Abstract
We report a case of intravesical metastasis of a clear cell renal cell carcinoma. In renal cell carcinoma 16% of patients present with metastatic disease. Renal cell carcinoma can metastasize to nearly every organ, although metastatic spread to the urinary bladder is rare, with fewer than 70 described cases. The route and pattern of metastatic spread is not yet fully understood and different pathways are suggested. Gross haematuria is the presenting symptom in the majority of cases. These intravesical metastases may be synchronous or metachronous and can be solitary or part of polymetastatic disease. No standard treatment can be suggested due to the rare nature of this phenomenon, and treatment varies from transurethral resection, partial or complete cystectomy to systemic therapy. Prognosis in patients with a solitary bladder lesion that developed metachronously is rather good, whereas poor prognosis can be expected in patients with synchronous and multiple metastases.
Keywords: intravesical, metachronous, metastasis, renal cell carcinoma
Introduction
Renal cell carcinomas (RCCs) are responsible for 80–85% of all primary renal neoplasms. Sixteen per cent of patients present with metastatic disease, while 30% of patients will experience local or distant recurrence after surgery of the primary renal mass.1 RCC is well known for its unpredictable mode of spread and can metastasize to nearly every organ rapidly or delayed; it most commonly metastasizes to regional lymph nodes, lung, liver, bone, adrenal gland, brain and skin.2 Metastasis to the urinary bladder, however, is uncommon, with fewer than 70 reported cases at present.
Case report
The case report concerns an 80-year-old female patient diagnosed with a large, polylobular mass with central necrosis at the left kidney (Figure 1), discovered on the occasion of a fall with left flank pain without haematuria. She underwent a radical nephrectomy by thoracoabdominal incision. Histological analysis revealed a clear cell renal cell carcinoma with tumour invasion of the left renal vein and with negative surgical margins. TNM-staging was pT3a pN0 pM0. Five years later the patient was admitted because of silent, gross haematuria. Cystoscopy showed a sessile polyp of 3 cm originating from the right ureteric orifice (Figures 2 and 3). The lesion was resected and was histologically found to be a metastasis of a clear cell renal cell carcinoma after review by an expert genitourinary pathologist (Figure 4). No other ureteral lesions or hydronephrosis were noted. Staging CT scan showed hypervascular, necrotic metastases at the level of the right lobe of the liver (Figure 5), histologically confirmed by percutaneous liver biopsy. The patient was initiated on targeted therapy, namely pazopanib 800 mg, an oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, and showed a very good therapeutic response with complete liquefaction of the liver metastases after 3 months, with acceptable side effects (Figure 6). After a total follow-up of 57 months, she is still in remission and in good general condition.
Figure 1.
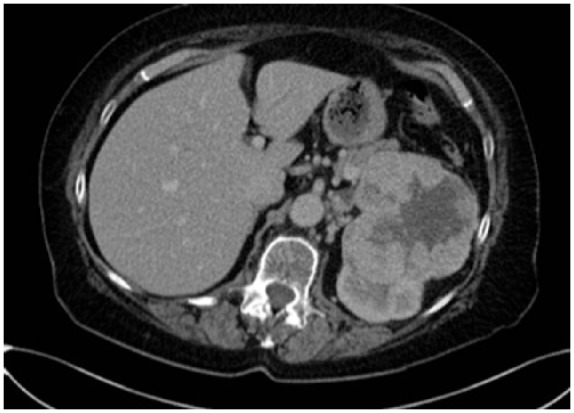
Large, polylobular mass with central necrosis at the left kidney.
Figure 2.
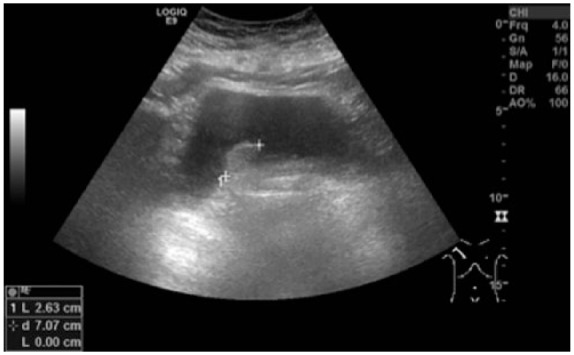
Sessile polyp of 3 cm originating from the right ureteric orifice.
