Abstract
MicroRNAs have been reported to play an important role in diverse biological processes and cancer progression. MicroRNA-7 has been observed to be downregulated in human gastric cancer tissues, but the function of microRNA-7 in gastric cancer has not been well investigated. In this study, we demonstrate that the expression of microRNA-7 was significantly downregulated in 30 pairs of human gastric cancer tissues compared to adjacent normal tissues. Enforced expression of microRNA-7 inhibited cell proliferation and migration abilities of gastric cancer cells, BGC823 and SGC7901. Furthermore, microRNA-7 targeted mTOR in gastric cancer cells. In human clinical specimens, mTOR was higher expressed in gastric cancer tissues compared with adjacent normal tissues. More interestingly, microRNA-7 also sensitizes gastric cancer cells to cisplatin (CDDP) by targeting mTOR. Collectively, our results demonstrate that microRNA-7 is a tumor suppressor microRNA and indicate its potential application for the treatment of human gastric cancer in future.
Keywords: miRNA-7, tumor growth, mTOR, cisplatin, gastric cancer
Introduction
Gastric cancer (GC) is one of the most common types of malignancies around the world. The incidence and mortality rate of GC have decreased worldwide over the past 20 years; however, it still ranks as the most common gastrointestinal malignancy and the second leading cause of cancer death in East Asia.1 Despite recent advances in gastrectomy, radiotherapy, and chemotherapy, more than half of all patients with advanced stage GC die of recurrence, even after undergoing curative gastrectomy.2 Thus, elucidation of the regulatory mechanisms modulating GC carcinogenesis and the molecular alterations underlying this disease is urgent for developing novel targeted therapies for GC.3
Previous studies have shown that the tumorigenesis of GC is involved in both genetic and epigenetic alterations, including the activation of oncogenes and/or the suppression of tumor suppressor genes.4–6 Mounting evidence suggests that noncoding microRNAs (miRNAs) play key roles in the development of GC. MicroRNAs s are a large class of endogenous noncoding RNAs, 21-23 nucleotides in length, that regulate about 30% of human gene expression.7 MicroRNAs can function through imperfect base pairing with specific sequences in the 3′ untranslated regions (3′-UTRs) of target messenger RNAs (mRNAs), leading to transcript degradation or translational inhibition.8
MicroRNAs have been associated with various types of cancer, which involved in many important physiological and pathological processes, including development, differentiation, and tumorigenesis. By binding to the 3′-UTR of mRNA, miRNA suppresses protein synthesis through mRNA degradation or translational repression. As a result, miRNAs can act as either tumor suppressors or oncogenes. As a tumor suppressor gene in several cancer types, microRNA-7 (miR-7) can affect tumor cell growth, migration, invasion, and apoptosis. To date, some genes have been identified as miR-7 target genes, including RelA, REGγ, RAF1, EGFR, CCNE1, and YY1.9–14
However, the function and the underlying mechanism of miR-7 in regulating gastric tumorigenesis are still to be further investigated. Interestingly, it has been reported that miR-7 regulates malignant behavior of GC cells by targeting insulin-like growth factor 1 receptor (IGF1R) and epidermal growth factor receptor (EGFR).15,16 In our study, we found that miR-7-targeted mTOR, a key downstream effector of the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway, has long been identified to play an important role in controlling cancer cell growth.17
It has been reported that miR-7 may play roles in GC via affecting different signaling pathways. In our study, we aimed to further identify the roles of miR-7 and its molecular as well as cellular mechanisms in GC. Overexpression of miR-7 inhibited cell proliferation and invasion of GC cells by suppressing a key target mTOR. We further defined miR-7-induced chemosensitivity of GC cells to cisplatin through mTOR suppression. Our data showed that the miR-7 expression was significantly downregulated in human GC tissues compared with the adjacent paired normal controls. Our results revealed a novel mechanism of miR-7 in GC, and it possessed a potential to be used as a novel strategy to develop miR-7-based therapeutics.
