Abstract
Motor cortex is important for motor skill learning, particularly the dexterous skills necessary for our favorite sports and careers. We are especially interested in understanding how plasticity in motor cortex contributes to skill learning. Although human studies have been helpful in understanding the importance of motor cortex in learning skilled tasks, animal models are necessary for achieving a detailed understanding of the circuitry underlying these behaviors and the changes that occur during training. We review data from these models to try to identify sites of plasticity in motor cortex, focusing on rodents as a model system. Rodent neocortex contains well-differentiated motor and sensory regions, as well as neurons expressing similar genetic markers to many of the same circuit components in human cortex. Furthermore, rodents have circuit mapping tools for labeling, targeting, and manipulating these cell types as circuit nodes. Crucially, the projection from rodent primary somatosensory cortex to primary motor cortex is a well-studied corticocortical projection and a model of sensorimotor integration. We first summarize some of the descending pathways involved in making dexterous movements, including reaching. We then describe local and long-range circuitry in mouse motor cortex, summarizing structural and functional changes associated with motor skill acquisition. We then address which specific connections might be responsible for plasticity. For insight into the range of plasticity mechanisms employed by cortex, we review plasticity in sensory systems. The similarities and differences between motor cortex plasticity and critical periods of plasticity in sensory systems are discussed.
Keywords: cortical circuits, cortical inhibition, motor cortex, motor learning, synaptic plasticity
INTRODUCTION
Clinicians, neuroscientists, and the laity hope to understand how the brain learns and generates the amazing range of complex motor behaviors necessary for dance, music, martial arts, and surgery. This is of interest not only as a pursuit of pure knowledge, but also because of the absence of effective ways to address the fragility of our brains in cases of aging, injury, and neurodegeneration. The major brain areas involved in motor control and procedural skill learning, including primary motor cortex (M1), have been identified via electrical microstimulation, lesions, neurophysiological recordings, imaging, and more targeted manipulations. Each brain area (see “Descending Motor Command Pathways for Reaching”) consists of multiple intermingled cell types connected in specific ways. Neuroscientists generally agree that motor skill learning (also called procedural learning) occurs via changes in the underlying circuitry, such as changes in circuit connectivity, synaptic strength, and neuronal excitability, but many specifics have thusfar been elusive. In part, this is because the underlying circuitry is not fully known.
M1 contains a vast array of pyramidal neuron types, organized by cortical layer and projection target, as well as many types of local inhibitory interneurons. The responses of these cell types change during learning. We hypothesize that changes in circuit connectivity in M1 contribute to the changes in neuronal responses during procedural learning. Identifying changes in connectivity and excitability of these cell types has been difficult since these changes may involve only a subset of neurons specifically involved in the behavior. Thus, our review focuses on the local and long-range connectivity of M1, as well as how it might change during motor skill learning.
Although humans and primates display a much richer diversity of skilled movements, rodent models present an advantageous model for elucidating relevant circuit changes during motor learning. Like primates, rodents have an agranular M1 and the rodent cortex contains the same major laminae as primate neocortex. But unlike primates, specific mouse lines exist to label, excite, and manipulate genetically defined cell types, including the major classes of interneurons and pyramidal cells. Adjacent forelimb M1 has similar local circuit connectivity to whisker M1 (Hooks et al., 2011; Lefort et al., 2009; Weiler et al., 2008) and thus both are attractive research areas. Furthermore, rodent M1 has prominent long-range input from many of the same brain areas as primate, including motor thalamus and somatosensory cortex. These projections are useful for studying cell-type specific connectivity of these areas. Thus, mouse M1 will provide much of the background for this review.
DEFINITION OF MOTOR SKILL LEARNING
Motor skill learning, also known as procedural learning, is the improvement in speed, accuracy, or consistency of a movement with training. For some tasks, this is preceded by perceptual training (to distinguish between cues) and learning of sensorimotor association (to link the stimulus to the proper behavioral output) that involve different circuits (Makino et al., 2016). Sensorimotor aspects include the integration of incoming sensory information (extero- or intero-receptive) into movement execution to produce a consistent movement or to adapt to changing demands online. During motor skill learning, forward models assist in generating an estimate of the body’s state during movement as well, though at a faster timescale than integration of sensory information (Scott, 2016).
Learning is strongly associated with changes in the brain’s processing. Learning dexterous movements, such as reaching, coincides with plasticity in motor cortex (M1). Coarsely, this plasticity includes increases in M1 representation area for the trained muscles associated with the skill. At the systems level, motor skill learning may result in changes in the locus of action selection from cortical to subcortical circuits. At the local circuits level, representation of movements in specific cell types, including corticospinal-projecting pyramidal cells, are enhanced, and reweighting of synaptic connections occurs, including changes in excitatory corticocortical and thalamocortical inputs as well as plasticity in local interneurons. Though these general principles seem to hold across different types of motor skill learning, procedural memories form over multiple timescales and different types of skills may require input from different structures or plasticity at different foci in M1.
