Abstract
In this study, we addressed the potential relationship between prominin-1 (prom1) and vascular endothelial growth factor (VEGFA) in diabetes-induced retinopathy. In total, we examined 28 retinas from 14 rats with streptozotocin-induced diabetes and 30 retinas from 15 untreated control rats. ELISA was used to measure the level of prom1 and VEGFA in retinal tissue homogenates. Immunohistochemical techniques were used with antibodies directed against prom1, VEGFA, and CASP-3. After 180 days of diabetes induction, we performed light and electron microscopy studies on rat eyes to evaluate histopathological changes and to estimate the de novo metric “Diabetic Retinopathy Histopathological Index” (DRHI). These changes were then correlated to the tissue and immunoexpression levels of prom1 and VEGFA. The data showed a significant upregulation of the tissue levels and optical densities (ODs) of VEGFA and prom1 immunoreactivity in diabetic retinas compared with controls. Both the tissue levels and OD values of prom1 and VEGFA correlated significantly with each other and to the diabetic structural changes as calculated by DRHI. Taken together, these data provide new insight into the potential role of prom1 and VEGFA in the development of diabetic retinopathy.
Keywords: hyperglycemia, prominin-1, rat model, retinopathy, VEGFA
Introduction
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes mellitus and is a leading cause of visual loss among individuals of working age.1,2 DR is influenced by vasculopathy and neuropathy. Vasculopathy mostly follows angiogenesis and vasculogenesis, which are dependent on several cytokines and chemokines and their associated tyrosine kinase or G protein-coupled receptors. A key player in both of these processes is vascular endothelial growth factor (VEGFA), also called vascular permeability factor.2 The underlying mechanism leading to the induction of diabetic neuropathy remains unknown, but hypoxia, ischemia, oxidative stress, and metabolic mechanisms have been implicated.3
Prominin-1 (prom1) was the first recognized gene of the penta-span transmembrane glycoprotein family and has been found in different epithelial and non-epithelial cells, including stem cells from various sources.4,5 In hematopoietic stem cells, endothelial progenitor cells (EPCs) and neuronal stem cells, prom1 is present in the extracellular matrix and is selectively sorted to plasma membrane protrusions.6,7 Recently, prom1 has been implicated in a number of genetic disorders that cause photoreceptor (PR) degeneration.8
Treatment of DR can only be achieved with an enhanced understanding of disease pathogenesis; however, because most structural, functional, and biochemical studies cannot be performed in human subjects, animal models are essential for studying the pathology of DR and thus for developing new and better treatments.
In this regard, the present study assessed the effect of hyperglycemia on the tissue and protein expression levels of prom1 and VEGFA in the retina of streptozotocin (STZ)-induced diabetic rats. This study reveals factors that could be altered in the retina to potentially promote regeneration.
Materials and Methods
Animal Model
A total of 30 albino rats, aged between 12 and 16 months and weighing 250–300 g, were used in this study. Animals were kept on a standard 12-hr light/dark cycle for 1 week before initiating the experiment and fed standard rodent chow. They were divided randomly into two groups: (1) control group (15 animals) receiving a 40 mg/kg intraperitoneal injection of a solution of 0.01 M citrate buffer, pH 4.5, and (2) diabetic group (15 animals) receiving 40 mg/kg STZ via intraperitoneal injection on 5 successive days to induce diabetes.9 Three days after the last injection of STZ, a blood sample was taken from the tail vein and rats with blood glucose above 200 mg/dl were considered STZ-induced diabetic rats.10 Animals from both groups were housed for at least 6 months under the same housing conditions. During this period, one of the diabetic animals died, leaving only 14 animals in the diabetic group. The experiments were performed in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals. The study was approved by the local institutional experimental research committee.
Specimen Collection
The right eyes were enucleated whole, dissected, and processed either for homogenate analysis using ELISA or transmission electron microscopy (TEM) analysis. The retinas of the left eyes were processed for histological and immunohistochemical analyses.
Tissue Extract for Homogenate Analysis Using ELISA
Using a dissecting microscope, the cornea was carefully sectioned, and all the contents of the anterior segment were removed in one piece to gain access to the posterior segment. Finally, the retina was exposed and collected. Half of the retinas were dissected immediately on ice to prevent degradation by proteases and subsequently processed for ELISA while the other half were processed for TEM.
The total tissue levels of prom1 and VEGFA in the retina were assessed using a sandwich enzyme immunoassay. Tissue extraction buffer was prepared (50 mM Tris, pH 7.5–300 mM NaCl–1 mM EDTA–1% Triton X-100–10 µg/ml aprotinin–0.1 mM benzethonium chloride, 1 mM benzamidine–0.1 mM phenylmethanesulfonyl fluoride). Retinal tissue was placed in a tube containing complete extraction buffer at a concentration of approximately 50 mg/ml and homogenized with an electric homogenizer. After washing the blade twice using the extraction buffer, constant agitation was maintained for 2 hr at 4C. Then, samples were centrifuged for 20 min at 13,000 rpm at 4C. An aliquot of the supernatant was collected (soluble protein extract) and assayed immediately using available commercial kits (cat. no SEB516Hu for prom1 and SEA143Ra for VEGFA; USCN, Houston, TX) with horseradish peroxide detection according to the manufacturer’s guidelines.11,12 The kits included standard with different concentrations, and the concentration of prom1 and VEGFA in the samples is determined by comparing the optical density (OD) of the samples to the standard curve.