Results
MicroRNA-7 Is Significantly Downregulated in GC Tissues and Cells
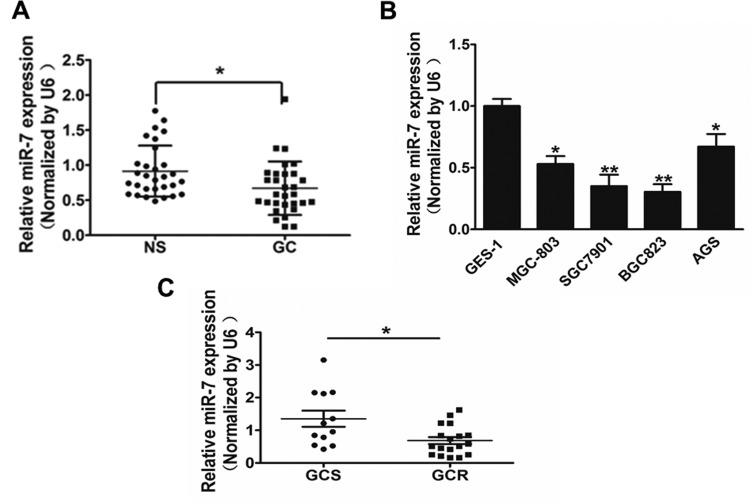
In our study, we assessed the miR-7 expression in 30 pairs of GC tissues and adjacent normal tissues, and Figure 1A showed that the miR-7 expression in tumor tissues was significantly lower compared with those controls. In addition, expression of miR-7 in 4 GC cell lines (MGC-803, AGS, SGC-7901, and BGC-823) was significantly decreased compared with the GES-1 cells, which is a normal gastric epithelial cell line that was chosen as a control (Figure 1B). To identify whether miR-7 affects the cisplatin effect, we classified the 30 specimens into cisplatin sensitivity (GCS) and resistance (GCR) and then assessed the miR-7 expression. Our results indicated that miR-7 was downregulated in GC tissues, cell lines, and GCR tissues.
Figure 1.
MicroRNA-7 (miR-7) is significantly downregulated in gastric cancer (GC) tissues and cells. A, Relative miR-7 expression levels were analyzed by qRT-PCR in 30 pairs of human GC tissues and adjacent normal tissues. U6 RNA level was used as an internal control. B, Relative miR-7 expression was analyzed in GES-1 cells (which is a normal gastric epithelial cell line that was chosen as a control) and 4 GC cell lines (MGC-803, SGC-7901, BGC-823, and AGS). C, Relative miR-7 expression levels were analyzed by qRT-PCR in 30 human GC tissues grouped to CDDP sensitive (GCS) and CDDP resistance (GCR). U6 RNA level was used as an internal control. Data represent mean (standard deviation) of 3 replicates. *Significant difference at P < .05. **Significant difference at P < .01.
Overexpression of miR-7 Inhibits Cell Proliferation and Cell Invasion of GC Cells
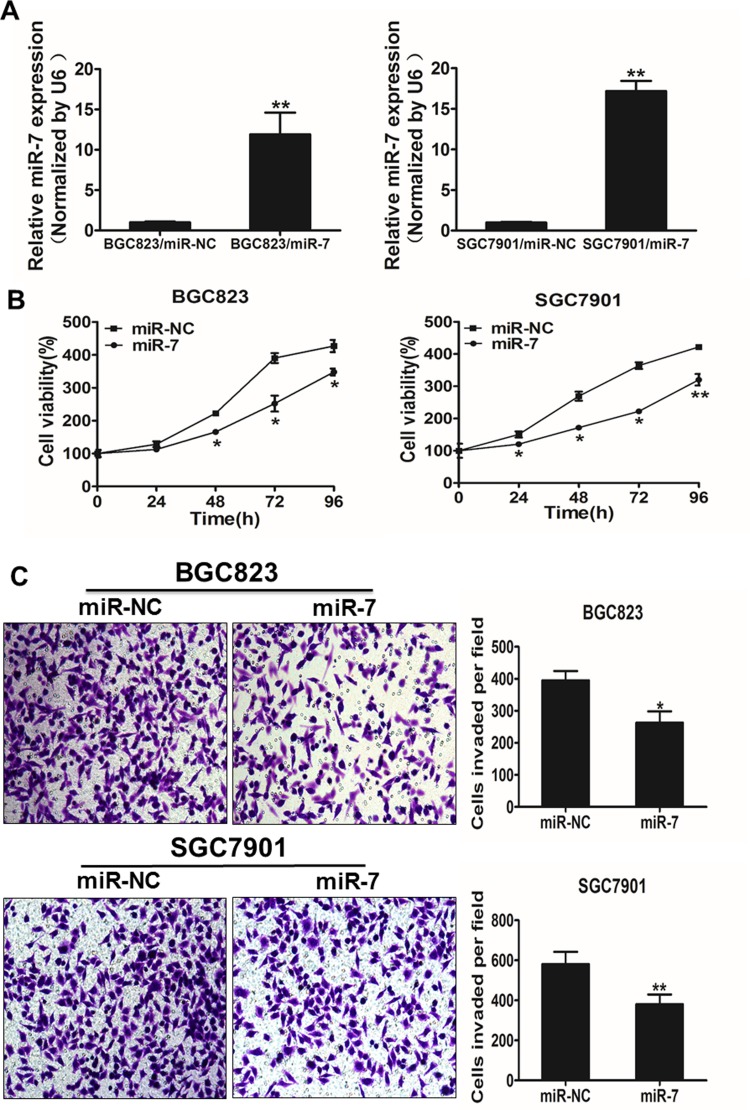
To explore the direct role of miR-7 in GC cells, we established stable cell lines by infecting BGC-823 and SGC7901 cells with lentiviral constructs harboring miR-7 or miR-7 negative control (miR-NC), then followed by selection of puromycin (Figure 2A). Cell viability assay indicated that the overexpression of miR-7 significantly reduced the rate of cell proliferation at 48 hours after the cell seeding (Figure 2B). Since invasion is key characteristics of malignant tumor, we next investigated the effects of miR-7 on invasion in vitro. Forced expression of miR-7 also markedly decreased the invasive ability of cancer cells (Figure 2C).
