Abstract
Hyperglycemia is very common in critically ill patients and interventional studies of intensive insulin therapy with the goal of returning ICU glycemia to normal levels have demonstrated mixed results. A large body of literature has demonstrated that diabetes, per se, is not independently associated with increased risk of mortality in this population and that the relationship of glucose metrics to mortality is different for patients with and without diabetes. Moreover, these relationships are confounded by preadmission glycemia; in this regard, patients with diabetes and good preadmission glucose control, as reflected by HbA1c levels obtained at the time of ICU admission, are similar to patients without diabetes. These data point the way toward an era when blood glucose targets in the ICU will be “personalized,” based on assessment of preadmission glycemia.
Keywords: blood glucose target, diabetes, hyperglycemia, intensive care unit, mortality, personalized glucose control
In 2001 a single-center randomized controlled trial (RCT) conducted in a postoperative cohort demonstrated improved morbidity and mortality with the use of continuous intravenous insulin administered to normalize blood glucose (BG).1 The critical care community was riveted by these findings and has remained so; this landmark trial has been cited in 10 389 published manuscripts (Google Scholar, accessed June 14, 2017). Three years later a single-center before and after implementation study corroborated these findings.2 However, a subsequent RCT conducted in a medical intensive care unit (ICU) in the same institution as the original trial demonstrated equivocally positive findings,3 and later trials were terminated prematurely due to unacceptably rates of severe hypoglycemia4 and failure to achieve adequate time in targeted BG range.5 Eight years after publication of the sentinel investigation the largest RCT of intensive insulin therapy (IIT), a multicenter international investigation involving 42 centers and 6104 patients, found higher 90-day mortality in the interventional arm6 leading guideline writers to abandon IIT in favor of moderate BG targets.7,8
How to explain these findings? Hypoglycemia—severe (BG <40 mg/dL) and mild to moderate (BG 40-69 mg/dL)—a “unifying complication” of the RCTs, has been demonstrated to be independently associated with increased risk of death in observational studies9-12 as well as in data from the RCTs.13,14 The “single-center” effect may also have played an important role. The lowest rates of hypoglycemia were seen in trials in which the clinical teams presumably had greater experience in learning how to manage the complex intervention of IIT.1-3 The failure to achieve adequate time in targeted BG range (TIR), due in part to potential analytic inaccuracies associated with monitoring technologies used in some of the trials, and low frequency of monitoring, with the likelihood of missed episodes of hyperglycemia and hypoglycemia, was likely a factor as well. Only one of these trials published this metric.4 It was quite low—42.8%4—and estimates of the other trials’ results suggested equally poor results.15 Finally, a meta-analysis of data from interventional trials suggested that nutritional strategy—enteral versus parenteral—was independently associated with mortality.16
However, an additional factor must be considered. Each of the RCTs targeted “euglycemia,” 80-110 mg/dL. Is it possible that this “one-size-fits-all” strategy was beneficial to some of the treated patients but deleterious to others? This review will explore this question and evaluate the burgeoning literature that has pointed the way toward an era of personalized glycemic control.
Hyperglycemia in Acute and Critical Illness: Nearly Universal
A detailed analysis of the pathophysiologic mechanisms underlying the hyperglycemia of acute and critical illness is beyond the scope of this review. In summary, hyperglycemia occurs commonly in critically ill patients, due to multiple causes—including, in part, the interplay of counterregulatory hormones, including cortisol, growth hormone, catecholamines and cytokines, as well as nutritional support and iatrogenic interventions such as administration of systemic corticosteroids.17 These factors lead to insulin resistance and increased hepatic glucose production. Nevertheless, while hyperglycemia is nearly ubiquitous in this population, numerous studies, including those to be detailed in this review, suggest that its deleterious impact is sustained by patients not “preconditioned” to levels of hyperglycemia.17,18
Is Diabetes a Risk Factor for Mortality in the Critically Ill? Data From Observational Studies
It is well known that diabetes is associated with a tremendous burden of morbidity and mortality worldwide, especially in developed nations. However, can the same be said of critically ill patients? Specifically, is preexisting diabetes a risk factor for mortality in patients admitted to the hospital with acute and critical illness?
