Abstract
Background and Aim:
Long noncoding RNA-plasmacytoma variant translocation 1 is identified to be highly expressed and exhibits oncogenic activity in a variety of human malignancies, including pancreatic cancer. However, little is known about the overall biological role and mechanism of plasmacytoma variant translocation 1 in pancreatic cancer so far. In this study, we investigated the effect of plasmacytoma variant translocation 1 on pancreatic cancer cell proliferation and migration as well as epithelial–mesenchymal transition.
Methods:
Pancreatic cancer tissue specimens and cell line were used in this study, with normal tissue and cell line acting as control.
Results:
It showed that plasmacytoma variant translocation 1 expression was significantly upregulated in pancreatic cancer tissues or cell line compared to normal groups. Plasmacytoma variant translocation 1 downregulation significantly inhibited zinc finger E-box-binding protein 1/Snail expression but promoted p21 expression, and it also inhibited the cell proliferation and migration. Additionally, p21 downregulation enhanced, and p21 overexpression repressed, zinc finger E-box-binding protein 1/Snail expression and cells proliferation in PANC-1 cells. However, p21 downregulation reversed the effect of plasmacytoma variant translocation 1 downregulation on zinc finger E-box-binding protein 1/Snail expression and cell proliferation and migration.
Conclusion:
Plasmacytoma variant translocation 1 promoted epithelial–mesenchymal transition and cell proliferation and migration through downregulating p21 in pancreatic cancer cells.
Keywords: lncRNA, PVT1, EMT, p21, pancreatic cancer
Introduction
Pancreatic cancer (PC) is a malignant carcinoma of digestive system, with PC being the fourth leading cause of cancer death in Western.1 Although surgical excision is the most effective therapy for it, half of patients with PC will survive no more than 6 months after the clinical diagnosis, a 5-year survival rate below 5%.2 Therefore, further explanation of the PC pathogenesis can contributed to clinical diagnosis, prevention, and treatment of PC.
Long noncoding RNAs (lncRNAs) are transcripts longer than 200 nucleotides with no protein-coding capacity.3 Recent studies have demonstrated that lncRNAs are involved in diverse biological processes, such as cell proliferation and apoptosis, as well as in cancer progression and metastasis.4 The human plasmacytoma variant translocation 1 (PVT1) oncogene, located at chromosome 8q24.21, is upregulated in a wide variety of cancers,5 including PC.6 It has been identified as an important regulator of PC carcinogenesis and development. You,7 using a piggyBac transposon-based genome-wide mutagenesis strategy, identified that PVT1 gene led to the increased sensitivity to gemcitabinein in human PC cells. Highly expressed PVT1 is attributed to tumor progression in pancreatic duct adenocarcinoma (PDAC). And it could act as a potential biomarker to predict PDAC prognosis.6 Further study has revealed that PVT1 significantly promotes the proliferation of non–small cell lung cancer cells by downregulating p15 and p21 gene expression.8
Epithelial–mesenchymal transition (EMT) is triggered by adherent epithelial cells lost their polarity, then converses into independent fibroblast-like morphology. The EMT events in the cancer cells lead to the acquisition of invasive and metastatic properties, which contributing to fibrosis and cancer pathological processes.9 Indeed, tumor cells convert from low- to high-grade malignancy partly through EMT.10,11 The EMT induction can be regulated by a diverse array of cytokines and growth factors, such as transforming growth factor (TGF)-β.12 Previous study showed that p21 is responsible for preventing TGF-β from inducing cell proliferation in cancerous cells.13,14 Zhang et al5 found that knockdown of p21, normal mammary epithelial cell line MCF10A cells undergo EMT and exhibit upregulation of EMT markers (Snail-1, Slug, and Twist). Thus, the sum of evidence led us to hypothesize that PVT1 regulates EMT and cell proliferation in PC possibly by regulating p21.
Although the functions of PVT1 in PC have been partly clarified until now, very limited studies report the role of p21 associated with PVT1 functions. The present study was designed to investigate whether p21 could serve as a regulator of PVT1 promoting EMT and cell proliferation in PC.
