Abstract
Oil bodies have multiple functions: oleosin-mediated freezing tolerance of seeds, direct interaction with glyoxysomes for lipid degradation in seedlings, and antifungal compound production in leaves.
Oil bodies are lipid (mainly triacylglycerols) storage compartments that occur primarily in seeds and senescing leaves. Seed oil bodies develop from the endoplasmic reticulum in embryo cells during seed maturation and are degraded during seed germination and subsequent seedling growth. Triacylglycerols are the major oils used for energy and metabolic substrates during seedling growth. Oil bodies also function as subcellular factories that produce antifungal compounds in senescing leaves and perilesional leaf areas infected with fungi. Here, we discuss plant oil body functions by focusing on three dynamic issues: size regulation of oil bodies in seeds, physical interconnections between oil bodies and glyoxysomes in seedlings, and the oil body defense system in senescing leaves.
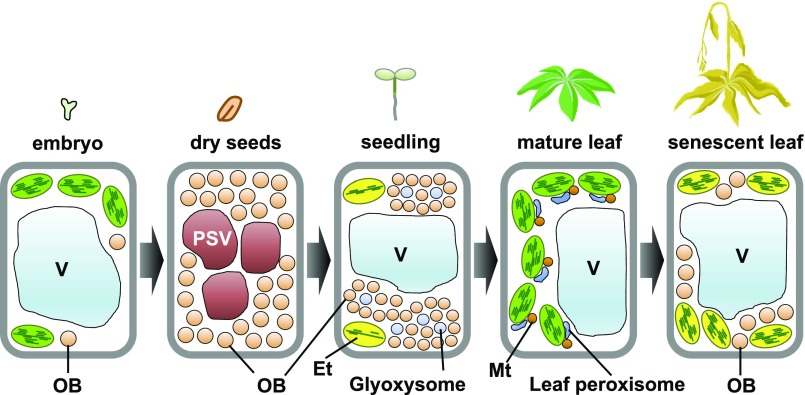
Oil bodies are actively biosynthesized in seed embryo cells and accumulate in dry seed cells (Fig. 1). In germinating seeds, oil bodies are degraded rapidly by glyoxysomes, which are unique peroxisomes for fatty acid β-oxidation and the glyoxylate cycle (Fig. 1; Hayashi et al., 2000; Graham, 2008). Storage lipids are degraded in oil bodies to obtain energy and carbon. Mature leaves contain few oil bodies, whereas senescent leaves contain numerous oil bodies at different stages of development (Fig. 1; Shimada et al., 2015). Environmental conditions such as dark and extreme temperature (heat and cold) induce oil body formation (Gidda et al., 2016), suggesting that leaf oil bodies function in stress responses.
Figure 1.
Schematic view of oil body (OB) formation and degradation during plant development. Oil bodies form in embryos and are abundant in dry seeds. During seed germination and subsequent seedling growth, glyoxysomal enzymes degrade oil bodies to release stored energy. Mature leaves contain few oil bodies, whereas oil bodies accumulate in senescent leaves. Et, Etioplast; Mt, mitochondria; PSV, protein storage vacuole; V, vacuole.
ABUNDANT OIL BODIES IN SEED CELLS: DYNAMIC FUNCTIONS OF MEMBRANE PROTEINS
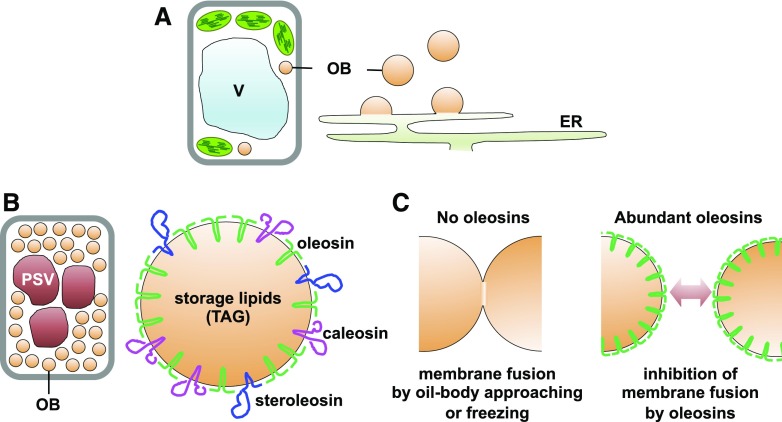
Oil body formation begins on the endoplasmic reticulum (ER) membrane (Fig. 2A; Chapman et al., 2012). Storage lipids are produced by lipid biosynthetic enzymes on the ER and then stored in the space between two leaflets of the ER phospholipid bilayer (Yen et al., 2008). Oil bodies bud off from the ER surface as a phospholipid monolayer (Fig. 2A). Triacylglycerols are a class of storage lipids used as an energy source during seed germination and seedling growth. Seed cells contain large triacylglycerol reserves in oil bodies. The seed oil body diameter is typically 0.5 to 2 µm (Tzen et al., 1993; Shimada et al., 2008). Seeds contain three oil body protein families: oleosins, caleosins, and steroleosins (Fig. 2B; Tzen et al., 1990; Chen et al., 1999; Lin et al., 2002; Shimada and Hara-Nishimura, 2010). Oleosins are the most abundant oil body proteins, with molecular masses between 15 and 30 kD (Tzen et al., 1990; Jolivet et al., 2004). Caleosins and steroleosins have peroxygenase and β-hydroxysteroid dehydrogenase activities, respectively (Lin et al., 2002; Hanano et al., 2006). Oleosins are targeted first to the ER membrane and then move to immature oil bodies fused to the ER; they have greater stability on the oil body membrane than the ER membrane (Abell et al., 1997, 2002, 2004). Finally, oil bodies containing oleosin proteins are separated from the ER.
Figure 2.
Specific proteins accumulate on the oil body (OB) membrane surface, and oleosins prevent oil bodies from fusing with each other in seeds. A, Oil bodies bud off from the ER. B, Seed oil bodies contain three membrane proteins: oleosin, caleosin, and steroleosin. Oleosins are the most abundant oil body membrane protein. C, Oleosins function to inhibit oil body fusion. Oleosin deficiency causes oil body fusion (left), whereas high expression of oleosin prevents oil body fusion (right). TAG, Triacylglycerol; V, vacuole.
