Abstract
Structural and functional roles of cellulose, xyloglucan, and pectins in cell wall enlargement are reappraised with insights from mechanics, atomic force microscopy, and other methods.
The primary wall of a growing cell is a versatile, subtle, and dynamic structure, with unique properties and functions in the life of the plant (Burton et al., 2010). When a cell grows, its wall stretches irreversibly as the cell enlarges in volume. Cells can start and stop this process quickly, in less than a minute in some cases, revealing that the molecular processes underlying irreversible wall expansion are dynamically controlled. Such dynamic behavior may be mediated, at least in part, by changes in wall pH (Hager, 2003; Barbez et al., 2017), which modulates the wall-loosening action of expansins (Cosgrove, 2015) and potentially other wall-modifying agents. Wall pH in turn is dynamically modulated by plasma membrane H+-ATPase activity (Haruta et al., 2014, 2015) and other processes in the wall. Because growing cell walls are thin and in close physical contact with plasma membranes, wall pH can be rapidly modulated (Bibikova et al., 1998; Monshausen et al., 2007; Barbez et al., 2017). As a result of the pH-dependent activity of expansins, the growing cell wall behaves like a “smart material”—one whose properties (extensibility in this case) reversibly and rapidly change with environment (e.g. pH). Slower changes in wall structure that influence the wall’s ability to expand also occur as part of the natural course of cell development, e.g. as cells are displaced through the elongation zone of a stem (Phyo et al., 2017), or in response to external perturbations, e.g. Sahaf and Sharon (2016). These slower changes may include changes in mechanics, such as wall stiffening, and in the density or accessibility of sites where expansins or other proteins can loosen the wall.
The wall itself is synthesized in a team effort: mobile cellulose synthesis complexes (Paredez et al., 2006; Li et al., 2016b) produce long, thin, strong, stiff cellulose microfibrils at the cell surface, while matrix polysaccharides and glycoproteins are deposited to the cell surface via the secretory apparatus (Zhu et al., 2015; Kim and Brandizzi, 2016). The cytoskeleton guides the wall synthesis machinery to supply wall components to appropriate locations on the cell surface (Szymanski and Staiger, 2017), where the components assemble to form an organized, mechanically strong structure that can withstand the in-plane tensile forces generated by the outward push of cell turgor pressure yet is able to expand in a controlled manner. The structural requirements for orderly expansion of the cell wall are not well defined at this time. Moreover, except with the possible exception of tip-growing cells (Dumais et al., 2006; Rojas et al., 2011), synthesis, secretion, and wall assembly are only distantly coupled to the wall extension process itself. For instance, cellulose synthesis in carbon-limited Arabidopsis (Arabidopsis thaliana) hypocotyls was temporally distinct from cell expansion (Ivakov et al., 2017); likewise, wall deposition did not keep up with cell expansion in dark-grown hypocotyls, resulting in substantial wall thinning (Refrégier et al., 2004). On the other hand, gradients in wall thickness in the growing trichome of Arabidopsis precisely matched predictions for mechanical stability of the wall, implying good coordination between local wall deposition and expansion (Yanagisawa et al., 2015). We still have much to learn about how plant cells build a stable yet extensible wall.
When the normal molecular assembly of the cell wall is disturbed, for instance, by mutations that affect synthesis of cellulose (Fagard et al., 2000), xyloglucan (Cavalier et al., 2008), or pectic polysaccharides (Mouille et al., 2007), cell expansion may be disrupted in unpredictable ways. Genetic results suggest that errors in wall assembly trigger surface receptor-like kinases such as FERONIA that may act as sensors of cell wall integrity (CWI; Humphrey et al., 2007; Höfte, 2015) and mechanosensation (Hamant and Haswell, 2017). The ensuing responses, which include production of reactive oxygen species and inactivation of the plasma membrane H+-ATPase, likely give rise to some of the complex phenotypes that originate from rather simple modifications of wall polysaccharides (Voxeur and Höfte, 2016). For example, growth defects stemming from mutation of a cellulose synthase gene in Arabidopsis were partially suppressed by mutation of THESEUS1 (Hématy et al., 2007), another member of the same receptor kinase family as FERONIA (Cheung and Wu, 2011; Li et al., 2016a). Evidently, CWI responses compound and confound the direct effects of cell wall defects. Defects in pectin metabolism appear particularly prone to trigger CWI responses that activate the brassinosteroid pathway, leading to diverse growth phenotypes (Wolf et al., 2012, 2014). On the other hand, FERONIA and its extracellular peptide ligand (“rapid alkalinization factor”) are also required for normal root growth and auxin responses (Haruta et al., 2014; Shih et al., 2014; Velasquez et al., 2016; Barbez et al., 2017). Cell expansion thus appears to be intimately linked to these wall sensor pathways in ways we are only beginning to fathom.
This Update focuses on the growing cell wall, in particular, the structural, mechanical, and physicochemical processes underlying irreversible wall enlargement during diffuse cell growth. Diffuse growth refers to surface expansion occurring on entire facets of cell walls, for instance, the side walls of elongating cells in the body of a growing root or stem. Diffuse growth may occur with or without a directional bias, which depends partly on wall structure and partly on patterns of mechanical stress in the wall (Baskin and Jensen, 2013). Its intensity may vary along a cell wall surface and on different cell wall facets. For instance, side walls of a hypocotyl cell may elongate rapidly, whereas its end walls may not enlarge much at all (Peaucelle et al., 2015). In the jigsaw-puzzle-like pavement cells of the Arabidopsis leaf epidermis, a complex pattern of local wall surface expansion occurs in the periclinal (outer epidermal) wall as well as in the anticlinal (side) walls (Szymanski, 2014; Armour et al., 2015). These complex expansion patterns have been linked to cytoskeletal dynamics within the cell and to spatial patterns of tensile stress (Szymanski and Cosgrove, 2009; Zhang et al., 2013; Sampathkumar et al., 2014a).
Diffuse growth is the dominant pattern for most cells in the plant body and is traditionally contrasted with tip growth, for instance, in pollen tubes and root hairs, where surface expansion is localized to limited regions of the hemispherical tip (Campàs et al., 2012; Sanati Nezhad and Geitmann, 2013; Velasquez et al., 2016). Despite differences in spatial patterning, tip growth may involve some of the same cell wall processes as occur in diffuse growth, but in a more intense, spatially localized manner. This point is supported by the stunted elongation of root hairs in plants with genetic lesions in expansin genes specifically expressed in root hairs (Yu et al., 2011). Although expansins have been characterized largely in the context of diffuse growth, they also are necessary for root hair growth. Leaf trichomes in Arabidopsis are fiber-like cells with a conical shape that arises from highly anisotropic diffuse growth; its tip-biased gradient in surface expansion has been related to spatial gradients in cell diameter, wall stress, and wall thickness (Yanagisawa et al., 2015).
In this Update, I begin with a review of the biophysical basis of cell wall growth, followed by our changing concepts of the role and interactions of cell wall components that compose the cell wall, and end with recent insights from atomic force microscopy (AFM), showing the details of microfibril organization and motions during wall enlargement.
