Abstract
Background:
Strict glycemic control is known to be a vital key in the management of diabetes mellitus (DM). However, traditional methods face limitations in their efficacy due to the pain and invasiveness of needle pricking which often discourages DM patients from performing the required number of tests per day. Saliva glucose (SG) sensing has long been considered a noninvasive alternative to blood glucose monitoring for diabetes management, however the sample preparation and sensor detection limit have been deemed as challenges to overcome. Herein, we describe a preliminary clinical validation of a disposable SG sensor without any requirement for sample preparation.
Methods:
The sensor utilizes glucose dehydrogenase flavine-adenine dinucleotide (GDH-FAD) in conjunction with disposable screen printed electrodes to measure glucose levels in saliva collected directly from 9 healthy subjects. Cyclic voltammetry and amperometric-time (Amp-it) assays were used to develop calibration curves and test subjects. Sensor calibration was performed using simulated saliva at 6.5 pH and 37ºC.
Results:
The lower limit of detection was determined to be 0.11 mg/dL. A lag time of 15 minutes with a positive correlation between SG and BG levels was found, which agrees with literature results. The detected SG ranges from 2.38 to 3.40 mg/dL over a BG range of 90 to 143 mg/dL.
Conclusion:
This is the first reported use of measuring SG with GDH-FAD without prior sample preparation. Upon optimization, the sensor has the potential to serve as a supplement to blood glucose monitoring.
Keywords: diabetes mellitus, biosensor, glucose dehydrogenase flavin-adenine dinucleotide, glucose monitoring, saliva glucose monitoring
Diabetes mellitus (DM) is a chronic disease resulting from a hormonal disorder that causes either the inadequate production of insulin, diminished tissue responses to insulin, or both.1 DM affects 347 million people worldwide and 29.1 million in the United States,2 which is expected to double by 2050.3 The projected expense of DM is estimated to reach $438 million by 2030.3 The average person with diabetes spends $13 700 on their supplies and treatment yearly, which is approximately 2.3 times higher than standard health-care-associated costs endured by a healthy person.4
Tight glycemic management is recognized as the best practice to avoid chronic complications in diabetes.5-7 Self-monitoring of blood glucose (SMBG) via disposable blood glucose test strips is currently the standard for glycemic control, allowing patients to adjust insulin dosage with progressive glucose readings.8 According to a 2014 report from the Center of Disease Control and Prevention, 28.7% of DM patients rely on insulin therapy and/or the combination of insulin and oral medication.9 However, due to the pain associated with finger pricking, people with diabetes often have difficulty complying with testing standards and meeting the number of required tests per day.10,11 Approximately 30% of people with diabetes fall short or barely meet the minimum of three BG tests per day as posted by the American Diabetes Association.11 Currently, there is a lack of devices capable of noninvasive means of SMBG that offer patients the ability to test at home, at more consistent intervals, and without pain. Therefore, a noninvasive alternative or supplemental means of monitoring glucose levels should help improve patient compliance.
DM is a rapidly expanding issue and has been the target of various forms of research and development. Aside from disposable blood glucose test strips, there are commercially available continuous blood glucose monitoring systems (CGMS) as well as research advancements in tattoo-based glucose monitors12 and glucose-sensing contact lenses.13 CGMS provides a valuable stream of glucose measurements for tight glycemic control, but the wide range of detection mechanisms have resulted in many different forms of CGMSs with varying accuracy, consistency, and even invasiveness, making normalization across patients difficult.14 Both glucose-sensing contact lenses and tattoo-based glucose sensors have obtained preliminary success but require further validation.12,13
SG monitoring was first investigated in the 1980s15-17 in an attempt to develop an alternative for BG measurement, as saliva samples are easily accessible and painless to obtain as compared to tear fluid and blood. Although the means by which glucose enters saliva remains inelucidated, it is commonly accepted that glucose transport follows the paracellular pathway as it progresses from the blood stream through the sublingual gland into the oral cavity. In support of the physiological mechanism behind glucose transport, specifically salivary flow rates and glucose reabsorption into the oral cavity, it has been confirmed that SG levels do remain much lower than BG levels,18 emphasizing the need for a sensitive detection technique. The research presented in this paper supports the presence of a correlation between BG and SG, and provides supporting evidence that SG detection is both possible and accurate for its proposed use.