Vincent and coinvestigators performed an analysis of the Sepsis Occurrence in Acutely Ill Patients observational study to address this question.19 This cohort included 3147 patients from 198 worldwide centers; insulin treated diabetes was present in 7.2%. Although patients with diabetes had higher severity of illness, as reflected by Sequential Organ Failure Assessment and SAP scores, than did those without diabetes, there was no difference in ICU or hospital mortality comparing these two groups.
Graham et al used two large US databases to evaluate the independent association of diabetes with mortality in critically ill patients.20 Among 36 414 patients admitted to the Mayo Clinic system between 1999 and 2007, mortality was slightly higher among patients with diabetes than among those without—10.31% versus 9.68%. However, multivariable analysis including age and severity of illness demonstrated that diabetes was independently associated with reduced risk of mortality—OR (95% CI) 0.88 (0.79-0.98), P = .022. In contrast, mortality was 8.79% versus 9.68% among patients with and without diabetes in 1.5 million patients admitted between 2003 and 2007 to hospitals in the University Health Consortium and, again, multivariable analysis including age and severity of illness demonstrated the strong independent association of diabetes with reduced risk of mortality—OR (95% CI) 0.75 (0.74-0.76), P < .001.
Finally, Siegelaar and coworkers performed a meta-analysis of 141 studies including 12.5 million acutely and critically ill patients, finding no overall association between diabetes and ICU, hospital or 30-day mortality.21 Subgroup analysis confirmed this lack of association among patients admitted to medical, surgical, trauma, or mixed ICUs; however, diabetes patients admitted to cardiovascular surgery units had increased mortality compared to those without diabetes.
The observational nature of these data precludes proof of causality; they can only be considered hypothesis-generating. Another important limitation is their absence of any data regarding glucose metrics during hospitalization.
Diabetes Status and Mortality in the Interventional Trials of Intensive Insulin Therapy
A guiding principle of clinical medicine is that risks and benefits of an intervention are not evenly distributed across a population. For example, an intervention that yields a 4% absolute reduction in mortality may have resulted from a 25% reduction in 20% of the cohort, no change in 70% and a 10% increase in mortality in the remaining 10%. A review of interventional trials of IIT, stratified by diabetes status, suggests that the intervention may have had such a differential effect.
Table 1 displays mortality, stratified by diabetes status, of patients in the conventional and interventional arms of the largest trials for which these data are available.22 For each, the relative risk for mortality associated with IIT is lower for patients without diabetes than for the diabetes patients. Notably, it can be seen that the mortality reduction due to IIT in the landmark first Leuven trial was nearly entirely attributable to that observed in patients without diabetes. In contrast, in the NICE-SUGAR trial the difference in relative risk for mortality associated with IIT comparing patients with and without diabetes was small (point estimates 1.15 and 1.09, respectively).
Table 1.
Mortality of Patients in Interventional Trials of Intensive Insulin Therapy, Stratified by Diabetes Status.
| Conventional arm |
Interventional arm |
RR for mortality: interventional arm* |
||||
|---|---|---|---|---|---|---|
| No diabetes | Diabetes | No diabetes | Diabetes | No diabetes | Diabetes | |
| Leuven 1 (Van den Berghe et al)1 | 57/680 (8.4%) | 6/103 (5.8) | 31/664 (4.7%) | 4/101 (4.0%) | 0.56 (0.36-0.85)a | 0.68 (0.20-2.33)b |
| Leuven 2 (Van den Berghe et al)3 | 208/508 (40.9%) | 34/97 (35.1%) | 180/409 (36.8%) | 42/106 (39.6%) | 0.90 (0.77-1.05)c | 1.13 (0.79-1.62)d |
| Stamford (Krinsley)2** | 399/2134 (18.7%) | 120/532 (22.6%) | 287/2121 (13.5%) | 111/578 (19.2%) | 0.72 (0.63-0.83)e | 0.85 (0.67-1/07)f |
| NICE-SUGAR (NICE-SUGAR Study Investigators)6 | 586/2416 (24.3%) | 165/596 (27.7%) | 634/2394 (26.5%) | 195/615 (31.7%) | 1.09 (0.99-1.20)g | 1.15 (0.96-1.36)h |
Relative risk and 95% CI. **Before and after nonrandomized trial. The other studies listed were RCTs.
