Abstract
Background:
Although tensile force on an immature tibial tuberosity is considered the main cause of Osgood-Schlatter disease (OSD), the relationship between bony maturity and the pathogenesis of OSD remains obscure.
Purpose:
To survey the bone maturation process of the tibial tuberosity by age and sex and clarify its relationship to OSD.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
A total of 731 Japanese basketball players aged 6 to 14 years were enrolled in this study. Ultrasonographic examination was performed in all participants (1462 knees) to evaluate the bony maturity of the tibial tuberosity by use of the Ehrenborg classification. The age- and sex-specific prevalence of each stage was investigated, and the prevalence of symptomatic OSD and its relationship with bony maturity were also assessed.
Results:
The process of bone maturation occurred 1 to 2 years earlier in female participants compared with male participants. Among female participants, 59.2% were already at the epiphyseal stage (stage E) by 10 years of age, and 47.4% were skeletally mature by 14 years. Among male participants, conversely, only 8.0% were at stage E by 10 years of age, and only 13.8% were skeletally mature by 14 years. The overall prevalence of symptomatic OSD was 6.8% (males, 6.4%; females, 7.2%), and the onset was 1 year earlier in the female participants. The prevalence of symptomatic OSD tended to increase with age and bony maturity, significantly increasing from the cartilaginous stage (stage C) to the apophyseal stage (stage A) (odds ratio, 9.48) and from stage A to stage E (odds ratio, 2.22).
Conclusion:
The tibial tuberosity matures earlier in female participants. The risk of OSD is greater in stage A than stage C and in stage E than stage A. The risk of OSD increases with age in males but not in females.
Keywords: Osgood-Schlatter disease (OSD), ultrasonography, bone maturation process, tibial tuberosity, adolescent, basketball
Osgood-Schlatter disease (OSD) is one of the major disorders affecting the knee joint in adolescent athletes. In 1903, Osgood20 and Schlatter25 separately reported the condition of OSD, which is a traction apophysitis of the tibial tuberosity caused by repetitive strain from the quadriceps muscle and chronic avulsion of the tibial tubercle. OSD occurs during early adolescence, which is an important phase for the improvement of an individual’s sports skills.24 Severe knee pain restricts young athletes from daily training and competition and can ultimately lead to a decrease in their performance level.8,23,24
In general, the prevalence of OSD is around 10% in adolescents who are active in sports.6,14 The treatment of OSD is commonly conservative, including medication, cryotherapy, and physical therapy.11,13 The prognosis of OSD has been reported to be excellent in most cases.2,10,11 However, Kaya et al13 reported that only approximately 50% of OSD patients are completely recovered at 2 years after their initial diagnosis. Even after conservative and surgical treatments, some OSD patients have residual pain or complications that persist into adolescence or adulthood.12,21
Although several studies have indicated that the degree of bone maturation is associated with OSD,10,14,19 the relationship between bony maturity and the pathogenesis of OSD is still obscure. Clarification of the bone maturation process of the tibial tuberosity might provide clues to the early diagnosis of OSD in adolescent athletes. The bone maturation status of the tibial tuberosity was evaluated and classified radiographically by Ehrenborg and Lagergren9 in the early 1960s and ultrasonographically by Ducher et al,8 Sailly et al,24 and Nakase et al.17 An ultrasonographic assessment is convenient and minimally invasive, and clinicians can use ultrasonography to observe the tibial tuberosity.5,11,13,15,17,24 The purpose of the present study was to investigate the bony maturation process of the tibial tuberosity by age and sex based on ultrasonography findings to clarify the relationship of the maturation process to OSD.
Methods
This study was conducted on 731 Japanese preadolescent and adolescent basketball players aged 6 to 14 years (350 males and 381 females) who participated in an annual preparticipation examination between January 2014 and January 2016. The players were recruited from local youth basketball clubs. The study comprised the following 3 items: a self-reported questionnaire, a physical examination of the knee, and an ultrasonographic examination of the tibial tuberosity. Self-reported questionnaire forms were distributed to the participants before an annual medical checkup and collected on the day of the checkup. Items on the questionnaire included the participant’s age, sex, total practice time (days and hours) per week, and current subjective knee pain.