WALL STRESS RELAXATION DRIVES CELL GROWTH
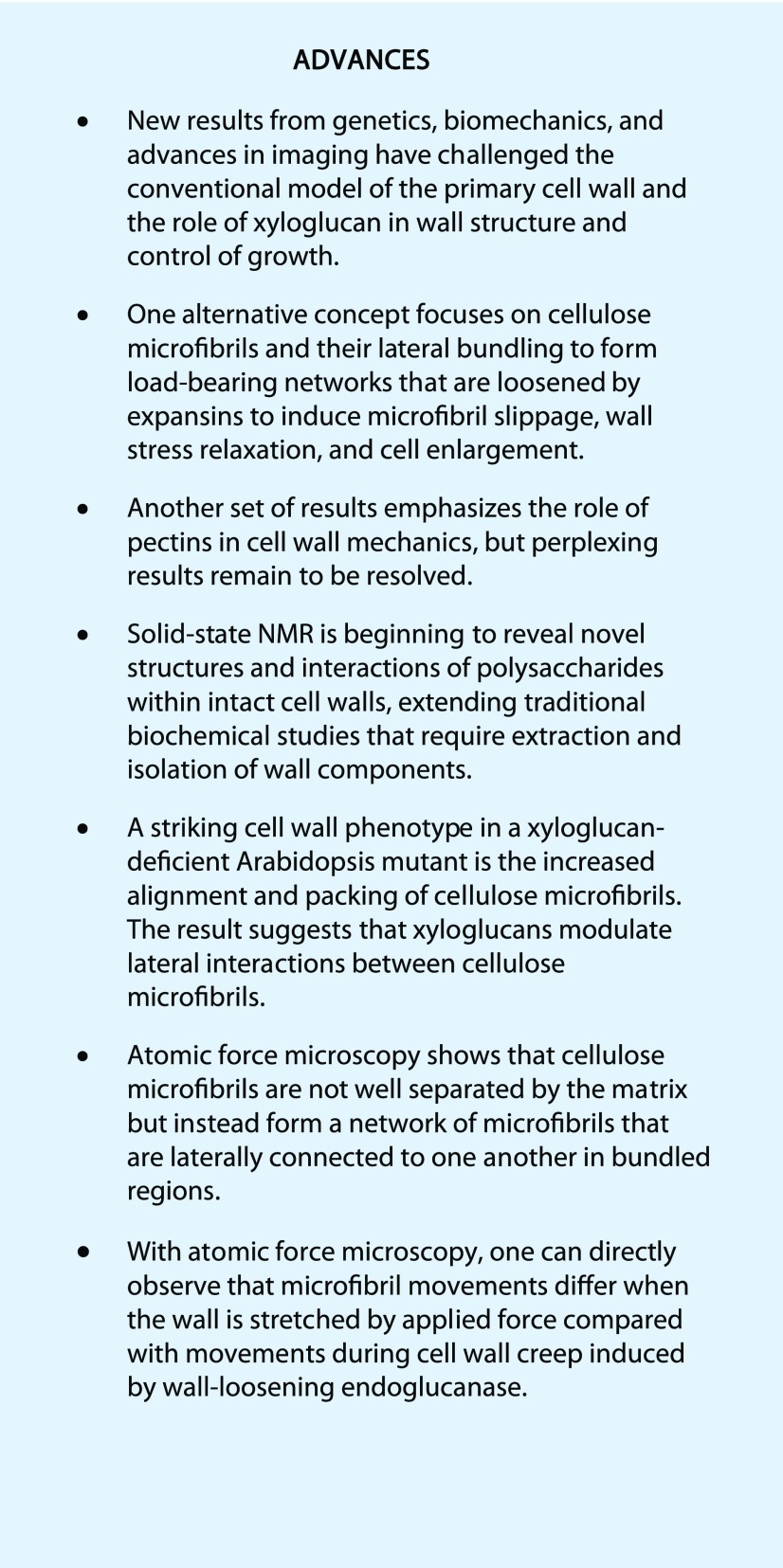
What happens when a cell grows? Cell volume increases as a result of water uptake, and wall surface area enlarges irreversibly by local separation of cell wall components (Cosgrove, 2016b). The phrase “turgor-driven growth” (or its variants) is often used to describe this process, but this phrasing can be misleading if it is taken to imply that growth is simply a mechanical stretching of an inherently pliant cell wall, like stretching a piece of putty. To understand this point better, it is useful to distinguish different patterns of cell wall stretching in response to an applied tensile force (Fig. 1). Elastic deformation is by definition reversible and instantaneous upon applied force. Small wall deformations are typically elastic, whereas after large, rapid, transient deformations, many cell walls do not fully return to their initial size. The irreversible part is a plastic deformation, which occurs when the wall is stretched beyond a yield point. When force is applied to a wall in a sustained manner, the stretch (“strain” in engineering terms) is partly elastic and partly plastic or partly viscoelastic and partly viscoplastic, depending on the time scales involved (Boudaoud, 2010; Moulia, 2013). These latter two terms refer to time-dependent deformations. Without sustained cell wall loosening, a constant force applied to a wall typically results in a time-dependent strain that approaches a nearly steady value in a few minutes, depending on the sample and its strain history (Hohl and Schopfer, 1992). Such deformation is a result of the polymeric nature of plant cell walls, but the exact structural basis for these mechanical properties is largely unknown and needs further theoretical development.
Figure 1.
Schematic comparisons of different strain patterns of cell walls (top) stretched with a uniaxial force (bottom). A, Purely elastic (reversible) strain. Cell walls often display a retarded elasticity because wall polymer motions are not instantaneous. B, A combination of elastic and plastic strain. Beyond the yield point strain is partly elastic and partly plastic. C, Viscoelastic and viscoplastic strains. Polymer motions require time to reach equilibrium. D, Wall loosening results in sustained time-dependent extension (creep).
These purely mechanical responses of walls differ in an essential way from the sustained wall expansion that occurs during cell growth (Cosgrove, 2016b; Zhang et al., 2017), which depends on continuous loosening by expansins or other wall-loosening agents (Cosgrove, 2016a). Wall loosening results in wall stress relaxation that drives cell growth. The significance of wall stress relaxation for plant growth was first recognized by Ray et al. (1972) and later solidified by detailed theory and experimental results (Cosgrove, 1993a, 1993b; Ortega, 2017). As a result of the complex interactions of cell wall components, there are various distinctive ways in which cell walls may become mechanically softer (meaning more easily deformed by mechanical force), but they do not necessarily result in an increase in wall relaxation and growth, and they therefore do not qualify as wall loosening processes (see below and Table I). For instance, with rare exceptions (Yuan et al., 2001), lytic enzymes may soften walls, but they do not stimulate cell growth or cause long-term cell wall extension (creep; Ruesink, 1969; Cosgrove and Durachko, 1994; Fleming et al., 1997). On the other hand, α-expansins cause stress relaxation and prolonged enlargement of cell walls, but they lack wall lytic activity and they do not soften the wall, as measured by tensile tests (see figure 8 in Yuan et al. [2001]). These are remarkable facts that seem counterintuitive to expectations based on conventional models of cell walls (Carpita and Gibeaut, 1993). To illustrate the point in another way: Xyloglucan-deficient hypocotyl walls from the xxt1,xxt2 mutant of Arabidopsis are more compliant (more easily stretched) in tensile tests compared with wild-type walls, yet xxt1,xxt2 hypocotyls grow more slowly than the wild type (Xiao et al., 2016). Moreover, despite their greater mechanical compliance, the xxt1,xxt2 walls extend more slowly in creep tests, exhibit less stress relaxation, and are less responsive to α-expansins compared with wild-type walls (Park and Cosgrove, 2012a). Another example illustrates the flip side of the coin: Low temperature strongly reduced cell wall expansion in Chara cells, but wall elasticity was hardly affected (Proseus et al., 2000). Many other examples have been documented where mechanical tests do not reliably report on the growth properties of the cell wall (Cosgrove, 2016b).
Table I. Brief explanation of terms related to wall mechanics and growth properties, as used here.
| Term | Meaning |
|---|---|
| Extensibility | General term for the ability of the cell wall to grow; in other contexts, this is the coefficient relating growth rate to turgor pressure (Cosgrove, 1993b); not elasticity and not a purely mechanical property, as it depends on wall loosening |
| Loosening | Molecular process causing wall stress relaxation, resulting in water uptake and cell growth; it confers irreversibility to wall strains; expansins are well-recognized as wall-loosening agents |
| Softening | A process that makes the wall more deformable to mechanical force |
| Weakening | A process that reduces the force or energy need to break walls |
| Elasticity | A measure of how readily the cell wall changes shape in response to a transient mechanical force |
| Plasticity | A measure of the irreversible component of wall deformation in response to a transient mechanical force |
| Compliance | The slope for strain/stress curves; it is the reciprocal of modulus or stiffness |
Despite this “inconvenient truth” [apologies to Al Gore (2006)], wall elasticity is frequently taken to be synonymous with cell wall growth properties. Sometimes this is a matter of convenience—elasticity is relatively straightforward to incorporate into simulations of growth (Fayant et al., 2010; Huang et al., 2015)—and numerous methods have been devised in recent years to measure elasticity of tissues and cells with mechanical devices (e.g. Routier-Kierzkowska et al., 2012; Nezhad et al., 2013; Sanati Nezhad et al., 2013; Beauzamy et al., 2015b; Vogler et al., 2015; Mosca et al., 2017). To be fair, there are indeed cases where elasticity roughly correlates with cell growth, as discussed below. Such correlation may indicate a change in wall structure that actually contributes to altered growth by amplifying wall stress relaxation (see Cosgrove [2016b]), or may be entirely coincidental, resulting from structural changes independent of wall extensibility. Elastic changes may also be a consequence of the altered growth. For instance, it has long been known that auxin treatment results in increased wall compliances in many cases (Heyn, 1932; Edelmann and Kohler, 1995; Braybrook, 2017). However, the onset of auxin-induced growth is fast and precedes the gradual change in wall mechanics (Cleland, 1984), which may reflect longer-term changes in wall structure induced by auxin or faster growth itself.
Another factor in the confusion between elasticity and extensibility may be the fuzzy definition of terms such as wall loosening, softening, and weakening (Table I). We do not have an established vocabulary to distinguish the many facets of wall properties. As discussed here, wall loosening refers to a shift or cut of a load-bearing part of the wall, relaxing tensile stress in the whole wall and simultaneously reducing cell turgor, which is the Newtonian counterbalance to wall stress. The reduction in turgor enables passive water uptake by osmosis, which elastically stretches the cell wall, restoring turgor. Such loosening-dependent polymer movement might be called a chemorheological flow (Dumais, 2013; Moulia, 2013; Cosgrove, 2016b). However, this term usually implies a chemical change in covalent bonding within the cell wall, which does not appear to be necessary for cell wall creep, i.e. α-expansin facilitates cell wall creep without evidence of hydrolysis or other covalent modification of wall polymers (Cosgrove, 2015, 2016a). I use wall “softening” to denote a process that changes wall stiffness, without the implication that the wall can grow more quickly. I use wall “weakening” to refer to cases where the wall breaking strength is reduced. Thus, xxt1,xxt2 hypocotyls are weaker than the wild type because their breaking strength is reduced (Cavalier et al., 2008). Despite being softer and weaker, xxt1,xxt2 hypocotyls are less extensible in assays of growth and cell wall creep. The key conclusion is that elasticity reports on wall structure, not the dynamic relaxation processes that determine wall extensibility and that drive cell wall growth. With the definitions used here, a stretchy rubber band has significant elasticity but no extensibility.