Materials and Methods
Patients and Tissue Samples
Between January 2015 and July 2016, a total of 30 PC tissue samples and 20 normal tissues were collected from the Third Affiliated Hospital of Suzhou University (Changzhou, Jiangsu, China). Fresh PC samples and normal tissues were obtained under sterile conditions, snap frozen in liquid nitrogen, and stored at −80°C refrigerator. The informed consent was obtained and this study was approved by the ethics committee of hospital.
Cell Lines and Cell Culture
The human PC cell line PANC-1, and a normal pancreatic ductal epithelial cell line HPDE6c7, were purchased from the American Type Culture Collection. Cells were cultured in Dulbecco’s modified Eagle medium (Gibco, Maryland) supplemented with 10% fetal bovine serum. They were all cultured in a humidified incubator at 37°C with 5% CO2.
Quantitative Real-Time Polymerase Chain Reaction Assay
Total RNA was extracted from tissues or cells using Trizol reagent (Invitrogen). RNA was reverse transcribed using reverse transcription kit (Promega, Madison, Wisconsin) according to the manufacturer’s instructions. Real-time polymerase chain reaction (PCR) was performed using the SYBR Green Master Mix (Takara, Japan). All assays were performed in triplicate. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control.
Cell Transfection
PANC-1 cells were transfected with interference sequences such as si-PVT1, si-p21, and scrambled small interfering RNA (siRNA), as well as overexpression vectors such as pcDNA-PVT1, pcDNA-p21, and empty vector using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instruction. At 48 hours after transfection, cells were harvested for quantitative real-time PCR or Western blot analysis.
Cell Proliferation Assays
Cells were seeded in a 96-well plate with 1 × 104 cells/well. Cell viability was assessed every 24 hours using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Roche Applied Science, Mannheim, Germany) according to the manufacture’s instruction. Experiments were repeated 3 times independently.
Western Blotting
Cells were lysed with Radio-Immunoprecipitation Assay (RIPA) protein extraction reagent containing 50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), sodium orthovanadate, sodium fluoride, ethylenediaminetetraacetic acid, and protease inhibitors (Beyotime, Beijing, China). The cell lysates were loaded on 10% SDS-polyacrylamide gel electrophoresis gel and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat milk and incubated with primary antibodies, anti-p21 antibody (1:1000, ab109520), anti-SNAIL antibody (1:500, ab180714), anti-ZEB1 antibody (1:1000, ab203829), and anti-GAPDH antibody (1:10 000, ab128915; Abcam, Cambridge, Massachusetts) at 4°C overnight. The membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG H&L horseradish peroxidase (HRP; 1/5000, ab97051) or anti-rabbit HRP (1/2000, ab6721; Abcam, Cambridge, Massachusetts) secondary antibodies at room temperature for 1 hour, followed by detecting the visualized immune complexes with an enhanced chemiluminescence detection kit (Supersignal West Pico Trial kit, Pierce, lL, USA).
Statistical Analysis
All quantitative data were expressed as mean value (standard deviation). The Student t test and analysis of variance were used to compare quantitative variables. χ2 test and Fisher exact test were used to compare categorical variables. Statistical analysis and graph presentation were performed using SPSS v.17.0 software (SPSS Inc, Chicago, Illinois) and GraphPad Prism 5 Software (GraphPad, San Diego, California). P < .05 was considered as statistically significant.
