Abstract
Treatment of patients with type 2 diabetes is directed against treating symptoms of hyperglycemia, minimizing the risk of hypoglycemia, and the risk of microvascular and macrovascular complications. The majority of patients with type 2 diabetes die from cardiovascular or cerebrovascular disease. Future therapies should therefore focus on reducing cardiovascular morbidity in this high-risk population. Glucagon-like peptide-1 receptor agonists (GLP-1RA) and sodium-glucose co-transporter 2 inhibitors (SGLT2-i) are two drug classes with proven antihyperglycemic effect in type 2 diabetes. However, these drugs seem to have other effects such as weight reduction, low risk of hypoglycemia, and blood pressure reduction. Emerging evidence suggests pleiotropic effects, which potentially could be important in reducing cardiovascular risk. Prompted by regulatory authorities demanding cardiovascular outcome trials (CVOTs) assessing the cardiovascular safety of new antihyperglycemic drug candidates, many CVOTs are ongoing and a few of these are finalized. Somewhat surprising recent CVOTs in both drug classes have shown promising data on cardiovascular morbidity and mortality in patients with a very high risk of cardiovascular events. It is uncertain whether this is a class effect of the two drug classes, and it is yet unproven whether long-term cardiovascular benefits of these drugs can be extrapolated to populations at lower risk of cardiovascular disease. The aim of the present review is to give an overview of our current knowledge of the GLP-1RA and SGLT2-i classes, with specific focus on mechanisms of action, effects on cardiovascular risk factors and cardiovascular morbidity and mortality from the CVOTs presently available. The clinical potential of these data is discussed.
Keywords: cardiovascular safety, type 2 diabetes, glucagon-like peptide-1 (GLP-1) receptor agonist, sodium-glucose co-transporter type 2 (SGLT2) inhibitor, cardiovascular events
Introduction
Type 2 diabetes mellitus (T2DM) is associated with considerable increases in cardiovascular morbidity and mortality.1 Although mortality rates in patients with T2DM seem to decrease, rates are still significantly elevated compared with the normal population. Therapies like lipid-lowering drugs,2,3 blood pressure reduction4 and antithrombotic therapy as secondary prevention5 have significantly contributed to the reduction in cardiovascular events and risk in this population. In accordance, combining these therapies as a multipharmacologic intervention in patients with T2DM with microalbuminuria is associated with substantial long-term cardiovascular risk reduction and mortality.6,7
Antihyperglycemic therapy is effective in reducing the microvascular complications of diabetes as nephropathy, retinopathy and neuropathy.8 However, previous studies with these antihyperglycemic drugs have not been successful in providing evidence for the same risk reduction in macrovascular complications as cardiovascular events.9–11 Newer studies have even created concern about adverse cardiovascular outcomes with certain types of antihyperglycemic drugs.12,13 In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, intensive glycemic control was associated with a marginal lower incidence of myocardial infarction, but an increased mortality rate.9 These results prompted both the United States (US) Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to require the pharmaceutical industry to conduct independent cardiovascular outcome studies (CVOTs) to specific assess cardiovascular safety of new antihyperglycemic drug during development. In the US FDA ‘guidance for industry’ (2008), there are demands that any new antihyperglycemic drug for the treatment of T2DM should be studied. When added to standard care these new drugs should be noninferior to placebo with regard to major cardiovascular events.
There are two new classes of antihyperglycemic drugs for the treatment of T2DM that have been marketed during recent years, before and after the new US FDA/EMA requirements: glucagon-like peptide-1 receptor agonists (GLP-1RAs) from 2005 and sodium-glucose co-transporter 2 inhibitors (SGLT2-is) from 2012. The list of the current marketed GLP-1RAs and SGLT2-is are depicted in Table 1. For drugs belonging to both these classes, CVOTs have been started and a few completed. For the GLP-1RA class, CVOTs evaluating lixisenatide, liraglutide, semaglutide and exenatide have been completed. For the SGLT2-i class, two CVOTs evaluating empagliflozin and canagliflozin have been completed at present. For all other drug candidates in the two classes, results of the CVOTs are pending (see Tables 2 and 3).
Table 1.
Currently approved, or soon to be evaluated, drug candidates of glucagon-like peptide-1 receptor agonists (GLP-1ra) (1) and sodium-glucose co-transporter 2 inhibitors (SGLT2-i) (2). Combination drugs containing GLP-1ra or SGLT2-i.
| Active ingredient | Status | Drug name |
|---|---|---|
| Exenatide (1) | Approved in EU (2006) | Byetta® |
| Liraglutide (1) | Approved in EU (2009) | Victoza® |
| Exenatide extended release (1) | Approved in EU (2011) | Bydureon® |
| Lixisenatide (1) | Approved in EU (2013) | Lyxumia® |
| Albiglutide (1) | Approved in EU (2014) | Eperzan®, Tanzeum® |
| Dulaglutide (1) | Approved in EU (2014) | Trulicity® |
| Liraglutide + Insulin degludec | Approved in EU (2016) | Xultophy® |
| Semaglutide (1) | Under development (phase III) | – |
| Dapagliflozin (2) | Approved in EU (2012) | Forxiga® |
| Canagliflozin (2) | Approved in EU i (2013) | Invokana® |
| Empagliflozin (2) | Approved in EU (2014) | Jardiance® |
| Dapagliflozin + Metformin | Approved in EU (2014) | Xigduo® |
| Canagliflozin + Metformin | Approved in EU (2014) | Vokanamet® |
| Empagliflozin + Metformin | Approved in EU (2015) | Synjardy® |
| Dapagliflozin + Saxagliptin | Approved in EU (2016) | Qtern® |
| Ipragliflozin (2) | Approved in Japan | (Suglat®) |
| Luseogliflozin (2) | Approved in Japan | (Lusefi®) |
| Tofogliflozin (2) | Approved in Japan | (Apleway®, Deberza®) |
| Sotagliflozin (2) | Under development (phase III) | – |
| Ertugliflozin (2) | Under development (phase III) | – |
| Remogliflozin etabonat (2) | Under development (phase IIb) | – |
EU, European Union; GLP-1ra, glucagon-like peptide-1 receptor agonist; SGLT2-i; sodium-glucose co-transporter 2 inhibitor.
Table 2.
Overview of cardiovascular outcome trials in the classes of drug candidates of glucagon-like peptide-1 receptor agonists (1) and sodium-glucose co-transporter 2 inhibitors (2).
