Abstract
Background:
Ultra-fast-acting insulins, such as fast-acting insulin aspart (faster aspart), have pharmacokinetic properties that may be advantageous for patients using continuous subcutaneous insulin infusion (CSII), provided that they are compatible with and safe to use in CSII.
Methods:
Randomized, double-blind, parallel-group, actively controlled trial evaluating compatibility, efficacy, and safety of faster aspart in adults with type 1 diabetes using their own MiniMed Paradigm pump with Quick-Set or Silhouette infusion sets. Following run-in, subjects were randomized (2:1) to faster aspart (n = 25) or insulin aspart (n = 12) for 6 weeks. Primary endpoint was the number of microscopically confirmed episodes of infusion-set occlusions.
Results:
No microscopically confirmed episodes of infusion-set occlusions were observed in either arm. Seven possible infusion-set occlusions were reported by five subjects (all faster aspart); none were prompted by a plug observed by the subject (prompted by unexplained hyperglycemia [n = 6] or leakage [n = 1]) and none were confirmed. Macroscopic and microscopic evaluation showed no color change or particle/crystal formation in the infusion sets. Premature infusion-set changes were reported in 44% and 16.7% of subjects in the faster aspart and insulin aspart groups, respectively. A nonsignificant trend toward better efficacy was observed with faster aspart (estimated treatment difference [ETD] [95% CI] in HbA1c change: –0.14% [–0.40, 0.11]). No new safety issues were found in either treatment group.
Conclusions:
Over 6 weeks of treatment, no microscopically confirmed infusion-set occlusions were observed for faster aspart or insulin aspart, indicating similar compatibility with CSII use.
Keywords: infusion set occlusion, insulin pump, rapid-acting insulin, safety, type 1 diabetes mellitus
Continuous subcutaneous insulin infusion (CSII) is a popular treatment option in people with type 1 diabetes (T1D).1 Short-acting human insulin or rapid-acting insulin analogs are typically administered via CSII to control both the basal and postprandial bolus insulin requirements of an individual. However, for postprandial plasma glucose (PPG) control, currently available rapid-acting insulin analogs (insulin lispro, insulin aspart, and insulin glulisine) are generally perceived as not being sufficiently fast by either patients or physicians, and the speed of onset and offset of action remain recognized barriers to optimal glycemic control with CSII.2
Fast-acting insulin aspart (faster aspart) contains the conventional insulin aspart molecule formulated with two well-known excipients (niacinamide and L-arginine).3 Using a euglycemic clamp in subjects with T1D, Heise and colleagues showed that, when injected subcutaneously, faster aspart had an earlier onset, higher early exposure, and a greater early glucose-lowering effect compared with conventional insulin aspart. This improved pharmacological profile translated into an improvement in PPG and HbA1c control in patients with T1D on a multiple daily injections (MDI) regimen in a large-scale trial under real-life conditions.4 Furthermore, a pharmacokinetics/pharmacodynamics (PK/PD) study in subjects with T1D using CSII demonstrated even greater pharmacological improvements with faster aspart over insulin aspart than that seen with subcutaneous injection administration.5 Faster aspart may thus be advantageous for patients using CSII, provided that it is compatible and safe to use in this application. A potential challenge with delivery of insulin via CSII is the physical stability of the insulin, which remains under ambient conditions (subject to greater physical agitation and temperature fluctuations) in the infusion system (infusion set and reservoir) for a relatively long period of time versus insulin stored under standard conditions. Infusion-set occlusions or insulin degradation may lead to hyperglycemia, ketosis, and diabetic ketoacidosis.6,7
The key objective of the onset 4 trial was to evaluate the compatibility, as well as the safety and short-term efficacy, of faster aspart and insulin aspart used in CSII therapy over a 6-week treatment period in adults with T1D.