CELL WALL MODELS NEED FURTHER REFINEMENT AND TESTING
This physical framework, in which wall stress relaxation initiates cell growth and couples water uptake with wall expansion, leaves numerous molecular details unresolved. What is the molecular nature of wall loosening? Which wall components of the wall are the targets of wall loosening, and which components limit the ensuing polymer motions (e.g. spreading of cellulose microfibrils)? Are there multiple ways to induce wall relaxation and loosen the wall? What are the molecular bases of wall elasticity and plasticity?
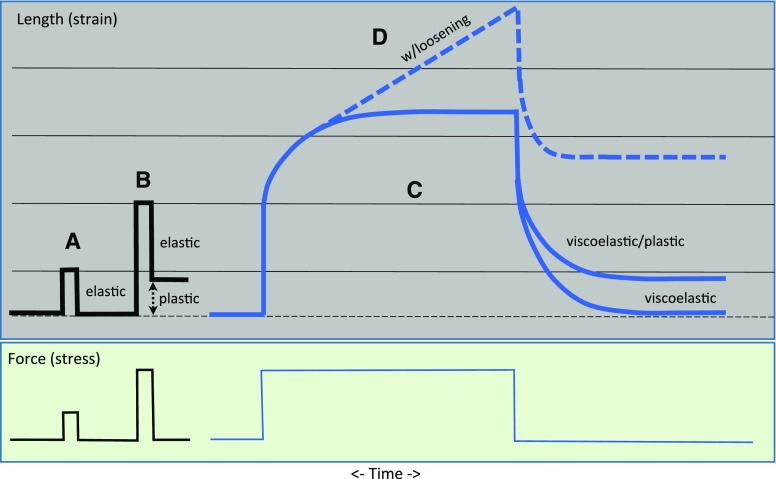
The answers to these questions require an accurate model of cell wall structure and a deep understanding of the molecular basis of wall enlargement. Notwithstanding textbook models, this remains a major unmet challenge. For many years, xyloglucan was imagined to function as a load-bearing tether linking well-separated cellulose microfibrils (Carpita and Gibeaut, 1993; Albersheim et al., 2011), with pectins functioning as a compliant, hydrated, gel-like matrix between the microfibrils. This concept entailed predictions of wall mechanics that were largely untested. Recent results, however, call for reevaluation of the roles of cellulose, xyloglucan, and pectins in wall structure and growth (Cosgrove, 2016b). For instance, extensive xyloglucanase treatment of isolated cell walls from cucumber (Cucumis sativus) and Arabidopsis did not induce cell wall extension (creep) or increase mechanical compliances (stress/strain behavior in tensile tests), even though at least half of the xyloglucan was removed (Park and Cosgrove, 2012b). On the other hand, low concentrations of bifunctional endoglucanases (able to cut both xyloglucan and cellulose) induced cell wall creep and mechanical softening (increase in tensile compliances). The extent of wall hydrolysis needed to induce these biomechanical actions was very small. Enigmatically, the combination of a xyloglucan-specific endoglucanase with a cellulose-specific endoglucanase did not mimic the bifunctional enzymes in their action on cell wall creep. To explain this enigma, we proposed that the bifunctional enzymes hydrolyze relatively inaccessible load-bearing junctions between cellulose microfibrils where xyloglucan and noncrystalline cellulose are entwined (Fig. 2). Digestion of these nexus points, dubbed “biomechanical hotspots,” releases some of the load-bearing junctions between the microfibrils, enabling stress relaxation and irreversible microfibril movements. Limited accessibility and kinetics account for the failure of combined xyloglucan-specific and cellulose-specific enzymes to cause cell wall creep.
Figure 2.
Conceptual depiction of structural features of primary cell walls. Cellulose microfibrils are represented as thick rods with hydrophobic (blue) and hydrophilic faces (orange). Xyloglucan (green) is found in solvated, coiled conformations and in extended conformations bound to the hydrophobic faces of cellulose, based on Zheng et al. (2017b). It is also depicted as entrapped between microfibrils. Pectins (yellow) are represented as coiled structures that fill the space between microfibrils and bind to the hydrophilic surfaces (based on solid-state NMR results). Microfibrils are bundled by direct contacts and at junctions where cellulose and xyloglucan intertwine. The red arrows point to cellulose-xyloglucan-cellulose junctions that are sites of wall loosening by bifunctional endoglucanases. This depiction is a synthesis based on the most recent results from AFM, FESEM, solid-state NMR, and mechanics.
The origin of these proposed junctions is uncertain. One possibility is that they form spontaneously by physical entrapment and self-assembly as cellulose and xyloglucan are deposited to the cell surface. Coordination of vesicle secretion with cellulose synthesis might facilitate their formation. Another possibility is that hotspots are formed enzymatically. Recent reports have identified enzymes (xyloglucan endotransglucosylase/hydrolase) that can cut noncrystalline cellulose and ligate xyloglucan onto the cellulose end (Hrmova et al., 2007; Simmons et al., 2015; Shinohara et al., 2017). The specific activity of these enzymes in performing such hetero-transglucosylations is very low, so the biological significance of such reactions is uncertain. Further work is needed to uncover the origin of biomechanical hotspots.
The hotspot concept gains support from a study that used a novel solid-state NMR strategy to characterize the target of expansin binding in complex walls from Arabidopsis seedlings (Wang et al., 2013). With use of a sensitivity-boosting technique called dipolar nuclear polarization to enhance 1H-13C cross-polarization, nuclear magnetic spins were selectively filtered through 15N,13C-labeled expansin protein to 13C in wall components within close proximity to expansin. The results showed that expansin binds to cellulose with a slightly different chemical shift than the bulk of the cellulose, indicating cellulose chains that are packed together differently than most of the cellulose. Moreover, there was evidence of xyloglucan in close proximity to expansin. These NMR characteristics resemble those that might be expected for the biomechanical junctions targeted by wall-loosening bifunctional endoglucanases (Park and Cosgrove, 2012b). Additional NMR characterization found that the expansin-binding sites are on the surface of cellulose microfibrils, yet are relatively distant from water, consistent with limited accessibility (Wang et al., 2016b).
The concept of wall structure emerging from these results emphasizes the importance of direct connectivity between cellulose microfibrils, as opposed to previous concepts where microfibrils were depicted as well separated by matrix polymers and connected only via tethers. As described below, AFM reveals the organization of cellulose microfibrils to be a network of laterally bundled microfibrils rather than a collection of well-spaced microfibrils connected only by matrix, as traditionally depicted in textbook models of primary cell walls.
Another cellulose-related topic emerging from recent work is the potential significance of the cross-sectional shape of cellulose microfibrils, which influences the proportion of hydrophilic and hydrophobic faces on the microfibril (Newman et al., 2013; Cosgrove, 2014; Wang and Hong, 2016). This issue is important for cell wall models because of the potential for these two faces to interact differently with matrix components. The hydrophilic faces of cellulose microfibrils are populated by hydroxyl groups extending from the sides of the Glc residues, whereas the hydrophobic faces are those that expose the Glc rings with their nonpolar −CH groups. X-ray crystallography studies show that expansins bind the hydrophobic face of cellulose chains (Georgelis et al., 2012). Computational studies indicate that xyloglucan likewise preferentially binds to the hydrophobic face of cellulose (Zhao et al., 2014). This computational result is supported by a recent field emission scanning electron microscopy (FESEM)-based study showing that xyloglucan indeed covers the hydrophobic faces of cellulose microfibrils in onion (Allium cepa) cell walls (Zheng et al., 2017b). Complementing these results, recent experimental and computational studies indicate that substituted xylans in secondary cell walls may bind the hydrophilic faces of cellulose (Simmons et al., 2016; Grantham et al., 2017; Pereira et al., 2017). Primary cell walls generally contain only small amounts of xylan, e.g. Zablackis et al. (1995), with the notable exception of cell walls from grass species (Carpita, 1996). Nevertheless, a recent study characterized a xylan in primary walls of Arabidopsis (Mortimer et al., 2015) that might selectively bind to the hydrophilic faces of microfibrils. Because β-expansin was recently shown to target xylans in grass cell walls (Wang et al., 2016a), it seems plausible that α- and β-expansins may loosen the connections between different faces of cellulose microfibrils in the wall.
Direct cellulose-cellulose contacts may be prevalent in primary walls, but whether such interactions are mediated via their hydrophilic or hydrophobic faces is uncertain and indeed may differ for primary and secondary cell walls. Some studies propose that microfibrils aggregate via contacts of their hydrophilic faces (Ding et al., 2012; Oehme et al., 2015), whereas an AFM analysis of microfibril patterns in onion cell walls suggested that microfibril bundling occurs via the hydrophobic faces of microfibrils (Zhang et al., 2016). In Arabidopsis hypocotyls of the xyloglucan-deficient xxt1,xxt2 mutant (Xiao et al., 2016), cellulose microfibrils were more aligned and closely packed than in the wild type, an indication that xyloglucan may promote dispersion of microfibrils within a lamella. It is also possible that these changes in cellulose organization are a consequence of CWI responses to wall defects.