| Drug | CVOT name | Patient enrollment | Inclusion criteria | Exclusion criteria | Trial duration | Primary outcome result |
|---|---|---|---|---|---|---|
| Lixisenatide (1) | ELIXA | 6068, randomization 1:1 vs. placebo | Type 2 diabetes and recent CV event (< 180 days before screening) | Age < 30 yr, eGFR < 30 ml/min, HbA1c < 5.5% and > 11.0%, planned coronary revascularization | 25 months | 13.4% lixisenatide group 13.2% placebo group HR 1.20 (95% CI 0.89 – 1.17), p=NS (superiority) |
| Liraglutide (1) | LEADER | 9340, randomization 1:1 vs. placebo | Type 2 diabetes (⩾ 50 yr) and coronary heart disease and/or cerebrovascular disease and/or peripheral vascular disease and/or chronic kidney disease or ⩾ 60 yr and ⩾ 1 CV risk factor | HbA1c < 7.0%, Acute CV event < 14 days before screening, type 1 diabetes, history of multiple endocrine neoplasia or medullary thyroid cancer | 45 months | 13.0% liraglutide group 14.9% placebo group HR 0.87 (95% CI 0.78 – 0.97), p=0.01 (superiority) |
| Semaglutide (1) | SUSTAIN 6 | 3297, randomization 1:1:1:1 (semaglutide 0.5 and 1.0 mg once-weekly) | Type 2 diabetes (⩾ 50 yr) and coronary heart disease and/or cerebrovascular disease and/or peripheral vascular disease and/or chronic kidney disease or ⩾ 60 yr and ⩾ 1 CV risk factor | HbA1c < 7.0%, recent acute CV event < 90 days before screening, planned coronary, carotid or peripheral artery vascularization, long-term dialysis | 25 months | 6.6% semaglutide group 8.9% placebo group HR 0.74 (95% CI 0.58 – 0.95), p=0.02 (superiority) |
| Empagliflozin (2) | EMPA-REG OUTCOME | 7020, randomization 1:1:1 (empagliflozin 10 and 25 mg once-daily | Type 2 diabetes, BMI ⩽ 45 kg/m2, eGFR ⩾ 30 ml/min, established CV disease | HbA1c < 7.0% or > 10.0% | 37 months | 10.5% empagliflozin group 12.1% placebo group HR 0.86 (95% CI 0.74 – 0.99), p=0.04 (superiority) |
| Canagliflozin (2) | CANVAS program (CANVAS and CANVAS-R) |
10142, canagliflozin 100 or 300 mg once-daily vs. placebo | Type 2 diabetes, eGFR ⩾ 30 ml/min, established CV disease or ⩾ 2 CV risk factors | HbA1c < 7.0% or > 10.5% | 43 months | 26.9 participant events per 1000 patient-years canagliflozin group 31.5 participant events per 1000 patient-years placebo group HR 0.86 (95% CI 0.75 – 0.97), p=0.02 (superiority) |
| Exenatide extended release (1) | EXSCEL | 14782, randomization 1:1 (exenatide 2 mg once-weekly) |
Type 2 diabetes, eGFR ⩾ 30 ml/min, 70% with established CV disease, 30% without CV disease | HbA1c < 6.5% or > 10.0% | 38 months | 11.4% exenatide group 12.2% placebo group HR 0.91 (95% CI 0.83-1.00), p=0.06 (NS) (superiority) |
BMI, body mass index; CI, confidence interval; CV, cardiovascular; CVOT, cardiovascular outcome trial; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HR, hazard ratio; NS, not significant.
Table 3.
Overview of pending cardiovascular outcome trials in the classes of drug candidates of glucagon-like peptide-1 receptor agonists (1) and sodium-glucose co-transporter 2 inhibitors (2).
| Drug | CVOT name | Estimated patient enrollment | Study start | Study completion | Expected rial duration | Status |
|---|---|---|---|---|---|---|
| Albiglutide (1) | HARMONY outcomes (Albiglutide 30-50 mg once-weekly vs. placebo) | 9400 | July 2015 | May 2019 | 3-5 years | Ongoing |
| Dulaglutide (1) | REWIND (dulaglutide 1.5 mg once-weekly vs. placebo) | 9622 | July 2011 | July 2018 | 6.5 years | Ongoing |
| Dapagliflozin (2) | DECLARE TIMI58 (dapagliflozin 10 mg once-daily vs. placebo) | 25880 | April 2013 | April 2019 | 6 years | Ongoing |
The present review focuses on the physiology, cardiovascular effects and the unexpected findings from the CVOTs, in which we have data available with drugs from the GLP-1RA and SGLT2-i classes. Further, it is discussed the potential benefits and implications for future treatment of patients with T2DM.
Physiology and pathophysiology in type 2 diabetes mellitus
GLP-1 and GLP-1 receptor agonists
Naturally occurring glucagon-like peptide-1 (GLP-1) is an intestinal insulinotropic hormone secreted from the enteroendocrine L-cells in the mucosa from duodenum, jejunum, ileum and colon.14 The effect of GLP-1 on glucose homeostasis is depicted in Figure 1. GLP-1 acts through the glucagon-like peptide-1 receptor (GLP-1R), which belongs to the superfamily of G protein-coupled receptors. This receptor has been shown to be expressed widely in different tissues both in humans and other primates. Thus, GLP-1R seems to be distributed in both the pancreas, the gastrointestinal tract, the kidneys, the lungs, the heart, the brain and the blood vessels of several organs.15,16 Together with glucose-dependent insulinotropic polypeptide (GIP), GLP-1 is responsible for the incretin effect. The incretin effect describes the effect of the incretin hormones secreted in response to ingested nutrients to augment insulin secretion. The potentiation of glucose-stimulated insulin secretion is thought to be the primary mode of GLP-1’s role in glucose homeostasis. However, GLP-1 also regulates glycemia by insulin-independent mechanisms. At the same time GLP-1 potentiates insulin secretion from the pancreatic β-cells; GLP-1 suppresses glucagon secretion from the pancreatic α-cells.17 Furthermore, GLP-1 inhibits gastric emptying rate. Thus, by this mechanism the entry of glucose and other nutrients to the gut is delayed, which contributes to the reduction in postprandial glucose excursions. The combined effect on the pancreatic insulin and glucagon secretion results in reduced (hyper) glycemia by enhanced glucose disposal in peripheral tissues and reduced hepatic glucose production. Accumulating evidence suggests that the effect on hepatic glucose metabolism may also be mediated by the central nervous system.17 Thus, administration of GLP-1 directly into the hypothalamus increases hepatic glycogen storage in mice,18 and GLP-1 receptors have been found on nerve terminals in the portal vein where GLP-1 signaling was observed affecting glycemia both fasting and after post-challenge in rats.19 Also mediated by the central nervous system, GLP-1 induces increasing satiety and reduced food intake resulting in weight loss.20 Finally GLP-1 may also increase resting energy expenditure and lower plasma concentrations of free fatty acids in humans.21
Figure 1.
Secretion of GLP-1 from the intestine in response to food and the effect on the pancreas and stomach on glucose homeostasis.
GLP-1, glucagon-like peptide-1.
