Abstract
The intestinal environment is linked to an array of conditions and diseases, including osteoporosis. Human and animal studies indicate that probiotics can benefit intestinal health and may provide a useful therapeutic to prevent and/or treat bone loss. Probiotics are defined as live microorganisms that when administered in adequate amounts will confer a health benefit on the host. In this review, we will focus on 1) probiotics (definition, history, nomenclature, types), 2) the effects of probiotics on bone health and 3) mechanisms of probiotic prevention of bone pathologies.
Introduction
Each year more than 2 million fractures occur because of osteoporosis [1]. Numerous therapies have been developed for the prevention and treatment of osteoporosis. As a first approach, patients are asked to make changes to their lifestyle (i.e., exercise, cessation of smoking) and diet (including vitamin D and calcium supplementation) [2]. For patients at a higher risk of fractures, pharmacologic treatments (drugs and biologics) are used to inhibit bone resorption or stimulate bone formation [3]. Despite the many treatment options, we have yet to stop the increase in osteoporosis fractures. This may be in part due to patient concerns about side effects (although rare) from many pharmaceutical/drug-based therapies [4]. Given that 67 million Americans are predicted to have low bone mass by 2020, it is important to continue to identify additional therapeutic approaches/targets for osteoporosis.
One therapeutic target receiving increasing attention is the intestinal microbiome, which is an important regulator of physiologic functions of many organs including bone. The intestinal microbiota accounts for 90% of the cells in our body and amounts to ~100 trillion microbes comprising ~1000 species and 28 different phyla [5]. In addition to outnumbering host cell number, the gut microbiota also express 100-fold more genes compared to the human genome. [5]. As the microbiome coevolve with us, changes in its composition can consequently influence our human health [6]. Dysbiosis (a microbial imbalance) is linked to disease and bone loss; however, more importantly, the reverse is also true: treatment with probiotics can beneficially modulate the gut microbiota to enhance health, including that of bone [7–10]. In this review we will focus on 1) probiotics (definition, history, nomenclature, types), 2) the overall effects of probiotics on bone health and 3) mechanisms of probiotic prevention of bone pathologies.
1) PROBIOTICS
Probiotic – Defined
The word “probiotic” is derived from the Latin word ‘pro’ and the Greek word ‘bios’ meaning “for life;” this contrasts with “antibiotic” meaning “against life” [11–20]. While “good for life” is a general definition of probiotics, the detailed definition of what constitutes a probiotic has been difficult to achieve and has changed over time. In the 1950s, Werner Kollath, a German scientist, used the word “probiotic” to be inclusive of all organic and inorganic supplements that restored the health of malnourished patients [11, 12, 19, 20]. Years later, probiotics were further defined as substances produced by one microorganism to promote growth of another microorganism [11, 12, 16, 18–26]. In the 1970s, Fujii and Cook described probiotics as compounds that build resistance to infection in the host but do not inhibit the growth of microorganisms in vitro [11, 18, 27]. In the 1980s and 1990s, there was a surge of different probiotic definitions. For example, in 1990 Parker defined probiotics as organisms or substances in feed supplements which contribute to intestinal microbial balance [11, 14, 18, 19, 22, 24, 28]. Parker’s general definition was unsatisfactory to many since the word “substances” included chemical supplements such as antibiotics [18, 28]. Most researchers cited the definition of Fuller, who, in 1989, defined probiotics as live microbial feed supplements [11, 18, 19, 22, 24, 25]. Fuller’s definition stressed the importance of live cells as an essential part of the effective probiotic [18]. His definition also stated that a probiotic or supplement will benefit the host by improving the intestinal microbial balance [11, 26]. Many thought this definition was not as applicable to humans as it was to animals [11]. Subsequently, in the early 1990s, the definition was broadened to include viable mono or mixed cultures of live microorganisms which, when given to humans or animals, benefits the host by improving the properties of the indigenous microflora [29]. In the late 1990s, Salminen offered the view of incorporating non-viable bacteria in the probiotic definition [11, 28]. Finally, in 2001, after consultation of international scientists working on behalf of the FAO/WHO (Food and Agricultural Organization/World Health Organization) probiotics were proposed to be defined “as live microorganisms that when administered in adequate amounts will confer a health benefit on the host” [11, 15, 19, 21, 24, 30, 31]. Misuse of the probiotic term became a major problem in the ensuing years. For this reason, the International Scientific Association for Probiotics and Prebiotics (ISAPP) organized a meeting of clinical and scientific experts on probiotics in October 2013 to re-examine the concept and definition of probiotics [31]. The ISAPP panel recommended that the definition of probiotic as defined by FAO/WHO in 2001 is broad enough to enable a wide range of products to be developed, and at the same time sufficiently narrow to impose some core requirements [24, 31]. Thus, probiotics currently remain defined as live microorganisms that when administered in adequate amounts will confer a health benefit on the host.
The History of Probiotic Discovery
Probiotic use can be traced back over 10,000 years ago [32]. During the Neolithic period of the Stone Age, animal domestication and husbandry developed [20]. Ancient oriental people, as well as Phrygian, Sarmatian, and Macedonian nomadic shepherds drank milk from cows, sheep, goats, horses, and camels. Traditional Egyptian fermented milk products (Laban Rayeb and Laban Khad) were consumed as early as 7000 BCE [11, 19, 32]. Both iconographic and written evidence from 3000 – 2000 BCE indicate that Hindi, Egyptians, Greeks, and Romans all used fermented milk products [11]. Fermenting milk was also evident in the Middle and Far East of Asia and spread throughout eastern Europe and Russia by the Tartars, Huns, and Mongols during their land conquests [11]. Fermented products other than milk, such as beer, bread, wine, kefir, kumis, and cheese were also consumed [32] since fermentation increased their long-term storage [11, 19, 20].
The ancient Ayurvedic texts, written between 400 and 200 BCE, linked a long and healthy life with the intake of milk and dairy products [20]. To store the milk, it was customary to use containers made from animal skins or stomachs [19, 20]. The containers were a source of bacteria, most likely ancestors of Lactobacillus acidophilus and bulgaricus, which came into contact with the milk [20]. One Turkish legend describes a shepherd, traveling the hot desert, who forgot he had milk in a goatskin bag. When he checked, the milk had transformed into a thick, creamy, and tasty custard; this new product was referred to as yogurt [20]. For the Turkish people, yogurt was the elixir of life, as they believed that this food gave physical and inner well-being and could prolong life [20].
The modern history of probiotics begins in the late nineteenth to early twentieth century. Elie Metchnikoff (a Nobel laureate), as well as Theodor Escherich, studied microbial communities in feces and described the need for a complex intestine (microbe-wise) [33]. Metchnikoff was a Kharkov/Ukrainian scientist working at the Pasteur Institute [19, 20]. Pasteur had identified the microorganisms responsible for fermentation but it was Metchnikoff who investigated the effects these microbes had on human health [20]. Metchnikoff associated the longevity of Bulgarian rural people (who had an average lifespan of 87 years) to their regular consumption of fermented dairy products such as yogurt [19, 20, 24, 34]. Metchnikoff described two bacteria types: one that leads to putrefying luminal contents and produces unhealthy waste products (NH3, H2S, amines), and another that ferments luminal contents and produces beneficial metabolic products (i.e, lactic acid) [35]. This was a key concept because probiotic bacteria secrete enzymes that are not produced by human intestinal cells. These enzymes can ferment non-digestible poly-carbohydrates (mainly dietary fiber) to produce energy for the bacteria as well as other factors such as short chain fatty acids (SCFA) and lactic acid which benefits the intestinal epithelium [36]. Metchnikoff theorized that the production of lactic acid would prevent the toxic effects of putrefying microbes. This further led Metchnikoff to suggest that lactobacilli may benefit gastrointestinal metabolism and counteract illness and aging [11, 20, 24]; thus, he considered lactobacilli a probiotic [20, 25, 37]. Thanks to Metchnikoff, the dairy industry began in France and subsequently spread throughout Europe, using fermented milk obtained from Bacillus bulgaricus, Streptococcus thermophiles and Lactobacillus delbruekii [19].
