Abstract
The present study used human lung fibroblast (HELF) cells as a test model to evaluate the role of oxidative stress (OS) and extracellular signal-regulated kinases 1/2 (ERK1/2) protein in HELF cell proliferation exposed to PCB118. Results from 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide demonstrated that PCB118 at lower concentrations stimulated proliferation of HELF cell and abrogate proliferative effect at higher dose concentrations and in a time-dependent manner. Moreover, reactive oxygen species, malondialdehyde (MDA), and superoxide dismutase showed a significant increase at higher concentrations of PCB118 than the lower concentrations with the passage of time. Antioxidant enzymes such as glutathione peroxidase exhibited decreasing trends in dose- and time-dependent manner. Lipid peroxidation assay resulted in a significant increase in MDA level in PCB118-treated HELF cells compared with controls, suggesting that OS plays a key role in PCB118-induced toxicity. Comet assay indicated a significant increase in genotoxicity at higher concentrations of PCB118 exposure than the lower concentrations. It was found that PCB118 showed expression of ERK1/2 protein after 4 hours, while after 48 hours, the protein expression was less, indicating PCB toxicity to MAPK protein of HELF cell. Oxidative stress, ERK1/2, and HELF cell proliferation exhibited correlation. The results will elaborate toxicological evaluation of PCB118 to HELF cells and will help to develop drug for PCB-induced diseases.
Keywords: hormesis, oxidative stress, xenobiotic and endocrine disruptor, MAPK, HELF cells
Introduction
The lung is a highly vascularized target organ for a wide variety of xenobiotic that may enter through inhalation and bloodstream; hence, lungs may be more vulnerable to chemical toxic effects.1 Among the chemical toxic effects, the highly reported are lung cancer,2,3 lung development process,4 and respiratory and asthma.5,6 It has been reported that the fine particulate matter (PM) with an aerodynamic diameter ≤2.5 µm (PM 2.5) in polluted air and contaminated food was associated with lung cancer and other health problems in humans and animals.7,8 Within these environmental polluting agents, polychlorinated biphenyls (PCBs), which are products of various industrial processes, are more concerned due to their persistent nature, lipophilic properties, and transfer into food chain.9 The PCBs are classified as 209 different congeners, and the toxicity of each PCB is structure-dependent mechanisms.
Fibroblast cells are the most common type of cells found in connective tissue and secrete collagen proteins that maintained a structural framework for many tissues and also help in wound healing.10,11 Recently, human lung fibroblast (HELF) cells have been used as a test model to evaluate metals toxic effects.12,13 However, to date, there is scarce information regarding PCBs to human lung cells in general and particularly to fibroblast cells. The studies of lung cells will help to understand how lung cells behave after exposure to PCBs. 2,3′,4,4′,5-pentachlorobiphenyl (PCB118) was selected because international agencies consider them suitable indicators of industrial pollution present in oil, fish, and so on.14
Oxidative stress (OS) characterized by the production of reactive oxygen species (ROS) in the cells is often triggered by exogenous substances.15,16 Normal cell signaling is activated by ROS, and deregulation (low or high) of ROS may alter the redox mechanisms which are responsible for expression of genes such as transcriptional factors that regulate cell differentiation, proliferation, and apoptosis. Among the consequences of enhanced ROS production is DNA damage.17 There are several antioxidant such as catalase, glutathione (GSH), and SOD that have been reported to keep ROS level as low as possible in organisms.18 Recently, it has been proved that OS may be linked to the hormetic effects19,20 and proposed one of the hormesis mechanistic approach, which are still lacking for most of the organisms in general and HELF cells particularly.
Many diverse extracellular stimuli, including chemicals, hormones, growth factors, osmolar shock, stress, and elevated in temperature, result in activation and phosphorylation of mitogen-activated protein kinases (MAPKs).21 The MAPKs (sometimes called extracellular signal-regulated kinases [ERKs] or JNK) comprise a family of related protein kinases that are themselves activated by phosphorylation on threonine and tyrosine residues. The MAPK-activating enzymes (MAPK/ERK or JNK kinases, or MEKs) are unusual in their ability to catalyze phosphorylation on both threonine and tyrosine residues.22,23 The ERK1/2 pathway typically transduces growth factor signals and involves in cell differentiation or proliferation.12 Several studies reported the activation of MAPK pathways in HELF cells exposed to Arsenic12 and human tissues.24 The activation of MAPK pathway has been studied for the Mytilus hemocytes exposed to coplanar and noncoplanar PCB congeners.25 However, studies regarding the activation of MAPK in HELF cell lines exposed to PCBs are scarce.
Previously, several studies have used HELF cells as a test model to demonstrate the chemicals toxic effects.12,13 However, studies regarding the toxic effect of PCBs and mechanism involved in HELF cells are still unclear.26 Hence, in the present study, we took HELF cells as a test model to figure out the involvement and relationship of OS and ERK1/2 protein in HELF cell proliferation exposed to PCB118.