Tissue Preparation for TEM
Specimens for TEM were immediately fixed in 2.5% phosphate-buffered glutaraldehyde (pH 7.4) and post-fixed in 1% osmium tetroxide in the same buffer at 4C. They were then dehydrated and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate. Sections were examined and photographed using a JEOL JEM 1010 electron microscope (Jeol Ltd; Tokyo, Japan).13
Tissue Preparation for Histochemical and Immunohistochemical Analyses
The left eyeballs were fixed in 4% paraformaldehyde at room temperature immediately after enucleation for 24 hr. The specimens were embedded in paraffin and sectioned at 4 µm. After deparaffinization and rehydration, sections were stained with H&E, Periodic acid–Schiff (PAS), and Masson’s Trichrome as described earlier.14 For immunohistochemistry, a standard immunoperoxidase technique was used. Endogenous peroxidase activity was quenched with 3% H2O2 for 30 min in the dark. Sections were subjected to antigen retrieval by microwaving the sections in 10 mM citrate buffer (pH 6.0) for 10 min at 95C–100C and allowed to cool for 1 hr at room temperature. After incubation for 10 min in a blocking solution (10× phosphate-buffered saline [PBS] and 2% normal horse serum) to control background staining (Histo-Plus kit; Zymed, San Francisco, CA), sections were incubated for 30 min with primary antibody rabbit polyclonal anti-CASP-3 antibody (diluted 1:100, cat. no PAA626Ra02, Uscn Life Science Inc, China), rabbit polyclonal anti-CD133 antibody (diluted 1:200; Abnova, cat. no PAB12663, Taipei, Taiwan), or rabbit polyclonal anti-VEGFA antibody (diluted 1:100; cat. no PAA143Ra71, Uscn). Dilutions were made with antibody diluent (TA-125-UD; Lab Vision Corporation, Medical laboratory, Fremont, CA). Peroxidase activity was demonstrated by incubation with the substrate chromogen DAB (3,30-diaminobenzidine tetrahydrochloride). The sections were rinsed in PBS and left overnight (1:200) in a humidified chamber at 4C. Then, they were washed twice in PBS. The secondary antibody was an antirabbit antibody kit (code no: Ko773, lot [code no: P0448]). Sections were covered with biotinylated secondary antibody for 30 min and then washed in PBS. Then, a peroxidase-labeled avidin/biotin solution reaction (Novo Stain Super ABC Kit; Novocastra, Newcastle upon Tyne, UK) was applied for 45 min, followed by washing with PBS. Finally, freshly prepared DAB (3,39-diaminobenzidine tetrahydrochloride; Sigma, St. Louis, MO) was added for 4 min followed by Mayer’s hematoxylin as a counterstain. The sections were washed, dehydrated, mounted, and examined. For the negative control, the same steps were followed, but the primary antibody was replaced by PBS.14
Image Acquisition and Assessment of the OD15
Images were captured using a Leica Q-Win digital camera CH-9435 DFC 290 coupled to a photomicroscope (Germany). Images were captured with 40× and 100× objective lenses.
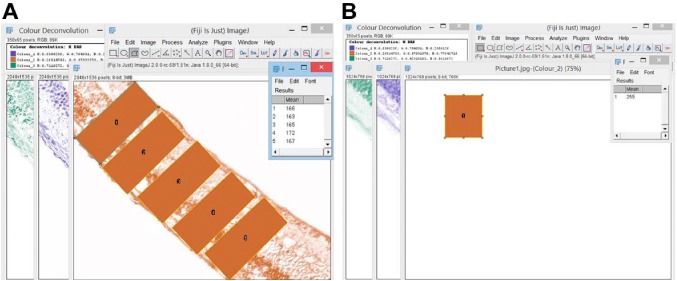
ImageJ v2. 35 (NIH) was used for brightness adjustment, to identify the presence and the extent of the expression of prom1 and VEGFA in the DAB images, and for data collection (Fig. 1). An ImageJ plugin was run to analyze the cytoplasmic staining by assigning a histogram profile for the deconvoluted DAB image using H-DAB-vector to yield three differently colored images: green, brown, and blue. Calibration of the DAB images (brown-colored) was performed by measuring the mean intensity of five non-overlapping areas of the stained tissue. The uniformity of section thickness was considered for legitimate comparisons using this approach. The empty areas were omitted from the measurements to not bias the outcomes. To convert the intensity numbers into OD, we applied the following formula:
Figure 1.
Screenshot of the method of measurement of the mean gray value of the DAB-stained sections using ImageJ software. Panel (A) represents the deconvolution of a prom1-positive DAB image. Panel (B) represents the deconvolution of a negative-stained DAB image. Abbreviation: DAB, diaminobenzidine tetrahydrochloride.
where the max intensity = 250; mean intensity = mean gray value.
Assessment of the Histopathological Changes of the Retina
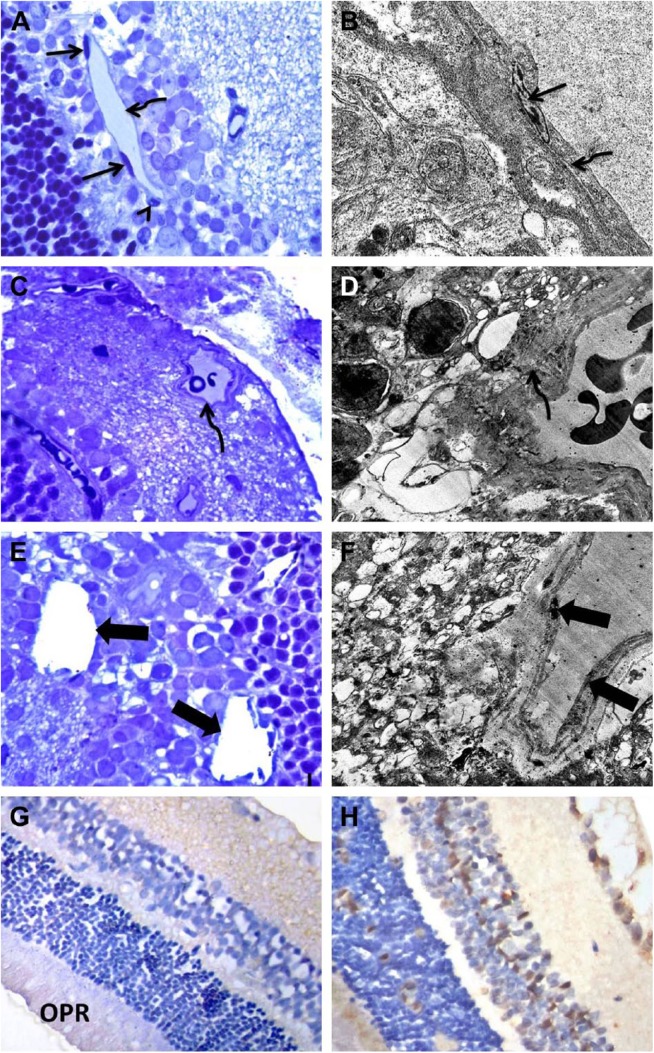
The common histopathological criteria associated with DR are (1) endothelial cell damage, (2) pericyte loss, (3) acellular degenerated capillaries representing basement membrane tubes lacking cell nuclei, (4) increased capillary basement membrane thickening, (5) decreased number of ganglion cells in the ganglion cell layer (GCL), (6) apoptosis of the inner nuclear layer (INL), and (7) apoptosis of the outer nuclear layer (ONL)16–21 (Fig. 2).