About the same time that Metchnikoff was making his discoveries of lactic acid-producing bacteria, French pediatrician Dr. Henry Tissier observed that children with diarrhea had a low number of ‘Y’-shaped bacteria in their stools [19, 24, 26]. Healthy children had an abundance of these bacteria. In 1905, he isolated the bacteria, Bacillus bifidus, and linked its presence in children to those who were breast-fed [33]. He suggested these bacteria could be administered to patients with diarrhea to help restore their healthy flora (eubiosis) and used it to recolonize the gut of children (14,19–21,27,28,33). As the health benefits of milk-associated bacteria became better known, fermented dairy products were appearing around the world. For example, in 1935 a Japanese Microbiologist, Dr. Shirota, isolated Lactobacillus casei and added it to a dairy drink that was eventually marketed. Today, food products containing probiotics are usually dairy, mainly due to the historical association of lactic acid bacteria with fermented milk [11, 20, 32].
Probiotic Nomenclature and Types
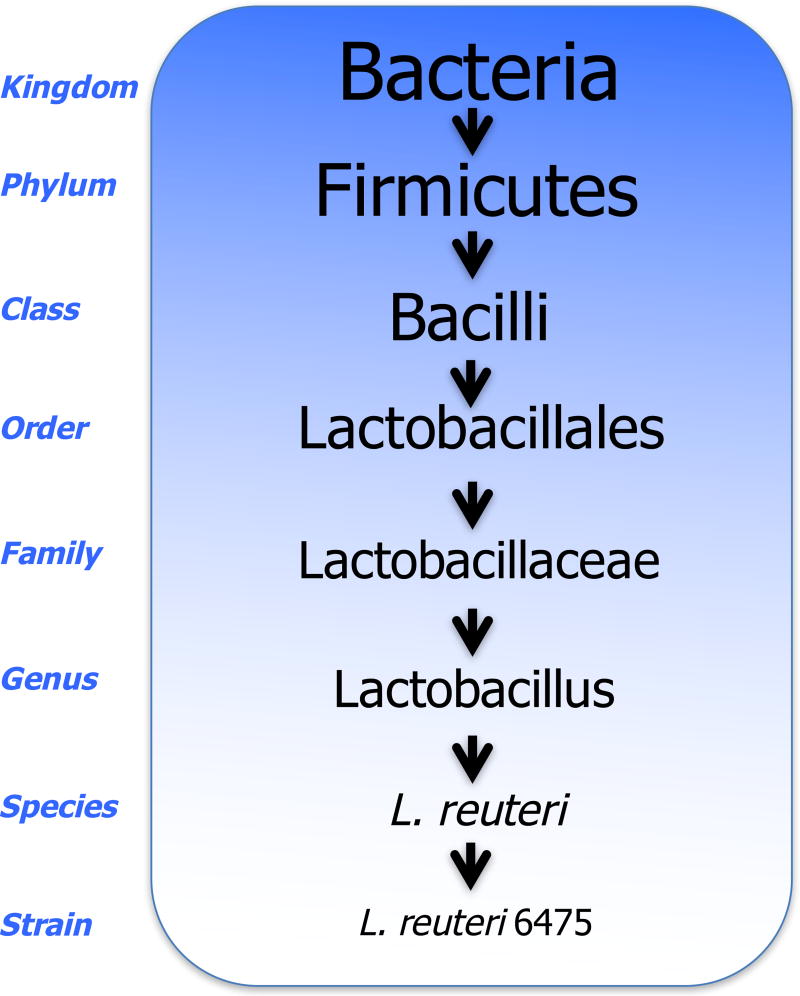
Probiotics are widely consumed and have a long history of safe use. Bacteria names are derived from descriptors of the bacteria (i.e., Lactobacillus, ‘lacto’ meaning “milk” and ‘bacillus’ meaning “rod-shaped”), a scientist’s name (i.e., Pasturella, found by Louis Pasteur), the place where found (i.e., Legionella longbeachiae, found in Long Beach California), or an organization (i.e., Legionella and the American Legion). In addition to a general name, the bacteria are described based on a taxonomic/genetic hierarchy [39]. Based on this system, bacteria are divided into phylum, class, order, family, genus, species and subspecies and/or strain (Figure 1). With more than 23 bacteria phyla, it is easy to see the abundance of specific probiotics and the complexity of their names. Current evidence indicates that the beneficial effect of probiotics are strain-specific [22]. It is also important to note that not all bacteria within a species act the same and/or can be regarded as a probiotic. Below, we discuss several of the most notable probiotics including lactic acid bacteria, Bifidobacteria and Enterococcus (also see Table 1).
Fig 1. Scientific Nomenclature.
An example of bacterial scientific nomenclature for the Lactobacillus reuteri 6475 strain.
Table 1.
Common Probiotic Bacteria
| Genus | Species | Genus | Species | Other |
|---|---|---|---|---|
| Lactobacillus | acidophilus | Bifidobacterium | longum | Enterococcus faecalis |
| crispatus | bifidum | Enterococcus faecium | ||
| johnsonii | Infantis | Lactococcus lactis | ||
| gasseri | animalis | Escherichia coli (Nissle 1917) | ||
| casei | adolescentis | Propionibacterium freudenreichii | ||
| rhamnosus | Lactis | Saccharomyces cerevisiae | ||
| reuteri | breve | Streptococcus thermophilus | ||
| plantarum | Bacillus cereus | |||
| fermentum | Bacillus subtilis | |||
| salivarius |
a) Lactic acid bacteria/ Lactobacillales
Lactic acid bacteria (also known as LAB) are one of the most important groups of bacteria/probiotics with health benefits that are thought to result in part from their production of lactic acid, their major fermentation product [11, 34, 42]. In general, they are gram-positive, acid-tolerant, asporogenous rods and cocci which are oxidase, catalase, and benzidine negative; they lack cytochromes, do not reduce nitrates to nitrite, are gelatinase negative, and are unable to utilize lactate [11, 38, 42]. Lactic acid bacteria obtained from fermented milk products have been used for centuries. Traditional fermented milk is a useful source of probiotics because it contains a complex composition of lactic acid bacterial species. In a recent study, 148 lactic acid bacterial strains were isolated from Kurut, a traditional naturally fermented yak milk from China [43]. Additional studies are evaluating these traditional fermented products as potential natural sources of probiotic bacteria [43].
Lactic acid bacteria, which consists of a diverse genera, are grouped as either homofermenters or heterofermenters based on the fermentation end product [38, 42]. Homofermenters produce lactic acid from glucose as a major product and heterofermenters produce a number of products such as carbon dioxide, acetic acid, ethanol as well as lactic acid [38, 42]. Homofermentive lactics include the genera Streptococcus which produces the L(+) lactate isomer and Pedicoccus which produces DL lactate [42]. Heterofermentive lactics consist of the genus Leucoostoc which produce D(−) lactate and a subgroup of the genus Lactobacillus, the Betabacteria which produce DL lactate [42].
Lactobacilli are ubiquitous in nature and are usually found in carbohydrate rich environments [11]. They also are a part of the normal flora in the intestinal tract of many animals. The genus Lactobacillus belongs to the phylum Firmicutes, class Bacilli, order Lactobacillales, family Lactobacillaceae [11]. The most commonly isolated species are Lactobacillus acidophilus, L. salivarius, L. casei, L. plantarum, L. fermentum, L. reuteri, L. rhamnousus, L. gasseri, L. reuteri and L. brevis from human intestine [11]. Several of these, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, and Lactobacillus reuteri have been extensively studied and well documented [44].
Lactobacillus acidophilus, which was first isolated from children’s feces by Ernst Moro in 1900, is capable of colonizing the human colon, has antimicrobial effects, and can be used to treat intestinal infections [26, 44]. Lactobacillus rhamnosus GG or Lactobacillus GG (LGG) is commonly used in dairy products marketed for infant and children’s consumption. Lactobacillus GG was isolated from human feces in 1983 and is indigenous to the human intestinal flora, has a tolerance to low pH environment and adheres to the gastrointestinal tract [44, 45]. LGG is effective in treating diarrhea [19, 46, 47]. Lactobacillus gasseri colonizes the gastrointestinal tract, oral cavity, and vagina in humans and is believed to contribute or potentiate probiotic activity in part by reducing fecal mutagenic enzymes as well as stimulate macrophages [44].
