Abstract
Purpose:
We recently developed a novel, open-source in vivo dosimetry that uses the electronic portal imaging device to detect dose delivery discrepancies. We applied our method on patients with rectal cancer treated on a belly board device.
Methods:
In vivo dosimetry was performed on 10 patients with rectal cancer treated prone on the belly board with a 4-field box arrangement. Portal images were acquired approximately once per week from each treatment beam. Our dosimetry method used these images along with the planning CT to reconstruct patient planar dose at isocenter depth.
Results:
Our algorithm proved sensitive to dose discrepancies and detected discordances in 7 patients. The majority of these were due to soft tissue differences between planning and treatment, present despite matching to bony anatomy. As a result of this work, quality assurance procedures have been implemented for our immobilization devices.
Conclusion:
In vivo dosimetry is a powerful quality assurance tool that can detect delivery discrepancies, including changes in patient setup and position. The added information on actual dose delivery may be used to evaluate equipment and process quality and to guide for adaptive radiotherapy.
Keywords: in vivo dosimetry, EPID dosimetry, belly board device, patient safety, adaptive radiotherapy
Introduction
In vivo dosimetry by transit electronic portal imaging device (EPID) images is a growing field in radiotherapy,1–3 which may be useful in treatment quality assurance (QA).4 We have recently developed a low-resource, open-source, in vivo dosimetry method by EPID, which is very simple to implement.5,6 In a preliminary study, we found evidence of higher than expected interfractional variability in patients with rectal cancer treated prone on the belly board.7
In radiotherapy for rectal cancer, the belly board is often used as an immobilization device to spare the small bowel.8 However, the use of belly boards is associated with reduced patient position reproducibility. Based on our center’s experience, we hypothesized that even after appropriate bony anatomy alignment, interfractional soft tissue discrepancies persist. These may be due to setup procedure, belly board position with respect to patient, belly board differences between simulation and treatment, or persistent bowel gas.
The purpose of this article is to demonstrate the utility of our dosimetry method by detecting, for the first time, dose discrepancies in vivo in rectal cancer treatment on the belly board. This study illustrates the power of EPID in vivo dosimetry to review new and existing treatment delivery techniques by detecting potential delivery errors, identifying the cause of error, and estimating its dosimetric impact. No additional equipment is required, and it may be applied as a QA study or for every treatment fraction (fx) to monitor machine performance and patient setup accuracy.
Methods
Ten consecutive patients with rectal cancer (2 females and 8 males treated in 2014-2015), who were prescribed 45 Gy in 25 fx with 3-dimensional conformal radiotherapy (3D-CRT) in a 4-field box arrangement, were enrolled. Collection of extra images was approved by the local ethics board and patients gave written consent. All patients were treated in a prone position with a full bladder on an in-house belly board device: a frame of rectangular cushions, mounted on hard plastic, which left a 37 × 42 cm2 (lateral by longitudinal) opening in the center, 8 cm deep. The abdomen is displaced into the opening by gravity. Patients were positioned with the iliac crest aligned to the lower end of the belly board aperture. This setup allows close alignment with the lumbosacral joint, allowing optimal small bowel displacement.9 Patient setup was verified by orthogonal kilovoltage (kV) image pairs for the first 3 fx and weekly per local protocol.
Patients 1, 2, 7, and 8 were treated on a Clinac 21EX, and patients 3-6 and 9-10 on a Trilogy (Varian Medical Systems, Palo Alto, California). Portal images were acquired with Varian aSi-1000 EPIDs in cine mode (ie, continuous acquisition) placed 50 cm downstream of isocenter, with the following parameters: patients 1-2: 15 frames/sec (f/s), 4 frames/image (f/i); patients 7-8: 7.825 f/s, 4 f/i; patients 3-6, 9-10: 7.825 f/s, 8 f/i. Each field produced 4 to 20 cine images, depending on monitor unit (MU) and imaging parameters. Of the 40 fields, 38 were 15 MV energy and 2 were 6 MV. EPID dosimetry was performed approximately once per week and resulted in 2 to 8 imaged fxs per patient. The fx numbers indicated in the article correspond to the fxs in which imaging was performed, not the actual fx number of the treatment course. Eight fields with enhanced dynamic wedges were excluded because cine imaging is susceptible to dose-rate modulation artifacts.10
Our in-house 2D in vivo dosimetry is based on a point-dose estimation first presented by Piermattei et al,11 which we expanded to calculate dose in the whole plane at isocenter depth. The details are available in a separate publication6 and the method is summarized below. First, correlation ratios between the isocenter dose at mid-depth of a slab phantom and the pixel intensity of the transit image (ie, through the phantom, using the same field) are collected. These correlation ratios are then used to process the patients’ in vivo cine images. Each patient cine set was processed in MATLAB (The MathWorks, Inc, Natick, Massachusetts). Each cine image was inverted, a flood field correction through 20 cm of water applied, beam-on artifacts removed, and beam-off frame loss corrected for. All cines of the field were summed into a single image. EPID backscatter was accounted for following the method proposed by Berry et al.12 The resolution was reduced to 512 × 384 to reduce computational load. From this image, dose was calculated inside the EPID-measured field (50% line) at isocenter depth. This calculation is performed off-line, requires approximately 10 seconds on a standard computer and makes use of the previously measured correlation ratios11 between EPID signal and isocenter dose. Also, it requires a projection of the patient’s planning computed tomography (CT) from the corresponding gantry angle, which was calculated in MATLAB before treatment. The CT projection provides the total attenuation, in terms of water equivalent thickness, along each ray line from the source to each detector pixel, inclusive of inhomogeneities.