Materials and Methods
Chemicals and Reagents
The PCB118 was purchased from AccuStandard, Inc (New Haven, Connecticut). The 2′,7′-dichlorofluorescein diacetate (DCFH-DA), 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Corp (St Louis, Missouri). The Dulbecco’s modified Eagle’s medium (DMEM) and Fetal Bovine Serum (FBS) were obtained from Gibco and Life Technologies (Grand Island, New York). The kits for the detection of glutathione peroxidase (GSH-Px) activity, MDA content, and SOD activity were purchased from Nanjing Jiancheng Biological Corp (New Jersey). All reagents used were of analytical grade. The PCB118 stock solution (20 µg/mL) was prepared in DMSO and further diluted to working concentrations. The concentration of DMSO in the culture medium was lower than 0.2% (vol/vol).
Cell Culture and Differentiation
The HELF cells were obtained from Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cells were cultured and maintained in DMEM enriched with 10% FBS and 100 U/mL of penicillin–streptomycin in 5% CO2 humidified at 37°C.
Cell Proliferation Assay
For the analysis of cell proliferation, cells were incubated at a density of 5 × 103 cells per well in 96-well plates. Then allowed the cells to adhere overnight and exposed to different concentration of PCB118 as control (CK), 10−5, 10−4, 10−3, 10−2, 10−1, 1, 5, 10, 20 μg/mL for 4, 12, 24, and 48 hours. After incubation, the medium was added 20 mL of MTT at 5 mg/mL in each well and further incubated for 4 hours at 37°C. Then culture medium was replaced and added 150 µL DMSO to dissolve the formazan violet crystals. The absorbance of each well was measured at 570 nm by a microplate reader (SpectraMax Plus 384, Sunnyvale, California).
Measurement of OS
Dimethyl sulfoxide was used to dissolve the probe DCFH-DA to make 50 mM as a stock solution. After treatment with PCB118 (control, 0.1, 0.5, 1, 5, or 10 μg/mL), HELF cells at a density of 1 × 105 cells/mL were suspended in 1 mL PBS with 100 μM of DCFH-DA for 1 hour at 37°C in the dark. Samples were washed with 1 mL PBS. Then absorbance was measured at 504 nm by SpectraMax Plus 384 microplate reader using the protocol for fluorometric measurement.27
Measurement of Antioxidant Enzymes
The measurement of lipid peroxidation (LPO) in the PCB118-treated HELF cells was done by using Jiancheng Biochemical assay kit (Nanjing, China). Briefly, an aliquot of the cell suspension (0.5 mL) was mixed with 2 mM sodium azide in 4.5 mL of 0.1 M PBS, pH 7.4. Then mixture was incubated at 37°C for 1 hour. After incubation, 2 mL of 28% wt/vol trichloroacetic acid was added, and the tube was vortexed and centrifuged at 1000g for 5 minutes. Then 4 mL supernatant was added to a tube that contains 1 mL of 1% wt/vol thiobarbituric acid. The mixture was boiled for 15 minutes and then immediately cooled. Absorbance was measured at 532 nm using a SpectraMax Plus 384 microplate reader.
The GSH-Px activities of the PCB118-treated HELF cells were measured by using the GSH-Px cellular assay (Jiancheng Biochemical). Briefly, an aliquot of the cell suspension (100 μL) was mixed with 700 μL reaction mixture containing 1 mM EDTA, 1 mM sodium azide, 0.2 mM NADPH, and 1 mM GSH in PBS, pH 7.2, and 100 μL containing 10 U GSH reductase. Then tubes were vortexed and incubated at room temperature for 5 minutes. After incubation, the absorbance was measured at 340 nm by using a SpectraMax Plus 384 microplate reader. The SOD activities in the PCB118-treated HELF cells were measured by the inhibition of pyrogallol autoxidation by SOD, as described by Jiancheng Biochemical Co, Ltd assay kit. Briefly, an aliquot of the cell suspension (200 μL) was mixed in 750 μL in assay mixture that contain 50 mM Tris–HCl, 1 mM EDTA, and 50 mM cacodylic acid with the addition of 250 μL of 2 mM pyrogallol. Then absorbance of the sample mixtures was measured at 550 nm by using a SpectraMax plus 384 microplate reader. The SOD’s one unit activity is defined as the amount of the enzyme required to prohibit the rate of pyrogallol autoxidation by 50%. The SOD activity was expressed as unit of enzyme/milligram of cell proteins.