PECTINS
As the idea of a direct cellulose network within primary cell walls has gained support, pectins have also attracted new attention as potential modifiers of wall enlargement. The case has been most cogently argued for pollen tubes (Parre and Geitmann, 2005; Rojas et al., 2011; Sanati Nezhad et al., 2014), where pectins dominate wall structure, and for the giant-celled alga Chara corallina (Boyer, 2016). In Arabidopsis cell walls, results from multidimensional solid-state NMR indicate extensive noncovalent cellulose-pectin interactions (Wang et al., 2012, 2015). This is surprising because such interactions are not observed in binding studies in vitro (Zykwinska et al., 2008a, 2008b). A recent study of cell wall properties along the axial growth gradient of the Arabidopsis stem gives additional clues (Phyo et al., 2017). Pectins in the apical (faster-growing and softer) region of the stem are more mobile, more hydrated, more esterified, and more branched compared with pectins in the lower (more slowly growing and stiffer) region of the stem. How these correlated structural changes in pectin properties influence pectin-cellulose interactions, cell wall mechanics, and growth needs further testing. In contrast, a very different concept of pectin was proposed in a study that characterized a proteoglycan with covalently linked pectin and xylan domains (Tan et al., 2013). This structure is reminiscent of the early macromolecular model of primary cell walls by Keegstra et al. (1973). The potential role of such proteoglycans in wall structure, mechanics, and growth remains to be evaluated.
In another vein, a series of elegant experiments with clear but perplexing results implicate localized de-esterification of homogalacturonan as a signature event in the auxin-induced patterning of the shoot apical meristem of Arabidopsis, resulting in elastically softer regions of the meristem surface, as measured by microindentation techniques, where leaf primordia emerge (Peaucelle et al., 2008, 2011; Braybrook and Peaucelle, 2013). Experiments with the auxin-transport pin1 mutant and genes encoding pectin methyl esterase inhibitor proteins suggest that auxin patterning of the shoot apical meristem requires pectin de-esterification.
The correlation of de-esterified pectin with softer meristem regions is perplexing because, in the broader context of cell wall properties, pectin de-esterification is commonly thought to result in stiffer, not softer walls, as a result of increased ability for calcium-mediated cross-linking of homogalacturonan. For instance, in the hemispherical tip of growing pollen tubes, de-esterified homogalacturonan is associated with stiffer walls and cessation of wall expansion (Geitmann and Parre, 2004; Sanati Nezhad et al., 2014). Likewise, pectin de-esterification is associated with the decline in the growth rate and increased wall stiffness along the apical-to-basal gradient of growing stems (Goldberg et al., 1986; Phyo et al., 2017). Moreover, wall loosening by expansin is hindered in the basal regions of growing stems where the extent of de-esterified pectin is high (Cosgrove, 1996), and this hindrance may be partially reversed by removal of pectins and calcium (Zhao et al., 2008). Hocq et al. (2017) have questioned the concept of wall stiffening by calcium cross-linking of pectins, yet older results with calcium chelators yielded a nuanced conclusion: that calcium cross-links are indeed load-bearing but are not broken during acid-induced (expansin-mediated) wall loosening (Virk and Cleland, 1990). Likewise, imaging with AFM shows that addition and removal of calcium reversibly stiffens pectins on the surface of onion epidermal cell walls (Zhang et al., 2016), yet parallel experiments show little effect of calcium on wall extension in vitro. Thus, more research is needed to clarify the perplexing observations about pectin esterification, calcium cross-linking, wall softening, auxin responses, and cell growth in the meristem. Are CWI responses complicating this story?
The perplexing results about pectin esterification reported for the shoot apical meristem raise the following question: Is cell wall enlargement in the meristem regulated in the same way as in subapical zones of rapid cell enlargement, or do walls in the meristem follow a different set of rules? Indeed, we know rather little about cell wall structure and wall extensibility in meristematic regions, other than the limited information inferred from immunohistochemistry (Yang et al., 2016) and osmomechanical probing of cell elasticity (Kierzkowski et al., 2012; Nakayama et al., 2012; Routier-Kierzkowska and Smith, 2013). In a recent multifaceted study of the swollen shoot apical meristems from the clavata3-2 mutant of Arabidopsis, wall composition was found to consist of approximately 30% cellulose, approximately 26% pectin, and 15% xyloglucan (Yang et al., 2016). This is unremarkable, as it is similar to the wall composition of whole Arabidopsis seedlings (White et al., 2014). Older studies also bear on the question of cell enlargement mechanisms in meristems: Local application of α-expansin protein to the surface of the shoot apical meristem resulted in an outgrowth resembling early stages of a leaf primordium (Fleming et al., 1999), and more pronounced outgrowth resulted from transient induction of an α-expansin on the flanks of the meristem (Pien et al., 2001), indicating cell walls in the meristem are sensitive to the loosening action of α-expansin. Moreover, α-expansin is endogenously expressed at the site of incipient leaf primordia before primordium outgrowth (Reinhardt et al., 1998), evidence that modulation of cell wall enlargement is similar to that of other plant tissues. Allowing for the high frequency of dividing cells in meristems and the unique wall synthesis machinery involved in cell plate formation during cell division (Gu et al., 2016), current results indicate meristem walls are similar in composition and growth mechanisms as documented in rapidly elongating cells that emerge from meristems. Thus, the enigmatic function of pectin de-esterification in the meristem remains an open question.
INSIGHTS FROM AFM OF EPIDERMAL CELL WALLS
Plant developmental biologists are probably most familiar with AFM from studies that have used the device to indent the surface of growing tissues to evaluate local stiffness (e.g. Peaucelle et al., 2011; Milani et al., 2014; Sampathkumar et al., 2014a). This is a complex topic beyond the scope of this review, but readers are referred to reviews that assess the varied approaches and interpretations of these stiffness measurements (Milani et al., 2013; Mosca et al., 2017). AFM-based stiffness maps of the shoot apical meristem have been compared with maps of cell shape, cell division, auxin flow, gene expression, and cytoskeletal patterns (Nakayama et al., 2012; Robinson et al., 2013; de Reuille et al., 2014; Sassi et al., 2014). These studies contribute to sophisticated models of meristem morphogenesis and phyllotaxis in which wall stress, mechanics, enlargement, and the cytoskeleton play interacting roles (Nakayama et al., 2012; Kierzkowski et al., 2013; Sampathkumar et al., 2014b), concepts rooted in the pioneering efforts of Paul Green to understand the biophysics of meristem dynamics (Green et al., 1996).
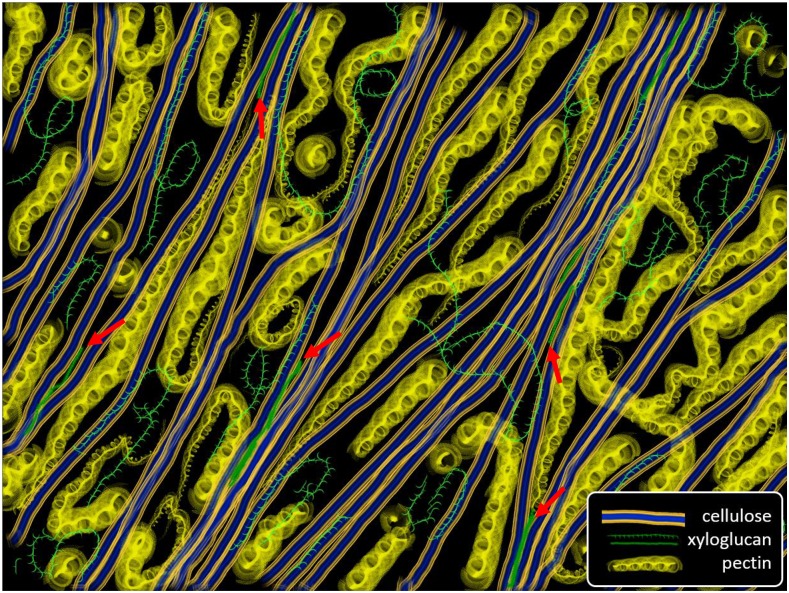
AFM can also be used to image cell wall surfaces at sufficiently high resolution to detect individual cellulose microfibrils (approximately 3 nm diameter; Fig. 3, A and B), revealing cellulose organization in unprecedented detail (Zhang et al., 2014). Note that fluorescence microscopy, even super resolution versions, lacks the resolution needed to see individual microfibrils in the complex and dense fibrillar network of cell walls. Transmission electron microscopy generally gives limited information about microfibril organization in cell walls, with the exception of the replica/shadowing method (McCann et al., 1990), which has been superseded by FESEM. FESEM has excellent resolving power for microfibril detection (Fujita and Wasteneys, 2014; Zheng et al., 2017a), but to attain high resolution, the wall must be dehydrated, which means wall polymers may become distorted or shift position as the water is removed. In contrast, AFM can be carried out under water, allowing imaging of walls in a near-native state. The surface topology can be measured with nm resolution and simultaneously probed mechanically to measure an indentation modulus (resistance to surface deformation).
Figure 3.
