Abstract
Background
Hypoglycemia is a known risk of intensive postoperative glucose control in cardiac surgery patients. However, neither the consequences of hypoglycemia relative to hyperglycemia, nor the possible interaction effects, have been well described. We examined the effects of postoperative hypoglycemia, hyperglycemia, and their interaction on short-term morbidity and mortality.
Methods
Single institution Society of Thoracic Surgeons (STS) database patient records from 2010–2014 were merged with clinical data, including blood glucose values measured in the intensive care unit (ICU). Exclusion criteria included fewer than 3 glucose measurements and absence of a STS predicted risk of morbidity or mortality score. Primary outcomes were operative mortality and composite major morbidity (permanent stroke, renal failure, prolonged ventilation, pneumonia or myocardial infarction). Secondary outcomes included ICU and postoperative length of stay. Hypoglycemia was defined as <70 mg/dL, and hyperglycemia as >180 mg/dL. Simple and multivariable regression models were used to evaluate outcomes.
Results
A total of 2,285 patient records met selection criteria for analysis. The mean postoperative glucose was 140.8 ± 18.8 mg/dL. Overall, 21.4% of patients experienced a hypoglycemic episode (n=488), and 1.05% (n=24) had a severe hypoglycemic episode (<40 mg/dL). The unadjusted odds ratio (UOR) for operative mortality for patients with any hypoglycemic episode compared to those without was 5.47 (95% CI 3.14–9.54), and the UOR for major morbidity was 4.66 (95% CI 3.55–6.11). After adjustment for predicted risk of morbidity or mortality and other significant covariates, the adjusted odds (AOR) of operative mortality are significant for patients with any hypoglycemia (AOR 4.88, 95% CI 2.67–8.92) and patients with both events (AOR 8.29 95% CI 1.83–37.5) but not hyperglycemia alone (AOR 1.62, 95% CI 0.56–4.69). The AOR of major morbidity for patients with both hypo- and hyperglycemic events is 14.3 (95% CI 6.50–31.4).
Conclusions
Postoperative hypoglycemia is associated with both mortality and major morbidity after cardiac surgery. The combination of both hyperglycemia and hypoglycemia represents a substantial increase in risk. Although it remains unclear whether hypoglycemia is a cause, an early warning sign, or a result of adverse events, this study suggests hypoglycemia may be an important event in the postoperative period after cardiac surgery.
Keywords: Glucose control, hypoglycemia, quality, perioperative care, regression
Hyperglycemia is associated with increased mortality and morbidity, particularly infections, in cardiac surgery patients both with and without diabetes. These findings are supported by a large and growing body of evidence that includes clinical trials1–3 and systematic reviews4 as well as observational cohorts.5,6 Consequently, adequate glycemic control remains an important quality measure for cardiac care teams, and is outlined in Society for Thoracic Surgery (STS) guidelines.7
The ideal or appropriate level of postoperative glycemic control following cardiac surgery has been a moving target for several years as data accumulate to support both intensive3,8 and more permissive9,10 glycemic management strategies. The use of intra- and postoperative insulin infusions to maintain serum glucose less than 180 mg/dL, and less than 150 mg/dL in select patients, is supported by level B evidence and STS guidelines, but the ideal level and duration of control remains undetermined.
Furthermore, it is becoming clear that glucose dysregulation beyond hyperglycemia may also pose a risk to patients. Critically ill patients also have an increased rate of adverse events relative to the number of hypoglycemic episodes they experience.6,11–13 Although newer computer-guided algorithms were designed, in part, to minimize variability in glucose levels, their primary objective is to manage hyperglycemia in acutely ill patients. We hypothesize that episodes of hypoglycemia are also associated with adverse events following cardiac surgery. To further elucidate the appropriate target range for postoperative glucose control, the objective of this study was to evaluate the relative effect of hyper- and hypoglycemic episodes on risk-adjusted outcomes after cardiac surgery.