Measurement of DNA Damage
The single-strand breaks in DNA after exposure to PCB118 for 24 hours were evaluated by alkaline single-cell gel electrophoresis (comet assay), as described previously.28 The HELF cells were treated with PCB118 with low concentration dose (control, 10−2, 5 µg/mL) alone for 24 hours or pretreated with low concentration of PCB118 for 24 hours, then treated with 20 µg/mL PCB118 for another 24 hours. Cells treated with 0.1% DMSO/media and 20 µg/mL PCB118 alone were used as negative and positive control, respectively. Normal melting agarose (NMA) and low-melting agarose (LMA) solutions were prepared in PBS. Sequentially, 100 μL 1% NMA was coated on warm frosted glass microscope slides. Then, cells after exposure were suspended in 0.7% LMA, and 75 μL of this solution was pipetted onto the first layer of gel. Finally, the third layer (80 μL of 0.5% LMA) was added. The precipitated “sandwich” gel was completely immersed into cooled (4°C) cell lysis buffer (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1% Triton X, 10% DMSO, pH 10.0) for 1 hour. After lysis, the slides were subjected to a horizontal gel electrophoresis in cooled alkaline electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, pH 12.5) at 25 V and 300 mA for 20 minutes. Then the slides were soaked with neutralization buffer (0.4 M Trizma base, pH 7.5, 4°C) for 8 minutes, twice, and air dried. The slides were stained with PI (20 μg/mL) and evaluated under a fluorescence microscope. Fifty randomly captured cells from each sample were measured using CASP software (University of Wroclaw, Poland) to quantify damage in DNA. Following 5 comet parameters were assessed—tail length (TL; distance from nuclear center to the end of the comet tail), tail DNA percentage (TD% [expressed by the percentage of fluorescent intensity in tail]), head DNA percentage (HD% [expressed by the percentage of fluorescent intensity in head]), tail moment (TM; product of TL and TD), and comet length. All of the experimental steps were performed under dim red light at 4°C to avoid additional DNA damage.
Protein Assay
Human lung fibroblast cell protein was measured as described previously.29 The absorbance of HELF cell protein was measured at 595 nm by SpectraMax Plus 384 microplate reader.
Western Blot Analysis
Human lung fibroblast cells were seeded in 6-well plates at a density of 1 × 105 cells per well for 24 hours. Then cells were treated with PCB118 at the concentrations of control, 0.001, 0.01, 1, 5, and 10 µg/mL for 4 and 48 hours, respectively. After treatment, the cells were collected, and cell homogenates were prepared by sonication of cells in 300 µL of ice-cold 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 5 mM NaF EDTA, 50 mM NaPPi, 1 µM PMSF, 1 mM DTT, 5 µg/mL leupeptin, 2 µg/mL aprotinin, and 1% NP-40. Homogenates were centrifuged at 12 000g for 30 minutes at 4°C. The protein levels were measured with the BCA (Bio-Rad, Hercules, California) assay. For Western blot analysis, protein samples were subjected to 10% SDS-PAGE with 8% spacer gel. Then proteins were transferred to a nitrocellulose membrane (Hybond ECL; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) at 330 mA for 1.5 hours, and the membrane was blocked with 5% nonfat milk. The membrane was incubated with antibodies (polyclonal rabbit antihuman cyclin E, CDK2, p21 cyclin D1, and caspases-9 dilution of 1:1000; Cell Signaling Technology, Inc, Beverly, Massachusetts) at 4°C at overnight. After washing, the secondary antibodies (dilution 1:5000) were incubated at room temperature for 2 hours. The protein densitometry analysis was measured by a Quantity One software.
Statistical Analyses
The statistical analysis was performed using SPSS 18.0 (SPSS Inc, Chicago, Illinois). One-way analysis of variance was used to analyze the data to compare the means of different PCB118 concentrations with control. The differences were considered statistically significant for P value less than .05. Results were presented as mean values (standard deviations [±SD]).
Results
Proliferation of HELF Cells Induced by PCB118
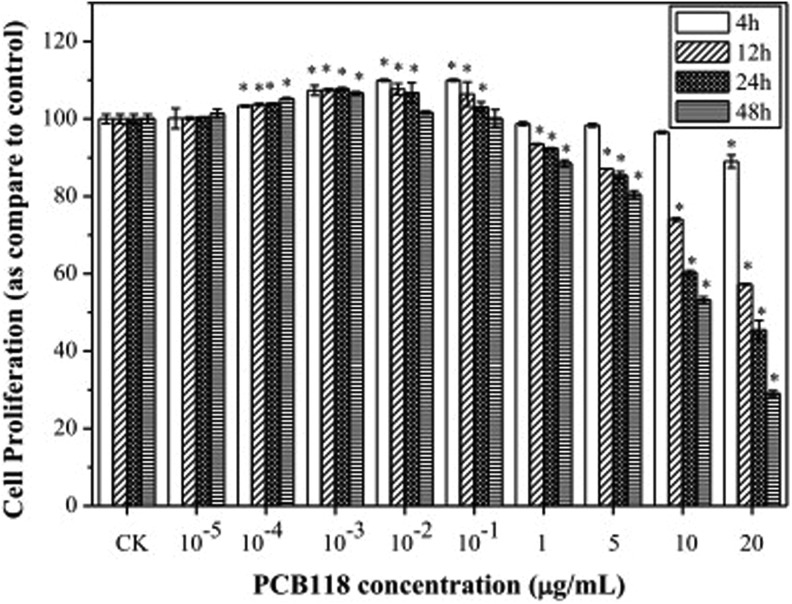
The effects of different concentrations of PCB118 on cell proliferation were examined by MTT assays following treatment with control, 10−5, 10−4, 10−3, 10−2, 10−1, 1, 5, 10, 20 μg/mL for 4, 12, 24, and 48 hours, respectively. Figure 1 shows that the cell proliferation was significantly increased in cells in dose- and time-dependent manner incubated with 10−4, 10−3, and 10−2 PCB118 μg/mL. In contrast, there was a marked decrease in cell proliferation in 1, 5, 10, and 20 μg/mL PCB118-exposed HELF cells (except 20 μg/mL for 4 hours).