AFM images showing the arrangement of cellulose microfibrils and matrix on the inner surface of the periclinal wall of onion epidermis. A, Large-scale (2 × 2 μm) peak force error map showing microfibril bundling and cross-lamellate organization of microfibrils. Image adapted from Zhang et al. (2014). B, Close-up of boxed region in A. Single microfibrils are seen to bundle into groups of two of more. Some microfibril details are obscured by relatively stiff matrix. C, Two-color merged image based on a height map (red), which highlights microfibrils, and a modulus map (green), which indicates stiffness (resistance to indentation). The area outlined in white contains regions of stiff matrix (bright green) closely associated with microfibrils. Other matrix regions are dark, indicating they are soft. D, Two-color merged image based on a height map (red, predominantly microfibrils) and a deformation map (green, predominantly matrix). Regions of soft matrix between microfibrils are obvious in this image. Images adapted from Zhang et al. (2016). E, Modulus map of onion wall in a relaxed state (small axial force); the predominance of blue color indicates low resistance to indentation. F, Modulus map of the same wall region upon application of axial force, stretching the wall in the direction indicated by the arrow. The predominance of red shows that microfibrils have been pulled taut by the axial force, indicating they are load bearing, as are matrix components, though to a lesser extent. Images adapted from Zhang et al. (2017).
We used AFM to characterize cellulose microfibril organization of the outer (periclinal) wall of onion scales (the fleshy leaf of the bulb). Epidermal peels from onion scales have been used in numerous studies to connect tissue-level mechanics, growth, and net cellulose orientation (Wilson et al., 2000; Hepworth and Bruce, 2004; Suslov et al., 2009; Beauzamy et al., 2015a). In previous studies, whole epidermal layers were prepared with intact (living) cells. We developed an alternative procedure to prepare epidermal strips in which the outer (periclinal) wall tears away from the rest of the cell. This exposes the inner (most recently synthesized) surface for imaging by AFM and provides a simpler material for mechanical studies (a sheet of outer epidermal walls rather than a layer of intact turgid cells with complex architecture).
Like the outer epidermal walls in many plant organs, including Arabidopsis hypocotyls (Crowell et al., 2011), the epidermal wall in onion is thick (2 or more μm, depending on which scale is used) and has a cross-lamellate construction. In AFM images, individual microfibrils in the surface lamella are seen to form a network with single microfibrils merging into and out of bundled regions where two or more microfibrils are laterally aligned and in close contact (Fig. 3, A and B; Zhang et al., 2016). Microfibrils are arranged roughly in a common direction in each lamella, and microfibril orientation shifts abruptly by 30° to 90° between adjacent lamellae, producing a wall comprised of many, highly anisotropic lamellae in a wide range of orientations. The result of this cross-lamellate structure is that the whole wall has much weaker net structural anisotropy than the individual lamellae that make up the wall. By combining height maps with modulus maps and deformation maps, we can visualize microfibrils in the context of rigid and soft regions of the matrix (Fig. 3, C and D). Moreover, by stretching the wall, we can detect microfibril motions and detect which components become more resistant to indentation, an indication that they bear some of the tensile force (Fig. 3, E and F).
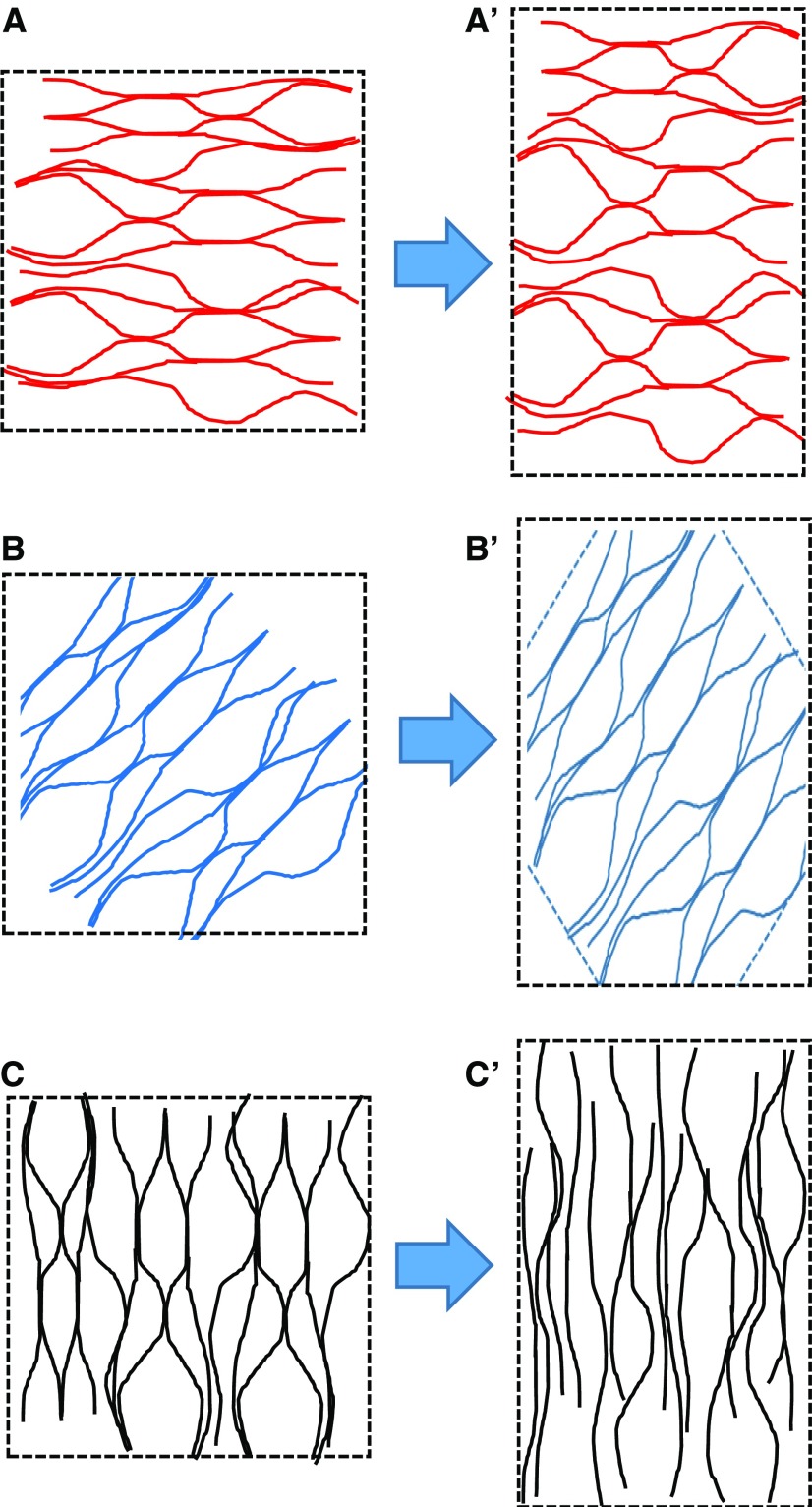
This cross-lamellate construction has important implications for the patterns of microfibril separation during cell growth. It is commonly accepted that cell walls expand preferentially in the direction at right angle to the net direction of cellulose alignment (Baskin, 2005; Suslov et al., 2009). Multicellular tissues present additional structural complications beyond the scope of this review (Crowell et al., 2010; Baskin and Jensen, 2013). For a cell wall with net transverse orientation, it is easy to imagine that the distance between microfibrils increases as the cell elongates axially. This is commonly illustrated by analogy with the way a wound spring elongates (like a Slinky toy). But what happens in a cross-lamellate wall where cellulose is organized in lamellae that are aligned in many orientations, axially, transversely, and at a spectrum of angles between these two orthogonal directions? Specifically, how do microfibrils move in lamellae where cellulose microfibrils are aligned parallel to the direction of maximal growth? In considering this question, I assume that microfibrils are too stiff to stretch appreciably and too strong to break, and also assume that adjacent lamellae do not slip past each other. With these assumptions, it seems that microfibrils in these axially aligned lamellae must have a mechanism of side-by-side gliding or axial shearing (Fig. 4). If axial shearing of this type requires more force than lateral separation of microfibrils in transversely aligned lamellae, then axial shearing between microfibrils aligned in the direction of cell growth may limit the rate of cell wall enlargement. This reasoning puts the focus of attention on the molecular nature of the lateral associations between microfibrils and the patterns of microfibril motions during wall expansion. We know rather little about this aspect of primary cell wall growth.
Figure 4.
Predicted patterns of microfibril movement in a cross-lamellate cell wall stretched uniaxially. Shown is cellulose alignment in single lamellae before (left) and after (right) stretching in the vertical direction. Axial elongation is accompanied by transverse shrinkage during elastic uniaxial extensions. A, In lamellae with cellulose oriented transverse to the direction of stretch, the axial distance between microfibrils will increase and cellulose microfibrils will bend or kink in the transverse direction. B, For lamellae with cellulose oriented at approximately 45°, microfibril angle will shift in the axial direction and distance between microfibrils will be reduced. C, For lamellae with cellulose oriented in the same direction as the axial stretch, microfibrils will become straighter, more closely packed, and will undergo side-by-side sliding. Figure is adapted from Zhang et al. (2016).