Patients and Methods
Study Population
The University of Virginia maintains a certified STS institutional adult cardiac surgery database into which all patient records are entered. We queried and retrieved records for all adult (>18 years) patients undergoing cardiac operations from 2010 through 2014. These records were merged with clinical data including all glucose measurements for a given hospitalization. All patients with a calculated predicted risk of mortality (PROM) and a surgery date in the stated timeframe were included. Postoperative glucose levels were obtained from our institution’s electronic medical record. Patients were excluded if there were fewer than 3 recorded glucose values or if STS records could not be matched to the clinical data or had conflicting information (e.g. two different operative dates); for patients with multiple cardiac procedures only the first of these was analyzed. Only post-operative glucose values were included in the analysis. Moderate hypoglycemia was defined as any blood glucose measurement less than 70 mg/dL, and severe hypoglycemia as less than 40 mg/dL. Hyperglycemic episodes were defined as a serum glucose of >180 mg/dL, given current guidelines. Insulin infusions are used to manage all patients while in the intensive care unit, and the target glucose range was 120–160 mg/dL. From 2010 through mid-August 2013, insulin infusions were titrated using a standard nomogram. In August 2013, the Glucommander™ protocol (Glytec, Waltham, MA) was implemented and insulin infusions were titrated hourly based on that algorithm.14
Statistical Analysis
The primary outcomes of interest were operative mortality and major morbidity, which was defined as a composite of stroke, renal failure, prolonged ventilation, pneumonia or myocardial infarction. Secondary outcomes included ICU and post-operative length of stay. Patient demographics, preoperative comorbidities and risk factors, operative characteristics and post-operative outcomes were compared between those patients experiencing any hypoglycemic episodes and those who did not. Categorical variables were compared using Pearson’s χ2 test, and continuous variables were tested using the Wilcoxon rank-sum test.
Multivariable regression models were used to test the relationship between hypoglycemia and the outcomes of interest while controlling for other covariates. Model variables were selected based upon univariate analyses or selected a priori. For logistic models, performance was assessed by the c-statistic and goodness of fit was tested using the Hosmer-Lemeshow method. The negative binomial distribution was used to model count outcomes including length of stay (LOS, in hours or days), as the data were over-dispersed relative to the Poisson distribution. In models where episodes of both hyperglycemia and hypoglycemia were evaluated together, this was expressed as an interaction term. Covariates used for risk adjustment in multivariable models included age, sex, diabetes status, end-stage renal disease, mean postoperative glucose, operation performed, urgency, year of surgery, and the STS predicted risk of morbidity or mortality.
All measures of central tendency are presented as medians with interquartile ranges (IQR) unless otherwise noted, and categorical variables are presented as a percentage of the group of origin. All hypothesis tests were two-sided, and statistical significance was set at α=0.05. Odds ratios (OR) with 95% confidence intervals are used to report results of logistic regression, and incidence rate ratios (IRR) with 95% confidence intervals are used to describe results from negative binomial regressions. Data analysis was performed using Stata version 13.1 (StataCorp, College Station, TX).
Results
Patient Characteristics and Risk Factors
A total of 3,584 patients underwent cardiac surgery during the study period, of whom 1,267 were excluded due to an operation that precludes PROM calculation. An additional 32 patients were excluded for incomplete or duplicate records, yielding a study population of 2,285 patients. Four hundred and eighty-eight patients (21.4%) experienced at least one episode of moderate hypoglycemia postoperatively, and 1,905 (83.3%) had at least one episode of hyperglycemia. Of patients experiencing hypoglycemia, approximately half had a single episode (253/488, 52.0%), 22.5% had two episodes, and the remaining 124 patients (25.4%) had three or more episodes. A total of 24 patients (1.05%) had at least one episode of severe hypoglycemia. Demographics, risk factors and operative characteristics of patients with and without any episode of hypoglycemia are presented in Table 1.
Table 1.