b) Bifidobacteria
Bifidobacteria are the predominant intestinal organism of breast-fed infants. These bacteria are rod-shaped, non-gas producing and anaerobic. Breast milk has been found to contain lactic acid bacteria as well as Bifidobacteria, both now included in formulas and foods targeted to pre-term and full-term infants [43]. Bifidobacteria are generally characterized as gram positive, non-spore forming, non-motile and catalase-negative anaerobes [11]. Initially they had been assigned to the genera Bacillus, Bacteroides, Mocardia, Lactobacillus and Corynebacterium, before being recognized as a separate genera in 1974 and included in the Actinomycetaceae family [11, 44]. This family consists of 5 genera: Bifidobacterium, Propionibacterium, Microbacetium, Corynebacterium, and Brevibacterium [11]. Currently there are 32 species in the genus Bifidobacterium, 12 are isolated from human sources, 15 from animal intestinal tracts or rumen, 3 from honeybees and the other 2 are found in fermented milk and sewage [11, 38]. Species found in humans are: Bifidobacteria. adolescentis, B. angulatum, B. bifidum, B. breve, B. catenulatum, B dentium, B. infactis, B. longum, ad B. pseudocatemulatum [11, 44]. These probiotic species can induce immunoglobulins, improve food nutritional value by assimilation of substrates not metabolized by the host, have potential anti-carcinogenic activity, and folic acid synthesis [44]. Specifically, Bifidobacterium infantis has been found to significantly improve symptoms in patients with irritable bowel disease [19].
c) Enterococcus
There are 37 species of Enterococcus which have been validated for use as probiotics [48]. Enterococci are singular, double or short chained gram positive cocci [44]. These bacteria occur in many habitats such as soil, surface water, ocean water, sewage, on plants and in the gastrointestinal tract of animals and humans, with E. faecalis being the most predominant [48]. Bacteria of the Enterococcus genus can also be used to treat diarrhea, irritable bowel syndrome, are considered to be an alternative for antibiotics, and are used for lowering cholesterol and immune regulation [44, 48].
d) Other probiotics
Besides the human gastrointestinal tract, the gastrointestinal tract of other animals such as pigs, rats, and poultry are also good sources of probiotics [43, 47]. Other probiotic strains have been discovered in marine and freshwater fish such as rainbow trout and shrimp [43], as well as in non-fermented foods such as meat and fruits [43]. Lactobacillus strains from brine of naturally fermented olives and from pickled juices have also demonstrated probiotic properties [43]. Other popular probiotics are Streptococcus thermophilus, Lactococcus lactis subsp. lactis, Leuconostoc mesenteroides, Propionibacterium freudenreichii, Pediococcus acidilactici, Sporolactobacillus inulinus, Escherichia coli, other bacteria of the Bacillus species, other lactic acid bacteria species, Saccharomyces cerevisiae and Saccharomyces boulardii yeasts. Many popular probiotics are added to dairy products and can have favorable effects on human health [11, 19, 21, 22, 34, 44]. There is a selection criteria regarding probiotic strains used in such products. There are several components of this criteria: a) the bacterium must be reported in the literature, b) concrete proof of assistance to health must exist, c) the bacterium must be able to colonize the gastrointestinal tract and have a regulatory role in microbial balance in that area, d) must be resistant to low pH values and bile salts in order to be able to sustain their viability, e) must posses natural antibiotic effect in order to prevent pathogen growth with their antimicrobial activity, f) must be safe to consume and show no antibiotic resistance, and g) must be suitable for commercialization [11, 22, 24, 30, 43, 44].
e) Commensal bacteria
Through co-evolution, humans not only tolerated the presence of the intestinal microbiota but also evolved to use the colonization of commensal microbes for immune development and function, intestinal barrier integrity, and overall health [49]. Commensal microbes comprise the resident bacteria that live on the human body and in the intestine amount to over 500 different strains including probiotic strains. The composition of intestinal microbes differs depending upon the intestinal region, with gradients existing both vertically and longitudinally (Figure 2) [50]. Along the longitudinal axis, the number of microbiota increases distally with the greatest level in the colon (~1012). Along the vertical axis, certain bacteria are found in the upper mucus layer above the epithelium while others prefer the lumen. Different microbes thrive in different regions because of the local environment, which is influenced by luminal dietary contents, bile, pH, mucus, other bacteria, etc…. Several of the major probiotic strains were originally isolated from humans include: Lactobacillus acidophilus, bifidobacteria, several LAB strains, [43], and Lactobacillus rhamnosus GG [44, 45]. In the intestine, the balance of beneficial bacteria with neutral or inflammatory bacteria is critical. Thus, intestinal dysbiosis (microbe imbalance) leads to a reduction in the beneficial commensal microbes and can contribute to disease [49]. Probiotic intake can help restore commensal microbe balance.
Fig 2. Regional bacterial changes of the intestine.
The intestine is a major source of commensal microbes containing more than 500 species. Along the longitudinal axis, the number of bacteria increases distally. Along the vertical axis the majority of bacteria are in the lumen with some in the top mucus layer. Microbes colonize different environments based on a number of factors including pH and the nutrients available.
2) PROBIOTICS AND BONE HEALTH
Probiotics Regulate the Gut-Bone Axis
Oral probiotics benefit the intestine as well as extra-intestinal organs including bone [8–10, 51, 52]. Bone is a dynamic organ that depends on a fine balance between the bone forming osteoblasts and bone resorbing osteoclasts. An imbalance in this process can lead to bone disease. Bone homeostasis can be regulated by hormones such as estrogen, parathyroid hormone as well as by immune cells [53–55]. The gastrointestinal system also plays a key role in bone health, most notably by regulating absorption of minerals such as calcium, phosphorous and magnesium as well as by being major producers of endocrine factors that signal to bone cells, such as incretins and serotonin. Therefore, agents/conditions that influence intestinal physiology can impact bone health. Recent studies, including some from our lab, indicate that in addition to mineral absorption, the intestinal microbiota can be a critical player in regulating bone physiology [7, 8, 52, 56, 57]. Thus, we and others have examined the influence of probiotics on gut microbiome and how this modulates bone health. The effect of probiotics on the gut-bone axis is determined by a variety of factors. In this sub-section we will discuss studies examining the effect of probiotics on bone during growth, aging, and menopause. In addition, we will discuss the role of sex in bone responses to probiotics as well as the safety of probiotics.
a) Probiotic Effects on Growth
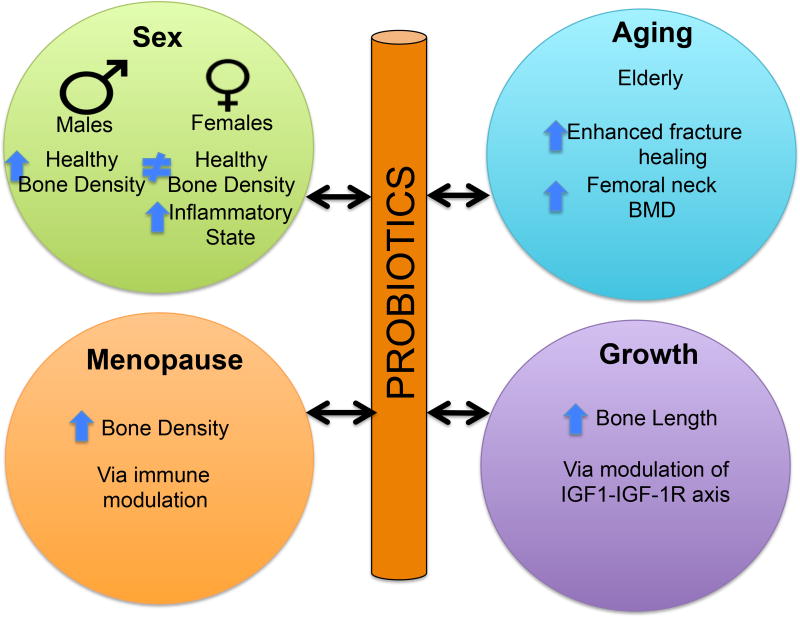
Stability of the intestinal microbiota composition is a critical regulator of intestinal homeostasis throughout life, from new born to adulthood. Increasing evidence also indicates that intestinal homeostasis plays a key role in the development of healthy strong bone during childhood and adolescence, which ultimately leads to a healthy adult skeleton [58]. By comparing microbiota from undernourished and healthy children from a Malawian birth cohort, Blanton et al [59] demonstrated that the microbiota is causally related to childhood nutrition. More importantly, the microbiota effects were functionally transmittable to germ-free mice (mice lacking a microbiome). Specifically, germ-free mice whose intestines were populated with microbiota from the undernourished children displayed reduced growth, altered bone morphology and metabolic dysfunction compared to mice populated with age-matched healthy microbiota [59]. Supplementation with two bacterial strains (Ruminococcus gnavus and Clostridium symbiosum) added to the microbiome from undernourished children ameliorated growth abnormalities in the mice, supporting a role for microbiome composition and by extension probiotics in growth regulation [59]. In support of these findings, Schwarzewr et al [60] show that undernourished mice supplemented with the probiotic Lactobacillus plantarum are able to maintain normal growth rates. Specifically, under-nutrition suppresses growth and bone growth parameters (femur length, cortical thickness, cortical bone fraction, and trabecular fraction of the femur) and these effects were prevented by L. plantarum treatment [60]. Importantly, and in agreement with Blanton et al [59], the presence and/or composition of microbiota during development was shown to be important for regulating mouse growth rates. By comparing wild-type and germ-free mice the group found that growth parameters were decreased in the germ-free mice which were 4% shorter and weighed less than the WT mice. This response was shown to be dependent on the IGF-1-IGF-1R axis (Fig 3). Analysis of growth hormone (GH), IGF-1, and IGFBP-3 levels indicated a significant decrease in germ free compared to wild type mice 56 days after birth while on under-nourished diet [60]. Supplementation with L. plantarum brought IGF-1 and IGFBP- 3 back to wild type levels, suggesting L. plantarum can recapitulate the beneficial effects of the microbiota on the IGF-1-IGF-1R axis [60]. Yan et al. [61] also demonstrate the important role of the gut microbiota in regulating IGF-1 expression, bone formation and growth in mice. These effects cross animal species and are seen in drosophila as well. Specifically, drosophila display growth suppression in response to undernutrition or lack of a microbiome [62]. When germ-free flies are repopulated with probiotic lactobacilli strains the flies regain their ability to grow at normal rates [62]; and the IGF axis is restored [63]. In humans, Steenhout et al examined the impact of probiotic-supplemented formulas on growth in both healthy and vulnerable populations [64]. They concluded that the probiotic Bifidobacterium lactis has a positive effect on growth in infants born to mothers with human immunodeficiency virus [64]. Taken together, these studies demonstrate that a healthy gut microbiome is important for bone growth during development.