It was early demonstrated by Nauck and colleagues22 that the incretin effect was significantly reduced in patients with T2DM. This observation has been confirmed more recently by others.23 However, most evidence suggests that it is not a primary abnormality in GIP and GLP-1 secretion that is responsible for the reduced incretin effect in T2DM.24,25 Further, the elimination rate of GIP and GLP-1 in patients with T2DM seems not to be different from healthy subjects.26 Thus, a reduced sensitivity of the pancreatic β-cells to GIP and GLP-1 may be the explanation for the reduced incretin effect in T2DM.17
Native GLP-1 is rapidly degraded by the enzyme dipeptidyl peptidase 4 (DPP-4) resulting in a very short half-life of approximately 2 min.26 It is therefore necessary to develop GLP-1RA with longer half-lives and resulting prolonged action in order to be used as antihyperglycemic drugs. Resistance towards degradation by DPP-4 in GLP-1RA has been obtained by introducing a naturally occurring peptide (exendin-4) developed from the saliva of the lizard Heloderma suspectum which activates GLP-1R with the same potency as native GLP-1, or by changing a few amino acids of the native GLP-1 molecule protecting it from DPP-4 degradation. The GLP-1RA is a heterogeneous class consisting of short-acting agonists like exenatide BID (Byetta®) and lixisenatide QD (Lyxumia®), and the continuous-acting agonists GLP-1RA like liraglutide QD (Victoza®), exenatide extended release QW (Bydureon®), dulaglutide QW (Trulicity®), albiglutide QW (Eperzan®, Tanzeum®) and semaglutide QW (to be marketed). The clinical efficacy of these drugs in reducing haemoglobin A1c (HbA1c) in patients with T2DM varies according to the drug, background therapy and baseline HbA1c, ranging from 4.3–18.6 mmol/mol (0.4–1.7%) in most long-term studies.27 Only a limited number of open label head-to-head comparisons between the different GLP-1RAs have been performed.28
SGLT2 and SGLT2-inhibitors
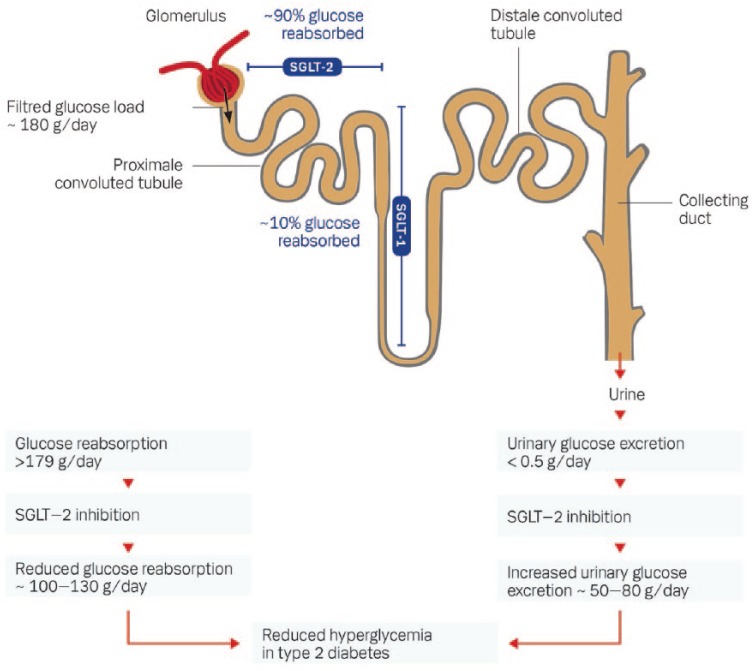
The kidneys filter approximately 180 liters of plasma per day corresponding to 160–180 grams of glucose filtered from the circulation in healthy subjects. At normoglycemia virtually all filtered glucose is reabsorbed (together with sodium) in the proximal convoluted tubule of the nephron to the circulation by the two glucose transporters sodium-glucose co-transporter-2 (SGLT-2) and -1 (SGLT-1).29 SGLT-2 is located in the first part of the proximal convoluted tubule and is responsible for 80–90% of the total glucose reabsorption to the circulation. SGLT-1 is located more distant in the straight segment of the proximal convoluted tubule and is only responsible for the remaining 10–20% of the glucose reabsorption (see Figure 2). This glucose reabsorption is a process independent of insulin. SGLT-2 is known primary to be expressed in the kidney, whereas SGLT-1 is also expressed in the small intestine, heart and lung tissue.29 In the small intestine SGLT-1 is responsible for the main part of the absorption of glucose and galactose. In glucose-tolerant individuals the maximal glucose reabsorption capacity in the kidneys is not exceeded, in contrast to diabetic individuals with hyperglycemia. At plasma levels above approximately 10 mmol/l the maximal capacity of SGLT-2 is exceeded, and the excess glucose which cannot be reabsorbed is excreted in the urine as glucosuria. Long-term hyperglycemia seems to upregulate SGLT-2 which increases the capacity and the threshold for glucose reabsorption.30 This upregulation seems to be reversible, since it can be normalized by antihyperglycemic treatment.31 The increased threshold in the kidneys for glucose reabsorption in T2DM patients with chronic hyperglycemia could have a potential role in the maintenance of hyperglycemia in these subjects. The co-transport of sodium results in decreased sodium excretion by the kidneys and increased total body sodium content, which potentially can contribute to development and deterioration of hypertension in patients with T2DM.32
Figure 2.
The renal tubular reabsorption of glucose and the effect of SGLT2-inhibition.
SGLT1, sodium-glucose co-transporter type 1; SGLT2, sodium-glucose co-transporter type 2.
Inhibition of SGLT-2 is well known from the clinical condition known as familial renal glucosuria, which is by phenotype a benign condition characterized by varying degrees of urinary glucose excretion at a normal level of blood glucose. SGLT-2 is encoded by the SLC5A2 gene. At least 44 different mutations in this gene is known, the loss-of-function mutations results in these rare cases of familial renal glucosuria.33 Most of the individuals with familial renal glucosuria are without symptoms, and only very rarely suffer from hypoglycemia, hypovolemia or genitourinary infections.33 SGLT-2 inhibitors (SGLT2-is) decrease the reabsorption of glucose and increase the excretion of glucose via a selective inhibition of SGLT-2. The glucosuria results in decreased plasma glucose levels and improved glycemic control measured by HbA1c in patients with T2DM.34 This effect is dependent of the plasma glucose level, but independent of β-cell function and insulin sensitivity. The risk of hypoglycemia and decreased effect due to secondary failure of β-cell function seems therefore very limited.35 The efficiency of the SGLT2-is is dependent of a normal or near-normal glomerular filtration rate (GFR). Thus, by decreasing GFR the effect on glucose metabolism of SGLT2-is is decreasing. At GFR levels below 30 ml/min the effect on glucosuria is insignificant.36 In general, SGLT2-is are not recommended at GFR levels below 45 ml/min. The first SGLT2-i discovered was phlorizin, a naturally occurring flavonoid found primarily in the bark from apple trees. Phlorizin was originally studied as a potential drug candidate, but was abandoned because of low bioavailability and nonselectivity regarding SGLT-1 and SGLT-2 inhibition.37 The different selective SGLT2-is currently available are apparent from Table 1. Use of the three SGLT2-is available in Europe [dapagliflozin (Forxiga®), canagliflozin (Invokana®) and empagliflozin (Jardiance®)] result in dose-dependent increases in glucosuria in both healthy subjects and patients with T2DM. In healthy subjects the maximal glucose excretion rate is approximately 62–74 mg/day.38
The SGLT2-is have a significant effect on hyperglycemia in T2DM. The largest meta-analysis with SGLT2-is containing 45 placebo-controlled trials and 13 trials with an active comparator, reported an effect in reducing HbA1c of approximately 7.2 mmol/mol (0.66%) versus placebo.39 The different SGLT2-is are not yet compared in head-to-head studies. All available SGLT2-is have significant antihyperglycemic effects on HbA1c both as monotherapy and as add-on to metformin, dipeptidylpeptidase 4-inhibitors (DPP4-inhibitors), sulfonylureas (SUs) and insulin.35,39–43
Effects on cardiovascular risk factors
GLP-1 receptor agonists
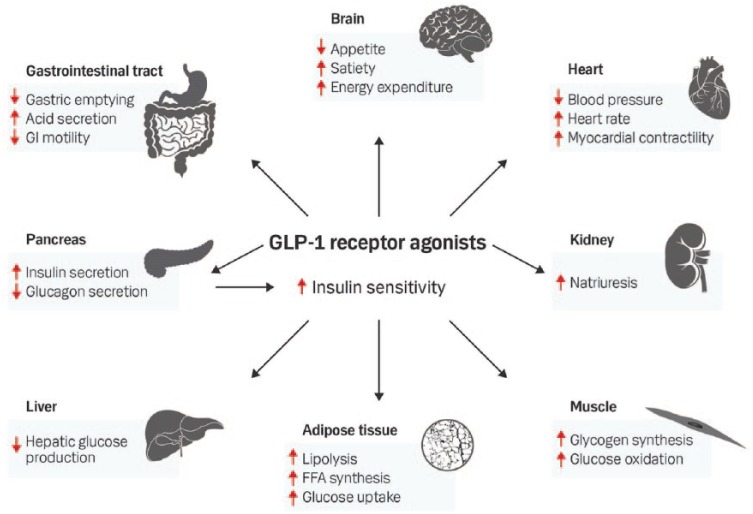
The presently known main effects of GLP-1 and GLP-1RA on different organ systems are summarized in Figure 3. It is well documented from several clinical studies that GLP-1RAs are reducing food intake and promotes a significant weight loss of approximately 4–6% in patients with T2DM.17,44 Apparently, partly independent of this weight loss and reduction in hyperglycemia, a reduction in systolic, and to a minor degree, diastolic blood pressure has been found.45 The mechanism for this effect has not been finally elucidated. However increased natriuresis by the kidneys, improved endothelial function, direct relaxation of vascular smooth muscle and indirect vasodilation by possible neurohormonal mechanisms have been proposed, together with weight loss, to be responsible for this effect.45–47 In contrast, an increase in heart rate during chronic and long-term treatment with GLP-1RA has consistently been found.45,47 Different hypothesis behind this effect have been proposed, but the mechanism is not clear. A direct effect on the heart is possible, since receptors for GLP-1 are present in the sinoatrial node.48 Anti-inflammatory effects of GLP-1 and GLP-1RA have been reported in both rodent and human studies, as well as an improvement in lipid profile and possible improvement in endothelial function.47 GLP-1RA may have also have beneficial cardioprotective effects in the clinical setting. Thus, treatment with native GLP-1 or exenatide improved ventricular function49 or reduced infarct size in patients with ST-segment elevation myocardial infarction.50 Supported by preclinical studies, earlier studies suggested an improved ventricular function in patients with chronic heart failure by native GLP-1 infusion.51 Newer placebo-controlled clinical studies with albiglutide and liraglutide have however not shown any improvement in ventricular function or clinical outcome in patients with chronic heart failure [New York Heart Association (NYHA) class II–III].52,53 Thus, current evidence does not support beneficial effects of GLP-1RAs in the treatment of patients with heart failure and impaired ventricular function.