Each physical examination was performed in a standardized manner by a single physical therapist. The participant's height and weight were assessed, and tenderness on the tibial tuberosity was investigated to evaluate for apophysitis of the tibial tuberosity. The diagnostic criteria for symptomatic OSD were tenderness at the tibial tuberosity and current subjective knee pain.
The ultrasonographic examination was carried out on both knees of all participants (1462 knees) by experienced clinicians who were blinded as to the physical examination results. Each participant was assessed by a single examiner. The devices used for this examination were the MyLab mobile ultrasound system (Esaote) and the Sonimage HS1 portable ultrasound system (Konica Minolta), with a 14- to 18-MHz high-frequency linear transducer. To assess the anterior aspect of the knee, the knee was flexed 90°. The linear transducer was placed on the tibial tuberosity to obtain an image that included both the tibial tuberosity and the distal patellar tendon. The ultrasonographic findings were evaluated based on a standardized protocol.
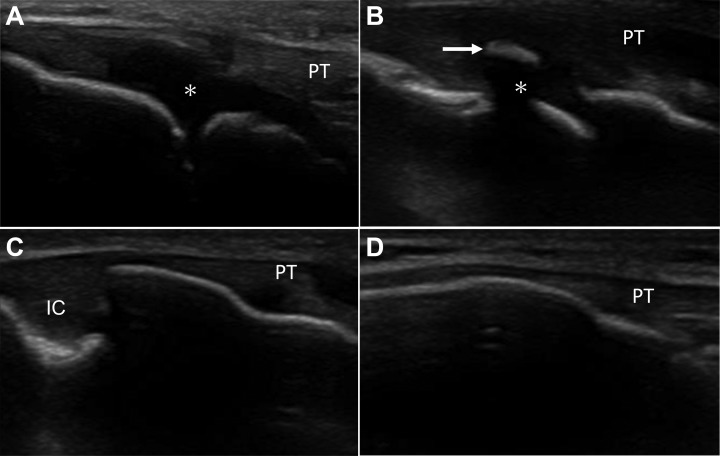
The morphologic characteristics of the tibial tuberosity were divided into 4 types according to bony maturity as classified by Ehrenborg and Lagergren9: the cartilaginous stage (stage C), the apophyseal stage (stage A), the epiphyseal stage (stage E), and the bony stage (stage B). Stage C is characterized by a large amount of apophyseal cartilage without a secondary ossification center. Stage A is characterized by the patellar tendon attaching to apophyseal cartilage, but secondary ossification centers are seen in the apophysis. Stage E is characterized by the patellar tendon attaching to the bone surface, and a thin layer of insertional cartilage is still present. Stage B is characterized by the patellar tendon attaching to the tubercle and the absence of any apophyseal cartilage (Figure 1).
Figure 1.
Ultrasonographic morphological characteristics of the tibial tuberosity (sagittal view) according to the Ehrenborg and Lagergren9 classification. (A) The cartilaginous stage (stage C) is characterized by a large amount of apophyseal cartilage (asterisk) without a secondary ossification center. (B) The apophyseal stage (stage A) is characterized by a cartilage attachment with secondary ossification center (arrow). (C) The epiphyseal stage (stage E) is characterized by the patellar tendon (PT) attaching to the bone surface, and a thin layer of insertional cartilage (IC) is still present. (D) The bony stage (stage B) shows mature attachment.
Written informed consent to participate in the study was obtained from the participants and their guardians. The study was approved by the ethics committee of our institute (No. 2160).
Statistical Analysis
Values are presented as mean ± standard deviation (SD). An unpaired Student t test was used to compare demographic data such as age, height, weight, and body mass index (BMI) between the male and female participants, both overall and according to each stage of bony maturity. The Cochrane-Armitage trend test was used to investigate trends in the prevalence of symptomatic OSD along with age and bony maturation stage. Age- and sex-adjusted logistic regression analyses were performed to examine the relationship between symptomatic OSD and bony maturation stage, and odds ratios (ORs) and 95% CIs were calculated for each group. All tests of statistical significance were 2-tailed. P values <.05 were considered significant. All analyses were conducted by use of JMP version 10.0.2 software (SAS Institute).
Results
Baseline Characteristics of the Study Population
The mean ± SD age of all participants was 11.2 ± 1.5 years, with no significant differences between sexes. Although the mean height and weight were significantly greater for male participants (P < .01), the BMI values showed no significant difference (Table 1).