SG is most commonly measured through optical spectroscopy19 and electrochemical assays.20 Electrochemical SG sensors are more favorable for SMBG as they are generally cheaper to produce, provide better sensitivity and are already employed in traditional BG detection mechanisms. Optical sensors, although hold promise for futuristic use, face limitations with the current technology. Spectroscopy faces problems associated with low signal-to-noise ratios, pH and temperature sensitivity, and inconsistent measurements caused by natural changes in biological media. In addition, the spectrum of glucose is analogous to other sugars present in the mouth causing lack of specificity in the response.21 Most electrochemical approaches employ the enzyme glucose oxidase,22-24 however, the use of glucose dehydrogenase flavine-adenine dinucleotide (GDH-FAD) presents distinct advantages in SG sensing, which we will demonstrate in later section. Recently developed disposable lab-on-a-chip SG biosensors for monitoring glucose levels of patients with DM23,25 using glucose oxidase suggest SG monitoring may be a potential alternative to BG monitoring. However, since a saliva sample may contain food particles, bacteria, cells, and other contaminates, sample preparation such as filtering or centrifuging is typically required,14,15,26 but such time consuming practices are not feasible for at-home users. This paper aims to illustrate the design and development of a disposable electrochemical SG sensor employing a higher specificity enzyme, GDH-FAD, that can detect SG without any sample preparation. It also investigates whether a rinsing protocol alone27 is sufficient to obtain clean signals in an effort to develop user-friendly SMBG sensors.
Material and Methods
Reagents and Chemicals
All chemical reagents were purchased from Sigma (St Louis, MO, USA) unless stated otherwise. The 10 mM phosphate buffer saline (PBS) tablets were purchased from Calbiochem (Gibbstown, NJ, USA), potassium hexacyanoferrate (III) from EMD Chemicals (Billerica, MA, USA). The redox probe reagent used was 100 mM potassium ferricyanide dissolved in pH 7.4 PBS. GDH-FAD is a kind donation from Amano Enzyme LLC.
Making of Simulated Saliva
In order to create a calibration curve most representative of human mouth conditions, a modified simulated saliva protocol was followed to prepare the simulated saliva.28-30 The preparation of simulated saliva can be found in the appendix.
Sensor Fabrication
The sensor employed in this work is a commercially available screen printed sensor known as Zensor (CH-Instrument, Texas). It has a carbon working electrode, a carbon counter electrode, and an Ag/AgCl reference electrode. The reagent was prepared by mixing 1 mL of 100 mM potassium ferricyanide with 1.5 mg of GDH-FAD enzyme. 100 mM potassium ferricyanide was prepared in pH 7.4 1X phosphate buffer saline (PBS). Dried sensors were then prepared by pipetting 27 uL of the reagent onto the sensing well to ensure uniform coverage of all 3 electrodes, a practice commonly used in the industry. The sensors were then placed in a dehydrator at 30°C for 25 minutes to dry the reagent completely. Completed sensors were carefully examined for visible defects, and if present, were not used for testing. Upon introduction of the sample, the enzyme is reconstituted in solution, permitting binding to the active site. Prepared sensors can be stored at room temperature for up to 12 weeks.