Figure 3.
Overview of the proposed effects of GLP-1 receptor agonists on different organ systems.
FFA: Free fatty acids; GI, gastrointestinal; GLP-1, glucagon-like peptide-1.
SGLT2-inhibitors
Treatment with SGLT2-is results in a net caloric loss of approximately 200–320 calories/day due to the increased urinary glucose excretion. The resulting mean weight loss with SGLT2-is is 1.8 kg versus placebo,39 varying from 1.6 to 2.8 kg weight loss with the different SGLT2-is.43 After 6 months of treatment, no further weight loss is observed, assumingly due to a compensatory increase in food intake, which is supported by experimental studies in obese rats.38 Studies using dual-energy
X-ray absorptiometry and computerized tomography scans have shown that two-thirds of the weight reduction is due to fat mass reduction and a slightly greater reduction in visceral versus subcutaneous adipose tissue.43,54
SGLT2-is provide a clinical significant reduction in both systolic and diastolic blood pressure. The mean reduction in systolic blood pressure is 3.8 mmHg versus placebo and 4.5 mmHg versus other antihyperglycemic treatment.39 The corresponding reduction in diastolic blood pressure is 1.8 mmHg versus placebo and 2.0 mmHg versus other treatment. This effect is observed with all three currently available SGLT2-is and is further substantiated by 24-hour blood pressure measurement. In long-term studies of 2–4 years this effect on blood pressure seems persistent.43,55 The blood pressure reduction with SGLT2-is seems to be additive to the blood pressure reduction in patients treated with antihypertensive drugs like angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists. The mechanism for blood pressure reduction is not entirely clear. It is only partly due to the weight reduction, and may be explained by the increased diuresis, nephron remodeling, natriuresis and reduction of arterial stiffness.56
A variety of other potential mechanisms for cardiovascular disease risk reduction with SGLT2-is have been suggested and is depicted in Figure 4. Recent studies have suggested increased insulin sensitivity in patients with T2DM due to increased glucosuria by SGLT2-inhibition by measurement of peripheral glucose uptake.57,58 By reducing insulin resistance and hyperinsulinemia the risk of atherosclerosis may be reduced.59 A study of patients with type 1 diabetes found reduction in arterial stiffness and no increase in heart rate with empagliflozin.60 Effects on reduction of urinary albumin excretion in patients with T2DM and renal impairment have been observed with all three SGLT2-is.61–63 How this change translate to cardiovascular risk remains however to be clarified. Increased uric acid have been potentially associated with cardiovascular mortality64 and heart failure,65 although the causal relationship is not clear. Treatment with SGLT2-is has consistently shown a significant reduction in uric acid levels in patients with T2DM.66,67 Minor changes in lipid levels have been observed with SGLT2-is. Thus, small increases in both high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, and small decreases in triglycerides have been found during treatment with SGLT2-is.68 Whether these minor changes in lipids are clinical relevant remains to be established. Finally, rodent studies have suggested that SGLT2-inhibition may reduce inflammation and oxidative stress.69,70 Currently no human studies exist to support these proposed beneficial effects.
Figure 4.
Overview of the proposed effects of SGLT2-inhibitors on different organ systems.
ACE, angiotensin converting enzyme; Ang, angiotensin; SGLT2, sodium-glucose co-transporter type 2.
Cardiovascular outcome studies
GLP-1 receptor agonists
CVOTs with four of the GLP-1RAs have now been finalized and published.71–74 Studies with other GLP-1RAs are emerging.
Lixisenatide, a short-acting GLP-1RA, was the first drug in this class to be investigated in a CVOT and results were published in 2015.71 In the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial, 6068 patients were enrolled with T2DM and recent myocardial infarction or unstable angina within 180 days before screening. The ELIXA trial was a multicenter, randomized, double-blind, placebo-controlled study, and was designed to assess the effects of lixisenatide on cardiovascular morbidity and mortality. The major exclusion criteria were an age less than 30 years, recent or planned coronary revascularization procedure (15 days before to 90 days after screening), renal insufficiency [estimated GFR (eGFR) less than 30 ml/min/1.73 m2] and a HbA1c less than 36.6 mmol/mol (5.5%) or more than 96.7 mmol/mol (11.0%). The patients were randomized to 20 µg of once-daily subcutaneous administrated lixisenatide versus placebo in addition to standard therapy. The primary endpoint was a composite of the first occurrence of either death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina. The trial was sufficiently powered to show noninferiority (96% power) and to show superiority with 90% power. The mean duration of the follow up was 25 months. The intervention group was at baseline well balanced with the control group. Lixisenatide had a significant, but modest reduction in HbA1c (0.6%), and a small weight reduction of 0.6 kg compared with placebo. A small, but significant reduction in systolic blood pressure of 0.8 mmHg was shown with lixisenatide. An expected increase in heart rate was found during the first 6 weeks of the study with lixisenatide, but this difference was not sustained throughout the study. The primary cardiovascular composite endpoint occurred in 13.4% of the patients treated with lixisenatide versus 13.2% of patients in the placebo group, hazard ratio (HR) 1.02, 95% confidence interval (CI), 0.89–1.17. Thus, noninferiority of lixisenatide to placebo was demonstrated, but not superiority. Also, there was no difference in hospitalization frequency for heart failure (4.0% in the lixisenatide group versus 4.2% in the placebo group), or death from any causes (7.0% versus 7.4%). Based on this study, it was concluded that treatment with lixisenatide in addition to conventional therapy is not altering the cardiovascular risk in patients with T2DM and recent acute coronary syndrome.71 It is noted however, that the follow-up time in this trial was relatively short and the inclusion criteria of the patients differed somewhat from the CVOTs of the two other GLP-1RAs72,73 (see Table 2). The most common adverse event reported was gastrointestinal events leading to discontinuation in 4.9% of patients treated with lixisenatide versus 1.2% in the placebo group.71 Serious adverse events were reported at a similar rate in the lixisenatide group (20.6%) and in the placebo group (22.1%). The incidence of microvascular outcomes was not reported in this trial.