P = .0068. bP = .5403. cP = .1815. dP = .5029. eP < .0001. fP = .1698. gP = .0760. hP = .1265.
Acute Glycemia, Diabetes Status, and Mortality in the Critically Ill
The first study that evaluated the relationship between glycemia during critical illness and mortality, stratified by diabetes status, included 5365 patients admitted to a single US center between 1999 and 2006. Approximately half were admitted before glycemic control was formalized and the later cohort were treated with BG target 80-140 mg/dL.23 There was a more than 4-fold increase in mortality when comparing nondiabetes patients with mean BG 70-99 mg/dL (9.2%) to those with mean BG ≥180 mg/dL (39.4%) during ICU stay. Among the diabetes patients the difference in mortality comparing these two bands of glycemia was much less marked: 13.0% and 25.5%.
Similarly, Egi et al studied 4946 patients admitted to two Australian ICUs between 2000 and 2004.24 The lowest mortality among nondiabetes patients was observed in those with mean glycemia 80-136 mg/dL; this rate increased substantially as mean glycemia increased further. In contrast, mortality was similar for diabetes patients across this entire range of glycemia, including for those with mean BG >200 mg/dL. In both studies, the few patients with mean glycemia <80 mg/dL sustained very high rates of mortality, regardless of diabetes status. In addition, Falciglia et al evaluated 259,040 admissions to 173 Veterans Administration US hospitals and found that the odds ratio for mortality increased more rapidly as mean ICU glycemia increased beyond the reference value of 70-110 mg/dL among patients without diabetes compared to those with diabetes.25
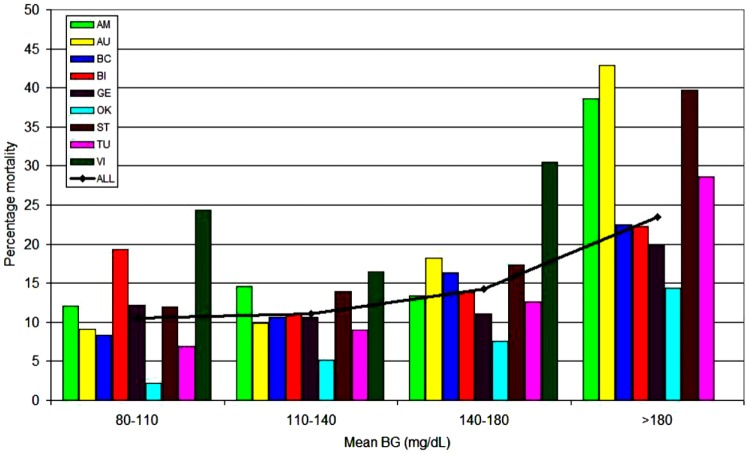
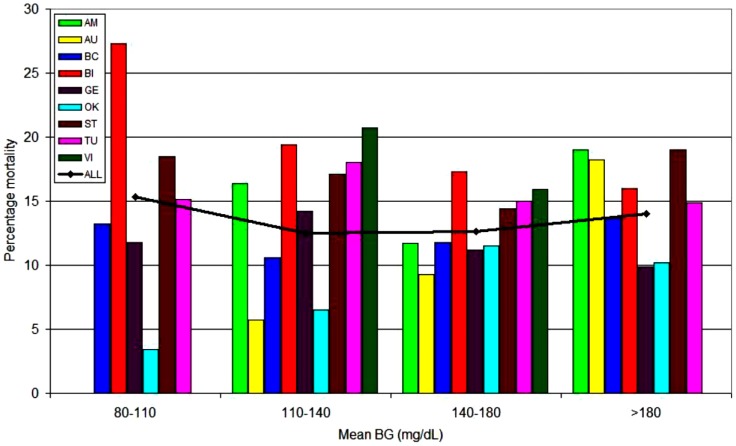
A 9-center 4-continent observational investigation including 44 964 critically ill patients evaluated the relationship of three “domains” of glucose control—mean glycemia, hypoglycemia and glucose variability—to mortality; results were stratified by diabetes status.26 Figures 1a and 1b illustrate the relationship of mean glycemia to mortality for patients without and with diabetes, respectively. There was a clear relationship between increasing mortality rates and mean glycemia above 80-140 mg/dL for patients without diabetes. In contrast, for patients with diabetes there was no relationship between mean glycemia and mortality across the entire range. Moreover, multivariable analysis demonstrated that mean BG 80-40 mg/dL was independently associated with decreased risk of mortality among patients without diabetes, but increased risk of mortality for those with diabetes, for whom the range of 140-180 mg/dL was independently associated with reduced risk of mortality. Hypoglycemia was associated with increased risk of mortality in all patients, whereas increased glucose variability, defined as coefficient of variation ≥20%, was independently associated with increased risk of mortality among patients without diabetes but not among diabetes patients. These results were largely corroborated in a 10 320 patient observational study by Sechterberger et al.27
Figure 1a.
The relationship between mean ICU glycemia and mortality: patients without diabetes. Adapted from Krinsley et al,26 with permission from BioMed Central.