Methods
Trial Design
Trial design was based on the methodology used in studies evaluating the efficacy, safety, and compatibility of rapid-acting insulin analogs in subjects undergoing CSII therapy,8,9 and also guidance from a US Food and Drug Administration (FDA) draft guideline for insulin pumps.10
This trial was a randomized, double-blind, parallel-group, two-center (Profil, Neuss, Germany and Atlanta Diabetes Associates, Atlanta, GA, USA), actively controlled trial with a 2-week run-in period, a 6-week treatment period, and a follow-up period. Subjects were eligible if they were diagnosed with T1D (≥12 months), aged ≥18 years, had an HbA1c ≤9.0% and a body mass index (BMI) 20-35 kg/m2. Eligible subjects had to be administering insulin aspart, insulin lispro, or insulin glulisine (with no other antidiabetic treatment) for 3 months prior to the trial, and using CSII via a MiniMed Paradigm pump (Medtronic, Northridge, CA, USA) over the past 6 months prior to the trial. Trial exclusion criteria are listed in the Supplementary Material. The trial was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) good clinical practice. Informed consent was obtained prior to initiating any trial activities. The study protocol, subject information, and consent forms were reviewed and approved by independent ethics committees and/or institutional review boards.
Treatment
During the 2-week run-in period, all subjects were switched from their previous rapid-acting insulin analog treatment to insulin aspart. Subjects’ knowledge on using an insulin pump, handling of infusion sets, and keeping a trial diary was reinforced. After the run-in period, subjects were randomized in a 2:1 ratio of faster aspart:insulin aspart for 6 weeks of treatment using CSII. Randomization was stratified according to infusion set. Subjects continued to use their own CSII MiniMed Paradigm pump, in combination with QuickSet or Silhouette infusion sets provided by Novo Nordisk (cannula length was not standardized across the two infusion sets) for the duration of the trial (Supplementary Table 1).
Subjects were free to choose their own meals, with no predetermined carbohydrate limit. Bolus insulin doses were adjusted during the trial by the subject based on a preprandial and bedtime glucose target <108 mg/dl and a 2-h PPG glucose target 140 mg/dl. The basal insulin infusion rate was optimized during the first week of treatment; thereafter, it was not adjusted unless considered clinically necessary by the investigator. The insulin:carbohydrate ratio, insulin sensitivity factors, and other pump settings were adjusted at the investigator’s discretion during weekly contacts.
Assessments
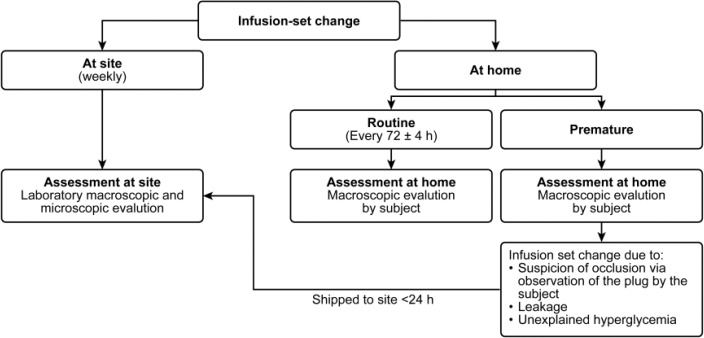
Subjects were instructed to change the infusion set and reservoir every 72 (±4) h, and only before this time if there was any suspicion of occlusion (based on patient self-assessment), leakage, unexplained hyperglycemia (without apparent medical, dietary, insulin dosage reason, or pump problem), infusion-site reaction, or other technical reason. If a premature change of an infusion set was prompted by suspicion of occlusion, leakage, or unexplained hyperglycemia, subjects were requested to send the infusion set and reservoir within 24 h to the site for further examination. Macroscopic evaluation of the infusion set and reservoir was conducted by the subject after a routine or premature change at home. Laboratory macro- and microscopic examinations of the reservoir and infusion set were performed at each weekly site visit and following receipt of a prematurely changed infusion set due to potential occlusion (Figure 1). Upon macroscopic evaluation, the subject or investigator would evaluate whether the insulin solution was clear and colorless, and whether any particles or crystal formation were present. An occlusion was only confirmed upon microscopic evaluation by the laboratory. A description of efficacy and safety assessments is included in the Supplementary Material.
Figure 1.
Evaluation of infusion sets.
Key Endpoints
The primary endpoint was the number of microscopically confirmed episodes of infusion-set occlusions during the 6 weeks of treatment. Secondary compatibility endpoints included number of possible infusion-set occlusions, number of premature infusion-set changes, and the number of infusion sets used per week. Episodes of possible infusion-set occlusions were defined as infusion sets changed due to suspicion of occlusion, leakage, or unexplained hyperglycemia. Additional secondary compatibility endpoints were the number of infusion sets with (1) color change in pump reservoir and/or distal tubing and (2) particles and/or crystal formation in pump reservoir and/or distal tubing; these were assessed by laboratory macro- and microscopic evaluation and also the subjects’ own macroscopic evaluation.
