Abstract
Background
We conducted a phase II trial to evaluate the efficacy of dasatinib, a multitargeted tyrosine kinase inhibitor, for adults with recurrent glioblastoma (GBM).
Methods
Eligibility requirements were Karnofsky performance status ≥60%; no concurrent hepatic enzyme-inducing anticonvulsants; prior treatment with surgery, radiotherapy, and temozolomide exclusively; and activation or overexpression of ≥2 putative dasatinib targets in GBM (ie, SRC, c-KIT, EPHA2, and PDGFR). Using a 2-stage design, 77 eligible participants (27 in stage 1, if favorable, and then 50 in stage 2) were needed to detect an absolute improvement in the proportion of patients either alive and progression-free patients at 6 months (6mPFS) or responding (any duration) from a historical 11% to 25%.
Results
A high rate of ineligibility (27%) to stage 1 precluded a powered assessment of efficacy, but there was also infrequent treatment-related toxicity at 100 mg twice daily. Therefore, the study was redesigned to allow intrapatient escalation by 50 mg daily every cycle as tolerated (stage 1B) before determining whether to proceed to stage 2. Escalation was tolerable in 10 of 17 (59%) participants evaluable for that endpoint; however, among all eligible patients (stages 1 and 1B, n = 50), there were no radiographic responses, median overall survival was 7.9 months, median PFS was 1.7 months, and the 6mPFS rate was 6%. The clinical benefit was insufficient to correlate tested biomarkers with efficacy. The trial was closed without proceeding to stage 2.
Conclusions
Intraparticipant dose escalation was feasible, but dasatinib was ineffective in recurrent GBM. Clinical trials.gov identified. NCT00423735 (available at http://clinicaltrials.gov/ct2/show/NCT00423735).
Keywords: chemotherapy, dasatinib, glioblastoma, phase II, tyrosine kinase inhibitor
Glioblastoma (GBM) has a poor prognosis after recurrence, and median overall survival (OS) is 4–6 months. Cytotoxic chemotherapy such as carmustine controls growth for ≥6 months in only 10%–20% of patients.1 Small molecule inhibitors are also generally ineffective.2–4 Bevacizumab, either alone or with irinotecan, is associated with 6-month progression-free survival (6mPFS) and response rates of ∼40%.5,6 However, there are concerns that bevacizumab may induce more invasive and treatment-refractory tumor biology.7,8 Recent results have also demonstrated that bevacizumab does not prolong OS when added to radiotherapy and temozolomide as part of first-line therapy.9,10 Therefore, an opportunity remains for testing novel agents at recurrence in the bevacizumab-naïve setting.
GBM is a molecularly complex disease. Potential explanations for lack of efficacy include inadequate target inhibition or improper patient selection. In addition, treatment failure with single agents that target one signaling abnormality may result from the uninhibited actions of other “bypass” molecular abnormalities or from the need to target more than one oncogenic signal simultaneously. Therefore, treatment with a single agent that could target several key signaling pathways, especially in the appropriate patient population, represents an attractive therapeutic approach. Dasatinib (Sprycel, Bristol-Myers Squibb, previously BMS-354825) is such an agent.
Dasatinib has inhibitory effects on at least 5 kinase families involved in human malignancies: SRC, KIT, PDGFR, and EPHA2, and BCR-ABL fusion.11–13 It is also FDA approved for BCR-ABL mutant hematological malignancies. Although BCR-ABL is not implicated in GBM, the other 4 targets may contribute to GBM progression or therapeutic resistance. For example, the majority of GBMs exhibit amplification/overexpression of SRC (∼60%), PDGFR (∼75%), and ephrin (∼90%); ∼50% exhibit c-KIT amplification.14–17 Mouse modeling by transgenic and somatic-cell gene transfer methods have further confirmed the importance of both SRC and PDGFR signaling in gliomagenesis as reviewed elsewhere.18 In addition, although imatinib is ineffective in GBM,19,20 dasatinib inhibits PDGFR more potently than imatinib.12,21 Preclinical data also suggest that dasatinib may be effective in glioma.22 Therefore, we hypothesized that dasatinib might be more effective for recurrent GBMs than other receptor tyrosine kinase inhibitors, including imatinib, because of its broader spectrum of molecular targets and increased potency against key targets.