Figure 2.
Overexpression of miR-7 inhibits the ability of cell proliferation and cell invasion in gastric cancer (GC) cells. A, Relative expression levels of microRNA-7 (miR-7) in BGC823/miR-7, BGC823/miR-NC, SGC7901/miR-7, and SGC7901/miR-NC stable cell lines were confirmed by RT-qPCR. B, Overexpression of miR-7 arrested cell proliferation in BGC823 and SGC7901 cells. C, The MiR-7 overexpression reduced cell invasion in BGC823 and SGC7901 cells. Data represent mean (standard deviation) of 3 replicates. *Significant difference at P < .05. **Significant difference at P < .01.
mTOR is a Target of miR-7
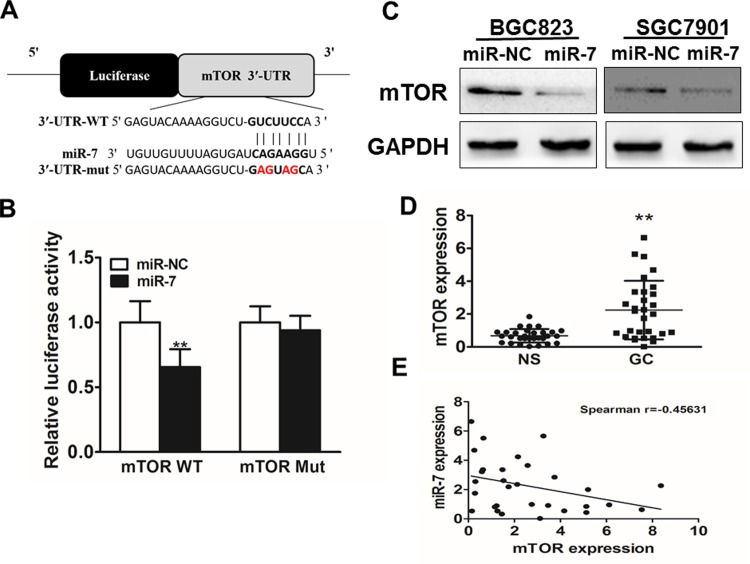
To analyze the underlying molecular mechanism of miR-7 in GC, TargetScan and miRanda (www.targetscan.org; www.microrna.org) were used to explore potential targets of miR-7 in GC. Figure 3A shows that the 3′-UTR of mTOR contained the binding site for the seed region of miR-7. To confirm mTOR was the direct target of miR-7 in GC, human mTOR 3′-UTR, containing either wild-type or mutant miR-7 binding sequence, then was cloned downstream of the firefly luciferase reporter gene in the pMIR-reporter vector. SGC7901 cells were transfected with 2 reporter plasmids, plus either miR-7 or miR-NC mimics. The luciferase activity of the reporter in the vector containing the mTOR 3′-UTR (wild type) was significantly reduced by miR-7, while the mTOR 3′-UTR (mutant) exhibited an insignificantly affected luciferase activity (Figure 3B). Western blotting analysis was conducted to determine the mTOR expression at the protein level. We found that the mTOR expression was downregulated at the protein level in miR-7-treated cells (Figure 3C). These results suggested that miR-7 directly targeted mTOR by binding to its 3′-UTRs in GC cells. Furthermore, we measured the mTOR expression at the mRNA level in human GC specimens and normal tissues. The results showed that the average expression level of mTOR was significantly higher in tumor tissues than that in normal tissues (Figure 3D). Then, we determine the correlation between mTOR levels and miR-7 expression levels in the same GC tissues. As shown in Figure 3E, Spearman rank correlation analysis showed that the expression levels of mTOR and miR-7 in 30 GC specimens were inversely correlated (Spearman correlation r = −.45631). Thus, these results suggested that mTOR is a target of miR-7.