Sensor Calibration
In order to create a calibration curve most representative of human mouth conditions, simulated saliva was modified to a pH of 6.5 using 12 M hydrochloric acid and a temperature of 37°C using a water bath.31 Simulated saliva batches contain ionic salts to obtain a solution similar to human saliva. Potassium chloride, magnesium chloride and calcium chloride are contained within the matrix. Additional interference testing was completed, which verified that the signal due to these compounds was negligible compared to that of glucose. Glucose was then added to the simulated saliva to construct standard glucose solutions of 0, 2.5, 5, and 10 mg/dL, covering the physiological range of saliva glucose.15,23,32,33 Using 120 µL of sample and an electrochemical analyzer (1230A, CH-Instrument), cyclic voltammetry (CV) was first conducted against 5 mg/dL of glucose in simulated saliva to determine a range of potentials suitable for amperometric-current-over-time (Amp i-t) assay. Standard glucose concentrations in simulated saliva were then tested against various potentials in Amp i-t to evaluate the resulting lower limit of detection (LLD), calculated by 3.3*stdev/slope where stdev is the standard deviation of the response. An analysis of correlation showed that the electrical current at t = 10 seconds is a good representation of the signal produced by the sample. After calculating the resulting LLDs, the bias potential of 0.35 V was chosen as it yields the lowest LLD among all voltages tested. Using 0.35 V, the Amp i-t signals of 0, 2.5, 5, and 10 mg/dL in simulated saliva at t = 10 seconds were taken as the calibration curve.
Clinical Testing
The preliminary clinical study consisted of 6 healthy male subjects and 3 healthy female subjects with ages ranging from 19 to 25 years. The study was approved by the Arizona State University Institutional Review Board (IRB) under the identification number of STUDY00002778. All procedures and tests were performed in compliance with IRB requirements. The sample collection steps are as follows. Each subject was asked to rinse his or her mouth with fresh water 3 times for 3 seconds each time. The subject was then asked to accumulate saliva for 30 seconds, and deposit it onto a sterilized metal lab spatula. A time of 10 seconds was consistently maintained to transfer the 120 µL saliva sample onto the sensor which was preconnected to the electrochemical analyzer. This mechanism was consistent across both the preclinical trial and generation of the calibration curve. The saliva sample did not undergo any preparation or purification steps. Immediately following the deposition of saliva, Amp i-t was performed at 0.35 V for 30 seconds and the current readings at t = 10 seconds were used as the representative signal for the SG measurement, as discussed previously. The electrical signals were then converted to SG using the calibration curve. Each SG measurement was accompanied by a BG measurement using a SMBG meter and test strip. The testing schematic can be seen in Figure 1.
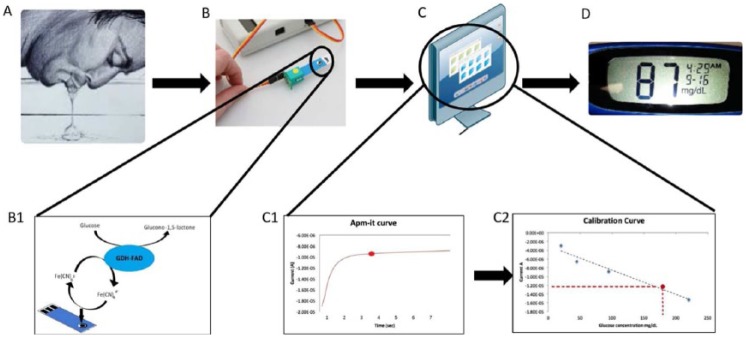
Figure 1.
Schematic representation of the glucose detection mechanism. (A) Collection of saliva by naturally salivating. (B) Transfer the sample onto the SG sensor in which (B1) the glucose is catalyzed by GDH-FAD enzyme the resulting electrons are detected by the sensor under an electron mediator, potassium ferricyanide. (C) Data processing, where (C1) the electrical current generated after a set amount of time is recorded into the system and matched against a (C2) calibration curve, which then calculates the glucose concentration and displays the result on (D) a monitor.
SG-BG Lag Time Study
A glucose tolerance test was performed on 9 healthy subjects by administering a 45 g glucose shot, orally. The subjects were asked to rinse their mouth following the above protocol, and their resting SG and BG values were measured immediately. After the subject swallowed the glucose shot at t = 0 minute, their saliva was collected and tested using the protocol described previously. The entire SG measuring process from sampling to obtaining a measurement was approximately 90 seconds. The time difference between a SG measurement and a BG measurement was controlled to be within 3 minutes. The SG and BG were measured every 15 minutes until t = 60 minutes, then were measured once every 30 minutes until t = 180 minutes using the same procedure described above.