We conducted a single-arm phase II trial of dasatinib as monotherapy, initially at 100 mg twice daily, for patients with bevacizumab-naïve recurrent GBM harboring overexpression/activity of SRC, PDGFR, EPHA2, and/or c-KIT, following radiotherapy and temozolomide.23
Materials and Methods
Eligibility Criteria
Major eligibility criteria included GBM (or subtype) histology confirmed centrally (by K.D.A.); prior treatment with surgery, radiotherapy, and temozolomide only; worsening disease by imaging or histological confirmation; Karnofsky performance status ≥ 60%24; age ≥18 years; and normal end-organ function (hepatic, renal, bone marrow; Supplementary material, Table S1). Concurrent use of H2 blockers or proton pump inhibitors was prohibited because of the potential effects on stomach pH and drug absorption. Patients taking antiplatelet agents, anticoagulants, or nonsteroidal anti-inflammatory drugs were excluded because of concerns about increased bleeding risk. Use of hepatic CYP450 enzyme-inducing antiepileptic drugs (EIAEDs) was also prohibited for ≥ 2 weeks before registration because of potential effects on dasatinib metabolism. Participants of child-bearing potential agreed to use contraception, and women were neither pregnant nor nursing mothers. Availability and testing of pretreatment tumor tissue was also required (described below). All participants (or appropriate representatives) signed a study-specific informed consent form approved by the local Institutional Review Board (or equivalent body) at the participating institution. The protocol was also approved by the American College of Radiology Institutional Review Board. This protocol is registered with clinicaltrials.gov (identifier NCT00423735).
Treatment
Dasatinib was initiated at 100 mg twice daily until disease progression or intolerable toxicity. A cycle was defined as 28 days, although treatment was continuous. Baseline evaluations included physical examination, blood chemistries, complete blood count, electrocardiogram, and brain imaging with contrast-enhanced MRI (or CT for patients unable to tolerate MRI). Evaluations during treatment included physical examinations every other week, complete blood counts and serum chemistries weekly, electrocardiograms as clinically indicated every cycle, and follow-up brain imaging every other cycle. The primary endpoint was a hybrid of 6mPFS rate and radiographic response rate (either 6mPFS or response of any duration). Objective responses were assessed by the Macdonald criteria.25 Partial response was defined as ≥ 50% decrease in size of enhancing tumor on consecutive brain MRI (or CT for those unable to tolerate MRI) scans at least 1 month apart, stable or reduced corticosteroid dosing, and no neurological deterioration. Complete response required total disappearance of all enhancing tumor on consecutive MRI (or CT) scans at least 1 month apart, discontinuation of corticosteroids, and no neurological deterioration. Progressive disease was defined as ≥25% increase in the size of enhancing tumor or any new tumor; or clinical progression not attributable to another cause. Other responses were classified as stable (eg, tumors between 50% smaller and 25% larger).
Toxicity was initially assessed by the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0. For agent-related (possibly, probably, or definitely) intolerable or severe toxicities, dose reductions were permitted to a minimum of 70 mg once daily. Dasatinib was supplied by the Pharmaceutical Management Branch of the NCI under a collaborative agreement with Bristol-Myers Squibb. Chemotherapy reviews were performed on all cases (A.B.L.).
Statistical Design
The trial was initially conceived as a single-arm phase 2 trial using a 2-stage design26 with a combined 6mPFS and response (either was sufficient to reduce delay in proceeding to stage 2 if responses were observed in stage 1) rate of 25% considered promising and requiring 77 eligible patients to achieve 95% power for detecting an increase over the estimated historical combined 6mPFS and response rate of 11% associated with ineffective therapy. Kaplan-Meier methodology was used to estimate median PFS and OS.27 Stage 1 planned to accrue 27 eligible patients. If ≥3 of 27 eligible patients in stage 1 achieved either 6mPFS or a radiographic response, then stage 2 would accrue 50 additional eligible participants.