Secondary efficacy endpoints included the change from baseline after 6 weeks of treatment in terms of HbA1c, fructosamine, fasting plasma glucose (FPG), 2-h PPG increment (measured from 7-9-7 self-measured plasma glucose [SMPG] profiles, see Supplementary Material), insulin dose, and 1,5-anhydroglucitol. Secondary safety endpoints included the number of treatment-emergent severe or blood glucose (BG)-confirmed hypoglycemic episodes (confirmed by plasma glucose <56 mg/dl), number of treatment-emergent hyperglycemic episodes (confirmed by plasma glucose value ≥300 mg/dl), and number of treatment-emergent adverse events (TEAEs).
Statistical Analysis
The trial was not powered to detect any prespecified differences between treatments. Sample size was not based on a formal calculation but on guidance from an FDA draft guideline for insulin pumps,10 which stipulates that 15-20 subjects need to be evaluated with the investigational insulin. Efficacy endpoints are presented using the full analysis set (FAS; all randomized subjects) and safety endpoints are summarized using the safety analysis set (SAS; all subjects receiving any infusion of faster aspart or insulin aspart). All statistical analyses were based on the FAS.
Change from baseline in FPG, 1-5-anyhydroglucitol, fructosamine, and HbA1c were analyzed using an analysis of variance (ANOVA) model. Change from baseline in 2-h PPG increment over all meals was analyzed using a mixed-effect model for repeated measurements. Treatment-emergent severe or BG-confirmed hypoglycemia was analyzed using a negative binomial regression model. For these endpoints, estimated mean treatment differences (or ratios) are shown together with two-sided 95% confidence intervals (95% CIs). All other endpoints are summarized using descriptive statistics. Further detail on statistical analyses is outlined in the Supplementary Material.
Results
Overall, 37 subjects (of 49 screened) completed the run-in period and were then randomized in a 2:1 ratio to faster aspart (n = 25) or insulin aspart (n = 12). After 17 days of treatment, one subject in the faster aspart group withdrew due to a nonserious, severe TEAE (worsening of rheumatoid arthritis) (Supplementary Figure 1).
A total of 27 subjects were enrolled in Germany and 10 in the USA. Baseline characteristics of the trial population are presented in Table 1. Both treatment groups were comparable in terms of the type of pump and infusion set used (Supplementary Table 1).
Table 1.
Baseline Characteristics at Randomization.
| Characteristics, n, FAS | Faster aspart (n = 25) | Insulin aspart (n = 12) |
|---|---|---|
| Age, years | 48.9 (14.6) | 34.7 (9.1) |
| Gender, n (%) | ||
| Male | 14 (56.0) | 8 (66.7) |
| Female | 11 (44.0) | 4 (33.3) |
| Race, n (%) | ||
| White | 24 (96.0) | 12 (100.0) |
| Black or African American | 1 (4.0) | 0 (0.0) |
| Body weight, kg | 84.2 (16.8) | 81.2 (17.5) |
| BMI, kg/m2 | 27.0 (3.8) | 25.7 (4.0) |
| Duration of diabetes, years | 25.9 (13.3) | 20.3 (9.5) |
| HbA1c, % | 7.3 (0.7) | 7.7 (0.7) |
| FPG, mg/dl | 149.9 (52.6) | 167.4 (71.6) |
| Fructosamine, μmol/l | 320.5 (42.3) | 333.5 (33.4) |
Values are mean (SD) unless stated otherwise. BMI, body mass index; FAS, full analysis set; FPG, fasting plasma glucose.
Compatibility
No microscopically confirmed episodes of infusion-set occlusions were observed in either of the treatment arms after 6 weeks of treatment. For this endpoint, 219 infusion sets (n = 147 [faster aspart], n = 72 [insulin aspart]) were examined macroscopically and microscopically in the laboratory after routine changes at site visits, along with three from the faster aspart group that had been shipped to the site following a premature change at home (two for unexplained hyperglycemia, one for leakage). In total, 379 infusion sets were evaluated during the trial in the faster aspart arm and 174 infusion sets in the insulin aspart arm (Table 2).