Demographics and Comorbidities
| Factor | No Hypoglycemia | Hypoglycemic Episodes | p-value |
|---|---|---|---|
| N=1797 | N=488 | ||
| Age | 68 (59, 76) | 70 (61, 77) | 0.025 |
| Female | 545 (30.3%) | 181 (37.1%) | 0.004 |
| BMI | 28.7 (25.4, 33.1) | 27.7 (24.3, 32.8) | 0.005 |
| Mean glucose, mean (SD) | 143.8 (17.3) | 147.9 (18.8) | <0.001 |
| Median glucose, median (IQR) | 137 (128.5, 148) | 141.25 (130, 154) | <0.001 |
| Diabetes | 639 (35.6%) | 297 (60.9%) | <0.001 |
| Baseline A1c | 6 (6, 7) | 6 (6, 8) | <0.001 |
| Dyslipidemia | 1479 (82.3%) | 433 (88.7%) | <0.001 |
| Hypertension | 1446 (80.5%) | 422 (86.5%) | 0.002 |
| Peripheral Arterial Disease | 262 (14.6%) | 104 (21.3%) | <0.001 |
| NYHA Classification | <0.001 | ||
| Class I | 12 (1.7%) | 2 (0.8%) | |
| Class II | 249 (34.6%) | 55 (21.2%) | |
| Class III | 306 (42.5%) | 115 (44.2%) | |
| Class IV | 153 (21.2%) | 88 (33.8%) | |
| Preoperative Creatinine, mean (SD) | 1.26 (1.08) | 1.42 (1.37) | 0.005 |
| End-Stage Renal Disease | 40 (2.2%) | 23 (4.7%) | 0.003 |
| Endocarditis | 61 (3.4%) | 17 (3.5%) | 0.92 |
| Heart Failure within 2 weeks | 720 (40.1%) | 260 (53.3%) | <0.001 |
| Ejection Fraction | 57 (47, 63) | 55 (37, 63) | <0.001 |
| Prior MI | 721 (40.1%) | 241 (49.4%) | <0.001 |
| Reoperation | 202 (11.2%) | 57 (11.7%) | 0.79 |
| Chronic Lung Disease | <0.001 | ||
| None | 1404 (78.1%) | 338 (69.3%) | |
| Mild Disease | 209 (11.6%) | 73 (15.0%) | |
| Moderate Disease | 134 (7.5%) | 41 (8.4%) | |
| Severe Disease | 50 (2.8%) | 36 (7.4%) | |
| Status | 0.060 | ||
| Elective | 1078 (60.0%) | 275 (56.4%) | |
| Urgent | 700 (39.0%) | 202 (41.4%) | |
| Emergent | 19 (1.1%) | 11 (2.3%) | |
| Procedure Type | 0.001 | ||
| AV Replacement | 449 (25.0%) | 101 (20.7%) | |
| AV Replacement + CAB | 200 (11.1%) | 70 (14.3%) | |
| CAB Only | 882 (49.1%) | 238 (48.8%) | |
| MV Repair | 122 (6.8%) | 20 (4.1%) | |
| MV Repair + CAB | 45 (2.5%) | 22 (4.5%) | |
| MV Replacement + CAB | 20 (1.1%) | 3 (0.6%) | |
| MV Replacement Only | 79 (4.4%) | 34 (7.0%) | |
| Reoperations | 202 (11.2%) | 57 (11.7%) | 0.79 |
| Number of Glucose Measurements, median (IQR) | 43 (32, 62) | 75 (49.5, 111) | <0.001 |
| Predicted risk of mortality | .017 (.008, .037) | .032 (.013, .061) | <0.001 |
Overall, patients who experienced postoperative hypoglycemia had higher operative risk. These patients were older (70 [16] vs. 68 [7], p=0.025), more likely to be female (37% vs 30%, p=0.004), diabetic (61% vs 36%, p<0.001), hypertensive (87% vs. 81%, p=0.002) and on dialysis (4.7% vs 2.2%, p=0.003). The predicted risk of mortality was 3.2% in the hypoglycemic patients vs. 1.7% in the control patients (p<0.001). There was no change in number of patients with hypoglycemic events over time (p=0.87) despite the change in glucose control protocols (Table 2).
Table 2.