Tissue Analyses
Among the multiple targets inhibited by dasatinib, there are at least 4 known targets of major importance in GBM biology: SRC, PDGFR-alpha, EPHA2, and KIT. We hypothesized that absence of expression or activity of these targets in tumor tissue would reduce the likelihood of benefit. Therefore, expression/activation of at least 2 of these potential targets was required for eligibility in an attempt to exclude those participants least likely to benefit. Using commercially available antibodies (eg, anti-PDGFR and antiphospho-PDGFR), immunohistochemistry (IHC) was performed and immunostaining was scored on a 4-point scale (0–3) analogous to that developed by others.28 Conditions for IHC of paraffin-embedded sections were applied as described previously.29 Antibodies and dilutions were as follows: Kit (Lab Vision # RB- 9038-R7, 1:500); EphA2 (Santa Cruz # sc 924, 1:200); p-Src (Tyr527, Cell Signaling # 2105, 1:100); and PDGFR-apha (Cell Signaling # 3164, 1:200). Tumors were separated into upper and lower halves based on target expression with the lower half having no or mild expression (ie, score 0–1) and the upper half having strong expression (ie, score 2–3). For each target, an IHC staining score of 2–3 was considered positive. Overall, staining was dichotomized as positive and negative based on an overall view of the tumor tissue relative to controls and established staining patterns specific to each protein. A score of positive required, at a minimum, focal robust and clear positivity of a substantial proportion of the tumor available for study. If the pattern was restricted to weak and diffuse staining only, this was not considered positive for this study. Appropriate positive and negative controls were included in each batch of immunohistochemical staining.
Pharmacokinetic Analyses
Blood samples for dasatinib pharmacokinetics (stage 1B only, described below) were collected in EDTA-anticoagulated vacutainers on cycle 1 day 1, cycle 3 day 1, and cycle 5 day 1. Samples were taken before, and 1, 2, 4, and 6–8 hours after the morning dose of dasatinib. Plasma was prepared by centrifugation and immediately frozen at −20°C. Plasma concentrations of dasatinib were quantitated with a validated LC-MS/MS assay as previously described.30
Plasma pharmacokinetic parameters, including area under the concentration versus time curve (AUC), of dasatinib were extracted from the data by noncompartmental methods with PK Solutions 2.0 (Summit Research Services).
Results
Stage 1
There were 21 patients (10 men, 11 women) who met both the clinical and molecular eligibility criteria. Median age was 51 years (range, 33y–81y, Table 1). Molecular analyses revealed that among the eligible patients, 8 (38%) had tumors that harbored 2 putative dasatinib targets, 10 (48%) had 3 targets, and 3 (14%) had all 4 targets (Table 2). However, among 29 participants registered, 8 were ineligible because of exclusionary concurrent medications, prior therapy, or laboratory results. Therefore, underaccrual of eligible patients (21 rather than the 27 planned) precluded the preplanned, appropriately powered assessment of efficacy. Moreover, toxicity was also much milder than anticipated with neither pleural effusions (reported as a concerning agent-related toxicity in other cancers) nor grade 4–5 agent-related toxicities (Table 3 and Supplementary material, Table S2). This led to the concern that participants were underdosed. Therefore, the protocol was amended to allow intrapatient dose escalation through stage 1B rather than reopening stage 1 to complete accrual of 27 eligible patients using the same dosing schedule that was potentially inadequate and before opening stage 2 with 50 additional participants with insufficient evidence of efficacy. In this design, participants would escalate dosing by 50 mg per day per cycle up to a maximum of 400 mg total per day, absent intolerable toxicity. Therefore, cycle 1 consisted of 100 mg twice daily; cycle 2 was 100 mg in the morning and 150 mg at night, cycle 3 was 150 mg twice daily; etc. Pharmacokinetic analyses were conducted. Accrual would continue to stage 2 (with 50 additional participants) if both escalation could be achieved safely (defined as a majority experiencing no dose-limiting toxicity during escalation) and if ≥ 3 of 27 eligible participants achieved 6mPFS or radiographical response. Because escalation would first occur during cycle 2, participants considered evaluable for escalation were those who completed ≥ 2 cycles without progression (or death) or those who discontinued after ≤ 2 cycles because of toxicity.
