Abstract
Background:
This study was performed to investigate the efficacy of Glucommander (GM) (Glytec®), a computer-based algorithm versus standard (paper form-based) continuous insulin infusion (CII) in the treatment of patients with diabetic ketoacidosis (DKA).
Methods:
This was a retrospective multicenter study involving 2665 patients with DKA treated with either GM (n = 1750) or standard protocols (n = 915) across 34 institutions in the United States. GM estimates the rate of CII using an insulin sensitivity factor referred to as a “multiplier” that ranges between 0.01 and 0.03. Outcomes of interest were differences in time to resolve DKA (blood glucose [BG] <200 mg/dL and bicarbonate < 18 mmol/L) and number of hypoglycemic events defined as a BG <70 mg/dl.
Results:
Treatment with GM was associated with lower rates of hypoglycemia during the time of the insulin drip (12.9% vs 35%, P = .001), faster time to normalization of blood glucose (9.7 ± 8.9 vs 10.97 ± 10.2 hours, P = .0001) and resolution of metabolic acidosis (13.6 ± 11.8 vs 17.3 ± 19.6 hours, P = .0001), and shorter hospital length of stay (3.2 ± 2.9 vs 4.5 ± 4.8 days, P = .01) compared to standard care. Best treatment outcomes were achieved with an initial multiplier of 0.01 and a glucose target range between 120 and 180 mg/dl.
Conclusion:
The GM algorithm in DKA treatment resulted in lower rates of hypoglycemia and faster DKA resolution over standard paper-based algorithms. Prospective randomized clinical trials comparing the efficacy and cost of computer-based algorithms versus standard CII regimens are warranted.
Keywords: electronic glucose management system, diabetic ketoacidosis, intravenous insulin, Glucommander
DKA is a serious metabolic complication of uncontrolled diabetes mellitus and a condition that invariably needs emergency care or hospitalization. The number of hospital discharges with DKA as first-listed diagnosis in the United States has been progressively rising since 1988 through 2009.1 This poses a huge burden on health care systems by utilization of emergency department services,2 with nearly half a million hospital days per year and an estimated annual direct and indirect cost of 2.4 billion USD.2 In addition, one study demonstrated that 16% of evaluated patients had a return of DKA during their hospital admission, further prolonging and complicating their hospital course, and contributing to increased cost.3
Continuous intravenous insulin infusion (CII) is widely accepted as the standard of care for the treatment of patients with diabetic ketoacidosis (DKA).4-7 A variety of standard or conventional (paper form-based) and computer-based algorithms have been shown to be effective in the management of hyperglycemia in critically ill patients. There have been comparisons of standard algorithms and Glucommander™ (GM) in the medical intensive care unit demonstrating comparable glucose control with fewer incidences of hypoglycemia with GM.8 Typically, regular human insulin is the preferred intravenous insulin however, there have been studies demonstrating the use of analog insulin in DKA therapy.9 There have been smaller retrospective studies in critically ill patients comparing nurse-driven computerized insulin infusion program and conventional paper-based protocol, which demonstrated faster achievement of target glucose and fewer hypoglycemic events.10 However, such data are not available in the management of DKA.
GM is commercially available proprietary glucose management software that is prescriber directed and utilizes evidence-based multivariate algorithms to provide care teams with intravenous and subcutaneous insulin dosing recommendations. The software continuously recalculates dosing and dynamically adjusts to each individual patient’s sensitivities and other clinical variables. The algorithm takes into account the following variable including the patients’ height, weight, blood glucose (BG), hemoglobin A1c values for initial insulin infusion rate, and the rate of change of glucose and carbohydrates consumed if any during the titration phase of the drip.
GM typically implemented and executed on a computer available at the patient’s bedside or nearby nursing station. A clinician orders GM by specifying initial parameters including target glucose and the multiplier or insulin sensitivity factor. The nurse caring for the patient enters the appropriate parameters and the point-of-care glucose. The GM then recommends an insulin infusion rate and a time to check the next BG. At the recommended time, the nurse checks the BG and enters the next glucose in the GM, which results in a recommendation to change or continue the insulin infusion rate and the time to check the next glucose. This process is repeated indefinitely till the software is discontinued based on clinicians discretion.