Figure 3.
mTOR is a target of microRNA-7 (miR-7). A, Sequence of the miR-7 binding site within the human mTOR 3′-UTR and a schematic diagram of the reporter construct showing the entire mTOR 3′-UTR sequence and the mutant mTOR 3′-UTR sequence. The mutant nucleotides of the mTOR 3′-UTR are labeled in red. B, Luciferase assay on SGC7901 cells, which were cotransfected with miR-NC or miR-7, and a luciferase reporter containing the full length of mTOR 3′-UTR (WT) or a mutant (MT) harboring 4 mutant nucleotides of the miR-7-binding site. Luciferase activities were measured 24-hour posttransfection. MicroRNA-7 markedly suppressed luciferase activity in mTOR 3′-UTR (WT) reporter constructs. The data represent mean (SD) for separate transfections (n = 4). C, The immunoblotting showed that expression levels of mTOR were decreased in cells with miR-7 overexpression. D, The expression of mTOR in adjacent normal tissues and human GC specimens was determined by quantitative RT-PCR analysis, and fold changes were obtained from the ratio of mTOR to GAPDH levels. E, Spearman correlation analysis was used to determine the correlation between the expression levels of mTOR and miR-7 in GC specimens. Data represent mean (standard deviation) of 3 replicates. **Significant difference at P < .01.
miR-7 Renders GC Cells More Sensitive to Cisplatin Treatment by Targeting mTOR
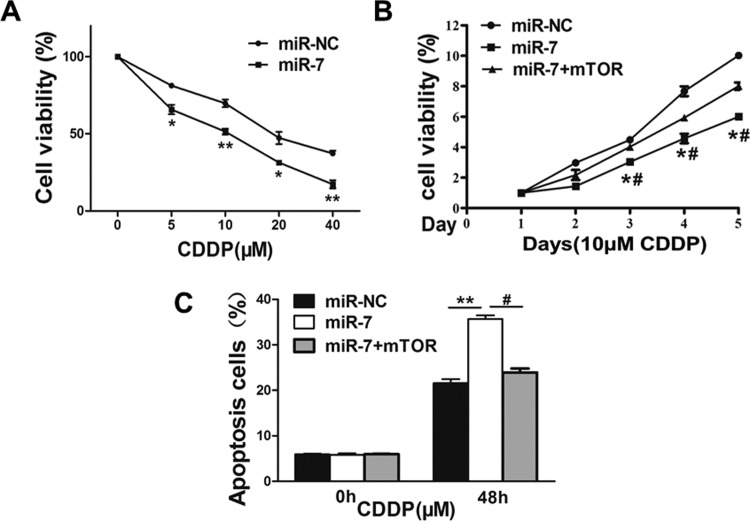
Resistance to cisplatin treatment is one of the major causes for the failure of chemotherapy in cancer treatment. Therefore, it is critical to discover new strategies to increase cisplatin effect for therapeutic purposes. Our results showed that overexpression of miR-7 significantly increased chemosensitivity to cisplatin treatment in SGC7901 cells (Figure 4A). Furthermore, cell growth rate was assayed using Cell Counting Kit-8 (CCK-8) proliferation assay in the presence of cisplatin at different time points, and we found that forced expression of mTOR resulted in more resistance to cisplatin treatment in miR-7-overexpressing cells (Figure 4B). To further test whether miR-7 and its target mTOR play a role in cellular apoptosis in the presence of cisplatin treatment, FACS (Fluorescence-activated cell sorting) analysis was performed. The combination of miR-7 and cisplatin treatment significantly induced cellular apoptosis, whereas forced expression of mTOR partially abolished the apoptotic effect induced by the combination of miR-7 and cisplatin treatments (Figure 4C). These results indicate that miR-7 renders GC cells more sensitive to cisplatin treatment for inducing apoptosis through targeting mTOR.
Figure 4.
MicroRNA-7 regulates cisplatin chemosensitivity by targeting mTOR in gastric cancer cells. A, SGC7901 cells stably expressing miR-7 negative control (miR-NC) or microRNA-7 (miR-7) were treated with different concentrations of cisplatin for 48 hours and analyzed by Cell Counting Kit-8 (CCK-8) assay. B, SGC7901 cells stably expressing miR-NC, miR-7, or miR-7 in combination with mTOR overexpression were treated with 10 μM of cisplatin for indicated time points. Cell proliferation was analyzed by CCK-8 assay, and cell apoptosis was analyzed by flow cytometry in SGC7901 cells. C, Data represent mean (standard deviation) from 3 replicates. *Significant difference at P < .05 compared to miR-NC control. **Significant difference at P < .01 compared to miR-NC control. #Significant difference at P < .05 compared to miR-7 and mTOR overexpression.