The Arabidopsis (Arabidopsis thaliana) genome contains 16 oleosin genes, including five seed-type oleosin genes (Kim et al., 2002). OLE1 is the most abundant oleosin in Arabidopsis seeds, followed by OLE2 (Shimada et al., 2008). Seeds of oleosin single mutants (ole1 and ole2) contain larger oil bodies than those of the wild type (Siloto et al., 2006; Shimada et al., 2008), and seeds of an oleosin double mutant (ole1 ole2) contain larger oil bodies than those of ole1 and ole2 single mutants (Shimada et al., 2008). Oleosin knockdown also causes larger oil bodies in soybean (Glycine max) and rice (Oryza sativa; Schmidt and Herman, 2008; Wu et al., 2010). These observations suggest that a reduction in oleosin content increases the oil body diameter, because a steric hindrance of oleosins on the oil body surface inhibits oil body fusion (Fig. 2C; Tzen and Huang, 1992). The ole1 ole2 double mutant has significantly lower oleosin content on the oil body surface than the wild type. Oil bodies with less abundant oleosins are easily fused to each other to form irregularly shaped oil structures in seed cells of the ole1 ole2 double mutant (Shimada et al., 2008). Oil bodies in ole1 or ole2 single mutant seeds are spherically shaped with larger diameters than those in the wild type (Shimada et al., 2008). These combined results suggest that oil body fusion is partially inhibited in single mutants, because they still produce residual levels of oleosins, unlike the double mutants.
Oil body development is critical for seed germination. Seeds of the ole1 ole2 double mutant exhibit severely defective germination (Shimada et al., 2008), suggesting that oleosins are essential for normal germination. Seeds of ole1 and ole2 single mutants germinate normally, although they have enlarged oil bodies (Siloto et al., 2006; Shimada et al., 2008). These combined results suggest that the abnormally enlarged oil bodies of ole1 ole2 seeds interfere with normal germination. Seeds of the ole1 and ole2 single mutants are sensitive to freezing stress (−30°C), whereas wild-type seeds are resistant to freezing stress (Shimada et al., 2008). Freezing stress promotes additional oil body fusion in ole1 seeds, which results in irregularly enlarged oil bodies and defective germination (Shimada et al., 2008). Higher oleosin levels in wild-type seeds inhibit freezing stress-induced oil body fusion (Fig. 2C). Therefore, oleosins function to enhance plant survival during winter.
Oil body sizes are essentially uniform in wild-type seeds (Tzen et al., 1993; Shimada et al., 2008). Preliminary data suggest that oleosins might be involved in oil body size regulation. We propose two hypotheses for the regulation of oil body size. (1) Oil bodies remain connected to the ER until their diameter reaches approximately 1 µm. During this connection, storage lipids are biosynthesized and oleosins accumulate on oil body membranes. Then, oleosins are involved in separating oil bodies from the ER when the diameter reaches approximately 1 µm. (2) Oil bodies are separated from the ER when their diameter is small and become cytosolic oil bodies. Then, cytosolic oil bodies undergo fusion to enlarge. Oleosins accumulate on the oil body surface during fusion. When oleosins become abundant on the surface, oil body fusion is inhibited. In ole1 seeds, freezing stress enhances the fusion of cytosolic oil bodies (Shimada et al., 2008). This result suggests that oil body fusion occurs when the oleosin content on the oil body surface is low. The ER proteins SEIPINs control oil body number and size (Cai et al., 2015), suggesting that these factors might be involved in oil body formation on the ER membrane.
Oil bodies, PSVs, nuclei, and other organelles have an orderly distribution pattern in wild-type seed cells. PSVs and nuclei are located in the cell center and surrounded by oil bodies. This localization might promote the rapid degradation and utilization of storage lipids and proteins to support seed germination. Oleosin-deficient mutants have enlarged oil bodies, which disrupt the normal localization pattern of PSVs and the nucleus (Shimada et al., 2008). PSVs are localized throughout the entire cytoplasm in seeds of ole1, ole2, and ole1 ole2 mutants (Shimada et al., 2008), suggesting that oil body enlargement affects PSV distribution. The irregularly enlarged oil bodies in ole1 ole2 seeds also disrupt nuclear shape and localization; the nucleus in these seed cells is compressed against the cell periphery (Shimada et al., 2008). This may result from the enlarged oil bodies displacing PSVs and the nucleus to a peripheral region. The irregular nuclei in ole1 ole2 seeds contribute to their observed germination defects (Shimada et al., 2008). These combined results indicate that oil body size significantly affects organelle distribution in seeds.
Several transcription factors (TFs) control storage lipid biosynthesis during seed maturation (Santos-Mendoza et al., 2008; Baud and Lepiniec, 2010), with LEAFY COTYLEDON2 (LEC2) as the primary TF (Santos Mendoza et al., 2005). LEC2 targets the downstream WRINKLED1 TF to up-regulate storage lipid biosynthesis (Cernac and Benning, 2004; Baud et al., 2007, 2009). LEC2 overexpression enhances oleosin expression and triacylglycerol accumulation (Stone et al., 2008; Che et al., 2009), suggesting that LEC2 essentially activates oil body production. LEC2 also promotes storage protein accumulation during seed maturation (Verdier and Thompson, 2008), and ectopic expression of LEC2 induces the expression of storage proteins and oleosins (Stone et al., 2001, 2008). These combined results suggest that LEC2 promotes seed maturation through the production of abundant storage lipids and proteins.
PHYSICAL INTERACTION BETWEEN OIL BODIES AND GLYOXYSOMES IN HETEROTROPHIC SEEDLINGS
Oil bodies in developing embryos and dry seed accumulate storage lipid triacylglycerols produced from photoassimilates. Therefore, we can consider oil bodies as sink organelles. During seed imbibition and germination, the physiological function of oil bodies changes dramatically from a sink to a source organelle. As a source organelle, oil bodies catabolize triacylglycerol into Suc, which provides an energy source to support heterotrophic seedling growth until the start of photosynthesis.
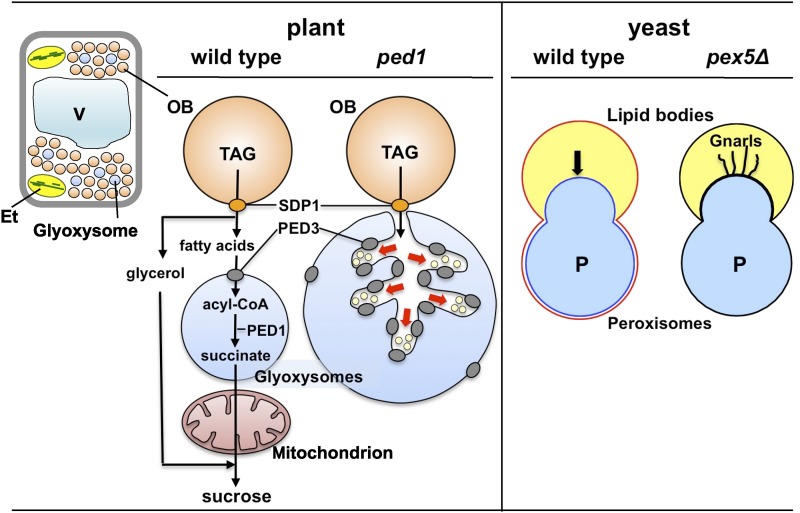
This unique gluconeogenesis is initiated by triacylglycerol hydrolysis into glycerol and free fatty acids (Fig. 3, plant wild type). The first step is initiated by a specific triacylglycerol lipase called SDP1, which hydrolyzes triacylglycerol into diacylglycerol and one fatty acid (Eastmond, 2006; Kelly et al., 2011). Complete liberation of two fatty acids from the diacylglycerol moiety requires diacylglycerol lipase and monoacylglycerol lipase. The monoacylglycerol lipase was identified recently as MAGL8 (Kim et al., 2016b). The diacylglycerol lipase remains to be identified. Finally, glycerol and fatty acids are converted into Suc by independent metabolic pathways (Hayashi and Nishimura, 2003; Quettier et al., 2008). The conversion of fatty acids into Suc has been studied extensively, because these are the main sources of Suc in germinating seeds. These studies have determined that acyl-CoA, an activated form of fatty acid, is metabolized to Suc by the cooperative action of enzymes in glyoxysomes, mitochondria, and cytosol (Baker and Graham, 2002; Hayashi and Nishimura, 2006; Theodoulou and Eastmond, 2012). There is still some debate regarding the first steps in triacylglycerol catabolism. Recent cytological studies suggest that the pathway is regulated not only by enzymatic reactions but also by physical interactions between oil bodies and glyoxysomes. Here, we discuss the relationship between triacylglycerol catabolism and oil body-glyoxysome interactions.
