Abstract
Interactions between receptor like kinases and guanyl nucleotide exchange factors together with identification of effector proteins reveal putative ROP GTPases signaling cascades.
Rho of Plants (ROP) proteins, also known as RACs, are the plant-specific subfamily of Rho small GTP-binding proteins, referred to here as small G proteins (Zheng and Yang, 2000a; Brembu et al., 2006; Eliáš and Klimeš, 2012). Like other members of the Ras superfamily of small G proteins, ROPs function as molecular switches due to changes in conformation upon GTP binding and hydrolysis (Berken and Wittinghofer, 2008). The conformational differences between the GTP- and GDP-bound states facilitate transient interactions with effector and regulatory proteins that, in turn, result in periodic activation/inactivation cycles of signaling cascades. Small G protein function is characterized by two central features: (1) due to inefficient GTP hydrolysis, these proteins remain in the GTP-bound active form for extended periods of time; and (2) due to the low dissociation coefficient of GDP, its release is inefficient and depends on enzymatic activity (Bourne et al., 1991; Vetter and Wittinghofer, 2001). Because of these features, the GTP-/GDP-dependent activation/inactivation cycles of small G proteins are regulated in time and space by GDP/GTP Exchange Factors (GEFs) that facilitate the release of GDP and GTPase-Activating Proteins (GAPs) that enhance GTP hydrolysis (Berken and Wittinghofer, 2008).
ROPs relay signaling from distinct plasma membrane domains; ROPs bind through posttranslational lipid modifications and interaction with membrane lipids (Bloch and Yalovsky, 2013). Thus, ROPs function as molecular switches that transduce intracellular and extracellular stimuli in a spatially regulated fashion, resulting in localized regulation of intracellular responses.
A third group of regulatory proteins, Rho GDP Dissociation Inhibitors (RhoGDIs), interact with high and low affinity with GDP- and GTP-bound Rho proteins, respectively, remove them from the membrane, and stabilize them in the cytoplasm until their reactivation by GEFs (Boulter and Garcia-Mata, 2010). The recycling induced by RhoGDIs is thought to maintain Rho proteins in distinct plasma membrane domains and to play an essential role in the establishment and maintenance of cell polarity (Johnson et al., 2011). As will be discussed below, in plants, RhoGDIs are critical for cell polarity maintenance.
Like Rho family proteins from fungi and mammalian cells, ROPs regulate the organization and dynamics of the actin and microtubule (MT) cytoskeleton (Box 1; Bashline et al., 2014; Hamada, 2014; Hashimoto, 2015; Henty-Ridilla et al., 2013; Pick, 2014), endocytosis and exocytosis, and the activation of NADPH oxidase and intracellular kinase cascades. Through effects on actin, MTs, vesicle trafficking, reactive oxygen species (ROS) production, and protein phosphorylation, ROPs regulate cell growth and shape, cytokinesis, subcellular protein localization, and responses to pathogens (Gu et al., 2004; Basu et al., 2008; Yalovsky et al., 2008; Yang, 2008; Nagawa et al., 2010; Bloch and Yalovsky, 2013; Kawano et al., 2014; Oda and Fukuda, 2014; Rivero et al., 2017). ROPs also are implicated in the regulation of abscisic acid (ABA) and auxin signaling and transport (Wu et al., 2011; Liao et al., 2017) and in the regulation of mRNA transcription (Zhang et al., 2016) and protein translation (Schepetilnikov et al., 2017) (Fig. 1; Table I).
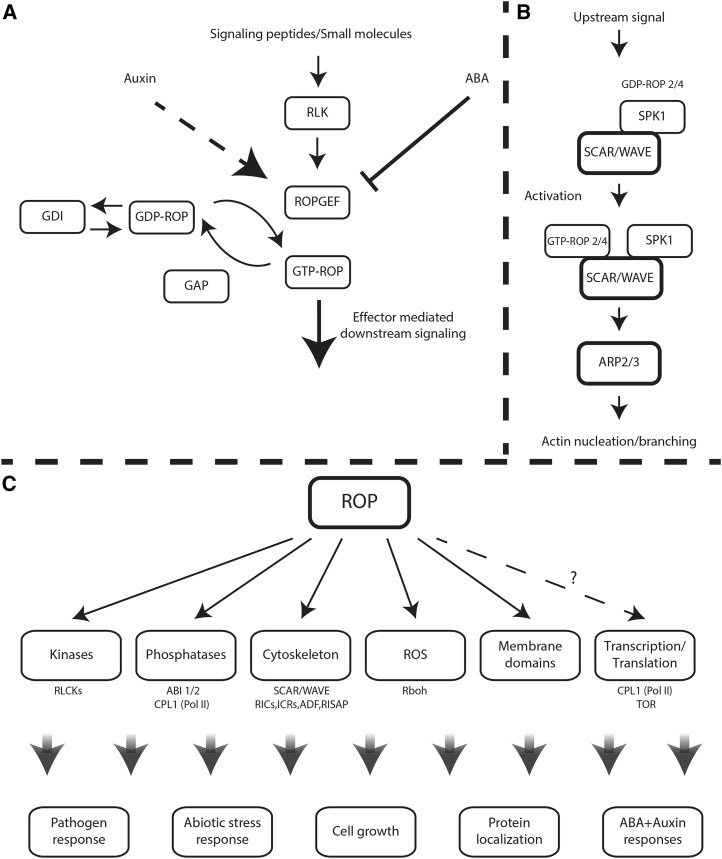
Figure 1.
ROP signaling pathways. A, ROP activation/deactivation cycles. ROPs cycle between a GTP-bound active state and a GDP-bound inactive state. Activation is regulated by ROP-specific GEFs, inactivation is enhanced by ROP-specific GAPs, and recycling of type I ROPs between the cytosol and the plasma membrane is facilitated by RhoGDIs. Some GEFs are activated by peptide-activated, plasma membrane-associated RLKs. ROPs also are up-regulated by auxin by an as yet unknown pathway (dashed arrow) and inactivated by ABA, which enhances the degradation of ROPGEFs. B, The Dock family GEF homolog SPK1 is associated with the WAVE complex. Upon activation, ROP-GTP interacts with the WAVE SRA1 subunit and possibly other subunits, resulting in WAVE activation. Activated WAVE activates actin nucleation/branching induced by Arp2/3. C, ROP downstream signaling. ROP regulated pathways and known effectors. The dashed arrow indicates uncertainty regarding the cellular targets of ROP. CPL1 is regulated by ROP signaling but does not interact physically with ROPs. TOR interacts weakly with GTP-bound ROP2 and more strongly with the GDP-bound form.
Table I. Regulators and effectors of ROP signaling.
At, Arabidopsis thaliana; Hv, Hordeum vulgare (barley); Lj, Lotus japonicus; Mt, Medicago trancatula; Nt, Nicotiana tabacum (tobacco); Os, Oryza sativa (rice); Sl, Solanum lycopersicum (tomato); Zm, Zea mays (maize).
| Name | Process | Cellular Function |
Species | Reference |
|---|---|---|---|---|
| ROPs | ||||
| AtROP1 | Pollen tube growth, root growth, response to endophytic fungus | At | Fu et al. (2001); Gu et al. (2005); Lee et al. (2008); Venus and Oelmüller (2013) | |
| AtROP2 | Pavement cell growth, root hair initiation and growth, stomata opening, phytochrome-mediated light responses, ABA responses, transcriptional and translational regulation | At | Li et al. (2001); Fu et al. (2002, 2005); Jones et al. (2002); Fischer et al. (2006); Jeon et al. (2008); Ikeda et al. (2009); Hwang et al. (2011); Stanislas et al. (2015); Zhao et al. (2015); Hong et al. (2016); Zhang et al. (2016); Schepetilnikov et al. (2017); Wang et al. (2017) | |
| AtROP3 | Pollen growth, embryo and root development, auxin distribution | At | Expression data; Huang et al. (2014) | |
| AtROP4 | Pavement cell growth, root hair initiation and growth, pathogen response | At | Molendijk et al. (2001, 2008); Fu et al. (2005) | |
| AtROP5 | Pollen tube growth | At | Feng et al. (2016) | |
| AtROP6 | Pavement cell growth, root hair growth, ROS production, inhibition of endocytosis, root and lateral root development, salicylic acid-regulated pathogen response | At | Molendijk et al. (2001); Fu et al. (2005, 2009); Sorek et al. (2010); Poraty-Gavra et al. (2013); Venus and Oelmüller (2013); Wang et al. (2015) | |
| AtROP7 | Vascular development | At | Brembu et al. (2005); Wang et al. (2017) | |
| AtROP8 | Pollen nuclei migration in the central cell | At | Kawashima et al. (2014) | |
| AtROP9 | ABA and auxin responses, migration of pollen nuclei in pollen tubes | At | Li et al. (2013); Nibau et al. (2013); Choi et al. (2014) | |
| AtROP10 | ABA responses | At | Zheng et al. (2002); Xin et al. (2005); Choi et al. (2014) | |
| AtROP11 | ABA signaling, secondary cell wall patterning, root hair growth, inhibition of endocytosis | At | Bloch et al. (2005, 2011a, 2011b); Li et al. (2012b); Li and Liu (2012); Oda and Fukuda (2012); Yu et al. (2012) | |
| ZmROP2 | Regulation of asymmetric cell division during stomata development | Zm | Humphries et al. (2011) | |
| ZmROP9 | Regulation of asymmetric cell division during stomata development | Zm | Humphries et al. (2011) | |
| OsRAC1 | Pathogen response, lignin biosynthesis, activation of RBOH | Os | Kawasaki et al. (2006); Wong et al. (2007); Akamatsu et al. (2013); Nagano et al. (2016) | |
| NtRAC5 | Pollen tube growth | Nt | Kost et al. (1999); Klahre et al. (2006); Klahre and Kost (2006); Sun et al. (2015) | |
| HvRACB | Susceptibility to powdery mildew, regulation of cell growth | Hv | Schultheiss et al. (2002, 2005); Pathuri et al. (2008) | |
| HVRAC1 | Susceptibility to powdery mildew, regulation of cell growth, ROS production | Hv | Pathuri et al. (2008) | |
| HvRAC2 | Susceptibility to powdery mildew, regulation of cell growth | Hv | Pathuri et al. (2008) | |
| MtROP10 | Regulation of symbiotic interaction with rhizobium | Mt | Lei et al. (2015) | |
| LjROP6 | Regulation of symbiotic interaction with rhizobium | Lj | Ke et al. (2012) | |
| Cytoskeleton-associated ROP effectors | ||||
| RIC1 | Pollen tube growth, pavement cell growth, cell wall sensing | Actin severing and capping, cortical MT severing by KTN1 | At | Fu et al. (2005, 2009); Lin et al. (2013a); Zhou et al. (2015); Higaki et al. (2017) |
| RIC3 | Pollen tube growth | Actin disassembly | At | Gu et al. (2005); Lee et al. (2008) |
| RIC4 | Pollen tube growth, pavement cell growth, root growth, response to endophytic fungus | Actin assembly and bundling | At | Fu et al. (2005, 2009); Gu et al. (2005); Lee et al. (2008); Venus and Oelmüller (2013) |
| RISAP | Pollen tube growth | Actin-dependent vesicle transport | Nt | Stephan et al. (2014) |
| NtADF1 | Pollen tube growth | Actin depolymerization | Nt | Chen et al. (2002, 2003) |
| SCAR1-4 | Pavement cell growth, trichome growth, stomata development and asymmetric division | Actin branching | At, Zm | Uhrig et al. (2007); Basu et al. (2008); Facette et al. (2015) |
| SPK1 | Pavement cell and trichome growth | ROPGEF | At | Basu et al. (2008) |
| SRA1 | Pavement cell and trichome growth | Actin branching | At | Uhrig et al. (2007); Basu et al. (2008) |
| ICR1 | Vesicle trafficking, cell polarity | MT organization (putative) | At | Lavy et al. (2007); Hazak et al., (2010, 2014) |
| ICR2 | Unknown | MT organization (putative) | At | Lavy et al. (2007) |
| MIDD1 | Secondary cell wall deposition in metaxylem cells | Localized MT depolymerization through AtKinesin-13A | At | Mucha et al. (2010); Oda et al. (2010); Oda and Fukuda (2012) |
| HvRBK1 | Basal resistance to pathogenic fungi | MT stability | Hv | Huesmann et al. (2012) |
| Non-cytoskeleton-associated ROP effectors | ||||
| RIC7 | Stomata movement | Vesicle trafficking | At | Hong et al. (2016) |
| HvRIC171 | Pathogen response | Fungal penetration susceptibility | Hv | Schultheiss et al. (2008) |
| RBK1 | Pathogen response | Receptor-like cytoplasmic kinase | At | Molendijk et al. (2008) |
| RBK2 | Pathogen response | Receptor-like cytoplasmic kinase | At | Molendijk et al. (2008) |
| NCRK | Pathogen response | Receptor-like cytoplasmic kinase | At | Molendijk et al. (2008) |
| AtRLCK VI_A3 | Pathogen response | Receptor-like cytoplasmic kinase | At | Reiner et al. (2015) |
| ABI1 | ABA signaling | PP2C phosphatases | At | Li et al. (2012a) |
| ABI2 | ABA signaling | PP2C phosphatases | At | Yu et al. (2012) |
| CPL1 | Transcriptional regulation | Pol II CTD phosphatase | At | Zhang et al. (2016) |
| TOR | Auxin response | Translation control | At | Schepetilnikov et al. (2017) |
| RBOHB/D | Pathogen response | NADPH oxidase | Os | Wong et al. (2007); Kosami et al. (2014); Nagano et al. (2016) |
| ROPGAPs | ||||
| MAGAP | Response to pathogenic fungi | Polar organization of cortical MT | Hv | Hoefle et al. (2011) |
| PHGAP1 | Cell division plane selection | PH domain ROPGAP: positioning of the cortical division zone regulator kinesin-12 POK1 | At | Stöckle et al. (2016) |
| PHGAP2 | Cell division plane selection | PH domain ROPGAP: positioning of the cortical division zone regulator kinesin-12 POK1 | At | Stöckle et al. (2016) |
| ROPGAP3 | Secondary wall pits in metaxylem | Local MT destabilization | At | Oda and Fukuda (2012) |
| RhoGAP1 | Pollen tube polar growth | Regulation of ROP activity | Nt | Klahre and Kost (2006); Sun et al. (2015) |
| REN1 | Pollen tube polar growth | Regulation of ROP activity | At | Hwang et al. (2008) |
| ROPGAP1 | Pollen tube polar growth | Actin organization | At | Fu et al. (2001); Hwang et al. (2010) |
| ROPGAP4 | Response to oxygen deprivation | Regulation of ROS levels | At | Baxter-Burrell et al. (2002) |
| ROPGEFs | ||||
| SPK1 | Pavement cells and trichome growth | Dock-like ROPGEF | At | Basu et al. (2008) |
| ROPGEFs1/8/9/12/14 | Pollen tube growth | Mediate PRK6 signaling and AtPRK2 signaling | At | Zhang and McCormick (2007); Chang et al. (2013) |
| ROPGEF4 | Secondary wall pits in metaxylem, ABA responses, cell wall sensing | MT destabilization | At | Li and Liu (2012); Oda and Fukuda (2012); Huang et al. (2013) |
| ROPGEF7 | Regulation of embryo and root development | Regulation of PLETHORA2 expression | At | Chen et al. (2011) |
| ROPGEF1 | Root hair and embryo development, ABA responses | Mediate FER signaling | At | Duan et al. (2010); Li and Liu (2012); Liu et al. (2017) |
| ROPGEF10 | ABA responses | Mediate FER signaling | At | Huang et al. (2013) |
| OsSWAP70 | Responses to pathogens | Putative Dbl-like ROPGEF | Os | Yamaguchi et al. (2012) |
| RhoGDIs | ||||
| AtRhoGDI1/SCN1 | Regulation of ROP2 and RBOHC distribution at root hair initiation sites, pollen tube growth | At | Carol et al. (2005); Hwang et al. (2010); Feng et al. (2016) | |
| AtRhoGDI2a | Pollen tube growth | At | Hwang et al. (2010); Feng et al. (2016) | |
| AtRhoGDI2b | Pollen tube growth | At | Hwang et al. (2010); Feng et al. (2016) | |
| RLKs | ||||
| FERONIA | Root hair growth, pollen tube rupture, defense responses | Cell wall sensor and a signaling scaffold, signals through ROPGEFs | At | Duan et al. (2010, 2014); Yu et al. (2012); Huang et al. (2013); Stegmann et al. (2017) |
| THESEUS | Defense responses | Cell wall sensor signaling through ROPGEFs | At | Höfte (2015); Qu et al. (2017a) |
| PRK6 | Pollen tube growth | LURE receptor signaling through ROPGEFs | At | Takeuchi and Higashiyama (2016) |
| AtPRK2 | Pollen tube growth | Plasma membrane LRK that signals through ROPGEFs | At | Chang et al. (2013) |
| SlPRK1 | Pollen tube germination and growth | Plasma membrane LRK signaling through ROGEFS | Sl | Kaothien et al. (2005) |
| SlPRK2 | Pollen tube germination and growth | Plasma membrane LRK signaling through ROGEFS | Sl | Kaothien et al. (2005) |
| OsCEBiP/OsCERK1 | Pathogen response | Plasma membrane LRK that senses chitin and signals through ROPGEFs | Os | Akamatsu et al. (2013) |
| MtNFP | Symbiotic interaction with rhizobium | Interacts with GTP-MtROP10 | Mt | Lei et al. (2015) |
| LjNFR5 | Symbiotic interaction with rhizobium | Interacts with GTP-LjROP6 | Lj |
Ke et al. (2012) |
In recent years, there have been a plethora of new studies on ROPs. Studies on pollen tube and root hair development and plant immune responses have indicated that ROPGEFs are activated by plasma membrane-bound Receptor-Like Kinases (RLKs). The next steps will be elucidation of ROP signaling pathways from identification of the upstream receptors to mechanisms of ROPGEF and ROP activation to identification of downstream effectors and their functions (Fig. 1). In this Update, we aim to summarize our current understanding of ROP structure/function and signaling with an emphasis on new findings that have not been subject to previous reviews. Because of space limitations and focus, we are unable to cover all published literature in the field, including the proposed functions of ROPs in regulating transcription and translation. Thus, we apologize to colleagues whose works are not cited.