SG-BG Correlation
The SG values excluding data points from malfunctioned sensors and traceable operator errors, are plotted against the BG values without a lag time adjustment. The data points at t = 0 min from the SG-BG lag time study are excluded due to potential contamination from administering the glucose challenge.
Results
Using the methods described above, the CV response and SG calibration curve can be seen in Figure 2. CV was performed against 5 mg/dL of glucose in simulated saliva (Figure 2A). The red circle indicates the expected current responses observed in Amp-it when 0.35 V is applied. The calibration curve (Figure 2B) was performed against 0, 2.5, 5, and 10 mg/dL of glucose with 3 replications at each concentration, covering the physiological SG range. The current response at t = 10 seconds was taken as the representative signal of the sample. The %RSDs for this physiological concentration ranging from low to high concentrations are 12%, 3%, 4%, and 8%, respectively. The correlation between the resulting current responses and SG concentrations was 0.986, and the calibration curve is described by , with y being the current in A, x being the concentration of glucose in mg/dL. The LLD was calculated to be 0.11 mg/dL.
Figure 2.
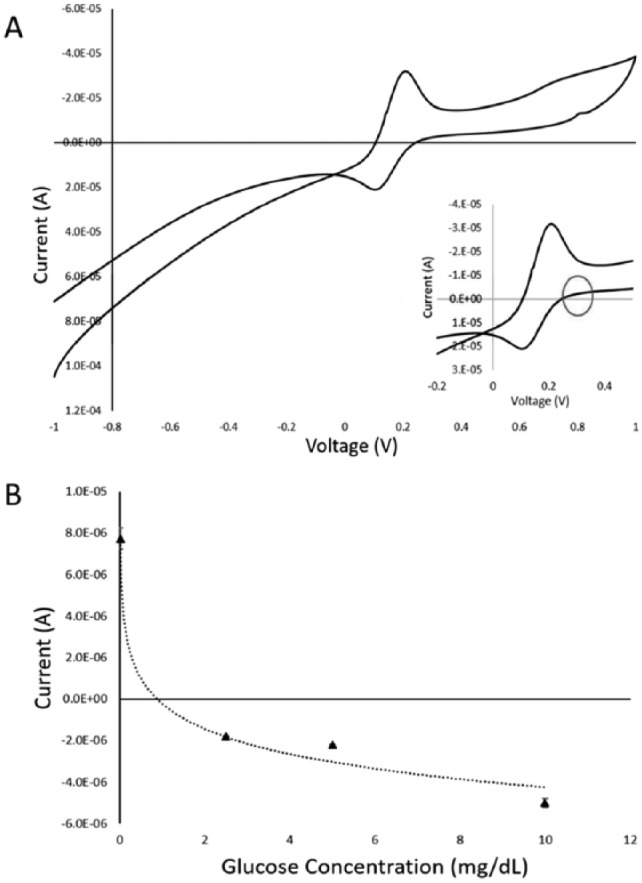
(A) CV of the GDH modified sensor in saliva with 5 mg/dL of glucose. The inset is the zoomed in view from −0.2 V to 0.4 V. The red circle indicates the expected current responses observed in Amp-it when 0.35 V is applied. (B) The calibration curve between glucose concentrations in saliva (mg/dL) and the current response. The relationship is characterized as Y = −1.75E-06 Ln(X) – 2.26E-07 with R2 value of .986. Each concentration was replicated 3 times. Error bars represent standard error.
Using the testing protocol described above, 9 healthy subject’s SG-BG tracking results were averaged and plotted in Figure 3. The healthy subject was given 15 g of oral glucose challenge at t = 0 min. The SG and BG track well with a lag time of 15 ± 15 minutes.
Figure 3.