Fig 3. Probiotics bone effects in different populations.
Probiotics benefit bone health across differing populations. The host bone responses are dependent upon factors such as sex, aging, menopause and growth.
b) Probiotic Effects on Aging Bone
Aging is associated with many complications including osteoporosis. The use of probiotics to benefit longevity and health dates back to ancient Ayurvedic texts (400 and 200 BCE) [20]. Given this, it is surprisingly that only recently research has begun to focus on the critical role and mechanisms of microbiome/probiotic regulation of aging conditions, such as osteoporosis. While there currently are several ongoing studies examining probiotic effects on bone health in the elderly, only a few studies have been published to date. In one study, Lactobacillus casei Shirota was given to elderly male and female patients (n=417); after 4 months of treatment these patients showed enhanced fracture healing (distal radius) compared to patients with placebo treatment (Fig 3) [65]. In a similar study, 50 postmenopausal women with osteopenia (50–72 years of age) were randomly assigned to take either GeriLact (7 probiotic bacteria species) or a placebo for 6 months. The multispecies probiotic GeriLact significantly decreased biomarkers of bone resorption in comparison with the placebo group, though no significant changes in bone mineral density were observed during this period of treatment [66]. Interestingly, the probiotic treatment did significantly decreased serum levels of parathyroid hormone and the pro-inflammatory marker TNF-α [66]. Another study, that saw an effect on bone density, involved the treatment of osteoporotic males (64–67 years of age) with Kefir fermented milk for 6 months. The group found a 5% increase in femoral neck bone mineral density measured by DEXA [67]. This study supports a benefit of probiotics on bone health, but it is important to recognize that only 24 subjects were studied and the contribution of calcium in the Kefir was not separated from the effects of the probiotic bacteria. While not directly examining bone, a recent study by Han et al. screened a library of c. elegan mutants to identify bacterial metabolites that influence lifespan and reduce aging complications [68]. The polysaccharide colonic acid was found to be involved in mediating longevity and reducing aging complications, supporting a role for intestinal microbes in regulating lifespan and health. Taken together, ancient texts and recent data indicate the potential for probiotics to maintain bone health throughout life.
c) Probiotic Effects on Menopausal Osteoporosis
The natural loss of estrogen due to menopause is the most important risk factor for osteoporosis in women. Women, over the course of their lifetime, lose about 50% of their trabecular bone and 30% of their cortical bone; about half of the bone loss occurs during the first 10 years after menopause [69]. Recent studies have examined the influence of the microbiota and probiotic treatment during osteoporosis especially under conditions of estrogen deficiency in animal models. For example, while we previously noted that intact healthy female mice do not display a bone response to L. reuteri, we found that L. reuteri treatment can prevent ovariectomy-induced bone loss in mice, suggesting that lack of estrogen may influence responsiveness to L. reuteri effects on bone (Fig 3) [70]. These findings were confirmed by others using similar or distinct probiotics [44,48,63]. In a recent study, Li et al.[71] demonstrated that microbiota is necessary for sex-steroid deficiency-induced bone loss. Female wild type and germ-free mice were given Lupron (ovarian sex steroid antagonist) to block the effect of estrogen in mice. While wild-type mice lost bone as expected, the germ-free mice did not lose bone, demonstrating that the microbiota may be essential for estrogen-deficiency induced bone loss [71]. While Lupron increased intestinal permeability in wild type mice, it did not affect permeability in the germ free mice. Supplementation of conventional mice with Lactobacillus rhamnosus GG (LGG) or VSL#3 reduced gut permeability, intestinal inflammation and protected mice against bone loss induced by ovariectomy induced estrogen deficiency [71].
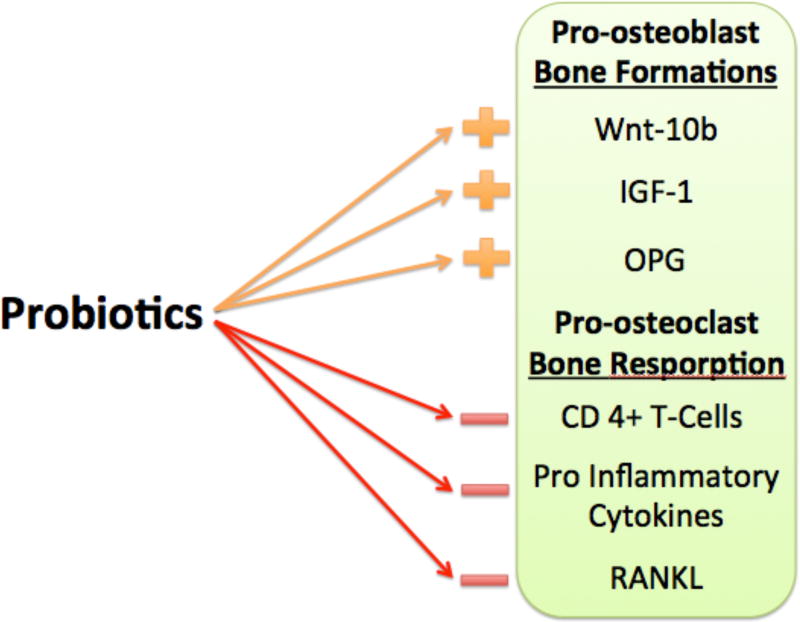
Probiotics have been proposed to function in multiple ways under estrogen deficient conditions. One important mechanism is through the suppression of osteoclastogenesis, an event that is upregulated during estrogen deficiency/menopause. Our studies showed that L. reuteri can suppress OVX-induced increases in bone marrow CD4+ T-lymphocytes, which are responsible for the overstimulation of osteoclasts (Fig 5) [52]. In addition, we have also shown that a 3kd fraction of the L. reuteri can inhibit osteoclastogenesis in vitro [72]. Similarly, Ohlson et al showed that the probiotics could affect pro-inflammatory cytokines such as TNFα and IL1β, as well as increase osteoprotegerin levels, all of which will decrease osteoclastogenesis. Similar attenuation of bone loss was also demonstrated with soymilk, that was supplemented with L. paracasei subsp. paracasei NTU101 or L. plantarum NTU 102 in ovariectomized mice [8]. Narva et al has also demonstrated a similar outcome with the use of fermented milk, valylprolyl-proline, and Lactobacillus helveticus LBK-16H in ovariectomized rats [73]. Finally, Rodrigues et.al showed that synbiotics, in this study a combination of prebiotics (Yacon flour) and probiotics (Bifidobacterium longum), increased bone mineral content in rats [51]. Together, these studies demonstrate an important role for oral probiotics in reversing estrogen-deficiency-induced bone loss.