These inferences about microfibril movements gain support from a recent study in which the nanoscale movements of cellulose microfibrils were directly monitored by AFM (Zhang et al., 2017). The cell wall was extended in a well-defined series of extensions that included elastic and plastic strains imposed by axial force as well as time-dependent creep induced by treatment with an endoglucanase with wall-loosening activity. For lamellae in which the microfibrils were oriented at 30° to 60° off the axis of applied tensile force, mechanical stretching resulted in passive reorientation of microfibrils in the direction of stretch, as predicted by multinet growth models (Preston, 1982). In addition, examples of axial shearing and lateral separation were observed, providing direct evidence for the microfibril movements inferred above from general considerations. When cell wall creep was induced by application of a wall-loosening endoglucanase, remarkably different patterns of microfibril movements were observed compared with those during elastic and plastic deformations. This difference was attributed to changes in microfibril connectivity, i.e. selective loosening at hotspots by endoglucanase action. Thus, the stretching of a wound spring does not seem an apt analogy for how microfibrils move during cell wall growth.
This AFM study documents an example where microfibril movements motivated by applied force differed from those that occurred when wall expansion was induced by wall loosening. The microfibrils in differently aligned lamellae displayed the large diversity of microfibril movements that are required for enlargement of cross-lamellate walls. It is possible that different patterns of microfibril movement involve different matrix components, e.g. xyloglucans, xylans, pectins, or even water alone, and are mediated by different wall-loosening agents.
PERSPECTIVE
As new tools and new approaches have been applied to investigate cell growth, it has become evident that our conventional model of the growing cell wall falls short. Xyloglucan seems to have rather different functional roles than those hypothesized in conventional depictions of the growing cell wall of the past 40 years. New evidence for the role of pectins in wall structure and morphogenesis are tantalizing, yet raise new questions and the biological responses evoked by CWI sensors complicate the interpretation of cell wall mutants. Finally, the new appreciation of the complexity of cellulose organization in the growing wall presents opportunities for rethinking the molecular control of diffuse growth. Experimental systems that enable studies of both pectin and cellulose networks are needed for future integration of these emerging ideas.
Footnotes
Work on expansins is supported by the U.S. Department of Energy (grant no. DE-FG2-84ER13179) and work on cell wall structure is supported as part of the Center for LignoCellulose Structure and Formation, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences (award no. DE-SC0001090).
Articles can be viewed without a subscription.
References
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A (2011) Plant Cell Walls. Garland, New York [Google Scholar]
- Armour WJ, Barton DA, Law AM, Overall RL (2015) Differential growth in periclinal and anticlinal walls during lobe formation in Arabidopsis cotyledon pavement cells. Plant Cell 27: 2484–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbez E, Dünser K, Gaidora A, Lendl T, Busch W (2017) Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc Natl Acad Sci USA 114: E4884–E4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21: 203–222 [DOI] [PubMed] [Google Scholar]
- Baskin TI, Jensen OE (2013) On the role of stress anisotropy in the growth of stems. J Exp Bot 64: 4697–4707 [DOI] [PubMed] [Google Scholar]
- Beauzamy L, Derr J, Boudaoud A (2015a) Quantifying hydrostatic pressure in plant cells by using indentation with an atomic force microscope. Biophys J 108: 2448–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauzamy L, Louveaux M, Hamant O, Boudaoud A (2015b) Mechanically, the shoot apical meristem of Arabidopsis behaves like a shell inflated by a pressure of about 1 MPa. Front Plant Sci 6: 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova TN, Jacob T, Dahse I, Gilroy S (1998) Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development 125: 2925–2934 [DOI] [PubMed] [Google Scholar]
- Boudaoud A. (2010) An introduction to the mechanics of morphogenesis for plant biologists. Trends Plant Sci 15: 353–360 [DOI] [PubMed] [Google Scholar]
- Boyer JS. (2016) Enzyme-less growth in Chara and terrestrial plants. Front Plant Sci 7: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braybrook SA. (2017) Analyzing cell wall elasticity after hormone treatment: an example using tobacco BY-2 cells and auxin. Methods Mol Biol 1497: 125–133 [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Peaucelle A (2013) Mechano-chemical aspects of organ formation in Arabidopsis thaliana: the relationship between auxin and pectin. PLoS One 8: e57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Gidley MJ, Fincher GB (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6: 724–732 [DOI] [PubMed] [Google Scholar]
- Campàs O, Rojas E, Dumais J, Mahadevan L (2012) Strategies for cell shape control in tip-growing cells. Am J Bot 99: 1577–1582 [DOI] [PubMed] [Google Scholar]
- Carpita NC. (1996) Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol 47: 445–476 [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M, et al. (2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20: 1519–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2011) THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr Opin Plant Biol 14: 632–641 [DOI] [PubMed] [Google Scholar]
- Cleland RE. (1984) The Instron technique as a measure of immediate-past wall extensibility. Planta 160: 514–520 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (1993a) How do plant cell walls extend? Plant Physiol 102: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (1993b) Wall extensibility: its nature, measurement and relationship to plant cell growth. New Phytol 124: 1–23 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (1996) Plant cell enlargement and the action of expansins. BioEssays 18: 533–540 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2014) Re-constructing our models of cellulose and primary cell wall assembly. Curr Opin Plant Biol 22: 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2015) Plant expansins: diversity and interactions with plant cell walls. Curr Opin Plant Biol 25: 162–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2016a) Catalysts of plant cell wall loosening. F1000Res 5: F1000 Faculty Rev-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2016b) Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J Exp Bot 67: 463–476 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ, Durachko DM (1994) Autolysis and extension of isolated walls from growing cucumber hypocotyls. J Exp Bot 45: 1711–1719 [DOI] [PubMed] [Google Scholar]
- Crowell EF, Gonneau M, Vernhettes S, Höfte H (2010) Regulation of anisotropic cell expansion in higher plants. C R Biol 333: 320–324 [DOI] [PubMed] [Google Scholar]
- Crowell EF, Timpano H, Desprez T, Franssen-Verheijen T, Emons AM, Höfte H, Vernhettes S (2011) Differential regulation of cellulose orientation at the inner and outer face of epidermal cells in the Arabidopsis hypocotyl. Plant Cell 23: 2592–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reuille PB, Robinson S, Smith RS (2014) Quantifying cell shape and gene expression in the shoot apical meristem using MorphoGraphX. Methods Mol Biol 1080: 121–134 [DOI] [PubMed] [Google Scholar]
- Ding SY, Liu YS, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338: 1055–1060 [DOI] [PubMed] [Google Scholar]
- Dumais J. (2013) Modes of deformation of walled cells. J Exp Bot 64: 4681–4695 [DOI] [PubMed] [Google Scholar]
- Dumais J, Shaw SL, Steele CR, Long SR, Ray PM (2006) An anisotropic-viscoplastic model of plant cell morphogenesis by tip growth. Int J Dev Biol 50: 209–222 [DOI] [PubMed] [Google Scholar]
- Edelmann HG, Kohler K (1995) Auxin increases elastic wall-properties in rye coleoptiles: implications for the mechanism of wall loosening. Physiol Plant 93: 85–92 [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H (2000) PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12: 2409–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayant P, Girlanda O, Chebli Y, Aubin CE, Villemure I, Geitmann A (2010) Finite element model of polar growth in pollen tubes. Plant Cell 22: 2579–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AJ, Caderas D, Wehrli E, McQueen-Mason S, Kuhlemeier C (1999) Analysis of expansin-induced morphogenesis on the apical meristem of tomato. Planta 208: 166–174 [Google Scholar]
- Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C (1997) Induction of leaf primordia by the cell wall protein expansin. Science 276: 1415 [Google Scholar]
- Fujita M, Wasteneys GO (2014) A survey of cellulose microfibril patterns in dividing, expanding, and differentiating cells of Arabidopsis thaliana. Protoplasma 251: 687–698 [DOI] [PubMed] [Google Scholar]
- Geitmann A, Parre E (2004) The local cytomechanical properties of growing pollen tubes correspond to the axial distribution of structural cellular elements. Sex Plant Reprod 17: 9–16 [Google Scholar]
- Georgelis N, Yennawar NH, Cosgrove DJ (2012) Structural basis for entropy-driven cellulose binding by a type-A cellulose-binding module (CBM) and bacterial expansin. Proc Natl Acad Sci USA 109: 14830–14835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R, Morvan C, Roland JC (1986) Composition, properties and localization of pectins in young and mature cells of the mung bean hypocotyl. Plant Cell Physiol 27: 417–429 [Google Scholar]
- Gore A. (2006) An Inconvenient Truth: The Planetary Emergency of Global Warming and What We Can Do About It. Rodale Books, New York [Google Scholar]
