Abstract
Purpose:
To explore the safety and efficacy of irreversible electroporation ablation in unresectable fibrous sarcoma with 2 electrodes.
Methods:
A 74-year-old woman with unresectable retroperitoneal malignant fibrous sarcoma was treated with percutaneous irreversible electroporation. Four ablations were performed on the mass, which measured 7.3 × 7.0 × 7.5 cm, with 2 electrodes.
Results:
A contrast-enhanced computed tomography scan 2 months postoperatively showed that the tumor had reduced to 5.1 × 4.0 × 5.2 cm, without obvious enhancement. Any adverse reactions were evaluated as level 1.
Conclusion:
In the short term, the treatment with 2 electrodes for fibrous sarcoma appears to be safe and effective.
Keywords: irreversible electroporation, percutaneous, 2-electrodes
Introduction
Soft-tissue sarcomas originate primarily from elements of the mesodermal embryonic layer, classified according to the adult tissue that they resemble. Each sarcoma presents in a different way, with unique therapeutic challenges. Current treatments of sarcomas, such as adjuvant chemotherapy, radiotherapy, and immunotherapy, have an unacceptable recurrence rate. A new method called irreversible electroporation (IRE) has recently been shown to be a more promising alternative to sarcoma ablation.1,2
Irreversible electroporation is an emerging nonthermal tissue ablation technology, which delivers short pulses of high-voltage, low-energy direct current to tumors to induce cell necrosis by creating cellular membrane disruption. Irreversible electroporation does not rely on tissue temperature changes as are radiofrequency or cryoablative procedures.3 Therefore, IRE could be able to preserve blood vessels, bile ducts, nerves, and other vital organs.4–6 Irreversible electroporation has been used in humans for pancreatic, liver, kidney, and prostate cancers.2,7 Here we report a case where a patient with fibrosarcoma received IRE treatment with 2-electrode arrays; follow-up examinations demonstrated complete tumor regression 2 months after IRE.
Case Report
A 74-year-old woman was found to have a soft-tissue mass in the peritoneum situated between the gallbladder and the left hepatic duct on an abdominal ultrasound in 1998. The tumor had a diameter of about 1.5 cm and a distinct boundary. The patient underwent retroperitoneal tumor resection during open abdominal surgery. Pathology of the tumor showed (retroperitoneal) malignant fibrosarcoma. She subsequently received systemic chemotherapy with doxorubicin, cisplatin, and fluorouracil, but this was discontinued after 4 cycles because of the lack of response.
She was admitted to our hospital with recurrence of the retroperitoneal malignant fibrous sarcoma in March 2016. At this time, the tumor was large (7.3 × 7.0 × 7.5 cm), and the surrounding tissue boundaries were unclear. She declined surgery because of the high risk associated with reoperation and decided to accept instead percutaneous IRE ablation.
Preoperative physical examination indicated body temperature 36.6°C, heart rate 69 per minute, respiration 18 per minute, blood pressure 104/65 mm Hg, functional status score 1; laboratory tests including blood, urine, and stool examinations were normal; liver and kidney functions were normal and she had a normal clotting time. Preoperative contrast-enhanced computed tomography (CT; CT SOMATOM Definition 64 AS; Siemens Medical Solutions, Forchheim, Germany) imaging revealed a mass of 7.3 × 7.0 × 7.5 cm in the retroperitoneum inferior to the vena cava near the portal triad, adjacent to the head of the pancreas and the left hepatic lobe, which were compressed, adherent, and being invaded (Figure 1).
Figure 1.
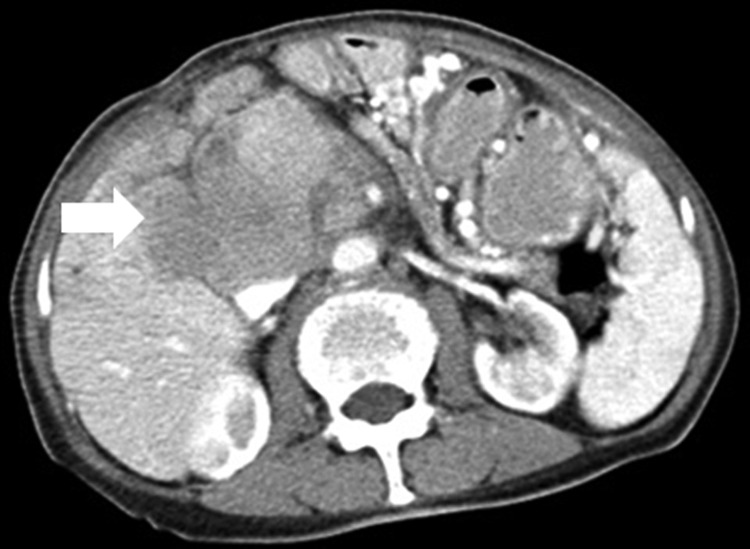
Preoperative contrast-enhanced computed tomography (CT) scan in a 74-year-old woman showing a 7.3 × 7.0 × 7.5 cm mass in retroperitoneal inferior vena cava near the portal triad (arrow), which was due to a malignant fibrous retroperitoneal sarcoma.