Figure 1b.
The relationship between mean ICU glycemia and mortality: patients with diabetes. Adapted from Krinsley et al,26 with permission from BioMed Central. Participating centers: AM, Amsterdam; AU, Austin; BC, Baycare; BI, Birmingham; GE, Geelong; OK, Okayama; ST, Stamford; TU, Tufts; VI, Vienna.
The different relationship between mean ICU glycemia and mortality when comparing patients with and without diabetes was further assessed by Lanspa and colleagues working in an eight hospital 12 ICU system.28 The 3529 patients included in the study were treated with one of two computerized insulin protocols, targeting BG 80-110 or 90-140 mg/dL. Among patients without diabetes the lower BG target was independently associated with reduced risk of mortality. In contrast, among diabetes patients the higher BG target was associated with reduced risk of mortality.
In addition, one single-center observational study has evaluated the interaction of diabetes status, time in BG range (70-140 mg/dL, TIR) and mortality in a mixed medical-surgical critically ill cohort of 3297 patients with ICU length of stay ≥24 hours, treated with a BG target of 90-120 mg/dL.29 The median (IQR) TIR for patients with and without diabetes were 55.0% (35.5-71.1) and 80.5% (61.4-94.0), P < .0001. Mortality for patients without diabetes above and below the median TIR was 8.47% versus 15.71% (P < .0001); among patients with diabetes there was no significant difference in mortality between patients above and below the median TIR. Multivariable analysis demonstrated that among patients without diabetes, TIR above the median value was independently associated with reduced risk of mortality (P = .0019), but among patients with diabetes there was no independent association of TIR with mortality.
Finally, a recently published two-center investigation evaluated the association of glucose metrics with mortality across the continuum of hospitalization, from ICU admission to hospital discharge, the first study to assess whether glucose control after ICU transfer to general medical wards was independently associated with mortality.30 Confirming previous work, mean BG 80-140 mg/dL during ICU and general ward care, compared to the 140-180 mg/dL range recommended by guideline groups,7,8 was independently associated with decreased risk of mortality for patients without diabetes but increased mortality for patients with diabetes. Hypoglycemia was independently associated with increased mortality for patients with and without diabetes in both settings. Increased glucose variability (coefficient of variation ≥20%) was independently associated with increased mortality in patients without diabetes in both settings, but not in patients with diabetes. These findings, though not proof of causality, raise the possibility that efforts to improve BG control in ICU survivors may increase their prospects for hospital survival. In addition, they raise the intriguing possibility that the results of the interventional trials of IIT may have been confounded by the participating centers’ unequal attention and success to safe and effective BG control after ICU discharge.