Table 2.
Evaluation of the Infusion Sets During the Trial.
| Type of change | Faster aspart | Insulin aspart | Total | Evaluation |
|---|---|---|---|---|
| Routine at home | 210 | 98 | 308 | Macroscopic by subject |
| Routine at site visit | 147 | 72 | 219 | Macroscopic and microscopic by laboratory |
| Premature | 21 | 4 | 25 | Macroscopic by subject; plus macroscopic and microscopic by laboratory if shippeda |
| Unclassified | 1 | 0 | 1 | Macroscopic by subject |
| Total | 379 | 174 | 553 |
Three of the seven infusion sets that were changed prematurely because of a possible occlusion were shipped to the laboratory for macroscopic and microscopic evaluation (all in the faster aspart group).
Seven possible but unconfirmed infusion-set occlusions, which led to a premature change of infusion set at home, were reported by five subjects in the faster aspart group. In none of these cases did the subject observe a plug that may have led to a suspicion of occlusion. One possible occlusion was prompted by leakage, while six changes were prompted by unexplained hyperglycemia (of these six changes, only one fulfilled the protocol-specified definition of hyperglycemia [plasma glucose >300 mg/dl]). No possible infusion-set occlusions were reported in the insulin aspart group. Laboratory macroscopic and microscopic evaluation of the infusion sets at the site after a possible occlusion was possible in three cases, and none showed color change or crystal formation.
Overall, there were 21 premature infusion-set changes reported by 11 (44%) subjects in the faster aspart group and four premature changes reported by two (16.7%) subjects in the insulin aspart group (Table 2). Technical issues (eg, empty reservoir, kinked, or dislodged infusion-set tubing) was the most commonly cited reason for a premature infusion-set change (for seven of the changes in the faster aspart group, and for three in the insulin aspart group).
Laboratory-based and the subjects’ own macroscopic examination of the reservoir and infusion set after a routine or a premature change did not reveal any color change or particle/crystal formation in the insulin solution in either treatment group. Laboratory microscopic evaluation revealed two instances of particle/crystal formation in the faster aspart group, both graded as minimal. These reservoir particle/crystal formations were described as either small silicone-like particles or gray-shadowed particles and were considered unlikely to be related to insulin. No evidence of insulin polymerization or fibrillation was observed with either faster aspart or insulin aspart. The number of infusion sets used per week was 2.50 ± 0.35 (mean ± SD) in the faster aspart group and 2.38 ± 0.18 in the insulin aspart group.
Efficacy
The point estimates for efficacy endpoints, except FPG, indicated better glycemic control with faster aspart compared with insulin aspart; however, these observations were not statistically significant (Table 3). SMPG profiles at baseline and week 6 are presented in Figure 2. A trend for improvement in the 2-h PPG increment (taken from the 7-9-7 SMPG profiles) was consistently observed across all meals (breakfast, lunch, main evening meal; Table 3).
Table 3.
Efficacy Results After 6 Weeks of Treatment.
| Faster aspart |
Insulin aspart |
ETD, faster aspart – insulin aspart (95% CI) | |||
|---|---|---|---|---|---|
| Baseline | 6 weeks | Baseline | 6 weeks | ||
| HbA1c, % | 7.3 (0.7) | 7.1 (0.7) | 7.7 (0.7) | 7.6 (0.7) | −0.14 (–0.40, 0.11) |
| Fructosamine, μmol/l | 320.5 (42.3) | 318.2 (44.9) | 333.5 (33.4) | 340.8 (28.5) | −11.30 (–26.39, 3.80) |
| FPG, mg/dl | 149.9 (52.6) | 148.8 (55.9) | 167.4 (71.6) | 140.6 (61.8) | 13.5 (–28.2, 55.2) |
| 1,5-anhydroglucitol, μg/ml | 5.2 (3.3) | 5.7 (3.7) | 3.7 (1.9) | 3.6 (2.3) | 0.45 (–0.26, 1.16) |
| 2-h PPG increment, mg/dla | |||||
| All meals | 16.4 (33.9) | 8.5 (30.9) | −3.0 (39.0) | 8.2 (47.2) | −13.94 (–37.05, 9.16) |
| Breakfast | 16.5 (37.4) | 4.3 (48.6) | −10.9 (82.2) | 4.1 (93.5) | −15.46 (–53.4, 22.48) |
| Lunch | 18.2 (67.4) | 11.2 (47.6) | 16.0 (59.0) | 36.2 (53.6) | −23.70 (–59.92, 12.53) |
| Dinner | 14.6 (46.8) | 14.6 (46.8) | −14.0 (59.7) | 4.1 (52.6) | −0.31 (–37.47, 36.85) |
Values are observed mean (SD). CI, confidence interval; ETD, estimated treatment difference; FPG, fasting plasma glucose; PPG, postprandial plasma glucose.