Fig 5. Mechanism of probiotics beneficial bone affects.
Probiotic treatment can modulate the differentiation and function of osteoblasts through changes in Wnt-10b, insulin like growth factor-1 and OPG as well as osteoclasts through modulation of CD4+ T-cells, pro-inflammatory cytokines and RANKL.
d) Influence of Sex on Probiotic Effectiveness
Sex hormones are known to play a critical role in regulating bone density [74]. For example, males have greater bone density than females mainly due to differences in cortical bone expansion and greater trabecular bone volume [75, 76]. In addition, studies indicate that some mouse models display gender differences in response to hormones, such as PTH, that regulate bone [77]. Similarly, in one of the earliest bone studies to identify sex-specific responses to probiotic use, our lab administered Lactobacillus reuteri ATCC PTA 6475 (L. reuteri) to healthy male and healthy female mice for 4 weeks [9]. L. reuteri increased bone volume fraction and bone mineral density in healthy male mice and this was associated with a suppression of intestinal inflammation (Fig 3) [9]. Surprisingly, these effects were not observed in female mice, demonstrating that L. reuteri treatment influences bone (and gut) in a sex-specific manner [9]. This is also consistent with studies that induce intestinal inflammation by infecting mice with, H. hepaticus; in these studies, the pathogenic bacteria caused intestinal inflammation and bone loss in male mice but did not have a significant effect in female mice [78]. Taken together the findings suggest that female mice do not respond to either “bad” or “good” bacteria. In later studies, we identified that intact female mice can respond to probiotic (L. reuteri) treatment, but only when they are put into mild inflammatory state through dorsal surgical incision [7], supporting a potential role for inflammatory cells and estrogen in regulating female responses to luminal bacteria.
e) Probiotic Safety Throughout Life
The above studies indicate that probiotics hold great promise for supporting bone health. While generally regarded as safe (GRAS), there are some situations where probiotics need to be used cautiously. Patients with compromised immune systems, significant intestinal barrier dysfunction or with severe/critical illness may be susceptible to adverse effects such as sepsis, fungemia and intestinal ischemia [79] under these conditions the concern is that the load of intestinal bacteria, even though beneficial, could lead to inflammation and cross-over into the blood system where immune cells may be compromised and unable to remove/kill the bacteria. Recent tolerability studies for one probiotic, lactobacillus rhamnosus GG (LGG), are very positive. Children with Crohn’s disease, which involves a barrier break, tolerate orally supplemented LGG and displayed a side effect profile comparable with placebo [80]. Similarly, elderly patients (66–80 years old) did not display serious adverse effects in response to probiotic (LGG) treatment [81]. Mild symptoms that can occur include bloating, gas and nausea during the adaptation to probiotic ingestion. As with any new therapy, it is important to carry out these safety and tolerability studies.
3) MECHANISMS OF PROBIOTIC PREVENTION OF BONE PATHOPHYSIOLOGY
Effect of probiotics in dysbiosis-induced bone loss
Dysbiosis is caused by an imbalance of gut microbiota composition/function [82]. While primarily an ailment of the gut, dysbiosis can have systemic effects due to increased permeability of the intestinal mucosa [83]. This can result in bacterial products such as lipopolysaccharide to enter systemic circulation resulting in systemic and local tissue inflammation at distant sites including the bone (Fig 4) [84, 85]. Our lab has shown that dysbiosis caused by an infectious H. hepaticus bacteria can induce gut inflammation as well as bone loss in male mice [86]. Long-term antibiotic treatment can also induce dysbiosis and has been shown to influence bone. Specifically, male mice treated with antibiotics (ampicillin and neomycin) from 4 to 16 weeks of age display decreased bone strength and reduced B and T cell populations [87]. In a periodontal model of dysbiosis, bone loss was observed [88]. Activation of NOD1 (Nucleotide-binding oligomerization domain containing 1) a receptor for immune function in the gut spared bone-loss in these mice, indicating that it could have important effects in similar cases in humans [88].
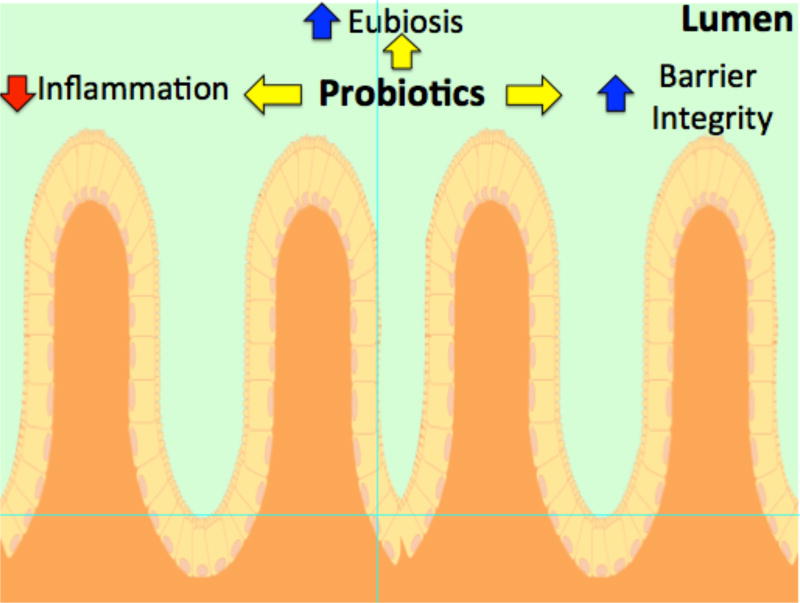
Fig 4. Model of Probiotic mechanistic signals regulating bone density.
A disruption in gut microbiota homeostasis can lead to increased inflammation and gut permeability resulting in systemic organ inflammation, including within bone. Prevention of local gut inflammation and permeability by promoting a healthy gut microflora (eubiosis) is one of the many ways probiotics can benefit bone health.
Probiotic treatment can benefit dysbiosis and gut health through maintaining intestinal barrier function and thereby preventing toxins from entering systemic circulation [89–93]. In a study causing enteropathogenic E.coli (EPEC)-induced dysbiosis, administration of probiotic E.coli Nissle 1917 increased specific claudin expression and prevented increases in intestinal permeability seen after infection with EPEC (Fig 4) [94]. While pathogenic dysbiosis can damage the intestinal barrier, several studies have shown that this barrier can be rescued through the use of specific probiotics [95–98]. These studies suggest that several conditions linked with gut dysbiosis can be improved through the proper treatment with probiotics. Along with treating the intestinal permeability observed in dysbiosis, probiotics have also been shown to have positive effects on bone health in dysbiosis models. Periodontal disease characterized by dysbiosis of the healthy oral bacterial flora leading to increased inflammation and subsequent bone loss was prevented with probiotic administration. Using this model, mice with periodontitis that were treated with Lactobacillus brevis CD2 displayed decreased bone loss and lower expression of pro-inflammatory cytokines such as tumor necrosis factor, interleukin-1β and -17A (Fig 5). Similar studies in a rat model of periodontal disease indicate that probiotics (Bacillus subtilus and Saccharomyces cerevisiae) can decrease bone resorption, increased bone density, and decreased inflammation [99, 100]; dysbiosis was also prevented by probiotic treatment [99].
Effect of probiotics in IBD-induced bone loss
Inflammatory Bowel Disease (IBD) can have detrimental effects on bone health by affecting the actions of osteoblasts and osteoclasts and promoting osteoporosis [101]. IBD is characterized by gut dysbiosis which generates an inflammatory response both locally and systemically, including within the bone marrow and bone [86]. Thus, IBD-induced intestinal inflammation is the primary pathology that leads to IBD-induced osteoporosis [101]. When the dysbiosis is recognized by the immune system, an inflammatory response occurs that includes the release of many pro-inflammatory cytokines such as TNF-α, interleukins IL-6, IL-11, IL-17, as well as prostaglandin E2 [102]. Cytokine expression is also elevated in bone [86, 103, 104]. The elevation of pro-inflammatory cytokines promotes osteoclast activity and also suppresses osteoblast activity; the latter occurs by decreasing maturation and increasing cell death. IBD also affects the RANK-RANKL-OPG pathway of bone metabolism and promote excessive bone loss [105]. Prostaglandin E2 promotes RANKL and inhibits OPG, which results in greater osteoclast activation. For a comprehensive review of how IBD affects bone, please refer to the chapter by Dr. Sylvester.