EPID-calculated dose was compared (pixel-by-pixel and gamma analysis) to that of the treatment planning system (TPS; Varian Eclipse 11.0.31, analytical anisotropic algorithm, 2.5 mm dose calculation grid). Each dose difference map was visually inspected; features of interest and regions of dose difference of approximately ±10% or more were investigated by in-depth analysis of the relevant EPID, kV (if available), and CT images. Gamma analysis was performed with 5%, 3 mm criteria as proposed previously.13–15 This threshold was chosen taking that we quantified the accuracy of our dose reconstruction in slab phantoms to be about ±3% and others set a ±5% threshold in its point dose in vivo implementation.6,7,14,16
Results and Discussion
One hundred sixty-four fields delivered to 10 patients were analyzed. Figure 1 shows results for a patient whose measured dose closely matched the planned dose in the isocenter plane. Relevant dose differences were detected in 7 patients. These were grouped into 5 sources of error: (1) soft tissue filling of the belly board opening, (2) belly board positioning (with respect to patient bony anatomy), (3) persistent gas, (4) patient bony anatomy setup, and (5) other. Observations are summarized in Table 1. Discordances in delivery were identified by our EPID in vivo dosimetry, while the explanations presented result from a more in-depth analysis of EPID, kV, and CT images.
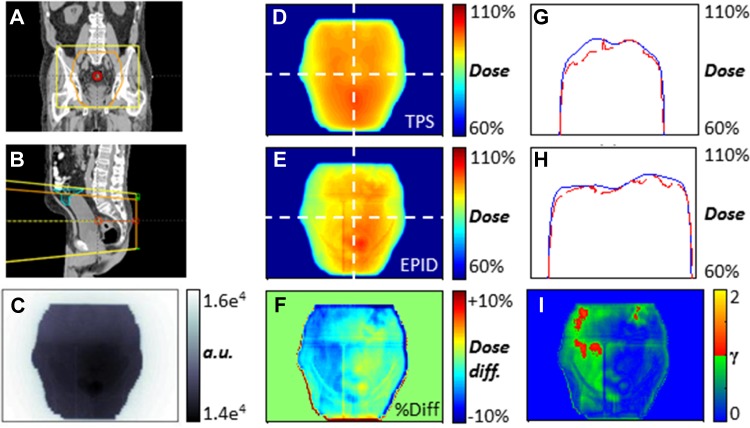
Figure 1.
Example of in vivo electronic portal imaging device (EPID) dose calculation at isocenter depth (patient 1, postero-anterior [PA] field, fraction 2). A and B, Coronal, sagittal views of planning CT and field with the small bowel contoured in cyan. C, Single cine frame. D, Planned dose map. E, EPID-reconstructed dose at isocenter depth. F, Point-to-point dose difference map. G and H, Cross-plane and in-plane dose profiles (blue, planned; dashed red, EPID) as overlaid on D and E. I, 5%, 3 mm gamma evaluation.
Table 1.