Dysglycemic Events Over Time
| Year Surgery Performed | Hypoglycemic Events | Hyperglycemic Events |
|---|---|---|
| 2010 (N=478) | 115 (24.1%) | 427 (89.3%) |
| 2011 (N=479) | 93 (19.4%) | 402 (83.9%) |
| 2012 (N=502) | 90 (17.9%) | 426 (84.9%) |
| 2013 (N=528) | 119 (22.5%) | 402 (76.1%) |
| 2014 (N=298) | 71 (23.8%) | 248 (83.2%) |
| Test for trend: | P = 0.87 | P < 0.001 |
Unadjusted Postoperative Outcomes
The unadjusted odds ratio (UOR) for operative mortality for patients with any hypoglycemic episode compared to those without was 5.47 (95% CI 3.14–9.54), and the UOR for major morbidity was 4.66 (95% CI 3.55–6.11). For patients with any hyperglycemic episode, the UOR were 1.94 (95% CI 0.77–4.90) and 5.36 (95% CI 2.82–10.2) for mortality and morbidity, respectively. Unadjusted and adjusted analyses are shown in Table 3. Patients with hypoglycemic episodes also had more wound infections (UOR 3.09, 95% CI 1.51–6.32) and longer ICU (70 [93] vs. 37 [47] hours, p<0.001) and overall post-procedural LOS (7 [6] vs. 5 [2] days, p<0.001). The unadjusted incidence rate ratio (IRR) for ICU hours was 2.53 (95% CI 2.25–2.85), representing an average of a 2.5-fold increase in the number of hours a patient with hypoglycemic episodes spends in the ICU relative to patients without. These patients also have a 69% increase in post-procedure hospital days (IRR 1.69, 95% CI 1.60–1.78), compared to patients experiencing hyperglycemic episodes who have a 38% increase in hospital days (IRR 1.38, 95% CI 1.29–1.48) and a 64% increase in ICU hours (IRR 1.64, 95% CI 1.40–1.91). Patients experiencing both hyper- and hypoglycemic events had a greatly increased risk of operative mortality (UOR 11.3, 95% CI 2.67–47.8), major morbidity (UOR 15.9, 95% CI 7.64–33.0), and wound infection (UOR 11.0, 95% CI 1.44–84) as well as prolonged ICU (IRR 3.19, 95% CI 2.68–3.80) and total (IRR 2.04, 95% CI 1.89–2.21) post-procedure LOS.
Table 3.
Logistic & Negative Binomial Regression
| Unadjusted Odds Ratio (95% CI) | p-value | Adjusted Odds Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Operative Mortality | ||||
| Hypoglycemia only | 5.47 (3.14–9.54) | p<0.001 | 4.88 (2.67–8.92) | p<0.001 |
| Hyperglycemia only | 1.94 (0.77–4.90) | p=0.162 | 1.62 (0.56–4.69) | p=0.37 |
| Hypo- and hyperglycemia | 11.3 (2.67–47.8) | p<0.001 | 8.29 (1.83–37.5) | p=0.006 |
| Major Morbidity | ||||
| Hypoglycemia only | 4.66 (3.55–6.11) | p<0.001 | 3.76 (2.77–5.09) | p<0.001 |
| Hyperglycemia only | 5.36 (2.82–10.2) | p<0.001 | 5.31 (2.62–10.8) | p<0.001 |
| Hypo- and hyperglycemia | 15.9 (7.64–33.0) | p<0.001 | 14.3 (6.50–31.4) | p<0.001 |
| Wound Infection | ||||
| Hypoglycemia only | 3.09 (1.51–6.32) | p=0.002 | 2.08 (0.97–4.46) | p=0.06 |
| Hyperglycemia only | 6.06 (0.82–44.6) | p=0.08 | 3.15 (0.39–25.7) | p=0.3 |
| Hypo- and hyperglycemia | 11.0 (1.44–83.8) | p=0.021 | 4.7 (0.54–41.1) | p=0.2 |
| Post-Operative Length of Stay | ||||
| Hypoglycemia only | 1.69 (1.60–1.78) | p<0.001 | 1.49 (1.41–1.57) | p<0.001 |
| Hyperglycemia only | 1.38 (1.29–1.48) | p<0.001 | 1.27 (1.18–1.36) | p<0.001 |
| Hypo- and hyperglycemia | 2.04 (1.89–2.21) | p<0.001 | 1.79 (1.65–1.95) | p<0.001 |
| Total ICU Hours | ||||
| Hypoglycemia only | 2.53 (2.25–2.85) | p<0.001 | 1.96 (1.75–2.20) | p<0.001 |