The cardiovascular effects of liraglutide were evaluated in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, which was finalized in December 2015.72 In this double-blinded trial, 9340 patients were randomized to either a maximum dose of 1.8 mg subcutaneously administrated liraglutide once-daily or placebo, in addition to other antihyperglycemic therapy (except for GLP-1RA, DPP-4 inhibitors or pramlintide). The patients enrolled were 50 years or older and having T2DM and established cardiovascular disease, either coronary heart disease, cerebrovascular disease, peripheral vascular disease, chronic kidney disease (stage 3 or greater) or chronic heart failure of NYHA class II–III. Patients over 60 years with at least one cardiovascular risk factor were also included. Major exclusion criteria were type 1 diabetes, history of multiple endocrine neoplasia type 2 or medullary thyroid cancer, occurrence of an acute cardiovascular event within 2 weeks before screening. The primary composite outcome was death of cardiovascular causes, nonfatal myocardial infarction or nonfatal stroke. The planned minimum follow-up time was 42 months, and the actual median follow-up time in the trial was 45 months. The patients were well balanced in the two groups, and the median daily dose of liraglutide was 1.78 mg throughout the trial. The difference in HbA1c between the groups was small (approximately 0.4%) throughout the study. Patients treated with liraglutide had a significant weight loss of 2.3 kg compared with placebo, and a reduction in systolic (1.2 mmHg) and diastolic (0.6 mmHg) blood pressure. As seen in other studies, liraglutide treatment was associated with an increase in resting heart rate of 3.0 beats per minute. Patients treated with liraglutide experienced fewer cardiovascular events. Thus, the primary composite outcome occurred in 13.0% of patients treated with liraglutide versus 14.9% of placebo-treated patients, HR 0.87 (95% CI, 0.78–0.97). All-cause mortality rates were also lower among liraglutide treated patients: 8.2% versus 9.6% among patients who received placebo, HR 0.85 (95% CI, 0.74–0.97), including death due to cardiovascular causes: 4.7% in the liraglutide group versus 6.0% in the placebo group, HR 0.78 (95% CI, 0.66–0.93). However, only an insignificant number of fewer cases of nonfatal myocardial infarction and stroke was found with liraglutide. Hospitalizations for heart failure were not different between the liraglutide (4.7%) and the placebo (5.3%) group. Interestingly, patients with more severe kidney disease (eGFR < 60 ml/min/1.73 m2) and patients aged 50 years or more with established cardiovascular disease may have greater benefit of liraglutide treatment in comparison with other patient groups (subgroup analysis).72 Thus, it was concluded from this trial that liraglutide treatment significantly reduced the rate of death from any cause and cardiovascular events in patients with T2DM at high risk for future cardiovascular events. The rate of gastrointestinal adverse events (nausea, vomiting, diarrhea and abdominal discomfort) was higher with liraglutide (0.2–1.6%) compared with placebo (0–0.4%). The serious adverse event rate was similar between liraglutide and placebo (49.7% versus 50.4%, respectively). The incidence of microvascular outcomes was significantly lower with liraglutide versus placebo, HR 0.84 (95% CI, 0.73–0.97), primarily driven by a lower rate of nephropathy.72 Retinopathy incidence rate was similar in this trial (0.6 versus 0.5 events per 100 patient-years, liraglutide versus placebo).
The third GLP-1RA, semaglutide, is not yet approved by the regulatory authorities in the US or Europe, but has been evaluated in a cardiovascular outcome study: Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 diabetes (SUSTAIN-6).73 This trial was a preapproval, randomized, double-blind, placebo-controlled, multicenter study conducted from 2013 to 2016. In this CVOT, 3297 patients with T2DM were randomized to two doses of semaglutide, 0.5 mg and 1.0 mg, administered once-weekly or placebo, in addition to standard therapy. The patient inclusion criteria in this trial were very similar to the LEADER trial.72 Major exclusion criteria in the trial were recent treatment with DPP-4 inhibitors, other GLP-1RAs or fast-acting insulin, a history of an acute coronary or cerebrovascular event within 90 days before screening, or long-term dialysis treatment. The primary composite cardiovascular outcome was also similar to LEADER: death from cardiovascular causes, nonfatal myocardial infarction or nonfatal stroke. The patients in the four groups were well balanced at baseline. The follow-up time was 25 months. During this period semaglutide 0.5 mg and 1.0 mg significantly reduced HbA1c levels by 0.7% and 1.0%, respectively. The mean body weight was 2.9 kg lower in patients receiving 0.5 mg semaglutide, and 4.3 kg lower in patients receiving 1.0 mg semaglutide compared with the placebo group (p < 0.001). Systolic blood pressure was reduced significantly in the group receiving semaglutide 1.0 mg by 2.6 mmHg compared with the placebo group (p < 0.001). A small elevation in heart rate of 2.0–2.5 beats per minute was also observed with semaglutide versus placebo in this study. Surprisingly, diabetic retinopathy complications was observed in 3.0% of the semaglutide-treated patients compared with 1.8% of patients in the placebo group, HR 1.76 (95% CI, 1.11–2.78). However, new onset or worsening of diabetic nephropathy was observed in significantly fewer patients in the semaglutide group: 3.8% versus 6.1% in the placebo group, HR 0.64 (95% CI, 0.46–0.88). The primary endpoint occurred in significantly fewer patients in the semaglutide group versus in the placebo group (6.6% versus 8.9%), HR 0.74 (95% CI, 0.58–0.95; p = 0.02 for superiority). For the different entities in the composite endpoint no significant differences were found, except for nonfatal stroke which occurred in 1.6% of the patients in the semaglutide group versus 2.7% in the placebo group, HR 0.61 (95% CI, 0.38–0.99). Hospitalization rate for heart failure was similar in the two groups: 3.6% versus 3.3% (semaglutide versus placebo). Although the number of patients were fewer and the follow-up time shorter than with liraglutide in the LEADER trial, this trial shows that treatment with semaglutide significantly reduced cardiovascular events in a population of high-risk patients with T2DM. As in the LEADER trial with liraglutide, the gastrointestinal adverse event rate was significantly higher with semaglutide. Rate of serious adverse events was similar in the semaglutide groups (33.6–35%) versus the placebo groups (36.1–39.9%). However, the rate of microvascular outcomes was different. Thus, the rate of retinopathy was higher in the semaglutide group (3.0%) versus the placebo group (1.8%), HR 1.76 (95% CI, 1.11–2.78). Newly detected or worsening of nephropathy was however lower with semaglutide (3.8%) compared with placebo (6.1%), HR 0.64 (95% CI, 0.46–0.88).73
Very recently, the results of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial was published.74 In this large, double-blind, pragmatic trial, 14752 patients with T2DM were randomized to either exenatide (extended release formulation) or placebo in addition to other antihyperglycemic therapy (any therapy allowed except GLP-1RAs). The design and inclusion criteria differed somewhat from the other CVOTs. It was designed as an event-driven trial with at least 1360 confirmed primary composite CV end points, defined as cardiovascular death, non-fatal myocardial infarction or non-fatal stroke. The patients included were more broadly defined.75 Thus, 30.4% of the patients having no known CVD at baseline and nearly 50% of the patients were treated with insulin. Patients from 21 to 92 years of age were included in the trial (mean age 62.7 years). Key exclusion criteria were repeating episodes of hypoglycemia, an estimated GFR < 30 ml/min/1.73 m2, a HbA1c below 48 mmol/mol (6.5%) or above 86 mmol/mol (10%), a family history of medullary thyroid cancer or MEN-2, calcitonin level above 40 ng/l and previous treatment with a GLP-1RA. A total of 1744 CV events occurred during the trial and the median follow-up period was 38 months. The difference in HbA1c between the exenatide group and the placebo group was significant, but narrowed during the trial, mean difference –0.53%. Body weight decreased significantly with exenatide: –1.27 kg vs. placebo. Systolic blood pressure, LDL cholesterol and triglycerides were also reduced with exenatide. The primary endpoint occurred in 11.4% of the patients treated with exenatide vs. in 12.2% of the patients in the placebo group, HR 0.91 (95% CI 0.83–1.00), p=0.06. There was no evidence of heterogeneity among subgroups of patients. Death from any cause occurred less frequent with exenatide than with placebo: 6.9% vs. 7.9%, HR 0.86 (95% CI 0.77–0.97). Thus, in contrast to the LEADER and SUSTAIN-6 trial, the incidence of CV events in this trial were not different among patients who received exenatide or placebo treatment. The apparent discrepancy of CV event reduction in this trial may be explained by the broader T2DM population studied as regard to age and CV risk, shorter follow-up period, lower HbA1c levels and the concomitant antihyperglycemic treatment (SGLT2-is more frequently used in the placebo group). The number of patients who presented with acute pancreatitis, cancers or medullary thyroid carcinomas were not different between the exenatide and the placebo group during the trial.74 Serious adverse events were similar between the exenatide (16.8%) and the placebo group (16.6%). Microvascular outcomes have not been reported so far.
