Abstract
Background:
Painful subcutaneous insulin injections may decrease treatment compliance. Improving injection comfort therefore represents a particular area of technological research in which steady progress has been made since the introduction of the insulin pen in 1985. Injection pain can be influenced by many variables, but relatively little is known about their impact. This study investigated the impact of injection volume (range 0-2250 µL), speed (range 0-800 µL/sec), and site (abdomen vs thigh) on pain sensation.
Method:
In random order, patients (n = 80) with type 1 or type 2 diabetes received 24 saline injections subcutaneously through a 27G ultra-thin-wall needle. Injections were performed in the abdomen (n = 19) and thigh (n = 5) with predefined speed-volume combinations. For each injected speed-volume combination, patients scored their pain sensation on a 100 mm visual analog scale (VAS).
Results:
The mean pain scores for speed-volume combinations were all in the lower part (<20 mm) of the VAS, indicating zero to mild pain. Pain sensation was statistically higher (P < .05) with the 2250 µL volume compared to other injection volumes (range 4.3-5.1 mm) and with thigh compared to abdomen injections (2.1 mm). Pain sensation did not change with increasing injection speed. Patient acceptance of the injection pain was high for all injections (range 93.7-98.7%).
Conclusions:
In summary, large volume and thigh injections are rated more painful, but the clinical impact of these findings is likely marginal considering the low absolute pain levels and high patient acceptance rates. Injection speed does not influence pain sensation.
Keywords: diabetes, dosing accuracy, injection pain, insulin pen, subcutaneous injection
Painful injections may present a barrier to treatment initiation and long-term adherence. Not surprisingly, research into alternative and pain-free ways of administering insulin, such as via the oral, buccal, nasal or pulmonary route, has gained interest for decades.1,2 Despite these efforts, research into alternative insulin administration has not yet produced any commercially viable products and subcutaneous injection remains the only option for almost all patients. Improving injection comfort represents a particular area of technological research. State-of-the-art delivery devices with smaller, single-use needles, reduced manual injection force and easier-to-use pen injectors have been designed over the past 30 years to improve injection comfort for patients with diabetes.3 Today, the vast majority of insulin users in Europe inject via modern pen devices, although their use in the United States is lagging behind.4 A number of studies have shown that improved injection comfort with prefilled pen injectors enhances treatment compliance and health outcomes in comparison to injections with a vial and syringe combination.5-8
When it comes to pain perception with subcutaneous drug injection, many different factors play a role: the design of the administration device including needle configuration (length, diameter, wall thickness, bevel type), injection technique (angle, pressure, speed), drug formulation (pH, viscosity, drug concentration), drug dose (volume), injection site (abdomen, thigh, upper arm), previous injection experience, and time of day, among others, influence pain perception.9-14 It is clear from the factors listed here that not all can be controlled by drug and device manufacturers, but improving individual components should contribute to reduce the burden of insulin injections for the patient. It is therefore important to investigate individual factors influencing pain perception in a systematic and controlled way. One such investigation recently reported how injection speed, volume, and site (abdomen vs thigh) impact on perceived pain.15 The findings showed that injection speeds between 150 µL/sec and 450 µL/sec do not influence pain perception, whereas injection volumes of 1200 µL or higher and injections in the thigh are considered more painful.15 The aim of the present study was to expand the existing knowledge by investigating pain perception over a wider range of speed and volume combinations that are relevant for current and future treatment of diabetes and other diseases.
Methods
In the study, subjects received a total of 24 injections during a 1-day visit to the clinic. The injections were performed in a randomized, double blind, crossover manner. After informed consent procedures, a screening visit was performed to check the eligibility of potential subjects. The in- and exclusion criteria limited the study population to male and female adult subjects with type 1 or type 2 diabetes who were receiving daily needle injection(s) with antidiabetes medication (ie, insulin or GLP-1), with a BMI between 18-30 kg/m2, who had a light skin color, were nonsmokers, and were without advanced sensory neurological deficits (grade 0/8, 1/8, or 2/8 from a Rydell-Seifer tuning fork test). Furthermore, intake of any pain-relieving or analgesic medication was not allowed within 7 days before the experimental study visit.
