Abstract
Purpose
Exposure of blood to foreign surfaces induces reciprocal conversion of the plasma proteins factor XII (fXII) and plasma prekallikrein (PPK) to the proteases α-fXIIa and α-kallikrein. This process, called contact activation, has a range of effects on host defense mechanisms, including promoting coagulation. The nature of the triggering mechanism for contact activation is debated. One hypothesis predicts that fXII has protease activity, either intrinsically or upon surface-binding, that initiates contact activation. We tested this by assessing the proteolytic activity of a recombinant fXII variant that cannot be converted to α-fXIIa.
Recent findings
The proteolytic activity of fXII-T (for “triple” mutant), a variant with alanine substitutions for arginine at activation cleavage sites (Arg334, Arg344, and Arg353) was tested with known α-fXIIa substrates. FXII-T activates PPK in solution, and the reaction is enhanced by polyphosphate, an inducer of contact activation released from platelets. In the presence of polyphosphate, fXII-T converts fXII to α-fXIIa, and also converts the coagulation protein factor XI to its active form.
Summary
The findings support the hypothesis that contact activation is initiated through activity intrinsic to single-chain fXII, and indicate that pre-existing α-fXIIa is not required for induction of contact activation.
Keywords: Factor XII, Plasma Prekallikrein, Factor XI, Contact Activation
Introduction
Exposure of blood to a variety of non-biologic surfaces and substances can lead to formation of a blood clot [1,2*,3*,4,5*]. Surface-induced coagulation is initiated by a process called contact activation. Contact activation is the first step in clot formation in the activated partial thromboplastin time (aPTT) assay widely used to evaluate plasma thrombin generation [6,7]. In the aPTT the plasma protease precursors factor XII (fXII) and plasma prekallikrein (PPK) undergo reciprocal proteolytic conversion to the fully active protease species α-factor XIIa (α-fXIIa) and α-kallikrein (α-kal) on an anionic surface such as silica particles (Figure 1) [8.9]. The glycoprotein high molecular weight kininogen (HK) serves as a cofactor by facilitating PPK binding to the activating surface [10–12]. α-FXIIa, its degradation product β-FXIIa (Figure 2), and α-kal can activate components of host defense processes, including the plasma coagulation and complement systems, inflammation, and fibrinolysis (Figure 1) [1–4,13]. FXII, PPK and HK are also referred to as the kallikrein-kinin system (KKS), because α-kallikrein, among its activities, cleaves HK to release the inflammatory peptide bradykinin [2*,3*].
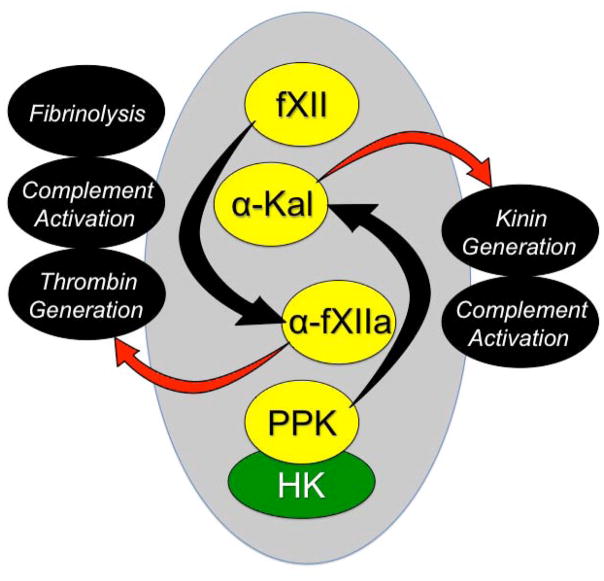
Figure 1. Contact Activation.
On a surface (represented by the gray oval), factor XII (fXII) undergoes autocatalytic conversion to the protease α-fXIIa, which in turn converts plasma prekallikrein (PPK) to the protease α-kallikrein (α-Kal). High molecular weight kininogen (HK) serves as a cofactor for contact activation by facilitation the interaction between PPK and the surface. Through their proteolytic capacities, α-fXIIa and α-kal can influence a number of host-defense systems (shown in black ovals). α-FXIIa promotes thrombin generation through conversion of factor XI to factor XIa. α-Kal can promote inflammation by cleaving HK to liberate the nanopeptide bradykinin.
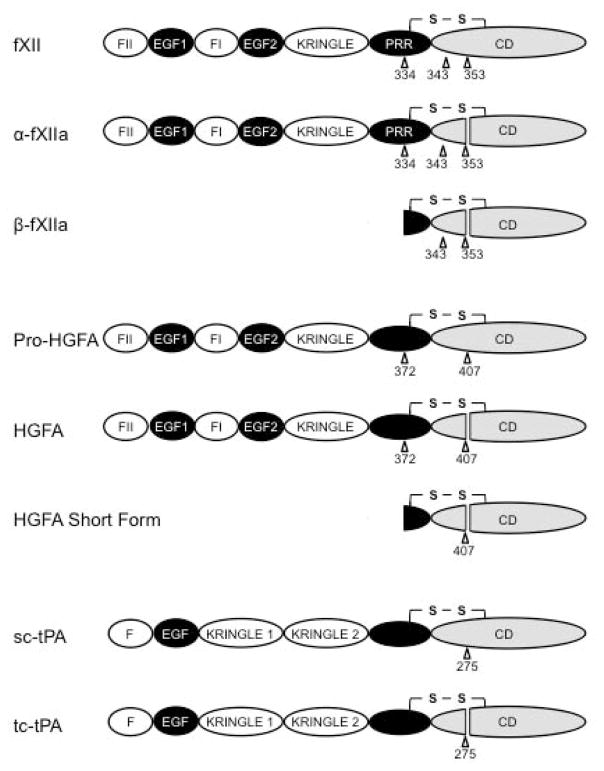
Figure 2. Domain organization of factor XII and related proteases.