- Grantham NJ, Wurman-Rodrich J, Terrett OM, Lyczakowski JJ, Stott K, Iuga D, Simmons TJ, Durand-Tardif M, Brown SP, Dupree R, et al. (2017) An even pattern of xylan substitution is critical for interaction with cellulose in plant cell walls. Nat Plants 3: 859–865 [DOI] [PubMed] [Google Scholar]
- Green PB, Steele CS, Rennich SC (1996) Phyllotactic patterns: a biophysical mechanism for their origin. Ann Bot (Lond) 77: 515–527 [Google Scholar]
- Gu F, Bringmann M, Combs JR, Yang J, Bergmann DC, Nielsen E (2016) Arabidopsis CSLD5 functions in cell plate formation in a cell cycle-dependent manner. Plant Cell 28: 1722–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A. (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116: 483–505 [DOI] [PubMed] [Google Scholar]
- Hamant O, Haswell ES (2017) Life behind the wall: sensing mechanical cues in plants. BMC Biol 15: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Gray WM, Sussman MR (2015) Regulation of the plasma membrane proton pump (H(+)-ATPase) by phosphorylation. Curr Opin Plant Biol 28: 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Höfte H (2007) A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Hepworth DG, Bruce DM (2004) Relationships between primary plant cell wall architecture and mechanical properties for onion bulb scale epidermal cells. J Texture Stud 35: 586–602 [Google Scholar]
- Heyn AJN. (1932) Further investigations of the mechanism of cell elongation and the properties of the cell wall in connection with elongation. Protoplasma 19: 78–97 [Google Scholar]
- Hocq L, Pelloux J, Lefebvre V (2017) Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci 22: 20–29 [DOI] [PubMed] [Google Scholar]
- Höfte H. (2015) The yin and yang of cell wall integrity control: brassinosteroid and FERONIA signaling. Plant Cell Physiol 56: 224–231 [DOI] [PubMed] [Google Scholar]
- Hohl M, Schopfer P (1992) Physical extensibility of maize coleoptile cell walls: apparent plastic extensibility is due to elastic hysteresis. Planta 187: 498–504 [DOI] [PubMed] [Google Scholar]
- Hrmova M, Farkas V, Lahnstein J, Fincher GB (2007) A barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-beta-D-glucans. J Biol Chem 282: 12951–12962 [DOI] [PubMed] [Google Scholar]
- Huang R, Becker AA, Jones IA (2015) A finite strain fibre-reinforced viscoelasto-viscoplastic model of plant cell wall growth. J Eng Math 95: 121–154 [Google Scholar]
- Humphrey TV, Bonetta DT, Goring DR (2007) Sentinels at the wall: cell wall receptors and sensors. New Phytol 176: 7–21 [DOI] [PubMed] [Google Scholar]
- Ivakov A, Flis A, Apelt F, Fünfgeld M, Scherer U, Stitt M, Kragler F, Vissenberg K, Persson S, Suslov D (2017) Cellulose synthesis and cell expansion are regulated by different mechanisms in growing Arabidopsis hypocotyls. Plant Cell 29: 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K, Talmadge KW, Bauer WD, Albersheim P (1973) The structure of plant cell walls. III. A model of the walls of suspension-cultured sycamore cells based on the interconnections of the macromolecular components. Plant Physiol 51: 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierzkowski D, Lenhard M, Smith R, Kuhlemeier C (2013) Interaction between meristem tissue layers controls phyllotaxis. Dev Cell 26: 616–628 [DOI] [PubMed] [Google Scholar]
- Kierzkowski D, Nakayama N, Routier-Kierzkowska AL, Weber A, Bayer E, Schorderet M, Reinhardt D, Kuhlemeier C, Smith RS (2012) Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science 335: 1096–1099 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Brandizzi F (2016) The plant secretory pathway for the trafficking of cell wall polysaccharides and glycoproteins. Glycobiology 26: 940–949 [DOI] [PubMed] [Google Scholar]
- Li C, Wu HM, Cheung AY (2016a) FERONIA and her pals: functions and mechanisms. Plant Physiol 171: 2379–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bashline L, Zheng Y, Xin X, Huang S, Kong Z, Kim SH, Cosgrove DJ, Gu Y (2016b) Cellulose synthase complexes act in a concerted fashion to synthesize highly aggregated cellulose in secondary cell walls of plants. Proc Natl Acad Sci USA 113: 11348–11353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MC, Wells B, Roberts K (1990) Direct visualization of cross-links in the primary plant cell wall. J Cell Sci 96: 323–334 [Google Scholar]
- Milani P, Braybrook SA, Boudaoud A (2013) Shrinking the hammer: micromechanical approaches to morphogenesis. J Exp Bot 64: 4651–4662 [DOI] [PubMed] [Google Scholar]
- Milani P, Mirabet V, Cellier C, Rozier F, Hamant O, Das P, Boudaoud A (2014) Matching patterns of gene expression to mechanical stiffness at cell resolution through quantitative tandem epifluorescence and nanoindentation. Plant Physiol 165: 1399–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104: 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Faria-Blanc N, Yu X, Tryfona T, Sorieul M, Ng YZ, Zhang Z, Stott K, Anders N, Dupree P (2015) An unusual xylan in Arabidopsis primary cell walls is synthesised by GUX3, IRX9L, IRX10L and IRX14. Plant J 83: 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca G, Sapala A, Strauss S, Routier-Kierzkowska AL, Smith RS (2017) On the micro-indentation of plant cells in a tissue context. Phys Biol 14: 015003. [DOI] [PubMed] [Google Scholar]
- Mouille G, Ralet MC, Cavelier C, Eland C, Effroy D, Hématy K, McCartney L, Truong HN, Gaudon V, Thibault JF, et al. (2007) Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J 50: 605–614 [DOI] [PubMed] [Google Scholar]
- Moulia B. (2013) Plant biomechanics and mechanobiology are convergent paths to flourishing interdisciplinary research. J Exp Bot 64: 4617–4633 [DOI] [PubMed] [Google Scholar]
- Nakayama N, Smith RS, Mandel T, Robinson S, Kimura S, Boudaoud A, Kuhlemeier C (2012) Mechanical regulation of auxin-mediated growth. Curr Biol 22: 1468–1476 [DOI] [PubMed] [Google Scholar]
- Newman RH, Hill SJ, Harris PJ (2013) Wide-angle x-ray scattering and solid-state nuclear magnetic resonance data combined to test models for cellulose microfibrils in mung bean cell walls. Plant Physiol 163: 1558–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezhad AS, Naghavi M, Packirisamy M, Bhat R, Geitmann A (2013) Quantification of the Young’s modulus of the primary plant cell wall using Bending-Lab-On-Chip (BLOC). Lab Chip 13: 2599–2608 [DOI] [PubMed] [Google Scholar]
- Oehme DP, Doblin MS, Wagner J, Bacic A, Downton MT, Gidley MJ (2015) Gaining insight into cell wall cellulose macrofibril organisation by simulating microfibril adsorption. Cellulose 22: 3501–3520 [Google Scholar]
- Ortega JKE. (2017) Dimensionless number is central to stress relaxation and expansive growth of the cell wall. Sci Rep 7: 3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491–1495 [DOI] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ (2012a) Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol 158: 465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ (2012b) A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol 158: 1933–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parre E, Geitmann A (2005) Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220: 582–592 [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H (2011) Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr Biol 21: 1720–1726 [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Louvet R, Johansen JN, Höfte H, Laufs P, Pelloux J, Mouille G (2008) Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr Biol 18: 1943–1948 [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Wightman R, Höfte H (2015) The control of growth symmetry breaking in the Arabidopsis hypocotyl. Curr Biol 25: 1746–1752 [DOI] [PubMed] [Google Scholar]
- Pereira CS, Silveira RL, Dupree P, Skaf MS (2017) Effects of xylan side-chain substitutions on xylan-cellulose interactions and implications for thermal pretreatment of cellulosic biomass. Biomacromolecules 18: 1311–1321 [DOI] [PubMed] [Google Scholar]
- Phyo P, Wang T, Kiemle SN, O’Neill H, Pingali SV, Hong M, Cosgrove DJ (2017) Gradients in wall mechanics and polysaccharides along growing inflorescence stems. Plant Physiol 175: 1593–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98: 11812–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston RD. (1982) The case for multinet growth in growing walls of plant cells. Planta 155: 356–363 [DOI] [PubMed] [Google Scholar]
- Proseus TE, Zhu GL, Boyer JS (2000) Turgor, temperature and the growth of plant cells: using Chara corallina as a model system. J Exp Bot 51: 1481–1494 [DOI] [PubMed] [Google Scholar]
- Ray PM, Green PB, Cleland R (1972) Role of turgor in plant cell growth. Nature 239: 163–164 [Google Scholar]
- Refrégier G, Pelletier S, Jaillard D, Höfte H (2004) Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis. Plant Physiol 135: 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Wittwer F, Mandel T, Kuhlemeier C (1998) Localized upregulation of a new expansin gene predicts the site of leaf formation in the tomato meristem. Plant Cell 10: 1427–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Burian A, Couturier E, Landrein B, Louveaux M, Neumann ED, Peaucelle A, Weber A, Nakayama N (2013) Mechanical control of morphogenesis at the shoot apex. J Exp Bot 64: 4729–4744 [DOI] [PubMed] [Google Scholar]
- Rojas ER, Hotton S, Dumais J (2011) Chemically mediated mechanical expansion of the pollen tube cell wall. Biophys J 101: 1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routier-Kierzkowska AL, Smith RS (2013) Measuring the mechanics of morphogenesis. Curr Opin Plant Biol 16: 25–32 [DOI] [PubMed] [Google Scholar]
- Routier-Kierzkowska AL, Weber A, Kochova P, Felekis D, Nelson BJ, Kuhlemeier C, Smith RS (2012) Cellular force microscopy for in vivo measurements of plant tissue mechanics. Plant Physiol 158: 1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesink AW. (1969) Polysaccharidases and the control of cell wall elongation. Planta 89: 95–107 [DOI] [PubMed] [Google Scholar]