A RANGE OF SKILL LEARNING TASKS
Motor skills vary in the precision and accuracy required to complete the task, the complexity of the planned movement sequence, and the musculature involved in movement execution. Thus, these skills may engage distinct neural mechanisms for learning and control. Motor skills are linked with behaviors requiring motor coordination, manual dexterity, or balance. Increased difficulty leads to longer training to a specified performance criterion, and the fewer individual subjects will reach that criterion across a population. Expertise in motor skill performance can be defined as a task that is so difficult that only a select few in a population can execute it, even after extensive training.
Different types of motor skills have evolved in mammals and they can be categorized by the ways in which the nervous system implements them. Locomotion is an example of a phylogenetically primitive motor skill, but is unique as it is achieved through specialized spinal cord central pattern generators (MacKay-Lyons, 2002). Despite this property, in rats and mice acrobatic tasks requiring balance and coordination (e.g. the rotarod task) have successfully been used to study motor skill learning (Carrillo et al., 2013; Jones et al., 1999; Lee et al., 2013; Shiotsuki et al., 2010). Another primitive motor skill is reaching, having evolved in mammals from behaviors such as scooping and wiping in frogs and mammals (Iwaniuk and Whishaw, 2000). Reaching is deceptively simple, involving both proximal and distal musculature, as well as grasping behavior (Whishaw and Pellis, 1990). With the evolution of increasing dexterity in the primate hand, came unique topographic organization of M1 (Rathelot and Strick, 2009), and the ability to perform skilled, complex motor sequences with the fingers, such as finger tapping (Yu and Tomonaga, 2015). This ability seems to be unique to primates; however, rodents can produce limited individuated digit movement (Alaverdashvili and Whishaw, 2008). The ability to learn ethologically relevant skilled motor sequences for reward has been demonstrated in pigeons (Helduser and Gunturkun, 2012) and rodents (Robbins, 2002). Working dogs and pets are also capable of learning to perform a series of tricks in order to earn a range of rewards.
In studying motor skill learning, Doyon and Ungerleider emphasize the distinction between sequential movements and motor adaptation (Doyon, 2002). Sequential movements require learning short sequence elements and chaining them together into an action sequence (Diedrichsen and Kornysheva, 2015) while motor adaptation requires the use of sensory and proprioceptive feedback to adapt to changing environmental conditions. Examples of motor skill tasks that have sequential movement components are finger tapping (Karni et al., 1995) and the serial reaction time task (Robertson 2007). Examples of motor adaptation tasks are prism adaptation, reaching in force fields, visuomotor adaptation, and grip force adaptation (Doyon, 2002). Reaching requires sequential coordination of distal and proximal muscles, including the muscles of the shoulder, arm, and hand, but its individual components can be made subject to motor adaptation (i.e. reaching in force fields). The synaptic loci of motor skill learning in M1 may differ depending on the requirements of sequential movements versus motor adaptation.
In a recent paper from the Olveczky laboratory, rats were trained to press a lever after an interval of 700 ms following an initial lever press, a skilled motor sequence task. Lesions of M1 after attaining asymptotic performance did not affect performance of this task. However, lesions made prior to learning prevented rats from learning the appropriate interval (Kawai et al., 2015). Interestingly, to learn the interval time, individual animals repeated different forelimb motor patterns in between the two lever presses, preventing comparison of stereotyped trajectories across animals. As the individual’s motor skill became more ‘procedural’ or ‘habitual’ with repeated across-session experience, the cortical contribution to execution may have diminished. Consistent with this, dopamine in M1 has been shown to be necessary for learning a skilled reaching task, but not performance of the task once learned (Hosp et al., 2011). Across-session training in the lever press task may have emphasized corticothalamic pathways, particularly those connecting M1 to dorsolateral striatum (Yin et al., 2009). The study by Kawai et al. establishes that M1 is required to learn but not execute motor sequences that do not involve dexterous movements; however, a different story emerges for the execution of dexterous tasks involving forelimb reaching.
An important property of motor skill learning is that it can be ‘cached’ into a stored stimulus-response sequence that follows the top-down command of M1. By this, it is meant that M1 can ‘release’ an action sequence (Redish, 2013) stored in the synaptic weights of motor pathways. In support of this view, Guo et al. found that optogenetically releasing M1 from inhibition often triggered a reaching event in well-trained mice even in the absence of sensory cues (Guo et al., 2015). Their results demonstrated that M1 activity is necessary and sufficient for executing a dexterous motor skill.