The study protocol was approved by an independent ethics committee (Ethik-Kommission der Landesärztekammer Rheinland-Pfalz) and performed in accordance with the Declaration of Helsinki16 and Guidelines of Good Clinical Practice.
Study Procedures and Assessments
At the experimental study visit, each subject received the same number and type of injections. Out of 24 injections, 19 injections were performed in the abdomen and 5 injections in the thigh. Two injections were needle-only insertions (one in the abdomen and one in the thigh). The remaining 22 injections were combinations of various injection speeds (125, 167, 250, 500, 800 μL/s) and volumes (100, 400, 800, and 2250 μL). One combination of injection speed (250 μL/s) and volume (800 μL) was given 3 times in the abdomen of each subject to investigate the within-subject variability of the injection pain sensation. The injection sites were rotated spatially to minimize any potential effect of altered pain sensation with an increasing number of injections received; the different speed-volume combinations were randomized to eliminate any order effect. For all injections a 0.9% NaCl solution was injected using a 27G ultra-thin-wall needle with 0.5 inch needle length (TSK Laboratory, Oirschot, Netherlands). The time between injections was 5 to 15 minutes.
Immediately after each injection, the subjects were asked to rate their perceived injection pain on an electronic visual analog scale (VAS) by marking a 100 mm line. The extreme left of the scale (0 mm) signifies no pain and the extreme right (100 mm) signifies the worst pain imaginable by the subject.
After each pain assessment, the subject was then asked to indicate whether the pain would be acceptable for his/her daily diabetes treatment (yes/no).
Any backflow from an injection was quantified within 3 minutes after completion of the injection. The wet spot on absorbent paper held in to liquid on the skin at the injection site was compared to a reference set of paper strips as described by Præstmark et al.17
The injection site was examined by the medical staff for any potential acute (10 minutes after each injection) and short-term (1 hour after the last injection) local reactions. During a follow-up telephone call the day after the injections, the subjects were asked to report any long-term local reactions and in case of a reaction asked to return to the clinical site for a medical examination.
Statistical Analysis
The sample size calculation was based on data from a previous exploratory trial to investigate pain during subcutaneous injections with different injection speed and volume combinations.15 Assuming a maximum between subject variability of 28.5 mm based on the results of that study, 80 subjects would give a power of about 85% to detect a difference of injection pain rated on a VAS of at least 10 mm between the different injections, given a significance level of 5%.
The primary endpoint, injection pain rated on a VAS (mm) after each injection, was analyzed using a mixed model analysis of variance (ANOVA) approach (main model) to identify the most important factors/covariates influencing the pain perception assessment. In the main model injection volume, speed, their interaction and injection site (abdomen versus thigh) entered the model as fixed effects and subject entered as a random effect, thereby including within subject and between subject variation in the model. Only secondary testing for differences between specific volume/speed combinations was performed. The analysis was also done with adjusting for needle-only injection by including “injection-site-specific, needle-only pain” as covariate in the main model. The random effects were assumed independent and identically distributed, and the residual errors were assumed independent of the random effects, identically and normally distributed. The result of the first parametric analysis are presented as
P values of significance testing of treatment effects on injection pain (injection area, injection volume, injection speed and injection volume by speed interaction) using type 1 F-tests
LS-means differences with two-sided 95% confidence intervals (CIs) and P values for treatment effects on injection pain: Comparison with reference injection (needle-only injection for each factor and factor level (in addition, in the case of a nonsignificant injection volume by speed interaction the presentation was also to be done for the model without interaction)
Additional statistical analyses were also performed including the following subject characteristics as factors/covariates in the main model as a main effect one at a time:
Age
Gender
BMI
Diabetes type
Diabetes duration
Antidiabetic treatment
HbA1c
Results
In total 91 subjects were screened and 80 subjects were included in the study. All 80 subjects completed the study and were included in the safety analysis. One subject was included in the trial despite failing to meet one of the inclusion criteria (diagnosed with diabetes at least 6 months before the screening visit). The data of this subject was excluded from the full analysis set.