ROP EVOLUTION, STRUCTURE, AND FUNCTION
Evolution
ROPs have been identified in red and green algae and are ubiquitous in embryophytes, where they form multimember protein families (Brembu et al., 2006; Eliáš and Klimeš, 2012). The number of ROP proteins varies: there are two in the lycophyte Selaginella moellendorffii, four in the moss Physcomitrella patens, four in the gymnosperm Pinus taeda (loblolly pine), seven in rice (Oryza sativa), nine in maize (Zea mays), nine in tomato (Solanum lycopersicum), 11 in Arabidopsis (Arabidopsis thaliana), and 13 in poplar (Populus spp.; Fowler, 2010). Phylogenetic analyses indicate that the expansion of the ROP family resulted from multiple gene duplication events (Winge et al., 2000; Fowler, 2010). Interestingly, ROPs and ROPGEFs or other Rho GTPases have not been identified in some green algae, including Chlamydomonas reinhardtii and Volvox carteri, indicating a loss of the ROP/Rho signaling module in these organisms. Phylogenetic analyses indicated that ROPs emerged prior to the diversification of the RAC and Cdc42 subfamilies and represent a unique subfamily of Rho GTPases (Zheng and Yang, 2000a; Vernoud et al., 2003; Brembu et al., 2006; Boureux et al., 2007; Rojas et al., 2012).
Analysis of Rho GTPases from divergent eukaryotes revealed that early phylogenetic analyses of the Rho GTPases were biased by a focus on the fungal and animal members of the family. For example, Cdc42, which plays a central role in the regulation of cell polarity (Johnson and Pringle, 1990; Woods and Lew, 2017), emerged with the opisthokonts (fungi and metazoa) but is absent in other eukaryotes. The amoeba Dictyostelium discoideum has proteins that have been historically called Racs but are as divergent from each other as are Rac, Cdc42, and Rho (Eliáš and Klimeš, 2012). The analysis by those authors further confirmed that ROPs are unique to the plant kingdom (Eliáš and Klimeš, 2012). Collectively, the phylogenetic analyses carried out by several groups justify the name Rho of Plants (Zheng and Yang, 2000a). As will be discussed in this Update, ROPs combine functions of Rac, Cdc42, and possibly other Rho proteins, as could be expected from their early divergence during evolution. Plants also possess a unique family of ROPGEFs (Berken et al., 2005; Gu et al., 2006; Elias, 2008) and forms of the Cdc42 Rac Interacting Binding (CRIB) domain containing ROPGAPs (Wu et al., 2000). Furthermore, most of the ROP effectors identified to date are unique to plants. Hence, it appears that many components of the ROP signaling module are unique to plants.
Structure/Function
ROPs have molecular masses of 21 to 24 kD. Like other members of the Ras superfamily, ROPs are composed of an N-terminal catalytic G-domain where nucleotide and effector binding take place and a C-terminal hypervariable domain (HVR), which is responsible for subcellular targeting (Fig. 2A). The G-domain contains five conserved sequence motifs known as the G-box motifs (G1–G5), where nucleotide binding, GTP hydrolysis, and Mg2+ binding take place. The G2 and G3 motifs also are known as the switch I and switch II loops; these regions have different conformations in GDP- and GTP-bound states and are essential for the transient interaction with effector and regulatory proteins. Within the G domain, ROPs also contain a helical domain unique to the Rho family known as the insert region or αi. The structural conservation of the G-domain has been confirmed by three-dimensional crystal structures of the GDP-bound Arabidopsis ROP9 (Sørmo et al., 2006), ROP4 in complex with the catalytic PRONE (for Plant-Specific ROP Nucleotide Exchanger) domain of ROPGEF3 (Thomas et al., 2007), ROP5 (Thomas and Berken, 2010; Protein Data Bank code 3BWD), the nucleotide-free ROP7 in complex with the PRONE domain of ROPGEF8 (Thomas et al., 2009), and GTP-bound rice RAC1 (Kosami et al., 2014; Fig. 2, B–D).
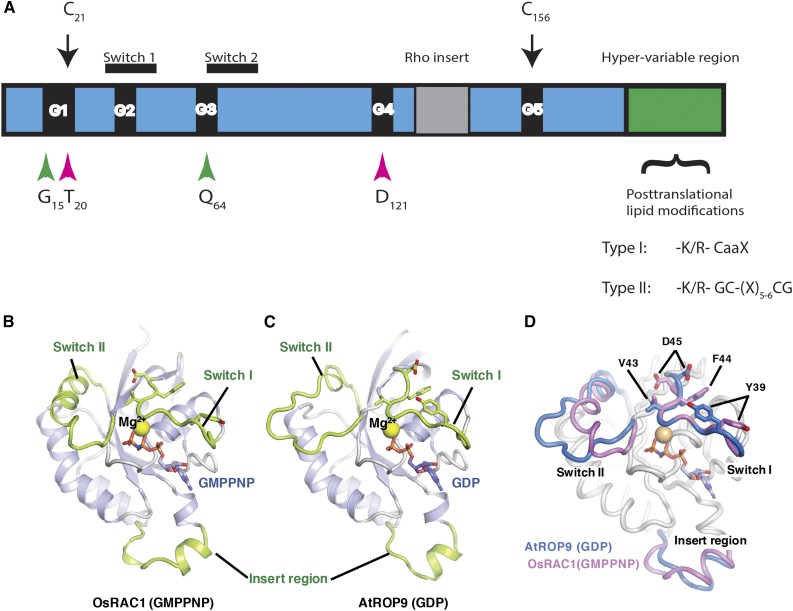
Figure 2.
Structures of ROPs. A, Schematic model highlighting the conserved G-box motifs (G1–G5), switch I and II domains, the insert region, and the HVR. The positions of the conserved residues mutated in the constitutively active and dominant-negative mutations are highlighted by green and magenta arrowheads, respectively. C21 and C156 are conserved G domain Cys residues that undergo activation-dependent transient S-acylation (black arrows). The HVR terminates with a canonical CaaX box geranylgeranylation motif and a PBR in type I ROPs or with a GC-CG box and a proximal PBR in type II ROPs. B, Three-dimensional structure of GTP-bound OsRAC1 [OsRAC1 (GMPPNP)]. C, Three-dimensional structure of the GDP-bound AtROP9 [AtROP9 (GDP)]. In B and C, the switch I, switch II, and insert regions are colored green. GMPPNP and GDP are shown as stick models (red, oxygen; blue, nitrogen; orange, phosphorus). The Mg2+ ions are shown as yellow spheres. D, Superimposed structures of OsRAC1 (GMPPNP) and AtROP9 (GDP). The main chains of OsRAC1 (GMPPNP) and AtROP9 (GDP) (chain B; Protein Data Bank code 2J0V) were superimposed using PyMOL. Regions of the OsRAC1 and AtROP9 proteins are colored blue and pink, respectively. The side chains of four key switch I residues in OsRAC1 (Val-43, Phe-44, Asp-45, and Tyr-39) and equivalent residues in AtROP9 (Val-39, Phe-40, Asp-41, and Tyr-35) are shown in stick representation. B to D were prepared and contributed by Izuru Ohki and Chojiro Kojima and are based on their article describing the structure of GTP-bound OsRAC1 (Kosami et al., 2014).
Based on structural conservation and known mutations in Ras (Feig, 1999), it was predicted and subsequently shown that point mutations in a G1 Gly (G15 in Arabidopsis ROP1–ROP7 and ROP9; G17 in ROP10 and ROP11; and G27 in ROP8) or G3 Gln (Q64 in Arabidopsis ROP1–ROP7 and ROP9; Q66 in ROP10 and ROP11; and Q76 in ROP8) would prevent GTP hydrolysis, resulting in GTP-locked, constitutively active (CA) mutants. Conversely, mutations in a G1 Thr (T20 in Arabidopsis ROP1–ROP6, ROP7, and ROP9; T22 in ROP10 and ROP11; and T32 in ROP8) or a G4 Asp (D121 in Arabidopsis ROP1–ROP6, ROP7, and ROP9; D123 in ROP10 and ROP11; and D133 in ROP8) are dominant negative (DN), reducing affinity for nucleotides and stabilizing the interaction with GEFs, thereby preventing ROP activation (Berken and Wittinghofer, 2008). In addition to the structural conservation of ROPs, the functional conservation of the constitutively active and dominant-negative mutations was confirmed by biochemical assays (Berken et al., 2005; Sorek et al., 2010; Kosami et al., 2014). The constitutively active and dominant-negative ROP mutants have been used extensively to study ROP function.
A comparison between the structure of the nonhydrolyzable GTP analog guanosine 5′-[β,γ-imido]triphosphate [Gpp(NH)p]-bound rice OsRAC1 and the GDP-bound AtROP9 revealed the conformational differences between the GDP- and GTP-bound states of ROPs (Fig. 2, B–D; Kosami et al., 2014). The structural analysis showed conformational differences in the switch I and switch II domains and the insert region (Fig. 2, B–D). However, the conformational changes in the switch I domain are the most critical for function. It was shown that GTP binding by OsRAC1 results in surface exposure of switch I Glu (D45) and a Tyr (Y39); both are required for its interaction with rice Respiratory Burst Oxidase Homolog (OsRBOHB) NADPH oxidase (Kosami et al., 2014). D45 and Y39 are highly conserved, and it will be interesting to examine whether they play central roles in the interaction of ROPs with other effectors. The structure of ROPs solved so far reveal differences in the structures of the switch II and insert regions (Berken and Wittinghofer, 2008; Thomas and Berken, 2010; Kosami et al., 2014). It is not known yet whether these differences also imply different substrate and regulatory specificities. Collectively, the sequence and structural and functional conservation of ROPs indicate that, regardless of their early divergence, they function in a similar fashion to the well-characterized Rho GTPases from fungi and metazoa.