Schematic diagrams showing single chain and cleaved forms of fXII (top), Pro-hepatocyte growth factor activator (Pro-HGFA), and tissue plasminogen activator (tPA) Sites of proteolysis during activation are indicated by arrows, with the numbers indicating the residue after which cleavage occurs. FXII is an 80 kDa polypeptide that may be cleaved at three locations. Cleavage after Arg353 converts fXII to α-fXIIa. Cleavage of α-fXIIa after Arg334 separates the non-catalytic and catalytic domains, forming βFXIIa. The importance of cleavage after Arg343 is not clear. The fXII non-catalytic domains are the fibronectin type 2 (F2), epidermal growth factor (EGF), fibronectin type 1 (F1), and kringle (K) domains, and proline–rich region (PRR). Pro-HGFA is converted to HGA by cleavage after Arg407. It can also be converted to a short form, similar to βFXIIa, by cleavage after Arg372. It has similar domain structure to fXII except that it lacks the PRR. Single chain tPA (sc-tPA) is converted to two chain tPA (tc-tPA) by cleavage after Arg275. Its non-catalytic portion contains domains similar to those found in fXII and Pro-HGFA.
Identifying the major physiologic roles of the KKS has been a challenge because deficiencies of its components are not associated with obvious phenotypes. A number of biologic substances, including polymers of orthophosphate (polyphosphate) [14–16], DNA [17,18], RNA [19], collagen [20] and misfolded protein aggregates [21] induce contact activation, and may represent physiologic or pathologic cofactors for the KKS in vivo. It has been proposed that the KKS contributes to the innate host response to invading microorganisms [5*,22–25]. Indeed, a process similar to contact activation may occur when KKS proteins assemble on cell membranes or cell walls of microorganisms, leading to production of bradykinin and antimicrobial peptides by HK cleavage [22–25]. This same capacity to assemble on “non-self” surfaces likely occurs when blood passes through extracorporeal circuits (cardiopulmonary bypass, extracorporeal membrane oxygenation, renal dialysis) or comes into contact with implantable devices (ventricular assist devices, artificial heart valves, and central venous catheters), contributing to thrombogenicity and inflammation [5,26–31].
While the major proteolytic reactions involved in contact activation are well described, there is uncertainty as to the event(s) that trigger fXII and PPK activation [32]. Plasma may normally contain traces of α-fXIIa or α-kal that prime the process once a surface is available [33–35]. Reciprocal fXII and PPK activation seems to occur at a measurable basal level in healthy mice, suggesting a continuous supply of α-fXIIa or α-kal [36]. Proteases from outside the KKS such as prolyl-carboxypeptidase (PRCP, activates PPK) [37–39] and factor XIa (fXIa, activates fXII) [40] may also serve as initiators. Another possibility is that fXII, rather than being a true zymogen (an inactive enzyme precursor), has proteolytic activity or expresses activity after binding to a surface [41–43]. Binding of certain proteins to the coagulation factor prothrombin (staphylocoagulase, von Willebrand factor binding protein, histone H4) [44–46] or the fibrinolytic protein plasminogen (streptokinase) [47] can produce conformational changes that confer protease activity on these zymogens. Surface binding may cause a similar effect with fXII. It has been difficult to sort out candidate mechanisms for initiating fXII activation, because it is difficult to exclude the possibility that fXII purified from plasma is contaminated with α-fXIIa. We addressed this recently by studying recombinant fXII variants that are not converted to α-fXIIa. Our work with one of these proteins and the implications of our findings are the subject of this review.
Factor XII Structure
Human fXII is an ~80 kDa polypeptide comprised of several domains that form a non-catalytic heavy chain and a C-terminal trypsin-like catalytic (light chain) domain (Figure 2) [13,48]. FXII is expressed in a number of cell types, but the protein that circulates in plasma comes primarily from hepatocytes [32,49]. The fXII gene has been identified in amphibian, reptile and mammal genomes, but not in fish, raising the possibility that fXII is adaptive for terrestrial environments [50,51]. For unknown reasons, the gene has been lost in birds and diving mammals (whales and porpoises). FXII is the result of a duplication event involving the more ancient gene for the protease Pro-hepatocyte growth factor activator (Pro-HGFA, Figure 2) [52,53]. Activation of Pro-HGFA (by thrombin) to the protease HGFA, like activation of fXII, is enhanced by surfaces, suggesting these homologs have similar surface-binding elements [54]. HGFA may be converted to a short form analogous to β-FXIIa [53,54]. For fXII, several lines of evidence indicate the N-terminal fibronectin type II domain is involved in surface binding, but other studies implicate the fibronectin type I and kringle domains, and the proline-rich region (Figure 2) [23,55–58]. It is possible that different domains contribute to binding, depending on the type of surface involved.