Pain perception was evaluated after a total of 1892 injections. Individual pain ratings ranged from 0 to 92 mm, but pain ratings were generally low with mean pain scores for volume and speed combinations all in the lower part (< 20 mm) of the VAS, indicating zero to mild pain (Figure 1). Pain sensation changed neither with increasing injection speed (P = .3939), nor with increasing injection volumes up to 800 μL (P ≥ .1664). Pain sensation was however statistically higher with the 2250 μL injection volume, from 4.3 mm compared to the 800 μL volume to 6.4 mm compared to needle-only insertions (P < .0001). In comparison to equivalent injections in the abdomen, injections in the thigh were consistently rated more painful (2.1 mm, P = .0013). Demographic parameters (age, gender, BMI), diabetes type, diabetes duration, HbA1c, and daily antidiabetes treatment dose did not significantly affect pain sensation.
Figure 1.
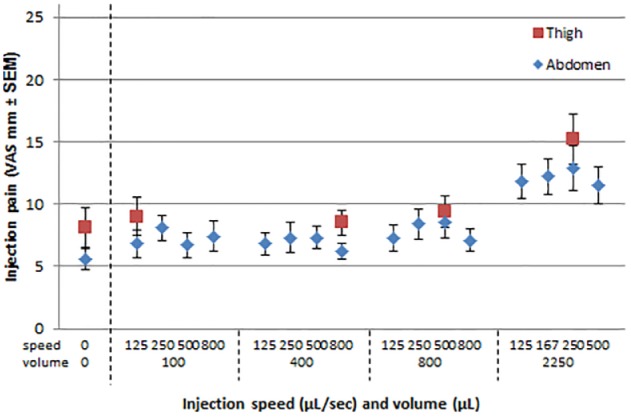
Mean injection pain for speed and volume combinations tested in the abdomen and thigh.
Table 1 gives an overview of the relative number of assessments for various pain intensities.18 This analysis shows that in addition to the low mean overall pain perception, very few individual injections caused moderate pain (n = 38 injections) and injections causing severe pain (n = 4 injections) were uncommon. One or more painful injections (≥45 mm) were reported by 13 of 79 subjects, with one subject reporting more than a quarter of all painful injections reported (9 moderate pain, 3 severe pain).
Table 1.
Individual Injections Listed by Pain Intensity According to the Ranges Defined by Jensen et al18 and the Number of Subjects Experiencing One or More Painful Injections.
| No pain (0-4 mm VAS) | Mild pain (5-44 mm) | Moderate pain (45-74 mm) | Severe pain (75-100 mm) | |
|---|---|---|---|---|
| Number of injections (% of total) | 947 (50.0%) | 906 (47.8%) | 38 (2.0%) | 4 (0.2%) |
| Number of subjects experiencing 1 or more injection of corresponding pain intensity (% of total) | 78 (98.7%) | 74 (93.7%) | 13 (16.5%) | 2 (2.5%) |
Pain ratings for the repeated 250 µL/sec-800 µL injection showed a high between-subject CV of 147%. The within-subject CV was still quite high at 69%, but such variation around a low mean pain rating of 8.4 mm will not result in any meaningful differences between subjects or injections.
Patient acceptance of the injection pain for daily diabetes treatment was high for all injections (range 93.7-98.7%), although the small number of injections with unaccepted levels of injection pain was more than double for the 2250 μL volume injections compared to the other volumes (odds ratio range 2.5-3.9, P < .05). Injections in the thigh were as well accepted as injections in the abdomen (P = .4448).
From 1869 injections that were eligible for backflow assessment, backflow or leakage was observed 621 times (33.2%). The number of backflow observations and mean volume significantly (P < .0001) increased with increasing injection volume, but not with increasing speed (P = .5387), and more (P < .0001) backflow was observed after injection in the thigh compared to the abdomen (Figure 2). Of all assessments after abdominal injection 27.4% showed some degree of backflow compared to 55.0% after thigh injection. Mean volumes observed were generally low (<5 μL) and for most injections (99.6%) the observed backflow fell within the ISO 11608-1:2000 specified dosing acceptance limits for pen injectors for medical use (Table 2).