Figure 1.
Human lung fibroblast (HELF) cell proliferation exposed to different concentrations of PCB118.
PCB118 showed stimulation in HELF cell proliferation at 5% to 30% and inhibition at 5% to 70%. The concentrations 10−2 and 10−1 μg/mL of PCB118 exhibited maximum stimulatory response in HELF cell, while 10 and 20 μg/mL of PCB118 exhibited more toxicity. Comparison among different time revealed that after 4 hours no significant toxic effects were observed exposed to PCB118. While after 12, 24, and 48 hours, significant reduction in HELF cell proliferation was observed exposed to PCB118.
Oxidative Stress Induced by PCB118
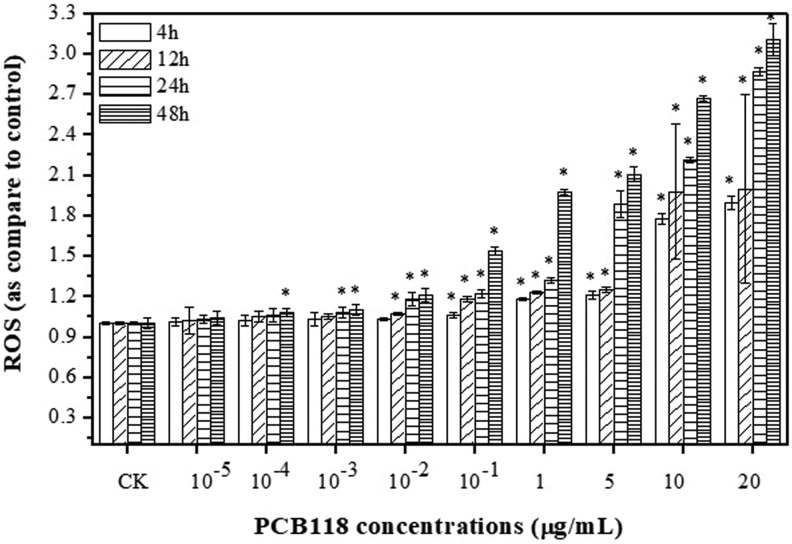
In order to understand the oxidative effects of PCB118, cells were incubated with DCFH-DA and the results showed increased oxidation for different time and doses after PCB118 treatments (Figure 2). Figure 2 revealed that low concentrations of PCB118 slightly increased the level of ROS, while higher concentrations exhibited significant increase in the level of ROS in time- and dose-dependent manner. The highest ROS level was recorded after 24 and 48 hours compared to the control (P < .05) for 10−1, 1, 5, 10, and 20 μg/mL of PCB118. The concentration 10−5, 10−4, 10−3, 10−2 μg/mL (24 and 48 hours) and 10−1 μg/mL (except 12, 24, and 48 hours) of PCB118 showed no significant increase in ROS production than the other doses and time in HELF cells. However, the 1, 5, 10, and 20 μg/mL of PCB118 indicated more ROS production after 12, 24, and 48 hours. The percentage of ROS in HELF cells exposed to 10 and 20 μg/mL of PCB118 after 12, 24, and 48 hours was 140% to 160%, 155% to 180%, and 170% to 200%, respectively.
Figure 2.
Reactive oxygen species (ROS) levels in human lungs fibroblast (HELF) cells exposed to PCB118.
Antioxidant Enzymes Induced by PCB118
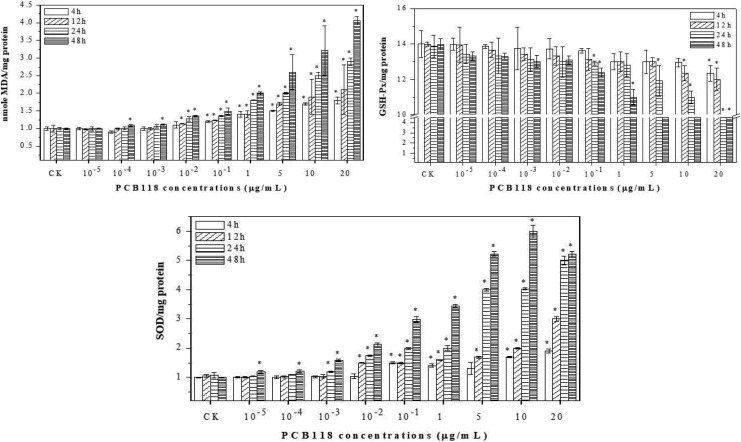
At lower concentrations of PCB118 such as 10−5, 10−4, 10−3, and 10−2, MDA level increased little; however, higher concentrations revealed a significant (P < .05) increasing response of MDA than the control. However, there was a little increase in MDA level after 4 and 12 hours with an increase in PCB118 concentrations. The results showed that PCB118 induced oxidative damage as evidenced by LPO at high doses. To examine a possible relationship between production of ROS and MDA levels by PCB118, we investigated the activities of major antioxidant enzymes, SOD and GSH-Px. As shown in Figure 3, the activities of GSH-Px were decreased, while the activities of SOD were increased (except 24 and 48 hours for 20 µg/mL of PCB118) in a concentration-dependent manner by PCB118. These results imply that increased production of ROS by PCB118 may be due to decreased cellular GSH-Px level and enhanced activities (except 24 and 48 hours for 20 µg/mL of PCB118) of SOD.