For the other approved GLP-1RAs no CVOT data are currently available, but trials are ongoing (see Table 3). A recent meta-analysis including data from nine randomized safety and efficacy trials with dulaglutide76 including a total of 6010 patients with T2DM. The primary measure was a four-component cardiovascular event endpoint of death due to cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke or hospitalization for unstable angina. In that study 0.67% of patients treated with dulaglutide compared with 1.18% experienced this primary endpoint, no statistical difference between the groups. Thus, the study suggests that dulaglutide does not increase the risk of cardiovascular events. The final answer of the effect on cardiovascular risk of dulaglutide and the other approved GLP-1RAs awaits the ongoing CVOTs.
SGLT2-inhibitors
The only finalized CVOTs evaluating SGLT2-is are the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG) and the CANagliflozin cardioVascular Assessment Study (CANVAS). The results of the EMPA-REG study evaluating empagliflozin is available.55 The CANVAS trial is finalized, and data has recently been reported.77 For dapagliflozin, the CVOT: DECLARE-TIMI58 is ongoing (see Table 3).
The EMPA-REG trial investigated the effect of empagliflozin on cardiovascular outcomes in patients with T2DM in addition to standard care in these individuals.55 A total of 7020 patients were randomized to either empagliflozin 10 mg or 25 mg or placebo treatment. Like the other CVOTs, this was a multicenter, double-blind and placebo-controlled trial. The study was conducted between 2010 and 2015. The patients included having T2DM and established cardiovascular disease, body mass index (BMI) ⩽ 45 kg/m2, eGFR ⩾ 30 ml/min/1.73 m2 and a HbA1c between 53–75 mmol/mol (7.0–9.0%; no other antihyperglycemic therapy), or HbA1c between 53–85.8 mmol/mol (7.0–10.0%; receiving other antihyperglycemic therapy). The study had a median follow-up time of 37 months. The primary composite outcome was similar to the GLP-1RA CVOTs: death from cardiovascular causes, nonfatal myocardial infarction or nonfatal stroke. The patients were at baseline well balanced according to demographical and clinical characteristics. The glycemic control was significantly reduced with empagliflozin versus placebo, however the difference in HbA1c between the empagliflozin group and the placebo group was slightly diminishing during the study: at 12 weeks the difference in HbA1c was 0.54%, and at week 94 the difference was 0.42%, and after 206 weeks the difference was 0.24% between the groups. Treatment with empagliflozin was associated with minor reductions in weight, waist circumference, uric acid level, systolic and diastolic blood pressure, but no increase in heart rate. Small increases in both LDL and HDL cholesterol was observed. The primary outcome occurred in significantly fewer patients treated with empagliflozin (10.5%) than in the placebo group (12.1%), HR 0.86 (95% CI, 0.74–0.99), p = 0.04 for superiority. Empagliflozin treatment resulted in a significantly lower risk of death from any cause, HR 0.68 (95% CI, 0.57–0.82) and death due to cardiovascular causes, HR 0.62 (95% CI, 0.49–0.77). Interestingly, the rate of hospitalization for heart failure was also significantly reduced with empagliflozin versus placebo, HR 0.65 (95% CI, 0.50–0.85). The benefit of empagliflozin on death from cardiovascular causes was consistently observed across all subgroups studied.55 It is concluded from the first CVOT of the SGLT2-i class, that treatment with empagliflozin in patients with T2DM at high risk for cardiovascular events is associated with lower risk of death of any cause, a lower risk of death from cardiovascular causes. Furthermore, that empagliflozin may have a benefit in worsening of heart failure in these patients. Notably, the difference in cardiovascular outcome between empagliflozin and placebo occurred very early in this trial after 3–6 months treatment. The exact mechanism for this phenomenon is currently not known, but may be multifactorial and most likely beyond glycemic control78 as depicted in Figure 4.
The cardiovascular outcome data for canagliflozin was recently reported in the CANVAS and CANVAS-R trials.77 In the CANVAS program, data from the two trials was integrated. A total of 10,142 patients with T2DM were randomized and completed the trial program. Patients were randomized to canagliflozin 100 mg or 300 mg daily versus placebo (CANVAS) or canagliflozin 100 mg with optional increase to 300 mg daily versus placebo (CANVAS-R). Data were reported for the integrated program and the two doses of canagliflozin. The program was conducted between 2009 and 2017. The T2DM patients included having a HbA1c between 53 and 91.3 mmol/mol (7.0% and 10.5%), 30 years or older with symptomatic atherosclerotic cardiovascular disease, or 50 years or older with at least two cardiovascular risk factors, and eGFR ⩾ 30 ml/min/1.73 m2. The mean follow up was 43.4 months. The primary outcome was similar to the CVOTs reported for the GLP-1RAs and the EMPA-REG trial: a composite of death from cardiovascular causes, nonfatal myocardial infarction and nonfatal stroke. The patients were well balanced at baseline according to demographic and clinical characteristics. The glycemic control was significantly reduced with canagliflozin versus placebo, with a difference in HbA1c of −0.58%. Similarly, reductions in body weight (−1.60 kg), systolic (−3.93 mmHg) and diastolic (−1.39 mmHg) blood pressure was found was found with canagliflozin versus placebo. Minor increases in both HDL and LDL cholesterol were found with an unchanged HDL to LDL cholesterol ratio. The primary outcome occurred in significantly fewer patients in the canagliflozin group versus placebo: 26.9 versus 31.5 patients with an event per 100 patient-years, HR 0.86 (95% CI, 0.75–0.97), p = 0.02 for superiority. The three components of the primary outcome showed the same trend, however not reaching statistical significance. The rate of hospitalization for heart failure was significantly reduced with canagliflozin: HR 0.67 (95% CI, 0.52–0.87), as observed with empagliflozin in the EMPA-REG trial. The rate of adverse events leading to discontinuation from the trial did not differ between the canagliflozin and the placebo group. However a higher risk of amputations of toes, feet or legs was observed with canagliflozin compared with placebo: 6.3 versus 3.4 amputations per 1000 patient-years, HR 1.97 (95% CI, 1.41–2.75). Further, an increase in fracture rate was found with canagliflozin versus placebo: 15.4 versus 11.9 patients with fracture per 1000 patient-years, HR 1.26 (95% CI, 0.99–1.52). For the other currently approved SGLT2-is only a meta-analysis of cardiovascular outcomes is available.39 In this meta-analysis based on 14 trials for dapagliflozin containing approximately 6300 patients, no signal of increase in cardiovascular events was found compared with placebo or active treatment [HR 0.73 (95% CI, 0.46–1.12)]. Similar results were obtained from a meta-analysis of 10 trials with canagliflozin versus placebo or an active comparator [a total of 10,474 patients; HR 0.95 (95% CI, 0.71–1.26)].39
Thus, results from ongoing CVOTs will provide final answers of potential benefits of the other SGLT2-is in this drug class.