TIME COURSE OF LEARNING
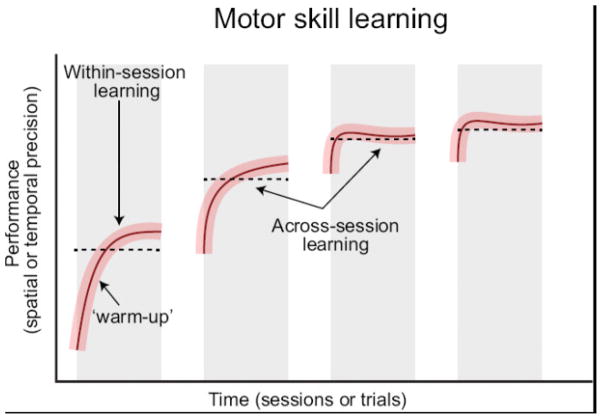
For complicated skills requiring multiple sessions of training to acquire, improvements can be observed both during a single session (within-session learning) as well as across sessions (across-session learning; Dayan and Cohen, 2011; Karni et al., 1998; Luft and Buitrago, 2005; Figure 1). Generally, smaller incremental improvements in performance are observed within-session while larger more stable improvements in performance are observed across-session. Depending on the difficulty of the task, within-session learning may only be observable during the first session (easy task), or across multiple early sessions (difficult task). Corresponding physiological changes presumably underlie these improvements, but these changes may occur at different connections within the circuit or in different brain structures.
Figure 1. Timecourse of motor skill learning.
During motor skill learning, the animal’s performance improves with an increase in spatial or temporal precision of the movement or success rate on the task. Improvement is not linear over all attempts, but shows certain variability in its timecourse. These variables include within-session learning (left), which may include a ‘warm-up’ effect. Within-session learning is expected to be the greatest during the initial training session (Karni et al., 1998). Across-session learning also occurs. This is believed to require rest or sleep to consolidate the gains of practice (Li et al., 2017; Yang et al., 2014). Within-session variation is possible, even after performance reaches a plateau due to factors such as attention and motivation.
For both the within- and across-session components, there is evidence that M1 plays a crucial role in learning. Recordings from mouse M1 during initial learning of a rotarod task show increased recruitment of task-related M1 neurons during the first session of training (Costa et al., 2004). During earlier sessions on a sequential finger tapping task, it was found that a larger extent of human M1 was activated by the first of two sequences in a trial, but that during later sessions BOLD activity in M1 was higher for the trained sequence regardless of whether it occurred first or second in the set (Karni et al., 1995). This work suggests that during earlier sessions a ‘habituation-like’ mechanism dominated M1 activation, resulting in reduction of activity from a second (similar) finger tapping sequence, but that this effect was replaced during later sessions as more M1 neurons were recruited into a network specific to the trained motor sequence. These results suggest that there are two phases of learning, a within-session component that is sequence-dependent and an across-session component that is sequence-independent. In the human finger tapping task, the disappearance of sequence-dependent activation occurred over a similar timescale to activity changes in areas projecting to M1, suggesting that the switch from sequence-dependent to -independent reflected a change in inputs to M1. Long-term potentiation (LTP) in M1 has been linked to the within-session component of motor skill learning. Within-session improvements in M1 TMS-evoked training-dependent movements were blocked by administration of an NMDA receptor blocker or GABAA receptor-activating drug (Butefisch et al., 2000), suggesting that within-session improvements in performance were due to LTP in M1. Drugs were administered systemically, and the effects in M1 could be a result of primary changes outside of M1, though the authors suggest that the similarities in training kinematics and baseline TMS-evoked potentials across conditions argue against the possibility of nonspecific global changes in cortical or subcortical excitability. Furthermore, reduction in local inhibition in M1 using the GABA antagonist bicuculine in anesthetized rats resulted in modification of movement evoked from intracortical electrical stimulation on a short timescale (Jacobs and Donoghue, 1991), similar to the reorganization seen after motor skill learning (see “Circuit Loci for Learning in Motor Cortex”). The expansion of motor maps as a result of motor skill learning persists in M1 for several days following skill acquisition, but is reversible (Nudo et al., 1996) and transient (Molina-Luna et al., 2008), suggesting that it is a labile process. Within-session learning effects may likewise be labile. For example, human subjects’ reaching performance in a resistive force field was impaired after training in a second force field, if training in the second condition occurred within 5 hours (Shadmehr and Brashers-Krug, 1997).
The within-session component of learning is limited by factors including attention, fatigue, and motivation. One difficulty in interpretation of within-session learning is ‘warm-up’ effects. These likely have physiological and attentional causes. An abrupt increase in activity from rest can perhaps cause physiological changes in response properties of stretch receptors in tendons or muscles that alter primarily proprioceptive feedback from Golgi organs or muscle spindles (Hoffman, 2002). The attentional component of motor skill learning is particularly important in tasks requiring coordination or timing, such as sequential finger tapping tasks or fixed-interval lever press tasks, but increased attention may not be beneficial for performance of other types of skilled learning tasks (Wulf, 2007). Attentional improvement versus detriment may parallel the classification of sequential movements versus motor adaptation. Motivation may likewise influence the time course of learning (Haibach, 2011; Mosberger et al., 2016).
In contrast to within-session learning, the across-session component of motor skill learning is hypothesized to be dependent on practice (physical repetition of trials or mental practice), consolidation during sleep, and physiological limitations on peak performance. Mental practice is thought to improve motor skill performance, depending on the task (Driskell, 1994). Sleep has been shown to lead to large improvements in a sequential finger-tapping task in a manner independent of the amount of practice-dependent learning (Walker et al., 2003). An equivalent amount of non-practice time without sleep did not lead to the improvement in performance that was observed following sleep (Walker et al., 2002). Over many repetitions of a motor task, subcortical habitual decision-making mechanisms in striatum may be engaged, which allow faster, more stereotyped movements (van der Meer et al., 2012). Indeed, a literature beyond the scope of this review exists that studies the differences in striatal processing as a function of training time (Lehericy et al., 2005; Miyachi et al., 2002; Yin et al., 2009).