| Hyperglycemia only | 1.64 (1.40–1.91) | p<0.001 | 1.31 (1.13–1.53) | p<0.001 |
| Hypo- and hyperglycemia | 3.19 (2.68–3.80) | p<0.001 | 2.44 (2.03–2.93) | p<0.001 |
Effects of Dysglycemia on Risk-Adjusted Outcomes
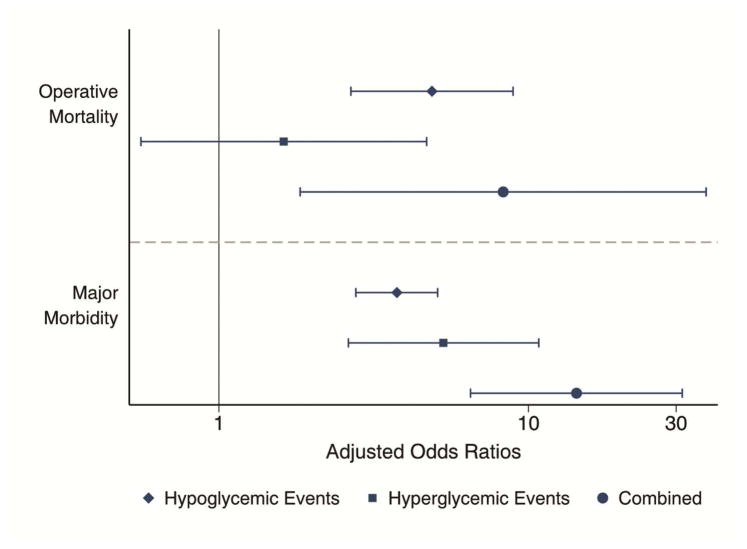
Given the baseline differences in populations between those patients experiencing hypoglycemic episodes and those who did not, multivariable regression modeling was used to explore the effects of experiencing hypoglycemic or hyperglycemic episodes as well as both combined. This was done while controlling for predicted risk of morbidity or mortality (in morbidity and LOS models) or the predicted risk of mortality (in mortality models) as well as mean postoperative glucose, diabetic status and other covariates as appropriate. The c-statistic for the morbidity and mortality models were 0.78 and 0.79 respectively. All adjusted outcomes are presented in Table 3, and complete models are available in appendix tables 1–5. The multivariable models demonstrate that, when adjusting for predicted risk of mortality, hyperglycemic episodes alone do not increase risk of mortality (AOR 1.62, 95% CI 0.56–4.69), but hypoglycemic episodes do (AOR 4.88, 95% CI 2.67–8.92) and there is a significant interaction in patients who experience both (AOR 8.29, 95% CI 1.83–37.5), which can be seen in Figure 1. In adjusted analyses examining major morbidity, hypoglycemia remains a significant independent predictor of morbidity (AOR 3.76, 95% CI 2.77–5.09). Again, the combination of hyper- and hypoglycemic events represents a significant increase beyond that seen with either anomaly alone (AOR 14.3, 95% CI 6.50–31.4). When stratified by diabetes status, the effect of hypoglycemia on major morbidity was relatively consistent across diabetics (AOR 3.28, 95% CI 2.14–5.02) and non-diabetics (AOR 4.65, 95% CI 3.06–7.07). Interestingly, the effects of hyperglycemia were only seen in the non-diabetic population (AOR 4.24, 95% CI 2.01–8.97); hyperglycemic episodes did not reliably result in increased morbidity in diabetic patients (AOR 6.82, 95% CI 0.64–72.4). Dysglycemia had no significant effect on adjusted odds of wound infection.
Figure 1.
Adjusted odds ratios of operative mortality, composite major morbidity, as well as incidence rate ratios for post-operative length of stay (days) and ICU hours. Hyperglycemic episodes alone were not associated with increased mortality risk, but patients with both hyper- and hypoglycemic events were at significantly increased risk for all adverse events.
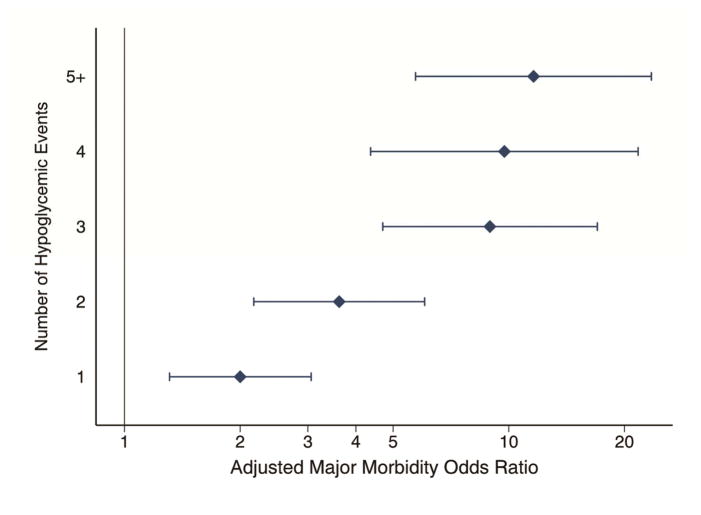
To determine whether there was a dose-response effect, we ran the multivariable models based on the total number of episodes of hypoglycemia. For both mortality and morbidity, even a single episode of hypoglycemia increases risk (AOR 2.71 95% CI 1.16–6.33; AOR 2.00 95% CI 1.31–3.06 respectively), and the odds increase with the number of hypoglycemia episodes. After adjusting for multiple comparisons, this trend is not statistically significant for mortality, but for major morbidity those patients with 3, 4, or 5+ hypoglycemic events had statistically significantly higher risk than those patients experiencing a single event (p=0.001, 0.005, and <0.001 respectively, Figure 2).