Figure 2.
Light and EM photographs of the retina of control and diabetic rats. Panels A and B: Control retinas show normal capillaries with intact pericytes (arrowhead) and flat endothelial cells (arrow) resting on a regular basement membrane (curved arrow). Panels C–F: Diabetic retinas show a capillary within the GCL with an irregularly thickened basement membrane (curved arrow). Notice the decreased number of cells in the GCL. Acellular blood vessels, endothelial cell damage, and pericyte loss (thick arrows) are also present. Panel G: Control retinas show weak expression of CASP-3 in the GCL, IPL, and OPR. Panel H: Diabetic retinas showed higher expression of CASP-3 in all retinal layers compared with control retinas. A, C, and E = toluidine blue; G and H = CASP-3 immunoperoxidase; B (TEM magnification = 25,000×), D (TEM magnification = 6000×), and F (TEM magnification = 8000×), uranyl acetate and lead citrate. Abbreviations: EM, electron microscopy; GCL, ganglion cell layer; IPL, inner plexiform layer; OPR, outer segment of the photoreceptor; TEM, transmission electron microscopy. Scale bar = 50 µm.
In the present study, we evaluated the histopathological changes in the diabetic group compared with controls. The first five structural changes were evaluated by examining 10 overlapping fields of the semi-thin sections at a magnification of 1000× and confirmed by TEM examination. Ganglion cells, which are characterized by a round or oval nucleus with condensed heterochromatin and at least one prominent nucleolus, were counted.22,23 According to Qin et al.,24 DR is characterized by −17% loss of ganglion cells compared with controls. In the present study, the mean ganglion cell count in the control group was 16 ± 0.34, and thus, <13 cells per field was considered a positive histopathological change.
For statistical analysis, to correlate the tissue levels and immunoexpression of prom1 and VEGFA in the retina with the histopathological changes due to hyperglycemia, a simple histopathological index, Diabetic Retinopathy Histopathological Index (DRHI), was calculated to provide an objective measure of the magnitude and extent of DR in the study animals.
First, a semiquantitative score was given based on the absence (= 0) or presence (= 1) of each of the histopathological features of DR. Then, the total of these scores as measured in the examined 10 fields was used to calculate the total case score and then applied to each study group.
DRHI was calculated using the following formula:
where 7 was the number of histopathological changes assessed in the retina of normoglycemic and hyperglycemic rats; 10 was the number of fields evaluated in each retina.25
Statistical Analysis
Results are expressed as the mean ± standard error (SE). Comparison of the studied variables between the control and diabetic groups was performed using an independent t-test. The p values less than 0.05 were considered statistically significant. Correlations among DRHI, prom1, and VEGFA were plotted, and the Pearson’s correlation coefficients were labeled.
Results
Survival Rate of Animals
The animals in the control group had no deaths, whereas one rat in the hyperglycemic group passed away 2 months following the final dose of STZ.
Assessment of the Tissue Levels of Prom1 and VEGFA
ELISA analysis of the retinal homogenates revealed that the tissue level of prom1 was significantly elevated in the samples from diabetic rats (19.7 ± 0.97 pg/ml) compared with control rats (12.6 ± 1.06 pg/ml; p<0.001). Furthermore, the tissue level of VEGFA was significantly elevated in the diabetic group (27.8 ± 1.22 pg/ml; p<0.001) compared with the control group (15.6 ± 1.03 pg/ml; p<0.001; Fig. 3).
Figure 3.
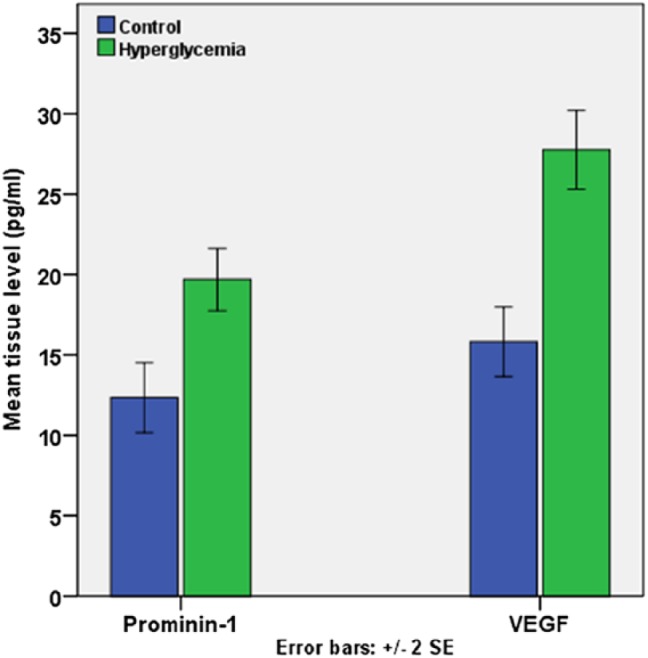
Histogram represents the mean tissue level of prom1 and VEGFA in the studied groups using ELISA analysis of homogenates. Abbreviation: VEGFA, vascular endothelial growth factor.