Quality Assurance Summary From In Vivo EPID Dosimetry on 10 Patients With Rectal Cancer.a
| Patient | No. Imaged Fields (Total: 164) | Soft Tissue Changes in Belly Board Opening | Belly Board Positioning Errors ≥ 1.5 cm | Bowel Gas | Patient Setup Errors ≥ 1 cm (Bony Anatomy) | Other |
|---|---|---|---|---|---|---|
| 1 | 8 (4 fields, 2 fx) | |||||
| 2 | 17 (3 fields, 6b fx) | Board misaligned by 2 cm S-I (AP field, fx 5) | Persistent gas (LAT fields, fx 2, 5, 6; Figure 4) | 1 cm S-I setup error (AP field, fx 4) | ||
| 3 | 12 (2 fields, 6 fx) | Stretched soft tissue caused +8% AP field dose (both fields, all fx; Figure 2) | Board misaligned by 2 cm S-I (both fields, fx 2, 5) | |||
| 4 | 20 (4 fields, 5 fx) | Stretched soft tissue caused +4% AP field dose (AP field, all fx) | Tissue reduction in posterior region (LAT fields, all fx; Figure 5). Unexplained +10%-15% dose areas (PA field, all fx) | |||
| 5 | 12 (2 fields, 6 fx) | Board misaligned by 2 cm S-I (both fields, fx 1) | ||||
| 6 | 16 (4 fields, 4 fx) | Board misaligned by 2 cm S-I (AP, PA fields, fx 3; Figure 3) | 1.5 cm L-R setup error (AP field, fx 2), probable VRT setup error (LAT fields, fx 2) | |||
| 7 | 21 (3 fields, 7 fx) | Stretched soft tissue caused +3% AP field dose (AP field, all fx) | ||||
| 8 | 16 (4 fields, 4 fx) | |||||
| 9 | 18 (3 fields, 6 fx) | |||||
| 10 | 24 (3 fields, 8 fx) | Genitals placed differently (AP field, all fx) |
Abbreviations: AP, antero-posterior; fx, fraction(s); LAT, lateral; L-R, left-right; PA, postero-anterior; S-I, superior-inferior; VRT, vertical.
aIn brackets are the imaged fields and fractions in which the dose discrepancy was visible (columns 3-7).
bAntero-posterior field fraction 6 is not usable due to imager misplacement.
Soft Tissue Filling of Belly Board Opening
In 3 patients (3, 4, 7), the EPID-measured dose was consistently higher in the superior region of the anteroposterior (AP) field. The region of increased signal coincides with the belly board opening (Figure 2D). This effect appears in almost all imaged fxs (not shown), so it is likely not due to random setup errors. Since a local signal increase is attributable to decreased attenuation, the most likely explanation is soft tissue displacement from the beam path, that is, reduced filling of the patients’ abdomen into the belly board opening. This may be a result of setup procedure, patient cooperation, or belly board differences between simulation and treatment units. This difference in abdomen drop was confirmed by the inspection of the lateral kV setup images (not shown). As a result, the superior region of the AP field received 8%, 4%, and 3% more dose than planned for patients 3 (Figure 2), 4, and 7, respectively. If the AP field contributes to approximately a quarter of the total dose, the maximum dose discrepancy of 8% accounts to approximately 2% of the total dose. This could result in increased dose to small bowel and resulting acute toxicity. Although it is probable that the small bowel contour of the planning CT is not an accurate representation of its location at treatment, judging by the large volume of the contour in the field (Figure 2D and E), it is likely that at least some small bowel received extra dose. Two patients presented grade 2 acute toxicity and the third grade 0 to 1, the sample size being too small to detect a specific result of increased toxicity.17
Figure 2.
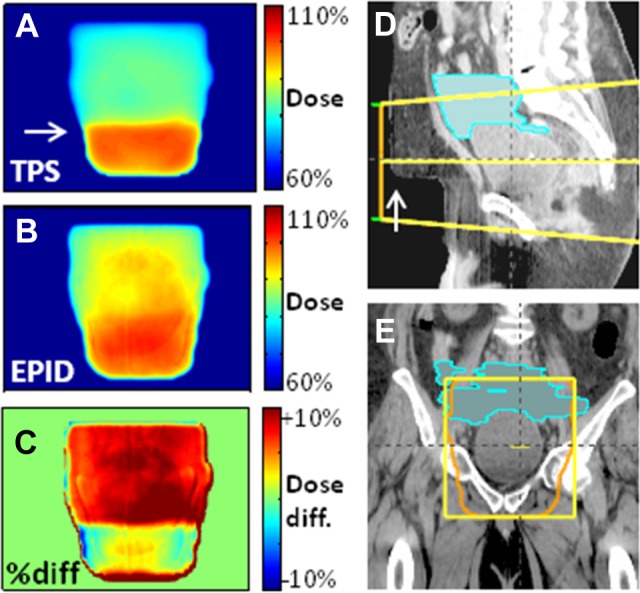
Variability in the amount of abdomen that fills the belly board opening affects dose in patient 3, anteroposterior (AP) field. The filling of the opening is “sharp” at planning (A, D: white arrow). B, The EPID-calculated dose map at isocenter depth, averaged over 6 fractions, displays a more gradual dose gradient. This is due to both different patient positioning and to the softer belly board cushion at the treatment unit (due to greater wear and tear), which in effect makes the hole in the board shallower. C, The dose discrepancy is especially clear in a point-to-point dose difference map. As a result, some of the small bowel (D, E: cyan) was likely overdosed by ∼8%. (Dose percentages with respect to AP field.)