Figure 3.
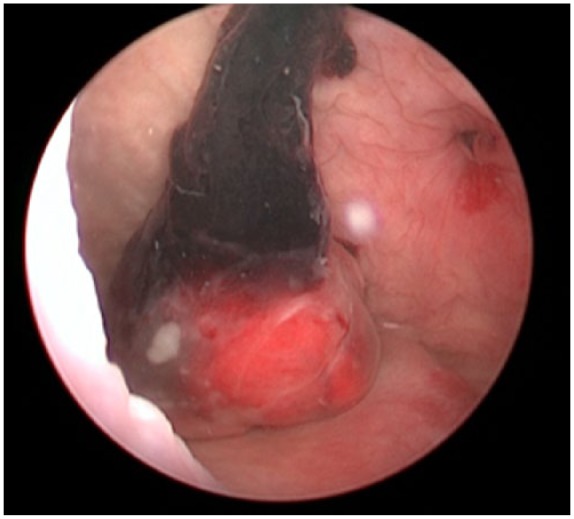
Sessile polyp of 3 cm originating from the right ureteric orifice (cystoscopic view).
Figure 4.
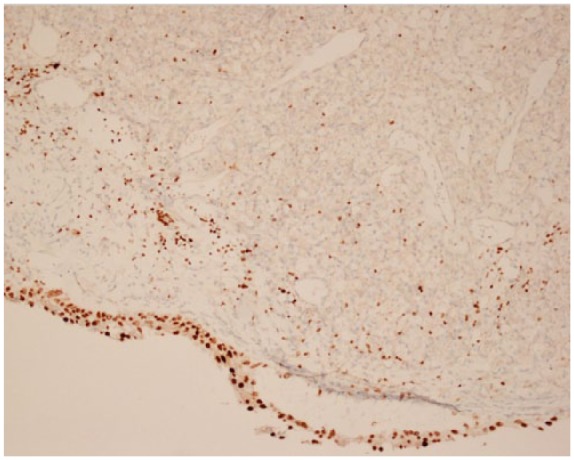
Section from the tumour shows carcinoma with clear cell morphology consistent with metastasis of previously diagnosed renal cell carcinoma. The cores of the superficial cell layer colours with GATA-3 staining, compatible with urothelium. The nuclei of the deep cell layer do not colour with GATA-3 staining, compatible with renal cell carcinoma.
Figure 5.
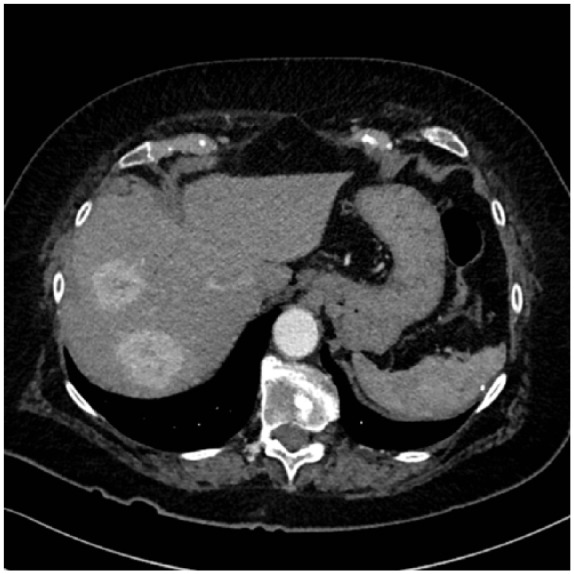
Biopsy-proven liver metastases of renal cell carcinoma.
Figure 6.