Results
PVT1 was Upregulated in PC Tissues or Cells
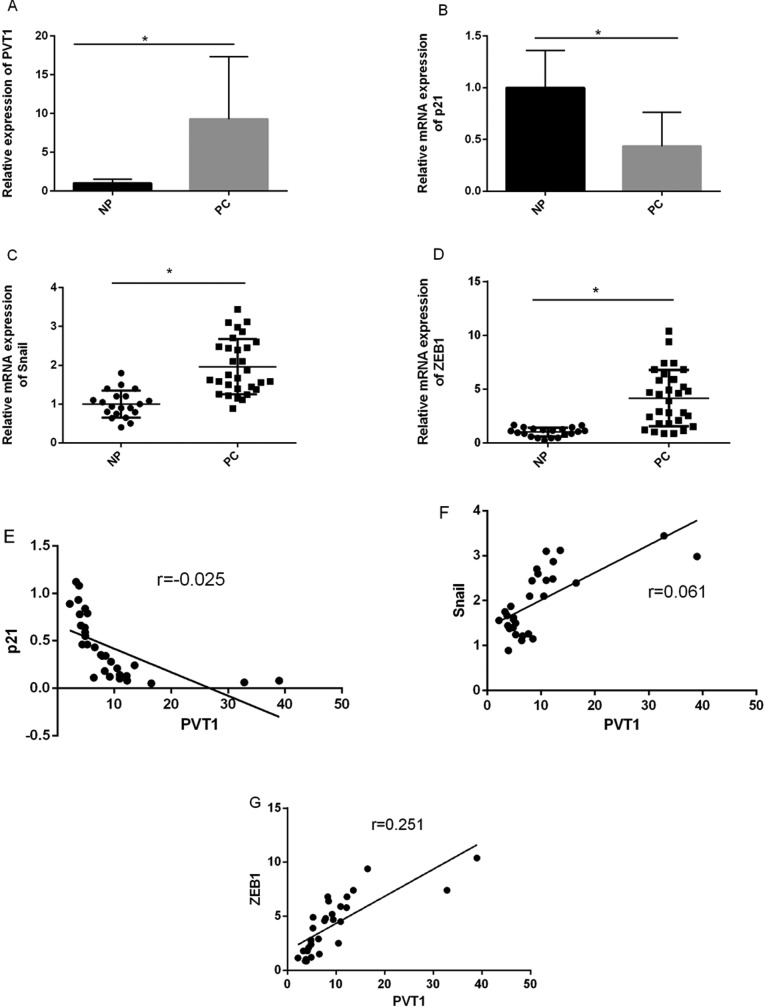
To investigate the role of PVT1 in PC progression, we detected PVT1 expression level in a total of 30 PC tissues and 20 normal tissues using qRT-PCR. The transcript levels of PVT1 were significantly upregulated in PC samples compared to normal pancreatic tissues (Figure 1A; P < .01). Next, we examined tissues expression levels of EMT transcription factors Snail and ZEB1, also showing upregulated expression levels (Figure 1C and D). In contrast, we found p21 expression was downregulated in PC samples compared to normal pancreatic tissues (Figure 1B). As shown in Figure 1F and G, Snail and ZEB1 expression levels were positively correlated with PVT1, while p21expression level was negatively correlated with PVT1.
Figure 1.
PCR analyses of PVT1, p21, Snail, and ZEB1 in pancreatic cancer (PC) tissues and normal pancreatic (NP) cancer tissues. PVT1 (A) and EMT-regulator Snail (C) and ZEB1 (D) levels were significantly upregulated compared to NP. B, Expression level of p21 was significantly downregulated. C, Expression level of p21 was negatively correlated with PVT1. Snail (F) and ZEB1 (G) expression positively correlated with PVT1. EMT indicates epithelial–mesenchymal transition; PCR, polymerase chain reaction; PVT1, plasmacytoma variant translocation 1.
In the 30 PC tissues, PVT1 expression levels were stratified according to the median level (low < median, high > median). As shown in Table 1, high PVT1 expression was associated with advanced clinical stage and lymph node metastasis. However, several other clinical pathological features were found not to be significant correlated with PVT1 expression, such as age, gender, tumor size, and histological differentiation.
Table 1.