Since within-session and across-session components of motor skill learning seem to occur on distinct timescales, it is likely that the underlying neural mechanisms and, possibly, the loci of plasticity are different. Many studies in mice are designed to better capture these longer timescale processes, as invasive assessments of changes in rodent circuitry generally occur at the end of training paradigms. The question of how stable representations are during performance and training has long been of interest (Chestek et al., 2007; Li et al., 2001; Padoa-Schioppa et al., 2004; Schmidt et al., 1976). More recently, chronic tracking of structural (Xu et al., 2009; Yang et al., 2009) and physiological changes (Chen et al., 2015; Huber et al., 2012; Peters et al., 2014) permit examination of these changes at multiple phases of learning.
DESCENDING MOTOR COMMAND PATHWAYS FOR REACHING
Contralateral forelimb M1 lesions impair forelimb dexterity in rodents, specifically forelimb reaching. This includes, in the rodent, an inability to pronate the paw over the food and an inability to supinate the paw to place food into the mouth. Perhaps surprisingly, in the Whishaw lesion studies grasping is spared (Whishaw, 2000; Whishaw et al., 1991). This contradicts the finding that inactivation of the rodent rostral forelimb area with a cooling probe (Brown and Teskey, 2014) produces specific deficits in grasping, independent of reaching deficits observed during inactivation of the caudal forelimb area. In the Whishaw (2000) study, increasing lesion size produces larger performance deficits, though all lesions targeted both rostral and caudal forelimb areas. In monkeys, an M1 lesion disrupts grasping in a more pronounced way than in rodent, though the deficit is dependent on the type of grasping (e.g. precision grasping versus power grasping; Savidan et al., 2017). Depending on the extent of cortical damage, partial recovery of reaching success can be achieved by use of compensatory movements of the shoulder and trunk (Cirstea and Levin, 2000; Whishaw, 2000). Use of mirror movements of the opposite hand can also facilitate recovery (Cauraugh and Summers, 2005; Whishaw, 2000). These compensatory movements may be more effective for shorter-distance reaches, as specific deficits in longer-distance reaches were observed following M1 lesions (Montoya et al., 1991).
Recent work suggests that the reticulospinal tract (originating in the brainstem pontine reticular formation) mediates this functional recovery after corticospinal tract damage (Baker, 2011), but see (Alstermark and Pettersson, 2014) for evidence that reticulospinal pathways are necessary and sufficient for successful reaching behavior in rodents. Alternately, side switching of ipsilateral corticospinal tract fibers by blocking Nogo-A in the cervical spinal cord followed by training on a forelimb reaching task results in nearly complete functional recovery (Wahl et al., 2014). Unilateral lesion of the corticospinal tract at the level of the medullary pyramid (pyramidal tract) in rats causes a permanent (~20–40%) decrease in skilled reaching performance (Whishaw et al., 1993). However, this lesion may have also disrupted cortical input to reticulospinal neurons (e.g. the pontine reticular formation). Similarly, in monkeys, lesion of the pyramidal tract (Figure 2) cause muscle weakness and permanent impairments to fine motor movements, including grasping and reaching (Lawrence and Kuypers, 1968; Lemon et al., 2012).
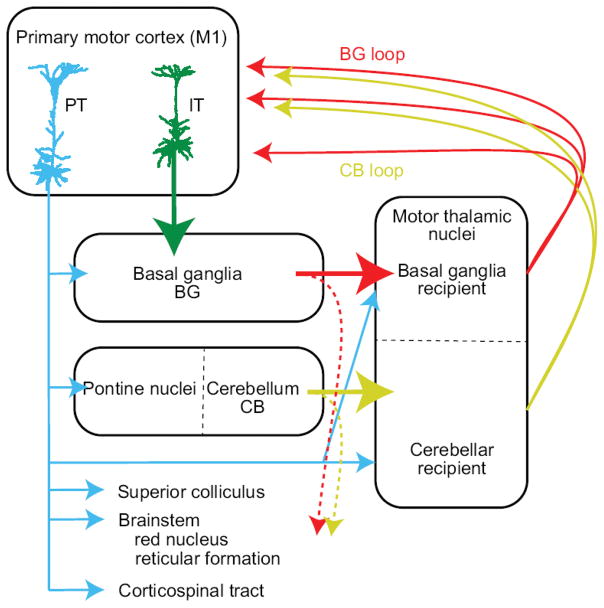
Figure 2. Major central nervous system regions involved in control of the motor system.