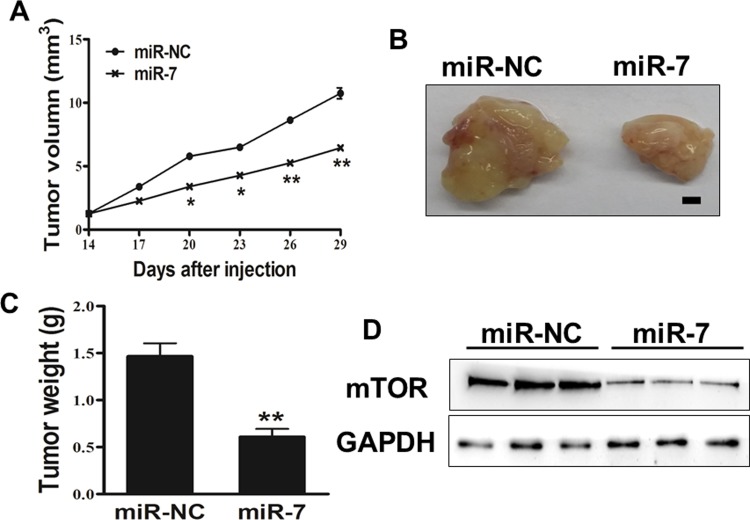
miR-7 Inhibits Tumor Growth In Vivo
To investigate the effect of miR-7 on tumor growth in vivo, SGC7901 cells overexpressing miR-7 or miR-NC were subcutaneously injected into both posterior flanks of nude mice (n = 6). Xenograft tumor volumes were determined every 2 days when they became visible. On day 14 postimplantation, the tumor growth of miR-7 overexpression group was significantly smaller than that of the control group (Figure 5A). Nude mice were killed on day 29 after the injection, and xenografts were collected. Figure 5B shows the representative xenograft tumors. The average tumor weight of the miR-7 overexpression group was markedly reduced by 60% compared with controls (Figure 5C). Total proteins from representative tumor samples were analyzed by Western blotting, and results showed that miR-7 suppressed the expressions of mTOR in vivo (Figure 5D). Thus, these findings suggested that miR-7 inhibited the tumor growth through targeting mTOR in vivo.
Figure 5.
MicroRNA-7 (miR-7) inhibits tumor growth in vivo. A, B, and C, Effect of miR-7 on the growth of SGC7901 cells inoculated into nude mice. Male BALB/cA nude mice were subcutaneously injected with 5 × 106 SGC7901 cells infected with lentiviruses harboring miR-7 negative control (miR-NC) or miR-7. Tumor volume and weight of 6 tumor tissues were monitored over time as indicated, and the tumor was excised and weighed after 14 days. MicroRNA-7 caused a decrease in tumor volume and weight. Bar = 2 mm. D, The expression levels of mTOR proteins from the 3 tumor tissues of miR-7 expressing group were lower than those of the miR-NC group. Data represent mean (standard deviation). *Significant difference at P < .05. **Significant difference at P < .01.
Discussion
Recent studies have demonstrated that miRNAs play important roles in carcinogenesis by a number of mechanisms, and certain miRNAs have been reported to be correlated with clinical characteristics and outcomes. The role of some miRNAs in GC and drug resistance has also been reported. For example, miR-508-5p regulates multidrug resistance by targeting ZNRD1 and ABCB1 in GC18; miR-34a modulated PI3K/AKT pathway and regulated cisplatin-induced GC cell death.19 MiR-15b and miR-16 have been reported to modulate multidrug resistance in human GC cells by targeting BCL2.20
Previous studies have shown that miR-7 is downregulated in several cancer types including GC. miR-7 have been reported to inhibit the invasion and metastasis ability of GC cells by suppressing EGFR expression.15 Furthermore, miR-7 functions as an antimetastatic miRNA by targeting IGF1R in GC.16 Meanwhile, other study also demonstrated that miR-7 targeted mTOR and inhibited proliferation and invasive behavior in GC cells. Here, consistent with previous studies, we found that miR-7 was downregulated in GC tissues compared with normal controls, and the miR-7 was decreased in human GC cell lines. More interestingly, we further found overexpression of miR-7 inhibited cell proliferation and cell invasion of GC cells. Thus, we demonstrated that miR-7 regulated GC growth, which provide new therapeutic strategies for GC prevention and treatment.