- Sahaf M, Sharon E (2016) The rheology of a growing leaf: stress-induced changes in the mechanical properties of leaves. J Exp Bot 67: 5509–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Krupinski P, Wightman R, Milani P, Berquand A, Boudaoud A, Hamant O, Jönsson H, Meyerowitz EM (2014a) Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 3: e01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Yan A, Krupinski P, Meyerowitz EM (2014b) Physical forces regulate plant development and morphogenesis. Curr Biol 24: R475–R483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanati Nezhad A, Geitmann A (2013) The cellular mechanics of an invasive lifestyle. J Exp Bot 64: 4709–4728 [DOI] [PubMed] [Google Scholar]
- Sanati Nezhad A, Naghavi M, Packirisamy M, Bhat R, Geitmann A (2013) Quantification of cellular penetrative forces using lab-on-a-chip technology and finite element modeling. Proc Natl Acad Sci USA 110: 8093–8098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanati Nezhad A, Packirisamy M, Geitmann A (2014) Dynamic, high precision targeting of growth modulating agents is able to trigger pollen tube growth reorientation. Plant J 80: 185–195 [DOI] [PubMed] [Google Scholar]
- Sassi M, Ali O, Boudon F, Cloarec G, Abad U, Cellier C, Chen X, Gilles B, Milani P, Friml J, et al. (2014) An auxin-mediated shift toward growth isotropy promotes organ formation at the shoot meristem in Arabidopsis. Curr Biol 24: 2335–2342 [DOI] [PubMed] [Google Scholar]
- Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB (2014) The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24: 1887–1892 [DOI] [PubMed] [Google Scholar]
- Shinohara N, Sunagawa N, Tamura S, Yokoyama R, Ueda M, Igarashi K, Nishitani K (2017) The plant cell-wall enzyme AtXTH3 catalyses covalent cross-linking between cellulose and cello-oligosaccharide. Sci Rep 7: 46099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons TJ, Mohler KE, Holland C, Goubet F, Franková L, Houston DR, Hudson AD, Meulewaeter F, Fry SC (2015) Hetero-trans-β-glucanase, an enzyme unique to Equisetum plants, functionalizes cellulose. Plant J 83: 753–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons TJ, Mortimer JC, Bernardinelli OD, Pöppler A-C, Brown SP, deAzevedo ER, Dupree R, Dupree P (2016) Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat Commun 7: 13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslov D, Verbelen JP, Vissenberg K (2009) Onion epidermis as a new model to study the control of growth anisotropy in higher plants. J Exp Bot 60: 4175–4187 [DOI] [PubMed] [Google Scholar]
- Szymanski DB. (2014) The kinematics and mechanics of leaf expansion: new pieces to the Arabidopsis puzzle. Curr Opin Plant Biol 22: 141–148 [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Cosgrove DJ (2009) Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol 19: R800–R811 [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Staiger C (2017) The actin cytoskeleton: functional arrays for cytoplasmic organization and cell shape control. Plant Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Eberhard S, Pattathil S, Warder C, Glushka J, Yuan C, Hao Z, Zhu X, Avci U, Miller JS, et al. (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25: 270–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez SM, Barbez E, Kleine-Vehn J, Estevez JM (2016) Auxin and cellular elongation. Plant Physiol 170: 1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk SS, Cleland RE (1990) The role of wall calcium in the extension of cell walls of soybean hypocotyls. Planta 182: 559–564 [PubMed] [Google Scholar]
- Vogler H, Felekis D, Nelson BJ, Grossniklaus U (2015) Measuring the mechanical properties of plant cell walls. Plants (Basel) 4: 167–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voxeur A, Höfte H (2016) Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology 26: 950–960 [DOI] [PubMed] [Google Scholar]
- Wang T, Chen Y, Tabuchi A, Cosgrove DJ, Hong M (2016a) The target of β-expansin EXPB1 in maize cell walls from binding and solid-state NMR studies. Plant Physiol 172: 2107–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hong M (2016) Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J Exp Bot 67: 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Park YB, Caporini MA, Rosay M, Zhong L, Cosgrove DJ, Hong M (2013) Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc Natl Acad Sci USA 110: 16444–16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Park YB, Cosgrove DJ, Hong M (2015) Cellulose-pectin spatial contacts are inherent to never-dried Arabidopsis primary cell walls: Evidence from solid-state nuclear magnetic resonance. Plant Physiol 168: 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Yang H, Kubicki JD, Hong M (2016b) Cellulose structural polymorphism in plant primary cell walls investigated by high-field 2D solid-state NMR spectroscopy and density functional theory calculations. Biomacromolecules 17: 2210–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zabotina O, Hong M (2012) Pectin-cellulose interactions in the Arabidopsis primary cell wall from two-dimensional magic-angle-spinning solid-state nuclear magnetic resonance. Biochemistry 51: 9846–9856 [DOI] [PubMed] [Google Scholar]
- White PB, Wang T, Park YB, Cosgrove DJ, Hong M (2014) Water-polysaccharide interactions in the primary cell wall of Arabidopsis thaliana from polarization transfer solid-state NMR. J Am Chem Soc 136: 10399–10409 [DOI] [PubMed] [Google Scholar]
- Wilson RH, Smith AC, Kacuráková M, Saunders PK, Wellner N, Waldron KW (2000) The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by Fourier-transform infrared spectroscopy. Plant Physiol 124: 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Mravec J, Greiner S, Mouille G, Höfte H (2012) Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr Biol 22: 1732–1737 [DOI] [PubMed] [Google Scholar]
- Wolf S, van der Does D, Ladwig F, Sticht C, Kolbeck A, Schürholz AK, Augustin S, Keinath N, Rausch T, Greiner S, et al. (2014) A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc Natl Acad Sci USA 111: 15261–15266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang T, Zheng Y, Cosgrove DJ, Anderson CT (2016) Xyloglucan deficiency disrupts microtubule stability and cellulose biosynthesis in Arabidopsis, altering cell growth and morphogenesis. Plant Physiol 170: 234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Desyatova AS, Belteton SA, Mallery EL, Turner JA, Szymanski DB (2015) Patterning mechanisms of cytoskeletal and cell wall systems during leaf trichome morphogenesis. Nat Plants 1: 15014. [DOI] [PubMed] [Google Scholar]
- Yang W, Schuster C, Beahan CT, Charoensawan V, Peaucelle A, Bacic A, Doblin MS, Wightman R, Meyerowitz EM (2016) Regulation of meristem morphogenesis by cell wall synthases in Arabidopsis. Curr Biol 26: 1404–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZM, Kang B, He XW, Lv SL, Bai YH, Ding WN, Chen M, Cho HT, Wu P (2011) Root hair-specific expansins modulate root hair elongation in rice. Plant J 66: 725–734 [DOI] [PubMed] [Google Scholar]
- Yuan S, Wu Y, Cosgrove DJ (2001) A fungal endoglucanase with plant cell wall extension activity. Plant Physiol 127: 324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablackis E, Huang J, Müller B, Darvill AG, Albersheim P (1995) Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol 107: 1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Mallery EL, Szymanski DB (2013) ARP2/3 localization in Arabidopsis leaf pavement cells: a diversity of intracellular pools and cytoskeletal interactions. Front Plant Sci 4: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Mahgsoudy-Louyeh S, Tittmann B, Cosgrove DJ (2014) Visualization of the nanoscale pattern of recently-deposited cellulose microfibrils and matrix materials in never-dried primary walls of the onion epidermis. Cellulose 21: 853–862 [Google Scholar]
- Zhang T, Vavylonis D, Durachko DM, Cosgrove DJ (2017) Nanoscale movements of cellulose microfibrils in primary cell walls. Nat Plants 3: 17056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zheng Y, Cosgrove DJ (2016) Spatial organization of cellulose microfibrils and matrix polysaccharides in primary plant cell walls as imaged by multichannel atomic force microscopy. Plant J 85: 179–192 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Yuan S, Wang X, Zhang Y, Zhu H, Lu C (2008) Restoration of mature etiolated cucumber hypocotyl cell wall susceptibility to expansin by pretreatment with fungal pectinases and EGTA in vitro. Plant Physiol 147: 1874–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Crespi VH, Kubicki JD, Cosgrove DJ, Zhong L (2014) Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: effects of surface hydrophobicity and side-chain variation. Cellulose 21: 1025–1039 [Google Scholar]
- Zheng Y, Cosgrove DJ, Ning G (2017a) High-resolution field emission scanning electron microscopy (FESEM) imaging of cellulose microfibril organization in plant primary cell walls. Microsc Microanal 23: 1048–1054 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wang X, Chen Y, Wagner E, Cosgrove DJ (2017b) Xyloglucan in the primary cell wall: assessment by FESEM, selective enzyme digestions and nanogold affinity tags. Plant J 10.1111/tpj.13778 [DOI] [PubMed] [Google Scholar]
- Zhu C, Ganguly A, Baskin TI, McClosky DD, Anderson CT, Foster C, Meunier KA, Okamoto R, Berg H, Dixit R (2015) The fragile Fiber1 kinesin contributes to cortical microtubule-mediated trafficking of cell wall components. Plant Physiol 167: 780–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zykwinska A, Thibault JF, Ralet MC (2008a) Competitive binding of pectin and xyloglucan with primary cell wall cellulose. Carbohydr Polym 74: 957–961 [Google Scholar]
- Zykwinska A, Thibault JF, Ralet MC (2008b) Modelling of xyloglucan, pectins and pectic side chains binding onto cellulose microfibrils. Carbohydr Polym 74: 23–30 [Google Scholar]