The cortical region most directly involved in the initiation and control of movement is the primary motor cortex (M1). Its subcortical descending pathways, originating in L5 pyramidal tract-type (PT-type, blue) neurons, contribute axons to the corticospinal tract which terminate on interneurons in the brainstem and spinal cord (and, in primates, may directly contact motoneurons that target the distal musculature controlling fingers). These axons also target subcortical motor nuclei, such as the red nucleus and brainstem reticular formation, which serve as the origin of the descending rubrospinal and reticulospinal tracts. Axonal collaterals target structures including the pontine nuclei, the superior colliculus, the basal ganglia, and the thalamus. Alternatively, intratelencephalic neurons (IT-type, green) in M1 target other cortical areas and ipsi- and contralateral striatum. Basal ganglia (red) and cerebellum (gold) form two major loops which via their output to motor thalamus can influence M1 and thus motor function. Arrows indicate the proposed laminae in M1 targeted by these areas (L2/3, L5A, and L5B, respectively).
The interplay between direct innervation of motoneurons and indirect motor commands via spinal interneurons may differentially mediate reaching behavior in rodent versus primate. In primates, propriospinal neurons receive convergent input from the four major descending motor pathways: the cortico-, rubro-, reticulo-, and tectospinal tracts (Alstermark and Isa, 2012). There is also direct cortical innervation of motoneurons (Landgren et al., 1962). In rodents, there is some evidence for direct cortical innervation of motoneurons (Grinevich et al., 2005; Hori et al., 2002; Liang et al., 1991), but these results are controversial (Alstermark et al., 2004; Yang and Lemon, 2003). A recent study suggests that direct cortical innervation of motoneurons exists in juvenile mice but that these connections are pruned during development. These connections persist into adulthood in a genetically modified strain of mouse that lacks PlexA1 signaling (Gu et al., 2017). PlexA1 mice were found to have superior manual dexterity than wild type controls. However, another study that severed corticospinal pathways at the level of the C1–C2 spinal segment suggests that reaching in rodents is not even dependent on the corticospinal tract at all, and may be wholly dependent on reticulospinal pathways (Alstermark and Pettersson, 2014). From this standpoint, disruption of M1 would affect reaching only indirectly, through modulation of reticulospinal brainstem pathways. Clearly further research is necessary to clarify the role of the rodent corticospinal pathways in reaching behavior.
In rodents, different cortical and subcortical circuits mediate the reach phase versus the grasp phase of skilled reaching. During the reach phase of forelimb reaching, limb position is regulated by feedback signals through cerebellar circuits. A subset of excitatory propriospinal interneurons in the C2-Th1 level of the spinal cord (V2a interneurons) innervate both motor neurons of the forelimb and, through an ascending pathway, the precerebellar lateral reticular nucleus. The Jessell lab (Azim et al., 2014) showed that ablation of these V2a interneurons impaired reaching but preserved digit extension and horizontal ladder walking movements in mice, revealing that skilled forelimb reaching includes an ascending cerebellar relay. In contrast, the grasp phase of forelimb reaching is mediated by Ia3 glutamatergic interneurons of the spinal cord, which receive cutaneous afferent input from the forelimb. Genetic removal of glutamate transmission from Ia3 interneurons results in deficits in grip strength in adults and the forepaw grasp reflex in neonatal animals (Bui et al., 2013). Descending motor commands from the medullary reticular formation ventral part (MvdM) have also been shown to be necessary for the grasp phase of skilled reaching. MvdM, which connects to specific forelimb motor neuron pools as well as cervical glutamatergic spinal interneurons (likely including Ia3 interneurons), sends descending excitatory input from L5 M1 cells, superior colliculus, red nucleus, and deep cerebellar nuclei. Silencing of glutamatergic output from MvdM results in decreased performance on accelerating rotarod and in the grasping phase of a single-pellet reaching task (Esposito et al., 2014). The differentiation between reach and grasp phases is preserved at different stages of the motor hierarchy, potentially including cortex. A dissociation of reaching and grasping was observed following inactivation of the rostral forelimb area (causing grasping impairment) versus the caudal forelimb area (causing reaching impairment) using a cooling probe (Brown and Teskey, 2014), further suggesting that different circuitry is required for reaching and grasping.