GM integrates with electronic medical record (EMR) systems and connected devices for streamlined use in inpatient and outpatient settings. In most of our hospitals the EMR used was EPIC®, which allowed for seamless integration of GM into the EPIC. GM can also be utilized as a stand-alone software or integrated into any EMR that allows for a third party application to be incorporated into their software in such a way that demographic, laboratory data and other variable can auto-populate from the hosting EMR into GM.
It is not known, however, if computer-based algorithms are superior to standard protocols in the management of patients with DKA. Furthermore, there are no intravenous protocols that have been specifically validated for the treatment of DKA and guidelines do not allude to using a specific protocol over another. We have previously published the use of GM in the emergency department (ED) setting in the context of DKA where we demonstrated its utility in distinguishing those patient who need admission to inpatient acute care versus those who can be safely discharged home after therapy in the ED.6 We have previously published a comparison of GM and standard basal-bolus insulin therapy in the inpatient setting.4 Comparisons of standard algorithm and GM in delivery of intravenous insulin therapy have demonstrated comparable glucose control with a low incidence of hypoglycemia.5 A study in black urban patients has shown that a protocol-based approach shows no difference in time to correction of hyperglycemia and ketoacidosis but a lower incidence of hypoglycemia compared to non-protocol-based treatment.7
Insulin use causing hypoglycemia or spontaneous hypoglycemia are associated with increased mortality among hospitalized patients.8,11 However, a casual association between hypoglycemia and mortality has not been clearly established in large studies.11 The incidence of hypoglycemia due to intravenous insulin in DKA treatment has not been well studied. We have previously published a study in the ED setting where were no episodes of hypoglycemia less than 40 mg/dl and the rate of hypoglycemia less than 70 mg/dl hypoglycemia (BG < 70 mg/dl) rate of 0.3% of the total glucose values measured.6
While there are general guidelines for the use of insulin infusion in treatment of hyperglycemia12 and specific guidelines by the American Diabetes Association in the management of hyperglycemic crises such as DKA,13 these guidelines do not prescribe well-defined targets as goals for glucose control but provides a spectrum of discretionary targets. The purpose of this study was to compare standard column-based or paper-based algorithm to the intravenous algorithm of GM in the management of insulin dosing as a component in the treatment of DKA. In this study, we also set out to determine the number of hypoglycemic events, the rate of correction of BG, and the best sensitivity factor or multiplier that is associated with a safe decline in BG while preventing hypoglycemia. We evaluated the rate and time to resolution of hyperglycemia (<200 mg/dl) and metabolic acidosis (bicarbonate >18 mmol/l), and frequency of hypoglycemia (<70 mg/dl) utilizing different multipliers ranging from 0.01 to 0.03 and targeting different BG targets between 120 and 180 mg/dL.
Methods
This was a multicenter, retrospective study using deidentified data collected at 34 academic and nonacademic institutions from 2013 to 2015 using the GM for clinical care, which was reviewed and approved by the Wake Forest Institutional Review Board. The version of GM studied in this project was the intravenous GM algorithm. GM software was commercially licensed by Glytec to sites and no modification to the algorithm was permitted or possible given the proprietary nature of the software. We were able to query central electronic databases from sites that were involved which transmitted data to a secure cloud-based system that hosted GM as well. Data sharing agreements existed between entities.
Patients were included if they were older than 18 years of age, admitted to intensive care unit or step-down floors with DKA, and treated with GM insulin delivery system or conventional protocol. All conventional protocols were column-based protocols or paper protocols based on previously validated intravenous protocols such as the Yale protocol14 and the Leuven protocol.15 None of the paper-based protocols were modeled specifically after the GM algorithm. During planned interruptions to EMR for events such as upgrades, sites are provided with ‘downtime’ forms to mirror GM algorithm. However, patients who had such interruptions were not included.