Figure 3.
Antioxidant and oxidant enzyme response in human lungs fibroblast (HELF) cells exposed to PCB118.
DNA Damage Induced by PCB118
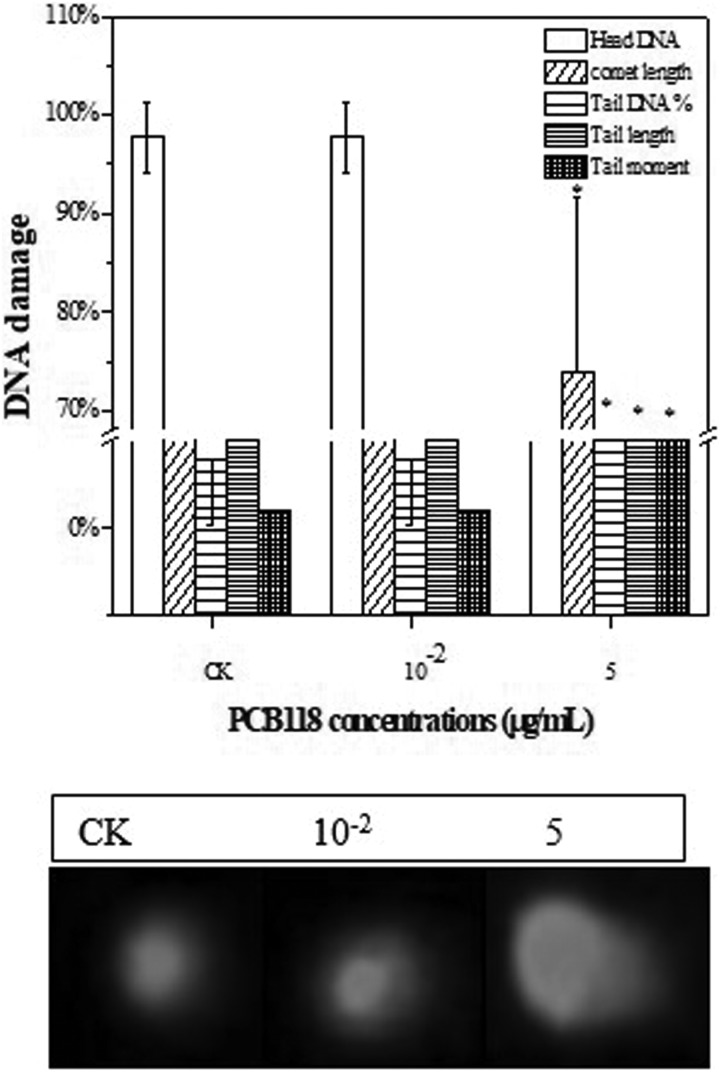
Single-cell gel electrophoresis assay was conducted to determine DNA damage induced by different concentrations of PCB118 (Figure 4). The extent of DNA strand breaks induced by different concentrations of PCB118 after 24 hours of exposure showed significant increase in DNA strand breaks as compared to control in HELF cells. However, DNA damage results indicated that there was no obvious increase in DNA breaks observed at lower concentrations of PCB118 as compared to the higher concentrations. In control samples, percentage of head DNA was more ∼97% and the comet length, tail DNA, TL, and TM were lower after 24 hours. However, at 5 µg/mL, percentage of head DNA decreased ∼76%, while the comet length, tail DNA, TL, and TM increased significantly compared to control and 10−2, respectively, suggesting that PCB118 may act as a genotoxic compound especially at the highest level of exposure.
Figure 4.
DNA damage in human lungs fibroblast (HELF) cell exposed to PCB118.
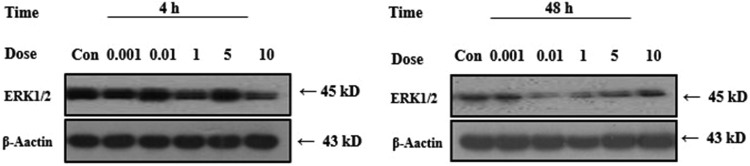
Response of MAPK Proteins Exposed to PCB118
The effects of PCB118 were also determined on the MAPK protein exposed for 4 and 48 hours (Figure 5). After 4 hours, ERK1/2 exhibited decreasing trend exposure to different concentrations of PCB118. We observed prominent bands of ERK1/2 protein after 4 hours for different concentrations of PCB118. While after 48 hours, exposure to different concentrations of PCB118 ERK1/2 expression decreased and bands were less prominent, suggesting that with the passage of time the different concentrations of PCB118 decreased the ERK1/2 protein.
Figure 5.
Effects of PCB118 on human lungs fibroblast (HELF) cell and extracellular signal-regulated kinase 1/2 (ERK1/2) protein expression.