ORGANIZATION OF EXCITATORY CONNECTIVITY IN MOTOR CORTEX
Local circuits
Differences in local circuit organization of primary motor and sensory cortices are of interest in understanding the degree to which different cortical areas are specialized for different tasks. Motor cortex is cytoarchitecturally distinct from adjacent posterior S1 due to the absence of a granular L4 (Shipp, 2005). This is in contrast to sensory areas, where L4 is the principal thalamorecipient layer (Sherman and Guillery, 2006). The major intracortical connections of sensory areas, including somatosensory (Lefort et al., 2009) and visual cortices (Binzegger et al., 2004; Douglas and Martin, 2004), are characterized by a powerful translaminar projection from L4 to L2/3. A modified version of the ascending L4 to L2/3 projection exists from RORβ+ neurons (a marker for L4 cells), which are present, though not tightly packed in a granular layer, in lateral motor cortical regions (Yamawaki et al., 2014). The local circuitry of M1 (Figure 3) is thus modified compared to S1 and other sensory areas, with the major translaminar projection originating in a relatively thin L2/3 (Brodmann, 1909) and projecting to L5 (Weiler et al., 2008). This recapitulates the L2/3 to L5 projection found across cortical areas (Hooks et al., 2011; Figure 3B). Local circuitry, however, is quite layer- and cell-type specific. The descending L2/3 projection targets lower L5A corticostriatal neurons and upper L5B corticospinal neurons (Anderson et al., 2010), but not the lower L5B neurons. These two classes of cells, also called intratelencephalic (IT-type) and pyramidal tract-type (PT-type; Shepherd, 2013) respectively, are hierarchically organized to funnel M1 signals to output neurons (PT-type cells). The distinct output pathways of IT- and PT-type neurons are shown in Figure 2. IT-type cells can be subdivided by layer, as neurons with IT-type projections are found in L2/3, L5A, L5B, and L6 (Figure 3C). IT-type neurons in L5A and L5B form connections with other IT-type neurons, and project to PT-type cells, but PT-type neurons do not connect to L5 IT-type cells (Kiritani et al., 2012). Collectively, this suggests that M1 is organized such that some local processing can occur in upper layers, before being fed forward to corticofugal output neurons. A similar local circuit funnels the output of local IT-type cells to L6 corticothalamic (CT-type) neurons.
Figure 3. Major local and long-range excitatory connections in M1.
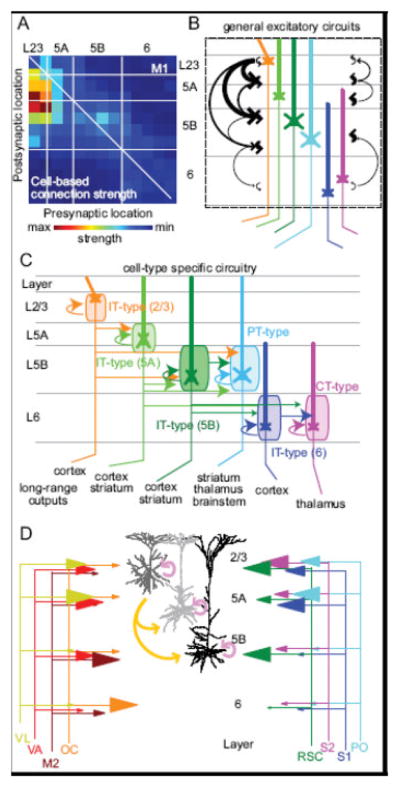
(A) Local excitatory connections between pyramidal neurons are illustrated in a connectivity matrix based on laser scanning photostimulation with glutamate uncaging (Hooks et al., 2011; Weiler et al., 2008). Presynaptic neurons are represented on the x-axis and postsynaptic pyramidal neurons are represented on the y-axis, with white lines marking rough laminar boundaries. The intense hotspot represents descending projections from L2/3 to L5A and L5B. (B) The excitatory-excitatory connectivity map is drawn as a cartoon with intralaminar and translaminar connection strength illustrated as arrow thickness. Pyramidal neurons are color-coded by projection type, matching panel (C). (C) Local circuits include substantial cell-type specificity. Pyramidal neuron cell-type is illustrated with different colors for different neurons within different layers. Cell-types send long-rang projections to specific targets (bottom). The major local projections are indicated as arrows. Orange arrows from L2/3 neurons indicate intralaminar connectivity as well as outputs to L5A and L5B neurons. IT-type neurons are subdivided into groups by layer (2/3, 5A, 5B, and 6). PT-type neurons and CT-type neurons generally receive input from other cell-types but do not send strong output back to these neurons in the local circuit. Specific connectivity of distinct neuron types is reviewed in (Shepherd, 2013). (D) L2/3, L5A, and L5B (shades of gray) illustrated in pink (intralaminar connections) and gold (translaminar connections). L6 neurons not illustrated. Long-range input from a number of thalamic and cortical projections is summarized. Sensory projections from parietal areas, including S1 (Mao et al., 2011), S2 (Suter and Shepherd, 2015), and retrosplenial cortex (RSC; Yamawaki et al., 2016), as well as sensory thalamus (Hooks et al., 2013) are shown in cool colors at right. Frontal cortex projections from secondary motor cortex (M2) and orbital cortex (OC) as well as motor thalamus (VA and VL; Hooks et al., 2013) are shown in hot colors at left. Size of arrowheads is proportional to amplitude of excitatory inputs to pyramidal neurons.