Inclusion criteria were admission diagnosis of DKA based on ICD-9 codes 250.10, 250.11, 250.12, and 250.13. This was corroborated with hyperglycemia was defined as BG >200 mg/dl with metabolic acidosis was defined as bicarbonate < 18 mmol/l and confirmed with an elevated anion gap of >12 mEq/l. Patients had to meet all the above criteria to be included in this study. Hypoglycemia defined as BG < 70 mg/dl. Hypoglycemia was assessed during the active insulin infusion time period and for a period of time when the intravenous insulin orders were active. Hypoglycemic events after the discontinuation of intravenous insulin orders and the cessation of the drip were not included. Hypoglycemia events before the initiation and events that occurred during the hospitalization after the discontinuation of intravenous insulin were not included.
In general, the target BG ranged from 100 mg/dl to 200 mg/dl but site-specific ranges were chosen by individual sites as directed by the order set and clinicians’ discretion based on the clinical picture. The specific targets have been described in the subsequent text. Patients with DKA were treated with continuous intravenous U-100 regular human insulin infusion through infusion pumps at the concentration of 1 unit per ml.
Measures
The goal of this study was to describe the time to resolution and glycemic control on patient receiving GM compared to conventional paper-based protocols with data collected retrospectively as an exploratory project.
The primary aim was to compare the efficacy of GM algorithm and conventional protocols in terms of hours to achieve target BG and hours for correction of bicarbonate level above 18 mmol/l. Time for resolution of DKA was defined as the time taken to achieve BG less than 250 mg/dl and a bicarbonate level of 18 mmol/l or higher. We chose 250 mg/dl as the cut off because BG values would often fluctuate around 200 mg/dl on their way down to target range but remained consistently below 250 mg/dl.
GM uses insulin sensitivity factor as determined by the provider based on the clinical circumstance to determine the initial insulin dose. This is referred to as a “multiplier” and determines the slope of correction of glucose toward the goal blood sugar. The insulin infusion is always according to the formula: Insulin per hour = multiplier × (BG – 60). The “multiplier” is automatically adjusted based on the glucose pattern and response to insulin based on a proprietary algorithm.
The secondary aims were to evaluate the efficacy and safety of different multipliers, ranging from 0.01 to 0.03 targeting different BG levels and median length of stay in the hospital. In analyzing the multiplier, patients treated with GM algorithm were on 0.01, 0.02, or 0.03 as the initial multiplier. For a patient with normal insulin sensitivity, a multiplier of 0.01 was the recommended initial choice. This was automatically adjusted upward by the algorithm based on subsequent glucose values. The target glucose choices that were available to health care providers were 100-140 mg/dl, 120-160 mg/dl, 140-180 mg/dl, and last, 160-180 mg/dl. We determined the time to target, defined as time to achieve target BG for each multiplier at each set target. The sites using conventional or paper-based protocols did not have a wide choice of glucose targets as available in GM but instead had one specific target ranging from 100-200 mg/dl based on the conventional practice at each site.
In addition, we compared frequency of hypoglycemia (BG ≤ 70 mg/dl) and severe hypoglycemia (BG ≤ 40 mg/dl) between patients in the GM group and the conventional therapy group. If there is a rapid decent of glucose approaching 100 mg/dl/hr, the GM recommends thirty minute glucose checks followed by a downward titration of the drip rate. If the glucose level falls below the prescribed threshold, then there is a rapid deescalation of drip rate with drip cessation for glucose below 70 mg/dl.
The discontinuation of insulin drip was based on clinician judgements which include the combination of resolution of hyperglycemia and acidosis with closure of anion gap. This was true for conventional protocols. Due to the integration of EMR with laboratory interfacing, GM is able to provide an alert for high Anion gap cautioning clinicians to prevent premature discontinuation of GM before resolution of DKA. Episodes of hypoglycemia are recognized within 30 min. Based on the GM algorithm, insulin is held 30 min and until the glucose level was >70 mg/dl. Previously published reports have validated the efficacy of the correction of hypoglycemia by the GM. A protocol for treating hypoglycemia has been incorporated into the GM since 1995 using intravenous dextrose based on a formula in the program for correction of hypoglycemia with 50% glucose levels = (100 − BG) × 0.2 g.16
Statistical Methodology
The mean with a measure of variability is reported. A sample preliminary test for the equality of variances indicates that the variances of the groups were significantly different. Therefore, a two- sample t-test was performed that does not assume equal variance. The P value from the t-tests of the observed sample groups determined statistical 95% significance. For all analyses, reported P values are two-sided, and P values < .05 were considered significant. Mountain States Health Alliance Quality Analysis using QI Macros SPC version 2010.06 performed all statistical analyses.