The patient gave written informed consent preoperatively. Irreversible electroporation was performed using the Angiodynamics Nanoknife system (Angiodynamics, Latham, New York). Treatment planning was based on preoperative CT imaging in which the tumor dimensions and morphology were measured. It is also helpful to know whether the lesion is visible or not with ultrasound. The treatment plan providing access points for the electrodes and the number of electrodes to be used was created and checked before the procedure. With this information, it is possible to calculate pulse amplitude that would need to be delivered. The treatment plan should also reflect the length of electrode that will be used. Percutaneous ablation was performed as 4 target sections to avoid the need for more than 2 electrodes to be placed at once.
The patient was administered general anesthesia in the supine position. Initially, the high voltage pulse caused muscle contractions and electrode displacement, so additional cisatracurium besilate for injection (Heng Rui Medicine Co. Ltd, Jiangsu, China) was given to achieve deep nerve muscle paralysis. All pulses were delivered in the ventricular absolute refractory period after a 0.05 seconds delay when an electrogastrogram synchronizer (an AccuSyneR synchronizer device) monitored the R wave from the patient and sent the sign to the IRE generator, thereby avoiding the occurrence of arrhythmias. Ultrasound (IU22; Philips Medical Systems, Bothell, WA, USA) was used to document accurate positioning of electrodes. The ablation zone was monitored and measured in real time with ultrasound.
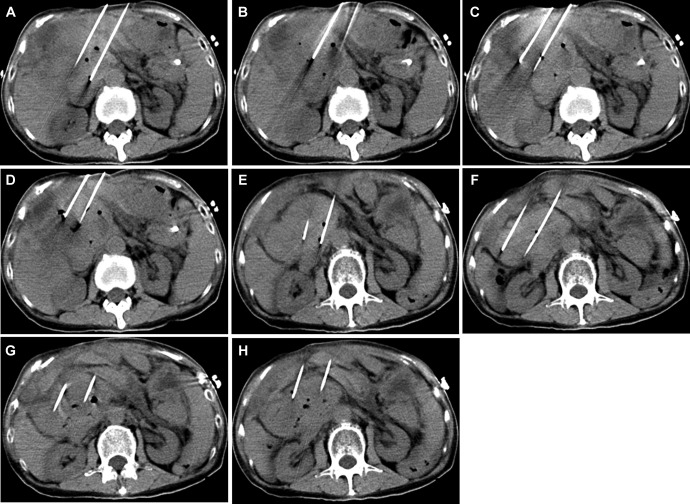
Two 15-cm monopolar electrodes (20400103 and 20400104; Nanoknife; AngioDynamics, Latham, New York) were placed in the first target section, and CT imaging was performed to further evaluate the electrode position relative to any vessels and to ensure an interprobe distance of 2 cm (Figure 2A). In the case of monopolar needle electrodes, the voltage setting for each electroporation was determined by the distance between each pair of needle electrodes, and the electrode terminal exposure deliver 1500 V/cm voltage-todistance ratio according to manufacturers’ recommendation. The active length of the electrodes was 2 cm. A standard default voltage of 1500 V/cm was initiated with the planned delivery of 90 pulses and a pulse width of 90 ms. The maximum current achieved was 48 A. The electrodes were then pulled back 1 cm to perform an overlapping ablation using a similar protocol, except that the voltage-to-distance ratio was lowered to 1300 V/cm (Figure 2B). The first target section was completed.
Figure 2.
Computed tomography (CT) images of the irreversible electroporation (IRE) treatment being performed (in all images, the electrode distance was 2 cm): (A) the first ablation; (B) the 2 electrodes pulled back 1 cm; (C) the second target section, with the 2 electrodes moved 2 cm toward the right; (D) the electrodes pulled back 1.2 cm; (E) the third target section with the 2 electrodes moved 2 cm toward to the left and down; (F) the last target section with the 2 electrodes moved 2 cm toward the right and down; (G and H) the electrodes pulled back 2 cm in the last target section.
Treatment of the second target section was carried out using the same parameters (Table 1) with the 2 electrodes moved 2 cm to the right (Figure 2C). Two pulse application sets, each composed of 90 microsecond pulses, were performed. The electrodes were then pulled back 1.2 cm to perform an overlapping ablation using the same parameters (Figure 2D). The last 2 target sections were treated using the same pulse parameters (Figure 2E-H). The duration of a single ablation was 1 to 2 minutes.