Recent studies have shown the protective effects of probiotics on IBD induced gut inflammation and on bone. Administration of a commercially available probiotic VSL#3 in a mouse model of ulcerative colitis led to decreased gut permeability and aided in treatment of inflammatory symptoms (Fig 5) [91]. Using other probiotics, such as L. reuteri (R2LC), in IL-10 deficient colitis models attenuated disease development, normalizing gut barrier function and reducing pro-inflammatory cytokines and histological disease score [106]. Consistent with these studies, DSS induced colitis caused increases in gut permeability in female BALB/c mice which was prevented with treatment of Bifidobacterium longum CCM 7952 (Bl) [107]. Additional studies indicate that the modulation of toll-like receptor 9 (TLR9) is necessary for the beneficial effects of probiotics in ulcerative colitis treatment [108].
Although these studies did not look at the direct effect of probiotics on bone, they do indicate that probiotics can have beneficial effects on IBD-induced gut inflammation, which is the one of the main components of IBD-induced bone loss. However, probiotics appear to have differential effects on bone inflammation. Treatment of bone marrow-derived dendritic cells from mice with VSL#3 showed increases in both pro and anti-inflammatory cytokine levels [109]. Taken together these studies show that probiotic treatment of IBD patients may be beneficial to correct the dysbiosis and reduce intestinal inflammation but further studies are needed to solidify the beneficial role of probiotics.
Effect of probiotics in Type-1 diabetes induced bone loss
Type-1 diabetes is a chronic autoimmune disease characterized by destruction of insulin-producing pancreatic β-cells, resulting in the requirement for exogenous insulin to control blood glucose levels. The consequent metabolic dysregulation has many deleterious consequences including bone loss. T1D-induced osteoporosis is thought to result primarily from the dysregulation of osteoblastic activity. Given that probiotics benefit bone health, probiotic treatment in this model has been examined. This is based on early studies indicating a role for the gut microbiome in T1D development. One of the original studies in non-obese diabetic mice (NOD) showed that NOD mice lacking MyD88 protein (adaptor for multiple innate immune receptors that recognize bacterial stimuli) did not develop T1D [110]. This protection is dependent on the commensal microbes because germ-free MyD88-negative NOD mice develop severe diabetes, whereas bacterial colonization attenuates T1D [110]. Thus, commensal bacteria maybe important to reduce disease susceptibility. Consistent with these findings, another group showed that early life antibiotics alters the gut microbiota and its metabolic capacities, intestinal gene expression and T-cell populations leading to accelerated T1D in NOD mice [111]. In addition, our lab has demonstrated that modulation of the gut microbiota with probiotic L. reuteri 6475 can prevent streptazotocin (STZ) induced T1D-mediated bone loss in mice. In this study, male (C57BL/6 14 weeks old) mice were given an STZ injection to induce type 1 diabetes which displayed a 35% reduction in bone volume fraction 4 weeks post injection [112]. Treatment with L. reuteri 6475 prevented this bone loss. This was further supported by trabecular bone data, which revealed that L. reuteri 6475 prevented the increase in trabecular spacing and reduction in trabecular number induced by T1D. STZ induced T1D bone loss comes from reduced osteoblast activity, which was consistent with decreased osteocalcin (bone formation) serum markers and decreases in mineral apposition rate (MAR) compared to controls. L. reuteri 6475 prevented decreases in both osteocalcin and MAR suggesting that probiotics specifically in this model, can have an anabolic effect on bone [112]. Additionally, part of T1D’s bone pathology is an increase in bone marrow adiposity, indicating an altered lineage commitment of bone marrow stromal cells toward the adipocyte over osteoblast lineage [113, 114]. In this study, consistent with benefiting bone health, L. reuteri 6475-treated T1D mice did not display increases in adipocyte number (86). Furthermore Wnt10b signaling which in mesenchymal precursor cells stimulates osteoblastogenesis and inhibits adipogenesis was decreased in T1D mouse bone (Fig 5). Treatment with probiotic L. reuteri 6475 fully restored whole bone Wnt10b gene expression back to normal levels (86). These findings suggest that probiotic use can prevent T1D bone loss by modulation of expression of Wnt10b in bone.
Conclusions
There are many studies supporting the role for the microbiome in the regulation of bone heath. Direct supplementation of beneficial probiotic bacteria can affect bone health by regulating aspects of gut such as preventing dysbiosis and/or increases in gut permeability and inflammation. However, more research is needed to understand the signaling pathways that link the gut microbiome to bone. Future studies should focus on identifying mechanisms in which probiotics/microbiome are able to regulate osteoblast/clast activities. These studies are important for developing future treatments for osteoporosis.
References
- 1.National Osteoporosis Foundation. https://www.nof.org/
- 2.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins F, L Rois-Arce N, Schepper J, Parameswaran N, McCabe LR. The Potential of Probiotics as a Therapy for Osteoporosis Microbiology Spectrum. 2016;5(4) doi: 10.1128/microbiolspec.BAD-0015-2016. doi: 10.1128/microbiolspec.BAD-0015-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla S, Shane E. A Crisis in the Treatment of Osteoporosis. J Bone Miner Res. 2016;31:1485–1487. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda S, Ohno H. Gut microbiome and metabolic diseases. Semin Immunopathol. 2014;36:103–114. doi: 10.1007/s00281-013-0399-z. [DOI] [PubMed] [Google Scholar]
- 6.Ley R, Turnbaugh P, Klein S, Gordon J. Human Gut microbes associated with obesity. Nature. 2006:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 7.Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, McCabe LR. Lactobacillus reuteri 6475 Increases Bone Density in Intact Females Only under an Inflammatory Setting. PLoS One. 2016;11:e0153180. doi: 10.1371/journal.pone.0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjögren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9:e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228:1793–8. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–82. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azizpour K, Bahrambeygi S, Mahmoodpour S, Azizpour A. History and basic of probiotics. Res J Biol Sci. 2009;4:409–426. [Google Scholar]
- 12.Hamilton-Miller JMT, Gibson GR, Bruck W. Some insights into the derivation and early uses of the word “probiotic”. Br J Nutr. 2003;90:845. doi: 10.1079/bjn2003954. [DOI] [PubMed] [Google Scholar]
- 13.Rijkers GT, de Vos WM, Brummer R-J, Morelli L, Corthier G, Marteau P. Health benefits and health claims of probiotics: bridging science and marketing. Br J Nutr. 2011;106:1291–1296. doi: 10.1017/S000711451100287X. [DOI] [PubMed] [Google Scholar]
- 14.Fuller R. Probiotics. J Appl Bacteriol Supplement. 1986:1S–7S. [PubMed] [Google Scholar]
- 15.FAO. Probiotics in food. Food Nutr Pap. 2001;85:71. [Google Scholar]
- 16.Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM. Health benefits of probiotics: a review. ISRN Nutr. 2013;2013:481651. doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morelli L, Capurso L. FAO / WHO Guidelines on Probiotics 10 Years Later. J Clin Gastroenterol. 2012;46:10–11. doi: 10.1097/MCG.0b013e318269fdd5. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan MG, Thornton G, O’Sullivan GC, Collins JK. Probiotic bacteria: myth or reality? Trends Food Sci Technol. 1992;3:309–314. [Google Scholar]
- 19.Gogineni VK. Probiotics: History and Evolution. J Anc Dis Prev Remedies. 2013;1:1–7. [Google Scholar]
- 20.Gasbarrini G, Bonvicini F, Gramenzi A. Probiotics History. J Clin Gastroenterol. 2016;50:S116–S119. doi: 10.1097/MCG.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 21.Senok AC, Ismaeel AY, Botta GA. Probiotics: Facts and myths. Clin Microbiol Infect. 2005;11:958–966. doi: 10.1111/j.1469-0691.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 22.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 23.Lilly Daniel M, Stillwell RH. Probiotics : Growth-Promoting Factors Produced by Microorganisms. Science (80-) 2017;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 24.Anukam K, Reid G. Probiotics: 100 years (1907–2007) after Elie Metchnikoff’s Observation. Commun Curr Res Educ Top trends Appl Microbiol. 2007:466–474. [Google Scholar]