The organization of exons encoding the Pro-HGFA and fXII catalytic domains indicate homology with the plasminogen activators urokinase (uPA) and tissue plasminogen activator (tPA, Figure 2) [54,59–62]. Miyazawa et al. proposed that fXII, Pro-HGFA, tPA and uPA form a protease family [52]. tPA and uPA express substantial proteolytic activity in their single-chain forms, a property referred to as low zymogenicity [63,64]. Trypsin-like proteases are usually secreted as inactive single-chain zymogens that require internal proteolysis after Arg15 (chymotrypsin numbering) for activity [65]. After cleavage, the new N-terminus of the catalytic domain (residue 16) forms a salt-bridge with Asp194, creating a substrate recognition site. The tPA catalytic domain has several features that likely stabilize a functional active site in the single-chain form (sc-tPA, Figure 3A). A salt bridge between Lys156 and Asp194 is key to maintaining a functional active site [64]. The carboxylate group of Asp194 forms hydrogen bonds with the main-chain nitrogen atoms of Gly142 and Cys191A that may stabilize the active conformation.
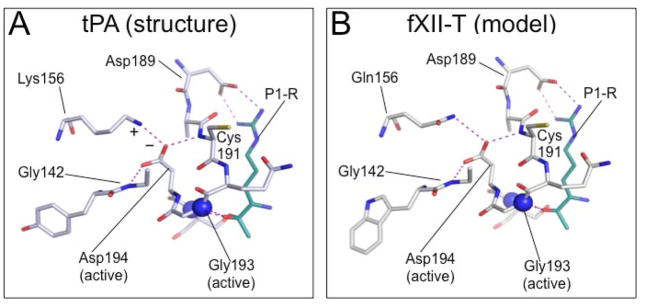
Figure 3. Model of the sc-fXII active site.
Shown are stick diagrams of S1 pocket structures with hydrogen bonds and electrostatic interactions shown as dotted lines (purple). The position of the oxyanion hole is indicated by the two blue spheres, which represent the nitrogen atoms of Ser195 and Gly193. (A) sc-tPA S1 pocket crystal structure (pdb:1BDA) showing Asp194 stabilized by a salt bridge formed with Lys156 (Indicated by + and − symbols), and by hydrogen bonds with the main-chain nitrogens of Gly142 and Cys191. The cyan stick figure represents the side-chain of the P1 arginine residue of the tPA inhibitor dansyl-Glu-Gly-Arg-chloromethyl-ketone. (B) Homology model (SWISS-MODEL [66]) of the predicted S1 pocket of fXII-T based on the sc-tPA structure. Gln156 forms a hydrogen bond with the Asp194 carboxylate group. The side-chain shown in cyan represents the P1 arginine of a substrate (PPK or fXI). Images with permission from Ivanov et al. (American Society of Hematology – Blood) [69].
Based on homology with tPA, we became interested in the possibility that fXII has activity in its single chain form (sc-fXII) that could serve as a trigger for contact activation. The glutamine at position 156 in fXII, unlike Lys156 in sc-tPA and single-chain urokinase (sc-uPA), would not form a salt bridge with Asp194 (Figure 3B), but it may provide weak stabilization through hydrogen bonding. A homology model of fXII based on the sc-tPA structure suggests fXII Asp194 may also form hydrogen bonds with Gly142 and Cys191A as in sc-tPA (Figure 3B) [66]. To assess the possibility that sc-fXII is a protease, we prepared and evaluated a form of fXII that cannot be converted to α-fXIIa.
Factor XII Activation
Griffin and Cochrane first raised the question of whether or not a proteolytic event is necessary to achieve fXII activity [67]. Opinions have differed. Silverberg et al. [33,34] and Tans et al. [35] concluded that α-fXIIa was the active form of fXII, noting that the sigmoidal progress curves for surface-dependent fXII autoactivation best fit a process initiated by α-fXIIa in fXII preparations. However, appreciating the difficulty of eliminating other possibilities with assays based on plasma fXII, Silverberg and Kaplan proposed that sc-fXII might have weak activity. They placed an upper limit on its ability to cleave the tripeptide substrate S-2302 at <4200-fold that of α-fXIIa [34]. In contrast, several groups have presented data that suggests the increased activity after fXII binds to a surface does not necessarily require conversion to α-fXIIa [41–43]. Most recently, Engel et al. showed that fXII binding to the polyanion polyphosphate results in a marked increase in capacity to cleave a tripeptide substrate without obvious conversion to α-fXIIa [43]. As discussed, a potential confounding factor in any study employing plasma fXII is α-fXIIa, either as an initial contaminant or as a product generated during a reaction. To address this, we prepared a recombinant variant of fXII that cannot be converted to α-fXIIa.
FXII is converted to α-fXIIa by autocatalysis, or by proteolysis mediated by α-kal or fXIa (a homolog of α-kal). During activation, the 80 kDa fXII polypeptide is cleaved, resulting in a 50 kDa non-catalytic heavy chain and a 30 kDa catalytic domain connected by a disulfide bond (Figure 2) [14]. FXII is cleaved at up to three sites within a 20 amino acid span during activation (after Arg334, Arg343, and/or Arg353, Figure 2). Cleavage after Arg353 (equivalent to Arg16 in chymotrypsin numbering, see previous section), converts fXII to α-fXIIa, while subsequent cleavage after Arg334 releases the heavy chain, forming β-FXIIa (Figure 2) [13,59,68]. The consequences of cleavage after Arg343 are not clear. To test the hypothesis that sc-fXII is a protease, we replaced Arg334, Arg343, and Arg353 with alanine [69**]. The resulting protein, designated fXII-T (for “triple” mutant), does not undergo autocatalysis to α-fXIIa in the presence of polyphosphate, and is resistant to cleavage by α-kal and fXIIa.