Histological Results
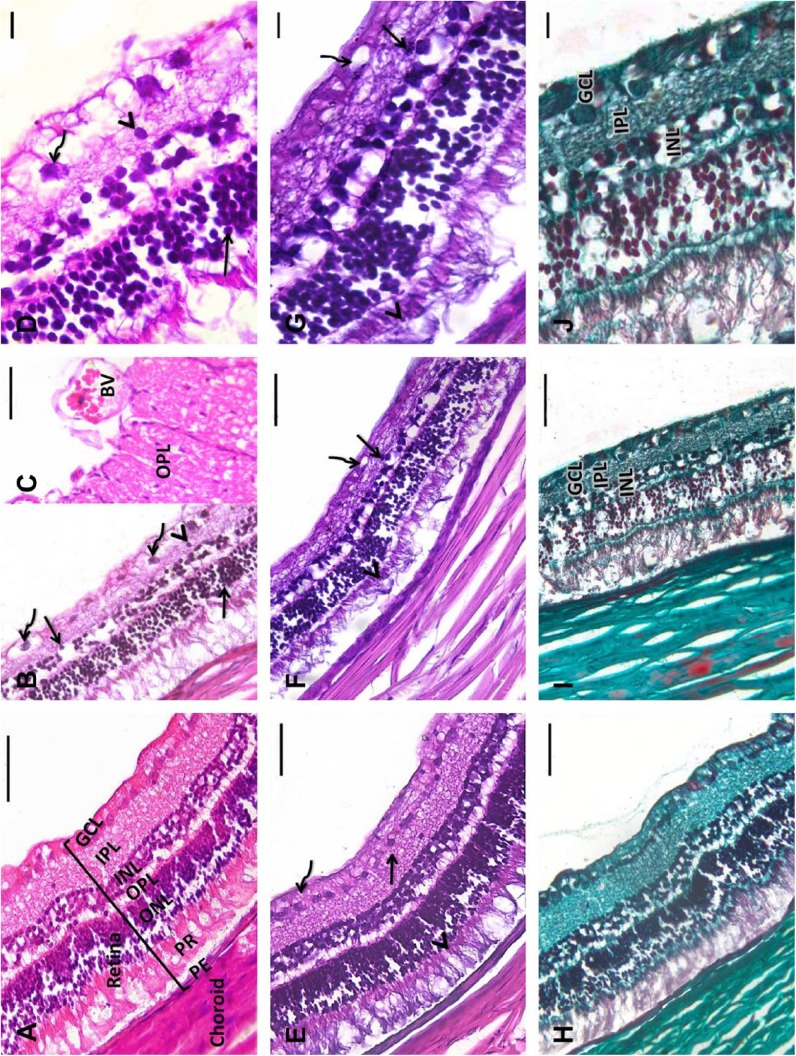
Control retinal tissues showed a normal arrangement of retinal layers. The retinas were arranged into pigmented epithelium (PE), PRs, an ONL, an outer plexiform layer (OPL), an INL, an inner plexiform layer (IPL), and a GCL (Figs. 4A and B). Compared with controls, hyperglycemic retinas showed apparent alterations in histological structure. There was a reduction in the number of ganglion cells, which contained shrunken pyknotic nuclei and disrupted PRs. The thin ONL and the INL showed desquamation of the cells with multiple pyknotic nuclei and widening of the intercellular space. Moreover, focal displacement of cells of the INL to IPL was apparent. The nuclei of vacuolated ganglionic cells were shrunken, pyknotic, and widely dispersed. Congested blood vessels were detected in the optic nerve layer (Figs. 4A–C).
Figure 4.
Representative retinal tissue sections from rats. A: Control retinas show normal arrangement of the retinal layers and choroid. Retinas are organized into PE, PR, ONL, OPL, INL, IPL, and GCL. B and C: Diabetic retinas show disrupted PR. Multiple pyknotic nuclei (arrows) and desquamation of cells with widening of the intercellular spaces between the cells of the thin ONL and INL. Focal displacement of cells of the INL to the IPL (arrowhead) is seen. The vacuolated ganglionic cell nuclei are shrunken, pyknotic, and widely dispersed (curved arrows). C: Large dilated congested blood vessels in the OP layer. D: A higher magnification of B. E: Control retinas show discrete glycogen granules throughout the retinal neuropil in both neurons and glial cells. F: Diabetic retinas reveal highly localized dense deposits of glycogen throughout the inner segments of the PR cells (arrowhead) and in small cell processes within the IPL (arrow). Glycogen deposits are seldom seen in cell bodies within the GCL (curved arrow). G: A higher magnification of F. H: Control retinas show few collagen fibrils distributed throughout the retinal layers. I: Diabetic retinas show many collagen fibers in the GCL, IPL, and INL. J: A higher magnification of I. A–D: H&E; E–G: PAS; H–J: Masson’s Trichrome. Abbreviations: PE, pigmented epithelium; PR, photoreceptor; ONL, outer nuclear layer, OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; OP, optic nerve; H&E, hematoxylin and eosin; PAS, Periodic acid–Schiff. Scale bar = 50 µm.
PAS-stained sections revealed discrete glycogen granules throughout the retinal neuropil in both the neurons and glial cells of the control group. The hyperglycemic group had highly localized dense deposits of glycogen that were consistently found throughout the inner segments of the PR cells and in small cell processes within the IPL. Glycogen deposits were occasionally found in cell bodies within the GCL (Figs. 4D and E).
Masson’s Trichrome stained sections in the control group showed a thin Bruch membrane and few collagen fibrils distributed throughout the retinal layers. The diabetic group showed thickening of the Bruch membrane and the appearance of many collagen fibers in the GCL, IPL, and INL (Figs. 4F and G).
Immunohistochemical Analysis
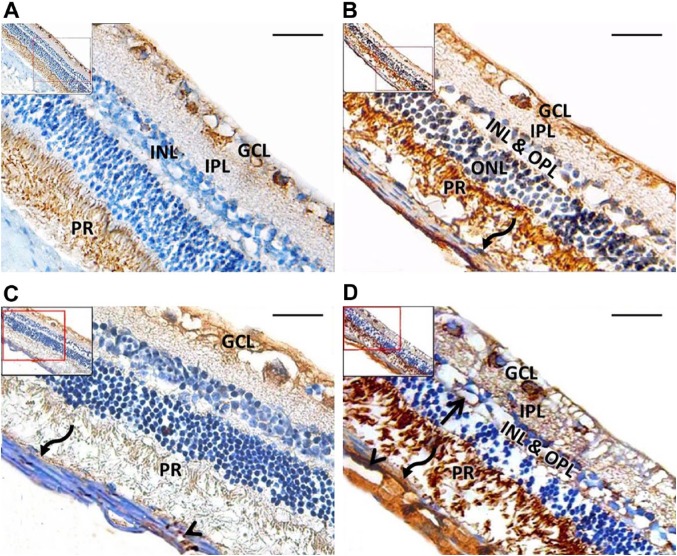
Immunohistochemical sections revealed moderate expression of prom1 in the GCL, IPL, and in the inner segment of the PRs. Prom1 was weakly expressed in the IPL and INL of the control group. Compared with the control group, the diabetic group showed robust expression of prom1 in the GCL, INL, inner segment of the PRs, and in the endothelium of blood vessels, but only moderate expression in the IPL and ONL. VEGFA-immunostained sections from the control group showed a strong reaction in the GCL and a weak reaction in the PR and vascular endothelium. Diabetic sections showed a strong VEGFA reaction in the GCL, IPL, INL, OPL, PR, and in the retinal and choroidal blood vessels (Fig. 5).