TABLE 1.
Anthropometric Data for Participantsa
| Variable | Total Sample (N = 731) | Male Participants (n = 350) | Female Participants (n = 381) | P Valueb |
|---|---|---|---|---|
| Age, y | 11.2 ± 1.5 | 11.2 ± 1.5 | 11.2 ± 1.6 | .83 |
| Height, cm | 146.3 ± 11.1 | 147.8 ± 11.9 | 145.1 ± 10.3 | <.01 |
| Weight, kg | 37.8 ± 9.2 | 38.9 ± 9.8 | 36.9 ± 8.6 | <.01 |
| Body mass index, kg/m2 | 17.4 ± 2.3 | 17.6 ± 2.3 | 17.3 ± 2.3 | .07 |
aValues are reported as mean ± SD.
bP values were compared between male and female participants.
Time Trend of the Bone Maturation Process of the Tibial Tuberosity
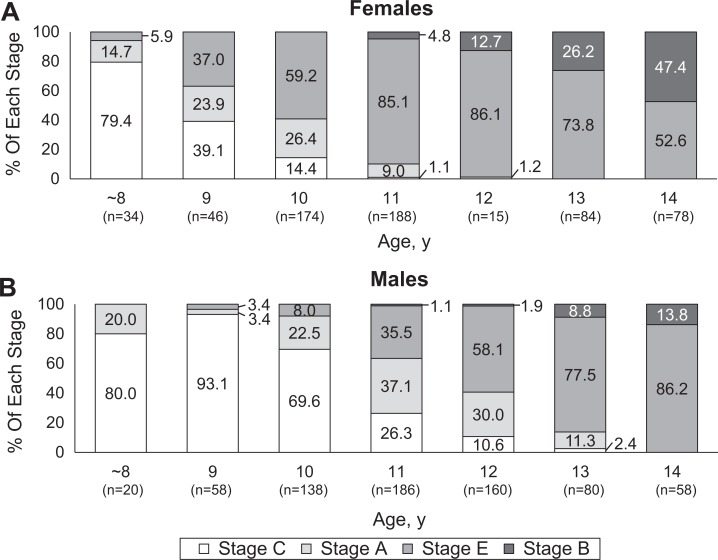
The ultrasonographic examinations demonstrated that for any given age, bone maturation in female participants occurred more quickly than male participants (Table 2). Among the female participants, 59.2% were already at stage E at 10 years of age; almost all of the females were at stage E or stage B at 12 years, and 47.4% were skeletally mature (stage B) at 14 years (Figure 2A). Among the male participants, only 8.0% were at stage E at 10 years of age; 40.6% were still at stage C or stage A at 12 years, and only 13.8% were skeletally mature at 14 years (Figure 2B).
TABLE 2.
Sex-Based Differences in Physical Characteristics According to Bony Maturity Stagea
| Stage | Sex | Age, y | P Value | Height, cm | P Value | Weight, kg | P Value |
|---|---|---|---|---|---|---|---|
| Cartilage stage | Male | 10.0 ± 1.0 | <.001 | 137.6 ± 7.2 | <.001 | 31.4 ± 5.1 | <.001 |
| Female | 8.9 ± 1.2 | 127.7 ± 6.1 | 25.3 ± 3.5 | ||||
| Apophyseal stage | Male | 11.1 ± 1.1 | <.001 | 145.2 ± 7.2 | <.001 | 36.3 ± 6.3 | <.001 |
| Female | 9.9 ± 0.8 | 137.0 ± 5.8 | 29.0 ± 3.5 | ||||
| Epiphyseal stage | Male | 12.2 ± 1.1 | <.001 | 155.7 ± 10.2 | <.001 | 45.3 ± 9.1 | <.001 |
| Female | 11.4 ± 1.3 | 146.9 ± 8.4 | 37.7 ± 7.0 | ||||
| Bony stage | Male | 13.2 ± 1.0 | .636 | 163.4 ± 9.0 | .002 | 52.4 ± 6.9 | .035 |
| Female | 13.0 ± 1.0 | 155.3 ± 4.8 | 47.5 ± 6.7 |
aValues are presented as mean ± SD.
Figure 2.