The C-terminal HVRs of ROPs are composed of Cys-containing sequence motifs, which undergo posttranslational lipid modifications, and proximal Arg-Lys-rich polybasic regions (PBRs). Type I ROPs (in Arabidopsis ROP1–ROP8) terminate with a canonical CaaL box motif (where C is Cys, a1 and a2 are aliphatic residues, and L is Leu) in which the Cys residue is modified by the C20 isoprenyl lipid geranylgeranyl. Type II ROPs terminate with GC-CG boxes (where G is Gly and C is Cys) in which the two Cys residues are separated by five or six aliphatic residues and undergo S-acylation by the C16 palmitate or C18 stearate fatty acids. Type II ROPs are likely not regulated by RhoGDIs, since geranylgeranylation is required for the interaction between Rho protein and RhoGDI. The PBRs in animal Rhos, RACs, and Cdc42 enhance membrane attachment by interaction with phosphatidylphosphoinositide (4,5) diphosphate (PIP2) and phosphatidylphosphoinositide (3,4,5) triphosphate (PIP3; Heo et al., 2006). The PBR is required for the plasma membrane attachment of type II ROPs (Lavy and Yalovsky, 2006), possibly by facilitating the interaction with PIPs. In addition, ROPs undergo activation-dependent transient S-acylation on conserved G-domain Cys residues (C21 and C156 in AtROP6). The transient S-acylation stabilizes the interaction of ROPs with the plasma membrane, leads to their accumulation in lipid rafts, and is required for their function (Sorek et al., 2010, 2011, 2017).
ROP REGULATORS: GEFS, GAPS, AND RHOGDI
GEFs
Plants have two major types of ROP GEFs: (1) a plant-unique family known as ROPGEFs that contain the PRONE catalytic domain (Berken et al., 2005; Gu et al., 2006); and (2) distantly related homologs of the animal Ced5, Dock180, Myoblast city (CDM) Zizimin Homology (CZH) RhoGEFs. The CZH RhoGEFs share three tandem Doc Homology Regions (DHRs), of which DHR2 has GEF activity. A single CZH RhoGEF homolog called SPIKE1 (SPK1) was identified in Arabidopsis (Qiu et al., 2002), and it was shown that either the full-length protein or its DHR2 domain can catalyze ROP nucleotide exchange (Basu et al., 2008). The rice OsSWAP70A and OsSWAP70B bear homology to Diffuse B cell lymphoma (Dbl)-type RhoGEFs that are prevalent in yeast and mammalian cells. OsSWAP70A and OsSWAP70B have GEF activity and were suggested to function as GEFs for ROPs (Yamaguchi et al., 2012).
ROPGEFs
ROPGEFs catalyze nucleotide exchange on ROPs but not on nonplant Rho GTPases (Berken et al., 2005). The specificity for ROPs is likely associated with the presence of a small Gly residue in the insert regions of ROPs at a position that, in nonplant ROPs, is occupied by a large Arg residue (Thomas et al., 2007; Berken and Wittinghofer, 2008). Structural studies revealed that ROPGEFs function as dimers and interact with two ROP molecules. Interestingly, it was found that the structure of ROPGEFs is different from that of the Dbl RhoGEFs, but the mechanism of guanyl nucleotide exchange is similar (Thomas et al., 2007). Furthermore, based on the cocrystal structure of ROP4 and PRONE8, it was predicted that the interaction between ROPs and ROPGEFs could be regulated by phosphorylation (Thomas et al., 2007; Berken and Wittinghofer, 2008; Thomas and Berken, 2010). The catalytic PRONE domain of ROPGEFs is constitutively active, and its function is regulated by the ROPGEF C-terminal domain (Berken et al., 2005; Gu et al., 2006). Studies in pollen tubes indicated that ROPGEFs interact with membrane-associated RLKs, which phosphorylate the C-terminal domain and relieve the autoinhibition of the PRONE domain, leading to ROPGEF activation (Kaothien et al., 2005; Zhang and McCormick, 2007; Chang et al., 2013). Similarly, studies in root hairs have demonstrated that ROPGEFs function downstream of the RLK FERONIA (FER; Duan et al., 2010; Yu et al., 2012; Huang et al., 2013). Thus, the activation of at least some ROPGEFs depends on intercellular signaling that involves phosphorylation by membrane-bound RLKs (Fig. 3).
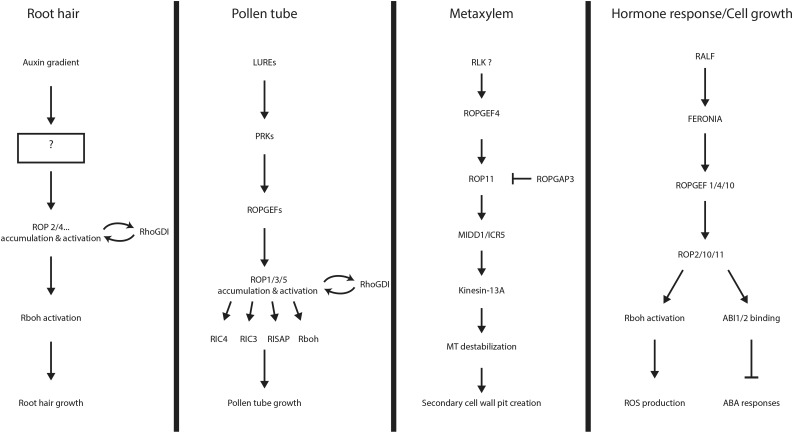
Figure 3.
ROP signaling pathways. A schematic overview of identified ROP signaling pathways is shown. Components of the signaling pathways mentioned in the text are noted, and their relationships to each other are indicated by arrows. The question mark notes an area of insufficient data. From top to bottom in each section are listed the upstream signal, the receptors that relay the signal into the cell, the regulation of ROP activity, and the downstream effectors that modulate the cellular response.
ROPGEFs have only been identified in red algae (Rhodophyta) and green plants (Chloroplastida) and appear to be a family of proteins unique to the plant kingdom (Archeaplastida/Plantae) that are not of a cyanobacterial origin (Elias, 2008). They form protein families with numbers ranging from two in S. moellendorffii, to six in P. patens, to 14 in Arabidopsis (Berken et al., 2005; Gu et al., 2006; Eklund et al., 2010). Arabidopsis ROPGEF mutants often display mild phenotypes (Li and Liu, 2012; Chang et al., 2013; Huang et al., 2013; Zhao et al., 2015; Wang et al., 2017), indicating that they likely function redundantly. Two reports indicated that ROPGEF1 and ROPGEF7 have specific functions during embryo development. ROPGEF7 RNA interference (RNAi) plants displayed compromised stem cell maintenance in embryo and roots (Chen et al., 2011). The ropgef1 mutants are characterized by defective embryo development and embryo auxin distribution and a mild gravitropic response phenotype (Liu et al., 2017), suggesting that the ROPGEF1 function in embryos is unique. However, ROPGEF1 might function in conjunction with other ROPGEFs in root gravitropic responses.
ROPGEFs are soluble proteins that are, however, observed in the plasma membrane, suggesting that they interact with components at the plasma membranes that facilitate their attachment to the membrane. Deletion of the AtROPGEF12 C-terminal domain abolished the membrane association following transient expression in tobacco (Nicotiana tabacum) pollen tubes. Upon transient expression in tobacco pollen tubes, several ROPGEF-GFP fusion proteins were detected at the pollen tube tip (Gu et al., 2006). In embryos, the AtROPGEF1-GFP fusion protein, driven by the ROPGEF1 prompter, was localized in a polarized manner (Liu et al., 2017). Taken together, these data indicate that ROPGEFs likely activate ROPs in the plasma membrane and that their spatial distribution influences the site or sites of ROP activation.
SPK1
SPK1 was discovered in a forward genetic screen in Arabidopsis for mutants with altered trichome development. spk1 mutants are seedling lethal and display a pleotropic phenotype that includes stunted plant size and abnormal leaf pavement cells and trichome growth, cotyledon shape, and cytoskeleton organization (Qiu et al., 2002). In in vitro pull-down assays, SPK1 interacts weakly with GDP-bound and nucleotide-free type I and type II ROPs but not with human RAC1 (Basu et al., 2008), and similar results were obtained in yeast two-hybrid assays (Uhrig et al., 2007). Coimmunoprecipitation and protein interaction assays indicated that SPK1 is associated with the Suppressor of cAMP Receptor (SCAR)/Wiskot-Aldrich syndrome protein-family Verprolin homology protein (WAVE) complex, which activates actin nucleation/branching by the Actin-Related Protein2/3 (Arp2/3) complex (Uhrig et al., 2007; Basu et al., 2008). GTP-bound type I ROPs interact with the SCAR/WAVE complex subunit SRA1 (also known as PIROGI/KLUNKER; Basu et al., 2004, 2008) or with other subunits of the complex (Uhrig et al., 2007). Importantly, analysis of SPK1, SRA1, and Arp2/3 mutants demonstrated that they function in the same pathway (Basu et al., 2008). Taken together, the genetic and biochemical analyses demonstrate that SPK1, type I ROPs (possibly ROP2 and ROP4), SCAR/WAVE, and Arp2/3 complexes regulate actin nucleation (Figs. 4 and 5).
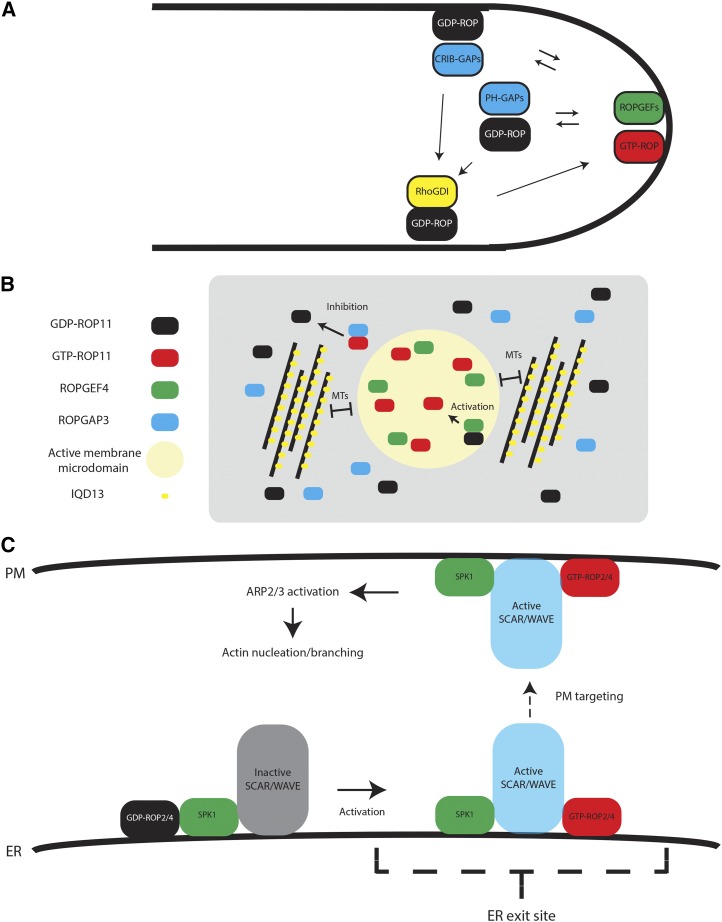
Figure 4.
Mechanisms of spatial ROP activity regulation. A, Local activation of ROPs in the pollen tube. ROPs are activated at the tip by the activation of ROPGEFs by associated RLKs. CRIB domain ROPGAPs inactivate ROPs at the shank to maintain polar growth. PH domain GAPs are localized in subapical cytoplasmic vesicles, restricting ROP active domains. RhoGDIs recycle ROPs from the membrane. B, Formation of active ROP domains in differentiating metaxylem. During metaxylem differentiation, ROP11 is coexpressed with ROPGEF4 and ROPGAP3, resulting in the formation of active ROP11 domains and global inactivation. The size of the ROP11 domains is restricted by MTs and the MT- and plasma membrane-associated protein IQD13. C, Activation of ROPs by SPK1 and the formation of active actin nucleation/branching domains. SPK1 is associated with the WAVE complex. Activation of ROPs by SPK1 causes their association with the SRA1 subunit of the WAVE complex and its activation. Active WAVE can then activate actin nucleation/branching by Arp2/3. ER, Endoplasmic reticulum; PM, plasma membrane.
Figure 5.
ROP regulation of cytoskeleton organization. A, Cortical MT reordering by ROP6, RIC1, and KTN1. RIC1 interacts physically with and promotes the MT severing by KTN1, leading to MT reordering. B, Localized cortical MT depolymerization. In developing metaxylem, MT depolarization takes place by the recruitment of MIDD1 and Kinesin13A to active ROP11 domains. C, Actin dynamics in tip-growing pollen tubes. ROP regulates actin assembly by two counteracting pathways. RIC4 promotes assembly, whereas RIC3 causes disassembly by stimulating Ca2+ influx into the cytoplasm. D, Actin nucleation/branching in trichomes and pavement cells. ROPs and the ROPGEF SPK1 interact with the SCAR/WAVE complex and promote actin filament nucleation/branching.
In developing trichomes, nucleation of actin at the tip depends on the WAVE-dependent function of Arp2/3 (Yanagisawa et al., 2015), which is likely locally regulated by SPK1. Thus, the combinatorial regulation of SPK1, ROPs, and WAVE subcellular distribution is very likely responsible for localized actin nucleation. It is not known how SPK1 function is regulated; like ROPGEFs, it may be activated by upstream signaling. It is also not known whether SPK1 regulates additional pathways. In P. patens, there are six SPK1 homologs, whereas in S. moellendorffii and Arabidopsis, SPK1 is encoded by a single gene (Eklund et al., 2010). Sequence analysis of SPK1 homologs from additional plant species will be required before it can be determined whether the reduction in SPK1 homologs followed an evolutionary trend. Genetic analysis in P. patens should reveal whether SPK1 function in the regulation of actin nucleation is evolutionarily conserved and whether it had additional functions that were lost during evolution.
OsSWAP70
The rice proteins OsSWAP70A and OsSWAP70B contain Pleckstrin Homology (PH) and Diffuse B cell Homology domains similar to many animal RhoGEFs and display similarity to human SWAP70 RhoGEF (Shinohara et al., 2002; Yamaguchi et al., 2012). OsSWAP70A displays GEF activity toward OsRAC1 and enhances OsRAC1-dependent ROS production. Surprisingly, however, OsSWAP70A interacts with both constitutively active and dominant-negative OsRAC1 and with Osrac7CA mutants but not with the dominant-negative form Osrac7DN. Furthermore, OsSWAP70B does not interact with any rice ROP (Yamaguchi et al., 2012). SWAP70 homologs have been identified in other plants (Yamaguchi et al., 2012). However, given that OsSWAP70A interacts with GTP-bound RAC1, which contradicts a long-held dogma regarding RhoGEF activity (Berken and Wittinghofer, 2008; Thomas and Berken, 2010), and in the absence of loss-of-function mutants to support the biochemical analysis, more work is required to determine whether the plant SWAP70 homologs are, indeed, ROPGEFs.