Figure 3.
The ped1 mutant reveals communication between oil bodies (OB) and glyoxysomes. Triacylglycerol (TAG) stored in oil bodies is catabolized during seedling heterotrophic growth. TAG is hydrolyzed by triacylglycerol lipase (SDP1) to produce glycerol and free fatty acids, which are ultimately converted into Suc. Suc is metabolized further to support seedling growth. Free fatty acids are imported into glyoxysomes by PED3, which is localized on the glyoxysomal membrane, and are subsequently activated to acyl-CoA forms. Then, acyl-CoA is converted to succinate in the glyoxysome via fatty acid β-oxidation and the glyoxylate cycle. PED1 is a 3-ketoacyl-CoA thiolase that catalyzes the last step of fatty acid β-oxidation. Succinate is converted into Suc by successive enzymatic reactions in mitochondria and the cytosol. Aberrantly enlarged glyoxysome morphologies are observed in ped1 seedlings. Dendritic tubules are formed by glyoxysomal membrane invaginations at the sites of oil body-glyoxysome contact. These tubules encompass small vesicles that may be derived from the oil body (red arrows). PED3 is significantly more enriched on the dendritic tubule membrane than on the outer glyoxysomal membrane. In yeast, peroxisomes invade and stably fuse with the lipid body. The lipid body monolayer membrane fuses with the peroxisomal membrane outer leaflet to form a single outer leaflet (red line), whereas the peroxisomal membrane inner leaflet still surrounds the peroxisomal matrix (blue line). Pexopodia invade the lipid body (arrow). In the yeast pex5Δ mutant, the lipid body often forms elongated, curled, and tangled tubules called gnarls. Et, Etioplast; V, vacuole.
SDP1 and MAGL8 are oil body membrane proteins. Therefore, all fatty acid molecules may be cleaved from triacylglycerol at the outer surface of the oil body membrane and subsequently imported into glyoxysomes for further catabolism. The glyoxysome is a functionally differentiated type of peroxisome that converts acyl-CoA into succinate via fatty acid β-oxidation and the glyoxylate cycle. The glyoxysomal membrane contains PED3 and long-chain acyl-CoA synthetase. PED3 (also known as CTS and PXA1) is a member of the full-size ABC transporter family, which transports substrates across membranes in an ATP-dependent reaction (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002b). Acyl-CoA synthetases such as Arabidopsis LACS6 and LACS7 activate fatty acids into acyl-CoA forms (Fulda et al., 2002; Hayashi et al., 2002a). These combined results suggest that fatty acids liberated at the oil body surface are captured and transported into the glyoxysome by CTS/PED3/PXA1 and then converted into acyl-CoA forms by the action of LACS6/7 at the inner surface of the glyoxysomal membrane. By contrast, some studies proposed a more complex model for fatty acid import into glyoxysomes, suggesting that PED3 does not import fatty acids but does import acyl-CoAs (Baker et al., 2015). In this model, fatty acids are first activated to acyl-CoAs in the cytosol. Then, acyl-CoAs are transported into glyoxysomes by CTS/PED3/PXA1. After import, acyl-CoAs are converted back into fatty acids by cleaving the CoA moiety; however, the fatty acids must be reactivated subsequently by LACS6/7 to acyl-CoA substrates for fatty acid β-oxidation. This model was based on results obtained by complementation analysis of yeast mutants with plant enzymes. However, yeast peroxisomes contain a heterodimer of the two half-size ABC transporters PXA1 and PXA2 as the counterparts of the full-size ABC transporter CTS/PED3/PXA1 in plant glyoxysomes. It is possible that fatty acid import into plant glyoxysomes differs from that into yeast peroxisomes.
Recent cytological analyses report remarkable differences between plant and yeast. Yeast cells contain lipid bodies and peroxisomes, which are equivalent to oil bodies and glyoxysomes in plant cells. In oleic acid-cultured Saccharomyces cerevisiae, lipid bodies stably adhere to peroxisomes by fusing their monolayer (half-unit) membrane to the outer leaflet of the peroxisomal bilayer (unit) membrane (Fig. 3, yeast wild type; Binns et al., 2006). The peroxisomal membrane inner leaflet forms protrusions called pexopodia, which extrude into the lipid body. Pexopodia are believed to be the site of fatty acid transfer across the two organelles. The yeast pex5Δ mutant has reduced fatty acid catabolism due to a defect in the peroxisomal protein import machinery and lipid bodies with aberrant morphologies (Fig. 3, yeast pex5Δ; Binns et al., 2006) and novel structures called gnarls, which may be formed due to the accumulation of free fatty acids. By contrast, several lines of evidence indicate that oil bodies and glyoxysomes in plant cells interact via a different mechanism. Arabidopsis PED1 encodes 3-ketoacyl-CoA thiolase, which catalyzes the last step of fatty acid β-oxidation (Fig. 3, plant; Hayashi et al., 1998). The ped1 mutant contains glyoxysomes with diameters up to 3 times larger than those in wild-type plants (Fig. 3, plant ped1). The enlarged glyoxysomes contain dendritic tubules, which are formed by glyoxysomal membrane invagination at oil body-glyoxysome contact sites (Hayashi et al., 2001). Unlike lipid bodies and peroxisomes in yeast, oil bodies and glyoxysomes in plants do not fuse with each other. The dendritic tubules encompass small vesicles that may originate from the oil body. Peroxisomal membrane invagination and vesicle formation originating from the lipid body have not been observed in the yeast pex5Δ mutant. Fatty acids may be transported from oil bodies to glyoxysomes via small vesicles originating from the oil body, rather than free diffusion through the cytosol. In support of this hypothesis, PED3 is enriched significantly on the dendritic tubule membrane where the vesicles may dock (Cui et al., 2016). A recent study observed the retrograde flow of membranous structures from glyoxysomes to oil bodies in plant cells (Thazar-Poulot et al., 2015). An analysis of GFP-SDP1 trafficking dynamics revealed that the SDP1 fusion protein first localized to the glyoxysomal membrane during early stages of seedling growth and then moved to tubular protrusions extending from the glyoxysome. The tubule eventually enwrapped the oil body, thereby facilitating GFP-SDP1 translocation. These observations reveal the importance of membrane dynamics for triacylglycerol catabolism. The mechanism underlying fatty acid import into glyoxysomes needs to be reconsidered from the viewpoint of membrane dynamics.