We used in vivo dosimetry results to guide a single ad hoc dose–volume histogram analysis. For patient 3 only, we simulated the observed effect in the TPS by increasing the MUs of the AP field by 8%. The volume of small bowel receiving 25 Gy increased by 155 cc. Banerjee et al reported that the volume of small bowel receiving 25 Gy is a good predictor of grade 3 acute gastrointestinal toxicity.18 Modest increases in dose may thus have a relevant effect on the dose–volume histogram and potentially increase toxicity. This is a good example of how EPID in vivo dosimetry can guide analysis to discern clinically significant differences in delivered dose.
In light of these in vivo dosimetry results, we tested the stiffness of the cushions of 3 boards (simulator and 2 treatment units) by applying various weights and measuring compression. The simulator belly board (with least wear) was the stiffest, that used for patients 1, 2, 7, and 8 was softer (2 mm extra compression), and that for patients 3-6, 9, 10 was drastically softer (8 mm extra). Also, stiffness was uneven between left and right cushions of the same board due to room setup (door on the left of board in 1 treatment unit, on the right in simulator and other unit). These differences cause variations in soft tissue contours and in anatomy rotation. As a result of this study, we are updating our center’s belly boards. These results demonstrate the need for regular QA on immobilization devices.
Belly Board Positioning With Respect to Patient
EPID in vivo dosimetry was able to detect 4 cases in which the belly board placement was suboptimal with respect to bony anatomy: patients 2, 3, 5 (not shown), and 6 (Figure 3). Typically, after the patient is set up and imaged, the treatment couch is shifted to line up bony anatomy. Depending on the initial setup, this may result in the belly board being in a different location. For the 4 cases, the variability in board placement was ∼2 cm, while the allowed variability in our center is ±1 cm. This incident was seen in only one of the imaged fxs for each patient, so it was likely a random event of limited clinical consequence.
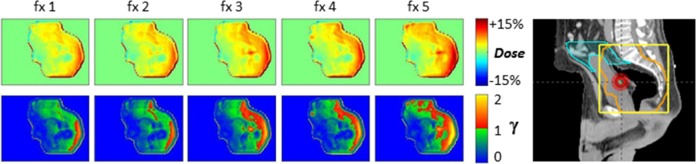
Figure 3.
Belly board positioning error (patient 6). Dose difference maps for 4 nonconsecutive fractions (fxs) show that on fx 3, the belly board was placed about 1.5 cm inferior with respect to planning CT (arrows). As a result, an extra ∼6 cm of attenuator are in the superior region of the field, causing dose reduction to the target and more small bowel to be in the field.9
Persistent Bowel Gas
In numerous instances, we found interfractional deviations in dose due to the presence of bowel gas. In particular, in patient 2, we found persistent bowel gas, with variations in quantity and location. Although soft tissue changes at the belly board opening would affect dose delivery from the AP field only, the presence of gas will mainly affect the dose deposited by the posteroanterior (PA) field. From the lateral images (Figure 4), we approximated the amount of tissue displaced by the gas and estimated that the dose to a small volume (<10 cc) of the small bowel increased by ∼5% of the PA field’s dose. This result suggests that, for this patient, the practice of contouring out gas and assigning it density of 1 g/cc (ie, assuming it is not present at treatment) does not accurately represent the treatment conditions. An example of using in vivo dosimetry for adaptive radiotherapy would be to identify patients in which gas is reproducible and replan without contouring it out (or, as a compromise, assign and intermediate density). Further study is needed to warrant clinical practice changes; this result is given to exemplify the power of EPID in vivo dosimetry for personalized, adaptive treatments.
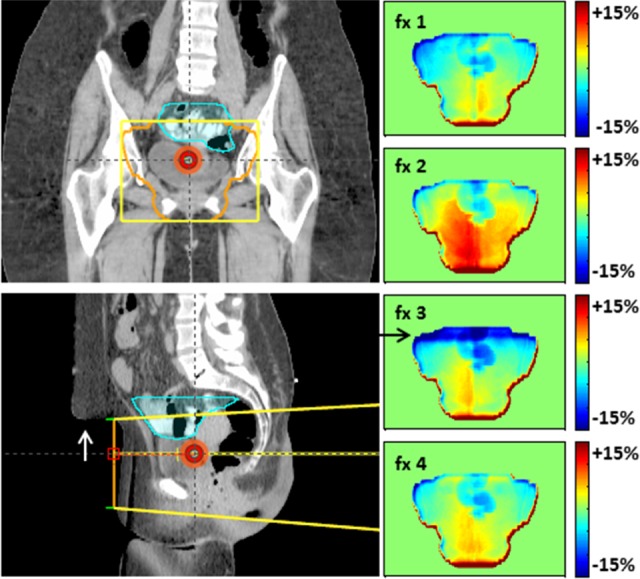
Figure 4.