ROP GAPs
Fungal and metazoan RhoGAPs share a conserved GAP domain that contains an invariable Arg residue required for the catalytic activity. In addition to the GAP domain, RhoGAPs usually contain additional domains that are required for activity and specificity (Lamarche and Hall, 1994; Rittinger et al., 1997; Graham et al., 1999). In plants, there are two types of GAPs for ROPs known as ROPGAPs and RENGAPs (ROP1 Enhancer GAPs; Wu et al., 2000; Hwang et al., 2008; Eklund et al., 2010).
ROPGAPs
ROPGAPs are characterized by a CRIB domain in addition to the GAP domain. The CRIB domain is found in Rho effectors such as WASP in animal cells and ROP Interacting CRIB-containing proteins (RICs) in plants and not in fungal and metazoan RhoGAPs. In ROPGAPs, the CRIB domain is required for activity, as it enhances the binding between ROPs and ROPGAPs (Wu et al., 2000; Schaefer et al., 2011). The CRIB domain also is required for the subcellular localization of ROPGAPs (Wu et al., 2000; Klahre and Kost, 2006). ROPGAPs function as dimers in a 2:2 stoichiometry with ROPs (Schaefer et al., 2011). Arabidopsis AtROPGAP2 has differential binding affinities for various ROPs (Schaefer et al., 2011), and phosphorylation regulates the interaction of tobacco RhoGAP1 and an interacting 14-3-3 protein (Klahre and Kost, 2006), suggesting how ROPGAP function might be regulated.
Following the transient expression of NtRhoGAP1 in tobacco pollen tubes, the protein was localized at the plasma membrane below the tip (Klahre and Kost, 2006; Sun et al., 2015). In agreement with this finding, the ectopic expression of Arabidopsis AtROPGAP1 in tobacco pollen tubes restricts the active ROP domain (Hwang et al., 2010; Fig. 4). ROPGAPs also are important rheostats of ROP signaling. In Arabidopsis, oxygen deprivation induces the ROP-regulated production of ROS. In turn, ROS induce the expression of ROPGAP4, which down-regulates ROP activity and contributes to plant tolerance under anoxic conditions (Baxter-Burrell et al., 2002). Gene expression studies in apple (Malus domestica) showed that ethylene coordinately down-regulates ROPs, ROPGAPs, and ROPGEFs, leading to altered apoplastic hydrogen peroxide levels, suggesting that the regulation of ROP activation status has an important function in the regulation of different abiotic stresses and physiological responses (Zermiani et al., 2015).
RENGAPs
RENGAPs were initially identified by a mutant screen in pollen overexpressing ROP1. RENGAPs contain a PIP-binding PH domain in addition to the GAP domain (Hwang et al., 2008). In pollen tubes, REN1 (also known as RENGAP1) is localized in subapical cytoplasmic vesicles, and its PH domain is not required for this localization. ren1 mutant pollen tubes are swollen, indicating that REN1 functions to restrict the active ROP domain to the pollen tube tips (Hwang et al., 2008; Fig. 4).
RENGAP2 and RENGAP3 interact with the mitosis-specific kinesin-12 POK1. During interphase, RENGAP2 and RENGAP3 are cytoplasmic, but during mitosis, they are localized to the cortical division zone (CDZ). The rengap2 rengap3 double mutant displays mild cell division reorientation, indicating that rapid inactivation of ROPs at the CDZ is required for the correct orientation of cell divisions (Stöckle et al., 2016). The division of labor between ROPGAPs and RENGAPs is currently unknown, and in some cases, such as during mitosis, they might function redundantly (Stöckle et al., 2016).
Formation of Active ROP Domains by the Combinatorial Function of ROPGEFs and ROPGAPs
During metaxylem development, the simultaneous expression of ROP11, ROPGEF4, and ROPGAP3 results in the formation of active ROP domains. The formation of the active ROP11 domain was recapitulated by transient coexpression of ROP11, the PRONE domain of ROPGEF4 (ROPGEF4PRONE), and ROPGAP3. Within the domain, ROP11 and ROPGEF4 are localized in the center and ROPGAP3 in the circumference (Oda and Fukuda, 2012). These experiments demonstrated that ROP-ROPGEF-ROPGAP modules have self-organization properties, suggesting that coordinated expression and localized activation of ROPGEFs together can result in cellular patterning (Figs. 3 and 4).
RhoGDIs
Plant RhoGDIs are structurally conserved (Bischoff et al., 2000). Structural and biochemical analyses have established that the interaction between Rho proteins and RhoGDI under physiological conditions depends on prenylation by geranylgeranyl of the GTPases and involves a hydrophobic pocket in the RhoGDI (Hoffman et al., 2000; DerMardirossian and Bokoch, 2005). In plants, type I ROPs are geranylgeranylated (Sorek et al., 2011, 2017), but type II ROPs do not contain a conserved geranylgeranylation CaaL box motif and are S-acylated but not prenylated (Lavy et al., 2002; Lavy and Yalovsky, 2006). Therefore, it is likely that type II ROPs are not regulated by RhoGDIs.
Transient expression of NtRhoGDI1 in tobacco pollen tubes indicated that it functions in the recycling of ROPs and in their focusing at the pollen tube tip (Klahre et al., 2006; Sun et al., 2015). Analysis of RhoGDI mutants in Arabidopsis showed their involvement in the maintenance of ROP activation domains and ROP stability (Carol et al., 2005; Feng et al., 2016). There are three RhoGDIs in Arabidopsis called RhoGDI1, RhoGDI2a, and RhoGDI2b. RhoGDI1 mutants, also known as SUPERCENTIPIDE1 (SCN1), develop multiple root hair (RH) initials instead of a single RH in each trichoblast. In scn1 mutants, GFP-ROP2 was observed along the trichoblast membrane and ROS production was detected at multiple sites, in contrast to wild-type plants, where ROP2 accumulation and ROS formation were detected specifically at the RH initiation sites (Carol et al., 2005). Interestingly, the phenotype of scn1 fits a mathematical model predicting that a loss of RhoGDI would result in traveling waves instead of a focal polar domain (Jilkine et al., 2007).
Silencing of RhoGDI2a by RNAi resulted in the development of shorter pollen tubes and expansion of ROP distribution; however, possible redundancy with other RhoGDIs was noted (Hwang et al., 2010). rhogdi1 rhogdi2a rhogdi2b triple mutants develop shorter and wider pollen tubes with higher levels of ROPs in the pollen tube tip plasma membranes and expanded ROP activation domains. In addition, protein immunoblots indicated that the overall ROP levels are lower in the RhoGDI triple mutants (Feng et al., 2016). In mammalian cells, reduction in RhoGDI1 levels by small interfering RNA resulted in higher levels of plasma membrane-associated Rho proteins concomitant with reduction in their levels (Boulter et al., 2010). It appears, therefore, that RhoGDIs have an evolutionarily conserved function in the regulation of homeostasis of Rho GTPases. Notably, although the RhoGDI triple mutant plants are less fertile than wild-type plants, they produce viable seeds and their sporophytes do not seem to be significantly different from wild-type controls (Feng et al., 2016). This indicates that type I ROPs are functional and, possibly, that there is redundancy between type I and type II ROPs.
ROP FUNCTION IN TIP AND DIFFUSE GROWTH
Regulation of Pollen Tube Tip Growth
Pollen tubes are characterized by highly polarized oscillatory tip growth that depends on differential cell wall composition, tip-focused vesicle transport, specialized actin organization, and Ca2+, ROS, and pH gradients (Cárdenas et al., 2008; Cheung and Wu, 2008; Chebli et al., 2012; Bloch et al., 2016; Michard et al., 2017). Actin organizes into multiple forms in different regions of pollen tubes, including thick long actin cables that are axially aligned in the shank, a mesh ring or funnel-like F-actin structure known as the actin fringe in the subapical region, and a group of fine and less abundant F-actin in the pollen tube apex. Various forms of F-actin structures participate in cytoplasmic streaming and vesicle trafficking to and from the tip and in the accumulation of exocytotic vesicles at the pollen tube apex (Cárdenas et al., 2008; Cheung and Wu, 2008; Qin and Yang, 2011; Rounds et al., 2014; Fu, 2015; Qu et al., 2017b). ROP signaling regulates pollen growth and was shown to affect actin organization, Ca2+ levels, and vesicle trafficking.
LUREs are ovule-derived small peptides that attract the pollen tube toward ovules (Dresselhaus and Franklin-Tong, 2013; Higashiyama and Takeuchi, 2015). Several LURE1 receptors have been identified (Takeuchi and Higashiyama, 2016; Wang et al., 2016). It was shown that Pollen-specific Receptor Kinase6 (PRK6) is a LURE1 receptor that localizes to the pollen tube tip and interacts with the regulatory C-terminal domain of pollen-expressed ROPGEF8/9/12/13 (Takeuchi and Higashiyama, 2016; Fig. 3). In agreement, ROPGEF8/9/12-GFP fusion proteins are localized at the pollen tube tip following transient expression in tobacco pollen tubes, and AtROPGEF9 has high specific activity toward the pollen-expressed AtROP1 (Gu et al., 2006). Tomato pollen PRK1 and PRK2 interact with a ROPGEF, originally called Kinase Protein Partner (Kaothien et al., 2005). Arabidopsis PRK2a interacts with ROPGEF12 in yeast through the C-terminal regulatory domain. Coexpression of PRK2a and ROPGEF12 in the tobacco pollen tube induces isotropic growth, and a phospho-mimicking mutation at the C-terminal domain activates ROPGEF12 (Zhang and McCormick, 2007). Taken together, the results from studies described in this section strongly suggest that, upon LURE perception, PRKs activate pollen-specific ROPGEFs that, in turn, activate pollen-specific ROPs. It should be noted that ROPGEF-dependent ROP activation by LUREs has yet to be demonstrated.
Ectopic expression of constitutively active ROP mutants results in isotropic pollen tube growth, whereas the expression of dominant-negative ROP mutant or ROPGAPs inhibits pollen tube growth and is associated with changes in actin organization (Fu et al., 2001; Klahre et al., 2006; Klahre and Kost, 2006). Several studies addressed the mechanisms by which ROPs regulate actin organization. Coexpression studies in the tobacco pollen tube suggest that the tobacco ROP NtRAC5 can lead to the phosphorylation and suppression of actin depolymerization activity of Actin Depolymerizing Factor (ADF; also known as Cofilin; Chen et al., 2003).
Three RIC proteins were implicated in the regulation of pollen tube growth. RIC3 function is associated with increased intracellular Ca2+ levels, leading to actin depolymerization, whereas RIC4 is implicated in actin polymerization. The antagonistic functions of RIC3 and RIC4 are suggested to regulate actin dynamics in pollen tubes (Fig. 5; Gu et al., 2005). The mechanisms of RIC3 and RIC4 function remain to be elucidated. RIC1 regulates pollen growth through its F-actin-severing and -capping activities. RIC1 displays oscillatory localization at the apical plasma membrane of the pollen tube tip, which depends on an intact CRIB domain. The severing activity of plasma membrane-localized RIC1 mediates the release of F-actin from the pollen tube apical plasma membrane into the cytoplasm. RIC1 also contributes to severing F-actin and capping the barbed ends in the cytoplasm (Zhou et al., 2015).
An effector of the tobacco ROP called RAC5 Interacting Subapical Pollen tube protein (RISAP) was identified in a yeast two-hybrid screen. RISAP-XFP fusion proteins localize to the trans-Golgi network and interact with actin and with the globular tail domain of a tobacco myosin XI through a protein called Domain of Unknown Function593. RISAP overexpression interferes with apical membrane trafficking and blocks tip growth. It was suggested that RISAP functions in tip-directed vesicle trafficking in a ROP-dependent fashion (Stephan et al., 2014). It would be of interest to analyze RISAP loss-of-function mutants to further examine its function. An Arabidopsis protein (At1g18990) is 43% identical to RISAP, and homologs also have been identified in other plant species. Thus, it should be possible to examine the conservation of RISAP function.
Using RabA4d as an exocytotic marker in transient expression assays in tobacco pollen tubes, it was shown that its accumulation displays an oscillatory pattern that precedes the growth phase and is regulated by F-actin (Lee et al., 2008). Analysis of the exocyst Sec3a subunit mutant demonstrated that polarized exocytosis is required for both the selection of the pollen tube initiation site and polarized growth (Bloch et al., 2016). It is not known whether ROPs are involved directly in the regulation of polarized exocytosis in pollen. An analysis of multiple pollen-expressed ROP mutants has not yet been described, and it is not known whether they are required for selection of the pollen tube germination sites, similar to the function of Cdc42 in the selection of the bud site in Saccharomyces cerevisiae (Johnson and Pringle, 1990; Woods and Lew, 2017).
Regulation of RH Tip Growth
RH tip growth is characterized by actin/myosin-dependent vesicle trafficking toward the growing tip and cell wall relaxation at the tip (Mendrinna and Persson, 2015). RHs form close to the rootward side of trichoblasts, and their initiation and growth require tip-focused cell wall relaxation that depends on ROS-dependent pH fluctuations (Monshausen et al., 2007) and the activity of cell wall expansins (Cho and Cosgrove, 2002). During RH growth, F-actin forms a fine meshwork at the tip and longitudinally oriented filaments axially along the RH shank. When growth ceases, the actin filaments also are detected at the RH tip (Jones et al., 2002; Bloch et al., 2005, 2011b). ROPs regulate both RH initiation and growth. Prior to RH initiation, ROP2/4/6 accumulate at the site of future RH formation in response to a local auxin gradient (Molendijk et al., 2001; Jones et al., 2002; Fischer et al., 2006; Ikeda et al., 2009; Fig. 4). The accumulation of ROPs at future RH formation sites was found to be altered in the procuste1 cellulose synthase mutant, in clasp and sabre mutants that affect MT organization, and in actin7 and actin interacting protein1-2 mutants, suggesting involvement of the cell wall MTs and F-actin in the regulation of the RH initiation sites (Singh et al., 2008; Pietra et al., 2013; Kiefer et al., 2015).