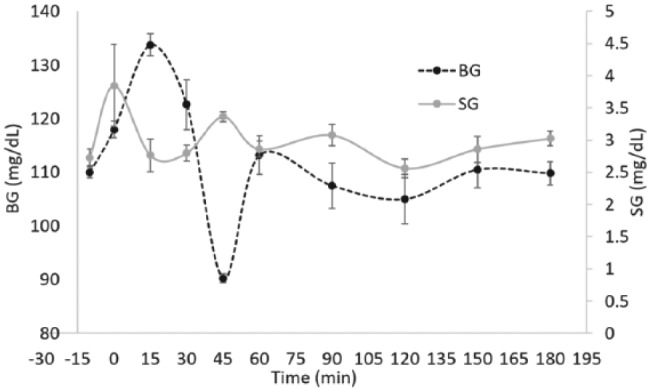
Shows how the saliva glucose tracks blood glucose in all subjects using disposable SG sensors and SMBG sensors. The dotted black line represents blood glucose measurements and trends. The solid grey line represents saliva glucose measurements and trends. The time stamps are −10, 0, 15, 30, 45, 60, 90, 120, 150, and 180 minutes. Glucose challenge was given at t = 0 minutes.
Using the criteria described above, the averaged SG and BG values are plotted against each other to show the SG-BG correlation of all subjects, as seen in Figure 4. The result is not lag time adjusted.
Figure 4.
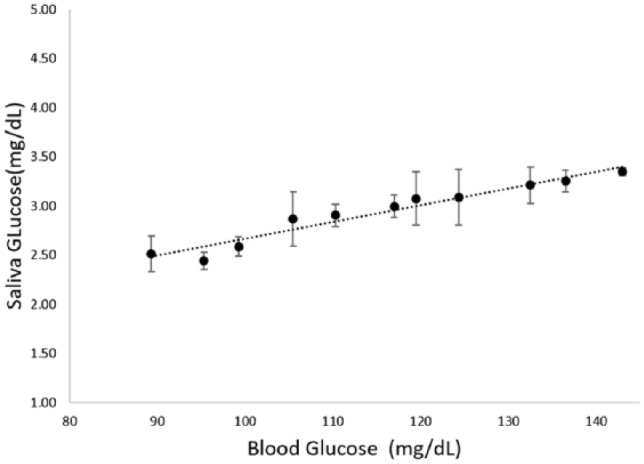
Shows the correlation of SG-BG among all subjects. Data points from faulty sensors and mishandling were removed. The slope is characterized by Y = 0.017X + 0.96 with R-square value of .94. The error bars represent standard error.
Discussion
The SG seems to track BG with a lag time of 15 ± 15 minutes, which is consistent with literature.15,25 The variance in lag time can be attributed to individual metabolic differences due to dietary patterns, lifestyles, and race.23 The BG drop in Figure 3 at t = 45 min can be attributed to increased insulin production and secretion as the BG peaks.34 Despite the correlation, changes in SG are much more subtle as compared to BG. The physiological mechanisms behind this observation further emphasize the need for a highly sensitive enzyme capable of detecting minute changes in concentration. For these reasons, employing GDH-FAD provided a unique advantage over glucose oxidase.
A previously conducted interference test verified the specificity of GDH-FAD to glucose and disproved its reactivity to other sugars except xylose, which is not present in the oral cavity.35 GDH-FAD also demonstrated a signal-to-noise ratio 9 times higher than that of glucose oxidase.36 Due to the small fluctuations in SG values that translate to relatively high changes in BG, the signal must be able to emerge from the surrounding noise. In addition, GDH-FAD has 25 times more enzymatic activity than glucose oxidase, which permits rapid glucose sensing.35 We have previously reported the use of a GDH-FAD modified sensor for the detection of tear glucose, which also contains very low glucose levels analogous to those of saliva, and have verified the performance of the sensors in an animal trial.36-39
The simplicity ofsensor fabrication and lack of sample preparation provide strong evidence in support of a potential noninvasive glucose monitor for patients with diabetes. Despite the advancements presented above, there remains a lack of consensus on the usefulness of SG in predicting BG. Some researchers have reported positive linear correlations15,40,41 while others have not.42-44 These differences may be a result of the variability in metabolic function across individuals as well as differences in health states, age and gender. Currently, the device is faced with some limitations, however, further validation and optimization may alleviate the reported inconsistencies.