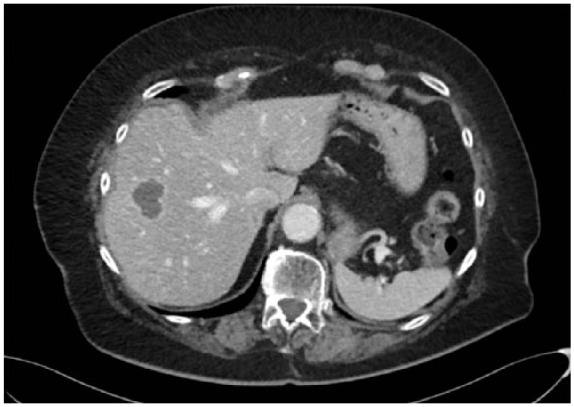
Therapeutic response with liquefaction of the liver metastases 3 months after initiating pazopanib.
Discussion
Renal cell carcinoma is well known for its unpredictable mode of spread and can metastasize to nearly every organ. Metastasis to the urinary bladder, however, is uncommon. These intravesical metastases may be synchronous or metachronous – that is, presenting respectively within 12 months or >12 months after nephrectomy,3 occurring respectively in 23% and 77% of reported cases.3 In case of metachronous metastases, the median time for metachronous bladder metastasis following the diagnosis of RCC is 33 months. Matsumoto and colleagues calculated in a retrospective analysis of 65 reported cases that intravesical metastases of renal cell carcinoma can present as a solitary lesion (62%) while additional metastatic sites are present in 38% of cases.3
In 75% of cases the diagnosis of intravesical metastasis is made following silent, gross haematuria. In the majority of cases, there is a documented history of RCC. However, infrequently, the primary renal tumour may present initially as a bleeding bladder lesion.4
The underlying mechanism of metastatic spread to the bladder is still a matter of debate. In general, four different pathways are suggested:
Drop metastasis or the direct extension and implantation of tumour cells down the urinary tract into the ureter and urinary bladder.5,6
Direct haematogenous route. This includes direct spread through the blood stream by invasion of the renal vein.4,5
Lymphatic spread. Cancer cells penetrate the lymphatic vessels and travel as emboli, facilitated by numerous interconnections between lymphatic and vascular channels.5
Retrograde venous route. This route suggests the embolism of tumour cells from the renal vein into the numerous venous connections of the left renal vein.5,7
Given the rare nature of metastatic renal cell carcinoma to the bladder, no guidelines can be made regarding standard treatment. In selected patients there might be a place for metastasectomy. Previous studies8 reported a significantly longer median overall survival or cancer-specific survival (CSS) following complete metastasectomy compared with incomplete and/or no metastasectomy. Moreover, studies have shown that the location of the metastatic lesion can be related to survival.9 The 2-year CSS of patients with solitary bladder metastases was found to be 71.1%, which is comparable to those patients with favourable solitary metastatic site treated with metastasectomy.3 In the past, transurethral resection or fulguration of the tumour, partial cystectomy, radical cystoprostatectomy, systemic immunotherapy and targeted therapy have been used. In the above cases, targeted therapy was initiated after finding systemic progression. After all, targeted therapy with sunitinib and pazopanib is now accepted as the first-line treatment for metastatic/advanced clear cell RCC.10 Since no guidelines can be made for this rare metastatic location, the suggestion for treatment in bladder metastasized renal cell carcinoma is to maintain a watchful attitude after performing transurethral resection, as a part of cytoreductive treatment, in those cases with a solitary bladder metastasis and to start with targeted therapy as soon as systemic metastatic spread is detected. The question can be raised whether patients with solitary bladder metastasis should be candidates for systemic targeted therapy.
In the past, the prognosis of bladder metastasis of renal cell carcinoma was considered very poor, with little chance of long-term survival. Matsumoto and colleagues concluded that only the presence of additional metastatic lesions at the time of diagnosis of the bladder metastasis and the development of bladder metastasis within a year after initial treatment of the RCC are independent prognostic factors. CSS was significantly higher in patients with solitary metastasis compared to their multi-metastatic counterparts (2-year CSS rates: 71.1% versus 25.8%; p = 0.007). The 2-year CSS rate for patients who developed bladder metastasis within a year was 58.4%, whereas this was 34.6% if diagnosis was made >1 year after initial treatment of the renal cell carcinoma (p = 0.063).3 In the past, however, there was no efficient systemic treatment available, while targeted therapy has now been proven to substantially increase life expectancy even in polymetastasized patients. In the above case, the patient is still alive and in good general condition, even though she was diagnosed with systemic metastatic spread. This finding supports the important benefit of targeted therapy in these metastatic patients.