Table 1.
Pretreatment characteristics
| Stage 1 (n = 21) | Stage 1B (n = 29) | |
|---|---|---|
| Age (years) | ||
| Median | 51 | 54 |
| Min–Max | 33–81 | 26–75 |
| <50 | 9 (43%) | 8 (28%) |
| ≥50 | 12 (57%) | 21 (74%) |
| Sex | ||
| Male | 10 (48%) | 17 (59%) |
| Female | 11 (52%) | 12 (41%) |
| Race | ||
| Asian | 0 (0%) | 1 (3%) |
| Black or African American | 1 (5%) | 2 (7%) |
| White | 20 (95%) | 26 (90%) |
| Ethnicity | ||
| Hispanic or Latino | 3 (14%) | 1 (3%) |
| Not Hispanic or Latino | 18 (86%) | 23 (79%) |
| Not reported | 0 (0%) | 5 (17%) |
| Karnofsky performance status | ||
| 60%–80% | 15 (71%) | 11 (38%) |
| 90%–100% | 6 (29%) | 18 (62%) |
| Neurological symptoms | ||
| None | 3 (14%) | 9 (31%) |
| Minor | 9 (43%) | 14 (48%) |
| Moderate but fully active | 6 (29%) | 1 (3%) |
| Moderate but required assistance | 3 (14%) | 5 (17%) |
| Initial extent of resection | ||
| Biopsy | 2 (10%) | 1 (3%) |
| Subtotal resection | 10 (48%) | 9 (31%) |
| Gross total resection | 9 (43%) | 18 (62%) |
| Other | 0 (0%) | 1 (3%) |
| Additional Surgery | ||
| None | 15 (71%) | 22 (76%) |
| Subtotal resection | 2 (10%) | 3 (10%) |
| Gross total resection | 4 (19%) | 4 (14%) |
| Corticosteroids | ||
| No | 5 (24%) | 15 (52%) |
| Yes | 16 (76%) | 14 (48%) |
| Anticonvulsants (non-enzyme inducing) | ||
| No | 9 (43%) | 8 (28%) |
| Yes | 12 (57%) | 21 (72%) |
Table 2.
Pretreatment molecular analysis among eligible patients
| Stage 1 (n = 21) | Stage1B (n = 29) | |
|---|---|---|
| p-SRC | ||
| No staining - negative | 12 (57%) | 8 (28%) |
| Strongly positive | 9 (43%) | 21 (72%) |
| PDGFR | ||
| No staining - negative | 10 (48%) | 14 (48%) |
| Mild/moderate - positive | 1 (5%) | 0 (0%) |
| Strongly positive | 10 (48%) | 15 (52%) |
| EPHA2 | ||
| No staining - negative | 2 (10%) | 5 (17%) |
| Strongly positive | 19 (91%) | 24 (83%) |
| c-KIT | ||
| No staining - negative | 2 (10%) | 7 (24%) |
| Strongly positive | 19 (91%) | 22 (76%) |
| Number of positive molecular markers | ||
| 2 | 8 (38%) | 10 (35%) |
| 3 | 10 (48%) | 14 (48%) |
| 4 | 3 (14%) | 5 (17%) |
Table 3.