The results of these data from observational and interventional investigations suggest that mean BG 80-140 mg/dL is independently associated with the lowest mortality among patients without diabetes, but the optimal mean BG for patients with diabetes, considered as a monolithic group, remains unknown.
Is the Diabetes Cohort Monolithic? The Important Relationship Between Acute and Chronic Glycemia and the Emerging Metric of Relative Glycemia
This review has explored the array of data demonstrating differences in the relationship of the domains of glycemic control to mortality when considering patients with and without diabetes. But is the diabetes cohort admitted to the ICU, in fact, monolithic? An emerging body of literature suggests that preadmission glycemia confounds the relationship between ICU glycemia and mortality among patients with diabetes.
Egi and coworkers were the first to investigate this question in their study of 415 diabetes patients admitted to two tertiary center ICUs.31 Mean BG during ICU stay was similar when comparing survivors and nonsurvivors. However, when outcomes were stratified by the patients’ HbA1c a different pattern emerged—diabetes patients with higher preadmission glycemia had higher mortality associated with lower ICU glycemia. Stated another way—if preadmission glycemic control was poor, reflected by high HbA1c levels, ICU survival was higher with higher ICU glycemia, and if preadmission BG control was good, reflected by HbA1c levels <7%, “tight” BG control during ICU stay was associated with higher survival.
Plummer and coinvestigators explored the interaction of acute and chronic hyperglycemia in 1000 patients admitted to a single mixed medical-surgical ICU.32 Among patients without diabetes and diabetes patients with HbA1c <6.5%, each 18 mg/dL increase in the highest BG level during the first 48 hours of ICU admission was independently associated with 20% increase in odds of death. In contrast, among diabetes patients with HbA1c ≥6.5% there was no relationship between peak glycemia and mortality.
In addition, the risk of hypoglycemia, and its consequences, may be modulated by preadmission glycemia. In a 3-center international study including 3084 patients, Egi et al found that the occurrence of hypoglycemia was related to HbA1c—among patients with HbA1c <6.5%, 6.5-7.9% and ≥8.0% mild-moderate hypoglycemia was observed in 3.8%, 11.1% and 16.4% and severe hypoglycemia was observed in 0.9%, 2.5% and 4.3%.33 Moreover, multivariable analysis demonstrated that hypoglycemia was independently associated with increased mortality only in the patients with HbA1c ≥8.0%.
These studies lead directly to the paradigm of relative glycemia. Roberts and coworkers evaluated 2290 patients admitted to a single tertiary center and defined critical illness as the need for ICU admission or hospital death.34 They defined “stress hyperglycemia ratio” (SHR) as the quotient of admission BG and preadmission mean BG as determined by a validated formula.35 Among patients with HbA1c <6.5% there was a strong linear relationship between admission BG and critical illness, corroborating numerous previous observational studies. In contrast, among patients with HbA1c ≥6.5% there was no clear relationship. However, each 0.1 increase in SHR was independently associated with 20% increase in odds of critical illness, regardless of HbA1c level. SHR was also found to be independently associated with major adverse cardiac events following percutaneous intervention in acute myocardial infarction.36
Similarly, Liao et al studied the “glycemic gap”—the difference, rather than the quotient, between admission and preadmission mean glycemia—in 518 patients admitted to a single tertiary center, demonstrating a striking linear relationship between glycemic gap and mortality.37 Moreover, glycemic gap was more predictive of mortality than was admission BG and the combination of glycemic gap and the APACHE II severity of illness score was more predictive of mortality than was the APACHE II score alone.
Multiple BG Targets in the ICU
Two small exploratory studies recently evaluated the use of moderate (108-180 mg/dL) and loose (180-252 mg/dL) BG targets in critically ill patients with diabetes. These demonstrated lower glucose variability38 and lower rates of “relative hypoglycemia,” defined as <30% predicted mean glycemia.39 Neither study was powered for any other clinically significant outcome.