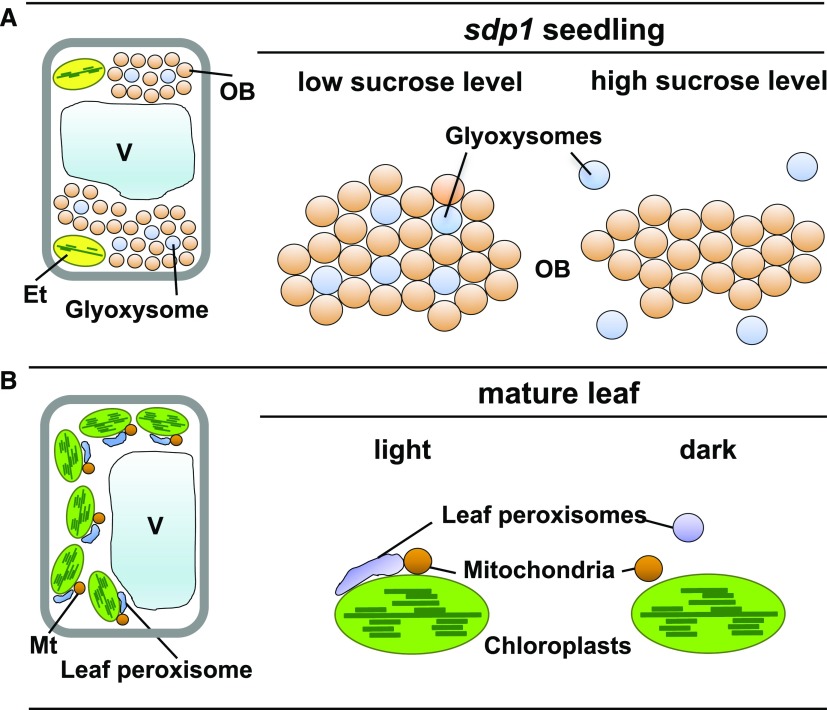
Cytological observations of the Arabidopsis specific triacylglycerol lipase (sdp1) mutant revealed that the close proximity of oil bodies and glyoxysomes is strictly regulated by the intracellular Suc concentration, which is an end product of triacylglycerol catabolism (Cui et al., 2016). Arabidopsis cells contain substantial oil body aggregates during early seedling growth. The sdp1 mutant has defective triacylglycerol catabolism, extremely low endogenous Suc concentrations, and cellular aggregates of oil bodies tethered physically to glyoxysomes (Fig. 4, sdp1 seedling, low Suc level). When the sdp1 mutant is supplied with exogenous Suc, glyoxysomes are not localized within the cellular aggregates (Fig. 4, sdp1 seedling, high Suc level) and triacylglycerol catabolism is attenuated. These combined results suggest that Suc negatively regulates triacylglycerol catabolism to prevent excess Suc production, possibly by dissociating glyoxysomes from oil bodies.
Figure 4.
Glyoxysomes and leaf peroxisomes differentially associate and disassociate with other organelles under specific conditions. A, Oil body (OB)-glyoxysome interactions in sdp1 mutant seedlings at the heterotrophic growth stage. When sdp1 seedlings are grown without Suc supplementation, many oil bodies and glyoxysomes associate physically with each other to form a mixed aggregation. By contrast, glyoxysomes dissociate from the aggregates when sdp1 seedlings are grown in the presence of exogenously supplied Suc. B, Interactions between leaf peroxisomes, chloroplasts, and mitochondria (Mt) in photosynthetic cells of mature leaves. Under light conditions (light), elongated leaf peroxisomes associate with chloroplasts and mitochondria. Under dark conditions (dark), leaf peroxisomes become spherical and dissociate from chloroplasts. Et, Etioplast; V, vacuole.
The dissociation of glyoxysomes and oil bodies in the developing seedling is physiologically important for autotrophic growth, which begins with the onset of photosynthesis. The pool of glyoxysomal enzymes is exchanged with leaf peroxisomal enzymes during seedling greening, which ultimately converts glyoxysomes into leaf peroxisomes (Hayashi and Nishimura, 2006). The activities in leaf peroxisomes, chloroplasts, and mitochondria are functionally coordinated to generate the photorespiratory pathway in photosynthetic cells (Titus and Becker, 1985; Nishimura et al., 1986). In situ femtosecond laser-induced impulsive force analyses indicate that these organelles establish physical contacts during photorespiration under light conditions (Fig. 4, mature leaf, light), whereas they are separated under dark conditions (Fig. 4, mature leaf, dark; Oikawa et al., 2015). Leaf peroxisomes are tethered to chloroplasts through a specialized membrane microdomain, a peroxisomal protrusion called a peroxule (Gao et al., 2016). After functional conversion of glyoxysomes into leaf peroxisomes, the organelle dissociates from oil bodies and changes its tethering partner(s) to chloroplasts and mitochondria. These studies reveal the importance of physical connections among plant organelles for establishing complex peroxisome-related metabolic pathways. The emerging evidence from different organisms suggests that peroxisomes are highly flexible in coordinating and communicating with other organelles (Shai et al., 2016), although the detailed mechanisms have not been determined. Future studies of these mechanisms will provide new insights into how plant cells regulate triacylglycerol catabolism at the organelle level during seedling growth.