ROP2 is expressed in RHs, and its overexpression induces ectopic RH initiation, resulting in higher RH density; expression of the dominant-negative ROP2 mutant reduces RH density. Importantly, overexpression of the activated ROP2CA also induces the formation of additional RHs, but the numbers were significantly lower than observed for wild-type ROP2 overexpressors (Jones et al., 2002). This suggests that ROP2 recycling between active and inactive states is essential for RH initiation. The rop2 null mutant develops shorter RHs, indicating that ROP2 is required for RH growth but that its function is redundant with other ROPs. Interestingly, ectopic expression of ROP6/7/11 did not increase RH initiation, suggesting that ROP2 and possibly ROP4 signaling in RH initiation is specific (Molendijk et al., 2001; Jones et al., 2002; Bloch et al., 2005). Interestingly, RHs developed in pluripetala (plp) mutant plants, which lack protein prenylation, suggesting that type II ROPs possibly also contribute to RH initiation (Running et al., 2004; Sorek et al., 2011; Chai et al., 2016). ROP2 activity also is regulated by Microtubule Associated Protein18, which interacts preferentially with GDP-bound ROP2 and competes with RhoGDI (Kang et al., 2017).
The expression of either type I or type II constitutively active ROP mutants results in RHs swelling, which is associated with disorganized actin filaments at the RH tips, inhibition of endocytosis, and nonpolar ROS distribution (Molendijk et al., 2001; Jones et al., 2002; Bloch et al., 2005; Sorek et al., 2010). ROPs activate RBOH-type NADPH oxidases during immune responses (Wong et al., 2007; Nagano et al., 2016). The SCN1/RhoGDI1-dependent and RHD2/AtRBOHC-dependent ROS accumulation at RH formation sites strongly suggest that ROP2/4 activates RBOHC during RH formation and growth (Carol et al., 2005). Consistent with the link between ROPs, ROS formation, and pH regulation, it was found that the ROP11CA-induced RH swelling was suppressed by the omission of nitrogen-containing compounds (either NH4+ or NO3−) from the growth medium. The omission or addition of NH4+ and NO3− affected intracellular and extracellular pH fluctuations and the overall pH at RH tips, implying a close correlation between ROP signaling and the pH at the RH tip (Bloch et al., 2011a, 2011b).
Upon activation, at least some ROPs undergo activation-dependent transient S-acylation on one or two G-domain Cys residues and, consequently, partition into detergent-resistant membranes, which is required for their function (Sorek et al., 2009, 2010, 2011, 2017). Consistently, ROP2 association with the plasma membrane is compromised in Protein S-Acyl Transferase4 (PAT4) mutants. pat4 RHs are shorter, and the multiple RH phenotype of the scn1/rhogdi1 mutant is suppressed in the pat4 background. Furthermore, ROPs activate rice RBOHB/H by recruiting them into lipid microdomains (Nagano et al., 2016). At the future RH initiation sites, ROP2/6 colocalize with PIP 4 phosphate 5 kinase 3, Dynamin Related Protein1A (DRP1A) and DRP2B, and the AGCVIII kinase D6 Protein Kinase (D6PK) in sterol-enriched domains (Stanislas et al., 2015). Auxin has been implicated in ROP activation (Tao et al., 2002; Wu et al., 2011; Miyawaki and Yang, 2014), yet the underlying mechanisms remain to be elucidated. PIN phosphorylation by D6PK regulates auxin transport (Barbosa et al., 2014; Zourelidou et al., 2014). Hence, the association of ROPs and D6PK in the RH initiation domain may provide a link between ROP signaling and auxin (Fig. 4; Box 2; Abo et al., 1991).
Signaling between cell wall extensibility and mechanisms that regulate cell growth is mediated in RHs by the RLK FER. FER is a member of the CrRLK1 (Catharanthus roseus RLK1) family that are thought to sense the cell through extracellular malectin-like domains that are presumed to interact with carbohydrates (Höfte, 2015; Li et al., 2016a; Voxeur and Höfte, 2016). RHs collapse or burst in fer mutant alleles. FER interacts with ROPGEF1 in yeast two-hybrid and bimolecular fluorescence complementation assays, and active ROP2 levels and ROS production are reduced in fer mutant plants (Duan et al., 2010). Hence, FER appears to regulate ROP function during the RH growth phase (Fig. 3).
To summarize, local auxin concentration gradients direct the formation of sterol-enriched ROP domains. The formation of single rather than multiple ROP domains depends of RhoGDI-dependent recycling of ROPs to and from the plasma membrane as well as cycling of ROPs between active and inactive states. During RH growth, ROP function is regulated by FER, which coordinates and adjusts cell wall composition in response to intracellular growth signals. Auxin signaling also is known to regulate RH growth (Knox et al., 2003), but it is unknown yet whether it signals through ROPs. Activated ROPs likely activate RBOHC, leading to ROS production and associated changes in intracellular and extracellular pH and intracellular Ca2+ levels (Foreman et al., 2003; Takeda et al., 2008). Through effectors, which have yet to be identified, ROPs regulate MTs and F-actin organization and dynamics (Fig. 3).
ROP Function during Diffuse Growth
With the exception of RHs and pollen tubes, plant cells grow by diffuse growth; this implies that their growth proceeds along the cell and is not limited to a single domain, as in tip growth. Three model systems have been used extensively to study ROP function during diffuse growth: trichomes, leaf epidermal pavement cells, and petal epidermal cells. Trichomes grow faster at the region closer to the tip, where the cell wall is thinner than at the basal side. Dense transverse MT arrays exist at the upper half but are absent from the tip and correspond to higher cell wall pressure and tension. Arp2/3-driven actin nucleation takes place at the MT clear zone at the tip and depends on an active WAVE complex (Yanagisawa et al., 2015). It is likely that ROPs are activated locally by SPK1 to promote WAVE activation. The expression of constitutively active rop2CA resulted in the development of trichomes with thicker stalks and branches and altered branch positions, while the expression of dominant-negative ROP mutants did not alter trichome growth (Fu et al., 2002). However, expression of the Clostridium difficile toxin B catalytic domain, which glycosylates and inhibits ROP function, alters trichome morphogenesis (Singh et al., 2013). It is expected that genetic analysis of loss-of-function ROP mutants will reveal which ROPs are involved in trichome development.
Pavement cells are interdigitated, characterized by their lobes and indentations. Mutual stresses operating between neighboring cells affect the orientation of the MTs and, in turn, the patterning of cell growth (Sampathkumar et al., 2014). Compromised isotropic pavement cells with reduced interdigitation are observed in plants with gain- and loss-of-function mutants in genes encoding ROP, ROP effectors, SPK1, and PLP and in transgenic plants expressing anti-ROP toxins (Fu et al., 2002, 2005, 2009; Qiu et al., 2002; Bloch et al., 2005; Lavy et al., 2007; Sorek et al., 2010, 2011; Poraty-Gavra et al., 2013; Singh et al., 2013). Based on the analysis of Arabidopsis ROP2, ROP4, and ROP6 loss-of-function and constitutively active mutants, it was proposed that these proteins coregulate pavement cell growth by affecting the organization of both cortical MTs and F-actin via different ROP effectors. ROP6 interacts with RIC1, a ROP effector and MT-binding protein. MT-associated RIC1 interacts with the P60 subunit of the MT-severing protein katanin (KTN1). RIC1 promotes the MT-severing activity of KTN1 and the formation of the transverse cortical MTs (Fig. 5; Fu et al., 2005, 2009; Lin et al., 2013a). Overexpression of RIC1 induced highly ordered transverse MTs that result in isomorphic cell growth. ric1 loss-of-function pavement cells exhibited more randomly oriented cortical MTs and a wider neck/indentation region than the wild type (Fu et al., 2009). ROP2 and possibly ROP4 were shown to promote the formation of actin microfilaments via another ROP effector, RIC4. ROP2 also was shown to recruit RIC1 to the plasma membrane and away from MTs, thereby inhibiting its action on MTs (Fu et al., 2005). As ric1 loss-of-function pavement cells are interdigitated (Fu et al., 2009), RIC1 function is likely redundant during pavement cell growth. Treating cotyledons with cellulase results in the development of isomorphic noninterdigitated pavement cells. Interestingly, the cellulase-induced isomorphic pavement cell growth phenotype was suppressed in a ric1 loss-of-function background (Higaki et al., 2017), suggesting that RIC1 could be part of a pathway that senses cell wall integrity.
The physical and signaling interaction between FER and ROPGEF1 established a connection between cell wall sensing and ROP signaling. It was recently reported that ROPGEF4 interacts with the cell wall-sensing CrRLK THESEUS1 during the defense response to Botrytis cinerea (Qu et al., 2017a). It will be interesting to study the function of RIC1 in these signaling cascades. Further work will be required to elucidate how ROP signaling is integrated with cell wall pressure and strain as well as the signals that activate and inactivate ROPs during pavement cell growth.
The abaxial petal epidermal cells are interdigitated. The interdigitation of the cells is compromised in the spk1 mutant and in the rop2 rop6 rop4RNAi mutant, and cells display transverse MT arrays. Furthermore, the levels of active ROP2 and ROP6 are lower in spk1 than in wild-type plants (Ren et al., 2016). Taken together, these results indicated that SPK1 is responsible for activating ROP2, ROP6, and likely ROP4, which together regulate the growth of petal epidermal cells. The adaxial petal epidermal cells have cone-like structures with sharp-angled tips and transverse MT arrays. In ktn1 mutants, the cone-shaped cells have wider tip angles and less ordered MT arrays. Similarly, spk1 and rop2 rop6 rop4RNAi mutants develop cells with wider tip angles. It was proposed that ROP2/6 and ROP4 regulate MTs via KTN1 (Ren et al., 2017). These new findings also suggest that ROP signaling networks play important and general roles in the regulation of cell expansion and cell shape; however, the precise regulatory mechanisms might be distinct in specific organs.
ROP FUNCTION IN SECONDARY WALL PATTERNING, CYTOKINESIS, AND NUCLEI MIGRATION
Regulation of Secondary Wall Patterning by ROPs
During vascular differentiation, the ROP effector Microtubule Depletion Domain1 (MIDD1), also known as Interactor of Constitutively Active ROP5 (ICR5) or ROP Interacting Partner3, is a regulator of secondary cell wall deposition in metaxylem. MIDD1 is an MT-binding protein that interacts with the MT-destabilizing protein KINESINE13A (KIN13A; Mucha et al., 2010). During metaxylem differentiation, MIDD1 promotes the depolymerization of cortical MTs, leading to the formation of secondary wall pits (Oda et al., 2010). During metaxylem differentiation, coexpression of ROP11, ROPGEF4, and ROPGAP3 results in the formation of activated ROP11 domains. The active ROP11, in turn, recruits MIDD1, which, in turn, recruits KIN13A, leading to local MT breakdown (Oda and Fukuda, 2012). It was demonstrated recently that the size of the active ROP domains in the metaxlyem is limited by a plasma membrane-associated protein designated IQD13, which affects MT orientation and dynamics (Fig. 5; Sugiyama et al., 2017).
The Function of ROPs in the Regulation of Cytokinesis
Mutant and protein localization studies have implicated ROPs in the regulation of cytokinesis. In dividing BY2 cells, GFP-AtROP4 is associated with the cell plate (Molendijk et al., 2001). During stomata development in maize, partial knockout of type I ROPs (rop2−/− rop9+/−) or treatment with a RAC inhibitor results in weak cell division polarization defects. ROP2/9 accumulate at the junctions between subsidiary mother cells (SMCs) and guard mother cells (GMCs), and their accumulation at these sites depends on the membrane RLK PANGLOS1 (PAN1). When expressed at high levels, GFP-ZmROP2 is not polarly localized in SMCs, and cell division polarization defects are observed. Furthermore, ROP accumulation in detergent-resistant membranes is lower in the pan1 mutant than in wild-type plants, suggesting that PAN1 is required for ROP activation. The accumulation of ROPs and PAN at the SMC/GMC junctions precedes the formation of actin patches at these locations (Humphries et al., 2011).
The first component known to accumulate at the SMC/GMC junctions is the SCAR/WAVE complex. PAN1 and PAN2 polarization and accumulation at the SMC/GMC junction is compromised in a brk1 SCAR/WAVE subunit mutant. Based on these results and on the interaction between SCAR/WAVE subunits and ROPs and SPK1, it was proposed that the SCAR/WAVE complex recruits both PAN1 and ROP2/9 and that the resulting ROP activation leads to the activation of SCAR/WAVE and, in turn, Arp2/3 activation and formation of the actin patch (Facette et al., 2015). It is not yet known whether SPK1 is associated with the SCAR/WAVE complex in maize, whether the PAN1-dependent ROP activation is associated with the catalytic activity of PAN1 on ROPs or ROPGEFs, or whether it is required for the recruitment of ROPs to the SMC/GMC junctions through a protein-protein interaction.
A role for ROP signaling in the regulation of cytokinesis polarity in the Arabidopsis root was established recently (Stöckle et al., 2016). Two PH domain-containing ROPGAPs designated PHGAP1/2 interact the PHRAGMOPLAST ORIENTING KINESIN1 (POK1). phgap1 phgap2 double mutants display a weak cell wall-positioning phenotype, and during cytokinesis, PHGAP1 and PHGAP2 proteins localize to the CDZ in a POK1-dependent fashion (Stöckle et al., 2016). Taken together, these data suggest that the down-regulation of ROPs at specific sites at the cell division zone is required for proper orientation of the cell division planes.
ROP Signaling and Nuclear Migration: ROP8 and Nuclei Migration in the Ovule Central Cell
The central cell contains actin filaments that grow from the plasma membrane toward the nucleus. Upon entry of the sperm nucleus, it is engulfed by actin filaments that make an astral-like structure that pull it toward the central cell nucleus. AtROP8 is expressed uniquely in the central cell and is localized at the plasma membrane. Expression of rop8DN disrupted the organization of the astral-like actin filaments and the migration of the sperm nuclei. Interestingly, the expression of rop8CA did not affect actin organization or pollen nucleus migration, suggesting that ROP8 is maintained in an active state in the central cell (Kawashima et al., 2014). Nuclei migration is a vital process in the differentiation of different cell types in plants, and it would be of interest to examine whether other ROPs have a broad role in other cell types.
The Regulation of Nuclei Migration during RH Formation by ROP Signaling
Prior to RH emergence, trichoblast nuclei are localized close to the inner cell membrane at the center of the long cell axis. Consequently, the nuclei migrate into the growing RHs. It was reported recently that the positioning of the nuclei close to the inner membrane and their migration are regulated by ROP and auxin signaling that synergistically regulate actin organization and dynamics (Nakamura et al., 2018).