Results
We deidentified data from 2,665 patients admitted to acute care with a diagnosis of DKA treated with either a computer-guided program (GM, n = 1750) or conventional protocols (n = 915). The sites utilizing conventional protocol were discrete from the sites utilizing GM or had transitioned from conventional protocol to using GM exclusively for dosing intravenous insulin. There were no sites using both paper-based protocol and GM contemporaneously. Data are represented as mean ± standard error of mean. The patient characteristics were as follows: the mean baseline BG for the GM group was 598 ± 255 mg/dl versus 425 ± 249 mg/dl for the conventional treatment group. The mean baseline bicarbonate for the GM group was 11 ± 4.5 mmol/l versus 14 ± 4.1 for the conventional treatment group. The mean baseline arterial pH for the GM group was 7.2 ± 2.5 versus 7.2 ± 1.9 for the conventional treatment group with no significant difference.
For the purpose of comparison of baseline demographics and data, we further divided the GM group and the conventional group into mild, moderate, and severe based on previously published diagnostic criteria for DKA.17 This is represented in Table 1.
Table 1.
Baseline Characteristics According to DKA Severity: Comparison of GM and Conventional Treatment Groups.
| Glucommander | Conventional protocol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild | SD | Moderate | SD | Severe | SD | Mild | SD | Moderate | SD | Severe | SD | |
| n Patients | 530 | 555 | 665 | 480 | 267 | 168 | ||||||
| n BG | 28770 | 25044 | 30270 | 19378 | 9679 | 6795 | ||||||
| Age, years | 50 | 17 | 44 | 18 | 40 | 17 | 57 | 19 | 48 | 24 | 31 | 22 |
| Gender | ||||||||||||
| Female, (%) | 51.0% | 49.0% | 54.0% | 54.0% | 53.0% | 53.0% | ||||||
| BMI (kg/m2) | 29.0 | 10.2 | 27.0 | 15.6 | 25.0 | 9.1 | 29.0 | 11.6 | 26.0 | 9.5 | 22.0 | 9.2 |
| Weight (kg) | 84.0 | 30.4 | 80.0 | 45.4 | 72.0 | 26.2 | 83.0 | 29.3 | 73.0 | 28.5 | 60.0 | 27.5 |
| Hemoglobin A1C | 10.5 | 5.2 | 10.7 | 5.3 | 11.2 | 5.3 | 9.0 | 4.9 | 9.1 | 4.9 | 10.7 | 5.7 |
| BG, mg/dl | 567 | 247 | 568 | 235 | 648 | 270 | 383 | 138 | 418 | 177 | 555 | 271 |
| Anion gap, mEq/l | 19.0 | 5.2 | 24.0 | 5.8 | 28.0 | 6.7 | 17.0 | 4.4 | 21.0 | 5.5 | 26.0 | 6.3 |
| Bicarbonate mEq/l | 17 | 1 | 12 | 1 | 6 | 2 | 17 | 1 | 12 | 1 | 7 | 2 |
| Osmolality, mOsm/kg | 306 | 105 | 306 | 100 | 310 | 90 | 300 | 85 | 303 | 104 | 305 | 96 |
| pH | 7.3 | 2.3 | 7.2 | 2.3 | 7.1 | 2.8 | 7.3 | 1.9 | 7.2 | 1.9 | 7.1 | 2.0 |