Discussion
In this study, we aimed at investigating the role of OS and ERK1/2 protein in HELF cell proliferation exposed to PCB118. Our results demonstrated that PCB118 induced HELF cell proliferation at low concentration (10−4 to 1 µg/mL for 4, 12, 24, and 48 hours) but inhibited cell growth at high concentration (5, 10, and 20 µg/mL for 12, 24, and 48 hours, respectively), as evidenced by the β-shaped dose–response curve. The stimulatory and inhibitory effects of cells after PCBs have been reported for SK-N-SH human neuronal cells,30 Vero cell,31 and human breast cancer cells.17 Similarly, Radice et al14 described the effects of PCB118 on MCF-7 cells and found that PCB118 exhibited significant hormetic effects to MCF-7 cell. The authors observed growth stimulatory responses at low doses of PCB118 while inhibitory responses at high doses compared to control. Therefore, it is proposed PCB118 may induce hormetic effects to the HELF cell proliferation.
Reactive oxygen species are well recognized for playing a dual role as both deleterious and beneficial species.32 However, recently Calabrese33 proposed that hormetic mechanism may follow 2 pathways after chemicals exposure: one is receptor-mediated and the other is cell signaling-mediated hormetic mechanisms. Several studies have been reported that low level of ROS stimulated the cell signaling-mediated hormetic mechanisms, which in turn cause the cell proliferation or apoptosis.20,34 In the present study, we observed that the PCB118 concentrations increased the ROS levels in a dose- and time-dependent manner, suggesting that production of ROS was triggered by PCB118, which may stimulate cell signaling-mediated hormetic mechanisms for cell proliferation and apoptosis. Several studies demonstrated that PCB101 and 118 treatments generate elevated intracellular ROS levels, and to abolish the ROS effects, antioxidants like GSH or antioxidant enzymes, such as SOD, produced,35,36 leading to the formation of DNA adducts. The ROS and antioxidant (GSH) levels in our study were in concordance with the previous studies for higher and lower chlorinated-like PCBs congener which induced OS in human breast cancer cells.17,36 Overall, the present study suggests that PCB118 produced more ROS and in turn induced more acute depletion of intracellular GSH in HELF cells.
The quantification of LPO is a biomarker for oxidative processes and also a well-established mechanism of cellular injury in animals and plants cells37; in this study, the level of MDA was measured as an indicator of OS in HELF cells exposed to PCB118. The present study suggests that PCB118 at higher doses produce LPO damage as compared to the lower doses and control. Similarly, Yang et al19 found that sodium arsenite damages LPO at higher doses exposed to HELF cells than the lower doses. The depletion of antioxidant GSH and induction of ROS and LPO have been implicated in oxidative damage of cell components for chemicals.38 In this study, there is significant correlation between ROS generation and GSH depletion, suggesting that higher production of ROS leads to increased membrane LPO with a concomitant decrease in antioxidants like GSH. Additionally, GSH is a cofactor of several detoxifying enzymes against OS. It scavenges free radicals to regenerate the homeostasis of intracellular redox status.39 The results suggested that this appears to be the underlying mechanism of PCB118-induced cytotoxicity mediated through OS in HELF cells.
Several studies assessing DNA damage as a consequence of PCB exposure to human cell lines have been conducted using the comet assay.36,40 We found that PCB118 at higher concentrations (10 and 20 µg/mL) after 24 hours induced DNA damage to HELF cells, while the lower concentrations exhibited less damage. The comet assay has been used to assess DNA damage in human MDA-MB-231 (MDA) and MCF-7 breast cancer cells exposed to PCB52 and 77. The mean comet TM and DNA damage were found to be significantly higher in coplanar PCB77 exposed to human cells.17 The results suggested that PCB118 at higher concentrations (5 µg/mL) induced DNA damage in HELF cells compared to control.
The possible molecular mechanisms involved in the effects of PCB118 on the HELF functions were investigated. Previous data demonstrated that the immune response of mussel hemocytes to bacterial challenge triggers a phosphorylative cascade involving the activation of tyrosine kinase-mediated cell signaling leading to a time-dependent increase in the phosphorylation of different MAPK families, the ERKs, p38, and JNKs.25,41 The effects of PCB118 on the activation state of different MAPK families were evaluated in HELF cell extracts subjected to SDS-PAGE and Western blot with specific anti-activated MAPK antibodies and the results are reported in Figure 5. The result here presented clearly show that HELF treatment with PCB118 results in a time-dependent decrease in the phosphorylation state of the stress-activated ERK1/2 MAPK. In particular, acute, short-term treatment of human neutrophils with PCB mixtures or different ortho-substituted PCB congeners can affect cell signaling, activating components involved in the transduction of extracellular stimuli, such as tyrosine kinases, ERK MAPKs, PLA 2, PLC, and calcium fluxes.42,43 In particular, we demonstrated that the phosphorylation state of ERK can be affected by different PCB, confirming and extending previous data obtained on neutrophils.43 Less prominent ERK1/2 bands were observed for the PCB118 after 48 hours, suggesting that PCB118 were toxic. We found a good correlation between OS, ERK1/2 protein, and cell proliferation, suggesting that OS stimulated cell proliferation through the cell signal pathway.