Summary of worst dasatinib-related adverse event per participant
| Adverse Event | CTCAE Grade | Stage 1 (n = 21) | Stage 1B (n = 29) |
|---|---|---|---|
| Worst nonhematological | 1 | 4 (19%) | 6 (21%) |
| 2 | 8 (38%) | 12 (41%) | |
| 3 | 8 (38%) | 5 (17%) | |
| 4 | 0 (0%) | 1 (3%) | |
| 5 | 0 (0%) | 0 (0%) | |
| Worst overall | 1 | 0 (0%) | 5 (17%) |
| 2 | 8 (38%) | 11 (38%) | |
| 3 | 12 (57%) | 8 (28%) | |
| 4 | 0 (0%) | 1 (3%) | |
| 5 | 0 (0%) | 0 (0%) |
Includes adverse events in which relationship to protocol treatment is missing.
Stage 1B
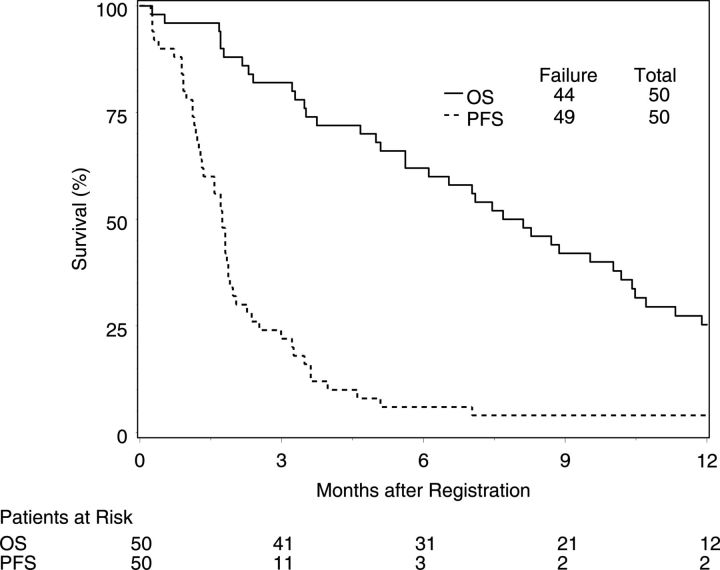
Twenty-nine eligible participants were accrued. Pretreatment characteristics were similar to those in stage 1 (Table 1). Intrapatient dose escalation was feasible: 10 (59%) participants escalated among 17 evaluable for that endpoint. Toxicity was also limited, although toxicities were more common than in stage 1 (Table 3 and Supplementary material, Table S2). The highest dose achieved was 350 mg per day (150 mg in the morning and 200 mg evening). However, efficacy was limited (Fig. 1, Table 5). Only 2 participants achieved 6mPFS, and there were no partial or complete responses. Therefore, accrual was terminated without proceeding to Stage 2.
Fig. 1.
Kaplan-Meier survival curves of all eligible patients (stage 1 + 1B combined). OS, overall survival; PFS, progression-free survival.
Table 5.
Survival
| Stage 1 | Stage 1B | Overall | |
|---|---|---|---|
| Median PFS (95% CI) | 1.7 months (1.0–2.0) | 1.8 months (1.2–2.0) | 1.7 months (1.3–1.9) |
| Median OS (95% CI) | 6.5 months (3.5–9.5) | 8.9 months (5.0–11.3) | 7.9 months (5.6–10.2) |
| 6mPFS rate (95%) CI) | 4.8% (0–14.7) | 6.9% (0–16.7) | 6.0% (0–12.8) |
Abbreviations: OS, overall survival; PFS, progression-free survival; 6mPFS, 6-month progression-free survival; CV%, coefficient of variation.
All 29 eligible participants in Stage 1B were studied for pharmacokinetics and had sufficient data to estimate all pharmacokinetic parameters on cycle 1day 1; parameters of 4 participants could be estimated on cycle 3 day 1, and one participant could be estimated on cycle 5 day 1 (Supplementary material, Fig. S1). The portion of the AUC extrapolated beyond the last time point was 17% on average (range, 6%–42%). Pharmacokinetic parameters on cycle 1 day 1 are presented in Table 4. The low number of participants with repeated pharmacokinetic sampling precluded a formal comparison of pharmacokinetic behavior over time.
Table 4.