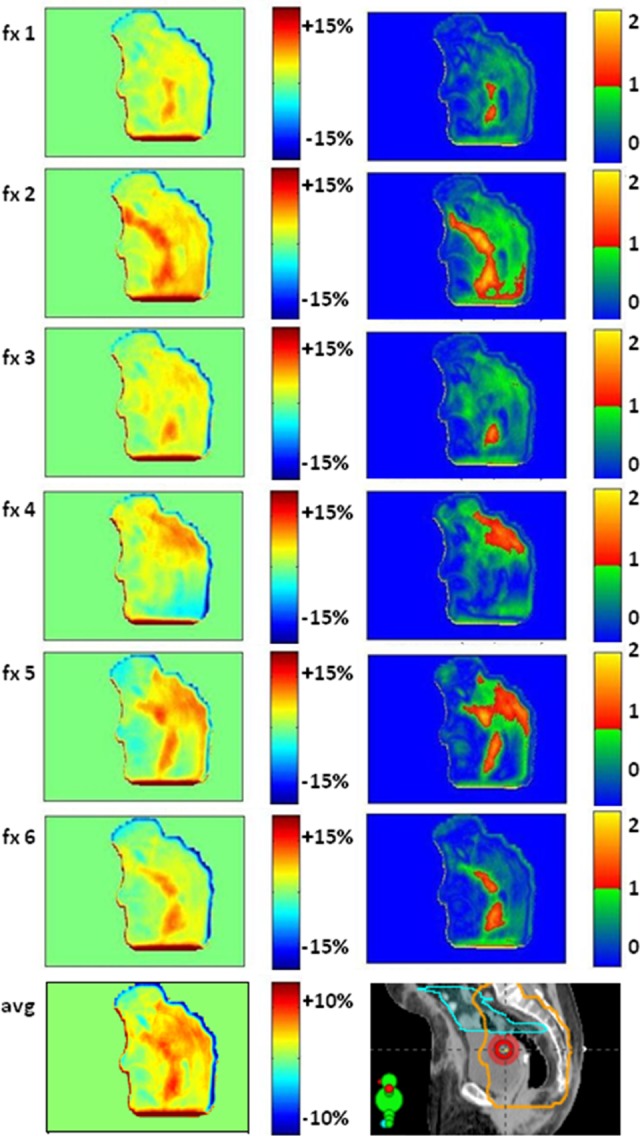
Persistent bowel gas affects dose (patient 2). Dose difference maps (left) and 5%, 3 mm gamma analysis (right) over 6 nonconsecutive treatment fractions for the left lateral field. The average dose difference map (bottom) indicates consistently more gas at treatment than simulation. There was likely some overdose to the small bowel (cyan contour on the planning CT). For patients like this, in vivo dosimetry may warrant adaptive treatment by repeating CT to account for consistent gas. (Percentages with respect to the lateral field’s dose.)
Bony Anatomy Setup Error
The in vivo dosimetry detected 2 cases of bony anatomy setup error (patients 2 and 6, not shown). In both cases, a dose difference map for 1 fx presented a region of underdosage at the location of the belly board opening similar to Figure 3, fx 3. Further inspection showed that the entire bony anatomy was ∼1 to 1.5 cm inferior with respect to the planned location. On those treatment days, there was no kV imaging. This is a good example of how in vivo EPID dosimetry allows evaluation of imaging protocols.
Other
We found 2 further cases of consistent dose differences, related to the prone treatment position. The dose maps obtained from the lateral fields of patient 4 (Figure 5) may be explained by a different patient position (eg, contracting the gluteus muscles or rotation of pelvis, due to uneven belly board stiffness) at treatment with respect to planning. In patient 10, different orientation of the genitals caused increased EPID signal in the inferior region of the field (not shown). These types of errors are only detectable by routine in vivo dosimetry.
Figure 5.
Systematic dose discrepancy indicative of muscle relaxation and/or anatomy rotation at treatment with respect to planning (patient 4). Dose difference maps (first row) and 5%, 3 mm gamma evaluation (second row).
Discussion on Belly Board Use for Rectal Cancer
The standard of care for stage II and III rectal cancer is radiation therapy with concurrent chemotherapy followed by surgery.19,20 Given typical pelvic anatomy, the small bowel often falls into the upper region of the treated volume and is the primary dose-limiting organ at risk. Acute grade 3 small bowel toxicity develops 2 to 3 weeks into treatment and can include diarrhea, abdominal pain, and nausea with reported incidence of 7% to 28%.20–23 Late effects, such as perforation or obstruction, may develop after months/years. A review of multiple studies found that partial small bowel irradiation of ∼50 Gy resulted in late obstruction or perforation rates in 2% to 9% of patients.24 This rate rose to 30% in patients treated with a larger field that extended superiorly to the level of the lumbar vertebrae.25 The volume of the small bowel that receives a relatively low dose is a good predictor of acute toxicity. In 2 studies, the greatest sensitivity for predicting early toxicity was associated with the small bowel volume receiving 15 to 25 Gy.18,26 Late toxicities, on the other hand, are better predicted by the small bowel volume receiving higher doses.24 In light of the high radiosensitivity of the small bowel, many centers make use of the belly board as it is inexpensive, customizable, increases patient comfort, and reduces the volume of small bowel in the treatment volume by 13 to 167 cc.8
The major challenge in using the belly board is patient position reproducibility. First, the prone position suffers from larger random and systematic setup errors with respect to supine.8 In addition, prone with belly board has larger mean positioning errors compared to prone without, with the greatest difference in the AP direction (4.4 vs 2.3 mm).8,27 Belly board setup is also susceptible to differences in rotation of the pelvis: a study28 found that the sacrum-to-S1-vertebra angle varies by approximately ±10° over multiple fxs and may drift over the course of treatment.