Figure 5.
Representative retinal tissue sections from rats. A: Control group shows moderate prom1 expression in the GCL and PR, with weak expression in the IPL and INL. B: Diabetic group shows strong prom1 expression in the GCL, INL, inner segment of the PR, and in the pigmented epithelium (curved arrow). Moderate expression is seen in the IPL and ONL. C: Control group shows a strong VEGFA reaction in the GCL and a weak reaction in the PR, pigmented epithelium (curved arrow), and vascular endothelium (arrowhead). D: Diabetic group shows a strong VEGFA reaction in the GCL, IPL, INL, OPL, PR, pigmented epithelium (curved arrow), and endothelium of retinal (arrow) and choroidal (arrowhead) blood vessels. A and B: Prom1 immunoperoxidase; C and E: VEGFA immunoperoxidase. Abbreviations: GCL, ganglion cell layer; PR, photoreceptor; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; OPL, outer plexiform layer; VEGFA, vascular endothelial growth factor. Scale bar = 50 µm.
In addition, statistical analysis of the data showed a significant increase in the OD of prom1 expression in the hyperglycemic retina (0.15 ± 0.005) compared with controls (0.09 ± 0.007; p<0.001). Furthermore, there was an increase in the OD of VEGFA expression in hyperglycemic retinas (0.1 ± 0.01) compared with controls (0.06 ± 0.007; p=0.031; Fig. 6).
Figure 6.
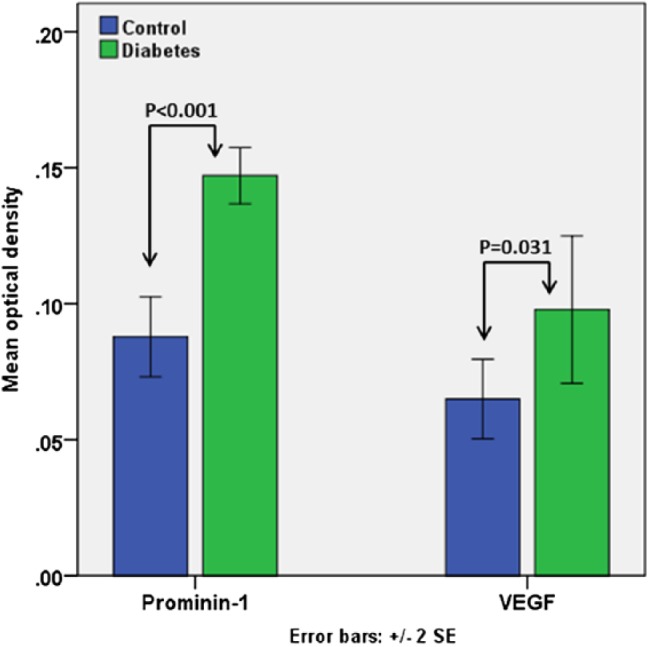
Histogram represents the OD of prom1 and VEGFA immunoreactivity in the studied groups. Abbreviations: OD, optical density; VEGFA, vascular endothelial growth factor.
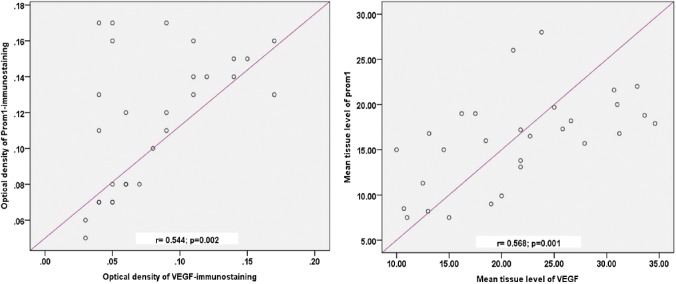
As shown in Fig. 7, there was a significant correlation between the ODs of prom1 and VEGFA immunostaining (r=0.544; p=0.002) and between their tissue levels (r=0.568; p=0.001).
Figure 7.
Correlation between the retinal tissue levels (left panel) and the OD (right panel) of prom1 and VEGFA in the control and diabetic groups. Abbreviations: OD, optical density; VEGFA, vascular endothelial growth factor.
Study of the DRHI
A descriptive analysis of different histopathological changes indicating retinopathy is shown in Table 1. The scores of the histopathological changes (= sum of scores in the 10 examined fields) were significantly higher (p<0.05) in the diabetic retinas compared with control retinas. In addition, DRHI was significantly higher in the diabetic group compared with the control group (p<0.001).
Table 1.
Score of the Histopathological Criteria of Diabetic Retinopathy in the Studied Rats.
| Histopathological Changes (mean ± SE) | Control Rats (n=15) | Hyperglycemic Rats (n=14) | p Value |
|---|---|---|---|
| Endothelial cell damage | 1.53 ± 0.26 | 4.57 ± 0.27 | <0.001* |
| Basement membrane thickening | 2.4 ± 0.21 | 3.29 ± 0.34 | 0.033* |
| Pericyte loss | 0.87 ± 0.22 | 3.5 ± 0.25 | <0.001* |
| Acellular degenerated capillaries | 1.07 ± 0.33 | 2.5 ± 0.29 | 0.003* |
| Apoptosis in INL | 1.53 ± 0.3 | 4.21 ± 0.47 | <0.001* |
| Apoptosis in ONL | 2.33 ± 0.29 | 4.36 ± 0.31 | <0.001* |
| Decrease in the number of cells in GCL | 2.53 ± 0.36 | 4 ± 0.39 | 0.011* |
| Total score | 12.27 ± 0.57 | 26.43 ± 0.2 | <0.001* |
| DRHI | 17.52 ± 0.82 | 37.74 ± 1.7 | <0.001* |
Observations were done with light microscope. Each histopathological value represents the mean ± SE of sum of 0/1 score in the 10 examined fields.
Abbreviations: DRHI, Diabetic Retinopathy Histopathological Index; INL, inner nuclear layer; ONL, outer nuclear layer; GCL, ganglion cell layer.
Significant p<0.05 for independent t-test.