mTOR, a serine/threonine protein kinase, comprises 2 different complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which are structurally similar but functionally different. mTOR is a downstream effector of PI3K/AKT. mTOR complex 1 is the target of rapamycin and rapamycin analogs, such as everolimus, and leads to cell anabolic growth by promoting mRNA translocation and protein synthesis,21,22 and also has roles in lipid synthesis and glucose metabolism. In our study, mTOR oncogene was experimentally validated as the novel target of miR-7 not only in vitro but also in vivo. Firstly, luciferase reporter assay showed that miR-7 directly recognized the 3′-UTR of mTOR transcripts. Secondly, the mTOR expression was significantly decreased in GC cells stably expressing miR-7. Thirdly, mTOR was upregulated in GC tissues. These results show that miR-7 is a tumor suppressor through targeting mTOR.
Recently, miRNAs have been proposed to play essential roles in the development of drug resistance.23–26 Resistance to chemotherapy is a result from a lot of factors including individual differences in patients and genetic or epigenetic changes in tumors, even those from the same tissue of origin.27 In this study, we found that forced overexpression of miR-7 promoted the inhibition effects of CDDP. Flow cytometer assay demonstrated that GC cells with miR-7 expression have CDDP higher sensitiveness to apoptosis. Therefore, it is important that an miR-7 restoration approach may offer a new modulation strategy to overcome chemoresistance to CDDP treatment in GC.
In summary, our study provided the first evidence that miR-7 played a significant role in suppressing GC cell growth through inhibition of mTOR. Although we confirmed that miR-7 could inhibit the phenotype of GC by targeting mTOR, there might be other targets of miR-7, which could also affect the growth of GC cells. Nonetheless, we showed that such effect was exerted through the suppression of mTOR. Therefore, future studies are required to identify additional targets and pathways of miR-7.
Materials and Methods
Cell Culture and Clinical Tissues
Human GC cell lines SGC7901, MGC-803, and BGC-823 were cultured in RPMI 1640 medium, AGS was cultured in F-12 K medium, GES-1 and HEK293 T cells were cultured in DMEM medium. All cells were incubated at 37°C in an atmosphere of 5% CO2. Gastric cancer tissues and the adjacent normal tissues were collected from clinical patients undergoing GC resection. All these tissues were immediately snap frozen in liquid nitrogen after surgery.
Lentiviral Packaging and Stable Cell Line Establishment
To stably overexpress miR-7 in GC cells, the lentiviral packaging kit was used (Thermo Fisher Scientific, Shanghai, China). Lentivirus carrying miR-7 or miR-NC was packaged following the manufacturer’s manual. Lentivirus was packaged in HEK293 T cells and secreted into the medium. Cells were infected by lentivirus carrying miR-7 or miR-NC with the presence of polybrene (Sigma-Aldrich, Shanghai, China) and selected by puromycin (Sigma-Aldrich, Shanghai, China) for 2 weeks to obtain stable cell lines.
Oligonucleotides and Cell Transfection
Cells were seeded into plates and incubated at 37°C and 5% CO2 overnight. Micro RNA-7 mimics and miR-NC were chemically synthesized by GenePharma. Cells at 50% to 70% confluence were transfected with miR-7 or miR-NC using Lipofectamine reagent (Invitrogen, Shanghai, China), according to the manufacturer’s instructions. Transfected cells were harvested at 24 or 48 hours after transfection.
RNA Extraction and Real-Time RT-PCR
RNA was extracted from cultured cells using TRIzol reagent (Invitrogen, Shanghai, China), and purified RNA was stored at −80°C prior to further analysis. Real-time RT-PCR analysis for mature miR-7 was carried out in triplicate using the RT Reagent Kit (Vazyme, Nanjing, China), according to the manufacturer’s instructions. Briefly, 500 ng total RNA was reversely transcribed into complementary DNA, and quantitative RT-PCR was performed using SYBR Green Master Mix (Vazyme, Nanjing, China) on a 7900HT system. The miR-7 expression in each group was determined relative to that of U6, and fold changes were calculated by relative quantification (2−▵▵Ct).
Cell Proliferation Assay
Cell counting Kit-8 (Dojindo Laboratories, Dalian, China) assay was used to determine cell viability. Cells were seeded at a density of 2000 per well in 96-well plates and cultured as described above for 48 hours after transfection. After 24, 48, 72, and 96 hours incubation, CCK-8 was added into each well, followed by 1 to 2 hours incubation. Absorbance at a wavelength of 450 nm was then determined. Experiments were carried out in triplicate.