Association Between PVT1 Expression and Clinicopathological Characteristics of Pancreatic Cancer.
| Clinicopatholigical Feature | PVT1 Expression | P Value | ||
|---|---|---|---|---|
| Total | Low | High | ||
| Age, Years | .4661 | |||
| <60 | 15 | 9 | 6 | |
| ≥60 | 15 | 6 | 9 | |
| Gender | .1086 | |||
| Male | 21 | 13 | 8 | |
| Female | 9 | 2 | 7 | |
| Tumor size, cm | .4172 | |||
| <2 | 11 | 4 | 7 | |
| ≥2 | 19 | 4 | 15 | |
| Clinical stage | .0253a | |||
| I + II | 17 | 12 | 5 | |
| III + IV | 13 | 3 | 10 | |
| Histological differentiation | 1 | |||
| Well | 4 | 1 | 3 | |
| Moderate and poor | 26 | 6 | 20 | |
| Lymph node metastasis | .0227a | |||
| No | 13 | 8 | 5 | |
| Yes | 17 | 3 | 14 | |
Abbreviation: PVT1, plasmacytoma variant translocation 1.
a P < .05.
Then, we performed Western blot and qRT-PCR to determine protein and mRNA expression level of p21, Snail, and ZEB1 in PC cells PANC-1, as well as in normal pancreatic duct epithelial cell HPDE6c7. Compared to HPDE6c7, the expression of PVT1, Snail, and ZEB1 was significantly higher in PANC-1 while p21expression was lower (Figure 2A and B). Taken together, these data suggested that PVT1, ZEB1, and Snail expression levels were highly upregulated in PC tissue or cells compared to normal cells, while p21expression was significantly downregulated.
Figure 2.
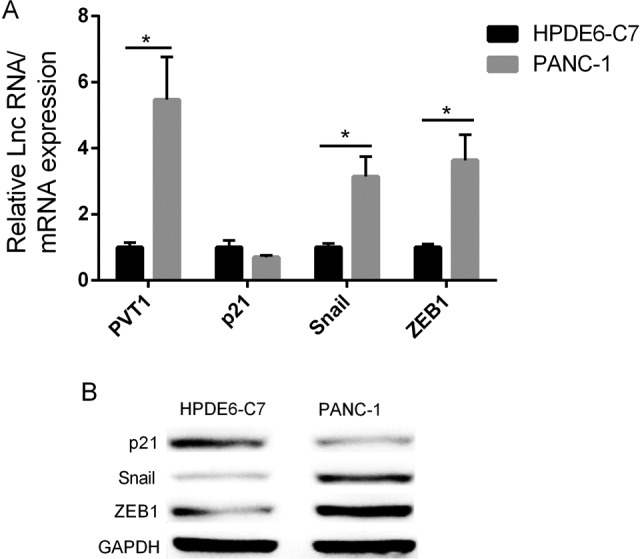
PVT1, p21, Snail, and ZEB1 expression in pancreatic cancer cell PANC-1 and normal pancreatic cell line HPDE6c7. A, PCR showed the elevated PVT1 and Snail/ZEB1 mRNA expressions, and the decreased expression of p21 in PANC-1. B, Western blot showed the elevated Snail/ZEB1 proteins expression, and decreased p21 protein expression. GAPDH was used as a loading control. PCR indicates polymerase chain reaction; PVT1, plasmacytoma variant translocation 1.