Conclusions
The present study concluded that PCB118 exhibited stimulatory effects at low doses and inhibitory effects at high doses on HELF cell proliferation. More cytotoxic effects were found for the PCB118 on HELF cells. Further, it was found that low doses of PCB118 induced ROS generation without cytotoxicity and suggest a cellular signal pathway mechanism for the stimulation of HELF cell proliferation. However, the higher doses of PCB118 produced marked ROS formation, cellular damage, as evidenced by LPO and comet assay, and inhibitory responses on the HELF cell proliferation. Activation of the ROS at low concentrations of PCB118 seems to play an important role in the biphasic/hormetic effect of PCB118 on cell proliferation.
Moreover, we observed that antioxidant GSH contents decreased with an increase in PCB118 concentrations in HELF cells. Further, results of the present study indicated that HELF cell proliferation exposed to PCB118 was correlated with varying ROS levels in a biphasic manner. The present study concluded that PCB118 significantly affected the mitogen-activated signaling pathway. It was found that PCB118 showed expression of ERK1/2 protein after 4 hours, while after 48 hours, the protein expression was less, indicating PCB toxicity to MAPK protein of HELF cell. However, the present study suggests that more detailed mechanisms in terms of growth factors and death factors should be investigated in future.
Acknowledgments
The first author is thankful to Chinese Government for providing scholarship for PhD studies.
Authors’ Note: M.Z.H. conceived the original idea. M.Z.H. designed the experiments. M.Z.H., J.H.S., and M.T. performed the experiments. M.Z.H. and C.S. analyzed the data. M.Z.H. and C.S. wrote and reviewed the paper.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was funded by The National Key Research and Development Plan (2016YFD0800207) and Science and Technology Foundation of Zhejiang Province, China (2017C33020). TWAS-COMSTECH Research Grant Award_15-384 RG/ENG/AS_C, HEC Start Up Research grant 21-700/SRGP/R&D/HEC/2015, Pakistan.
References
- 1. Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11(1):1–15. [DOI] [PubMed] [Google Scholar]
- 2. Pope CR, Burnett RT, Turner MC, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119(11):1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Recio-Vega R, Mendez-Henandez A, Jacobo-Avila A, et al. Potentially estrogenic polychlorinated biphenyls congeners serum levels and its relation with lung cancer. J Appl Toxicol. 2013;33(9):906–914. [DOI] [PubMed] [Google Scholar]
- 4. Miller MD, Marty MA. Impact of environmental chemicals on lung development. Environ Health Perspect. 2010;118(8):1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belessis Y. University of New South Wales. 2010.
- 6. Grigg J. Particulate matter exposure in children: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(7):564–569. [DOI] [PubMed] [Google Scholar]
- 7. Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the harvard six cities study. Am J Respir Crit Care Med. 2006;173(6):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pope CA, III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norström K, Czub G, McLachlan MS, Hu D, Thorne PS, Hornbuckle KC. External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ Int. 2010;36(8):855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cekanova M, Masi T, Plummer HK, 3rd, Majidi M, Fedorocko P, Schuller HM. Pulmonary fibroblasts stimulate the proliferation of cell lines from human lung adenocarcinomas. Anticancer Drugs. 2006;17(7):771–781. [DOI] [PubMed] [Google Scholar]
- 11. Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183(4):225–237. [DOI] [PubMed] [Google Scholar]
- 12. He XQ, Chen R, Yang P, Li AP, Zhou JW, Liu QZ. Biphasic effect of arsenite on cell proliferation and apoptosis is associated with the activation of JNK and ERK1/2 in human embryo lung fibroblast cells. Toxicol Appl Pharmacol. 2007;220(1):18–24. [DOI] [PubMed] [Google Scholar]
- 13. Jiang G, Duan W, Xu L, Song S, Zhu C, Wu L. Biphasic effect of cadmium on cell proliferation in human embryo lung fibroblast cells and its molecular mechanism. Toxicol In Vitro. 2009;23(6):973–978. [DOI] [PubMed] [Google Scholar]
- 14. Radice S, Chiesara E, Fucile S, Marabini L. Different effects of PCB101, PCB118, PCB138 and PCB153 alone or mixed in MCF-7 breast cancer cells. Food Chem Toxicol. 2008;46(7):2561–2567. [DOI] [PubMed] [Google Scholar]
- 15. Guo B, Liang YC, Zhu YG, Zhao FJ. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut. 2007;147(3):743–749. [DOI] [PubMed] [Google Scholar]
- 16. Razinger J, Dermastia M, Koce JD, Zrimec A. Oxidative stress in duckweed (Lemna minor L.) caused by short-term cadmium exposure. Environ Pollut. 2008;153(3):687–694. [DOI] [PubMed] [Google Scholar]
- 17. Lin CH, Huang CH, Chuang MC, et al. Protective role of estrogen receptor-alpha on lower chlorinated PCB congener-induced DNA damage and repair in human tumoral breast cells. Toxicol Lett. 2009;188(1):11–19. [DOI] [PubMed] [Google Scholar]