Invasion Assay
The effect of miR-7 on cell invasion was investigated using 24-well BD Matrigel invasion chambers (BD Biosciences, Shanghai, China), according to the manufacturer’s instructions. The transfected cells (5 × 104) were seeded in the upper well of the invasion chamber containing serum-free RPMI-1640, and RPMI-1640 containing 10% FBS was applied to the lower chamber. After 18 to 24 hours, any noninvading cells on the top well were removed with a cotton swab, while cells in the bottom well were fixed with 3% paraformaldehyde and stained with 0.1% crystal violet. Images were captured in 3 independent fields. The membrane was air-dried, soaked with 33% acetic acid for 15 minutes at room temperature, and then transferred to a 96-well plate. The absorbance was recorded at 570 nm. Results were obtained from 3 independent experiments.
Western Blot
Cells were treated as previously described, and cells were harvested after 24 hours and lyzed in radioimmunoprecipitation assay buffer supplemented with protease inhibitors on ice for 30 minutes. After 15-minute centrifugation, protein concentrations were determined by the BCA method (Beyotime, Nanjing, China) and then separated by 10% SDS-PAGE. Subsequently, protein was electrically transferred onto a nitrocellulose membrane (Whatman). The membrane was incubated with mTOR antibody (1:1000; Cell Signaling Technology, Shanghai, China) and GAPDH (1:5000; Bioworld Technology, Nanjing, China) at 4°C overnight.
Luciferase Reporter Assay
TargetScan software was used to predict miR-7 binding sites. A fragment of 3′-UTR of mTOR containing the putative miR-7 binding site was amplified by PCR. To generate a construct containing the mutant miR-7 binding site, 2 nucleotides corresponding to the 5′-seeding region of the miR-7 binding site on the wild-type fragment were substituted. Its complementary sequence in the 3′-UTR of mTOR (GUCUUCC) was replaced by GAGUAGC. The PCR products were digested using HindIII and SacI, then inserted into pMIR-reporter and validated by DNA sequencing. Constructs were transfected into SGC7901 cells in 24-well plates and cotransfected with miR-7 or miR-NC. Luciferase assays were performed at 24 hours posttransfection using the Dual Luciferase Reporter Assay System (Promega).
In Vitro Chemosensitivity Array
Cancer cells were seeded in a 96-well plate overnight at a density of 4000 cells per well. Freshly prepared cisplatin solution (Sigma-Aldrich) was added into medium to obtain final culture concentrations ranging from 5 to 40 μM. Cell viability was assayed using CCK-8 kit 48 hours later.
Apoptosis Assay
Apoptosis assay was measured by flow cytometry. For annexin V staining, 5 μL phycoerythrin–annexin V, 5 μL propidium iodide, and 400 μL 1× binding buffer were added to the cell samples and incubated for 15 minutes at room temperature in the dark. Samples were analyzed by flow cytometry (BD Biosciences) within 1 hour. Three experiments were performed in triplicate.
Xenograft Studies
For tumor growth assay, male nude mice (6 weeks old) were purchased from Shanghai Laboratory Animal Center, and animals were maintained under special pathogen-free conditions. Aliquots of cells (5 × 106) were suspended in 150 μL of FBS-free RPMI 1640 medium and subcutaneously injected into each side of the posterior flank of nude mice. Tumor size was measured every 2 days using vernier caliper when they became visible, and the tumor volume was calculated according to the formula: Volume = 0.5 × Length × Width2.
Statistical Analysis
All experiments were performed in triplicate, and data were analyzed with GraphPad Prism 5 (La Jolla, California). The correlation between miR-7 expression and mTOR levels in gastric tissues was analyzed using Spearman rank test. Statistical evaluation for data analysis was determined by t test. P < .05 was considered as statistically significant.