Time trend of the bone maturation process of the tibial tuberosity in (A) female and (B) male participants. Among female participants, 59.2% were already in stage E by 10 years of age; almost all females were in stage E or B by 12 years, and 47.4% were skeletally mature (stage B) at 14 years. Among male participants, conversely, only 8.0% were in stage E at 10 years of age; 40.6% of males were still in stage C or stage A by 12 years, and 86.2% were in still in stage E at 14 years.
Prevalence of Symptomatic OSD and Its Relation to Age, Sex, Total Practice Time, and Bony Maturity
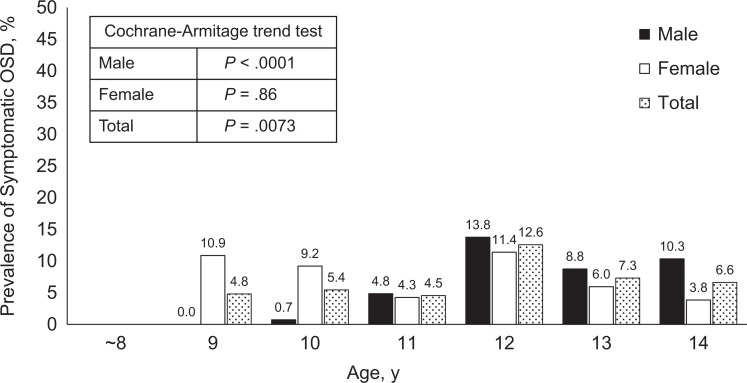
According to our criteria, 100 of 1462 knees (6.8%) were diagnosed as symptomatic OSD. By age, the prevalence of symptomatic OSD peaked at approximately 12 years in both sexes (males, 13.8%; females, 11.4%), whereas another peak was observed in the female participants at 9 to 10 years (9.2%-10.9%) and in the male participants at 14 years (10.3%) (Figure 3). The prevalence of symptomatic OSD increased significantly with older age for all participants and for the male participants (P < .01). In contrast, we did not find a trend for increased OSD prevalence with older age in female participants.
Figure 3.
Prevalence of symptomatic Osgood-Schlatter disease (OSD) by age and sex. Prevalence peaked at approximately 12 years of age in both sexes (males, 13.8%; females, 11.4%), whereas another peak was observed in female participants at 9 to 10 years of age (9.2%-10.9%) and in male participants at 14 years (10.3%). The prevalence of symptomatic OSD increased significantly with older age in male participants and in total (P < .01). No increasing trend was noted for prevalence of OSD in female participants.
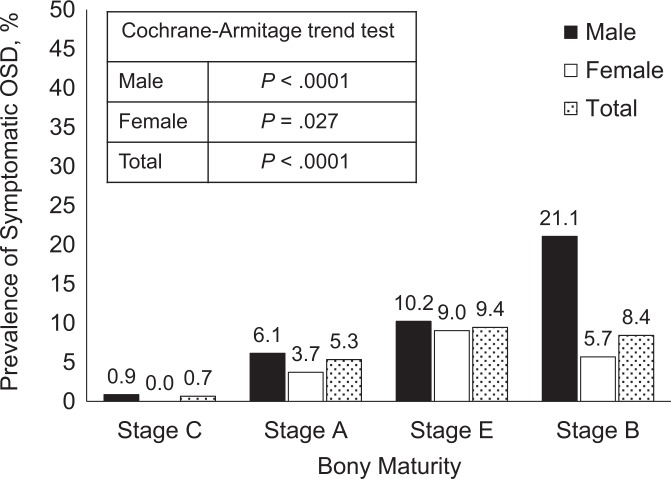
The prevalence of symptomatic OSD increased significantly with the progression of bony maturity (Figure 4). Symptomatic OSD occurred most commonly at stage C in male participants and at stage A in female participants. The age- and sex-adjusted logistic regression analysis revealed significantly high ORs for being diagnosed with OSD from stage C to stage A (OR, 9.48; 95% CI, 2.54-61.48) and from stage A to stage E (OR, 2.22; 95% CI, 1.19-4.45) (Table 3). In contrast, we found no significant increase in OSD occurrence from stage E to stage B (OR, 1.02; 95% CI, 0.44-2.12).
Figure 4.