- 25.Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Krabshuis J, Thomson A, Lemair T. Probiotics and prebiotics. Probiotics prebiotics-World Gastroenterol Organ Glob Guidel. 2011:1–28. [Google Scholar]
- 26.McFarland LV. From yaks to yogurt: The history, development, and current use of probiotics. Clin Infect Dis. 2015;60:S85–S90. doi: 10.1093/cid/civ054. [DOI] [PubMed] [Google Scholar]
- 27.Fujii A, Bush JH, Shores KE, Johnson RG, Garascia RJ, Cook ES. Probiotics: Antistaphylococcal Activity of 4- Aminocyclohexanecarboxylic Acid, Aminobenzoic Acid, and Their Derivatives and Structure?Activity Relationships. J Pharm Sci. 1977;66:844–848. doi: 10.1002/jps.2600660628. [DOI] [PubMed] [Google Scholar]
- 28.Salminen S, Ouwehand A, Benno Y, Lee YK. Probiotics: How should they be defined? Trends Food Sci Technol. 1999;10:107–110. [Google Scholar]
- 29.Huis in’t Veld JHJ, Havenaar R. Probiotics and Health in Man and Animal. J Chem Technol Biotechnol. 1991;51:562–567. [Google Scholar]
- 30.FAO, WHO. Guidelines for the evaluation of probiotics in food. 2002:1–11. [Google Scholar]
- 31.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:9. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 32.Ozen M, Dinleyici EC. The history of probiotics: the untold story. Benef Microbes. 2015;6:159–165. doi: 10.3920/BM2014.0103. [DOI] [PubMed] [Google Scholar]
- 33.Calatayud GA, Suárez JE. A new contribution to the history of probiotics. Benef Microbes. 2017;8:323–325. doi: 10.3920/BM2017.x002. [DOI] [PubMed] [Google Scholar]
- 34.Masood MI, Qadir MI, Shirazi JH, Khan IU. Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol. 2011;37:91–98. doi: 10.3109/1040841X.2010.536522. [DOI] [PubMed] [Google Scholar]
- 35.Brüssow H. Microbiota and healthy ageing: observational and nutritional intervention studies. Microb Biotechnol. 2013;6:326–34. doi: 10.1111/1751-7915.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lino PA, Martins MAP, Silva ME de S e, de Abreu MHNG. Anxiolytics, Sedatives, and Hypnotics Prescribed by Dentists in Brazil in 2010. Biomed Res Int. 2017;2017:1–5. doi: 10.1155/2017/2841549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metchnikoff E. The prolongation of life; optimistic studies. G.P. Putnam’s Sons; New York: 1908. [Google Scholar]
- 38.Soccol Carlos Ricardo, Vandenberghe Luciana Porto de Souza, Spier Michele Rigon, Medeiros Adriane Bianchi Pedroni, Yamaguishi Caroline Tiemi, Lindner Juliano De Dea, Pandey Ashok, Thomaz-Soccol V. The Potential of Probiotics: A Review. Food Technol Biotechnol. 2010;48:413–434. [Google Scholar]
- 39.Wiley J. Bergey’s Manual of Systematics of Archaea and bacteria. Bergey’s Man. Trust 2001 [Google Scholar]
- 40.Babel W, Endo I, Enfors S-O, Fiechter A, Hoare M, Hu W-S, Mattiasson B, Nielsen J, Schugerl K, Stephanopoulos G, Von Stockar U, Tsao GT, Ulber R, Wandrey C, Zhong J-J, De Vrese M, Schrezenmeir J. Probiotics, Prebiotics, and Synbiotics. Adv Biochem Engin/Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 41.Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002;82:279–289. [PubMed] [Google Scholar]
- 42.Carr FJ, Chill D, Maida N. The lactic acid bacteria: a literature survey. Crit Rev Microbiol. 2002;28:281–370. doi: 10.1080/1040-840291046759. [DOI] [PubMed] [Google Scholar]
- 43.Fontana L, Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr. 2013;109(Suppl):S35–50. doi: 10.1017/S0007114512004011. [DOI] [PubMed] [Google Scholar]
- 44.Yerlikaya O. Starter cultures used in probiotic dairy product preparation and popular probiotic dairy drinks. Food Sci Technol. 2014;34:221–229. [Google Scholar]
- 45.Gorbach SL. Probiotics and gastrointestinal health. Am J Gastroenterol. 2000;95:2–4. doi: 10.1016/s0002-9270(99)00806-0. [DOI] [PubMed] [Google Scholar]
- 46.Tuohy KM, Probert HM, Smejkal CW, Gibson GR. Using probiotics and prebiotics to improve gut health. Drug Discov Today. 2003;8:692–700. doi: 10.1016/s1359-6446(03)02746-6. [DOI] [PubMed] [Google Scholar]
- 47.Williams NT. Probiotics. Am. J. Heal. Pharm. 2010;67 doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 48.Franz CMAP, Huch M, Abriouel H, Holzapfel W, Gálvez A. Enterococci as probiotics and their implications in food safety. Int J Food Microbiol. 2011;151:125–140. doi: 10.1016/j.ijfoodmicro.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18:2. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues FC, Castro ASB, Rodrigues VC, Fernandes SA, Fontes EAF, de Oliveira TT, Martino HSD, de Luces Fortes Ferreira CL. Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food. 2012;15:664–70. doi: 10.1089/jmf.2011.0296. [DOI] [PubMed] [Google Scholar]
- 52.Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri Treatment Prevents Bone Loss in a Menopausal Ovariectomized Mouse Model. J Cell Physiol. 2014;229:1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–37. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hock JM, Gera I, Fonseca J, Raisz LG. Human Parathyroid Hormone-(l–34) Increases Bone Mass in Ovariectomized and Orchidectomized Rats. Endocrinology. 1988;122:2899–2904. doi: 10.1210/endo-122-6-2899. [DOI] [PubMed] [Google Scholar]
- 55.Yamada C. Role of incretins in the regulation of bone metabolism. Nihon Rinsho. 2011;69:842–7. [PubMed] [Google Scholar]
- 56.Ohlsson C, SjÖgren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015;26:69–74. doi: 10.1016/j.tem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei ANM, Abdul-Majeed S, Ahmad Z, Ibrahim Z, Jamaluddin R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. Biomed Res Int. 2015;2015:1–10. doi: 10.1155/2015/897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sommer F, Bäckhed F. The gut microbiota — masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 59.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan Y-M, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016 Feb 19;351(6275) doi: 10.1126/science.aad3311. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016 Feb 19;351(6275):854–7. doi: 10.1126/science.aad8588. 2016. [DOI] [PubMed] [Google Scholar]
- 61.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113:E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. Lactobacillus plantarum Promotes Drosophila Systemic Growth by Modulating Hormonal Signals through TOR-Dependent Nutrient Sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Hyun S. Body size regulation and insulin-like growth factor signaling. Cell Mol Life Sci. 2013;70:2351–2365. doi: 10.1007/s00018-013-1313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steenhout PG, Rochat F, Hager C. The Effect of Bifidobacterium lactis on the Growth of Infants: A Pooled Analysis of Randomized Controlled Studies. Ann Nutr Metab. 2009;55:334–340. doi: 10.1159/000248992. [DOI] [PubMed] [Google Scholar]
- 65.Lei M, Hua L-M, Wang D-W. The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomised clinical trial. Benef Microbes. 2016;7:631–637. doi: 10.3920/BM2016.0067. [DOI] [PubMed] [Google Scholar]
- 66.Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J Am Coll Nutr. 2017:1–10. doi: 10.1080/07315724.2017.1318724. [DOI] [PubMed] [Google Scholar]
- 67.Tu M-Y, Chen H-L, Tung Y-T, Kao C-C, Hu F-C, Chen C-M. Short-Term Effects of Kefir-Fermented Milk Consumption on Bone Mineral Density and Bone Metabolism in a Randomized Clinical Trial of Osteoporotic Patients. PLoS One. 2015;10:e0144231. doi: 10.1371/journal.pone.0144231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han B, Sivaramakrishnan P, Lin C-C, Neve L, He J, Tay L, wei R, Sowa J, Sizovs A, Du G, Wang J, Herman C, Wang M. Microbial Genetic Composition Tunes Hosts Longevity. Cell. 2017;169:1249–1262. doi: 10.1016/j.cell.2017.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–8. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri Treatment Prevents Bone Loss in a Menopausal Ovariectomized Mouse Model. J Cell Physiol. 2014;229:1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li JY, Chassaing B, Tyagi AMA, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126:1–15. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229:1822–30. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Narva M, Rissanen J, Halleen J, Vapaatalo H, Väänänen K, Korpela R. Effects of Bioactive Peptide, Valyl-Prolyl-Proline (VPP), and Lactobacillus helveticus Fermented Milk Containing VPP on Bone Loss in Ovariectomized Rats. Ann Nutr Metab. 2007;51:65–74. doi: 10.1159/000100823. [DOI] [PubMed] [Google Scholar]