Downregulation of PVT1 Alters the Levels of p21, Snail/ZEB1 and Inhibits PC Cells Proliferation and Migration
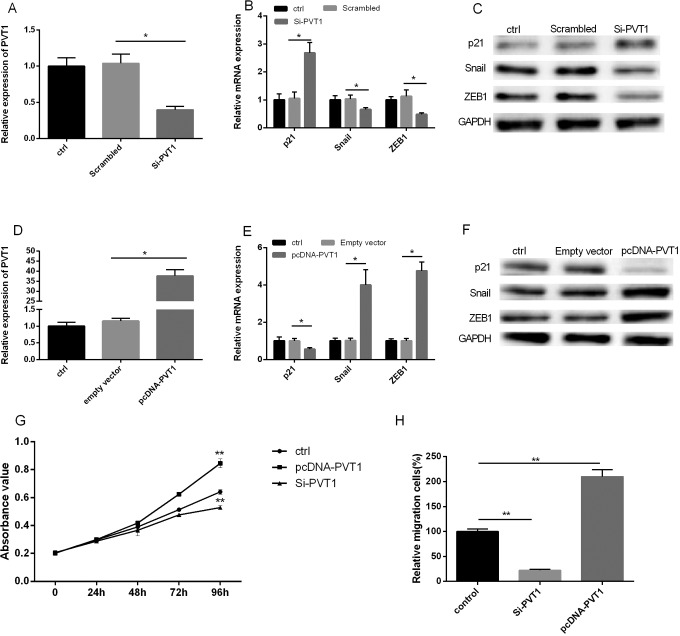
In order to gain insight into the function of PVT1 in PC cells, we established stable down- or overexpressed PVT1 using siRNA or pcDNA3.1 in PANC-1 cells (Figure 3A and D). The mRNA expression and protein levels of p21, Snail, and ZEB1 were analyzed with qRT-PCR and Western blot. The expression of p21 was found to be significantly upregulated in PANC-1 cells transfected with PVT1 siRNA. Conversely, the expressions were downregulated than did control cells (Figure 3B). Similarly, in cells transfected with pcDNA3.1-PVT1, p21 expression was found to be significantly downregulated, while Snail and ZEB1 expression upregulated (Figure 3E). Next, as shown in Figure 3G, MTT assays and transwell migration assay showed that downregulation of PVT1 expression significantly inhibited PANC-1 cells proliferation and migration, and overexpression PVT1 significantly promoted proliferation and migration compared to vector-only controls (Figure 3G and H). Results suggested that overexpression of PVT1 promoted the proliferation and migration capability of PC cells.
Figure 3.
PVT1 enhanced Snail/ZEB1 expression and proliferation in PANC-1 cells. Downregulation (A) or overexpression (D) of PVT1 by siRNA or pcDNA3.1 on cells. Downregulation of PVT1 decreased the levels of Snail/ZEB1 and increased p21 level in PANC-1 cells assayed by PCR (B) and Western blot (C). PVT1 overexpression elevated Snail/ZEB1levels and decreased p21 level in PANC-1 cells assayed by PCR (E) and Western blotting (F). G, Downregulation of PVT1 expression inhibited cell proliferation (MTT assays). H, PVT1 overexpression promoted cell migration. GAPDH was used as a loading control. MTT indicates 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PCR, polymerase chain reaction; PVT1 plasmacytoma variant translocation 1.
Negative Effects of p21 on Snail/ZEB1 Expression, Cell Proliferation, and Migration
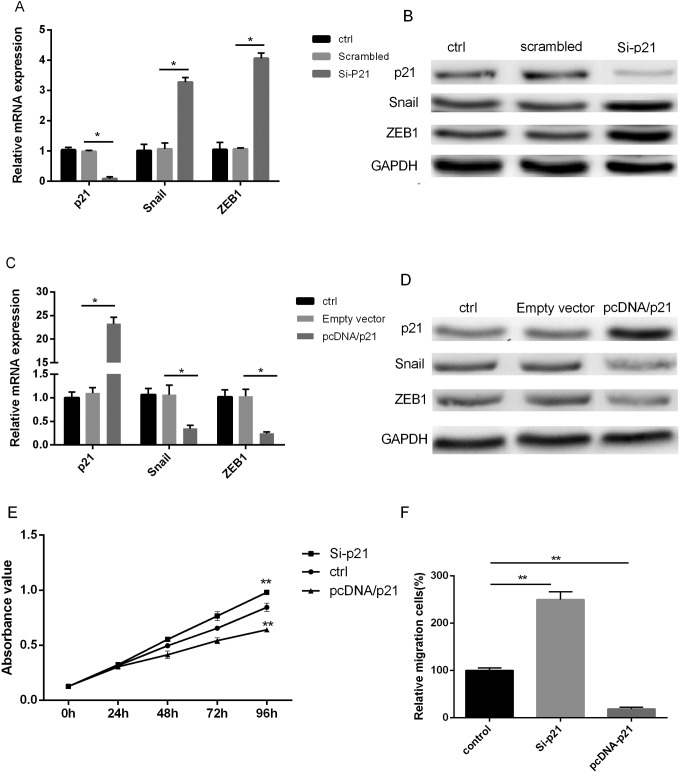
We have already confirmed that overexpression of PVT1 resulted in increase of Snail and ZEB1 expression, accompanied with a significantly decrease of p21 expression. Whether p21 can directly regulate Snail and ZEB1 was still unclear. Thus, we elucidated the effect of p21 on Snail and ZEB1 expression in PC cells. PANC-1 cells were transfected with P21 siRNA or pcDNA-p21 in order to reveal the gain-of-function effect or lose-of-function effect of p21 on Snail and ZEB1 expression. The qRT-PCR and Western blot assays supported that p21 suppression significantly promoted Snail and ZEB1 expression of PANC-1 cells (Figure 4A and B), while this situation would be rescued by p21 upregulation (Figure 4C and D). Accordingly, these results supported the idea that p21 suppressed PC cell proliferation, migration, and EMT.