In conclusion, bladder metastasis of RCC is a rare phenomenon that can develop as a solitary lesion or be part of polymetastatic disease. Treatment is still under debate and prognosis depends on the number of metastatic lesions and time of onset of the bladder metastasis.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Ruben De Groote, Department of Urology, OLV Hospital Aalst, Moorselbaan 164, 9300 Aalst, Belgium.
Alessandro Larcher, Department of Urology, OLV Hospital Aalst, Aalst, Belgium Division of Oncology/Unit of Urology; URI; IRCCS Ospedale San Raffaele, Milan, Italy.
Marijn Goossens, Department of Urology, OLV Hospital Aalst, Aalst, Belgium.
De Raeve Hendrik, Department of Pathology, OLV Hospital Aalst, Aalst, Belgium.
Van Der Steen Kris, Department of Pathology, OLV Hospital Aalst, Aalst, Belgium.
Vincent De Coninck, Department of Urology, OLV Hospital Aalst, Aalst, Belgium.
Geert De Naeyer, Department of Urology, OLV Hospital Aalst, Aalst, Belgium.
Peter Schatteman, Department of Urology, OLV Hospital Aalst, Aalst, Belgium.
Frederiek D’Hondt, Department of Urology, OLV Hospital Aalst, Aalst, Belgium.
Alexandre Mottrie, Department of Urology, OLV Hospital Aalst, Aalst, Belgium.
References
- 1. Frank I, Blute ML, Cheville JC, et al. Multifactorial postoperative surveillance model for patients with surgically treated clear cell renal cell carcinoma. J Urol 2003; 170: 2225–2232. [DOI] [PubMed] [Google Scholar]
- 2. McAchran SE, Williams DH, MacLennan GT. Renal cell carcinoma metastasis to the bladder. J Urol 2010; 184: 726–727. [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto K, Hayakawa N, Nakamura S, et al. Bladder metastasis from renal cell carcinoma: retrospective analysis of 65 reported cases. Clin Exp Metastasis 2015; 32: 135–141. [DOI] [PubMed] [Google Scholar]
- 4. Gelister JS, Falzon M, Crawford R, et al. Urinary tract metastasis from renal carcinoma. Br J Urol 1992; 69: 250–252. [DOI] [PubMed] [Google Scholar]
- 5. Zhang M, Wah C, Epstein JI. Metastatic renal cell carcinoma to the urinary bladder: a report of 11 cases. Am J Surg Pathol 2014; 38: 1516–1521. [DOI] [PubMed] [Google Scholar]
- 6. Raviv S, Eggener SE, Williams DH, et al. Long-term survival after ‘drop metastases’ of renal cell carcinoma to the bladder. Urology 2002; 60: 697. [DOI] [PubMed] [Google Scholar]
- 7. Abeshouse BS. Metastasis to ureters and urinary bladder from renal carcinoma: report of two cases. J Int Coll Surg 1956; 25: 117–126. [PubMed] [Google Scholar]
- 8. Petralia G, Roscigno M, Zigeuner R, et al. Complete metastasectomy is an independent predictor of cancer-specific survival in patients with clinically metastatic renal cell carcinoma. Eur Urol Suppl 2010; 9: 162. [Google Scholar]
- 9. Kavolius JP, Mastorakos DP, Pavlovich C, et al. Resection of metastatic renal cell carcinoma. J Clin Oncol 1998; 16: 2261–2266. [DOI] [PubMed] [Google Scholar]
- 10. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369: 722–731. [DOI] [PubMed] [Google Scholar]