Plasma dasatinib pharmacokinetics on cycle 1 day 1 after 100 mg of oral dasatinib
| PK Parameter | Unit | Mean (CV%) | Median | Geometric Mean | Published |
|---|---|---|---|---|---|
| Geometric Mean (CV%)13 | |||||
| Cmax | ng/mL | 151 (74) | 144 | 120 | 56 (118) |
| Tmax | h | 1.5 (63) | 1.0 | 1.3 | 1.5 |
| t1/2 | h | 2.2 (42) | 1.7 | 2.0 | 4.3 (40) |
| AUC0-inf | ng·h/mL | 458 (53) | 393 | 391 | 218 (102) |
| Cl/F | L/h | 318 (80) | 255 | 256 | 667 (81) |
| Vd/F | L | 1030 (106) | 655 | 753 | 4224 (84) |
Abbreviation: CV%, coefficient of variation.
Combined Results
Molecular analyses were performed on 94 potentially eligible patients. Immunostaining demonstrated that 2 (2%) participants harbored none of the putative dasatinib targets, 10 (11%) harbored 1, 22 (23%) harbored 2, 45 (48%) harbored 3 (including one not able to be tested for c-KIT), and 15 (16%) harbored all 4. Therefore almost all (83 participants, 88%) met the molecular criteria.
Best response among the 50 eligible participants (combined stages 1 and 1B) was stable disease in 12 (24%) and progression in 36 (72%). There were no responses. Median OS (Table 5, Fig. 1) was 7.9 months (95% CI, 5.6–10.2 months), median PFS was 1.7 months (95% CI, 1.3–1.9 months), and the 6mPFS rate was 6.0% (95%, 0%–12.8%).
Discussion
Dasatinib failed to demonstrate efficacy as monotherapy for recurrent GBM despite attempts to enrich the population and increase the dose. Two phase I trials tested dasatinib in combination with lomustine (which was excessively toxic)31 or erlotinib (well tolerated),32 and neither demonstrated any therapeutic benefit. A retrospective study showed limited toxicity but no activity when dasatinib was combined with bevacizumab following bevacizumab failure. The Alliance for Clinical Trials in Oncology recently completed accrual to a trial (NCCTG N0877) adding dasatinib to radiotherapy and temozolomide for newly diagnosed GBM, and results are pending.
It is possible that we observed both limited efficacy and toxicity because of inadequate dosing despite the relatively high starting dose of 100 mg twice daily (FDA approved dose is 100–140 mg once daily). However, pharmacokinetic results do not support this conclusion. For example, the dasatinib AUC and Cmax values observed were ∼400 ng·h/mL and 120 ng/mL, respectively, which is higher than published data from other studies.13 Dasatinib exposure is known to be quite variable within and between patients, with coefficients of variation of up to 100% for both AUC and Cmax.13,33 It is therefore difficult to assess the pharmacokinetic effects observed in this study in more than a semiquantitative fashion. The Cmax was approximately triple that of published levels (Table 4), yet the AUC was only double because the half-life was also shorter (2.0 vs 4.3 h geometric mean) than prior reports. Dasatinib discontinuation or dose reduction because of disease progression or toxicity precluded collection of sufficient samples following dose escalation above the starting dose to make meaningful conclusions about the effects on AUC or Cmax. Despite the high Cmax and AUC, it is possible that toxicities were masked by concurrent use of corticosteroids, which is more common in patients with gliomas (Table 1) than other cancers. This trial did not incorporate a surgical arm through which participants received treatment before tumor resection. Therefore, it is possible that tumor penetration into brain was insufficient for antineoplastic effect despite increased daily dosing. Preclinical data suggested that p-glycoprotein and related molecules limit accumulation of dasatinib into the brain, and brain tumors, through active efflux.34,35
We required activation or overexpression of 2, 3, or 4 of 4 putative dasatinib targets in an attempt to enrich the population for those most likely to benefit. Based on literature estimates of expression of each marker, we hypothesized that only 50% of participants who were clinically eligible would also meet this molecular eligibility criterion. However, 2, 3, or 4 of 4 putative dasatinib targets were detected in nearly all (88%) tumors. Therefore, it was not an effective strategy to preselect enrollment. A more effective approach may have been to require expression of all 4 of 4 targets in archival tissue. It is also possible that restricting accrual to cases with only a single, presumably driver target with significant signaling activity would have proven more effective. Finally, the molecular profile of dasatinib targets in archival tumor resected at GBM diagnosis may differ from the profile at disease recurrence. Absence of any responses precluded correlation with the tumor molecular profile, although there was no suggestion of correlation between the number of positive markers observed in those with stable disease versus those with progressive disease (Supplementary material, Table S3).