ROP FUNCTION IN BIOTIC AND ABIOTIC STRESS RESPONSES
Biotic Responses
The roles of ROP GTPases in plant pathogen and biotic responses have been the subject of recent detailed reviews (Bhagatji et al., 2010; Kawano et al., 2014; Rivero et al., 2017). This section will briefly highlight certain relevant aspects. Class VI Receptor-Like Cytoplasmic Kinases (RLCKs) belong to the large superfamily of RLKs, which are involved in a variety of cellular processes, including plant growth, development, and immune responses (Jurca et al., 2008; Lin et al., 2013b). RLCKs of the VI class appear to be downstream ROP effectors that regulate the pathogen response (Molendijk et al., 2008; Huesmann et al., 2012; Reiner et al., 2015). Barley (Hordeum vulgare) HvRBK1 is a type VI_A RLCK that is activated by HvRACB upon infection by the fungal pathogen Blumeria graminis f. sp. hordei (Bgh; Huesmann et al., 2012). Transient knockdown of HvRBK1 influences the stability of the cortical MTs in barley epidermal cells. HvRACB also regulates a RIC family member, RIC171, in the pathogen defense response. Overexpression of RIC171, similar to overexpression of constitutively activated RACB, renders epidermal cells more susceptible to penetration by Bgh. Additionally, RIC171 accumulates at sites of fungal attack, suggesting an enhanced ROP activity at sites of attempted fungal penetration (Schultheiss et al., 2008). HvRACB signaling is regulated by the barley MICROTUBULE-ASSOCIATED ROPGAP1 (HvMAGAP1), limiting the susceptibility of barley to Bgh (Hoefle et al., 2011).
In rice, OsRAC1 plays a critical role in defense responses induced by various microbe-associated molecular patterns, including chitin. OsRAC1 is activated by chitin perception mediated by OsCERK1, an RLK that forms a complex with OsCEBiP, a chitin-binding protein (Shimizu et al., 2010). In the presence of chitin, OsCERK1 phosphorylates and activates OsRACGEF. In turn, OsRAC1 activation activates lignin biosynthesis by activating Cinnamoyl CoA Reductase and ROS production by the activation of NADPH oxidase (Wong et al., 2007; Akamatsu et al., 2013; Nagano et al., 2016).
In Medicago trancatula and Lotus japonicus ROPs, MtROP10 and LjROP6 interact with the RLKs MtNFP and LjNFR5 in the membrane, respectively, and are required for rhizobium-induced nodule formation and for tip growth (Ke et al., 2012, 2016; Lei et al., 2015; Rivero et al., 2017). It is not known yet whether ROPGEFs colocalize with the respective ROPs and RLKs.
The expression of dominant-negative Atrop6DN under the regulation of the ROP6 promoter induced a salicylic acid-dependent systemic acquired resistance (SAR) response characterized by constitutive expression of pathogenesis-related genes. The rop6DN plants are small, have twisted leaves with rectangular pavement cells, and have altered actin and MT organization. rop6DN plants with mutations in SA biosynthesis and signaling factors demonstrated that the developmental and SAR phenotypes are separable. Interestingly, regardless of the constitutive SAR response, the rop6DN plants did not display enhanced resistance to Golovinomyces orontii powdery mildew (Poraty-Gavra et al., 2013). Possibly, the rop6DN-induced changes in cytoskeleton organization and cell wall influence the SAR response. In the future, it will be important to carry out gene expression analyses because, in ROP mutants, as this analysis could potentially reveal, hidden phenotypes that affect the plant would otherwise remain undetectable.
ROPs and Abiotic Signaling Responses
Accumulating evidence indicates that ROPs and ABA form negative feedback loops in which ABA signaling suppresses ROP activation and ROP signaling suppresses ABA responses (Lemichez et al., 2001; Zheng et al., 2002; Xin et al., 2005; Li et al., 2012a, 2012b, 2016; Li and Liu, 2012; Yu et al., 2012). ROP10 and ROP11 inhibit ABA signaling by interacting physically with ABA negative regulators, ABA Insensitive1 (ABI1) and ABI2 PP2C phosphatases. It was postulated that the interactions with ROPs prevent ABI1/2 ABA-dependent inactivation by PYR/PIL ABA receptors (Li et al., 2012b; Yu et al., 2012). ABA inhibits ROP activation by promoting the degradation of several ROPGEFs. ROPGEF1 interacts with ABI1 in yeast and in vitro and is degraded in response to ABA. In the abi1 abi2 hab1 pp2CA quadruple mutant, it is localized to prevacuolar compartments and undergoes degradation (Li et al., 2016b). Thus, ABI1 and possibly other phosphatases may form regulatory hubs that interact with both ROPGEFs and ROPs, forming a positive feedback loop that suppresses ABA responses. Upon an increase in ABA levels, PP2C is sequestered by PYR1/PYL ABA receptors, leading to ROPGEF degradation and ROP inactivation. Oxygen deprivation induces the expression of ROPGAP4, and ropgap4 mutant plants displayed lower oxygen deprivation tolerance due to ROS-induced overproduction of alcohol dehydrogenase (Baxter-Burrell et al., 2002). Hence, ROP activation/inactivation loops play an important function in abiotic stress tolerance and ABA signaling.
A recent report indicates that ROPGEF2 is activated directly or indirectly by phyB and, in turn, activates ROP2 and ROP7 to suppress red light-induced stomatal opening (Wang et al., 2017). Nucleotide exchange assays presented in this work suggest that ROPGEF2 catalyzes nucleotide activity on Cdc42. Yet, it is well documented that ROPGEFs are specific to plant ROPs and cannot catalyze GDP/GTP exchange on nonplant Rho GTPases (Berken et al., 2005; Thomas et al., 2007; Thomas and Berken, 2010). Hence, some of the data presented in this work might have to be revisited.
ROP signaling is involved in the regulation of abiotic stress via regulation of the cytoskeleton. The biphasic reorganization of MTs (rapid MT disassembly followed by reassembly of new MTs) induced by high salinity is required for plant tolerance to salt stress. The ROP2-RIC1 signaling pathway was found to modulate MT reorganization in response to salt stress in Arabidopsis (Li et al., 2017). During salt stress, activation of ROP2 leads to the removal of RIC1 from MTs, leading to enhanced MT reassembly and, in turn, an increase in plant tolerance to salt stress (Li et al., 2017).
Acknowledgments
Figure 2 panels B, C, and D were prepared and contributed by Dr. Izuru Ohki and Dr. Chojiro Kojima.
Footnotes
The research was supported by Israel Academy of Sciences (grant nos. ISF 827/15 and ISF-NCSF 1125/13) and by the Israel Center for Research Excellence on Plant Adaptation to Changing Environment (grant no. I-CORE 757-12) to S.Y. and the Natural Science Foundation of China to Y.F. (grant nos. 31325001 and 31361140354).
Articles can be viewed without a subscription.
References
- Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW (1991) Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353: 668–670 [DOI] [PubMed] [Google Scholar]
- Akamatsu A, Wong HL, Fujiwara M, Okuda J, Nishide K, Uno K, Imai K, Umemura K, Kawasaki T, Kawano Y, et al. (2013) An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity. Cell Host Microbe 13: 465–476 [DOI] [PubMed] [Google Scholar]
- Barbosa IC, Zourelidou M, Willige BC, Weller B, Schwechheimer C (2014) D6 PROTEIN KINASE activates auxin transport-dependent growth and PIN-FORMED phosphorylation at the plasma membrane. Dev Cell 29: 674–685 [DOI] [PubMed] [Google Scholar]
- Bashline L, Li S, Gu Y (2014) The trafficking of the cellulose synthase complex in higher plants. Ann Bot 114: 1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, El-Assal Sel-D, Le J, Mallery EL, Szymanski DB (2004) Interchangeable functions of Arabidopsis PIROGI and the human WAVE complex subunit SRA1 during leaf epidermal development. Development 131: 4345–4355 [DOI] [PubMed] [Google Scholar]
- Basu D, Le J, Zakharova T, Mallery EL, Szymanski DB (2008) A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc Natl Acad Sci USA 105: 4044–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028 [DOI] [PubMed] [Google Scholar]
- Berken A, Thomas C, Wittinghofer A (2005) A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 436: 1176–1180 [DOI] [PubMed] [Google Scholar]
- Berken A, Wittinghofer A (2008) Structure and function of Rho-type molecular switches in plants. Plant Physiol Biochem 46: 380–393 [DOI] [PubMed] [Google Scholar]
- Bhagatji P, Leventis R, Rich R, Lin CJ, Silvius JR (2010) Multiple cellular proteins modulate the dynamics of K-ras association with the plasma membrane. Biophys J 99: 3327–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F, Vahlkamp L, Molendijk A, Palme K (2000) Localization of AtROP4 and AtROP6 and interaction with the guanine nucleotide dissociation inhibitor AtRhoGDI1 from Arabidopsis. Plant Mol Biol 42: 515–530 [DOI] [PubMed] [Google Scholar]
- Bloch D, Lavy M, Efrat Y, Efroni I, Bracha-Drori K, Abu-Abied M, Sadot E, Yalovsky S (2005) Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol Biol Cell 16: 1913–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch D, Monshausen G, Gilroy S, Yalovsky S (2011a) Co-regulation of root hair tip growth by ROP GTPases and nitrogen source modulated pH fluctuations. Plant Signal Behav 6: 426–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch D, Monshausen G, Singer M, Gilroy S, Yalovsky S (2011b) Nitrogen source interacts with ROP signalling in root hair tip-growth. Plant Cell Environ 34: 76–88 [DOI] [PubMed] [Google Scholar]
- Bloch D, Pleskot R, Pejchar P, Potocký M, Trpkošová P, Cwiklik L, Vukašinović N, Sternberg H, Yalovsky S, Žárský V (2016) Exocyst SEC3 and phosphoinositides define sites of exocytosis in pollen tube initiation and growth. Plant Physiol 172: 980–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch D, Yalovsky S (2013) Cell polarity signaling. Curr Opin Plant Biol 16: 734–742 [DOI] [PubMed] [Google Scholar]
- Boulter E, Garcia-Mata R (2010) RhoGDI: a rheostat for the Rho switch. Small GTPases 1: 65–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K (2010) Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol 12: 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boureux A, Vignal E, Faure S, Fort P (2007) Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol 24: 203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature 349: 117–127 [DOI] [PubMed] [Google Scholar]
- Brembu T, Winge P, Bones AM (2005) The small GTPase AtRAC2/ROP7 is specifically expressed during late stages of xylem differentiation in Arabidopsis. J Exp Bot 56: 2465–2476 [DOI] [PubMed] [Google Scholar]
- Brembu T, Winge P, Bones AM, Yang Z (2006) A RHOse by any other name: a comparative analysis of animal and plant Rho GTPases. Cell Res 16: 435–445 [DOI] [PubMed] [Google Scholar]
- Cárdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146: 1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L (2005) A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Chai S, Ge FR, Feng QN, Li S, Zhang Y (2016) PLURIPETALA mediates ROP2 localization and stability in parallel to SCN1 but synergistically with TIP1 in root hairs. Plant J 86: 413–425 [DOI] [PubMed] [Google Scholar]
- Chang F, Gu Y, Ma H, Yang Z (2013) AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol Plant 6: 1187–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y, Kaneda M, Zerzour R, Geitmann A (2012) The cell wall of the Arabidopsis pollen tube: spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol 160: 1940–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Cheung AY, Wu HM (2003) Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth. Plant Cell 15: 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu HM, Cheung AY (2002) The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 14: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Liu H, Kong J, Yang Y, Zhang N, Li R, Yue J, Huang J, Li C, Cheung AY, et al. (2011) RopGEF7 regulates PLETHORA-dependent maintenance of the root stem cell niche in Arabidopsis. Plant Cell 23: 2880–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59: 547–572 [DOI] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 14: 3237–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Lee Y, Hwang JU (2014) Arabidopsis ROP9 and ROP10 GTPases differentially regulate auxin and ABA responses. J Plant Biol 57: 245–254 [Google Scholar]
- DerMardirossian C, Bokoch GM (2005) GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 15: 356–363 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Franklin-Tong N (2013) Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant 6: 1018–1036 [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY (2014) Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun 5: 3129. [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM (2010) FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA 107: 17821–17826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund DM, Svensson EM, Kost B (2010) Physcomitrella patens: a model to investigate the role of RAC/ROP GTPase signalling in tip growth. J Exp Bot 61: 1917–1937 [DOI] [PubMed] [Google Scholar]
- Elias M. (2008) The guanine nucleotide exchange factors Sec2 and PRONE: candidate synapomorphies for the Opisthokonta and the Archaeplastida. Mol Biol Evol 25: 1526–1529 [DOI] [PubMed] [Google Scholar]
- Eliáš M, Klimeš V (2012) Rho GTPases: deciphering the evolutionary history of a complex protein family. Methods Mol Biol 827: 13–34 [DOI] [PubMed] [Google Scholar]
- Facette MR, Park Y, Sutimantanapi D, Luo A, Cartwright HN, Yang B, Bennett EJ, Sylvester AW, Smith LG (2015) The SCAR/WAVE complex polarizes PAN receptors and promotes division asymmetry in maize. Nat Plants 1: 14024. [DOI] [PubMed] [Google Scholar]
- Feig LA. (1999) Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol 1: E25–E27 [DOI] [PubMed] [Google Scholar]
- Feng QN, Kang H, Song SJ, Ge FR, Zhang YL, Li E, Li S, Zhang Y (2016) Arabidopsis RhoGDIs are critical for cellular homeostasis of pollen tubes. Plant Physiol 170: 841–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Ikeda Y, Ljung K, Serralbo O, Singh M, Heidstra R, Palme K, Scheres B, Grebe M (2006) Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr Biol 16: 2143–2149 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Fowler JE. (2010) Evolution of the ROP GTPase signaling module. In Yalovsky S, Baluska F, Jones A, eds, Integrated G Protein Signaling in Plants. Springer-Verlag, Berlin, pp 305–327 [Google Scholar]