| Hct, % | 38.8 | 19.3 | 40.2 | 19.9 | 42.3 | 21.8 | 36.9 | 16.5 | 37.8 | 17.5 | 40.2 | 17.3 |
| Glucose (mg/dl) | ||||||||||||
| Min BG | 89 | 38 | 84 | 37 | 80 | 34 | 91 | 40 | 84 | 34 | 83 | 34 |
| Max BG | 594 | 242 | 597 | 226 | 675 | 269 | 412 | 140 | 450 | 206 | 585 | 263 |
| Time to resolution | ||||||||||||
| BG < 250 mg/dl, hours | 9.9 | 8.7 | 8.6 | 6.0 | 7.9 | 4.8 | 12.4 | 13.9 | 9.8 | 9.8 | 8.4 | 5.0 |
| BG < 200 mg/dl, hours | 13.6 | 14.9 | 10.8 | 7.9 | 9.9 | 7.8 | 20.0 | 24.1 | 15.2 | 19.5 | 12.6 | 11.7 |
| HCO3 > 15 mEq/l, hours | 2.0 | 6.2 | 12.8 | 14.8 | 18.0 | 26.8 | 1.9 | 6.8 | 15.7 | 18.0 | 20.6 | 18.0 |
| HCO3 > 18 mEq/l, hours | 15.7 | 22.2 | 19.7 | 20.7 | 28.0 | 33.6 | 25.8 | 31.5 | 29.6 | 34.9 | 30.6 | 24.3 |
| % of patients with hypoglycemia | ||||||||||||
| BG < 70 mg/dl | 11.0% | 14.0% | 13.0% | 33.0% | 36.0% | 39.0% | ||||||
| BG < 40 mg/dl | 0.0% | 0.4% | 0.2% | 6.3% | 7.5% | 6.0% | ||||||
| # of episodes of hypoglycemia | ||||||||||||
| BG < 70 mg/dl | 0.7% | 1.0% | 0.8% | 2.9% | 3.3% | 3.6% | ||||||
| BG < 40 mg/dl | 0.0% | 0.0% | 0.0% | 0.3% | 0.4% | 0.3% | ||||||
| Hospital length of stay | ||||||||||||
| Hospital # days | 7.4 | 14.1 | 5.8 | 9.3 | 4.9 | 5.8 | 8.7 | 13.2 | 8.1 | 9.7 | 5.5 | 6.7 |
| Median LOS | 3.5 | 3.0 | 3.2 | 5.1 | 4.4 | 3.2 | ||||||
The results of the primary outcome between the two groups showed that the time to reach BG less than 250 mg/dl was 9.1 hours for the GM group and 10.9 hours for conventional therapy group. The time for bicarbonate level to reach 18 mmol/L was 13.6 ± 11.8 hours for the GM group and 17.3 ± 19.6 hours for conventional therapy group. There was also a difference in length of hospital stay (LOS) for patients treated with GM than conventional therapy 3.2 ± 2.9 vs 4.5 ± 4.8 days. All three measures were statistically significant with P values < .001. The time to correct hyperglycemia and metabolic acidosis was 9.7 ± 8.9 hours and 19.6 ± 18.7 hours (P < .001) in the GM group and conventional therapy group, respectively.
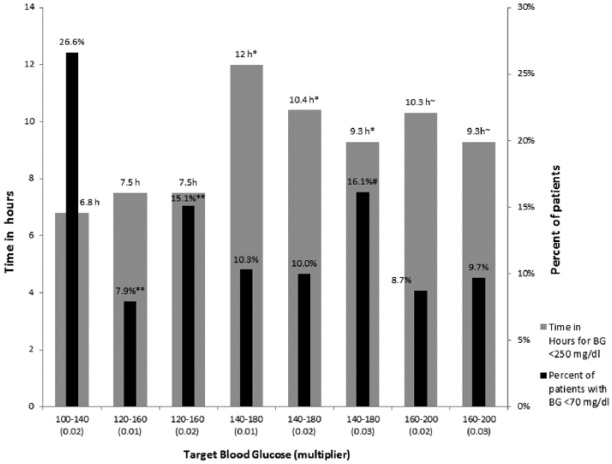
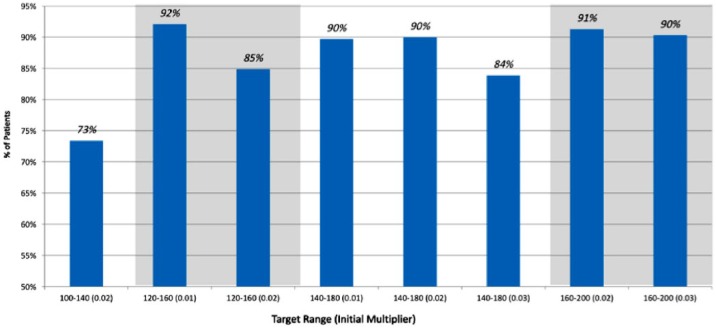
There were 225 (12.9%) of patients with BG under 70 mg/dl in the GM group as compared to 317 (35%) the standard treatment group. The rate of severe hypoglycemia with blood sugar under 40 mg/dl was 8 (0.46%) in the GM group as compared to the 60 (6.6%) in conventional treatment group. In the GM group the best treatment outcomes were achieved with an initial multiplier of 0.01 and a glucose target range between 