The Activity of fXII-T
We studied fXII-T activation of the α-fXIIa substrates PPK, fXII itself, and factor XI (fXI), the precursor of fXIa. When wild type fXII (fXII-WT) is mixed with PPK at plasma concentrations there is reciprocal conversion of the proteins to α-fXIIa and α-kal (Figure 4A) [69**,70]. Initiation of this process is often attributed to traces of α-fXIIa in fXII preparations. However, when fXII-WT is replaced by fXII-T, PPK is still converted to α-kal, although at a lower rate than with fXII-WT [69**]. PPK activation by fXII-T turns out to be four orders of magnitude slower than with α-fXIIa. The reaction requires a functional fXII catalytic apparatus, as fXII lacking the key active site serine residue Ser557 (fXII-S554A) [59] does not activate PPK (Figure 4A). These data show that sc-fXII has proteolytic activity even in the absence of a cofactor/surface. However, as expected with a contact reaction, PPK activation by fXII-T is accelerated by polyphosphate (Figure 4B). Most PPK in plasma circulates in complex with HK [11], which enhances surface-dependent PPK activation by α-fXIIa. In preliminary studies we observed that HK also enhances fXII-T activation of PPK in the presence of polyphosphate.
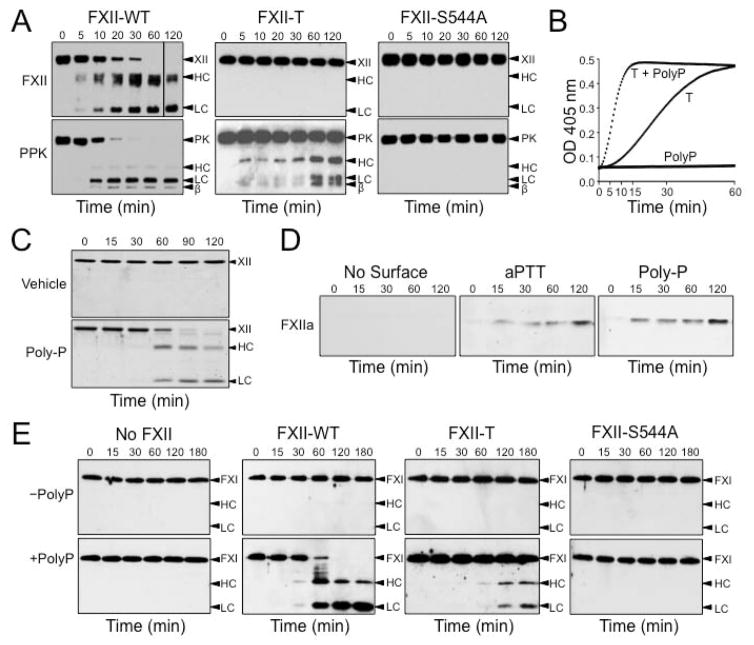
Figure 4. Proteolytic activity of single-chain fXII (sc-fXII).
(A) Reciprocal activation of fXII and PPK in the absence of a surface. FXII (200 nM) species and PPK (200 nM) were incubated at 37 °C. At indicated times, samples were removed into reducing sample buffer and analyzed by western blotting using polyclonal IgG to FXII (top) or PPK (bottom). Positions of standards for fXII (XII) and the heavy (HC) and light (LC) chains of α-fXIIa; and standards for PPK (PK), the heavy (HC) and light (LC) chains of α-kallikrein, and a fragment of the α-kallikrein heavy chain (β) are indicated at the right the images. (B) PPK Activation by fXII-T. Generation of kallikrein from PPK (200 nM) was followed by continuous monitoring of S-2302 (200 μM) cleavage at OD 405 nm in the presence or absence of 70 μM polyphosphate (Poly-P), and in the presence or absence of 200 nM FXII-T (T). (C) FXII Autoactivation in the presence of polyphosphate. FXII (200 nM) was incubated without (top) or with (bottom) 70 μM Poly-P. At indicated times samples were removed into reducing sample buffer, size fractionated by SDS-PAGE, and stained with Coomassie blue. Markers on the right are as in panel A. (D) FXII-S554A cleavage by fXII-T. Western blots of mixtures of FXII-T (200 nM) and FXII-S544A (200 nM) alone, or in the presence of silica aPTT reagent (10% final volume) or 70 μM Poly-P. At indicated times samples were removed into non-reducing sample buffer, and analyzed by western blot using an antibody that recognizes the fXIIa active site. (E) FXI-S557A cleavage by fXII-T. FXI-S557A (30 nM) was incubated with 200 nM fXII-WT, fXII-T or fXII-S544A in the absence (top) or presence (bottom) of 70 μM Poly-P. At indicated times samples were removed into non-reducing sample buffer, and analyzed by western blot using a goat-anti-human FXI polyclonal IgG. Images adapted with permission from Ivanov et al. (American Society of Hematology – Blood) [69].
FXII undergoes autoactivation to α-fXIIa in the presence of polyphosphate (Figure 4C) [14]. We wanted to determine if fXII-T catalyzes this reaction, but could not use fXII-WT as a substrate as it autoactivates on its own. Instead we use fXII-S554A, which is cleaved after Arg353 to form the equivalent of α-fXIIa, but lacks activity once cleaved because of the absence of the active site serine. In figure 4D, conversion of fXII-S554A to fXIIa-S554A is followed by western blot using an antibody specific for the fXIIa active site [70]. The α-fXIIa signal increases with time in the presence of silica or polyphosphate, consistent with fXII-T cleaving fXII-S554A after Arg353.