- Fu Y. (2015) The cytoskeleton in the pollen tube. Curr Opin Plant Biol 28: 111–119 [DOI] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120: 687–700 [DOI] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wu G, Yang Z (2001) Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol 152: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Xu T, Zhu L, Wen M, Yang Z (2009) A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr Biol 19: 1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Eccleston JF, Lowe PN (1999) The conserved arginine in rho-GTPase-activating protein is essential for efficient catalysis but not for complex formation with Rho.GDP and aluminum fluoride. Biochemistry 38: 985–991 [DOI] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z (2005) A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol 169: 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li S, Lord EM, Yang Z (2006) Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell 18: 366–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Wang Z, Yang Z (2004) ROP/RAC GTPase: an old new master regulator for plant signaling. Curr Opin Plant Biol 7: 527–536 [DOI] [PubMed] [Google Scholar]
- Hamada T. (2014) Microtubule organization and microtubule-associated proteins in plant cells. Int Rev Cell Mol Biol 312: 1–52 [DOI] [PubMed] [Google Scholar]
- Hashimoto T. (2015) Microtubules in plants. The Arabidopsis Book 13: e0179, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazak O, Bloch D, Poraty L, Sternberg H, Zhang J, Friml J, Yalovsky S (2010) A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol 8: e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazak O, Obolski U, Prat T, Friml J, Hadany L, Yalovsky S (2014) Bimodal regulation of ICR1 levels generates self-organizing auxin distribution. Proc Natl Acad Sci USA 111: E5471–E5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henty-Ridilla JL, Li J, Blanchoin L, Staiger CJ (2013) Actin dynamics in the cortical array of plant cells. Curr Opin Plant Biol 16: 678–687 [DOI] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T (2006) PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314: 1458–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki T, Takigawa-Imamura H, Akita K, Kutsuna N, Kobayashi R, Hasezawa S, Miura T (2017) Exogenous cellulase switches cell interdigitation to cell elongation in an RIC1-dependent manner in Arabidopsis thaliana cotyledon pavement cells. Plant Cell Physiol 58: 106–119 [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66: 393–413 [DOI] [PubMed] [Google Scholar]
- Hoefle C, Huesmann C, Schultheiss H, Börnke F, Hensel G, Kumlehn J, Hückelhoven R (2011) A barley ROP GTPase ACTIVATING PROTEIN associates with microtubules and regulates entry of the barley powdery mildew fungus into leaf epidermal cells. Plant Cell 23: 2422–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GR, Nassar N, Cerione RA (2000) Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell 100: 345–356 [DOI] [PubMed] [Google Scholar]
- Höfte H. (2015) The yin and yang of cell wall integrity control: brassinosteroid and FERONIA signaling. Plant Cell Physiol 56: 224–231 [DOI] [PubMed] [Google Scholar]
- Hong D, Jeon BW, Kim SY, Hwang JU, Lee Y (2016) The ROP2-RIC7 pathway negatively regulates light-induced stomatal opening by inhibiting exocyst subunit Exo70B1 in Arabidopsis. New Phytol 209: 624–635 [DOI] [PubMed] [Google Scholar]
- Huang GQ, Li E, Ge FR, Li S, Wang Q, Zhang CQ, Zhang Y (2013) Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth. New Phytol 200: 1089–1101 [DOI] [PubMed] [Google Scholar]
- Huang JB, Liu H, Chen M, Li X, Wang M, Yang Y, Wang C, Huang J, Liu G, Liu Y, et al. (2014) ROP3 GTPase contributes to polar auxin transport and auxin responses and is important for embryogenesis and seedling growth in Arabidopsis. Plant Cell 26: 3501–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesmann C, Reiner T, Hoefle C, Preuss J, Jurca ME, Domoki M, Fehér A, Hückelhoven R (2012) Barley ROP binding kinase1 is involved in microtubule organization and in basal penetration resistance to the barley powdery mildew fungus. Plant Physiol 159: 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JA, Vejlupkova Z, Luo A, Meeley RB, Sylvester AW, Fowler JE, Smith LG (2011) ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell 23: 2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JU, Jeon BW, Hong D, Lee Y (2011) Active ROP2 GTPase inhibits ABA- and CO2-induced stomatal closure. Plant Cell Environ 34: 2172–2182 [DOI] [PubMed] [Google Scholar]
- Hwang JU, Vernoud V, Szumlanski A, Nielsen E, Yang Z (2008) A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr Biol 18: 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JU, Wu G, Yan A, Lee YJ, Grierson CS, Yang Z (2010) Pollen-tube tip growth requires a balance of lateral propagation and global inhibition of Rho-family GTPase activity. J Cell Sci 123: 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Men S, Fischer U, Stepanova AN, Alonso JM, Ljung K, Grebe M (2009) Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat Cell Biol 11: 731–738 [DOI] [PubMed] [Google Scholar]
- Jeon BW, Hwang JU, Hwang Y, Song WY, Fu Y, Gu Y, Bao F, Cho D, Kwak JM, Yang Z, et al. (2008) The Arabidopsis small G protein ROP2 is activated by light in guard cells and inhibits light-induced stomatal opening. Plant Cell 20: 75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilkine A, Marée AF, Edelstein-Keshet L (2007) Mathematical model for spatial segregation of the Rho-family GTPases based on inhibitory crosstalk. Bull Math Biol 69: 1943–1978 [DOI] [PubMed] [Google Scholar]
- Johnson DI, Pringle JR (1990) Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol 111: 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Jin M, Lew DJ (2011) Symmetry breaking and the establishment of cell polarity in budding yeast. Curr Opin Genet Dev 21: 740–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Shen JJ, Fu Y, Li H, Yang Z, Grierson CS (2002) The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurca ME, Bottka S, Fehér A (2008) Characterization of a family of Arabidopsis receptor-like cytoplasmic kinases (RLCK class VI). Plant Cell Rep 27: 739–748 [DOI] [PubMed] [Google Scholar]
- Kang E, Zheng M, Zhang Y, Yuan M, Yalovsky S, Zhu L, Fu Y (2017) The microtubule-associated protein MAP18 affects ROP2 GTPase activity during root hair growth. Plant Physiol 174: 202–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaothien P, Ok SH, Shuai B, Wengier D, Cotter R, Kelley D, Kiriakopolos S, Muschietti J, McCormick S (2005) Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J 42: 492–503 [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kaneko-Kawano T, Shimamoto K (2014) Rho family GTPase-dependent immunity in plants and animals. Front Plant Sci 5: 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Koita H, Nakatsubo T, Hasegawa K, Wakabayashi K, Takahashi H, Umemura K, Umezawa T, Shimamoto K (2006) Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc Natl Acad Sci USA 103: 230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Maruyama D, Shagirov M, Li J, Hamamura Y, Yelagandula R, Toyama Y, Berger F (2014) Dynamic F-actin movement is essential for fertilization in Arabidopsis thaliana. eLife 3: 04501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke D, Fang Q, Chen C, Zhu H, Chen T, Chang X, Yuan S, Kang H, Ma L, Hong Z, et al. (2012) The small GTPase ROP6 interacts with NFR5 and is involved in nodule formation in Lotus japonicus. Plant Physiol 159: 131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke D, Li X, Han Y, Cheng L, Yuan H, Wang L (2016) ROP6 is involved in root hair deformation induced by Nod factors in Lotus japonicus. Plant Physiol Biochem 108: 488–498 [DOI] [PubMed] [Google Scholar]
- Kiefer CS, Claes AR, Nzayisenga JC, Pietra S, Stanislas T, Hüser A, Ikeda Y, Grebe M (2015) Arabidopsis AIP1-2 restricted by WER-mediated patterning modulates planar polarity. Development 142: 151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U, Becker C, Schmitt AC, Kost B (2006) Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant J 46: 1018–1031 [DOI] [PubMed] [Google Scholar]
- Klahre U, Kost B (2006) Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell 18: 3033–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K, Grierson CS, Leyser O (2003) AXR3 and SHY2 interact to regulate root hair development. Development 130: 5769–5777 [DOI] [PubMed] [Google Scholar]
- Kosami K, Ohki I, Nagano M, Furuita K, Sugiki T, Kawano Y, Kawasaki T, Fujiwara T, Nakagawa A, Shimamoto K, et al. (2014) The crystal structure of the plant small GTPase OsRac1 reveals its mode of binding to NADPH oxidase. J Biol Chem 289: 28569–28578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH (1999) Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol 145: 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche N, Hall A (1994) GAPs for rho-related GTPases. Trends Genet 10: 436–440 [DOI] [PubMed] [Google Scholar]
- Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S (2007) A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol 17: 947–952 [DOI] [PubMed] [Google Scholar]
- Lavy M, Bracha-Drori K, Sternberg H, Yalovsky S (2002) A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. Plant Cell 14: 2431–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Yalovsky S (2006) Association of Arabidopsis type-II ROPs with the plasma membrane requires a conserved C-terminal sequence motif and a proximal polybasic domain. Plant J 46: 934–947 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Szumlanski A, Nielsen E, Yang Z (2008) Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol 181: 1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei MJ, Wang Q, Li X, Chen A, Luo L, Xie Y, Li G, Luo D, Mysore KS, Wen J, et al. (2015) The small GTPase ROP10 of Medicago truncatula is required for both tip growth of root hairs and nod factor-induced root hair deformation. Plant Cell 27: 806–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua NH (2001) Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev 15: 1808–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lu H, Li W, Yuan M, Fu Y (2017) A ROP2-RIC1 pathway fine-tunes microtubule reorganization for salt tolerance in Arabidopsis. Plant Cell Environ 40: 1127–1142 [DOI] [PubMed] [Google Scholar]
- Li C, Wu HM, Cheung AY (2016a) FERONIA and her pals: functions and mechanisms. Plant Physiol 171: 2379–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen JJ, Zheng ZL, Lin Y, Yang Z (2001) The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol 126: 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Blanchoin L, Staiger CJ (2015a) Signaling to actin stochastic dynamics. Annu Rev Plant Biol 66: 415–440 [DOI] [PubMed] [Google Scholar]
- Li S, Lei L, Yingling YG, Gu Y (2015b) Microtubules and cellulose biosynthesis: the emergence of new players. Curr Opin Plant Biol 28: 76–82 [DOI] [PubMed] [Google Scholar]
- Li S, Zhou LZ, Feng QN, McCormick S, Zhang Y (2013) The C-terminal hypervariable domain targets Arabidopsis ROP9 to the invaginated pollen tube plasma membrane. Mol Plant 6: 1362–1364 [DOI] [PubMed] [Google Scholar]
- Li Z, Kang J, Sui N, Liu D (2012a) ROP11 GTPase is a negative regulator of multiple ABA responses in Arabidopsis. J Integr Plant Biol 54: 169–179 [DOI] [PubMed] [Google Scholar]
- Li Z, Li Z, Gao X, Chinnusamy V, Bressan R, Wang ZX, Zhu JK, Wu JW, Liu D (2012b) ROP11 GTPase negatively regulates ABA signaling by protecting ABI1 phosphatase activity from inhibition by the ABA receptor RCAR1/PYL9 in Arabidopsis. J Integr Plant Biol 54: 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu D (2012) ROPGEF1 and ROPGEF4 are functional regulators of ROP11 GTPase in ABA-mediated stomatal closure in Arabidopsis. FEBS Lett 586: 1253–1258 [DOI] [PubMed] [Google Scholar]
- Li Z, Waadt R, Schroeder JI (2016b) Release of GTP exchange factor mediated down-regulation of abscisic acid signal transduction through ABA-induced rapid degradation of RopGEFs. PLoS Biol 14: e1002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Tang R, Zhang X, Luan S, Yu F (2017) FERONIA receptor kinase at the crossroads of hormone signaling and stress responses. Plant Cell Physiol 58: 1143–1150 [DOI] [PubMed] [Google Scholar]
- Lin D, Cao L, Zhou Z, Zhu L, Ehrhardt D, Yang Z, Fu Y (2013a) Rho GTPase signaling activates microtubule severing to promote microtubule ordering in Arabidopsis. Curr Biol 23: 290–297 [DOI] [PubMed] [Google Scholar]
- Lin W, Ma X, Shan L, He P (2013b) Big roles of small kinases: the complex functions of receptor-like cytoplasmic kinases in plant immunity and development. J Integr Plant Biol 55: 1188–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dong Q, Kita D, Huang JB, Liu G, Wu X, Zhu X, Cheung AY, Wu HM, Tao LZ (2017) RopGEF1 plays a critical role in polar auxin transport in early development. Plant Physiol 175: 157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrinna A, Persson S (2015) Root hair growth: it’s a one way street. F1000Prime Rep 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Simon AA, Tavares B, Wudick MM, Feijó JA (2017) Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol 173: 91–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki KN, Yang Z (2014) Extracellular signals and receptor-like kinases regulating ROP GTPases in plants. Front Plant Sci 5: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K (2001) Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J 20: 2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk AJ, Ruperti B, Singh MK, Dovzhenko A, Ditengou FA, Milia M, Westphal L, Rosahl S, Soellick TR, Uhrig J, et al. (2008) A cysteine-rich receptor-like kinase NCRK and a pathogen-induced protein kinase RBK1 are Rop GTPase interactors. Plant J 53: 909–923 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104: 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha E, Hoefle C, Hückelhoven R, Berken A (2010) RIP3 and AtKinesin-13A: a novel interaction linking Rho proteins of plants to microtubules. Eur J Cell Biol 89: 906–916 [DOI] [PubMed] [Google Scholar]
- Nagano M, Ishikawa T, Fujiwara M, Fukao Y, Kawano Y, Kawai-Yamada M, Shimamoto K (2016) Plasma membrane microdomains are essential for Rac1-RbohB/H-mediated immunity in rice. Plant Cell 28: 1966–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagawa S, Xu T, Yang Z (2010) RHO GTPase in plants: conservation and invention of regulators and effectors. Small GTPases 1: 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Claes AR, Grebe T, Hermkes R, Viotti C, Ikeda Y, Grebe M (2018) Auxin and ROP GTPase signaling of polar nuclear migration in root epidermal hair cells. Plant Physiol 176: 378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibau C, Tao L, Levasseur K, Wu HM, Cheung AY (2013) The Arabidopsis small GTPase AtRAC7/ROP9 is a modulator of auxin and abscisic acid signalling. J Exp Bot 64: 3425–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2012) Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 337: 1333–1336 [DOI] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2014) Emerging roles of small GTPases in secondary cell wall development. Front Plant Sci 5: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Iida Y, Kondo Y, Fukuda H (2010) Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Curr Biol 20: 1197–1202 [DOI] [PubMed] [Google Scholar]