120 and 180 mg/dl, with only 7.9% of patients experiencing hypoglycemia and a time to target (TTT) of 7.5 hours. Figure 1 represents the various target glucose ranges chose in GM and the time taken for the BG to reach below 250 mg/dl. Figure 2 represents the percentage of patients who have resolution of DKA without hypoglycemia. The highest percentage of patient achieving resolution without significant hypoglycemia was in the group of patients who had an initial multiplier of 0.01 and target glucose of 120-160 mg/dl. The group of patients whose initial multiplier was 0.02 and a target blood sugar of 100-140 mg/dl demonstrated the highest percentage of hypoglycemia with 72% of patient achieving goal without hypoglycemia. In comparison, standard protocols had 65% of patients achieve goal without hypoglycemia.
Figure 1.
This chart demonstrates the various multiplier groups in GM group with percentage of patients with BG < 70 mg/dl, hours taken for blood sugar to reach below 250 mg/dl. Comparing similar target ranges we were able to show a statistical significance. *, #, **P < .05.
Figure 2.
Proportion of patients with DKA resolution without hypoglycemia across various multipliers and selected glucose target ranges.
There were no differences in the time to resolution of hyperglycemia or in the number of hypoglycemic events using a multiplier of 0.01 or 0.02 when BG target was between 120 and 180 mg/dl. Initial multiplier of 0.03 or a lower BG target of 100-140 mg/dl resulted in higher rates of hypoglycemia (16.1% and 26.6%), respectively.
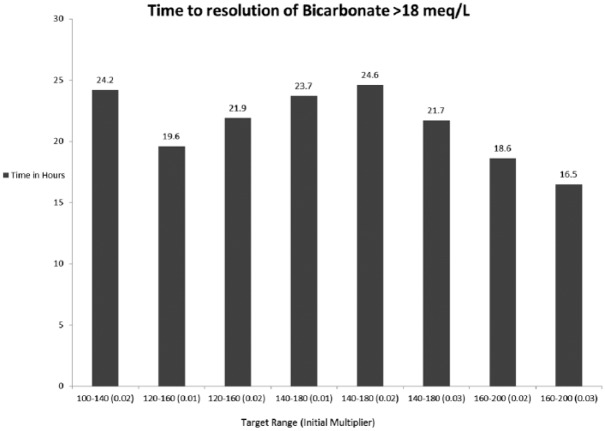
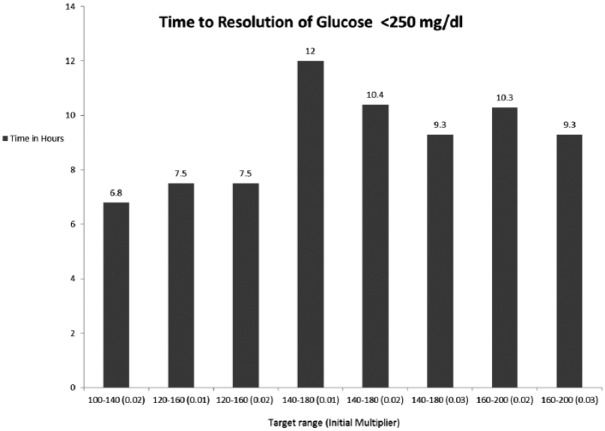
Within the GM treated group, we analyzed the time taken for BG to reach target and the time to bicarbonate to stabilize above 18 mmol/L between the various glucose targets and the multipliers. Figures 3 and 4 represent the data for bicarbonate and glucose, respectively. Figure 3 demonstrates the time taken for bicarbonate normalization. The group that was assigned a target of 160-200 mg/dl with a multiplier of 0.03 was the most rapid in reaching bicarbonate of above 18 mmol/l (16.5 hours) and the group that was assigned a target of 140-180 mg/dl and multiplier 0.02 reached the target in 24.6 hours. Figure 4 shows that the group that was assigned a target of 100-140 mg/dl with a multiplier of 0.01 was most rapid in reaching the BG goal (6.8 hours) and the group that was assigned a target of 140-180 mg/dl and multiplier 0.02 reached the target in 12 hours.