- 74.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9:699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, Börjesson AE, Ohlsson C. Sex steroid actions in male bone. Endocr Rev. 2014;35:906–60. doi: 10.1210/er.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nieves JW, Formica C, Ruffing J, Zion M, Garrett P, Lindsay R, Cosman F. Males Have Larger Skeletal Size and Bone Mass Than Females, Despite Comparable Body Size. J Bone Miner Res. 2004;20:529–535. doi: 10.1359/JBMR.041005. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Sakata T, Elalieh HZ, Munson SJ, Burghardt A, Majumdar S, Halloran BP, Bikle DD. Gender differences in the response of CD-1 mouse bone to parathyroid hormone: potential role of IGF-I. J Endocrinol. 2006;189:279–87. doi: 10.1677/joe.1.06351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am. J. Physiol. - Gastrointest. Liver Physiol. 2009;296(5):G1020–9. doi: 10.1152/ajpgi.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11:4745–67. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, Goldin B, Hartigan L, Kugathasan S, Levy J, Murray KF, Oliva-Hemker M, Rosh JR, Tolia V, Zholudev A, Vanderhoof JA, Hibberd PL. A Randomized, Double-blind Trial of Lactobacillus GG Versus Placebo in Addition to Standard Maintenance Therapy for Children with Crohn’s Disease. Inflamm Bowel Dis. 2005;11(9):833–9. doi: 10.1097/01.mib.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 81.Hibberd PL, Kleimola L, Fiorino A-M, Botelho C, Haverkamp M, Andreyeva I, Poutsiaka D, Fraser C, Solano-Aguilar G, Snydman DR. No evidence of harms of probiotic Lactobacillus rhamnosus GG ATCC 53103 in healthy elderly-a phase I open label study to assess safety, tolerability and cytokine responses. PLoS One. 2014;9:e113456. doi: 10.1371/journal.pone.0113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li M, Liang P, Li Z, Wang Y, Zhang G, Gao H, Wen S, Tang L. Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front Microbiol. 2015;6:692. doi: 10.3389/fmicb.2015.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA) – A novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses. 2012;79:104–112. doi: 10.1016/j.mehy.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 84.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 85.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes. 2007;56(7):1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 86.Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. Colitis induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis. 2013;19(8):1586–97. doi: 10.1097/MIB.0b013e318289e17b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guss JD, Horsfield MW, Fontenele FF, Sandoval TN, Luna M, Apoorva F, Lima SF, Bicalho RC, Singh A, Ley RE, van der Meulen MC, Goldring SR, Hernandez CJ. Alterations to the Gut Microbiome Impair Bone Strength and Tissue Material Properties. J Bone Miner Res. 2017;32(6):1343–1353. doi: 10.1002/jbmr.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaves de Souza JA, Frasnelli SCT, Curylofo-Zotti F de A, Ávila-Campos MJ, Spolidório LC, Zamboni DS, Graves DT, Rossa C. NOD1 in the modulation of host-microbe interactions and inflammatory bone resorption in the periodontal disease model. Immunology. 2016;149:374–385. doi: 10.1111/imm.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenfeldt V, Benfeldt E, Valerius NH, Pærregaard A, Michaelsen KF. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr. 2004;145:612–616. doi: 10.1016/j.jpeds.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 90.Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, Giakoumatou A, Petrohilou V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev. 2007;83:575–579. doi: 10.1016/j.earlhumdev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–91. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 92.Zareie M, Johnson-Henry K, Jury J, Yang P-C, Ngan B-Y, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–60. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bron PA, Kleerebezem M, Brummer R-J, Cani PD, Mercenier A, MacDonald TT, Garcia-Ródenas CL, Wells JM. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117:93–107. doi: 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKC-zeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 95.Anderson RC, Cookson AL, McNabb WC, Kelly WJ, Roy NC. Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol Lett. 2010;309(2):184–92. doi: 10.1111/j.1574-6968.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- 96.Resta–Lenert S, Barrett KE. Probiotics and Commensals Reverse TNF-α– and IFN-γ–Induced Dysfunction in Human Intestinal Epithelial Cells. Gastroenterology. 2006;130:731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 97.Qin H, Zhang Z, Hang X, Jiang Y. L. plantarum prevents Enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009;9:63. doi: 10.1186/1471-2180-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moorthy G, Murali MR, Devaraj SN. Lactobacilli facilitate maintenance of intestinal membrane integrity during Shigella dysenteriae 1 infection in rats. Nutrition. 2009;25:350–358. doi: 10.1016/j.nut.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Messora MR, Oliveira LFF, Foureaux RC, Taba M, Zangerônimo MG, Furlaneto FaC, Pereira LJ. Probiotic therapy reduces periodontal tissue destruction and improves the intestinal morphology in rats with ligature-induced periodontitis. J Periodontol. 2013;84:1818–26. doi: 10.1902/jop.2013.120644. [DOI] [PubMed] [Google Scholar]
- 100.Garcia VG, Knoll LR, Longo M, Novaes VCN, Assem NZ, Ervolino E, de Toledo BEC, Theodoro LH. Effect of the probiotic Saccharomyces cerevisiae on ligature-induced periodontitis in rats. J Periodontal Res. 2016;51:26–37. doi: 10.1111/jre.12274. [DOI] [PubMed] [Google Scholar]
- 101.Armour KE, Van’T Hof RJ, Grabowski PS, Reid DM, Ralston SH. Evidence for a pathogenic role of nitric oxide in inflammation-induced osteoporosis. J Bone Miner Res. 1999;14:2137–42. doi: 10.1359/jbmr.1999.14.12.2137. [DOI] [PubMed] [Google Scholar]
- 102.Ott SJ, Plamondon S, Hart A, Begun A, Rehman A, Kamm MA, Schreiber S. Dynamics of the mucosa-associated flora in ulcerative colitis patients during remission and clinical relapse. J Clin Microbiol. 2008;46:3510–3. doi: 10.1128/JCM.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am. J. Physiol. - Gastrointest. Liver Physiol. 2009;296(5):G1020–9. doi: 10.1152/ajpgi.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Irwin R, Raehtz S, Parameswaran N, McCabe LR. Intestinal inflammation without weight loss decreases bone density and growth. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2016;311(6):R1149–R1157. doi: 10.1152/ajpregu.00051.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5:98–104. doi: 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- 106.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–14. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 107.Srutkova D, Schwarzer M, Hudcovic T, Zakostelska Z, Drab V, Spanova A, Rittich B, Kozakova H, Schabussova I. Bifidobacterium longum CCM 7952 Promotes Epithelial Barrier Function and Prevents Acute DSS-Induced Colitis in Strictly Strain-Specific Manner. PLoS One. 2015;10:e0134050. doi: 10.1371/journal.pone.0134050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–8. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 109.Mariman R, Kremer B, Koning F, Nagelkerken L. The probiotic mixture VSL#3 mediates both pro- and anti-inflammatory responses in bone marrow-derived dendritic cells from C57BL/6 and BALB/c mice. Br J Nutr. 2014;112:1088–1097. doi: 10.1017/S000711451400169X. [DOI] [PubMed] [Google Scholar]
- 110.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Livanos AE, Greiner TU, Vangay P, Pathmasiri W, Stewart D, McRitchie S, Li H, Chung J, Sohn J, Kim S, Gao Z, Barber C, Kim J, Ng S, Rogers AB, Sumner S, Zhang X-S, Cadwell K, Knights D, Alekseyenko A, Backhed F, Blaser MJ. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol. 2016;1:16140. doi: 10.1038/nmicrobiol.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Botolin S, Faugere M-C, Malluche H, Orth M, Meyer R, McCabe LR. Increased Bone Adiposity and Peroxisomal Proliferator-Activated Receptor-gamma2 Expression in Type I Diabetic Mice. Endocrinology. 2005;146:3622–3631. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Motyl KJ, Raetz M, Tekalur SA, Schwartz RC, McCabe LR. CCAAT/enhancer binding protein beta-deficiency enhances type 1 diabetic bone phenotype by increasing marrow adiposity and bone resorption. AJP Regul Integr Comp Physiol. 2011;300:R1250–R1260. doi: 10.1152/ajpregu.00764.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]