2-h PPG increment taken from 7-9-7 self-measured plasma glucose profiles.
Figure 2.
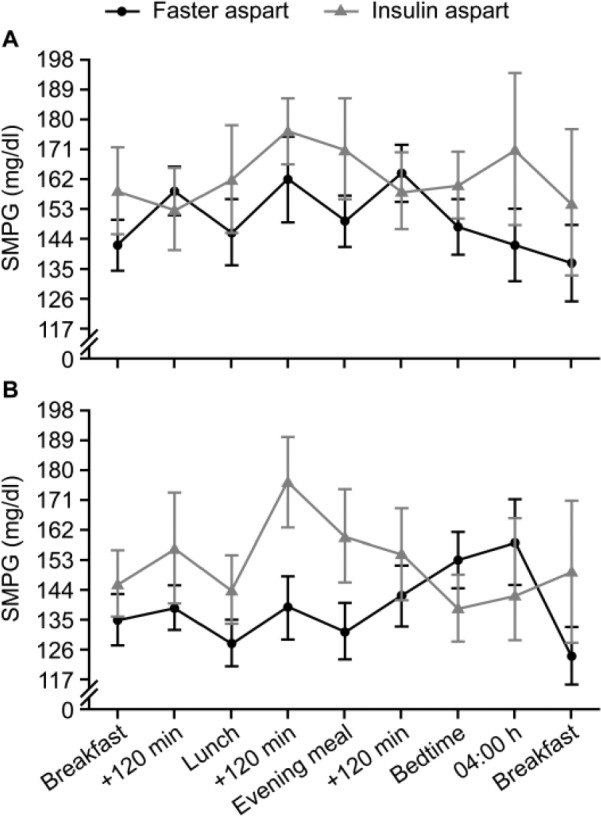
Nine-point SMPG profiles at (A) baseline and (B) week 6. SMPG, self-measured plasma glucose.
Safety
No episodes of severe hypoglycemia were reported in either treatment group. During the run-in period, there was an imbalance in the incidence of BG-confirmed hypoglycemia (46.8 [faster aspart] vs 18.5 [insulin aspart] events per patient-year of exposure), which was sustained throughout the trial (Table 4). The treatment ratio for severe or BG-confirmed hypoglycemic episodes, faster aspart/insulin aspart (95% CI), after 6 weeks of treatment was 1.81 (0.76, 4.32). However, after adjusting for the imbalance in severe or BG-confirmed hypoglycemia reported in the run-in period, the resulting treatment ratio after 6 weeks of treatment was 0.98 (0.47, 2.07) (post hoc analysis), indicating a similar risk of hypoglycemia with faster aspart compared with insulin aspart. Episodes of BG-confirmed hyperglycemia were reported at similar rates during the study (Table 4). Mean total bolus and basal insulin doses were consistently maintained throughout the study (Supplementary Material).
Table 4.
Incidence of Hypoglycemia and Hyperglycemia.
| Faster aspart (n = 25) |
Insulin aspart (n = 12) |
|||
|---|---|---|---|---|
| n (%) | R | n (%) | R | |
| Hypoglycemia reported during the run-in perioda | ||||
| Severe | 0 | 0.0 | 0 | 0.0 |
| BG-confirmed | 14 (56) | 46.8 | 5 (41.7) | 18.5 |
| Treatment-emergent hypoglycemia | ||||
| Severe | 0 | 0.0 | 0 | 0.0 |
| BG-confirmed overall | 19 (76) | 49.5 | 8 (66.7) | 23.4 |
| Treatment-emergent hyperglycemiab | ||||
| Overall | 18 (72) | 25.4 | 8 (66.7) | 35.5 |
| Unexplainedc | 10 (40) | 9.6 | 3 (25) | 11.3 |
BG-confirmed: plasma glucose value <56 mg/dl. Treatment-emergent is defined as an event that has an onset up to 1 day after the last day of randomized treatment and excluding events occurring in the run-in period. %, percentage of subjects; BG, blood glucose; n, number of subjects with at least one event; R, number of events per patient-year of exposure.