Figure 4.
Negative effects of p21 on Snail/ZEB1 expression and cell proliferation. Downregulation p21 evaluated the mRNA and protein expression of Snail/ZEB1 assayed by PCR (A) and Western blotting (B). Overexpression p21 decreased the mRNA and protein expression of Snail/ZEB1 assayed by PCR (C) and Western blotting (D). E, Overexpression p21 inhibited, and downregulation promoted proliferation of PANC-1 cells. F, Downregulation p21 promoted PANC-1 cell migration. GAPDH was used as a loading control. PCR indicates polymerase chain reaction.
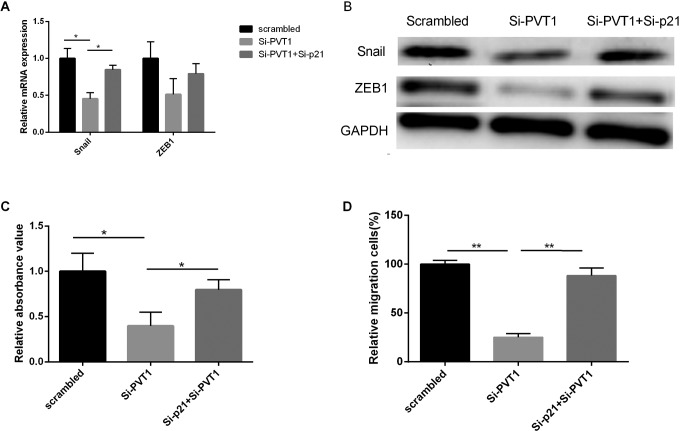
To better understand the mechanisms by which p21 engaged in PC development and progression, we investigated whether PVT1 affected PC cell proliferation, migration, or EMT via p21. PANC-1 cells were cotransfected with PVT1 siRNA and p21 siRNA or only transfected with PVT1 siRNA. The transfected cells were then subjected to identify protein expression of p21, ZEB1, and Snail by Western blot and mRNA expression by qRT-PCR. Our data showed that si-p21 partially recovered Snail and ZEB1 expressions, also rescued cell proliferation and migration which were inhibited by PVT1 siRNA (Figure 5C and D). Therefore, we confirmed the idea that the function of PVT1 promotes PC cell proliferation, migration, and EMT partially by regulating p21 expression.
Figure 5.
The functions of PVT1 regulated Snail/ZEB1 expression and cells proliferation were affected by p21. PANC-1 cells were cotransferred with PVT1 siRNA and p21 siRNA or only transferred with PVT1 siRNA. The cotransferred PVT1 siRNA and p21 siRNA partially recovered Snail/ZEB1 expression confirmed by PCR (A) and Western blotting (B), also rescued cell (C) proliferation and (D) migration which was inhibited by PVT1 siRNA (MTT assay). GAPDH was used as a loading control. PCR indicates polymerase chain reaction; PVT1, plasmacytoma variant translocation 1; TT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Discussion
As mentioned before, PVT1 has been identified as an important regulator of cell proliferation and embryonic stem cells. Recently, salivary PVT1 could regard as a novel noninvasive biomarker to detect early PC.15 However, the possible role and molecular mechanism of PVT1 in human PC remain to be clarified.