Finally, α-fXIIa promotes coagulation by converting fXI to fXIa by a process accelerated by polyanions [12,71]. Like fXII, fXI undergoes autoactivation if polyphosphate is present [72], so we tested the ability of fXII-T to cleave fXI lacking an active site serine (fXI-S557A) to take autoactivation out of the equation [73]. FXII-T cleaves fXI-S557A to a form that migrates with fXIa on western blot (figure 4E). The prolonged aPTT clotting time of fXII-deficient plasma (327±30 sec) is restored to normal by adding fXII-WT (40±2 sec). FXII-T had a measurable effect on the aPTT (164±19, p<0.0001), while fXII-S554A (which lacks a functional active site) did not (302±6 sec, p=0.2), consistent with the premise that fXII-T has proteolytic activity that can convert fXI to fXIa.
Conclusions and Future Considerations
In standard models of plasma coagulation used in clinical laboratories, thrombin generation is initiated either by contact activation (the intrinsic pathway of coagulation) or by the factor VIIa/tissue factor complex (the extrinsic pathway). The trigger for the extrinsic pathway seems clear, as up to 4% of factor VII in plasma is in the form of the protease factor VIIa [74]. While coagulation through the intrinsic pathway begins with fXII activation, the mechanism that initiates the process has been uncertain. The data presented here suggest that fXII expresses sufficient proteolytic activity in its single-chain form to initiate surface-dependent contact activation [69**]. Given the low level of activity relative to α-fXIIa, sc-fXII activity is probably most important early in contact activation. As α-fXIIa and α-kal accumulate they would quickly dominate the process, and are almost certainly responsible for the physiologic and pathologic effects of fXII and PPK. Our findings do not exclude other mechanisms for initiating fXII activation. However, the data do indicate that pre-existing α-fXIIa is not a requirement.
Polyphosphate enhances fXII-T activation of macromolecular substrates, but fXII-T demonstrates activity even in the absence of this polyanion. FXII-T cleaves the tripeptide S-2303 with ~3000-fold lower catalytic efficiency than α-fXIIa [69**], a value in reasonable agreement with the maximum for sc-fXII predicted earlier by Silverberg and Kaplan [34]. Interestingly, in contrast to results reported for plasma fXII [43], polyphosphate did not increase fXII-T cleavage of S-2302 [69**]. Taken as a whole, these data indicate that the catalytic domain in sc-fXII is in an active conformation while circulating (unbound) in plasma that is not as efficient catalytically as that of α-fXIIa. FXII-T also activates PPK in the absence of polyphosphate. Here, binding to a macromolecular substrate may contribute to formation of a functional (or more active) sc-fXII active site, as has been reported for other protease-substrate interactions [75]. The cofactor effect provided by polyphosphate, silica and other “surfaces” may enhance sc-fXII activity by altering the conformation of the protease, the substrate, or both, and also likely serves to concentrate enzymes and substrates, promoting catalysis.
Our findings have some interesting physiologic and pathologic implications. There appears to be a measurable basal rate of reciprocal FXII and PPK activation in healthy mice [36]. While activation of PPK by fXII-T is several orders of magnitude less efficient than activation by α-fXIIa, the plasma PPK concentrations (~600 nM) is near Km for activation by fXII-T, and the plasma fXII concentration (~400 nM) is likely several orders of magnitude higher than that of α-fXIIa under most circumstances. The high plasma fXII level may compensate for the weak specific activity of sc-fXII toward PPK, and the reaction could contribute to fXII and PPK turnover in vivo. The effect of PPK activation by this mechanism is probably limited by serpins such as C1-inhibitor that neutralize α-kal and fXIIa shortly after they form. In addition, we observed that fXII-T cleavage of PPK is inhibited by C1-inhibitor, suggesting sc-fXII activity toward PPK is controlled under normal circumstances. However, the reaction may contribute to the disease process in patients with hereditary angioedema who lack C1-inhibitor [76]. Clinical use of implantable devices and extracorporeal circuits present foreign surfaces to blood that can lead to thromboembolic events and inflammation [5*,26**,27**–31]. Contact activation appears to play a role in complications associated with such devices, and it will be interesting to see if the materials they are made of potentiate the activity of sc-fXII in a manner similar to polyphosphate and silica.
Finally, the observation that fXII has proteolytic activity raises questions of terminology. The current nomenclature system for plasma coagulation factors uses Roman numerals to indicate unactivated forms of coagulation proteins, and Roman numerals followed by a lower case “a” to indicate forms converted to active species by proteolysis [77,78]. By this convention, the term fXIIa indicates species of fXII cleaved after Arg353 (α-fXIIa, β-fXIIa), distinguishing them from uncleaved fXII. Given its measurable proteolytic activity, fXII could be considered a form of fXIIa, however, this would not be inconsistent with the rules of the current system. Perhaps the designation sc-fXII, following the convention for sc-tPA and sc-uPA, may be preferable, at least when discussing protease enzymology.
KEY POINTS.
The plasma protein factor XII participates in activation of several host defense mechanisms.
A variety of substances and surfaces support factor XII conversion to its fully active form α-factor XIIa, but the triggering mechanism for this process has been debated.
Factor XII has proteolytic activity in its single-chain “precursor” form that may trigger initial conversion of factor XII to α-factor XIIa when blood is exposed to a surface.
Acknowledgments
This work was support by awards HL58837 and HL81326 from the National Heart, Lung and Blood Institute.
The authors wish to thank Drs. Ingrid Verhamme, Jonas Emsely, Qiufang Cheng, Kent Dickeson and Mao-fu Sun for their contributions to the work described in this paper.
FINANCIAL SUPPORT AND SPONSORSHIP
This work was supported by the National Heart, Lung and Blood Institute, and the Ernest W. Goodpasture Chair in Experimental Pathology and Translational Research at Vanderbilt University.