- Pathuri IP, Zellerhoff N, Schaffrath U, Hensel G, Kumlehn J, Kogel KH, Eichmann R, Hückelhoven R (2008) Constitutively activated barley ROPs modulate epidermal cell size, defense reactions and interactions with fungal leaf pathogens. Plant Cell Rep 27: 1877–1887 [DOI] [PubMed] [Google Scholar]
- Pick E. (2014) Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task. Small GTPases 5: e27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra S, Gustavsson A, Kiefer C, Kalmbach L, Hörstedt P, Ikeda Y, Stepanova AN, Alonso JM, Grebe M (2013) Arabidopsis SABRE and CLASP interact to stabilize cell division plane orientation and planar polarity. Nat Commun 4: 2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poraty-Gavra L, Zimmermann P, Haigis S, Bednarek P, Hazak O, Stelmakh OR, Sadot E, Schulze-Lefert P, Gruissem W, Yalovsky S (2013) The Arabidopsis Rho of plants GTPase AtROP6 functions in developmental and pathogen response pathways. Plant Physiol 161: 1172–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Yang Z (2011) Rapid tip growth: insights from pollen tubes. Semin Cell Dev Biol 22: 816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu JL, Jilk R, Marks MD, Szymanski DB (2002) The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell 14: 101–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Zhang X, Song Y, Lin J, Shan X (2017a) THESEUS1 positively modulates plant defense responses against Botrytis cinerea through GUANINE EXCHANGE FACTOR4 signaling. J Integr Plant Biol 59: 797–804 [DOI] [PubMed] [Google Scholar]
- Qu X, Zhang R, Zhang M, Diao M, Xue Y, Huang S (2017b) Organizational innovation of apical actin filaments drives rapid pollen tube growth and turning. Mol Plant 10: 930–947 [DOI] [PubMed] [Google Scholar]
- Reiner T, Hoefle C, Huesmann C, Ménesi D, Fehér A, Hückelhoven R (2015) The Arabidopsis ROP-activated receptor-like cytoplasmic kinase RLCK VI_A3 is involved in control of basal resistance to powdery mildew and trichome branching. Plant Cell Rep 34: 457–468 [DOI] [PubMed] [Google Scholar]
- Ren H, Dang X, Cai X, Yu P, Li Y, Zhang S, Liu M, Chen B, Lin D (2017) Spatio-temporal orientation of microtubules controls conical cell shape in Arabidopsis thaliana petals. PLoS Genet 13: e1006851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Dang X, Yang Y, Huang D, Liu M, Gao X, Lin D (2016) SPIKE1 activates ROP GTPase to modulate petal growth and shape. Plant Physiol 172: 358–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittinger K, Walker PA, Eccleston JF, Smerdon SJ, Gamblin SJ (1997) Structure at 1.65 A of RhoA and its GTPase-activating protein in complex with a transition-state analogue. Nature 389: 758–762 [DOI] [PubMed] [Google Scholar]
- Rivero C, Traubenik S, Zanetti ME, Blanco FA (2017) Small GTPases in plant biotic interactions. Small GTPases June 23: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas AM, Fuentes G, Rausell A, Valencia A (2012) The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol 196: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds CM, Hepler PK, Winship LJ (2014) The apical actin fringe contributes to localized cell wall deposition and polarized growth in the lily pollen tube. Plant Physiol 166: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Ori N, Yalovsky S (2004) Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc Natl Acad Sci USA 101: 7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Krupinski P, Wightman R, Milani P, Berquand A, Boudaoud A, Hamant O, Jönsson H, Meyerowitz EM (2014) Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 3: e01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Höhner K, Berken A, Wittinghofer A (2011) The unique plant RhoGAPs are dimeric and contain a CRIB motif required for affinity and specificity towards cognate small G proteins. Biopolymers 95: 420–433 [DOI] [PubMed] [Google Scholar]
- Schepetilnikov M, Makarian J, Srour O, Geldreich A, Yang Z, Chicher J, Hammann P, Ryabova LA (2017) GTPase ROP2 binds and promotes activation of target of rapamycin, TOR, in response to auxin. EMBO J 36: 886–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss H, Dechert C, Kogel KH, Hückelhoven R (2002) A small GTP-binding host protein is required for entry of powdery mildew fungus into epidermal cells of barley. Plant Physiol 128: 1447–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss H, Hensel G, Imani J, Broeders S, Sonnewald U, Kogel KH, Kumlehn J, Hückelhoven R (2005) Ectopic expression of constitutively activated RACB in barley enhances susceptibility to powdery mildew and abiotic stress. Plant Physiol 139: 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss H, Preuss J, Pircher T, Eichmann R, Hückelhoven R (2008) Barley RIC171 interacts with RACB in planta and supports entry of the powdery mildew fungus. Cell Microbiol 10: 1815–1826 [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, Minami E, Okada K, Yamane H, Kaku H, et al. (2010) Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J 64: 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, et al. (2002) SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature 416: 759–763 [DOI] [PubMed] [Google Scholar]
- Singh MK, Ren F, Giesemann T, Dal Bosco C, Pasternak TP, Blein T, Ruperti B, Schmidt G, Aktories K, Molendijk AJ, et al. (2013) Modification of plant Rac/Rop GTPase signalling using bacterial toxin transgenes. Plant J 73: 314–324 [DOI] [PubMed] [Google Scholar]
- Singh SK, Fischer U, Singh M, Grebe M, Marchant A (2008) Insight into the early steps of root hair formation revealed by the procuste1 cellulose synthase mutant of Arabidopsis thaliana. BMC Plant Biol 8: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek N, Bloch D, Yalovsky S (2009) Protein lipid modifications in signaling and subcellular targeting. Curr Opin Plant Biol 12: 714–720 [DOI] [PubMed] [Google Scholar]
- Sorek N, Gutman O, Bar E, Abu-Abied M, Feng X, Running MP, Lewinsohn E, Ori N, Sadot E, Henis YI, et al. (2011) Differential effects of prenylation and S-acylation on type I and II ROPS membrane interaction and function. Plant Physiol 155: 706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek N, Poraty L, Sternberg H, Buriakovsky E, Bar E, Lewinsohn E, Yalovsky S (2017) Activation status coupled transient S-acylation determines membrane partitioning of a plant Rho-related GTPase. Mol Cell Biol 37: e00333-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek N, Segev O, Gutman O, Bar E, Richter S, Poraty L, Hirsch JA, Henis YI, Lewinsohn E, Jürgens G, et al. (2010) An S-acylation switch of conserved G domain cysteines is required for polarity signaling by ROP GTPases. Curr Biol 20: 914–920 [DOI] [PubMed] [Google Scholar]
- Sørmo CG, Leiros I, Brembu T, Winge P, Os V, Bones AM (2006) The crystal structure of Arabidopsis thaliana RAC7/ROP9: the first RAS superfamily GTPase from the plant kingdom. Phytochemistry 67: 2332–2340 [DOI] [PubMed] [Google Scholar]
- Stanislas T, Hüser A, Barbosa IC, Kiefer CS, Brackmann K, Pietra S, Gustavsson A, Zourelidou M, Schwechheimer C, Grebe M (2015) Arabidopsis D6PK is a lipid domain-dependent mediator of root epidermal planar polarity. Nat Plants 1: 15162. [DOI] [PubMed] [Google Scholar]
- Stegmann M, Monaghan J, Smakowska-Luzan E, Rovenich H, Lehner A, Holton N, Belkhadir Y, Zipfel C (2017) The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355: 287–289 [DOI] [PubMed] [Google Scholar]
- Stephan O, Cottier S, Fahlén S, Montes-Rodriguez A, Sun J, Eklund DM, Klahre U, Kost B (2014) RISAP is a TGN-associated RAC5 effector regulating membrane traffic during polar cell growth in tobacco. Plant Cell 26: 4426–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckle D, Herrmann A, Lipka E, Lauster T, Gavidia R, Zimmermann S, Müller S (2016) Putative RopGAPs impact division plane selection and interact with kinesin-12 POK1. Nat Plants 2: 16120. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Wakazaki M, Toyooka K, Fukuda H, Oda Y (2017) A novel plasma membrane-anchored protein regulates xylem cell-wall deposition through microtubule-dependent lateral inhibition of Rho GTPase domains. Curr Biol 27: 2522–2528.e4 [DOI] [PubMed] [Google Scholar]
- Sun J, Eklund DM, Montes-Rodriguez A, Kost B (2015) In vivo Rac/Rop localization as well as interaction with RhoGAP and RhoGDI in tobacco pollen tubes: analysis by low-level expression of fluorescent fusion proteins and bimolecular fluorescence complementation. Plant J 84: 83–98 [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531: 245–248 [DOI] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Wu HM (2002) Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14: 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Berken A (2010) Structure and function of ROPs and their GEFs. In Yalovsky S, Baluska F, Jones AM, eds, Integrated G Protein Signaling in Plants. Springer-Verlag, Berlin, pp 49–68 [Google Scholar]
- Thomas C, Fricke I, Scrima A, Berken A, Wittinghofer A (2007) Structural evidence for a common intermediate in small G protein-GEF reactions. Mol Cell 25: 141–149 [DOI] [PubMed] [Google Scholar]
- Thomas C, Fricke I, Weyand M, Berken A (2009) 3D structure of a binary ROP-PRONE complex: the final intermediate for a complete set of molecular snapshots of the RopGEF reaction. Biol Chem 390: 427–435 [DOI] [PubMed] [Google Scholar]
- Uhrig JF, Mutondo M, Zimmermann I, Deeks MJ, Machesky LM, Thomas P, Uhrig S, Rambke C, Hussey PJ, Hulskamp M (2007) The role of Arabidopsis SCAR genes in ARP2-ARP3-dependent cell morphogenesis. Development 134: 967–977 [DOI] [PubMed] [Google Scholar]
- Venus Y, Oelmüller R (2013) Arabidopsis ROP1 and ROP6 influence germination time, root morphology, the formation of F-actin bundles, and symbiotic fungal interactions. Mol Plant 6: 872–886 [DOI] [PubMed] [Google Scholar]
- Vernoud V, Horton AC, Yang Z, Nielsen E (2003) Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol 131: 1191–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions. Science 294: 1299–1304 [DOI] [PubMed] [Google Scholar]
- Voxeur A, Höfte H (2016) Cell wall integrity signaling in plants: “to grow or not to grow that’s the question.” Glycobiology 26: 950–960 [DOI] [PubMed] [Google Scholar]
- Wang C, Xu X, Hong Z, Feng Y, Zhang Z (2015) Involvement of ROP6 and clathrin in nodulation factor signaling. Plant Signal Behav 10: e1033127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liang L, Xue Y, Jia PF, Chen W, Zhang MX, Wang YC, Li HJ, Yang WC (2016) A receptor heteromer mediates the male perception of female attractants in plants. Nature 531: 241–244 [DOI] [PubMed] [Google Scholar]
- Wang W, Liu Z, Bao LJ, Zhang SS, Zhang CG, Li X, Li HX, Zhang XL, Bones AM, Yang ZB, et al. (2017) The RopGEF2-ROP7/ROP2 pathway activated by phyB suppresses red light-induced stomatal opening. Plant Physiol 174: 717–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge P, Brembu T, Kristensen R, Bones AM (2000) Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics 156: 1959–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, et al. (2007) Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 19: 4022–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods B, Lew DJ (2017) Polarity establishment by Cdc42: key roles for positive feedback and differential mobility. Small GTPases Mar 28: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Li H, Yang Z (2000) Arabidopsis RopGAPs are a novel family of rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for rop-specific GTPase stimulation. Plant Physiol 124: 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Hazak O, Cheung AY, Yalovsky S (2011) RAC/ROP GTPases and auxin signaling. Plant Cell 23: 1208–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Zhao Y, Zheng ZL (2005) Transcriptome analysis reveals specific modulation of abscisic acid signaling by ROP10 small GTPase in Arabidopsis. Plant Physiol 139: 1350–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Bloch D, Sorek N, Kost B (2008) Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol 147: 1527–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Imai K, Akamatsu A, Mihashi M, Hayashi N, Shimamoto K, Kawasaki T (2012) SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant J 70: 389–397 [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Desyatova AS, Belteton SA, Mallery EL, Turner JA, Szymanski DB (2015) Patterning mechanisms of cytoskeletal and cell wall systems during leaf trichome morphogenesis. Nat Plants 1: 15014. [DOI] [PubMed] [Google Scholar]
- Yang Z. (2008) Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol 24: 551–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Qian L, Nibau C, Duan Q, Kita D, Levasseur K, Li X, Lu C, Li H, Hou C, et al. (2012) FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc Natl Acad Sci USA 109: 14693–14698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermiani M, Zonin E, Nonis A, Begheldo M, Ceccato L, Vezzaro A, Baldan B, Trentin A, Masi A, Pegoraro M, et al. (2015) Ethylene negatively regulates transcript abundance of ROP-GAP rheostat-encoding genes and affects apoplastic reactive oxygen species homeostasis in epicarps of cold stored apple fruits. J Exp Bot 66: 7255–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yang G, Chen Y, Zhao Y, Gao P, Liu B, Wang H, Zheng ZL (2016) C-terminal domain (CTD) phosphatase links Rho GTPase signaling to Pol II CTD phosphorylation in Arabidopsis and yeast. Proc Natl Acad Sci USA 113: E8197–E8206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McCormick S (2007) A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 18830–18835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Wu Y, He Y, Wang Y, Xiao J, Li L, Wang Y, Chen X, Xiong W, Wu Y (2015) RopGEF2 is involved in ABA-suppression of seed germination and post-germination growth of Arabidopsis. Plant J 84: 886–899 [DOI] [PubMed] [Google Scholar]
- Zheng ZL, Nafisi M, Tam A, Li H, Crowell DN, Chary SN, Schroeder JI, Shen J, Yang Z (2002) Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 14: 2787–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZL, Yang Z (2000a) The Rop GTPase: an emerging signaling switch in plants. Plant Mol Biol 44: 1–9 [DOI] [PubMed] [Google Scholar]
- Zheng ZL, Yang Z (2000b) The Rop GTPase switch turns on polar growth in pollen. Trends Plant Sci 5: 298–303 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Shi H, Chen B, Zhang R, Huang S, Fu Y (2015) Arabidopsis RIC1 severs actin filaments at the apex to regulate pollen tube growth. Plant Cell 27: 1140–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zourelidou M, Absmanner B, Weller B, Barbosa IC, Willige BC, Fastner A, Streit V, Port SA, Colcombet J, de la Fuente van Bentem S, et al. (2014) Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. efe 3: 02860. [DOI] [PMC free article] [PubMed] [Google Scholar]