The SGLT2-is may be associated with euglycemic ketoacidosis. Although some cases have occurred in patients with type 1 diabetes, a recent analysis of US FDA adverse event reporting or a large database of commercially insured patients, suggested a two to seven-fold increase in risk of developing ketoacidosis in patients with T2DM treated with SGLT2-is.79,80 This may be a result of increased noninsulin-dependent glucose clearance, decreased renal clearance of ketone bodies, volume depletion and elevated glucagon concentrations.81,82 However neither in the EMPA-REG trial or the CANVAS program an increased rate of ketoacidosis was observed.
GLP-1 receptor agonists and SGLT2-inhibitors
There are currently no cardiovascular outcome studies investigating the combination of GLP-1RA and SGLT2-i therapy. While the two drug classes do exert their effects due to very different mechanisms, it may be expected that the combination of the two could potentially have additive benefits on cardiovascular risk reduction. A few studies have been investigating combination therapy of dapagliflozin and exenatide83,84 in patients with or without T2DM, observing a reduction in cardiovascular risk factors. A similar suggested benefit on cardiovascular risk factor and reduction in fat mass has been observed in a Japanese study investigating luseogliflozin (SGLT2-i) and liraglutide.85
It is currently not known whether the proposed increased risk of ketoacidosis associated with SGLT2-i treatment may be affected by concurrent use of GLP-1RA.
Discussion
The two relatively new drug classes of antihyperglycemic therapy, GLP-1RA and SGLT2-i, have been shown to exert clinical relevant effects on lowering hyperglycemia in patients with T2DM, at the same time with a low risk of hypoglycemia. In addition to this effect, both drug classes seem to have beneficial effects on different cardiovascular risk factors such as high blood pressure, overweight, and dyslipidemia. Both drug classes seem to have pleiotropic characteristics promoting these cardiovascular benefits, despite very different mode of actions. Thus, both drug classes may have effects on improving cardiac function and increasing natriuresis. Only the GLP-1RA class has an effect on increasing heart rate, and only the SGLT2-i class have an effect on reducing uric acid levels.
Until recently, cardiovascular outcome data on these drugs were lacking. However, after 2008 where the regulatory authorities began to require these data for new drugs, many studies are emerging. For the GLP-1RAs lixisenatide, liraglutide, semaglutide and exenatide, we have now robust cardiovascular outcome data available. The two trials, studying liraglutide and semaglutide, show consistently cardiovascular benefits in T2DM patients at very high risk of future cardiovascular events. For lixisenatide, a neutral effect was found on cardiovascular risk also in a high-risk population of T2DM. The characteristics of lixisenatide (short-acting) are somewhat different from liraglutide and semaglutide (continuous-acting), the patients studied were different and the follow-up time was shorter than in studies with liraglutide and semaglutide. For exenatide, a trend towards a cardiovascular benefit was observed, hovewer this was not significant. This discrepancy compared to the data obtained for liraglutide and semaglutide may be due to the broader type 2 diabetes population studied exerting somewhat lower cardiovascular risk, shorter follow-up period and a disproportionate use of SGLT2-is in the placebo group. Differences among the members of the GLP-1RA class in this regard is however not excluded. For exenatide, a trend towards a cardiovascular benefit was observed, hovewer this was not significant. This discrepancy compared to the data obtained for liraglutide and semaglutide may be due to the broader type 2 diabetes population studied exerting somewhat lower cardiovascular risk, shorter follow-up period and a disproportionate use of SGLT2-is in the placebo group. Differences among the members of the GLP-1RA class in this regard is however not excluded. The other incretin hormone therapy principle, the DPP4-inhibitors, do have a similar neutral effect on cardiovascular outcomes in different trials,86 however a recent meta-analysis of more than 95,000 patients showed an apparent increase in the rate of heart failure.87 The GLP-1RA class does not seem to have this unfavorable effect.
For the SGLT2-is, we have currently two robust cardiovascular outcome trials for empagliflozin and canagliflozin showing a consistent benefit on cardiovascular risk and death. Additionally, this drug (class) seems to have a substantial benefit in reducing hospitalizations and worsening of heart failure in a high-risk population of patients with T2DM. The two trials suggest that these benefits may be a class effect of these drugs. There is currently no indication that these effects should not be expected in other SGLT2-is. The observed increased rate of peripheral amputations and fractures during treatment with canagliflozin has not been observed with empagliflozin. The explanation and mechanisms for this apparent difference is unclear.
Important future trials will further elucidate the cardiovascular effects on other candidates in the two drug classes, including those with different characteristics and receptor profiles. This will potentially increase our understanding of the mechanisms behind the proposed pleiotropic characteristics. It is important to investigate the cardiovascular effect of these drugs in lower risk populations as well. The potential benefit of these drugs in patients with T2DM at lower risk of cardiovascular disease may have some support from recent observational and real-world studies as well as meta-analyses of randomized studies, both for GLP-1RAs76,88 and SGLT2-is.39,89 Further, since the mechanisms behind the cardiovascular effects of the two drug classes seem different, it is important to investigate if additional effects and an acceptable safety profile exist if drug candidates from the GLP-1RA and the SGLT2-i classes are combined in clinical therapy.
Finally, the cardiovascular outcome data emerging among the different GLP-1RAs and SGLT2-is, together with a low risk of hypoglycemia, suggests that these two drug classes should be preferred in certain high-risk patients with T2DM as second-line treatment in addition to metformin therapy. These drugs really have the potential to improve the prognosis and reduce mortality, in at least in a group of patients with T2DM and macrovascular complications or multiple cardiovascular risk factors.