Nevertheless, a positive correlation between SG and BG was identified, which is consistent with literature.15,40,41 The average SG values ranging from 2.38 mg/dL to 3.40 mg/dL do correlate to a BG range from 90 mg/dL to 143 mg/dL, which are in agreement with published SG values.15,23,32,33 However, given the variation of SG lag time among individuals, a personalized calibration curve is recommended to make SG an ideal, accurate, noninvasive alternative or supplement to BG monitoring. This study validates that the implementation of a rinsing protocol alone is possible to detect SG without the need of sample preparation, drastically reducing the entire SG measuring process to under a minute dependent upon user salivary rate. By utilizing GDH-FAD, the proposed sensor design does not rely on the use of nanotubes or nanoparticles to increase sensitivity, which increase the complexity of manufacturing and subsequently affect the approval process from Food and Drug Administration.
The subtle change in SG could be the side effect of no sample preparation, as the interference from untreated saliva may hinder the overall signal. Perhaps a nafeon coating, a standard industry practice to filter large molecules from blood on BG test strips, can also be applied to a future prototype of the SG sensor. Alternatively, mesoporous carbon (MPC) functionalized with GDH-FAD can also be used to strengthen the signal and filter out the interference of large molecules. Our lab has previously reported a screen printed carbon sensor employing glucose oxidase-functionalized MPC (fabricated in-house) working electrode capable of detecting glucose in whole blood.45 MPC can help filter out larger molecules by size exclusion and the porous structure increases the sensing surface area dramatically to an average of 1500 m2/g, However, since the MPC sensor has yet to be optimized to detect ranges below 10 mg/dL, and MPC synthesis adds increased complexity to a supposedly manufacturing-friendly design, it was not implemented in the reported study. A combination of commercially available MPC and GDH-FAD may offer a more sensitive SG sensor than the current approach.
Conclusion
In summary, an easy-to-use, rapid, and disposable SG sensor prototype employing GDH-FAD without the need of sample preparation is successfully developed. Initial test results indicate that the measured SG values and SG-BG lag time are consistent with literature, suggesting the potential of this approach. By not having to prepare the saliva sample, the overall SG measurement time and logistics are similar to that of BG, which is vital for the user’s acceptance. Additional optimization and personalized calibration are still recommended to improve the potential of this approach. Once fully developed, the technology can be implemented to serve as a supplement or alternative to traditional means of glycemic management.
Acknowledgments
The authors would like to thank TekCapital PLC and Belluscura Ltd for financial support; Amano Enzyme LLC for the kind donation of GDH-FAD enzyme; as well as Jonus Reyna, Cael Muggeridge, and Susan Sheffield for assisting with data collection.
Appendix
Preparation of Simulated Saliva
This protocol is adopted from the works of McKnight-Hanes and Whitford (1992),28 Levine et al (1987),29 and Gallapanayut (2004).30
Dissolve 2 g of methyl-p-hydroxybenzoate in 800 mL of DI water (solution A).
Take 20 mL of solution A and store separately for chemical solvent later (solution C).
Dissolve 10 g of sodium carboxymethyl cellulose in 200 mL of boiled DI water (solution B).
Mix 780 mL of solution A with 200 mL of solution B until it starts becoming sticky (solution D).
Dissolve 0.625 g of potassium chloride, 0.059 g of magnesium chloride hexahydrate, 0.166 g of calcium chloride dihydrate, 0.804 g of potassium phosphate dibasic, and 0.326 g of potassium phosphate monobasic with solution C. Mix well then add to solution D.
The completed simulated saliva is approximately 7.1 pH and should be stored at room temperature.
Footnotes
Abbreviations: Ag/AgCl, silver/silver chloride; Amp-it, amperometric-current-over-time; BG, blood glucose; CGMS, continuous glucose monitoring system; CV, cyclic voltammetry; DM, diabetes mellitus; GDH-FAD, glucose dehydrogenase flavin-adenine dinucleotide; LLD, lower limit of detection; MPC, mesoporous carbon; RSD, relative standard deviation; SG, saliva glucose; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Provisional patent of the saliva glucose sensor (no. 62/288,747) has been granted. An international application has also been filed (no. PCT/US2017/015434).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by TekCapital PLC and Belluscura, Ltd.