In our center, we had significant concerns regarding the quality of treatment on the belly board device. This dosimetry tool allowed us to quantify the impact of these concerns to the multidisciplinary tumor group. As a result of this process, we are making improvements to our belly boards by testing cushion stiffness and replacing foam regularly. These results also emphasized the need for a more detailed setup procedure (eg, climb onto belly board from specific side, shift posteriorly to fill board opening). Also, it was noticed that most discrepancies are highlighted by the AP and PA fields, which are the fields that go through the belly board itself. It is likely that the dose delivery from the AP field (which is perturbed by the belly board upstream of the patient) is the most affected, supporting the use of a lighter weighting of the AP field. These results have encouraged our groups to consider implementation of other treatment techniques (specifically: 3-field and volumetric-modulated arc therapy [VMAT] supine) for eligible patients.
Limitations
As this study is demonstration of the power and utility of in vivo dosimetry to QA delivery techniques, the small sample size and qualitative nature of the analysis limit to our institution the applicability of specific improvements. Also, further development is needed to extend our dosimetry technique to dynamic treatments (ie, intensity-modulated radiation therapy [IMRT], VMAT). Extension to IMRT may be achieved by summation of all cine images into an integrated image. Extension to VMAT would be more laborious, as it would require dose estimation for multiple angles of the treatment arc and 3D summation of the resulting planar dose maps. In either case, dose accuracy may suffer from the smaller, irregularly shaped subfields present in many dynamic treatments.
The limited accuracy of our in-house in vivo dosimetry must be considered while analyzing results. First, a characteristic limitation of using the planning CT to reconstruct dose via EPID images is that the CT may not reflect accurately the patient’s setup or anatomy at treatment, producing a dose calculation error.29 As a result, dose differences should mainly be interpreted as flags warranting further investigation. Second, we make use of correlation ratios obtained through slab phantoms and thus do not model the differences in scatter due to inhomogeneities. This may lead to systematic errors of up to 7% in the presence of lung.5 The effect of inhomogeneities on the primary beam, however, is taken into account by means of CT data. Globally, in slab phantom tests6, we found an accuracy of ±3%, and in their in vivo point-dose implementation, Fidanzio et al set an automated tolerance level of ±5% for pelvic treatments and ±6% for thoracic treatments. 30
When estimating dose discrepancies to the small bowel, the variability in internal organ motion must be taken into account. For example, in Table 1, we report on belly board positioning errors of up to 2 cm. To put this in perspective, Nuyttens et al 31 measured the distance from the bones of the posterior pelvis to the closest small bowel loop in 10 preoperative patients with rectal cancer and found that the average standard deviation of repeated measurements over the course of treatment was 2.7 cm. Although this is a limitation of the object of measurement, rather than of the measurement technique, it poses an intrinsic limit to the conclusions that one may draw from results.
The routine use of the EPID for in vivo dosimetry may raise the question of potential radiation damage to the imager, shortening its life span. Studies have found that amorphous Silicon diodes are remarkably resistant to radiation,32,33 with the only probable effect an increase in the necessary dark field correction.34–36 The electronics linked to the imaging panel are known to be more radiosensitive,37 so care must be taken to ensure only the panel itself is irradiated. For some large fields this may not be possible, so transit dosimetry may not be possible for all fields. Also, some fields with large couch angles may not allow extension of the imager arm due to possible collisions.
Conclusions
We applied a novel, simple, open-source EPID in vivo dosimetry method to verify dose delivery in patients with rectal cancer treated with 3D-CRT while prone on the belly board. The results have guided QA of the equipment and revision of the setup and treatment processes for rectal treatments in our center. Electronic portal imaging device in vivo dosimetry is a powerful tool to detect errors and evaluate the quality of treatment and ought to be more widely implemented in the clinic.