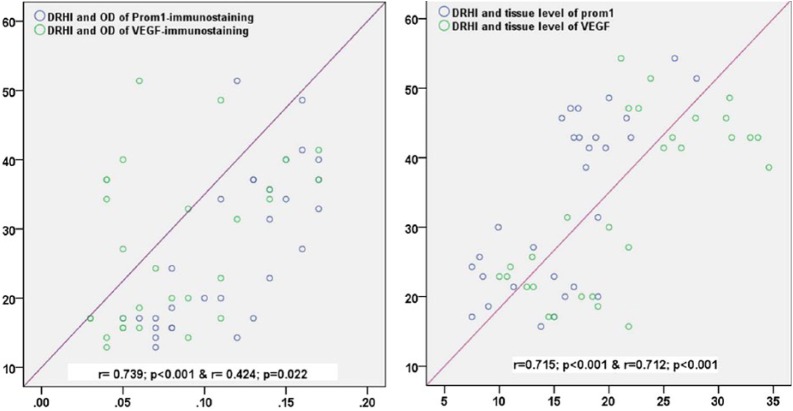
Among the control retinal tissues, DRHI was correlated significantly with the OD of prom1 (r=0.729; p<0.001) and the OD of VEGFA (r=0.416; p=0.025). Likewise, significant correlations were detected between the DRHI and the tissue levels of prom1 (r=0.715; p<0.001) and VEGFA (r=0.712; p<0.001) (Fig. 8).
Figure 8.
Correlation of the DRHI to the OD (A) and the retinal tissue levels (B) of prom1 and VEGFA in the control and diabetic groups. Abbreviations: DRHI, Diabetic Retinopathy Histopathological Index; OD, optical density; VEGFA, vascular endothelial growth factor.
Discussion
Our results provide new insight regarding the modulation of the tissue levels and immunoexpression of prom1 and VEGFA in the retina of STZ-induced diabetic rats with regard to the regulation of cellular and extracellular microenvironments.
Histopathological examination of hyperglycemic retinas revealed multiple structural alterations in all layers, including the neural and vascular structures. These changes indicate the development of retinopathy related to neuropathy and vasculopathy due to hyperglycemia and were quantitatively interpreted as a significant increase in the total histopathological score and DRHI in diabetic retinas compared with control retinas.
The predominant vascular changes include the thickening of the basement membrane and damage of the endothelial cells of the retinal capillaries. Those findings are consistent with those reported in a previous study of Curtis et al.,26 which related these changes to the development of retinal ischemia.
Neuropathy is a prominent feature in diabetic retinas and manifests as an increase in the apoptotic activity of neurons as evidenced by strong CASP-3 immunoreactivity in the retinal ganglion cells and in the inner and ONLs. Abu El-Asrar et al.27,28 reported that diabetic retinas initiate some molecular remodeling involving mechanisms that regulate neuronal survival and apoptosis through the expression of many antiapoptotic and proapoptotic mediators as well as through the upregulation of some mitochondrial proteins.
An examination of the VEGFA-immunostained sections revealed immunopositivity in most of the layers of the retina and in the endothelium of the blood vessels of control and hyperglycemic specimens. Furthermore, the tissue level of VEGFA was significantly higher in hyperglycemic rats compared with controls; the cells of the retina over-expressed VEGFA. These findings are in agreement with many recent studies.29–31 Moreover, the observed significant correlation between DRHI and both the tissue levels and immunoreactivity of this growth factor supports these findings.
Upregulation of VEGFA in the diabetic retina can be explained by retinal ischemia secondary to retinal vasculopathy. Other mechanisms that have been shown to be involved include inflammation, tissue hypoxia,32 oxidative stress,33 and impaired function of EPCs.34,35
VEGFA is thought to drive the vascular changes associated with hyperglycemia. It promotes endothelial cell proliferation leading to angiogenesis and neovascularization in ischemic tissues. In addition, it activates EPCs, which take part in pathological neovascularization and the production of other angiogenic factors.36,37 Thus, this may explain the endothelial dysfunction observed during hyperglycemia.
Examination of the diabetic sections revealed the presence of new blood vessels together with increased collagen fibers. Glial cells mostly have neuroprotective and supportive functions in the retina while angiogenesis is important in tissue healing. However, under conditions of inflammation and hypoxia-driven neovascularization, glial cells contribute to fibrotic scar formation that may lead to sub-retinal scarring or traction detachment of the retina.38 Thus, fibrosis is linked to the vascular changes due to hyperglycemia. Thus, the prevention of diabetic vasculopathy is an appropriate target to preserve retinal structure and function.
In the present study, we characterized the localization of prom1 in different retinal layers of control and diabetic rats. Prom1 is expressed in the PR cells, INL, and in the vascular endothelium. Our findings are largely in agreement with recent studies describing the localization of prom1 in non-epithelial cells, such as PR cells39 and in many differentiated epithelia.5,40 Accordingly, an important function for prom1 in the retina has been proposed.
Zacchigna et al.40 reported an essential role of prom1 in regulating the integrity of PRs in different species. Accumulating evidence has suggested the specific localization of prom1 in the membrane evaginations of rod cells. These evaginations represent essential precursors in the biogenesis of PR disk membranes that are continually renewed through adult life.41,42 Corbeil et al.42 highlighted the interaction between prom1 and membrane cholesterol in the generation of plasma membrane evaginations at the base of the outer segment of rod PR cells. Thus, prom1 could be considered as a neuroprotective element for the retina. In accordance with this hypothesis, previous studies have reported that mutations in prom1 cause some retinal diseases, such as retinitis pigmentosa.43
The present study found significantly higher prom1 tissue levels and immunoreactivity in diabetic rats compared with controls. Both the tissue level and OD of prom1 were significantly correlated with the pathological changes of the retina as revealed by DRHI. These findings indicate that prom1 changes along with alterations in the microenvironment. In support of the hypothesis of increased prom1 in diabetic retinas, Chen et al.44 reported the continuous release of prom1 from the plasma membrane into the cytoplasm in both complete and low glucose media, whereas Yang et al.43 reported that prom1 is related to cell metabolism as a glucose responsive gene in myotubes. Consequently, the intrinsic functions of prom1 could be affected by the metabolic changes evoked by hyperglycemia and may be involved in the associated neuropathy.