Long range inputs
During motor skill learning, procedural memories are thought to be encoded in changes to the synaptic weights of M1 inputs as well as its local circuits. Although long-range inputs to M1 have been studied for many decades (Strick, 1970), the advent of ChannelRhodopsin-2 (ChR2; Boyden et al., 2005) for circuit mapping has made the quantification of functional input strength for specific classes of input quite accessible. ChR2 can be introduced into neurons by viral vectors, in utero electroporation, or transgenic expression. Specificity can be achieved by either stereotaxic injections or Cre-dependent mechanisms combined with Cre-driver lines (Atasoy et al., 2008). Following sufficient opsin expression and axonal transport, long-range projections are excitable even in in vitro slice preparations (Petreanu et al., 2007) and can be quantified to assess synaptic connectivity. This is especially useful in cortical areas, as these areas contain a variety of cell types in different layers and a wide range of cortical and thalamic inputs. Recordings from individual neurons are helpful in elucidating the specific connectivity since, for many cell types, the strength of connectivity is highly specific and not simply proportional to the overlap of axons and dendrites (Shepherd et al., 2005). Defining baseline strength is somewhat difficult for cell types whose inputs show a great deal of paired-pulse facilitation and depression (Beierlein et al., 2003). Thus it is useful to have some idea of the short-term plasticity of the connections as well.
With the exception of brainstem neuromodulatory systems, virtually all long-range input to M1 originates in thalamus or other cortical areas, and is exclusively glutamatergic. A number of retrograde tracing studies have helped to identify the major inputs to M1. In rodents, these thalamic inputs include motor thalamic nuclei such as VA-VL, posterior sensory nuclei including PO, and ventromedial (VM) thalamus (Deschenes et al., 1998; Herkenham, 1980; Kuramoto et al., 2009; Kuramoto et al., 2015; Ohno et al., 2012). These are of interest because they are the main conduit through which the output of subcortical motor learning systems such as basal ganglia and cerebellum will influence cortical computations (Alexander et al., 1986; Kuramoto et al., 2009; Middleton and Strick, 2000). These axons differentially target distinct layers of M1 (Herkenham, 1980; Hooks et al., 2013; Kuramoto et al., 2009; Yamawaki and Shepherd, 2015), with weights indicated for pyramidal neurons in Figure 3. Synaptic weights for VM input are not included. In general, sensory projections (PO) target upper layers (L2/3 and L5A), while motor thalamic output also targets deeper layers (L5B; Hooks et al., 2013). As will be detailed below, thalamocortical inputs have different plasticity rules than corticocortical inputs. Although only a subset of these inputs have been studied in learning paradigms, they, along with cortical inputs described below, are included in our figure of putative sites for synaptic plasticity in M1 during motor skill learning (Figure 3).
Corticocortical inputs to M1 include posterior-originating cortical inputs thought to convey somatosensory information, the most numerous originating in primary (Mao et al., 2011) and secondary (Suter and Shepherd, 2015) somatosensory cortex. Spatial information from dorsal hippocampus can reach M1 via retrosplenial and posterior parietal cortex (Yamawaki et al., 2016). This information may be useful for aiming during reaching by processing proprioceptive feedback (Desmurget et al., 1999). Visual information also reaches rodent M1, including Area A of the dorsal stream (Wang et al., 2012). As in primate, frontal cortex projects to primary motor areas in rodents, including dorsal regions as well as orbitofrontal ones (Reep et al., 1990; Rouiller et al., 1993). Of interest, these frontal areas specifically target different pyramidal neuron populations than sensory inputs, including cell types conveying descending output to the thalamus, brainstem, and spinal cord (Hooks et al., 2013). It is worth noting that these frontal areas (M2 and OC in Figure 3) are hierarchically similar to premotor areas in primate, though not quite equivalent to secondary somatosensory (S2) or visual (V2) areas in sensory processing. M1 integrates a wide range of cortical and thalamic inputs, which impinge on excitatory (and potentially inhibitory) cell types in each layer and offers many putative sites for plasticity during motor learning.
CIRCUIT LOCI FOR LEARNING IN MOTOR CORTEX
One major means by which we hypothesize motor learning is stored in motor circuitry is via long-lasting changes in the strength and connectivity of synapses (Kandel and Spencer, 1968). Circuit mapping helps provide putative sites of changes, but identifying the actual loci of these changes is difficult. The challenges include (1) identifying the specific connection to examine and (2) accurately identifying the pre- and postsynaptic cell types by genetic means, laminar position, projection pattern, or response pattern. An additional hurdle is that changes may be specific to subsets of neurons involved in the task, not general to all neurons of a given type, which makes identifying the neuron’s response type or task involvement important. Thus tools that allow the labeling and manipulation of specific subsets of neurons defined by their pattern of activity will be valuable (Barth et al., 2004; Fosque et al., 2015; Hayashi-Takagi et al., 2015; Ramirez et al., 2015; Sorensen et al., 2016; Wang et al., 2017). Furthermore, while following response patterns over time is possible with long-term imaging or chronic recording methods, tracking changes in connectivity as a function of learning is difficult, since whole cell recording in vivo is only possible for a short period of time. Insight into M1 plasticity at the systems level is possible without methods that address plasticity at the synaptic level, however. Large scale changes in M1 representations occur with substantial manipulations or injuries, resulting in cortical remapping to alter the representation of the affected body (Wittenberg, 2010). Below we discuss mechanisms of cortical remapping in M1 as a result of task-specific manipulations.