Figure 2.
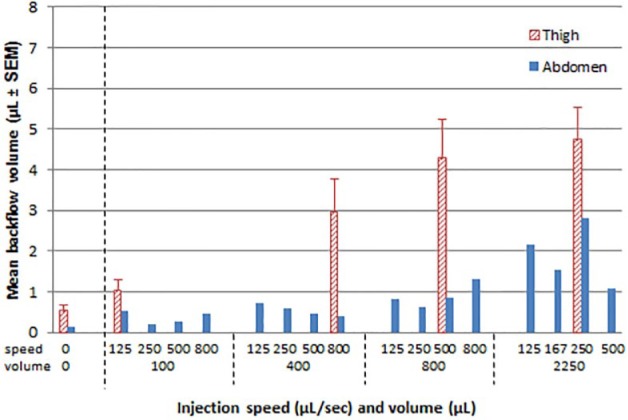
Mean backflow volumes for speed and volume combinations tested in the abdomen and thigh.
Table 2.
Backflow Measurements Exceeding the ISO 11608-1:2000 Accepted Dose Volume Deviation.
| Injection volume |
||||
|---|---|---|---|---|
| 100 µL | 400 µL | 800 µL | 2250 µL | |
| ISO 11608-1:2000 accepted dose volume deviation range (µL) | ±10 | ±20 | ±40 | ±112.5 |
| Number of injections with backflow greater than ISO dose volume deviation | 1 | 4 | 2 | 0 |
| Backflow (µL) (% of dose volume, injection site) | 13 (13%, thigh) | 27 (6.8%, thigh) 30 (7.5%, thigh) 35 (8.8%, thigh) 35 (8.8%, thigh) |
50 (6.3%, thigh) 60 (7.5%, abdomen) |
— |
Standard requirements allow deviations of up to 10 μL for injected volumes of 200 μL or below and deviations of up to 5% of the dose volume for volumes greater than 200 μL.
All 80 subjects experienced acute injection site reactions, predominantly hemorrhage and erythema. Most reactions (94.1%) were rated as barely perceptible and only a small amount was rated as well-defined (5.8%) or moderate and severe (0.1%). All reactions had disappeared within one hour after the injection and no new injection site reactions were recorded the day after the study visit. Observed reactions were similar between the abdomen and thigh and neither injection speed nor volume had an impact on the number of abnormal injection site assessments.
Discussion
The aim of this study was to quantify the impact of injection speed, volume, and site (abdomen vs thigh) on perceived pain. Our findings show that the speed of injection does not have any impact on perceived pain, whereas injection of a large volume (2250 µL) and thigh injections are statistically more painful than respectively injections with smaller volumes (800 µL or below) and abdomen injections. The general findings of the current study are in agreement with previous results15 and these studies together provide a robust dataset on the impact of injection speed, volume, and site on perceived pain.
Injection speeds were tested over a wide range from 125 to 800 µL/sec in the current study versus 150-450 µL/sec previously.15 The range was chosen based on the injection speeds that can be achieved with marketed pen injection devices, such as Sanofi’s SoloSTAR®. This device injects with a speed depending on the pressure applied by the patient and is generally believed to be in the region of 100-400 μL/sec. For future devices higher injection speeds are of interest to make the overall injection process quicker for patients, especially when administering large injection volumes. We also argued that if injection speed does impact on injection pain, we would have a better chance of detecting its impact with the larger differences in injection speed in our trial. No result in our trial indicated that injection speeds up to 800 µL/sec had an impact on injection pain perception. A possible explanation for this finding is that the injected fluid is distributed equally in the subcutaneous space, independent of injection speed.19 It is worth pointing out that injection speed might still influence perceived pain for particular drug formulations, for example, those known to cause pain upon injection such as heparin. In one crossover study, patients experienced subcutaneous injection of a heparin solution as less painful when the injection speed was 3 times slower.20 In our study, physiological saline was used to test for the distinct injection volumes and speeds. It remains uncertain if injection fluids with different attributes like divergent pH ranges or viscosity might turn out with a divergent pain perception with regard to the investigated ranges of injection volumes or speeds.