Prevalence of symptomatic Osgood-Schlatter disease (OSD) by bone maturity stage of the tibial tuberosity. The prevalence of symptomatic OSD increased significantly with the progression of bony maturity in both sexes (P < .05).
TABLE 3.
Odds Ratios for Osgood-Schlatter Disease by Bony Maturity Stagea
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Stage C to stage A | 9.48 | 2.54-61.48 | <.001 |
| Stage A to stage E | 2.22 | 1.19-4.45 | .012 |
| Stage E to stage B | 1.02 | 0.44-2.12 | .945 |
aObtained by use of an age- and sex-adjusted logistic regression analysis. A, apophyseal; B, bony; C, cartilage; E, epiphyseal.
The participants’ total practice time (days and hours per week) was 4.4 ± 1.3 days and 10.1 ± 5.0 hours in the symptomatic OSD group and 4.3 ± 1.9 days and 9.8 ± 4.6 hours in the nonsymptomatic group. No significant differences were found in these values between the two groups.
Discussion
To the best of our knowledge, this is the first report to detail the bone maturation status of the tibial tuberosity based on ultrasonographic findings by age and sex. Previous studies using radiographic evaluation have shown that the ossification process of the tibial tuberosity began earlier in female compared with male participants3 and that OSD also occurred 1 to 2 years earlier in female compared with male participants (8-14 years in females and 10-15 years in males), especially among adolescents who actively participated in sports.2,10,13,14 In the present study, bone maturation started at a significantly younger age and furthermore developed faster from stages C to E in female compared with male participants. Clinicians should consider these sex-based differences when diagnosing and treating juvenile OSD patients.
Although the prevalence of symptomatic OSD is reported to be 9.8% to 12.9%,6,14 only 6.8% of the participants in this study were diagnosed as having OSD. Overuse injuries including OSD tend to increase with age.26,27 The reason that the prevalence of OSD in the present study is lower may be because this study included a younger cohort (mean age, 11.2 years; range, 6-14 years) than previous studies (mean age, 13.1-13.7 years; range, 12-15 years).6,14 In this study, despite considering OSD an overuse injury, we found that the participants’ total practice time (days and hours per week) had no influence on the prevalence of symptomatic OSD. Other risk factors for OSD have been reported, such as quadriceps femoral and hamstring muscle tightness, body weight, and muscle strength during knee extension.6,18 Although these factors might have influenced the prevalence of OSD in this study, the influences of these risk factors were not investigated.
OSD is clinically characterized by local pain, swelling, and tenderness of the tibial tuberosity.2,11,15 These symptoms develop gradually with mild and intermittent pain, but the pain becomes more severe and continuous in the acute phase.11,13 OSD is usually diagnosed based on clinical signs and symptoms, and radiography is often used for confirmation and to exclude other conditions such as fracture, infection, or tumor.2,11,24 Although some studies have reported that the presence of a fragmented secondary ossification center causes pain and is indicative of OSD,6,15 secondary ossification centers have not yet appeared at stage C. It is sometimes difficult to detect the early ossification center abnormalities at stage A only by radiography.
Ultrasonographic examinations have been reported to be useful to visualize the cartilage and to detect tiny bony abnormalities and ossification, but it might be difficult to distinguish between normal and abnormal states in skeletally immature youth, especially during the transition from stage C to stage A. As well, investigators have reported that fragmentation of the tibial tuberosity was observed in both symptomatic and asymptomatic knees and thus cannot be used to distinguish the abnormal state from the normal one.3,8,15,22 Considering these clinical points, we defined the criteria of symptomatic OSD based on only subjective symptoms (current knee pain) and physical examination results (tenderness on the tibial tuberosity), not using radiography or ultrasonography.
According to our criteria, 0.7% of the stage C participants and 5.3% of the stage A participants were diagnosed with symptomatic OSD. This result indicated that information about only bony morphological changes would lead to an underestimation of OSD. However, the risk of developing OSD was increased by approximately 9.5 times as the bony maturation transitioned from stage C to stage A, and the risk increased by 2.2 times from stage A to stage E. This result suggested that the transition from stage C to stage E is the time of highest risk for the development of OSD. In light of these findings, radiography does not fully evaluate the tibial tuberosity in these less mature stages; a careful physical examination and a detailed assessment of surrounding soft tissue would be necessary to achieve an early diagnosis.