Table 1.
Physical and Pulse Parameters.a
| Pulse Application Set | Separation Between Electrodes (cm) | Voltage (V) | Repetition Rate (ms) |
|---|---|---|---|
| 1/1 | 2 | 3000 | 90 |
| 1/2 | 2 | 2600 | 90 |
| 2/1 | 2 | 2600 | 90 |
| 2/2 | 2 | 2600 | 90 |
| 3/1 | 2 | 2600 | 90 |
| 4/1 | 2 | 2600 | 90 |
| 4/2 | 2 | 2600 | 90 |
| 4/3 | 2 | 2600 | 90 |
aAll sets had pulse length of 90 μs.
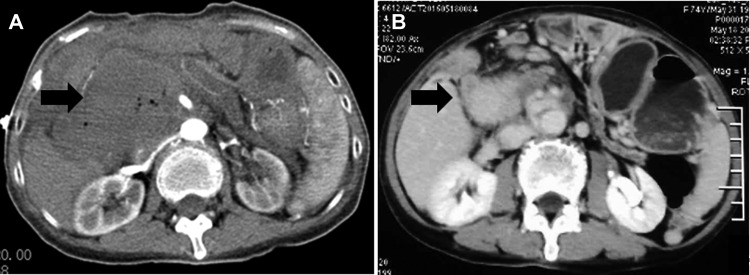
After the 2 electrodes had been removed, a contrast-enhanced CT was performed immediately and demonstrated a hyperdense ablation zone containing gas bubbles (Figure 3A); the gas bubbles were caused by the electrolysis of water by the electric current passing through the tissue.8 The portal vein and surrounding arteries were potent, and no leaky gut was observed.
Figure 3.
Contrast-enhanced computed tomography (CT) images showing: (A) a hypodense ablation zone containing gas bubbles immediately postoperatively (arrow); (B) the tumor narrowed to 5.1 × 4.0 × 5.2 cm, with no obvious enhancement 2 months postoperatively.
The patient awakened uneventfully in the intensive care unit. The next day she was transferred to the ward and experienced some pain from the puncture point but did not require analgesic medications during her admission. Any adverse reactions were evaluated as level 1 (National Cancer Institute Common Toxicity Criteria, version 3.0).9 There was no significant treatment-related bleeding or other serious complications. By day 4 postoperatively, the patient had made a good recovery and was able to leave the hospital.
A contrast-enhanced CT scan 2 months postoperatively showed that the tumor had reduced in size to 5.1 × 4.0 × 5.2 cm, and there was no obvious enhancement (Figure 3B). According to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines, the patient achieved complete response (CR).
Discussion
Irreversible electroporation is a new technology in clinical practice for tumor ablation. Compared with conventional thermal ablation techniques, such as radiofrequency, microwave, and cryoablation, IRE has following characteristics: (1) IRE ablation induces cell apoptosis,10,11 (2) IRE ablation success is not impaired by heat-sinking12 and prevents adjacent blood vessel damage,13 (3) IRE ablation can produce a sharp boundary between the affected and unaffected tissues and can reduce the damage to nontumor tissue, and (4) IRE ablation can effectively induce tissue necrosis and nonthermal cell death in microseconds to milliseconds.14 Beyer et al found that stereotactic IRE demonstrated a significant reduction of procedure length and higher accuracy compared to conventional IRE.15 For the above reasons, IRE ablation may be suitable for the treatment of tumors near the portal triad.
Retroperitoneal sarcoma near the liver blood vessels and portal triad can surround and compress the vessel lumens and other important organs, making it difficult to resect. In 2011, Neal et al reported the case of a female Labrador with a sarcoma on the left pelvic limb that was treated by IRE ablation; contrast-enhanced CT and histopathology showed complete tumor ablation after 6 months.16 This result suggested that IRE ablation was feasible and safe for soft-tissue sarcoma.
In our case, the patient had an unresectable retroperitoneal malignant fibrous sarcoma adjacent to the portal triad that was chemotherapy resistant, so we decided to trial IRE ablation with 2 electrodes. No significant treatment-related bleeding or other serious complications were noted in the perioperative period. A contrast-enhanced CT scan 2 months postoperatively showed the tumor had reduced from 7.3 × 7.0 × 7.5 to 5.1 × 4.0 × 5.2 cm, with no obvious enhancement of the tumor, suggesting the tumor had been completely ablated; according to mRECIST, the patient achieved CR. Our findings indicate that IRE ablation with 2 electrodes was safe and effective for this retroperitoneal malignant fibrous sarcoma.