Although these interpretations do not provide a complete understanding of the function of this protein, they do suggest that retinal neurons possess protective mechanisms to guard against apoptosis. Increased prom1 levels indicate that retinal neurons tend to be maintained for the entire life span of an individual by promoting neuroprotective elements, such as prom1. Similarly, retinal ganglion cells invoke a survival response to several growth factors after axotomy through an intracellular signaling (PI3-kinase/Akt) pathway that mediates and regulates the cell cycle.45,46
Interestingly, prom1 has been described as a marker to identify immature EPCs.47 Thus, another possible explanation for prom1 upregulation in diabetic retinas is the increased number of immature EPCs honing to the retina as one of the sites of injury in diabetes to promote tissue healing, although many other factors could affect their reparative ability.48
However, Mohammad et al.10 stated that diabetic neuropathy possibly results from loss of neuroprotective factors such as prom1 and from injury to the blood-retinal barrier as well. In contrast to our results, they reported a significant decrease in prom1 in retinal extracts from STZ-induced diabetics due to a proposed interaction between prom1 and gelatinase B/matrix metalloproteinase-9. Further investigations are necessary to distinguish the functions of prom1 in hyperglycemia and normoglycemia, which might lead to better therapeutics.
Importantly, prom1 is lately considered a marker of stem cells originating from various sources.4,5 Consequently, stem cells that were previously described in the retinal PE49 can express prom1. The increased tissue level and immunoreactivity of prom1 in the diabetic retina could be a marker for the activation of resident stem cells triggered by hyperglycemia to produce the new cells needed to regenerate damaged tissue.
Our data revealed a significant correlation between the tissue levels and OD of prom1 and VEGFA in all specimens. Consistent with our data, recent studies have reported a positive feedback regulation between VEGFA and prom1. Specific localization of prom1 in endothelial cells supports the hypothesis of Adini et al.,50 who demonstrated the ability of prom1 to interact and potentiate the antiapoptotic and angiogenic properties of VEGFA and also demonstrated a direct interaction between prom1 with VEGFA on endothelial cells. They also described a subpopulation of VEGFR2-GFP (VEGFR2–glial fibrillary protein) endothelial cells that co-express prom1, thus substantiating the interconnection between prom1 and VEGFA reported in the present study.
In conclusion, our data revealed the upregulation of retinal tissue levels and immunoreactivity of VEGFA and prom1 during hyperglycemia that could contribute to the development of diabetic vasculopathy and neuropathy. This study provides an experimental basis for future therapeutics.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SAM conceived of the study, participated in the design of the study, carried out the histological staining procedure, participated in the sequence alignment, drafted the manuscript, and performed the statistical analysis. EKH participated in the design of the study, carried out the histological staining procedure, participated in its design and coordination, and helped to draft the manuscript. RAE participated in the design of the study and patient’s selection, participated in the sequence alignment, and drafted the manuscript. ZAH participated in the design of the study and patient’s selection, participated in the sequence alignment, and drafted the manuscript. All authors read and approved the final manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Shaima M. Almasry, Department of Anatomy, Al-Mansoura University, Mansoura, Egypt Department of Anatomy, Taibah University, Medina, Saudi Arabia.
Eman K. Habib, Department of Anatomy, Ain Shams University, Cairo, Egypt
Rasha A. Elmansy, Department of Anatomy, Ain Shams University, Cairo, Egypt
Zeinab A. Hassan, Department of Anatomy, Taibah University, Medina, Saudi Arabia; Department of Histology and Cell Biology, Zagazig University, Zagazig, Egypt.
Literature Cited
- 1. Kocur I, Resnikoff S. Visual impairment and blindness in Europe and their prevention. Br J Ophthalmol. 2002;86(7):716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009;284(38):25602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82(964):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fargeas CA, Fonseca AV, Huttner WB, Corbeil D. Prominin-1 (CD133): from progenitor cells to human diseases. Future Lipidol. 2006;1(2):213–25. [Google Scholar]
- 5. Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D’Angelica M, Kemeny N, Lyden D, Rafii S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133– metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118(6):2111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–12. [PubMed] [Google Scholar]
- 7. Harris JR. Cholesterol binding and cholesterol transport proteins: structure and function in health and disease. Subcellular biochemistry book series (SCBI, volume 51). Dordrecht: Springer; 2010. [Google Scholar]
- 8. Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12(4):238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Descamps FJ, Martens E, Ballaux F, Geboes K, Opdenakker G. In vivo activation of gelatinase B/MMP-9 by trypsin in acute pancreatitis is a permissive factor in streptozotocin-induced diabetes. J Pathol. 2004;204(5):555–61. [DOI] [PubMed] [Google Scholar]
- 10. Mohammad G, Vandooren J, Siddiquei MM, Martens E, Abu El-Asrar AM, Opdenakker G. Functional links between gelatinase B/matrix metalloproteinase-9 and prominin-1/CD133 in diabetic retinal vasculopathy and neuropathy. Prog Retin Eye Res. 2014;43:76–91. [DOI] [PubMed] [Google Scholar]
- 11. Seki M, Fukuchi T, Tanaka T, Nawa H, Takei N, Abe H. Quantitative analyses of mRNA and protein levels of neurotrophin-3 in the rat retina during postnatal development and aging. Jpn J Ophthalmol. 2004;48(5):460–4. [DOI] [PubMed] [Google Scholar]
- 12. Eldomiaty MA, Almasry SM, Desouky MK, Algaidi SA. Voluntary running improves depressive behaviours and the structure of the hippocampus in rats: a possible impact of myokines. Brain Res. 2016;1657:29–42. [DOI] [PubMed] [Google Scholar]
- 13. Amelinckx S, Van Dyck D, Van Landuyt J, Van Tendeloo G. Electron microscopy: principles and fundamentals. John Wiley & Sons; 2007. doi: 10.1002/9783527614561. [DOI] [Google Scholar]