Figure 3.
This figure demonstrates the time taken in hours for bicarbonate to reach the set target and the time taken for bicarbonate level to stabilize above 18 meq/L comparing the various target glucose ranges as set in the GM algorithm.
Figure 4.
This figure demonstrates the time taken in hours for glucose to reach the set target and the time taken for glucose level to drop below 250 mg/dl among the various target blood sugars as set in the GM algorithm.
Discussion
This study is a retrospective comparison of a computer decision support system (Glucommander) for insulin dosing, versus conventional intravenous insulin protocol in DKA management. It demonstrates comparable efficacy in lowering glucose along with a faster normalization of bicarbonate levels. The glucose lowering seen with GM was associated with a lower hypoglycemia rate and very little severe hypoglycemia. The initial BG was significantly higher in the GM group but the time for resolution of DKA was faster with GM.
Since the implementation of electronic health records across a majority of hospitals and the use of computerized DKA order sets and protocols, there is improved compliance18,19 with the 2009 American Diabetes Association guidelines on management of DKA.17 There is one study evaluating the safety and efficacy of GM but this was a pediatric study. It did not show significant adverse events in comparison to manually titrated insulin infusion.20 Our study is one of the few studies compares a computerized decision support system with conventional protocols in adults admitted to the hospital.
The caveats of this study are that this is a retrospective analysis and suffers the drawback of not having a randomization or a controlled treatment. While both comparison groups were treated arms, the GM group received a very standardized therapy based on validated algorithms for intravenous insulin16 as compared to conventional arm of the study, which was not a single treatment protocol and a nonuniform treatment. The focus of this study was limited to insulin therapy and their effects on DKA resolution. It is however well understood that fluid resuscitation, electrolyte therapy and other supportive care have an impact on DKA resolution. These were however not variables that were adjusted in this study.
The optimal treatment outcomes as defined by the safest means to reach target BG without significant hypoglycemia and normalization of bicarbonate levels were achieved with an initial multiplier of 0.01 and a glucose target range of 120-180 mg/dl; a conservative initial multiplier (0.01) was safe and effective in treating patients with DKA. On the other hand, a glucose target range of 100-140 mg/dl and multiplier of 0.02 were associated with the highest hypoglycemia rate (26.6%). A glucose target range of 140-180 mg/dl with a multiplier choice of 0.01 took the longest to achieve target range. These are intuitive observations and were as expected; a tighter target range has been shown to be associated increased risk of hypoglycemia and a more relaxed target range took a longer time for BG normalization. While this was not a controlled study and the types of fluid therapy in DKA resuscitation were not the same across all protocols, we compared well-validated protocols to the GM algorithm.
Conclusion
We conclude that this exploratory project comparing GM algorithm versus conventional column-based or paper-based protocols of intravenous insulin administration in the treatment of DKA indicates safety in terms of lower hypoglycemia. This also demonstrates glucose and bicarbonate normalization was faster and was associated with a shorter length of stay. This may have ramifications for hospital systems and health care organizations in cost saving benefit in addition to promoting patient safety with use of GM as a means to titrate insulin for treatment of DKA. The validity of our finding will have to be demonstrated by prospective trials.