PRODUCTION OF DEFENSE COMPOUNDS ON LEAF OIL BODIES
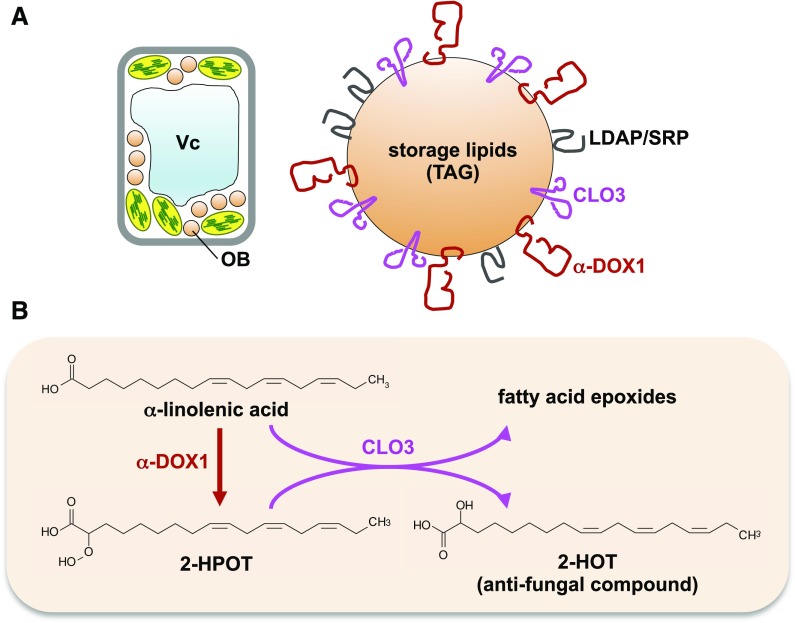
Oil bodies are rarely observed in mature young leaves, but they are enriched in senescent leaves (Fig. 1; Shimada et al., 2015). Leaf oil bodies contain the following proteins: caleosins, α-dioxygenase, and lipid droplet-associated proteins (LDAPs; Fig. 5A; Shimada et al., 2014; Gidda et al., 2016). Genes involved in oleosin biosynthesis are not expressed in leaves (Kim et al., 2002), suggesting that leaf oil body components differ from those of seed oil bodies. Consequently, leaf oil bodies may have different functions than seed oil bodies.
Figure 5.
Leaf oil body (OB) proteins and their functions. A, Oil bodies in senescent leaves contain CLO3, α-DOX1, and LDAP/SRPP proteins. B, CLO3 and α-DOX1 cooperatively biosynthesize the antifungal compound 2-HOT on the oil body membrane. 2-HPOT, 2-Hydroperoxy-octadecatrienoic acid; TAG, triacylglycerol; Vc, vacuole.
Seed oil bodies also contain caleosins (Chen et al., 1999). Caleosin proteins have one EF-hand calcium-binding domain (Naested et al., 2000) and peroxygenase activity, which produces hydroxyl fatty acids (oxylipins; Hanano et al., 2006). Eight caleosin genes have been identified in Arabidopsis (Hanano et al., 2006). CALEOSIN1 (CLO1) and CLO2 are expressed in seeds, whereas CLO3/RESPONSIVE TO DESSICATION20/PEROXYGENASE3 and CLO4 are expressed in leaves (Shen et al., 2014). CLO3 expression is stimulated by stress-responsive pathways induced by salt, drought, abscisic acid, and jasmonic acid (Takahashi et al., 2000). CLO3 also accumulates in response to infection by the pathogenic fungus Colletotrichum higginsianum (Shimada et al., 2014). CLO3 is localized on oil bodies in senescent leaves, and in leaves infected with C. higginsianum (Shimada et al., 2014), confirming that CLO3 is a leaf oil body protein (Fig. 5A).
Proteomics analyses of leaf oil bodies indicate that CLO3 function is correlated strongly with that of another leaf oil body protein, α-dioxygenase1 (α-DOX1; Shimada et al., 2014), and α-DOX1 and CLO3 have similar localization and expression patterns (Shimada et al., 2014). C. higginsianum infection induces α-DOX1 expression and localization on leaf oil bodies (Shimada et al., 2014), indicating that α-DOX1 is a leaf oil body protein (Fig. 5A). The catabolism of α-linolenic acid proceeds via α-DOX1 to produce 2-hydroperoxy-octadecatrienoic acid, which is an unstable compound (Fig. 5B; Hamberg et al., 1999). Then, CLO3 converts 2-hydroperoxy-octadecatrienoic acid to 2-hydroxy-octadecatrienoic acid (2-HOT; Fig. 5B; Shimada et al., 2014; Shimada and Hara-Nishimura, 2015). These reactions may occur cooperatively and rapidly on leaf oil bodies. 2-HOT has antifungal activity against Colletotrichum spp. and is induced by C. higginsianum infection and senescence (Shimada et al., 2014). These results suggest that leaf oil bodies containing CLO3 and α-DOX1 efficiently produce the antifungal compound 2-HOT. Thus, leaf oil bodies may function as an intracellular factory to produce lipid compounds for plant defense responses.
CLO4 is considered as a leaf oil body protein because CLO4 mRNA is expressed in leaves and CLO4 has been localized on oil bodies in transient expression assays of CLO4 (Kim et al., 2011). Physiological analyses of the clo4 mutant indicate that CLO4 is involved in seed germination, abscisic acid pathways, and stomatal closure (Kim et al., 2011). CLO4 also has peroxygenase activity, which produces hydroxyl fatty acids (Blée et al., 2012). These combined results suggest that caleosins and their products function in stress response pathways. Proteomics analyses of leaf oil bodies detect abundant levels of CLO3 and α-DOX1 but not CLO4 (Shimada et al., 2014; Brocard et al., 2017), suggesting that leaf oil bodies containing CLO3 and α-DOX1 differ from those containing CLO4. Leaf oil bodies containing CLO3 and α-DOX1 are induced in senescent leaves (Shimada et al., 2014), whereas the developmental pattern of leaf oil bodies containing CLO4 is unknown. Analysis of the clo4 mutant (Kim et al., 2011) suggests that leaf oil bodies containing CLO4 may function in abscisic acid responses during seed germination and stomatal closure.
The Arabidopsis α-DOX1 is a pathogen-induced α-dioxygenase (Hamberg et al., 1999; Shimada et al., 2014). Arabidopsis T-DNA insertion mutants of α-DOX1 are susceptible to green peach aphid (Myzus persicae; Avila et al., 2013). In pepper (Capsicum annuum), virus-induced gene silencing of α-dioxygenase expression confers susceptibility to the bacterial pathogen Xanthomonas axonopodis and suppression of the hypersensitive response (Hong et al., 2017). These results suggest that an α-dioxygenase-mediated oxylipin pathway is involved in plant defense against aphids and bacteria. A deficiency in α-dioxygenase causes defective growth in tomato (Solanum lycopersicum), Nicotiana attenuata, and pepper (Bannenberg et al., 2009; Steppuhn et al., 2010; Hong et al., 2017). By contrast, the Arabidopsis α-dox1 α-dox2 double mutant does not exhibit growth defects (Bannenberg et al., 2009). These combined results suggest that α-dioxygenases contribute differentially to plant growth in different species. In Arabidopsis, α-DOX1 deficiency reduces the 2-HOT content, whereas α-DOX2 deficiency does not significantly affect 2-HOT content (Shimada et al., 2014). The localization of α-DOX1 on leaf oil bodies may be crucial for 2-HOT production, as α-DOX2 is localized on the ER (Shimada et al., 2014). The subcellular localization of α-dioxygenases may determine differences in the effects of α-dioxygenase deficiency in different plant species.