- 14. Jones L. Connective tissues and stains. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. 5th ed. Philadelphia: Churchill Livingstone Publishers; 2002. p. 139–62. [Google Scholar]
- 15. Varghese F, Bukhari AB, Malhotra R, De A. IHC profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE. 2014;9(5):e96801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roy S, Maiello M, Lorenzi M. Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest. 1994;93(1):438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97(12):2883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46(9):1260–8. [DOI] [PubMed] [Google Scholar]
- 19. Stewart MW. Pathophysiology of diabetic retinopathy. In: Browning DJ. editor. Diabetic retinopathy: evidence-based management. New York: Springer; 2010. p. 1–30. doi: 10.1007/978-0-387-85900-2. [DOI] [Google Scholar]
- 20. Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(2):1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lai AKW, Lo ACY. Animal models of diabetic retinopathy: summary and comparison. J Diabetes Res. 2013;2013:106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quigley HA, Cone FE, Gelman SE, Yang Z, Son JL, Oglesby EN, Pease ME, Zack DJ. Lack of neuroprotection against experimental glaucoma in c-Jun N-terminal kinase 3 knockout mice. Exp Eye Res. 2011;92(4):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlamp CL, Montgomery AD, Mac Nair CE, Schuart C, Willmer DJ, Nickells RW. Evaluation of the percentage of ganglion cells in the ganglion cell layer of the rodent retina. Mol Vis. 2013;19:1387–96. [PMC free article] [PubMed] [Google Scholar]
- 24. Qin Y, Xu G, Wang W. Dendritic abnormalities in retinal ganglion cells of three-month diabetic rats. Curr Eye Res. 2006;31(11):967–74. [DOI] [PubMed] [Google Scholar]
- 25. El-Tarhouny SA, Almasry SM, Elfayomy AK, Baghdadi H, Habib FA. Placental growth factor and soluble Fms-like tyrosine kinase 1 in diabetic pregnancy: a possible relation to distal villous immaturity. Histol Histopathol. 2014;29(2):259–72. [DOI] [PubMed] [Google Scholar]
- 26. Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond). 2009;23(7):1496–508. [DOI] [PubMed] [Google Scholar]
- 27. Abu El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45(8):2760–6. [DOI] [PubMed] [Google Scholar]
- 28. Abu El-Asrar AM, Dralands L, Missotten L, Geboes K. Expression of antiapoptotic and proapoptotic molecules in diabetic retinas. Eye (Lond). 2007;21(2):238–45. http://www.ncbi.nlm.nih.gov/pubmed/16424911. [DOI] [PubMed] [Google Scholar]
- 29. Iacono P, Battaglia Parodi M, Bandello F. Antivascular endothelial growth factor in diabetic retinopathy. Dev Ophthalmol. 2010;46:39–53. [DOI] [PubMed] [Google Scholar]
- 30. Ye X, Xu G, Chang Q, Fan J, Sun Z, Qin Y, Jiang AC. ERK1/2 signaling pathways involved in VEGF release in diabetic rat retina. Invest Ophthalmol Vis Sci. 2010;51(10):5226–33. [DOI] [PubMed] [Google Scholar]
- 31. Kusari J, Zhou SX, Padillo E, Clarke KG, Gil DW. Inhibition of vitreoretinal VEGF elevation and blood-retinal barrier breakdown in streptozotocin-induced diabetic rats by brimonidine. Invest Ophthalmol Vis Sci. 2010;51(2):1044–51. [DOI] [PubMed] [Google Scholar]
- 32. Gupta N, Mansoor S, Sharma A, Sapkal A, Sheth J, Falatoonzadeh P, Kuppermann B, Kenney M. Diabetic retinopathy and VEGF. Open Ophthalmol J. 2013;7(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang RK, An L, Francis P, Wilson DJ. Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Opt Lett. 2010;35(9):1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–6. [DOI] [PubMed] [Google Scholar]
- 35. Lois N, McCarter RV, O’Neill C, Medina RJ, Stitt AW. Endothelial progenitor cells in diabetic retinopathy. Front Endocrinol (Lausanne). 2014;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de, Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26(9):2140–6. [DOI] [PubMed] [Google Scholar]
- 37. Costa C. Endothelial progenitor cells and the diabetic paradox current knowledge and therapeutic perspectives. Open Circ Vasc J. 2010;3:10–16. [Google Scholar]
- 38. Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117:576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maw MA, Corbeil D, Koch J, Hellwig A, Wilson-Wheeler JC, Bridges RJ, Kumaramanickavel G, John S, Nancarrow D, Röper K, Weigmann A, Huttner WB, Denton MJ. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9(1):27–34. [DOI] [PubMed] [Google Scholar]
- 40. Zacchigna S, Oh H, Wilsch-Bräuninger M, Missol-Kolka E, Jászai J, Jansen S, Tanimoto N, Tonagel F, Seeliger M, Huttner WB, Corbeil D, Dewerchin M, Vinckier S, Moons L, Carmeliet P. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci. 2009;29(7):2297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boesze-Battaglia K, Schimmel R. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J Exp Biol. 1997;200(Pt 23):2927–36. [DOI] [PubMed] [Google Scholar]
- 42. Corbeil D, Röper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2(2):82–91. [DOI] [PubMed] [Google Scholar]
- 43. Zhang Q, Zulfiqar F, Xiao X, Riazuddin SA, Ahmad Z, Caruso R, MacDonald I, Sieving P, Riazuddin S, Hejtmancik JF. Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum Genet. 2007;122(3–4):293–9. [DOI] [PubMed] [Google Scholar]
- 44. Chen H, Luo Z, Dong L, Tan Y, Yang J, Feng G, Wu M, Li Z, Wang H. CD133/prominin-1-mediated autophagy and glucose uptake beneficial for hepatoma cell survival. PLoS ONE. 2013;8(2):e56878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci. 2002;43(10):3319–26. [PubMed] [Google Scholar]
- 46. Weishaupt JH, Rohde G, Pölking E, Siren AL, Ehrenreich H, Bähr M. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–22. [DOI] [PubMed] [Google Scholar]
- 47. Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86(12):1198–202. [DOI] [PubMed] [Google Scholar]
- 48. Calzi SL, Neu MB, Shaw LC, Grant MB. Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy: treatment concept to correct diabetes-associated deficits. EPMA J. 2010;1(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang SZ, Yan RT. The retinal pigment epithelium: a convenient source of new photoreceptor cells? J Ophthalmic Vis Res. 2014;9(1):83–93. [PMC free article] [PubMed] [Google Scholar]
- 50. Adini A, Adini I, Ghosh K, Benny O, Pravda E, Hu R, Luyindula D, D’Amato RJ. The stem cell marker prominin-1/CD133 interacts with vascular endothelial growth factor and potentiates its action. Angiogenesis. 2013;16(2):408–16. [DOI] [PubMed] [Google Scholar]