It is also possible, if not likely, that wild-type target expression (regardless of number or level) may be insufficient for tumor response. For example, EGFR amplification does not correlate with response to EGFR tyrosine kinase inhibitors.36 Instead, response to dasatinib in GBM may require as yet unidentified driver mutations in dasatinib targets, analogous to BCR-ABL fusion in leukemia or kinase mutations in other cancers as reviewed elsewhere.37 For example, mutations in discoidin domain receptor-2 (DDR2)38,39 and BRAF40 were recently reported to predict response to dasatinib in lung cancer but were unknown when this trial accrued. A phase 2 trial is currently in progress (NCT01514864) for patients with non-small cell lung cancer harboring these specific mutations. If any future glioma studies of dasatinib are conducted, it would be prudent to consider prescreening for DDR2 or BRAF mutations in addition to increased daily or intermittent dosing41 to increase brain penetration.
Despite these limitations, we were able to escalate the dasatinib dose and perform centralized molecular pre-screening in all patients in real time without delaying registration in a multicenter cooperative group setting.
Funding
This projected was supported by Radiation Therapy Oncology Group (RTOG) grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI) and Bristol-Myers Squibb. Pharmacokinetic analyses were conducted using the University of Pittsburgh Cancer Institute Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and were supported in part by Bristol-Myers Squibb and award P30-CA47904. This manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI.
Supplementary Material
Acknowledgments
Lewis Strauss, MD, and Naoko Takebe, MD, PhD, provided extensive and thoughtful input during study design, conduct, and publication.
Conflict of interest statement. A.B.L.: consultant or advisory board member for (last 12 months): Foundation Medicine, Genentech, Midatech, Celgene, Novartis Heron; current research support from Aeterna Zentaris, Pfizer, Amgen, Genentech, Merck, Novartis, Bayer, AbbVie, GlaxoSmithKline, Stemline, Northwest Biotherapeutics, Plexxicon, Millenium, Novocure, Celldex, Agenus, Karyopharm, and Boehringer Ingelheim. M.R.G.: advisory board member for Genentech, Novartis, Merck, Abbvie, EMD Serono; honoraria from Merck, Genentech, Roche; research support from Genentech, Merck, and Glaxo Smith Kline. JHB: research support from Bristol-Myers Squibb and Novartis. MPM: consultant for Abbott, Bristol-Myers Squibb, Celldex, Novelos, Novocure, Phillips, Roche; stock options from Accuray, Pharmacyclics; board of directors for Pharmacyclics; speaker for Institute for Medical Education, Research To Practice, Serono Foundation; research funding from Novocure.