Abbreviations
- AP
antero-posterior
- 3D-CRT
3D conformal radiotherapy
- CT
computed tomography
- EPID
electronic portal imaging device
- fx
fraction
- IMRT
intensity-modulated radiation therapy
- kV
kilovoltage
- MU
monitor unit
- PA
postero-anterior
- QA
quality assurance
- VMAT
volumetric-modulated arc therapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Stefano Peca, PhD http://orcid.org/0000-0001-5940-6046
References
- 1. Mijnheer BJ, González P, Olaciregui-ruiz I, Rozendaal RA, Herk M, Van Mans A. Overview of 3-year experience with large-scale electronic portal imaging device-based 3-dimensional transit dosimetry. Pract Radiat Oncol. 2015;5(6):e679–e687. doi:10.1016/j.prro.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 2. Woodruff HC, Fuangrod T, Van Uytven E, et al. First experience with real-time EPID-based delivery verification during IMRT and VMAT sessions. Int J Radiat Oncol Biol Phys. 2015;93(3):516–522. doi:10.1016/j.ijrobp.2015.07.2271. [DOI] [PubMed] [Google Scholar]
- 3. Van Uytven E, Van Beek T, McCowan PM, Chytyk-Praznik K, Greer PB, McCurdy BMC. Validation of a method for in vivo 3D dose reconstruction for IMRT and VMAT treatments using on-treatment EPID images and a model-based forward-calculation algorithm. Med Phys. 2015;42(12):6945–6954. doi:10.1118/1.4935199. [DOI] [PubMed] [Google Scholar]
- 4. Brouwers PJAM, Lustberg T, Borger JH, et al. Set-up verification and 2-dimensional electronic portal imaging device dosimetry during breath hold compared with free breathing in breast cancer radiation therapy. Pract Radiat Oncol. 2015;5(3): e135–e141. doi:10.1016/j.prro.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 5. Peca S, Brown DW. Two-dimensional in vivo dose verification using portal imaging and correlation ratios. J Appl Clin Med Phys. 2014;15(4):117–128. doi:10.1120/jacmp.v15i4.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peca S, Brown DW, Smith WL. A simple method for 2-D in vivo dosimetry by portal imaging. Technol Cancer Res Treat. 2017; doi:10.1177/1533034617711354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peca S, Brown D, Smith WL. In vivo EPID dosimetry detects interfraction errors in 3D-CRT of rectal cancer. In: Jaffray D, eds. IFMBE Proceedings World Congress on Medical Physics and Biomedical Engineering, 7–12 June 2015, Toronto, Canada, vol. 51 Cham: Springer. [Google Scholar]
- 8. Wiesendanger-Wittmer EM, Sijtsema NM, Muijs CT, Beukema JC. Systematic review of the role of a belly board device in radiotherapy delivery in patients with pelvic malignancies. Radiother Oncol. 2012;102(3):325–334. doi:10.1016/j.radonc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 9. Koelbl O, Vordermark D, Flentje M. The relationship between belly board position and patient anatomy and its influence on dose–volume histogram of small bowel for postoperative radiotherapy of rectal cancer. Radiother Oncol. 2003;67(3):345–349. doi:10.1016/S0167-8140(03)00164-6. [DOI] [PubMed] [Google Scholar]
- 10. Greer PB. 3D EPID based dosimetry for pre-treatment verification of VMAT – methods and challenges. J Phys Conf Ser. 2013;444(1):12010 doi:10.1088/1742-6596/444/1/012010. [Google Scholar]
- 11. Piermattei A, Fidanzio A, Stimato G, et al. In vivo dosimetry by an aSi-based EPID. Med Phys. 2006;33(11):4414–4422. doi:10.1118/1.2360014. [DOI] [PubMed] [Google Scholar]
- 12. Berry SL, Polvorosa CS, Wuu CS. A field size specific backscatter correction algorithm for accurate EPID dosimetry. Med Phys. 2010;37(6):2425–2434. doi:10.1118/1.3468578. [DOI] [PubMed] [Google Scholar]
- 13. Fidanzio A, Greco F, Mameli A, et al. Breast in vivo dosimetry by EPID. J Appl Clin Med Phys. 2010;11(4):249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cilla S, Fidanzio A, Greco F, et al. Correlation functions for Elekta aSi EPIDs used as transit dosimeter for open fields. J Appl Clin Med Phys. 2011;12(1):218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piermattei A, Greco F, Fidanzio A, et al. Real-time dose reconstruction for wedged photon beams: a generalized procedure. J Appl Clin Med Phys. 2011;12(4):124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piermattei A, Greco F, Azario L, et al. A National project for in vivo dosimetry procedures in radiotherapy: first results. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms. 2012;274:42–50. doi:10.1016/j.nimb.2011.12.004. [Google Scholar]