All subjects were using insulin aspart only. bConfirmed by plasma glucose value ≥300 mg/dl. cNo apparent medical, dietary, insulin dosage reason, or pump problem.
The most frequently reported TEAEs (>5%) by preferred term were nasopharyngitis (0.9 events per patient-year of exposure [PYE] overall), cough and back pain (both 0.7 per PYE overall) (Supplementary Table 2). One severe TEAE occurred in the faster aspart group: a worsening of rheumatoid arthritis resulting in trial withdrawal. Two subjects (8%) in the faster aspart group each reported two infusion-site reactions; these were considered nonserious, mild, and possibly or unlikely to be related to faster aspart. There were no clinically relevant differences from baseline to end of trial or between treatment groups in clinical or laboratory assessments. There were no deaths, serious adverse events (AEs), or cardiovascular events in either of the treatment groups. Very few changes were made to the pump settings in both treatment groups during the trial (data not shown).
Discussion
It is recognized that CSII can help improve glycemic control and reduce the risk of hypoglycemia compared with an MDI regimen, while also offering lifestyle advantages for patients.11,12 In the USA, approximately 40% of subjects with T1D use CSII,13 and the uptake and technological sophistication of CSII systems continues to increase over time.1,14,15 A persistent challenge in CSII therapy is that currently available rapid-acting insulin analogs remain insufficiently rapid to fully address the rise in postprandial glucose that occurs after meals.16 Accordingly, there is a need to match CSII pumps with compatible ultra-fast-acting prandial insulin alternatives, such as faster aspart, to further optimize glycemic coverage and safety profiles in subjects using CSII.
The phase 3a trial described here was designed to evaluate the compatibility of faster aspart compared with insulin aspart in CSII in patients with T1D, based on the FDA draft guideline for insulin pumps.10 Over a 6-week treatment period, neither faster aspart nor insulin aspart were associated with any microscopically confirmed infusion-set occlusions. There was no evidence of infusion-set occlusions or insulin degradation after subjects’ or laboratory-based evaluation of the infusion set following a premature set change. No evidence of insulin polymerization or fibrillation was observed; however, the study was not primarily designed to assess these characteristics. Overall, the results of our study are in alignment with reports where conventional insulin aspart showed the greatest chemical and physical stability in the insulin pump, with the lowest rates of overall occlusion in comparison with insulins lispro and glulisine.9
Unexplained hyperglycemia has been used previously as a surrogate marker for possible infusion-set occlusions.6,17 In our trial, the unexplained hyperglycemia event rate was similar for faster aspart compared with insulin aspart. In the faster aspart group, 10 subjects reported unexplained hyperglycemia, but only six possible infusion-set occlusions were reported by four subjects following a premature change due to unexplained hyperglycemia; three of these episodes were reported by the same subject (who had a history of infusion-site issues) and five of the episodes did not meet the protocol-defined criteria for confirmed unexplained hyperglycemia. In the insulin aspart group, three subjects reported unexplained hyperglycemia, but none of these three subjects documented a possible infusion-set occlusion. The numerically lower number of subjects reporting unexplained hyperglycemia in the insulin aspart group, largely due to the 2:1 randomization, is likely to have contributed to the observed differences in possible infusion-set occlusions. Although no laboratory-based or subjects’ own evaluation revealed crystals, particles, or color changes of the insulin solution, four infusion sets were not evaluated by the laboratory (not shipped); thus, there is a possibility that occlusions may have been missed. Reports of silicone-like particles were documented, but this is most likely to be explained by the cutting procedure of the silicone tubing as part of the microscopic examination.18 A higher number of premature infusion-set changes were observed with faster aspart compared with insulin aspart and this may be partially accounted for by the 2:1 randomization ratio, as opposed to reflecting a problem with the tolerability of faster aspart.