Footnotes
Abbreviations: BG, blood glucose; BMI, body mass index; CII, continuous insulin infusion; DKA, diabetic ketoacidosis; ED, emergency department; EMR, electronic medical record; GM, Glucommander; HCO3, bicarbonate; Hct, hematocrit; LOS, length of stay; Max BG, maximum glucose; Min BG, minimum glucose; SD; standard deviation; TTT, time to target.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors Jagdeesh Ullal, Joseph A. Aloi, David Reyes-Umpierrez, Francisco J. Pasquel, Marina Rabinovich, Guillermo E. Umpierrez declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.Author Raymie McFarland is an employee of Glytec, Greenville, SC
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 2. Ginde AA, Pelletier AJ, Camargo CA., Jr National study of U.S. emergency department visits with diabetic ketoacidosis, 1993-2003. Diabetes Care. 2006;29(9):2117-2119. [DOI] [PubMed] [Google Scholar]
- 3. Jun AH, Rabinovich M, Johnson S. Evaluation of the diabetic ketoacidosis/hyperosmolar hyperglycemic state protocol at a large academic institution. Crit Care Med. 2015; 43(12):235.25514711 [Google Scholar]
- 4. Aloi J, Bode BW, Ullal J, et al. Comparison of an electronic glycemic management system versus provider-managed subcutaneous basal bolus insulin therapy in the hospital setting. J Diabetes Sci Technol. 2017;11(1):12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newton CA, Smiley D, Bode BW, et al. A comparison study of continuous insulin infusion protocols in the medical intensive care unit: computer-guided vs. standard column-based algorithms. J Hosp Med. 2010;5(8):432-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ullal J, McFarland R, Bachand M, Aloi J. Use of a computer-based insulin infusion algorithm to treat diabetic ketoacidosis in the emergency department. Diabetes Technol Ther. 2016;18(2):100-103. [DOI] [PubMed] [Google Scholar]
- 7. Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med. 1997;157(6):669-675. [PubMed] [Google Scholar]
- 8. Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85(3):217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umpierrez GE, Jones S, Smiley D, et al. Insulin analogs versus human insulin in the treatment of patients with diabetic ketoacidosis: a randomized controlled trial. Diabetes Care. 2009;32(7):1164-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouw JW, Campbell N, Hull MA, Juneja R, Guzman O, Overholser BR. A retrospective cohort study of a nurse-driven computerized insulin infusion program versus a paper-based protocol in critically ill patients. Diabetes Technol Ther. 2012;14(2):125-130. [DOI] [PubMed] [Google Scholar]
- 11. Kagansky N, Levy S, Rimon E, et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med. 2003;163(15):1825-1829. [DOI] [PubMed] [Google Scholar]
- 12. Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251-3276. [DOI] [PubMed] [Google Scholar]
- 13. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153. [DOI] [PubMed] [Google Scholar]
- 14. Shetty S, Inzucchi SE, Goldberg PA, Cooper D, Siegel MD, Honiden S. Adapting to the new consensus guidelines for managing hyperglycemia during critical illness: the updated Yale insulin infusion protocol. Endocr Pract. 2012;18(3):363-370. [DOI] [PubMed] [Google Scholar]
- 15. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359-1367. [DOI] [PubMed] [Google Scholar]
- 16. Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28(10):2418-2423. [DOI] [PubMed] [Google Scholar]
- 17. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Devi R, Zohra T, Howard BS, Braithwaite SS. Target attainment through algorithm design during intravenous insulin infusion. Diabetes Technol Ther. 2014;16(4):208-218. [DOI] [PubMed] [Google Scholar]
- 19. Laliberte B, Yeung SYA, Gonzales JP. Impact of diabetic ketoacidosis management in the medical intensive care unit after order set implementation. Int J Pharm Pract. 2017;25(3):238-243. [DOI] [PubMed] [Google Scholar]
- 20. Fort A, Narsinghani U, Bowyer F. Evaluating the safety and efficacy of Glucommander, a computer-based insulin infusion method, in management of diabetic ketoacidosis in children, and comparing its clinical performance with manually titrated insulin infusion. J Pediatr Endocrinol Metab. 2009;22(2):119-125. [DOI] [PubMed] [Google Scholar]