Footnotes
CONFLICTS OF INTEREST
D.G. receives consultant fees from the following companies (Bayer Pharma, Bristol-Myers Squibb, Ionis, Janssen, Merck, Novartis, and Ono Pharmaceuticals). I.I and A. M. have no conflicts to report.
BIBLIOGRAPHY
- 1.Colman RW, Schmaier AH. The contact activation system: biochemistry and interactions of these surface-mediated defense reactions. Crit Rev Oncol Hematol. 1986;5:57–85. doi: 10.1016/s1040-8428(86)80053-1. [DOI] [PubMed] [Google Scholar]
- 2*.Long AT, Kenne E, Jung R, et al. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost. 2016;14:427–437. doi: 10.1111/jth.13235. Excellent recent review of contact activation. [DOI] [PubMed] [Google Scholar]
- 3*.Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14:28–39. doi: 10.1111/jth.13194. Excellent recent review of contact activation. [DOI] [PubMed] [Google Scholar]
- 4.Danese E, Montagnana M, Lippi G. Factor XII in hemostasis and thrombosis: active player or (innocent) bystander? Semin Thromb Hemost. 2016;42:682–688. doi: 10.1055/s-0036-1571338. [DOI] [PubMed] [Google Scholar]
- 5*.Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost. 2015;13(suppl 1):S72–S81. doi: 10.1111/jth.12961. Excellent recent review of the effects of artificial surfaces used in clinical setting on blood coagulation. [DOI] [PubMed] [Google Scholar]
- 6.Van Cott EM, Roberts AJ, Dager WE. Laboratory Monitoring of Parenteral Direct Thrombin Inhibitors. Semin Thromb Hemost. 2017;43:270–276. doi: 10.1055/s-0036-1597297. [DOI] [PubMed] [Google Scholar]
- 7.Marlar RA, Clement B, Gausman J. Activated Partial Thromboplastin Time Monitoring of Unfractionated Heparin Therapy: Issues and Recommendations. Semin Thromb Hemost. 2017;43:253–260. doi: 10.1055/s-0036-1581128. [DOI] [PubMed] [Google Scholar]
- 8.Bates SM, Weitz JI. Coagulation Assays. Circulation. 2005;112:53–60. doi: 10.1161/CIRCULATIONAHA.104.478222. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler AP, Gailani D. The Intrinsic Pathway of Coagulation as a Target for Antithrombotic Therapy. Hematol Oncol Clin North Am. 2016;30(5):1099–114. doi: 10.1016/j.hoc.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiggins RC, Bouma BN, Cochrane CG, Griffin JH. Role of high-molecular-weight kininogen in surface-binding and activation of coagulation Factor XI and prekallikrein. Proc Natl Acad Sci USA. 1977;74:4636–40. doi: 10.1073/pnas.74.10.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandle RJ, Colman RW, Kaplan AP. Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc Natl Acad Sci USA. 1976;73:4179–83. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov I, Shakhawat R, Sun MF, et al. Nucleic acids as cofactors for factor XI and prekallikrein activation: Different roles for high-molecular-weight kininogen. Thromb Haemost. 2017;117:671–681. doi: 10.1160/TH16-09-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tans G, Rosing J. Structural and functional characterization of factor XII. Semin Thromb Hemost. 1987;13:1–14. doi: 10.1055/s-2007-1003471. [DOI] [PubMed] [Google Scholar]
- 14.Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SA, Choi SH, Davis-Harrison R, et al. Polyphosphate exerts differential effects on blood clotting, depending on polymer size [published correction appears in Blood. 2011;117(12):3477] Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labberton L, Kenne E, Long AT, et al. Neutralizing blood-borne polyphosphate in vivo provides safe thromboprotection. Nat Commun. 2016;7:12616. doi: 10.1038/ncomms12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould TJ, Vu TT, Swystun LL, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 19.Kannemeier C, Shibamiya A, Nakazawa F, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci USA. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Meijden PE, Munnix IC, Auger, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114:881–90. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 21.Maas C, Govers-Riemslag JW, Bouma B, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–18. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frick IM, Akesson P, Herwald H, et al. The contact system--a novel branch of innate immunity generating antibacterial peptides. EMBO J. 2006 Nov 29;25(23):5569–78. doi: 10.1038/sj.emboj.7601422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frick IM, Björck L, Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost. 2007;98:497–502. [PubMed] [Google Scholar]
- 24.Oehmcke S, Herwald H. Contact system activation in severe infectious diseases. J Mol Med (Berl) 2010;88:121–6. doi: 10.1007/s00109-009-0564-y. [DOI] [PubMed] [Google Scholar]
- 25.Sonesson A, Nordahl EA, Malmsten M, Schmidtchen A. Antifungal activities of peptides derived from domain 5 of high-molecular-weight kininogen. Int J Pept. 2011;2011:761037. doi: 10.1155/2011/761037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau JW, Liao P, Fredenburgh JC, et al. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood. 2014;123:2102–2107. doi: 10.1182/blood-2013-12-540872. [DOI] [PubMed] [Google Scholar]
- 27.Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. doi: 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]
- 28.Plötz FB, van Oeveren W, Bartlett RH, Wildevuur CR. Blood activation during neonatal extracorporeal life support. J Thorac Cardiovasc Surg. 1993;105:823–32. [PubMed] [Google Scholar]