Our study demonstrated that PVT1 expression was markedly increased in PC tissues compared to nontumor tissues. The high expression level of PVT1 in patients with PC was associated with clinical stage and lymph node metastasis. Previous studies have reported that several clinical parameters are also correlated with PVT1 expression in other cancer, such as tumor node metastases staging, deeper invasion depth in gastric cancer progression,16 and advanced T-stage in non–small cell lung cancer.8
Also, we identified the role of PVT1 in PC cells. Overexpression of PVT1 in PANC-1 cells promoted proliferation, migration, and EMT. Our results are consistent with others early findings. For example, Kong et al16 reported PVT1 expression was markedly increased in gastric cancer tissues, associating with a poor prognosis. To sum up, amplification of PVT1 is one of the most frequent events in many malignant tumors, such as colorectal cancer,17 ovarian and breast cancer,18 bladder cancer,19 and prostate cancer,20 and has been associated with reduced survival duration in patients.
To our knowledge, the cyclin-dependent kinase (CDK) inhibitor p21 (also known as p21WAF1/Cip1) promotes cell cycle arrest by inhibiting CDK2 and CDK1 activity in response to many stimuli.21 Numerous evidences showed that p21 expression is downregulated in various human cancers, such as colorectal,22 cervical,23 head and neck,24 and lung cancers.25 Liu et al26 reported p21 attenuated Ras- and c-Myc-induced breast tumor EMT. So what role may p21 play in PC? We assessed the effect of PVT1 on p21 expression in PC cells. After suppressing PVT1 in PANC-1 cells, we observed a significantly increase in the expression of the tumor suppressor p21 in transcriptional level. Furthermore, we showed that altering expression of p21 was associated with PANC-1 cell growth, indicating that PVT1 could regulate cell proliferation and migration by affecting p21.
Emerging evidence suggests that molecular mechanisms of PVT1 are diverse. They have been shown to regulate gene expression at multiple levels, including chromatin modification, transcription, and posttranscriptional processing. For example, PVT1 regulate gene transcription through recruiting transcription factors to their target gene promoters, therefore activating gene expression. Zhou et al27 reported PVT1 contributed to proliferation and arrested cell cycle at G0/G1 stage in thyroid cancer via recruiting zeste homolog 2 and regulating thyroid-stimulating hormone receptor expression. Wang et al28 found PVT1 promoted proliferation, cell cycling, and stem cell-like potential in hepatocellular carcinoma cells by stabilizing NOP2 nucleolar protein (NOP2) protein, an RNA-binding protein that binds to PVT1.
In conclusion, we first found p21 directly regulated ZEB1 and Snail expression in PC cells. Also, our data provided strong evidence that downregulation of p21 was involved in the function of PVT1 in modulating proliferation, migration, and EMT. Taken together, PVT1 regulated p21 expression, while p21 could downregulate ZEB1 and Snail expression. So we believe p21 may present as a mediator for PVT1-induced EMT, migration, and proliferation. Thus, a further study is needed to elucidate the regulation mechanisms of PVT1 on p21 in PC, and prove the regulatory relationships between them in vivo.