Figure 2.
Adjusted odds ratios of major morbidity as a function of number of hypoglycemic episodes. Even a single episode of hypoglycemia is significantly associated with increased morbidity risk, but additional episodes were associated with increasing risk.
Comment
This study is among the first to examine the relative effects of hyper- and hypoglycemic episodes on outcomes after cardiac surgery. Although hyperglycemic episodes alone were not associated with mortality when controlling for mean glucose, hypoglycemic episodes are, and there was a significant interaction effect in patients experiencing both events. Both hyper- and hypoglycemic events were associated with risk-adjusted major morbidity, and there was also a significant interaction effect for this outcome as well. Interestingly, there was no decrease in hypoglycemic episodes after the computerized insulin infusion algorithm was instituted, and only a modest decrease in hyperglycemic events.
Desai and colleagues randomized patients after coronary artery bypass graft surgery to either liberal (120–181 mg/dL) or strict (90–120 mg/dL) glycemic control strategies and found that there was a 3-fold increase in episodes of hypoglycemia (<60 mg/dL) in the strict control group versus the liberal group, but no statistically significant difference in severe hypoglycemia (<40 mg/dL).9 There were not any significant differences in adverse outcomes, but the event rate was low due to both the risk profile of the population and the size of the study. This would seem to be contradicted by the results of Giakoumiadakis et al in 2013, who conclude based on their prospective trial that “intensive” glucose control was favorable when compared to their control group.3 They assigned 212 patients quasi-randomly to intensive or standard glucose control strategies, and found a significant decrease in in-hospital mortality during the study period. However, they defined intensive control as a goal of 120–160 mg/dL, which in fact is more in line with Desai’s liberal protocol rather than the strict protocol. This highlights the importance of standardizing definitions, as studies vary widely in defining hypoglycemia as well as glucose control strategies.
There are limited data to inform our understanding of postoperative hypoglycemia after cardiac surgery and its effects. In a retrospective single-center study examining mixed medical and surgical ICU patients, Hermanides and colleagues found increased risk-adjusted mortality among patients who had experienced even a single episode of hypoglycemia (≤45 mg/dL).11 Although 67% (4012/5961) of patients had undergone cardiac surgery, they were not analyzed as a separate sub-group. In contrast, Stamou et al. found that hypoglycemia following cardiac surgery was associated with increased respiratory complications and ICU LOS, but not increased mortality.15 Finally, others have suggested that large changes in glucose values, or glycemic variability, may be more indicative of risk and adverse outcomes than absolute glucose values alone.16–19 Although this study does not address the issue of glycemic variability directly, we do find that patients with both hyper- and hypoglycemic episodes have significantly higher risk of adverse events, and the role of variability in our patient cohort is a topic of ongoing investigation by our group.
This study has several limitations. Foremost among these is our inability to determine whether hypoglycemia is cause or consequence of the increased risk of adverse events demonstrated here. Despite a dose-response relationship between number of hypoglycemic episodes and major morbidity, our data cannot determine the time course of these events. This may also be related to the limitation that the measurements in this study were largely taken in the ICU, and patients with complications and thus longer ICU lengths of stay are more likely to have a recorded hypoglycemic episode just by chance alone based on the increased number of measurements taken. The question of hypoglycemia as a harbinger versus an inherent risk in itself will be difficult to determine, but prospective studies with greater attention to the timing of adverse events relative to hypoglycemic episodes may be helpful. Second, this is an observational single-center study and results therefore may be subject to selection bias and results may not be generalizable to other clinical scenarios or centers. Finally, our analysis was restricted to short-term outcomes and does not provide insight on the effects of dysglycemia on long-term endpoints.