LDAPs have been identified as oil body proteins in avocado (Persea americana) mesocarp (Horn et al., 2013). Avocado LDAPs exhibit homology to small rubber particle proteins (SRPPs) of Pará rubber tree (Hevea brasiliensis; Oh et al., 1999). SRPPs are localized on the rubber particle membrane in rubber-accumulating plants (Berthelot et al., 2014). Rubber particles are surrounded by a phospholipid monolayer and store polyisoprene (Yamashita et al., 2016). The structure of rubber particles is similar to that of oil bodies. The Arabidopsis genome contains three LDAP/SRPP genes: LDAP1/SRP1, LDAP2/SRP2, and LDAP3/SRP3 (Gidda et al., 2016; Kim et al., 2016a). Proteomic analyses of leaf oil bodies in senescent Arabidopsis leaves indicate that LDAP1 is localized on oil bodies (Fig. 5A; Brocard et al., 2017). LDAP overexpression increases the abundance of dark-induced leaf oil bodies, whereas LDAP deficiency reduces their abundance (Gidda et al., 2016). Oil bodies in LDAP-overexpressing seedling hypocotyls are larger than those in wild-type seedlings, and LDAP-overexpressing plants have longer roots and are more resistant to drought stress than wild-type plants (Kim et al., 2016a). These combined results suggest that LDAPs enhance oil body formation, lipid biosynthesis, plant growth, and drought resistance. Yeast two-hybrid analysis indicates that H. brasiliensis LDAP/SRPP binds rubber elongation factor (REF; Yamashita et al., 2016). H. brasiliensis cis-prenyltransferases (HRTs) interact with HRT1-REF bridging protein, which binds REF and HRT for efficient rubber biosynthesis (Yamashita et al., 2016). LDAPs may recruit a complex for lipid biosynthesis, although they do not interact with seed oil bodies containing oleosins (Gidda et al., 2016), suggesting that LDAPs are antagonistic to oleosins in oil body targeting. LDAP is a unique protein involved in the physiological function of leaf oil bodies. A recent study shows that LDAP-interacting protein (LDIP) also localizes to oil bodies and regulates oil body size in Arabidopsis seeds and leaves (Pyc et al., 2017). LDAP and LDIP may coordinately maintain oil body formation.
CONCLUDING REMARKS
Oil bodies are surrounded by a monolayer of phospholipids and associated membrane proteins. Seed cells contain abundant oil bodies, and the most abundant oil body membrane protein oleosin prevents the homotypic fusion of oil bodies with each other and controls oil body size. Oleosin-dependent size regulation confers freezing tolerance for oil seeds and enables them to survive during the winter season. Oleosin also is required for the normal distribution of other organelles, including PSVs and nuclei in seed cells. It remains to be determined how oil body development controls organellar distribution in embryonic seed cells (see Outstanding Questions). In seedlings, physical interactions between oil bodies and glyoxysomes trigger the degradation of oil body lipids; these interactions are suppressed by Suc, which is a product of lipid catabolism. The molecular mechanism underlying the physical interaction of oil bodies and glyoxysomes remains to be determined (see Outstanding Questions). Although oil bodies are rare in young leaves, they are observed in senescing leaves and in the perilesional area of leaves infected with fungi. However, the physiological roles of leaf oil bodies are not fully understood. One function of leaf oil bodies is as a subcellular factory producing antifungal oxylipins for defense. Senescent leaves result in leaf litter enriched in antifungal compounds, which could protect seedlings from fungal infection. Leaf oil bodies also may function to provide bioactive secondary compounds. To further elucidate the roles of leaf oil bodies, it will be crucial to identify lipids other than triacylglycerols that are stored in these important sink organelles (see Outstanding Questions).
Footnotes
This work was supported by Grants-in-Aid for Scientific Research (I.H.-N., no. 15H05776; T.L.S., no. 16K18834) and by Specially Promoted Research of Grant-in-Aid for Scientific Research to I.H-.N. (no. 22000014) from the Japan Society for the Promotion of Science.
These authors contributed equally to the article.
Articles can be viewed without a subscription.
References
- Abell BM, Hahn M, Holbrook LA, Moloney MM (2004) Membrane topology and sequence requirements for oil body targeting of oleosin. Plant J 37: 461–470 [DOI] [PubMed] [Google Scholar]
- Abell BM, High S, Moloney MM (2002) Membrane protein topology of oleosin is constrained by its long hydrophobic domain. J Biol Chem 277: 8602–8610 [DOI] [PubMed] [Google Scholar]
- Abell BM, Holbrook LA, Abenes M, Murphy DJ, Hills MJ, Moloney MM (1997) Role of the proline knot motif in oleosin endoplasmic reticulum topology and oil body targeting. Plant Cell 9: 1481–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila CA, Arevalo-Soliz LM, Lorence A, Goggin FL (2013) Expression of α-DIOXYGENASE 1 in tomato and Arabidopsis contributes to plant defenses against aphids. Mol Plant Microbe Interact 26: 977–986 [DOI] [PubMed] [Google Scholar]
- Baker A, Carrier DJ, Schaedler T, Waterham HR, van Roermund CW, Theodoulou FL (2015) Peroxisomal ABC transporters: functions and mechanism. Biochem Soc Trans 43: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A, Graham I (2002) Plant Peroxisomes. Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- Bannenberg G, Martínez M, Rodríguez MJ, López MA, Ponce de León I, Hamberg M, Castresana C (2009) Functional analysis of α-DOX2, an active α-dioxygenase critical for normal development in tomato plants. Plant Physiol 151: 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Lepiniec L (2010) Physiological and developmental regulation of seed oil production. Prog Lipid Res 49: 235–249 [DOI] [PubMed] [Google Scholar]
- Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50: 825–838 [DOI] [PubMed] [Google Scholar]
- Baud S, Wuillème S, To A, Rochat C, Lepiniec L (2009) Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J 60: 933–947 [DOI] [PubMed] [Google Scholar]
- Berthelot K, Lecomte S, Estevez Y, Peruch F (2014) Hevea brasiliensis REF (Hev b 1) and SRPP (Hev b 3): an overview on rubber particle proteins. Biochimie 106: 1–9 [DOI] [PubMed] [Google Scholar]
- Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, Gilpin C, Chapman KD, Anderson RG, Goodman JM (2006) An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol 173: 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blée E, Flenet M, Boachon B, Fauconnier ML (2012) A non-canonical caleosin from Arabidopsis efficiently epoxidizes physiological unsaturated fatty acids with complete stereoselectivity. FEBS J 279: 3981–3995 [DOI] [PubMed] [Google Scholar]