Abbreviations
- BCA
The bicinchoninic acid assay
- CCK-8
Cell counting Kit-8
- EGFR
epidermal growth factor receptor
- FBS
fetal bovine serum
- GAPDH
Glyceraldehyde-3-Phosphate Dehydrogenase
- GC
gastric cancer
- GCS
CDDP sensitive of gastric cancer
- GSR
CDDP resistance of gastric cancer
- IGF1R
insulin-like growth factor 1 receptor
- miRNAs
microRNAs
- miR-7
microRNA-7
- mRNA
messenger RNA
- mTOR
Mechanistic Target Of Rapamycin
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- miR-NC
miR-7 negative control
- PI3K
phosphoinositide 3-kinase
- RT-PCR
real-time polymerase chain reaction
- UTRs
untranslated regions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14(4):249–258. [PubMed] [Google Scholar]
- 2. Kim SJ, Wang YG, Lee HW, et al. Up-regulation of neogenin-1 increases cell proliferation and motility in gastric cancer. Oncotarget. 2014;5(10):3386–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ren J, Huang HJ, Gong Y, Yue S, Tang LM, Cheng SY. MicroRNA-206 suppresses gastric cancer cell growth and metastasis. Cell Biosci. 2014;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo J, Yu W, Su H, Pang X. Genomic landscape of gastric cancer: molecular classification and potential targets. Sci China Life Sci. 2017;60(2):126–137. [DOI] [PubMed] [Google Scholar]
- 5. Jia ZF, Zhang SL, Cao XY, Zhou BS, Jiang J. Interaction between Helicobacter pylori and host genetic variants in gastric carcinogenesis. Future oncol. 2016;12(18):2127–2134. [DOI] [PubMed] [Google Scholar]
- 6. Tsai MM, Wang CS, Tsai CY, et al. Potential diagnostic, prognostic and therapeutic targets of microRNAs in human gastric cancer. Int J Mol Sci. 2016;17(6). pii: E945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giles KM, Brown RA, Ganda C, et al. microRNA-7-5p inhibits melanoma cell proliferation and metastasis by suppressing RelA/NF-kappaB. Oncotarget. 2016;7(22):31663–31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z, Liu Y, Li L, et al. MiR-7-5p is frequently downregulated in glioblastoma microvasculature and inhibits vascular endothelial cell proliferation by targeting RAF1. Tumour Biol. 2014;35(10):10177–10184. [DOI] [PubMed] [Google Scholar]
- 11. Shi Y, Luo X, Li P, et al. MiR-7-5p suppresses cell proliferation and induces apoptosis of breast cancer cells mainly by targeting REGγ. Cancer Lett. 2015;358(1):27–36. [DOI] [PubMed] [Google Scholar]
- 12. Zhang N, Li X, Wu CW, et al. MicroRNA-7 is a novel inhibitor of YY1 contributing to colorectal tumorigenesis. Oncogene. 2013;32(42):5078–5088. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Hu S, Zhang X, et al. MicroRNA-7 arrests cell cycle in G1 phase by directly targeting CCNE1 in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2014;443(3):1078–1084. [DOI] [PubMed] [Google Scholar]
- 14. Zhou X, Hu Y, Dai L, et al. MicroRNA-7 inhibits tumor metastasis and reverses epithelial-mesenchymal transition through AKT/ERK1/2 inactivation by targeting EGFR in epithelial ovarian cancer. PloS One. 2014;9(5):e96718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie J, Chen M, Zhou J, et al. MiR-7 inhibits the invasion and metastasis of gastric cancer cells by suppressing epidermal growth factor receptor expression. Oncolo Rep. 2014;31(4):1715–1722. [DOI] [PubMed] [Google Scholar]
- 16. Zhao X, Dou W, He L, et al. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32(11):1363–1372. [DOI] [PubMed] [Google Scholar]
- 17. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer cell. 2007;12(1):9–22. [DOI] [PubMed] [Google Scholar]
- 18. Shang Y, Zhang Z, Liu Z, et al. MiR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33(25):3267–3276. [DOI] [PubMed] [Google Scholar]
- 19. Cao W, Yang W, Fan R, et al. miR-34a regulates cisplatin-induce gastric cancer cell death by modulating PI3K/AKT/survivin pathway. Tumour Biol. 2014;35(2):1287–1295. [DOI] [PubMed] [Google Scholar]
- 20. Xia L, Zhang D, Du R, et al. MiR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J cancer. 2008;123(2):372–379. [DOI] [PubMed] [Google Scholar]
- 21. Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804(3):433–439. [DOI] [PubMed] [Google Scholar]
- 22. Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62(20):5645–5650. [PubMed] [Google Scholar]
- 23. Chen S, Chen X, Xiu YL, Sun KX, Zong ZH, Zhao Y. MicroRNA 490-3P enhances the drug-resistance of human ovarian cancer cells. J Ovarian Res. 2014;7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong L, Han Y, Zhang Y, et al. MicroRNA-21: a therapeutic target for reversing drug resistance in cancer. Expert Opin Ther Targets. 2013;17(9):1073–1080. [DOI] [PubMed] [Google Scholar]
- 25. Liu R, Liu X, Zheng Y, et al. MicroRNA-7 sensitizes non-small cell lung cancer cells to paclitaxel. Oncol Lett. 2014;8(5):2193–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rolfo C, Fanale D, Hong DS, et al. Impact of microRNAs in resistance to chemotherapy and novel targeted agents in non-small cell lung cancer. Curr Pharm Biotechnol. 2014;15(5):475–485. [DOI] [PubMed] [Google Scholar]
- 27. Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]