Injection volumes tested ranged from 100 µL to 2250 µL, also covering a wider range than the 400 to 1600 µL volumes previously tested.15 In both studies, no statistical different pain ratings were observed for injection volumes of 800 µL or less. Perceived pain did increase statistically with injection volumes above 1200 µL15 and up to the 2250 µL volume investigated in the current study. Although most patients with diabetes never have to inject volumes larger than 800 µL, which typically corresponds to a 80 unit insulin dose and is usually the maximum dose that can be given with a pen injection device, this finding is highly relevant for those patients in other therapeutic areas requiring larger volume injections. A possible explanation for the increased pain sensation with larger volumes is a different spatial distribution of injected fluid in the subcutaneous space, with more fluid placed in the more densely innervated fascia separating the deep and superficial subcutaneous fat layers.19
Injections in the thigh were rated more painful than identical injections in the abdomen, a result consistent with findings from previous studies.15 The average difference of 2.1 mm in pain rating between thigh and abdomen was small however and did not lead to a statistical difference in acceptance of the injection pain.
We observed no effect of demographic parameters (age, gender, BMI), diabetes type, diabetes duration, HbA1c, and daily antidiabetes treatment dose on pain sensation. But our data does show that there are great individual differences in pain perception with a between-subject CV of 147% for the 250 µL/sec-800 µL injection and considering that a small number of subjects is responsible for reporting all the painful injections. One could say that the tested speed-volume combination injections are generally painless and well-accepted, but there are individual exceptions, such as the subject reporting 12 out of 24 injections administered as painful.
Backflow measurements were performed to evaluate whether speed and volume of the injection have an impact on the dose accuracy. Although some degree of backflow or leakage was observed in almost one out of three injections and backflow increased with increasing injection volume and with thigh injections, the amount of backflow was generally low for all injections and only exceeded the ISO specified dose volume deviation in 7 instances (0.4% of all injections with backflow measurements). We can thus state that under the current experimental conditions backflow is usually not of clinical significance.
A strength of the current study design is that the impact of individual factors on pain perception is investigated in a systematic way under standardized conditions. The design and sample size with multiple injections enables detection of small differences in pain perception for different speed, volume, and site injections. Although each individual factor per se may not have a clinically significant impact on pain, in combination the effects on increased pain can be additive as we have shown for large volume injections in the thigh. Together with available literature on needle design and insertion technique, the impact of many individual factors on injection pain perception, backflow, and site reactions is now known.13,14,21-23 The available data should support device development and guide best practice for injection therapy to reduce the burden of insulin injections for the patient. Some limitations of the current study design have already been mentioned and include the use of saline as injection product and the testing of volumes larger than needed for diabetes therapy.
In summary, large volume and thigh injections are rated more painful, but the clinical impact of these findings is likely marginal considering the low absolute pain levels and high patient acceptance rates. Backflow after subcutaneous injection is not uncommon, but volumes are low enough that dosing accuracy is generally not compromised. Injection speed does neither influence pain sensation nor backflow.
Footnotes
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; CI, confidence interval; CV, coefficient of variation; GLP-1, glucagon-like peptide 1; ISO, international organization for standardization; LS, least square; VAS, visual analog scale.
Authors’ Note: EZ is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Results of this trial were presented at the following international scientific conference in poster format: 9th International Conference on Advanced Technologies & Treatments for Diabetes, Milan, Italy, February 3-6, 2016.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employees of Profil. CK is a shareholder within the company. Profil has collaborations with several pharmaceutical and biotechnology companies.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This investigator-initiated trial was supported by Sanofi.