Although this study only demonstrates an association, rather than a causal relationship, between hypoglycemia and adverse events we believe it represents an important first step in examining the role of hypoglycemia in risk-adjusted outcomes after cardiac surgery. Ultimately the question of what is the ideal range for glycemic control remains unanswered. However, given the results here and other studies that demonstrate a strong association with hypoglycemia and adverse events, hypoglycemia should be avoided in the postoperative period when possible, even at the expense of mild hyperglycemia.
Supplementary Material
Acknowledgments
Set acknowledgement box: This work was supported in part by grant UM1 HL088925, Network for Cardiothoracic Surgical Investigations in Cardiovascular Medicine. Article contains MMC.
Footnotes
Presented at the Poster Session of the Fifty-second Annual Meeting of The Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016. Winner of the Blue Ribbon as the top Critical Care Poster.
The Appendix Tables can be viewed in the online version of this article [insert doi number] on http://www.annalsthoracicsurgery.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109(12):1497–1502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- 2.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. The Journal of Thoracic and Cardiovascular Surgery. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 3.Giakoumidakis K, Eltheni R, Patelarou E, et al. Effects of intensive glycemic control on outcomes of cardiac surgery. Heart & Lung: The Journal of Acute and Critical Care. 2013;42(2):146–151. doi: 10.1016/j.hrtlng.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Boreland L, Scott-Hudson M, Hetherington K, Frussinetty A, Slyer JT. The effectiveness of tight glycemic control on decreasing surgical site infections and readmission rates in adult patients with diabetes undergoing cardiac surgery: A systematic review. Heart & Lung: The Journal of Acute and Critical Care. 2015;44(5):430–440. doi: 10.1016/j.hrtlng.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010;112(4):860–871. doi: 10.1097/ALN.0b013e3181d3d4b4. [DOI] [PubMed] [Google Scholar]
- 6.Thiessen S, Vanhorebeek I, Van den Berghe G. Glycemic control and outcome related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. 2015;29(2):177–187. doi: 10.1016/j.bpa.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons Practice Guideline Series: Blood Glucose Management During Adult Cardiac Surgery. ATS. 2009;87(2):663–669. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Group TAC. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. New England Journal of Medicine. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 9.Desai SP, Henry LL, Holmes SD, et al. Strict versus liberal target range for perioperative glucose in patients undergoing coronary artery bypass grafting: A prospective randomized controlled trial. The Journal of Thoracic and Cardiovascular Surgery. 2012;143(2):318–325. doi: 10.1016/j.jtcvs.2011.10.070. [DOI] [PubMed] [Google Scholar]
- 10.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, et al. Intensive versus Conventional Glucose Control in Critically Ill Patients. New England Journal of Medicine. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 11.Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Critical Care Medicine. 2010;38(6):1430–1434. doi: 10.1097/CCM.0b013e3181de562c. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Kim D-G, Suh GY, et al. Mild hypoglycemia is independently associated with increased risk of mortality in patients with sepsis: a 3-year retrospective observational study. Crit Care. 2012;16(5):R189. doi: 10.1186/cc11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Critical Care Medicine. 2007;35(10):2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 14.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28(10):2418–2423. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 15.Stamou SC, Nussbaum M, Carew JD, et al. Hypoglycemia with intensive insulin therapy after cardiac surgery: predisposing factors and association with mortality. The Journal of Thoracic and Cardiovascular Surgery. 2011;142(1):166–173. doi: 10.1016/j.jtcvs.2010.09.064. [DOI] [PubMed] [Google Scholar]
- 16.Hermanides J, Vriesendorp TM, Bosman RJ. Glucose variability is associated with intensive care unit mortality*. Critical care …. 2010 doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- 17.Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: Effects of intensive insulin therapy and relative association with mortality*. Critical Care Medicine. 2010;38(4):1021–1029. doi: 10.1097/CCM.0b013e3181cf710e. [DOI] [PubMed] [Google Scholar]
- 18.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Critical Care Medicine. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 19.Dossett LA, Cao H, Mowery NT, Dortch MJ. Blood glucose variability is associated with mortality in the surgical intensive care unit. The American …. 2008 doi: 10.1177/000313480807400802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.