In our patient, percutaneous IRE ablation was performed under CT and ultrasound guidance, using the 2 electrodes at a time, with the tumor having been divided into sections that were sequentially ablated. Using 2 electrodes only has some advantages. First, the method allows the ablation range to be judged accurately and can avoid the artifacts that multiple probes produce in CT images during percutaneous puncture. Second, the method can significantly reduce the probability of vessels being punctured. Third, with the probe being moved less than 1.5 cm, the ablation areas can be integrated, thereby avoiding the leaving of a nonablation zone. Fourth, intraoperative ultrasound can accurately monitor the size of the treatment effect in real time. Finally, intraoperatively, 2 electrodes are more easily fixed than multiple electrodes. The disadvantage, however, is that this method requires repeated ablations to be performed and, therefore, compared with the multielectrode arrays, using 2 electrodes only will increase the number of ablations.
In conclusion, this case report describes a technically successful percutaneous IRE procedure using 2 electrodes at a time for unresectable retroperitoneal malignant fibrous sarcoma. In the short term, this treatment appears to be safe and effective. For ablation of retroperitoneal sarcoma, 2-electrode approach also seems safe and effective.
Abbreviations
- CR
complete response
- CT
computed tomography
- IRE
irreversible electroporation
- mRECIST
Modified Response Evaluation Criteria in Solid Tumors
Footnotes
Authors’ Note: Zilin Qin and Jianying Zeng contributed equally to this work and share the first authorship. Informed consent was provided by the patient.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: International Scientific Fund of Fuda Cancer Hospital, Guangzhou (Y2015-ZD -001).
References
- 1. Yu Z, Zhang X, Ren P, Zhang M, Qian J. Therapeutic potential of irreversible electroporation in sarcoma. Expert Rev Anticancer Ther. 2012;12(2):177–184. [DOI] [PubMed] [Google Scholar]
- 2. Scheffer HJ, Nielsen K, de Jong MC, et al. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25(7):997–1011. [DOI] [PubMed] [Google Scholar]
- 3. Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255(2):426–433. [DOI] [PubMed] [Google Scholar]
- 4. Kos B, Voigt P, Miklavcic D, Moche M. Careful treatment planning enables safe ablation of liver tumors adjacent to major blood vessels by percutaneous irreversible electroporation (IRE). Radiol Oncol. 2015;49(3):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi JW, Lu DS, Osuagwu F, Raman S, Lassman C. Assessment of chronological effects of irreversible electroporation on hilar bile ducts in a porcine model. Cardiovasc Intervent Radiol. 2014;37(1):224–230. [DOI] [PubMed] [Google Scholar]
- 6. Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC., II Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107(5):544–549. [DOI] [PubMed] [Google Scholar]
- 7. van Gemert MJ, Wagstaff PG, de Bruin DM, et al. Irreversible electroporation: just another form of thermal therapy? Prostate. 2015;75(3):332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faroja M, Ahmed M, Appelbaum L, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology. 2013;266(2):462–470. [DOI] [PubMed] [Google Scholar]
- 9. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. [DOI] [PubMed] [Google Scholar]
- 10. Lee YJ, Lu DS, Osuagwu F, et al. Irreversible electroporation in porcine liver: acute computed tomography appearance of ablation zone with histopathologic correlation. J Comput Assist Tomogr. 2013;37(2):154–158. [DOI] [PubMed] [Google Scholar]
- 11. Xiao D, Yao C, Liu H, et al. Irreversible electroporation and apoptosis in human liver cancer cells induced by nanosecond electric pulses. Bioelectromagnetics. 2013;34(7):512–520. [DOI] [PubMed] [Google Scholar]
- 12. Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223–231. [DOI] [PubMed] [Google Scholar]
- 13. Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6(4):307–312. [DOI] [PubMed] [Google Scholar]
- 14. Lee EW, Wong D, Prikhodko SV, et al. Electron microscopic demonstration and evaluation of irreversible electroporation-induced nanopores on hepatocyte membranes. J Vasc Interv Radiol. 2012;23(1):107–113. [DOI] [PubMed] [Google Scholar]
- 15. Beyer LP, Pregler B, Niessen C, et al. Stereotactically-navigated percutaneous Irreversible Electroporation (IRE) compared to conventional IRE: a prospective trial. PeerJ. 2016;4:e2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neal RE, II, Rossmeisl JH, Jr, Garcia PA, Lanz OI, Henao-Guerrero N, Davalos RV. Successful treatment of a large soft tissue sarcoma with irreversible electroporation. J Clin Oncol. 2011;29(13):e372–e377. [DOI] [PubMed] [Google Scholar]