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152(1):27-38. [DOI] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589. [DOI] [PubMed] [Google Scholar]
- 7. Langer O, Levy J, Brustman L, Anyaegbunam A, Merkatz R, Divon M. Glycemic control in gestational diabetes mellitus-how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol. 1989;161(3):646-653. [DOI] [PubMed] [Google Scholar]
- 8. Katz LB, Macleod K, Grady M, Cameron H, Pfützner A, Setford S. A comprehensive evaluation of strip performance in multiple blood glucose monitoring systems. Expert Rev Med Devices. 2015;12(3):263-271. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 10. Ong WM, Chua SS, Ng CJ. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer Adherence. 2014;8:237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heinemann L. Finger pricking and pain: a never ending story. J Diabetes Sci Technol. 2008;2(5):919-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bandodkar AJ, Jia W, Yardımcı C, Wang X, Ramirez J, Wang J. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal Chem. 2014;87(1):394-398. [DOI] [PubMed] [Google Scholar]
- 13. Liao Y-T, Yao H, Lingley A, Parviz B, Otis BP. A 3-CMOS glucose sensor for wireless contact-lens tear glucose monitoring. J Solid-State Circuits IEEE. 2012;47(1):335-344. [Google Scholar]
- 14. Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for continuous glucose monitoring: current problems and future promises. J Diabetes Sci Technol. 2010;4(6):1540-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamaguchi M, Mitsumori M, Kano Y. Noninvasively measuring blood glucose using saliva. Eng Med Biol Mag IEEE. 1998;17(3):59-63. [DOI] [PubMed] [Google Scholar]
- 16. Marchetti P, Tognarelli M, Giannarelli R, et al. Decreased salivary glucose secretory rate: usefulness for detection of diabetic patients with autonomic neuropathy. Diabetes Res Clin Pract. 1989;7(3):181-186. [DOI] [PubMed] [Google Scholar]
- 17. Mandel ID. Sialochemistry in diseases and clinical situations affecting salivary glands. CRC Crit Rev Clin Lab Sci. 1980;12(4):321-366. [DOI] [PubMed] [Google Scholar]
- 18. Takai N, Yoshida Y, Kakudo Y. Secretion and re-absorption of glucose in rat submandibular and sublingual saliva. J Dent Res. 1983;62(10):1022-1025. [DOI] [PubMed] [Google Scholar]
- 19. Jurysta C, Bulur N, Oguzhan B, et al. Salivary glucose concentration and excretion in normal and diabetic subjects. BioMed Res Int. 2009;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klasner SA, Price AK, Hoeman KW, Wilson RS, Bell KJ, Culbertson CT. Paper-based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Anal Bioanal Chem. 2010;397(5):1821-1829. [DOI] [PubMed] [Google Scholar]
- 21. McNichols RJ, Cote GL. Optical glucose sensing in biological fluids: an overview. J Biomed Opt. 2000;5(1):5-16. [DOI] [PubMed] [Google Scholar]
- 22. Abikshyeet P, Ramesh V, Oza N. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab Syndr Obes. 2012;5:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang W, Du Y, Wang ML. Noninvasive glucose monitoring using saliva nano-biosensor. Sens Bio-Sens Res. 2015;4:23-29. [Google Scholar]
- 24. Gupta S, Sandhu SV, Bansal H, Sharma D. Comparison of salivary and serum glucose levels in diabetic patients. J Diabetes Sci Technol. 2015;9(1):91-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du Y, Zhang W, Wang M. An on-chip disposable salivary glucose sensor for diabetes control. J Diabetes Sci Technol. 2016;10(6):1344-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pronovost AD, inventor; assignee. Salivary glucose monitoring. United States patent US20080020477 A1; 2008. January 24. [Google Scholar]