- 18. Zhu H, Santo A, Li Y. The antioxidant enzyme peroxiredoxin and its protective role in neurological disorders. Exp Biol Med (Maywood). 2012;237(2):143–149. [DOI] [PubMed] [Google Scholar]
- 19. Yang P, He XQ, Peng L, et al. The role of oxidative stress in hormesis induced by sodium arsenite in human embryo lung fibroblast (HELF) cellular proliferation model. J Toxicol Environ Health A. 2007;70(11):976–983. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Shen G, Yu Y, Zhu H. The hormetic effect of cadmium on the activity of antioxidant enzymes in the earthworm eisenia fetida. Environ Pollut. 2009;157(11):3064–3068. [DOI] [PubMed] [Google Scholar]
- 21. Gómez N, Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991;353(6340):170–173. [DOI] [PubMed] [Google Scholar]
- 22. Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1995;92(17):7686–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alessandrini A, Crews CW, Erikson R. Phorbol ester stimulates a protein-tyrosine/threonine kinase that phosphorylates and activates the Erk-1 gene product. Proc Natl Acad Sci U S A. 1992;89(17):8200–8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. [DOI] [PubMed] [Google Scholar]
- 25. Canesi L, Lorusso LC, Ciacci C, Betti M, Zampini M, Gallo G. Environmental estrogens can affect the function of mussel hemocytes through rapid modulation of kinase pathways. Gen Comp Endocrinol. 2004;138(1):58–69. [DOI] [PubMed] [Google Scholar]
- 26. Hashmi MZ, Naveedullah Shen H, Zhu S, Yu C, Shen C. Growth, bioluminescence and shoal behavior hormetic responses to inorganic and/or organic chemicals: a review. Environ Int. 2014;64:28–39. [DOI] [PubMed] [Google Scholar]
- 27. DiPietrantonio AM, Hsieh TC, Juan G, Traganos F, Darzynkiewicz Z, Wu JM. Fenretinide-induced caspase 3 activity involves increased protein stability in a mechanism distinct from reactive oxygen species elevation. Cancer Res. 2000;60(16):4331–4335. [PubMed] [Google Scholar]
- 28. Tice R, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35(3):206–221. [DOI] [PubMed] [Google Scholar]
- 29. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 30. Lee YS, Jin DQ, Park SH, et al. 2, 3, 7:8-Tetrachlorobenzo-p-dioxin inhibits proliferation of SK-N-SH human neuronal cells through decreased production of reactive oxygen species. Free Radic Res. 2002;36(12):1283–1289. [DOI] [PubMed] [Google Scholar]
- 31. Chen Y, Shen K, Shen C, Chen L, Chen X. Comparison of structure-dependent hormetic cytotoxicity induced by coplanar and non-coplanar PCB congeners. J Hazard Mater. 2010;180(1-3):773–776. [DOI] [PubMed] [Google Scholar]
- 32. Sturrock A, Cahill B, Norman K, et al. Transforming growth factor-β1 induces Nox4 NAD (P) H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L661–L673. [DOI] [PubMed] [Google Scholar]
- 33. Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol. 2013;43(7):580–606. [DOI] [PubMed] [Google Scholar]
- 34. Lin PH, Lin CH, Huang CC, Chuang MC, Lin P. 2, 3, 7:8-Tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress, DNA strand breaks, and poly (ADP-ribose) polymerase-1 activation in human breast carcinoma cell lines. Toxicol Lett. 2007;172(3):146–158. [DOI] [PubMed] [Google Scholar]
- 35. Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85(7):743–750. [DOI] [PubMed] [Google Scholar]
- 36. Lin CH, Lin PH. Induction of ROS formation, poly (ADP-ribose) polymerase-1 activation, and cell death by PCB126 and PCB153 in human T47D and MDA-MB-231 breast cancer cells. Chem Biol Interact. 2006;162(2):181–194. [DOI] [PubMed] [Google Scholar]
- 37. Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004;11(9):1163–1182. [DOI] [PubMed] [Google Scholar]
- 38. Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Twaroski TP, O’Brien ML, Robertson LB. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: implications for oxidative stress. Biochem Pharmacol. 2001;62(3):273–281. [DOI] [PubMed] [Google Scholar]
- 40. Hassoun EA, Li F, Abushaban A, Stohs SJ. Production of superoxide anion, lipid peroxidation and DNA damage in the hepatic and brain tissues of rats after subchronic exposure to mixtures of TCDD and its congeners. J Appl Toxicol. 2001;21(3):211–219. [DOI] [PubMed] [Google Scholar]
- 41. Canesi L, Scarpato A, Betti M, Ciacci C, Pruzzo C, Gallo G. Bacterial killing by Mytilus hemocyte monolayers as a model for investigating the signaling pathways involved in mussel immune defence. Mar Environ Res. 2002;54(3-5):547–551. [DOI] [PubMed] [Google Scholar]
- 42. Brown GE, Stewart MQ, Bissonnette SA, Elia AE, Wilker E, Yaffe MB. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. J Biol Chem. 2004;279(26):27059–27068. [DOI] [PubMed] [Google Scholar]
- 43. Olivero J, Ganey PE. Role of protein phosphorylation in activation of phospholipase A 2 by the polychlorinated biphenyl mixture Aroclor 1242. Toxicol Appl Pharmacol. 2000;163(1):9–16. [DOI] [PubMed] [Google Scholar]