This trial was not powered to detect differences between treatments; however, safety and efficacy objectives were assessed. No severe hypoglycemia was reported, and although the rate of BG-confirmed hypoglycemia was greater with faster aspart compared with insulin aspart during the treatment period, a post hoc analysis showed that the numerical imbalance in hypoglycemic events between treatments appeared to be derived primarily from the imbalance in run-in hypoglycemia. The duration of the trial was too short to properly assess the impact of treatment on glycemic control using HbA1c; however, the reported HbA1c point estimates combined with other glycemic measures (fructosamine, 1,5-anhydroglucitol) indicate a general, nonsignificant trend for improved glycemic control with faster aspart. Furthermore, there is potential that the observed differences in the efficacy endpoints were partially accounted for by changes in the insulin aspart group, as opposed to improvements in the faster aspart group. The statistical analysis was adjusted for baseline HbA1c differences between groups to mitigate the potential impact of differences in glycemic control at baseline on the study outcomes. The observed baseline difference is likely due to chance, a common occurrence in randomized trials with a small sample size such as this. Pharmacological studies in subjects with T1D, particularly in those using CSII (assessed via euglycemic clamp), have shown that faster aspart has an improved time–action profile, with a greater early glucose-lowering effect compared with insulin aspart.5 An improved glucose-lowering effect with faster aspart after 14 days of CSII has also been demonstrated compared with insulin aspart in subjects with T1D after a standardized meal test, with the findings confirmed by continuous glucose monitoring for all meals.19 However, larger, long-term studies of faster aspart in CSII are warranted.
Conclusions
Over 6 weeks of treatment, no microscopically confirmed infusion-set occlusions were observed for faster aspart or insulin aspart, indicating similar compatibility with CSII use. Faster aspart demonstrated a nonsignificant trend toward improved glycemic control with no new safety issues. A phase 3b study, onset 5, is currently underway (ClinicalTrials.gov NCT02825251) and aims to confirm the efficacy and safety of faster aspart in a CSII setting in a larger population and longer study duration than the present study. With pump technology becoming increasingly sophisticated, and with the emergence of glucose-responsive feedback control (“closed loop”) systems, faster-acting pump insulins are likely to play an increasingly important role in CSII therapy.
Supplementary Material
Acknowledgments
Editorial assistance was provided by Steven Barberini, Liam Gillies, and Helen Marshall of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc, funded by Novo Nordisk.
Footnotes
Abbreviations: ANOVA, analysis of variance; BG, blood glucose; BMI, body mass index; CI, confidence interval; CSII, continuous subcutaneous insulin infusion; ETD, estimated treatment difference; FAS, full analysis set; faster aspart, fast-acting insulin aspart; FDA, US Food and Drug Administration; FPG, fasting plasma glucose FPG; MDI, multiple daily injections; PD, pharmacodynamics; PK, pharmacokinetics; PPG, postprandial plasma glucose; PYE, patient-year of exposure; SAS, safety analysis set; SD, standard deviation; SMPG, self-measured plasma glucose; TEAE, treatment-emergent adverse event; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EZ has received travel grants and speaker fees from Dance Biopharm, Novo Nordisk, and Roche Diabetes Care. MD and TG are employees of Novo Nordisk and hold shares with the company. TH is shareholder of Profil, a private research institute that received research funds from Adocia, Biocon, Dance Pharmaceuticals, Eli Lilly, Johnson & Johnson, Julphar, Medimmune, Mylan, Nordic Bioscience, Novo Nordisk, Poxel, Roche Diagnostics, Saniona, Sanofi, Senseonics, SkyPharma, and Zealand Pharma. In addition, TH received speaker honoraria from Eli Lilly and Novo Nordisk and fees for the participation in Advisory Boards from Novo Nordisk. LN has no potential conflicts of interest to disclose. BB holds shares with Aseko; has received speaker fees from AstraZeneca, Eli Lilly/Boehringer Ingelheim, Insulet, Janssen, Medtronic, Novo Nordisk, and Sanofi; has acted as a consultant to Adocia, Janssen, Medtronic, Mannkind, Novo Nordisk, and Sanofi; and receives grant and research support from Abbott, Becton Dickinson, DexCom, GSK, Janssen, Lexicon, Eli Lilly/Boehringer Ingelheim, Medtronic, NIH, Novo Nordisk, Sanofi, and Senseonics.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The trial was funded by Novo Nordisk.