Finally, Krinsley et al recently published the results of a two year before and after interventional study of two BG targets in 1979 patients admitted to a single mixed-medical ICU.40 In the first year the BG target was 90-120 mg/dL for all patients. In the second year the BG target was 80-140 mg/dL for patients without diabetes and for diabetes patients with HbA1c <7.0% and 110-160 mg/dL for diabetes patients with HbA1c ≥7.0%. Among patients without diabetes there was no change in mortality or severity-adjusted mortality between the two eras. However, among patients with diabetes there was a reduction in severity-adjusted mortality in the second era, driven largely by the reduction in severity-adjusted mortality among diabetes patients treated with the looser target. This hypothesis-generating investigation provides the rationale for further testing of multiple BG targets in critically ill patients, based on preadmission glycemia. Finally, while preadmission glycemia is best represented by measurement of HbA1c, this metric has important limitations; it may be confounded by hematologic conditions such as anemia, hemolysis and hemoglobinopathies; various medications such as dapsone and erythropoietin; mechanical heart valves; hypothyroidism; and variation in individual rates of protein glycation.41
Conclusions
Hyperglycemia is very common in critically ill patients, but a large body of observational as well as interventional studies suggest that its impact is deleterious predominantly in patients without preexisting diabetes. Moreover, an emerging body of literature suggests that the critically ill diabetes cohort is not monolithic, and that the relationship between glucose metrics and outcomes in this population is conditioned by preadmission glycemia. While the BG target of 80-140 mg/dL is associated with the lowest mortality among patients without diabetes in multiple investigations, the appropriate target for patients with diabetes remains unclear, and is likely based on assessment of preadmission glycemia. Further studies will be needed to test these hypotheses and to determine the safe and effective target for every critically ill patient. The era of personalized glycemic control is approaching.
Footnotes
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BG, blood glucose; CV, coefficient of variation; ICU, intensive care unit; IIT, intensive insulin therapy; RCT, randomized controlled trial; SHR, stress hyperglycemia ratio; TIR, targeted BG range.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Consulting/advisory boards—Edwards Life Sciences, Medtronic, OptiScan Biomedical, Roche Diagnostics.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. [DOI] [PubMed] [Google Scholar]
- 2. Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill patients. Mayo Clinic Proc. 2004;79:992-1000. [DOI] [PubMed] [Google Scholar]
- 3. Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461. [DOI] [PubMed] [Google Scholar]
- 4. Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139. [DOI] [PubMed] [Google Scholar]
- 5. Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Int Care Med. 2009;35:1738-1748. [DOI] [PubMed] [Google Scholar]
- 6. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. Nog Engl J Med. 2009;360:1283-1297. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ichai C, Preiser JC. International recommendations for glucose control in adult non-diabetic critically ill patients. Crit Care. 2010;14:R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35:2262-2267. [DOI] [PubMed] [Google Scholar]
- 10. Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med. 2010;38:1430-1434. [DOI] [PubMed] [Google Scholar]
- 12. Krinsley JS, Schultz MJ, Spronk PE, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Crit Care. 2011;15:R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38:1021-1029. [DOI] [PubMed] [Google Scholar]
- 14. NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108-1118. [DOI] [PubMed] [Google Scholar]
- 15. Krinsley JS. Glycemic control in the critically ill: what have we learned since NICE-SUGAR? Hosp Prac. 2015;43:191-197. [DOI] [PubMed] [Google Scholar]
- 16. Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU. A systemic review and metaanalysis. Chest. 2010;137:544-551. [DOI] [PubMed] [Google Scholar]
- 17. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycemia. Lancet. 2009;373:1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krinsley JS, Fisher M. The diabetes paradox: diabetes is not independently associated with mortality in critical illness. Hosp Prac. 2012;40:31-35. [DOI] [PubMed] [Google Scholar]