Abbreviations
- CDK
cyclin-dependent kinase
- EMT
epithelial–mesenchymal transition
- HRP
horseradish peroxidase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NOP2
NOP2 nucleolar protein
- PC
pancreatic cancer
- PCR
polymerase chain reaction
- PDAC
pancreatic duct adenocarcinoma
- PVT1
plasmacytoma variant translocation 1
- SDS
sodium dodecyl sulfate
- TGF
transforming growth factor
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2. Pericleous M, Rossi RE, Mandair D, Whyand T, Caplin M. Nutrition and pancreatic cancer. Anticancer Res. 2014;34(1):9–21. [PubMed] [Google Scholar]
- 3. Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7. [DOI] [PubMed] [Google Scholar]
- 4. Cheetham SW, Gruhl FJ, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108(12):2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Yan W, Jung YS, Chen X. PUMA cooperates with p21 to regulate mammary epithelial morphogenesis and epithelial-to-mesenchymal transition. PloS One. 2013;8(6):e66464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Yu W, Wang Q, et al. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med. 2015;106(3):143. [PubMed] [Google Scholar]
- 7. You L, Chang D, Du HZ, Zhao YP. Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem Biophys Res Commun. 2011;407(1):1. [DOI] [PubMed] [Google Scholar]
- 8. Cui D, Yu CH, Liu M, Xia Q, Zhang Y, Jiang W. Long non-coding RNA PVT1 as a novel biomarker for diagnosis and prognosis of non-small cell lung cancer. Tumor Biol. 2015;37(3):4127. [DOI] [PubMed] [Google Scholar]
- 9. Acloque H, Adams MS, Fishwick KJ, Bronnerfraser M, Nieto MA. Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–746. [DOI] [PubMed] [Google Scholar]
- 11. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. [DOI] [PubMed] [Google Scholar]
- 12. Cano CE, Motoo Y, Iovanna JL. Epithelial-to-mesenchymal transition in pancreatic adenocarcinoma. Scientific World J. 2010;10:1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bachman KE, Blair BG, Brenner K, et al. p21(WAF1/CIP1) mediates the growth response to TGF-beta in human epithelial cells. Cancer Biol Ther. 2004;3(2):221. [DOI] [PubMed] [Google Scholar]
- 14. Hartland SN, Prost S. TGFbeta induces apoptosis and EMT in primary mouse hepatocytes independently of p53, p21 Cip1 or Rb status. BMC Cancer. 2008;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie Z, Chen X, Li J, et al. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer. Oncotarget. 2016;7(18):25408–25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong R, Zhang EB, Yin DD, et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer. 2015;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahashi Y, Sawada G, Kurashige J, et al. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan Y, Kuo W, Stilwell JL, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13(19):5745–5755. [DOI] [PubMed] [Google Scholar]
- 19. Zhuang C, Li J, Liu Y, et al. Tetracycline-inducible shRNA targeting long non-coding RNA PVT1 inhibits cell growth and induces apoptosis in bladder cancer cells. Oncotarget. 2015;6(38):41194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh AL, Tuzova AV, Bolton E, Lynch TH, Perry AS. Long noncoding RNAs and prostate carcinogenesis: the missing ‘linc’? Trends Mol Med. 2014;20(8):428–436. [DOI] [PubMed] [Google Scholar]
- 21. Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhai H, Fesler A, Schee K, Fodstad O, Flatmark K. Clinical significance of long intergenic noncoding RNA-p21 in colorectal cancer. Clin Colorectal Cancer. 2013;12(4):261–266. [DOI] [PubMed] [Google Scholar]
- 23. Lo KWK, Yu MMY, Yim SF, Poon CS, Chung TKH, Wong YF. Aberrant expression of p21(WAF1/CIP1) and p27(KIP1) in cervical carcinoma. Cancer Lett. 2001;172(1):93. [DOI] [PubMed] [Google Scholar]
- 24. Park J, Kim J, Park JK, et al. Association of p21-activated kinase-1 activity with aggressive tumor behavior and poor prognosis of head and neck cancer. Head Neck. 2015;37(7):953–963. [DOI] [PubMed] [Google Scholar]
- 25. Teramen H, Tsukuda K, Tanaka N, et al. Aberrant methylation of p21 gene in lung cancer and malignant pleural mesothelioma. Acta Med Okayama. 2011;65(3):179. [DOI] [PubMed] [Google Scholar]
- 26. Liu M, Casimiro MC, Wang C, et al. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci U S A. 2009;106(45):19035–19039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Q, Chen J, Feng J, Wang J. Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR). Tumor Biol. 2015;37(3):3105. [DOI] [PubMed] [Google Scholar]
- 28. Wang F, Yuan J, Wang S, et al. Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology. 2014;60(4):1278–1290. [DOI] [PubMed] [Google Scholar]