kirchner S, Koop HU, Krupinska K (2012) Recombinant Whirly1 translocates from transplastomic chloroplasts to the nucleus. FEBS Lett 586: 85–88 [DOI] [PubMed] [Google Scholar]
- Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, Ohsumi Y, Hanson MR, Mae T (2008) Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol 148: 142–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, Wada S, Makino A, Ishida H (2010) The autophagic degradation of chloroplasts via rubisco-containing bodies is specifically linked to leaf carbon status but not nitrogen status in Arabidopsis. Plant Physiol 154: 1196–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, Hidema J, Wada S, Kondo E, Kurusu T, Kuchitsu K, Makino A, Ishida H (2015) Establishment of monitoring methods for autophagy in rice reveals autophagic recycling of chloroplasts and root plastids during energy limitation. Plant Physiol 167: 1307–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR (1997) Exchange of protein molecules through connections between higher plant plastids. Science 276: 2039–2042 [DOI] [PubMed] [Google Scholar]
- Köhler RH, Hanson MR (2000) Plastid tubules of higher plants are tissue-specific and developmentally regulated. J Cell Sci 113: 81–89 [DOI] [PubMed] [Google Scholar]
- Kong SG, Arai Y, Suetsugu N, Yanagida T, Wada M (2013) Rapid severing and motility of chloroplast-actin filaments are required for the chloroplast avoidance response in Arabidopsis. Plant Cell 25: 572–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg TB. (2017) Distributing signaling proteins in space and time: the province of cytonemes. Curr Opin Genet Dev 45: 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz B, Jeske H, Kleinow T (2012) The induction of stromule formation by a plant DNA-virus in epidermal leaf tissues suggests a novel intra- and intercellular macromolecular trafficking route. Front Plant Sci 3: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz B, Wege C, Jeske H (2010a) Cell-free construction of disarmed Abutilon mosaic virus-based gene silencing vectors. J Virol Methods 169: 129–137 [DOI] [PubMed] [Google Scholar]
- Krenz B, Windeisen V, Wege C, Jeske H, Kleinow T (2010b) A plastid-targeted heat shock cognate 70kDa protein interacts with the Abutilon mosaic virus movement protein. Virology 401: 6–17 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2003) Microfilaments and microtubules control the morphology and movement of non-green plastids and stromules in Nicotiana tabacum. Plant J 35: 16–26 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2004a) Stromules and the dynamic nature of plastid morphology. J Microsc 214: 124–137 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2004b) GFP-labelled Rubisco and aspartate aminotransferase are present in plastid stromules and traffic between plastids. J Exp Bot 55: 595–604 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2004c) In vivo analysis of interactions between GFP-labeled microfilaments and plastid stromules. BMC Plant Biol 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR (2004d) Plastids and stromules interact with the nucleus and cell membrane in vascular plants. Plant Cell Rep 23: 188–195 [DOI] [PubMed] [Google Scholar]
- Machettira AB, Groß LE, Tillmann B, Weis BL, Englich G, Sommer MS, Königer M, Schleiff E (2012) Protein-induced modulation of chloroplast membrane morphology. Front Plant Sci 2: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Radhamony R, Sinclair AM, Donoso A, Dunn N, Roach E, Radford D, Mohaghegh PS, Logan DC, Kokolic K, et al. (2010) mEosFP-based green-to-red photoconvertible subcellular probes for plants. Plant Physiol 154: 1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medgyesy P, Fejes E, Maliga P (1985) Interspecific chloroplast recombination in a Nicotiana somatic hybrid. Proc Natl Acad Sci USA 82: 6960–6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrshahi P, Johnny C, DellaPenna D (2014) Redefining the metabolic continuity of chloroplasts and ER. Trends Plant Sci 19: 501–507 [DOI] [PubMed] [Google Scholar]
- Mehrshahi P, Stefano G, Andaloro JM, Brandizzi F, Froehlich JE, DellaPenna D (2013) Transorganellar complementation redefines the biochemical continuity of endoplasmic reticulum and chloroplasts. Proc Natl Acad Sci USA 110: 12126–12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan SK, Sullivan JA, Gray JC (2005) Stromules: A characteristic cell-specific feature of plastid morphology. J Exp Bot 56: 787–797 [DOI] [PubMed] [Google Scholar]
- Newell CA, Natesan SK, Sullivan JA, Jouhet J, Kavanagh TA, Gray JC (2012) Exclusion of plastid nucleoids and ribosomes from stromules in tobacco and Arabidopsis. Plant J 69: 399–410 [DOI] [PubMed] [Google Scholar]
- Onfelt B, Nedvetzki S, Yanagi K, Davis DM (2004) Cutting edge: Membrane nanotubes connect immune cells. J Immunol 173: 1511–1513 [DOI] [PubMed] [Google Scholar]
- Onfelt B, Purbhoo MA, Nedvetzki S, Sowinski S, Davis DM (2005) Long-distance calls between cells connected by tunneling nanotubules. Sci STKE 2005: pe55. [DOI] [PubMed] [Google Scholar]
- Pérez-Bermúdez P, Blesa J, Soriano JM, Marcilla A (2017) Extracellular vesicles in food: Experimental evidence of their secretion in grape fruits. Eur J Pharm Sci 98: 40–50 [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Schmid SL (2008) Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135: 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed ML, Wilson SK, Sutton CA, Hanson MR (2001) High-level expression of a synthetic red-shifted GFP coding region incorporated into transgenic chloroplasts. Plant J 27: 257–265 [DOI] [PubMed] [Google Scholar]
- Rothenberg M, Hanson MR (1988) A functional mitochondrial ATP synthase proteolipid gene produced by recombination of parental genes in a petunia somatic hybrid. Genetics 118: 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H (2013) The biology of strigolactones. Trends Plant Sci 18: 72–83 [DOI] [PubMed] [Google Scholar]
- Schattat M, Barton K, Mathur J (2011) Correlated behavior implicates stromules in increasing the interactive surface between plastids and ER tubules. Plant Signal Behav 6: 715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Barton KA, Mathur J (2015) The myth of interconnected plastids and related phenomena. Protoplasma 252: 359–371 [DOI] [PubMed] [Google Scholar]
- Schattat MH, Griffiths S, Mathur N, Barton K, Wozny MR, Dunn N, Greenwood JS, Mathur J (2012a) Differential coloring reveals that plastids do not form networks for exchanging macromolecules. Plant Cell 24: 1465–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Klösgen RB (2011) Induction of stromule formation by extracellular sucrose and glucose in epidermal leaf tissue of Arabidopsis thaliana. BMC Plant Biol 11: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat MH, Klösgen RB, Mathur J (2012b) New insights on stromules: stroma filled tubules extended by independent plastids. Plant Signal Behav 7: 1132–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak JC, Hayden CC, Sasaki DY (2010) Steric confinement of proteins on lipid membranes can drive curvature and tubulation. Proc Natl Acad Sci USA 107: 7781–7786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismans G, van der Meer T, Langevoort O, Schreuder M, Bouwmeester H, Peisker H, Dörman P, Ketelaar T, van der Krol A (2016) Low-phosphate induction of plastidal stromules is dependent on strigolactones but not on the canonical strigolactone signaling component MAX2. Plant Physiol 172: 2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SG, Hongladarom T, Honda SI (1962) Chloroplasts and mitochondria in living plant cells: cinephotomicrographic studies. Science 138: 434–436 [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]