- 19. Vincent JL, Preiser JC, Sprung CL, Moreno R, Sakr R. Insulin-treated diabetes is not associated with mortality in critically ill patients. Crit Care. 2010;14:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graham BB, Keniston A, Gajic O, et al. Diabetes mellitus does not affect outcomes from a critical illness. Crit Care Med. 2010;38:16-24. [DOI] [PubMed] [Google Scholar]
- 21. Siegelaar SE, Hickman M, Hoekstra JB, Holleman F, DeVries JH. The effect of diabetes on mortality in critically ill patients: a systemic review and meta-analysis. Crit Care. 2011;15:R205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krinsley JS, Meyfroidt G, van den Berghe G, Egi M, Bellomo R. The impact of premorbid diabetic status on the relationship between the 3 domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care. 2012;15:151-160. [DOI] [PubMed] [Google Scholar]
- 23. Krinsley JS. Glycemic control, diabetic status and mortality in a heterogeneous population of critically ill patients before and during the era of tight glycemic control. Semin Thorac Cardiovasc Surg. 2006;18:317-325. [DOI] [PubMed] [Google Scholar]
- 24. Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249-2255. [DOI] [PubMed] [Google Scholar]
- 25. Falciglia M, Freyber R, Almenoff PL, D’Alessio D, Render M. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37:3001-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krinsley JS, Egi M, Kiss A, et al. Diabetic status and the relationship of the 3 domains of glycemic control to mortality in critically ill patients: an international multi-center cohort study. Crit Care. 2013;17:R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sechterberger MK, Bosman RJ, Oudemans-van Straaten HM, et al. The effect of diabetes mellitus on the association between measures of glycaemic control and ICU mortality: a retrospective cohort study. Crit Care. 2013;17:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanspa MJ, Hirshberg EL, Phillips GD, Holmen J, Stoddard G, Orme J. Moderate glucose control is associated with increased mortality compared with tight glucose control in critically ill patients without diabetes. Chest. 2013;143:1226-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krinsley JS, Preiser JC. Time in targeted blood glucose range 70-140 mg/dL is strongly associated with survival in non-diabetic critically ill adults. Crit Care 2015;19:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krinsley JS, Maurer P, Holowisnki S, et al. Glucose control, diabetes status and mortality in critically ill patients: the continuum from intensive care unit admission to hospital discharge. Mayo Clin Proc. 2017;92:1019-1029. [DOI] [PubMed] [Google Scholar]
- 31. Egi M, Bellomo R, Stachowski E, et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39:105-111. [DOI] [PubMed] [Google Scholar]
- 32. Plummer MP, Bellomo R, Cousins CE, et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Int Care Med. 2014;40:973-980. [DOI] [PubMed] [Google Scholar]
- 33. Egi M, Krinsley JS, Maurer P, et al. Pre-morbid glycemic control modulates the interaction between acute hypoglycemia and mortality. Int Care Med. 2016;42:562-571. [DOI] [PubMed] [Google Scholar]
- 34. Roberts Quinn SJ, Valentine N, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endo Met. 2015;100:4490-4497. [DOI] [PubMed] [Google Scholar]
- 35. Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008; 31:1473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang Y, Kim TH, Yoon KH, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Card. 2017;241:57-63. [DOI] [PubMed] [Google Scholar]
- 37. Liao WI, Wang JC, Chang WC, et al. Usefulness of glycemic gap to predict ICU mortality in critically ill patients with diabetes. Medicine 2015;94:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kar P, Plummer MP, Bellomo R, et al. Liberal glycemic control in critically ill patients with type 2 diabetes: an exploratory study. Crit Care Med. 2016;44:1695-1703. [DOI] [PubMed] [Google Scholar]
- 39. Di Muzio F, Presello B, Glassford NJ, et al. Liberal versus conventional glucose targets in critically ill diabetic patients: an exploratory safety cohort assessment. Crit Care Med. 2016;44:1683-1691. [DOI] [PubMed] [Google Scholar]
- 40. Krinsley JS, Preiser JC, Hirsch IB. Safety and efficacy of personalized glycemic control in critically ill patients: a two year before and after interventional trial. Endo Prac. 2017;23:318-330. [DOI] [PubMed] [Google Scholar]
- 41. Rubinow KB, Hirsch IB. Reexamining metrics for glucose control. JAMA. 2011;305:1132-1133. [DOI] [PubMed] [Google Scholar]