References
- 1. Zijlstra E, Heinemann L, Plum-Morschel L. Oral insulin reloaded: a structured approach. J Diabetes Sci Technol. 2014;8(3):458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heinemann L, Jacques Y. Oral insulin and buccal insulin: a critical reappraisal. J Diabetes Sci Technol. 2009;3(3):568-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah RB, Patel M, Maahs DM, Shah VN. Insulin delivery methods: past, present and future. Int J Pharm Investig. 2016;6(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcus A. Diabetes care—insulin delivery in a changing world. Medscape J Med. 2008;10(5):120. [PMC free article] [PubMed] [Google Scholar]
- 5. Slabaugh SL, Bouchard JR, Li Y, Baltz JC, Meah YA, Moretz DC. Characteristics relating to adherence and persistence to basal insulin regimens among elderly insulin-naive patients with type 2 diabetes: pre-filled pens versus vials/syringes. Adv Ther. 2015;32(12):1206-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seggelke SA, Hawkins RM, Gibbs J, Rasouli N, Wang CC, Draznin B. Effect of glargine insulin delivery method (pen device versus vial/syringe) on glycemic control and patient preferences in patients with type 1 and type 2 diabetes. Endocr Pract. 2014;20(6):536-539. [DOI] [PubMed] [Google Scholar]
- 7. Xie L, Zhou S, Wei W, Gill J, Pan C, Baser O. Does pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15(3):230-236. [DOI] [PubMed] [Google Scholar]
- 8. Ayyagari R, Wei W, Cheng D, Pan C, Signorovitch J, Wu EQ. Effect of adherence and insulin delivery system on clinical and economic outcomes among patients with type 2 diabetes initiating insulin treatment. Value Health. 2015;18(2):198-205. [DOI] [PubMed] [Google Scholar]
- 9. Aronson R. The role of comfort and discomfort in insulin therapy. Diabetes Technol Ther. 2012;14(8):741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glynn CJ, Lloyd JW. The diurnal variation in perception of pain. Proc R Soc Med. 1976;69(5):369-372. [PMC free article] [PubMed] [Google Scholar]
- 11. Laursen T, Hansen B, Fisker S. Pain perception after subcutaneous injections of media containing different buffers. Basic Clin Pharmacol Toxicol. 2006;98(2):218-221. [DOI] [PubMed] [Google Scholar]
- 12. Norman JJ, Prausnitz MR. Improving patient acceptance of insulin therapy by improving needle design. J Diabetes Sci Technol. 2012;6(2):336-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23(1-2):37-43. [DOI] [PubMed] [Google Scholar]
- 14. Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3(1):41-49. [DOI] [PubMed] [Google Scholar]
- 15. Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16(10):971-976. [DOI] [PubMed] [Google Scholar]
- 16. World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 17. Præstmark KA, Stallknecht B, Jensen ML, Sparre T, Madsen NB, Kildegaard J. Injection technique and pen needle design affect leakage from skin after subcutaneous injections. J Diabetes Sci Technol. 2016;10(4):914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen MP, Engel JM, McKearnan KA, Hoffman AJ. Validity of pain intensity assessment in persons with cerebral palsy: a comparison of six scales. J Pain. 2003;4(2):56-63. [DOI] [PubMed] [Google Scholar]
- 19. Thomsen M, Rasmussen CH, Refsgaard HH, et al. Spatial distribution of soluble insulin in pig subcutaneous tissue: effect of needle length, injection speed and injected volume. Eur J Pharm Sci. 2015;79:96-101. [DOI] [PubMed] [Google Scholar]
- 20. Zaybak A, Khorshid L. A study on the effect of the duration of subcutaneous heparin injection on bruising and pain. J Clin Nurs. 2008;17(3):378-385. [DOI] [PubMed] [Google Scholar]
- 21. Wittmann A, Kover J, Kralj N, et al. Insulin leakage value in relation to pen needle length and administered dose after subcutaneous injection. Diabetes Technol Ther. 2010;12(8):587-590. [DOI] [PubMed] [Google Scholar]
- 22. Ignaut DA, Fu H. Comparison of insulin diluent leakage postinjection using two different needle lengths and injection volumes in obese patients with type 1 or type 2 diabetes mellitus. J Diabetes Sci Technol. 2012;6(2):389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bailey TS. Analysis and perspective: comparison of insulin diluent leakage post-injection using two different needle lengths and injection volumes in obese patients with type 1 or type 2 diabetes mellitus. J Diabetes Sci Technol. 2012;6(2):394-395. [DOI] [PMC free article] [PubMed] [Google Scholar]