Soft tissue assessment using ultrasonography has attracted attention with regard to improving the accuracy of OSD diagnosis.7,8,15,24 Ultrasonography is useful for assessing the morphological changes of soft tissue surrounding the tibial tuberosity as well as bone. It can detect swelling of the patellar tendons and cartilage and the infrapatellar bursa; moreover, a color-enhanced Doppler ultrasonographic examination can detect increasing blood flow as well as neovascularization surrounding the apophysis of the tibial tuberosity. Previous studies have indicated that neovascularization occurs at a late phase of tendon disrepair and degeneration, and neurovascular ingrowth into the tendon could be responsible for pain generation.1,4,16 Sailly et al24 reported that increased blood flow was significantly associated with knee pain. Ultrasonography may yield improved accuracy in the diagnosis of OSD. Additionally, reaching full maturation of the tibial tuberosity seems to protect females against OSD but results in increased incidence in males.
There are 3 limitations in this study. The first is our study design; we demonstrated the prevalence of OSD at each stage of bony maturity and by age, but the exact time course of bony maturity of the tibial tuberosity and the prevalence of OSD were unclear since our study was a cross-sectional and not longitudinal study. A cohort study is required to investigate the exact time course. The second limitation involves our criteria for symptomatic OSD. In this study, the diagnosis of symptomatic OSD was made based on subjective symptoms and physical examination results without radiological findings, because it was difficult to assess the bony abnormality in skeletally immature participants, even with the use of ultrasonography. Detailed soft tissue assessments such as those obtained by color Doppler methods are needed to improve the accuracy of OSD diagnosis. The third limitation is interobserver reliability. Since the palpation of the tibial tuberosity and the ultrasonographic examination were carried out by several staff members, the detection of tenderness and the staging of ultrasonographic findings may have varied due to interobserver differences.
Conclusion
In this study, we found that the tibial tuberosity matured earlier in female compared with male participants and that OSD risk was higher during stage A than stage C and higher during stage E than stage A. Additionally, we noted that the risk of OSD increased with age in male but not in female participants.
Acknowledgment
The authors thank and acknowledge the following people for their collaboration and assistance with this study: Tomohiko Shigihara, Takanori Kashimura, and the members of the Fukushima Physical Therapy Association Medical Support Team.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from the Research Ethics Committee of Fukushima Medical University, Fukushima, Japan.
References
- 1. Bjur D, Alfredson H, Forsgren S. The innervation pattern of the human Achilles tendon: studies of the normal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res. 2005;320:201–206. [DOI] [PubMed] [Google Scholar]
- 2. Blankstein A, Cohen I, Heim M, et al. Ultrasonography as a diagnostic modality in Osgood-Schlatter disease: a clinical study and review of the literature. Arch Orthop Trauma Surg. 2001;121:536–539. [DOI] [PubMed] [Google Scholar]
- 3. Bloom RA, Gomori J, Milgrom C. Ossicles anterior to proximal tibia. Clin Imaging. 1993;17:137–141. [DOI] [PubMed] [Google Scholar]
- 4. Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–416. [DOI] [PubMed] [Google Scholar]
- 5. Czyrny Z. Osgood-Schlatter disease in ultrasound diagnostics—a pictorial essay. Med Ultrason. 2010;12:323–335. [PubMed] [Google Scholar]
- 6. de Lucena GL, dos Santos Gomes C, Guerra RO. Prevalence and associated factors of Osgood-Schlatter syndrome in a population-based sample of Brazilian adolescents. Am J Sports Med. 2011;39:415–420. [DOI] [PubMed] [Google Scholar]