- 27. Dawes C, Tsang R, Suelzle T. The effects of gum chewing, four oral hygiene procedures, and two saliva collection techniques, on the output of bacteria into human whole saliva. Arch Oral Biol. 2001;46(7):625-632. [DOI] [PubMed] [Google Scholar]
- 28. McKnight-Hanes C, Whitford GM. Fluoride release from three glass ionomer materials and the effects of varnishing with or without finishing. Caries Res. 1992;26(5):345-350. [DOI] [PubMed] [Google Scholar]
- 29. Levine M, Aguirre A, Hatton M, Tabak L. Artificial salivas: present and future. J Dent Res. 1987;66(suppl 2):693-698. [DOI] [PubMed] [Google Scholar]
- 30. Gallapanayut P. Effect of intensive fluoride varnish application on reducing enamel dissolution [MS thesis]. Thailand: Prince of Songkla University; 2004. [Google Scholar]
- 31. Baliga S, Muglikar S, Kale R. Salivary pH: A diagnostic biomarker. J Indian Soc Periodontol. 2013;17(4):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ravindran R, Gopinathan DM, Sukumaran S. Estimation of salivary glucose and glycogen content in exfoliated buccal mucosal cells of patients with type II diabetes mellitus. J Clin Diagn Res. 2015;9(5):ZC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soni A, Jha SK. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens Bioelectron. 2015;67:763-768. [DOI] [PubMed] [Google Scholar]
- 34. Suckale J, Solimena M. Pancreas islets in metabolic signaling-focus on the beta-cell. Front Biosci. 2008;13:7156-7171. [DOI] [PubMed] [Google Scholar]
- 35. Ferri S, Kojima K, Sode K. Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J Diabetes Sci Technol. 2011;5(5):1068-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lan K, McAferty K, Shah P, et al. A disposable tear glucose biosensor—part 3: assessment of enzymatic specificity. J Diabetes Sci Technol. 2011;5(5):1108-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bishop DK, La Belle JT, Vossler SR, Patel DR, Cook CB. A disposable tear glucose biosensor—part 1: design and concept testing. J Diabetes Sci Technol. 2010;4(2):299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. La Belle JT, Bishop DK, Vossler SR, Patel DR, Cook CB. A disposable tear glucose biosensor—part 2: system integration and model validation. J Diabetes Sci Technol. 2010;4(2):307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. La Belle JT, Engelschall E, Lan K, et al. A disposable tear glucose biosensor—part 4 preliminary animal model study assessing efficacy, safety, and feasibility. J Diabetes Sci Technol. 2014;8(1):109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reuterving C, Reuterving G, Hägg E, Ericson T. Salivary flow rate and salivary glucose concentration in patients with diabetes mellitus influence of severity of diabetes. Diabetes Metab. 1986;13(4):457-462. [PubMed] [Google Scholar]
- 41. Karjalainen K, Knuuttila M, Käär M. Salivary factors in children and adolescents with insulin-dependent diabetes mellitus. Pediatr Dent. 1995;18(4):306-311. [PubMed] [Google Scholar]
- 42. Ben-Aryeh H, Cohen M, Kanter Y, Szargel R, Laufer D. Salivary composition in diabetic patients. J Diabet Complications. 1988;2(2):96-99. [DOI] [PubMed] [Google Scholar]
- 43. Forbat L, Maskell G, Collins R, Sönksen P. Glucose concentrations in parotid fluid and venous blood of patients attending a diabetic clinic. J R Soc Med. 1981;74(10):725-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carda C, Mosquera-Lloreda N, Salom L, Gomez de, Ferraris M, Peydró A. Structural and functional salivary disorders in type 2 diabetic patients. Med Oral Patol Oral Cirugia Bucal. 2006;11(4):209. [PubMed] [Google Scholar]
- 45. Dai M, Maxwell S, Vogt BD, La Belle JT. Mesoporous carbon amperometric glucose sensors using inexpensive, commercial methacrylate-based binders. Anal Chim Acta. 2012;738:27-34. [DOI] [PubMed] [Google Scholar]