- 29.Wendel HP, Jones DW, Gallimore MJ. FXII levels, FXIIa-like activities and kallikrein activities in normal subjects and patients undergoing cardiac surgery. Immunopharmacology. 1999;45:141–144. doi: 10.1016/s0162-3109(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 30.van Montfoort ML, Meijers JC. Recent insights into the role of the contact pathway in thrombo-inflammatory disorders. Hematology Am Soc Hematol Educ Program. 2014;2014:60–5. doi: 10.1182/asheducation-2014.1.60. [DOI] [PubMed] [Google Scholar]
- 31.Key NS. Epidemiologic and clinical data linking factor XI and factor XII to thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014:66–70. doi: 10.1182/asheducation-2014.1.66. [DOI] [PubMed] [Google Scholar]
- 32.Naudin C, Burillo E, Blankenberg S, Butler L1, Renné T. Factor XII Contact Activation. Semin Thromb Hemost. doi: 10.1055/s-0036-1598003. in press. [DOI] [PubMed] [Google Scholar]
- 33.Silverberg M, Dunn JT, Garen L, Kaplan AP. Autoactivation of human Hageman factor. Demonstration utilizing a synthetic substrate. J Biol Chem. 1980;255:7281–7286. [PubMed] [Google Scholar]
- 34.Silverberg M, Kaplan AP. Enzymatic activities of activated and zymogen forms of human Hageman factor (factor XII) Blood. 1982;60:64–70. [PubMed] [Google Scholar]
- 35.Tans G, Rosing J, Griffin JH. Sulfatide-dependent autoactivation of human blood coagulation factor XII (Hageman factor) J Biol Chem. 1983;258:8215–8222. [PubMed] [Google Scholar]
- 36.Revenko AS, Gao D, Crosby JR, et al. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–5311. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Matafonov A, Madkhali H, et al. Prolylcarboxypeptidase independently activates plasma prekallikrein (fletcher factor) Curr Mol Med. 2014;1:1173–85. doi: 10.2174/1566524014666141015153519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shariat-Madar Z1, Rahimy E, Mahdi F, Schmaier AH. Overexpression of prolylcarboxypeptidase enhances plasma prekallikrein activation on Chinese hamster ovary cells. Am J Physiol Heart Circ Physiol. 2005;289(6):H2697–703. doi: 10.1152/ajpheart.00715.2005. [DOI] [PubMed] [Google Scholar]
- 39.Shariat-Madar Z1, Mahdi F, Schmaier AH. Recombinant prolylcarboxypeptidase activates plasma prekallikrein. Blood. 2004 Jun 15;103:4554–61. doi: 10.1182/blood-2003-07-2510. [DOI] [PubMed] [Google Scholar]
- 40.Bane CE, Jr, Ivanov I, Matafonov A, et al. Factor XI Deficiency Alters the Cytokine Response and Activation of Contact Proteases during Polymicrobial Sepsis in Mice. PLoS One. 2016 Apr 5;1:e0152968. doi: 10.1371/journal.pone.0152968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiggins RC, Cochrane CC. The autoactivation of rabbit Hageman factor. J Exp Med. 1979;150:1122–1133. doi: 10.1084/jem.150.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratnoff OD, Saito H. Amidolytic properties of single-chain activated Hageman factor. Proc Natl Acad Sci USA. 1979;76:1461–1463. doi: 10.1073/pnas.76.3.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engel R, Brain CM, Paget J, et al. Single-chain factor XII exhibits activity when complexed to polyphosphate. J Thromb Haemost. 2014;12:1513–1522. doi: 10.1111/jth.12663. [DOI] [PubMed] [Google Scholar]
- 44.Friedrich R, Panizzi P, Fuentes-Prior P, et al. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–9. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 45.Kroh HK, Panizzi P, Bock PE. Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proc Natl Acad Sci USA. 2009;106:7786–91. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barranco-Medina S, Pozzi N, Vogt AD, Di Cera E. Histone H4 promotes prothrombin autoactivation. J Biol Chem. 2013;288:35749–57. doi: 10.1074/jbc.M113.509786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verhamme IM, Panizzi PR, Bock PE. Pathogen activators of plasminogen. J Thromb Haemost. 2015;13(Suppl 1):S106–14. doi: 10.1111/jth.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.de Maat S, Maas C. Factor XII: form determines function. J Thromb Haemost. 2016;14:1498–506. doi: 10.1111/jth.13383. Excellent recent review of fXII structure-function relationships. [DOI] [PubMed] [Google Scholar]
- 49.Gordon EM, Gallagher CA, Johnson TR, et al. Hepatocytes express blood coagulation factor XII (Hageman factor) J Lab Clin Med. 1990;115:463–9. [PubMed] [Google Scholar]
- 50.Ponczek MB1, Gailani D, Doolittle RF. Evolution of the contact phase of vertebrate blood coagulation. J Thromb Haemost. 2008;6:1876–83. doi: 10.1111/j.1538-7836.2008.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doolittle RF. Step-by-step evolution of vertebrate blood coagulation. Cold Spring Harb Symp Quant Biol. 2009;74:35–40. doi: 10.1101/sqb.2009.74.001. [DOI] [PubMed] [Google Scholar]
- 52.Miyazawa K, Wang Y, Minoshima S, et al. Structural organization and chromosomal localization of the human hepatocyte growth factor activator gene--phylogenetic and functional relationship with blood coagulation factor XII, urokinase, and tissue-type plasminogen activator. Eur J Biochem. 1998;258:355–61. doi: 10.1046/j.1432-1327.1998.2580355.x. [DOI] [PubMed] [Google Scholar]
- 53.Shimomura T1, Kondo J, Ochiai M, et al. Activation of the zymogen of hepatocyte growth factor activator by thrombin. J Biol Chem. 1993;268:22927–32. [PubMed] [Google Scholar]