- Brocard L, Immel F, Coulon D, Esnay N, Tuphile K, Pascal S, Claverol S, Fouillen L, Bessoule JJ, Bréhélin C (2017) Proteomic analysis of lipid droplets from Arabidopsis aging leaves brings new insight into their biogenesis and functions. Front Plant Sci 8: 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Goodman JM, Pyc M, Mullen RT, Dyer JM, Chapman KD (2015) Arabidopsis SEIPIN proteins modulate triacylglycerol accumulation and influence lipid droplet proliferation. Plant Cell 27: 2616–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40: 575–585 [DOI] [PubMed] [Google Scholar]
- Chapman KD, Dyer JM, Mullen RT (2012) Biogenesis and functions of lipid droplets in plants. Thematic Review Series: Lipid Droplet Synthesis and Metabolism: From Yeast to Man. J Lipid Res 53: 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che N, Yang Y, Li Y, Wang L, Huang P, Gao Y, An C (2009) Efficient LEC2 activation of OLEOSIN expression requires two neighboring RY elements on its promoter. Sci China C Life Sci 52: 854–863 [DOI] [PubMed] [Google Scholar]
- Chen JC, Tsai CC, Tzen JT (1999) Cloning and secondary structure analysis of caleosin, a unique calcium-binding protein in oil bodies of plant seeds. Plant Cell Physiol 40: 1079–1086 [DOI] [PubMed] [Google Scholar]
- Cui S, Hayashi Y, Otomo M, Mano S, Oikawa K, Hayashi M, Nishimura M (2016) Sucrose production mediated by lipid metabolism suppresses the physical interaction of peroxisomes and oil bodies during germination of Arabidopsis thaliana. J Biol Chem 291: 19734–19745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ. (2006) SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18: 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham I, Baker A, Holdsworth M (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21: 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda M, Shockey J, Werber M, Wolter FP, Heinz E (2002) Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid beta-oxidation. Plant J 32: 93–103 [DOI] [PubMed] [Google Scholar]
- Gao H, Metz J, Teanby NA, Ward AD, Botchway SW, Coles B, Pollard MR, Sparkes I (2016) In vivo quantification of peroxisome tethering to chloroplasts in tobacco epidermal cells using optical tweezers. Plant Physiol 170: 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidda SK, Park S, Pyc M, Yurchenko O, Cai Y, Wu P, Andrews DW, Chapman KD, Dyer JM, Mullen RT (2016) Lipid Droplet-Associated Proteins (LDAPs) are required for the dynamic regulation of neutral lipid compartmentation in plant cells. Plant Physiol 170: 2052–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham IA. (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59: 115–142 [DOI] [PubMed] [Google Scholar]
- Hamberg M, Sanz A, Castresana C (1999) Alpha-oxidation of fatty acids in higher plants: identification of a pathogen-inducible oxygenase (piox) as an alpha-dioxygenase and biosynthesis of 2-hydroperoxylinolenic acid. J Biol Chem 274: 24503–24513 [DOI] [PubMed] [Google Scholar]
- Hanano A, Burcklen M, Flenet M, Ivancich A, Louwagie M, Garin J, Blée E (2006) Plant seed peroxygenase is an original heme-oxygenase with an EF-hand calcium binding motif. J Biol Chem 281: 33140–33151 [DOI] [PubMed] [Google Scholar]
- Hayashi H, De Bellis L, Hayashi Y, Nito K, Kato A, Hayashi M, Hara-Nishimura I, Nishimura M (2002a) Molecular characterization of an Arabidopsis acyl-coenzyme A synthetase localized on glyoxysomal membranes. Plant Physiol 130: 2019–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Nishimura M (2003) Entering a new era of research on plant peroxisomes. Curr Opin Plant Biol 6: 577–582 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nishimura M (2006) Arabidopsis thaliana: a model organism to study plant peroxisomes. Biochim Biophys Acta 1763: 1382–1391 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nito K, Takei-Hoshi R, Yagi M, Kondo M, Suenaga A, Yamaya T, Nishimura M (2002b) Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid β-oxidation. Plant Cell Physiol 43: 1–11 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Kato A, Mano S, De Bellis L, Hayashi-Ishimaru Y, Yamaguchi K, Hayashi H, Nishimura M (2000) Functional transformation of plant peroxisomes. Cell Biochem Biophys 32: 295–304 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M (1998) 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10: 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Hayashi M, Hayashi H, Hara-Nishimura I, Nishimura M (2001) Direct interaction between glyoxysomes and lipid bodies in cotyledons of the Arabidopsis thaliana ped1 mutant. Protoplasma 218: 83–94 [DOI] [PubMed] [Google Scholar]
- Hong CE, Ha YI, Choi H, Moon JY, Lee J, Shin AY, Park CJ, Yoon GM, Kwon SY, Jo IH, et al. (2017) Silencing of an α-dioxygenase gene, Ca-DOX, retards growth and suppresses basal disease resistance responses in Capsicum annum. Plant Mol Biol 93: 497–509 [DOI] [PubMed] [Google Scholar]
- Horn PJ, James CN, Gidda SK, Kilaru A, Dyer JM, Mullen RT, Ohlrogge JB, Chapman KD (2013) Identification of a new class of lipid droplet-associated proteins in plants. Plant Physiol 162: 1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet P, Roux E, D’Andrea S, Davanture M, Negroni L, Zivy M, Chardot T (2004) Protein composition of oil bodies in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 42: 501–509 [DOI] [PubMed] [Google Scholar]
- Kelly AA, Quettier AL, Shaw E, Eastmond PJ (2011) Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiol 157: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Park KY, Seo YS, Kim WT (2016a) Arabidopsis small rubber particle protein homolog SRPs play dual roles as positive factors for tissue growth and development and in drought stress responses. Plant Physiol 170: 2494–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HU, Hsieh K, Ratnayake C, Huang AH (2002) A novel group of oleosins is present inside the pollen of Arabidopsis. J Biol Chem 277: 22677–22684 [DOI] [PubMed] [Google Scholar]
- Kim RJ, Kim HJ, Shim D, Suh MC (2016b) Molecular and biochemical characterizations of the monoacylglycerol lipase gene family of Arabidopsis thaliana. Plant J 85: 758–771 [DOI] [PubMed] [Google Scholar]
- Kim YY, Jung KW, Yoo KS, Jeung JU, Shin JS (2011) A stress-responsive caleosin-like protein, AtCLO4, acts as a negative regulator of ABA responses in Arabidopsis. Plant Cell Physiol 52: 874–884 [DOI] [PubMed] [Google Scholar]
- Lin LJ, Tai SS, Peng CC, Tzen JT (2002) Steroleosin, a sterol-binding dehydrogenase in seed oil bodies. Plant Physiol 128: 1200–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naested H, Frandsen GI, Jauh GY, Hernandez-Pinzon I, Nielsen HB, Murphy DJ, Rogers JC, Mundy J (2000) Caleosins: Ca2+-binding proteins associated with lipid bodies. Plant Mol Biol 44: 463–476 [DOI] [PubMed] [Google Scholar]