Supplemental Material: Supplementary material is available for this article online.
References
- 1. Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz-Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev. 2016;32(1):21-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cengiz E, Bode B, Van Name M, Tamborlane WV. Moving toward the ideal insulin for insulin pumps. Expert Rev Med Devices. 2016;13(1):57-69. [DOI] [PubMed] [Google Scholar]
- 3. Heise T, Hövelmann U, Brøndsted Adrian CL, Nosek L, Haahr H. Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015;17(7):682-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russell-Jones D, Bode B, de Block C, et al. Double-blind mealtime faster-acting insulin aspart vs insulin aspart in basal–bolus improves glycemic control in T1D: the onset® 1 trial. Diabetes. 2016;65(suppl 1):A77. [Google Scholar]
- 5. Heise T, Zijlstra E, Nosek L, Rikte T, Haahr H. Pharmacological properties of faster-acting insulin aspart versus insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion: a randomized, double-blind, crossover trial. Diabetes Obes Metab. 2016;19:208-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Bon AC, Bode BW, Sert-Langeron C, DeVries JH, Charpentier G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2011;13(6):607-614. [DOI] [PubMed] [Google Scholar]
- 7. Phillips BD, Aurand LA, Bedwell MM, Levy JR. A novel approach to preventing diabetic ketoacidosis in a patient treated with an insulin pump. Diabetes Care. 2003;26(10):2960-2961. [DOI] [PubMed] [Google Scholar]
- 8. Bode B, Strange P. Efficacy, safety, and pump compatibility of insulin aspart used in continuous subcutaneous insulin infusion therapy in patients with type 1 diabetes. Diabetes Care. 2001;24(1):69-72. [DOI] [PubMed] [Google Scholar]
- 9. Bode B. Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid-acting insulin analogues-aspart, lispro, and glulisine. Endocr Pract. 2011;17:271-280. [DOI] [PubMed] [Google Scholar]
- 10. FDA Division of Metabolism and Endocrine Drug Products, Office of Biologics Research Review Center for Drugs and Biologics, FDA Division of Gastroenterology-Urology and General Use Devices, Office of Device Evaluation, Center for Devices and Radiologic Health. Draft requirements proposed for pump insulins and insulin pumps. February 20, 1985. [Google Scholar]
- 11. Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;20:CD005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters JE, Mount E, Huggins CE, Rodda C, Silvers MA. Insulin pump therapy in children and adolescents: changes in dietary habits, composition and quality of life. J Paediatr Child Health. 2013;49(4):E300-E305. [DOI] [PubMed] [Google Scholar]
- 13. Pickup J. Insulin pumps. Int J Clin Pract Suppl. 2011;(170):16-19. [DOI] [PubMed] [Google Scholar]
- 14. UK Health and Social Care Information Centre. National diabetes insulin pump audit report, 2013-15. April 2016. Available at: http://content.digital.nhs.uk/catalogue/PUB20436/nati-diab-insu-pump-audi-rep-2013-15_R.pdf.
- 15. Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists/American College of Endocrinology Insulin Pump Management Taskforce. Endocr Pract. 2014;20(5):463-489. [DOI] [PubMed] [Google Scholar]
- 16. Home PD. Plasma insulin profiles after subcutaneous injection: how close can we get to physiology in people with diabetes? Diabetes Obes Metab. 2015;17(11):1011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kerr D, Wizemann E, Senstius J, Zacho M, Ampudia-Blasco FJ. Stability and performance of rapid-acting insulin analogs used for continuous subcutaneous insulin infusion: a systematic review. J Diabetes Sci Technol. 2013;7(6):1595-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dewan PA, Owen AJ, Ashwood PJ, Terlet J, Byard RW. An in vitro study of silicone migration from intravenous fluid tubing. Pediatr Surg Int. 1997;12(1):49-53. [DOI] [PubMed] [Google Scholar]
- 19. Bode B, Johnson JA, Hyveled L, Tamer SC, Demissie M. Improved postprandial glycemic control with faster-acting insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Diabetes Technol Ther. 2017;19:25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.