- 54.Miyazawa K1, Shimomura T, Kitamura A, et al. Molecular cloning and sequence analysis of the cDNA for a human serine protease responsible for activation of hepatocyte growth factor. Structural similarity of the protease precursor to blood coagulation factor XII. J Biol Chem. 1993;268:10024–8. [PubMed] [Google Scholar]
- 55.Pixley RA, Stumpo LG, Birkmeyer K, et al. A monoclonal antibody recognizing an icosapeptide sequence in the heavy chain of human factor XII inhibits surface-catalyzed activation. J Biol Chem. 1987;262:10140–5. [PubMed] [Google Scholar]
- 56.Clarke BJ, Côté HC, Cool DE, et al. Mapping of a putative surface-binding site of human coagulation factor XII. J Biol Chem. 1989;264:11497–502. [PubMed] [Google Scholar]
- 57.Citarella F1, Ravon DM, Pascucci B, et al. Structure/function analysis of human factor XII using recombinant deletion mutants. Evidence for an additional region involved in the binding to negatively charged surfaces. Eur J Biochem. 1996;238:240–9. doi: 10.1111/j.1432-1033.1996.0240q.x. [DOI] [PubMed] [Google Scholar]
- 58.Citarella F, te Velthuis H, Helmer-Citterich M, Hack CE. Identification of a putative binding site for negatively charged surfaces in the fibronectin type II domain of human factor XII--an immunochemical and homology modeling approach. Thromb Haemost. 2000;84:1057–65. [PubMed] [Google Scholar]
- 59.Cool DE, Edgell CJ, Louie GV, et al. Characterization of human blood coagulation factor XII cDNA. Prediction of the primary structure of factor XII and the tertiary structure of beta-factor XIIa. J Biol Chem. 1985;260:13666–76. [PubMed] [Google Scholar]
- 60.Cool DE, MacGillivray TA. Characterization of the human blood coagulation factor XII gene. J Biol Chem. 1987;262:13662–73. [PubMed] [Google Scholar]
- 61.Ny T, Elgh F, Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci USA. 1984;81:5355–59. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dobrovolsky AB1, Titaeva EV. The fibrinolysis system: regulation of activity and physiologic functions of its main components. Biochemistry. 2002;67:99–108. doi: 10.1023/a:1013960416302. [DOI] [PubMed] [Google Scholar]
- 63.Tate KM, Higgins DL, Holmes WE, et al. Functional role of proteolytic cleavage at arginine-275 of human tissue plasminogen activator as assessed by site-directed mutagenesis. Biochemistry. 1987;26:338–343. doi: 10.1021/bi00376a002. [DOI] [PubMed] [Google Scholar]
- 64.Renatus M, Engh RA, Stubbs MT, et al. Lysine 156 promotes the anomalous proenzyme activity of tPA: X-ray crystal structure of single-chain human tPA. EMBO J. 1997;16:4797–4805. doi: 10.1093/emboj/16.16.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halfon S, Craik CS. In: Handbook of Proteolytic Enzymes. Barrett AJ, Rawlings ND, Woessner JF, editors. San Diego, CA: Academic Press; 1998. pp. 12–21. [Google Scholar]
- 66.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 67.Griffin JH, Cochrane CG. Mechanisms for the involvement of high molecular weight kininogen in surface-dependent reactions of Hageman factor. Proc Natl Acad Sci USA. 1976;73:2554–2558. doi: 10.1073/pnas.73.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90:3819–3843. [PubMed] [Google Scholar]
- 69**.Ivanov I, Matafonov A, Sun MF, et al. Proteolytic properties of single-chain factor XII: a mechanism for triggering contact activation. Blood. 2017;129:1527–1537. doi: 10.1182/blood-2016-10-744110. Primary publication for the data presented in this review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kokoye Y, Ivanov I, Cheng Q, et al. A comparison of the effects of factor XII deficiency and prekallikrein deficiency on thrombus formation. Thromb Res. 2016;140:118–24. doi: 10.1016/j.thromres.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gailani D, Gruber A. Factor XI as a Therapeutic Target. Arterioscler Thromb Vasc Biol. 2016;36:1316–22. doi: 10.1161/ATVBAHA.116.306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geng Y, Verhamme IM, Smith SA, et al. Factor XI anion-binding sites are required for productive interactions with polyphosphate. J Thromb Haemost. 2013;11:2020–8. doi: 10.1111/jth.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matafonov A, Sarilla S, Sun MF, et al. Activation of factor XI by products of prothrombin activation. Blood. 2011;118:437–45. doi: 10.1182/blood-2010-10-312983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McVey JH. The role of the tissue factor pathway in haemostasis and beyond. Curr Opin Hematol. 2016;23:453–61. doi: 10.1097/MOH.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 75.Lechtenberg BC, Johnson DJ, Freund SM, Huntington JA. NMR resonance assignments of thrombin reveal the conformational and dynamic effects of ligation. Proc Natl Acad Sci U S A. 2010;107:14087–14092. doi: 10.1073/pnas.1005255107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zuraw BL, Christiansen SC. HAE Pathophysiology and Underlying Mechanisms. Clin Rev Allergy Immunol. 2016;51:216–29. doi: 10.1007/s12016-016-8561-8. [DOI] [PubMed] [Google Scholar]
- 77.Wright IS. The nomenclature of blood clotting factors. Canad Med Ass J. 1962;86:373–374. [PMC free article] [PubMed] [Google Scholar]
- 78.Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]