- Nishimura M, Yamaguchi J, Mori H, Akazawa T, Yokota S (1986) Immunocytochemical analysis shows that glyoxysomes are directly transformed to leaf peroxisomes during greening of pumpkin cotyledons. Plant Physiol 81: 313–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SK, Kang H, Shin DH, Yang J, Chow KS, Yeang HY, Wagner B, Breiteneder H, Han KH (1999) Isolation, characterization, and functional analysis of a novel cDNA clone encoding a small rubber particle protein from Hevea brasiliensis. J Biol Chem 274: 17132–17138 [DOI] [PubMed] [Google Scholar]
- Oikawa K, Matsunaga S, Mano S, Kondo M, Yamada K, Hayashi M, Kagawa T, Kadota A, Sakamoto W, Higashi S, et al. (2015) Physical interaction between peroxisomes and chloroplasts elucidated by in situ laser analysis. Nat Plants 1: 15035. [DOI] [PubMed] [Google Scholar]
- Pyc M, Cai Y, Gidda SK, Yurchenko O, Park S, Kretschmar FK, Ischebeck T, Valerius O, Braus GH, Chapman KD, et al. (2017) Arabidopsis lipid droplet-associated protein (LDAP)-interacting protein (LDIP) influences lipid droplet size and neutral lipid homeostasis in both leaves and seeds. Plant J 92: 1182–1201 [DOI] [PubMed] [Google Scholar]
- Quettier AL, Shaw E, Eastmond PJ (2008) SUGAR-DEPENDENT6 encodes a mitochondrial flavin adenine dinucleotide-dependent glycerol-3-P dehydrogenase, which is required for glycerol catabolism and post germinative seedling growth in Arabidopsis. Plant Physiol 148: 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Mendoza M, Dubreucq B, Baud S, Parcy F, Caboche M, Lepiniec L (2008) Deciphering gene regulatory networks that control seed development and maturation in Arabidopsis. Plant J 54: 608–620 [DOI] [PubMed] [Google Scholar]
- Santos Mendoza M, Dubreucq B, Miquel M, Caboche M, Lepiniec L (2005) LEAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Lett 579: 4666–4670 [DOI] [PubMed] [Google Scholar]
- Schmidt MA, Herman EM (2008) Suppression of soybean oleosin produces micro-oil bodies that aggregate into oil body/ER complexes. Mol Plant 1: 910–924 [DOI] [PubMed] [Google Scholar]
- Shai N, Schuldiner M, Zalckvar E (2016) No peroxisome is an island: peroxisome contact sites. Biochim Biophys Acta 1863: 1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Xie J, Liu RD, Ni XF, Wang XH, Li ZX, Zhang M (2014) Genomic analysis and expression investigation of caleosin gene family in Arabidopsis. Biochem Biophys Res Commun 448: 365–371 [DOI] [PubMed] [Google Scholar]
- Shimada TL, Hara-Nishimura I (2010) Oil-body-membrane proteins and their physiological functions in plants. Biol Pharm Bull 33: 360–363 [DOI] [PubMed] [Google Scholar]
- Shimada TL, Hara-Nishimura I (2015) Leaf oil bodies are subcellular factories producing antifungal oxylipins. Curr Opin Plant Biol 25: 145–150 [DOI] [PubMed] [Google Scholar]
- Shimada TL, Shimada T, Takahashi H, Fukao Y, Hara-Nishimura I (2008) A novel role for oleosins in freezing tolerance of oilseeds in Arabidopsis thaliana. Plant J 55: 798–809 [DOI] [PubMed] [Google Scholar]
- Shimada TL, Takano Y, Hara-Nishimura I (2015) Oil body-mediated defense against fungi: from tissues to ecology. Plant Signal Behav 10: e989036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada TL, Takano Y, Shimada T, Fujiwara M, Fukao Y, Mori M, Okazaki Y, Saito K, Sasaki R, Aoki K, et al. (2014) Leaf oil body functions as a subcellular factory for the production of a phytoalexin in Arabidopsis. Plant Physiol 164: 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siloto RM, Findlay K, Lopez-Villalobos A, Yeung EC, Nykiforuk CL, Moloney MM (2006) The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 18: 1961–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gaquerel E, Baldwin IT (2010) The two alpha-dox genes of Nicotiana attenuata: overlapping but distinct functions in development and stress responses. BMC Plant Biol 10: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Braybrook SA, Paula SL, Kwong LW, Meuser J, Pelletier J, Hsieh TF, Fischer RL, Goldberg RB, Harada JJ (2008) Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: implications for somatic embryogenesis. Proc Natl Acad Sci USA 105: 3151–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98: 11806–11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Katagiri T, Yamaguchi-Shinozaki K, Shinozaki K (2000) An Arabidopsis gene encoding a Ca2+-binding protein is induced by abscisic acid during dehydration. Plant Cell Physiol 41: 898–903 [DOI] [PubMed] [Google Scholar]
- Thazar-Poulot N, Miquel M, Fobis-Loisy I, Gaude T (2015) Peroxisome extensions deliver the Arabidopsis SDP1 lipase to oil bodies. Proc Natl Acad Sci USA 112: 4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoulou FL, Eastmond PJ (2012) Seed storage oil catabolism: a story of give and take. Curr Opin Plant Biol 15: 322–328 [DOI] [PubMed] [Google Scholar]
- Titus DE, Becker WM (1985) Investigation of the glyoxysome-peroxisome transition in germinating cucumber cotyledons using double-label immunoelectron microscopy. J Cell Biol 101: 1288–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzen J, Cao Y, Laurent P, Ratnayake C, Huang A (1993) Lipids, proteins, and structure of seed oil bodies from diverse species. Plant Physiol 101: 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzen JT, Huang AH (1992) Surface structure and properties of plant seed oil bodies. J Cell Biol 117: 327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzen JT, Lai YK, Chan KL, Huang AH (1990) Oleosin isoforms of high and low molecular weights are present in the oil bodies of diverse seed species. Plant Physiol 94: 1282–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier J, Thompson RD (2008) Transcriptional regulation of storage protein synthesis during dicotyledon seed filling. Plant Cell Physiol 49: 1263–1271 [DOI] [PubMed] [Google Scholar]
- Wu YY, Chou YR, Wang CS, Tseng TH, Chen LJ, Tzen JT (2010) Different effects on triacylglycerol packaging to oil bodies in transgenic rice seeds by specifically eliminating one of their two oleosin isoforms. Plant Physiol Biochem 48: 81–89 [DOI] [PubMed] [Google Scholar]
- Yamashita S, Yamaguchi H, Waki T, Aoki Y, Mizuno M, Yanbe F, Ishii T, Funaki A, Tozawa Y, Miyagi-Inoue Y, et al. (2016) Identification and reconstitution of the rubber biosynthetic machinery on rubber particles from Hevea brasiliensis. eLife 5: e19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr (2008) Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49: 2283–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Silva ID, Bartel B (2001) The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol 127: 1266–1278 [PMC free article] [PubMed] [Google Scholar]