- 7. Demirag B, Ozturk C, Yazici Z, Sarisozen B. The pathophysiology of Osgood-Schlatter disease: a magnetic resonance investigation. J Pediatr Orthop B. 2004;13:379–382. [DOI] [PubMed] [Google Scholar]
- 8. Ducher G, Cook J, Spurrier D, et al. Ultrasound imaging of the patellar tendon attachment to the tibia during puberty: a 12-month follow-up in tennis players. Scand J Med Sci Sports. 2010;20:e35–e40. [DOI] [PubMed] [Google Scholar]
- 9. Ehrenborg G, Lagergren C. Roentgenologic changes in the Osgood-Schlatter lesion. Acta Chir Scand. 1961;121:315–327. [PubMed] [Google Scholar]
- 10. Flowers MJ, Bhadreshwar DR. Tibial tuberosity excision for symptomatic Osgood-Schlatter disease. J Pediatr Orthop. 1995;15:292–297. [DOI] [PubMed] [Google Scholar]
- 11. Gholve PA, Scher DM, Khakharia S, et al. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19:44–50. [DOI] [PubMed] [Google Scholar]
- 12. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Magnetic resonance imaging of Osgood-Schlatter disease: the course of the disease. Skeletal Radiol. 2002;31:334–342. [DOI] [PubMed] [Google Scholar]
- 13. Kaya DO, Toprak U, Baltaci G, et al. Long-term functional and sonographic outcomes in Osgood-Schlatter disease. Knee Surg Sports Traumatol Arthrosc. 2013;21:1131–1139. [DOI] [PubMed] [Google Scholar]
- 14. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes: retrospective study of incidence and duration. Am J Sports Med. 1985;13:236–241. [DOI] [PubMed] [Google Scholar]
- 15. Lanning P, Heikkinen E. Ultrasonic features of the Osgood-Schlatter lesion. J Pediatr Orthop. 1991;11:538–540. [DOI] [PubMed] [Google Scholar]
- 16. Ljung BO, Forsgren S, Fridén J. Sympathetic and sensory innervations are heterogeneously distributed in relation to the blood vessels at the extensor carpi radialis brevis muscle origin of man. Cell Tissues Organs. 1999;165:45–54. [DOI] [PubMed] [Google Scholar]
- 17. Nakase J, Aiba T, Goshima K, et al. Relationship between the skeletal maturation of the distal attachment of the patellar tendon and physical features in preadolescent male football players. Knee Surg Sports Traumatol Arthrosc. 2014;22:195–199. [DOI] [PubMed] [Google Scholar]
- 18. Nakase J, Goshima K, Numata H, Oshima T, Takata Y, Tsuchiya H. Precise risk factors for Osgood-Schlatter disease. Arch Orthop Trauma Surg. 2015;135:1277–1281. [DOI] [PubMed] [Google Scholar]
- 19. Ogden JA, Southwick WO. Osgood-Schlatter’s disease and tibial tuberosity development. Clin Orthop Relat Res. 1976;116:180–189. [PubMed] [Google Scholar]
- 20. Osgood RB. Lesions of the tibial tubercle occurring during adolescence. Boston Med Surg J. 1903;148:114–117. [PubMed] [Google Scholar]
- 21. Pihlajamäki HK, Mattila VM, Parviainen M, et al. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men. J Bone Joint Surg Am. 2009;91:2350–2358. [DOI] [PubMed] [Google Scholar]
- 22. Rosenberg ZS, Kawelblum M, Cheung YY, et al. Osgood-Schlatter lesion: fracture or tendinitis? Scintigraphic, CT, and MR imaging features. Radiology. 1992;185:853–858. [DOI] [PubMed] [Google Scholar]
- 23. Ross MD, Villard D. Disability levels of college-aged men with a history of Osgood-Schlatter disease. J Strength Cond Res. 2003;17:659–663. [DOI] [PubMed] [Google Scholar]
- 24. Sailly M, Whiteley R, Johnson A. Doppler ultrasound and tibial tuberosity maturation status predicts pain in adolescent male athletes with Osgood-Schlatter’s disease: a case series with comparison group and clinical interpretation. Br J Sports Med. 2013;47:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schlatter C. Verletzungen des schnabelformigen Fortsatzes der oberen Tibiaepiphyse. Beitr Klin Chir. 1903;38:874–887. [Google Scholar]
- 26. Stracciolini A, Casciano R, Levey Friedman H, Meehan WP, III, Micheli LJ. Pediatric sports injuries: an age comparison of children versus adolescents. Am J Sports Med. 2013;41:1922–1929. [DOI] [PubMed] [Google Scholar]
- 27. Stracciolini A, Casciano R, Levey Friedman H, Stein CJ, Meehan WP, III, Micheli LJ. Pediatric sports injuries: a comparison of male versus female subjects. Am J Sports Med. 2014;42:965–972. [DOI] [PubMed] [Google Scholar]