Footnotes
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Matheus AS de M, Tannus LR M, Cobas RA, et al. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens 2013; 2013: 653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364: 685–696. [DOI] [PubMed] [Google Scholar]
- 3. Cholesterol Treatment Trialists’ (CTT) Collaboration, Fulcher J, O’Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015; 385: 1397–1405. [DOI] [PubMed] [Google Scholar]
- 4. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313: 603–615. [DOI] [PubMed] [Google Scholar]
- 5. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. [DOI] [PubMed] [Google Scholar]
- 7. Gaede P, Lund-Andersen H, Parving H-H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591. [DOI] [PubMed] [Google Scholar]
- 8. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS)Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 9. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364: 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ADVANCE Collaborative Group, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 11. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 12. Goldfine AB. Assessing the cardiovascular safety of diabetes therapies. N Engl J Med 2008; 359: 1092–1095. [DOI] [PubMed] [Google Scholar]
- 13. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356: 2457–2471. [DOI] [PubMed] [Google Scholar]
- 14. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 15. Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett 1995; 358: 219–224. [DOI] [PubMed] [Google Scholar]
- 16. Pyke C, Heller RS, Kirk RK, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014; 155: 1280–1290. [DOI] [PubMed] [Google Scholar]
- 17. Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 2015; 95: 513–548. [DOI] [PubMed] [Google Scholar]
- 18. Knauf C, Cani PD, Perrin C, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 2005; 115: 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vahl TP, Tauchi M, Durler TS, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 2007; 148: 4965–4973. [DOI] [PubMed] [Google Scholar]
- 20. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 2014; 124: 4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pannacciulli N, Bunt JC, Koska J, et al. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am J Clin Nutr 2006; 84: 556–560. [DOI] [PubMed] [Google Scholar]
- 22. Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986; 63: 492–498. [DOI] [PubMed] [Google Scholar]
- 23. Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 24. Calanna S, Christensen M, Holst JJ, et al. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care 2013; 36: 3346–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calanna S, Christensen M, Holst JJ, et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia 2013; 56: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vilsbøll T, Agersø H, Krarup T, et al. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 2003; 88: 220–224. [DOI] [PubMed] [Google Scholar]
- 27. Courtney H, Nayar R, Rajeswaran C, et al. Long-term management of type 2 diabetes with glucagon-like peptide-1 receptor agonists. Diabetes Metab Syndr Obes 2017; 10: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab 2016; 18: 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011; 91: 733–794. [DOI] [PubMed] [Google Scholar]
- 30. Kamran M, Peterson RG, Dominguez JH. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J Am Soc Nephrol 1997; 8: 943–948. [DOI] [PubMed] [Google Scholar]
- 31. Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest 1951; 30: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Felicetta JV, Sowers JR. Systemic hypertension in diabetes mellitus. Am J Cardiol 1988; 61: 34H–40H. [DOI] [PubMed] [Google Scholar]
- 33. Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 2010; 5: 133–141. [DOI] [PubMed] [Google Scholar]
- 34. Hummel CS, Lu C, Loo DDF, et al. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol, Cell Physiol 2011; 300: C14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther 2014; 8: 1335–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Macha S, Mattheus M, Halabi A, et al. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab 2014; 16: 215–222. [DOI] [PubMed] [Google Scholar]
- 37. Ehrenkranz JRL, Lewis NG, Kahn CR, et al. Phlorizin: a review. Diabetes Metab Res Rev 2005; 21: 31–38. [DOI] [PubMed] [Google Scholar]
- 38. Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol 2015; 309: F889–F900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159: 262–274. [DOI] [PubMed] [Google Scholar]
- 40. Fioretto P, Giaccari A, Sesti G. Efficacy and safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in diabetes mellitus. Cardiovasc Diabetol 2015; 14: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013; 36: 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liakos A, Karagiannis T, Athanasiadou E, et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2014; 16: 984–993. [DOI] [PubMed] [Google Scholar]
- 43. Scheen AJ. SGLT2 inhibition: efficacy and safety in type 2 diabetes treatment. Expert Opin Drug Saf 2015; 14: 1879–1904. [DOI] [PubMed] [Google Scholar]
- 44. Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017; 19: 524–536. [DOI] [PubMed] [Google Scholar]
- 45. Sivertsen J, Rosenmeier J, Holst JJ, et al. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol 2012; 9: 209–222. [DOI] [PubMed] [Google Scholar]
- 46. Lovshin JA, Barnie A, DeAlmeida A, et al. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 2015; 38: 132–139. [DOI] [PubMed] [Google Scholar]
- 47. Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab 2016; 24: 15–30. [DOI] [PubMed] [Google Scholar]
- 48. Nakatani Y, Kawabe A, Matsumura M, et al. Effects of GLP-1 receptor agonists on heart rate and the autonomic nervous system using Holter electrocardiography and power spectrum analysis of heart rate variability. Diabetes Care 2016; 39: e22–e23. [DOI] [PubMed] [Google Scholar]
- 49. Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004; 109: 962–965. [DOI] [PubMed] [Google Scholar]
- 50. Lønborg J, Vejlstrup N, Kelbæk H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012; 33: 1491–1499. [DOI] [PubMed] [Google Scholar]
- 51. Sokos GG, Nikolaidis LA, Mankad S, et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006; 12: 694–699. [DOI] [PubMed] [Google Scholar]
- 52. Lepore JJ, Olson E, Demopoulos L, et al. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail 2016; 4: 559–566. [DOI] [PubMed] [Google Scholar]
- 53. Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016; 316: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cefalu WT, Leiter LA, Yoon K-H, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 55. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 56. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015; 9: 48–53. [DOI] [PubMed] [Google Scholar]
- 57. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014; 124: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014; 124: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Howard G, O’Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation 1996; 93: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 60. Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yale J-F, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013; 15: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014; 85: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369–384. [DOI] [PubMed] [Google Scholar]
- 64. Ndrepepa G, Braun S, King L, et al. Prognostic value of uric acid in patients with type 2 diabetes mellitus and coronary artery disease. Clin Sci 2013; 124: 259–268. [DOI] [PubMed] [Google Scholar]
- 65. Huang H, Huang B, Li Y, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail 2014; 16: 15–24. [DOI] [PubMed] [Google Scholar]
- 66. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 375: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 67. Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012; 35: 1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16: 457–466. [DOI] [PubMed] [Google Scholar]
- 69. Tahara A, Kurosaki E, Yokono M, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol 2013; 715: 246–255. [DOI] [PubMed] [Google Scholar]
- 70. Osorio H, Coronel I, Arellano A, et al. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med Cell Longev 2012; 2012: 542042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257. [DOI] [PubMed] [Google Scholar]
- 72. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 74. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. Epub ahead of print 14 September 2017. DOI: 10.1056/NEJMoa1612917. [DOI] [PubMed] [Google Scholar]
- 75. Mentz RJ, Bethel MA, Gustavson S, et al. Baseline characteristics of patients enrolled in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL). Am Heart J 2017; 187: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ferdinand KC, Botros FT, Atisso CM, et al. Cardiovascular safety for once-weekly dulaglutide in type 2 diabetes: a pre-specified meta-analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol 2016; 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 78. Marx N, McGuire DK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur Heart J 2016; 37: 3192–3200. [DOI] [PubMed] [Google Scholar]
- 79. Blau JE, Tella SH, Taylor SI, et al. Ketoacidosis and SGLT2 inhibitor treatment: analysis of FAERS data. Diabetes Metab Res Rev. Epub ahead of print 24 July 2017. DOI: 10.1002/dmrr.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med 2017; 376: 2300–2302. [DOI] [PubMed] [Google Scholar]
- 81. Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015; 38: 1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100: 2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4: 1004–1016. [DOI] [PubMed] [Google Scholar]
- 84. Lundkvist P, Pereira MJ, Katsogiannos P, et al. Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: sustained reductions in body weight, glycaemia and blood pressure over 1 year. Diabetes Obes Metab 2017; 19: 1276–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Seino Y, Yabe D, Sasaki T, et al. Sodium-glucose cotransporter-2 inhibitor luseogliflozin added to glucagon-like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52-week, open-label, single-arm study. J Diabetes Investig. Epub ahead of print 14 May 2017. DOI: 10.1111/jdi.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Scheen AJ. Cardiovascular outcome studies with incretin-based therapies: comparison between DPP-4 inhibitors and GLP-1 receptor agonists. Diabetes Res Clin Pract 2017; 127: 224–237. [DOI] [PubMed] [Google Scholar]
- 87. Udell JA, Cavender MA, Bhatt DL, et al. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2015; 3: 356–366. [DOI] [PubMed] [Google Scholar]
- 88. Toulis KA, Hanif W, Saravanan P, et al. All-cause mortality in patients with diabetes under glucagon-like peptide-1 agonists: a population-based, open cohort study. Diabetes Metab 2017; 43: 211–216. [DOI] [PubMed] [Google Scholar]
- 89. Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. Epub ahead of print 3 August 2017. DOI: 10.1016/S2213-8587(17)30258-9. [DOI] [PubMed] [Google Scholar]