- 17. Bosset JF, Calais G, Daban A, et al. ; EORTC Radiotherapy Group. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: Assessment of acute toxicity and treatment compliance: report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. Eur J Cancer. 2004;40(2):219–224. doi:10.1016/j.ejca.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 18. Banerjee R, Chakraborty S, Nygren I, Sinha R. Small bowel dose parameters predicting grade ≥3 acute toxicity in rectal cancer patients treated with neoadjuvant chemoradiation: an independent validation study comparing peritoneal space versus small bowel loop contouring techniques. Int J Radiat Oncol Biol Phys. 2013;85(5):1225–1231. doi:10.1016/j.ijrobp.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 19. Sauer R, Fietkau R, Wittekind C, et al. ; German Rectal Cancer Group. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis. 2003;5(5):406–415. doi:10.1046/j.1463-1318.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- 20. Sauer R, Becker H, Hohenberger W, et al. ; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi:10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 21. Bosset JF, Collette L, Calais G, et al. ; EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi:10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 22. Braendengen M1, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26(22):3687–3694. doi:10.1200/JCO.2007.15.3858.A. [DOI] [PubMed] [Google Scholar]
- 23. Robertson JM, Söhn M, Yan D. Predicting grade 3 acute diarrhea during radiation therapy for rectal cancer using a cutoff-dose logistic regression normal tissue complication probability model. Int J Radiat Oncol Biol Phys. 2010;77(1):66–72. doi:10.1016/j.ijrobp.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 24. Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76(suppl 3):101–107. doi:10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 25. Mak AC, Rich TA, Schultheiss TE, Kavanagh B, Ota DM, Romsdahl MM. Late complications of postoperative radiation therapy for cancer of the rectum and rectosigmoid. Int J Radiat Oncol Biol Phys. 1994;28(3):597–603. doi:10.1016/0360-3016(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 26. Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52(1):176–183. doi:10.1016/S0360-3016(01)01820-X. [DOI] [PubMed] [Google Scholar]
- 27. Allal AS, Bischof S, Nouet P. Impact of the “belly board” device on treatment reproducibility in preoperative radiotherapy for rectal cancer. Strahlenther Onkol. 2002;178(5):259–262. doi:10.1007/s00066-002-0889-8. [DOI] [PubMed] [Google Scholar]
- 28. Kasabasic M, Faj D, Ivkovic A, Jurkovic S, Belaj N. Rotation of the sacrum during bellyboard pelvic radiotherapy. Med Dosim. 2010;35(1):28–30. doi:10.1016/j.meddos.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 29. Rozendaal RA, Mijnheer BJ, Hamming-Vrieze O, Mans A, van Herk M. Impact of daily anatomical changes on EPID-based in vivo dosimetry of VMAT treatments of head-and-neck cancer. Radiother Oncol. 2015;116(1):70–74. doi:10.1016/j.radonc.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 30. Fidanzio A, Azario L, Greco F, Cilla S, Piermattei A. Routine EPID in-vivo dosimetry in a reference point for conformal radiotherapy treatments. Phys Med Biol. 2015;60(8): N141–N150. doi:10.1088/0031-9155/60/8/N141. [DOI] [PubMed] [Google Scholar]
- 31. Nuyttens JJ, Robertson JM, Yan D, Martinez A. The position and volume of the small bowel during adjuvant radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2001;51(5):1271–1280. doi:10.1016/S0360-3016(01)01804-1. [DOI] [PubMed] [Google Scholar]
- 32. Munro P, Bouius DC. X-ray quantum limited portal imaging using amorphous silicon flat-panel arrays. Med Phys. 1998;25(5):689–702. doi:10.1118/1.598252. [DOI] [PubMed] [Google Scholar]
- 33. Antonuk LE. Electronic portal imaging devices: a review and historical perspective of contemporary technologies and research. Phys Med Biol. 2002;47(6): R31–R65. doi:10.1088/0031-9155/47/6/201. [PubMed] [Google Scholar]
- 34. Louwe RJW, McDermott LN, Sonke JJ, et al. The long-term stability of amorphous silicon flat panel imaging devices for dosimetry purposes. Med Phys. 2004;31(11):2989–2995. doi:10.1118/1.1803751. [DOI] [PubMed] [Google Scholar]
- 35. Nijsten SMJJG, van Elmpt WJC, Jacobs M, et al. A global calibration model for a-Si EPIDs used for transit dosimetry. Med Phys. 2007;34(10):3872–3884. doi:10.1118/1.2776244. [DOI] [PubMed] [Google Scholar]
- 36. Nijsten SMJJG, Mijnheer BJ, Dekker ALJ, Lambin P, Minken AWH. Routine individualised patient dosimetry using electronic portal imaging devices. Radiother Oncol. 2007;83(1):65–75. doi:10.1016/j.radonc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 37. Winkler P, Georg D. An intercomparison of 11 amorphous silicon EPIDs of the same type: implications for portal dosimetry. Phys Med Biol. 2006;51(17):4189–4200. doi:10.1088/0031-9155/51/17/005. [DOI] [PubMed] [Google Scholar]