References
- 1. Brandes AA, Tosoni A, Amista P, et al. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology. 2004;63(7):1281–1284. [DOI] [PubMed] [Google Scholar]
- 2. Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. [DOI] [PubMed] [Google Scholar]
- 4. Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 6. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 10. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunter T. Oncoprotein networks. Cell. 1997;88(3):333–346. [DOI] [PubMed] [Google Scholar]
- 12. Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–6661. [DOI] [PubMed] [Google Scholar]
- 13. Demetri GD, Lo Russo P, MacPherson IR, et al. Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res. 2009;15(19):6232–6240. [DOI] [PubMed] [Google Scholar]
- 14. Takenaka N, Mikoshiba K, Takamatsu K, et al. Immunohistochemical detection of the gene product of Rous sarcoma virus in human brain tumors. Brain Res. 1985;337(2):201–207. [DOI] [PubMed] [Google Scholar]
- 15. Nister M, Libermann T, Betsholtz C, et al. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988;48(14):3910–3918. [PubMed] [Google Scholar]
- 16. Joensuu H, Puputti M, Sihto H, et al. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207(2):224–231. [DOI] [PubMed] [Google Scholar]
- 17. Wykosky J, Gibo DM, Stanton C, et al. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–551. [DOI] [PubMed] [Google Scholar]
- 18. Lassman AB, Holland EC. Central Nervous System Tumors. In: Holland EC, ed. Mouse Models of Human Cancer. New York: Wiley-Liss; 2004: 199–213. [Google Scholar]
- 19. Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res. 2006;12(16):4899–4907. [DOI] [PubMed] [Google Scholar]
- 20. Raymond E, Brandes AA, Dittrich C, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26(28):4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105(7):2640–2653. [DOI] [PubMed] [Google Scholar]
- 22. Ahluwalia MS, de Groot J, Liu WM, et al. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett. 2010;298(2):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 24. Karnofsky DA, Abelmann WH, Carver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma with particular reference to bronchogenic carcinoma. Cancer. 1948;1(4):634–656. [Google Scholar]
- 25. Macdonald DR, Cascino TL, Schold SC, Jr., et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 26. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. [DOI] [PubMed] [Google Scholar]
- 27. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;54(282):457–481. [Google Scholar]
- 28. Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63(11):2742–2746. [PubMed] [Google Scholar]
- 29. Simmons ML, Lamborn KR, Takahashi M, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61(3):1122–1128. [PubMed] [Google Scholar]
- 30. Twardowski PW, Beumer JH, Chen CS, et al. A phase II trial of dasatinib in patients with metastatic castration-resistant prostate cancer treated previously with chemotherapy. Anticancer Drugs. 2013;24(7):743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franceschi E, Stupp R, van den Bent MJ, et al. EORTC 26083 phase I/II trial of dasatinib in combination with CCNU in patients with recurrent glioblastoma. Neuro Oncol. 2012;14(12):1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reardon DA, Vredenburgh JJ, Desjardins A, et al. Phase 1 trial of dasatinib plus erlotinib in adults with recurrent malignant glioma. J Neurooncol. 2012;108(3):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dai G, Pfister M, Blackwood-Chirchir A, et al. Importance of characterizing determinants of variability in exposure: application to dasatinib in subjects with chronic myeloid leukemia. J Clin Pharmacol. 2008;48(11):1254–1269. [DOI] [PubMed] [Google Scholar]
- 34. Tang SC, de Vries N, Sparidans RW, et al. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) gene dosage on plasma pharmacokinetics and brain accumulation of dasatinib, sorafenib, and sunitinib. J Pharmacol Exp Ther. 2013;346(3):486–494. [DOI] [PubMed] [Google Scholar]
- 35. Agarwal S, Mittapalli RK, Zellmer DM, et al. Active efflux of Dasatinib from the brain limits efficacy against murine glioblastoma: broad implications for the clinical use of molecularly targeted agents. Mol Cancer Ther. 2012;11(10):2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. [DOI] [PubMed] [Google Scholar]
- 37. Sawyers CL. Making progress through molecular attacks on cancer. Cold Spring Harb Symp Quant Biol. 2005;70:479–482. [DOI] [PubMed] [Google Scholar]
- 38. Pitini V, Arrigo C, Di Mirto C, et al. Response to dasatinib in a patient with SQCC of the lung harboring a discoid-receptor-2 and synchronous chronic myelogenous leukemia. Lung Cancer. 2013;82(1):171–172. [DOI] [PubMed] [Google Scholar]
- 39. Hammerman PS, Sos ML, Ramos AH, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1(1):78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sen B, Peng S, Tang X, et al. Kinase-impaired BRAF mutations in lung cancer confer sensitivity to dasatinib. Sci Transl Med. 2012;4(136):136ra170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clarke JL, Pao W, Wu N, et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99(2):283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.