Following training on a skilled reaching task, the caudal forelimb area of rat M1 shows an increase in the representation of the digit and wrist areas in response to intracortical microstimulation (Kleim et al., 1998). Similar expansion in the monkey forelimb area (at the expense of digit representation) of M1 has been observed following training on a key-turning task, whereas increased digit expansion is observed (at the expense of forearm representation) following a small object retrieval task requiring manual dexterity (Nudo et al., 1996). Depending on the parameters of this intracortical microstimulation, simple or complex movements may be elicited (Bonazzi et al., 2013; Brown and Teskey, 2014; Kleim et al., 2002a). Blocking intracortical glutamate transmission prevents elicitation of complex movements from intracortical microstimulation without eliminating basic motor map topography (Harrison et al., 2012). It is not known whether intracortical microstimulation activates synaptic afferents to M1 neurons (Jankowska et al., 1975; Tehovnik, 1996; Tolias et al., 2005), directly activates M1 neurons (Histed et al., 2009), or, more likely, both at differing ratios depending on stimulus parameters such as stimulus intensity, frequency, and electrode placement. Future testing with optogenetic methods combined with biophysical modeling may be able to clarify this point. Importantly, the expansion in M1 representation is not simply a result of increased use, as lever-pressing (Kleim et al., 1998) and wheel running (Kleim et al., 2002b) or reaching for an unattainable pellet (Kleim et al., 2004) do not result in increased forelimb representation in M1.
Additionally, ablation of basal forebrain cholinergic inputs prevents map reorganization and impairs learning on a skilled reaching task (Conner et al., 2003). This data suggests that motor skill learning is accompanied by an expansion in the somatotopically-related M1 region in an acetylcholine-dependent manner, though it is still unclear how this functional organization might represent a motor engram for procedural learning, as has been suggested (Whishaw, 2000; Monfils et al., 2005). It may be that a greater percentage of the total computational power of M1 is brought to bear during learning a new task. The increased output from M1 L5 might, in turn, be necessary for driving plasticity in downstream structures or for initiating consolidation. The role of complex movement elicitation, while unclear, seems more consistent with M1 ‘releasing’ a stored action (Guo et al., 2015) when the cortex is released from inhibition.
It also remains to be seen whether expansion of motor cortical maps is a short term phenomena or whether it persists and is intimately tied to long term gains in performance. Our prediction is that previously established expansions will be ‘masked’ following retraining on a different task but ‘re-emerge’ quickly following renewed training on a previously learned task (Linkenhoker and Knudsen, 2002; Linkenhoker et al., 2005). It seems possible that M1 can handle multiple competing representations simultaneously depending on the current demands placed on the animal, and that it would be able to flip back and forth between learned states, expanding its representational space (and therefore its processing power) to flexibly adapt to changing demands.
Hyperpolarization-activated Cyclic Nucleotide–gated (HCN) channels (Ih current), which are highly expressed in L5 pyramidal cells, especially corticospinal neurons (Sheets et al., 2011), may be necessary to induce a dynamic cortical state where cortical representations become more labile during learning, allowing functional expansion of cortical motor maps. Infusion of an HCN blocker into the caudal forelimb area of rat M1 causes an increase in errors in a skilled reaching task, as well as eliciting more complex movements as a result of intracortical microstimulation (Boychuk et al., 2017). The errors were primarily in the reach advance and grasping phases, with errors in grasping potentially suggesting that the rostral forelimb area was also impacted by the HCN blocker. It is not known whether these errors were a result of an inability to learn the proper reaching sequence or if HCN blockers would have impaired performance of pre-trained animals as well, since infusion of saline and drug was done in a counterbalanced manner across days and not sequentially. We suggest that the HCN blockade interfered with learning a skilled reaching sequence by preventing ‘fine tuning’ of synaptic weights onto L5 M1 corticospinal neurons. Implicit in this suggestion is that increased cortical representations, in fact, hide more complex dynamics, revealed by an evaluation of spine plasticity.
The mechanisms underlying M1 remapping may include changes in dendritic or axonal arborization and connectivity. Large scale changes in dendritic arborization have been shown in L2/3 and L5 pyramidal neuron populations during motor learning (Greenough et al., 1985; Withers and Greenough, 1989), and these changes are specific to the pyramidal neurons involved in the task (Wang et al., 2011; Figure 4A). However, these are difficult to integrate into our understanding of circuit changes since the associated changes in connectivity are not clear. However, structural correlates of motor learning certainly do suggest changes in connectivity. Specifically, dendritic spines are among the likely structural substrate of changes in excitatory connectivity subserving long-term memories (Yang et al., 2009). The ability to image spines chronically during learning has led to the insight that new spine formation is associated with motor learning. New spines form and persist on pyramidal neurons involved in motor tasks across a range of skills, from reaching and grasping (Xu et al., 2009) to lever pressing (Peters et al., 2014) to locomotion on the rotarod (Hayashi-Takagi et al., 2015; Yang et al., 2009). Note that this contrasts with the motor map expansion data which does not show an increase in represented area during lever pressing (Kleim, et al, 1998). One explanation is that the degree of large scale reorganization varies with the demands of the task, and that changes in connectivity associated with spine dynamics are sufficient to support learning some skills, such as lever pressing, but not others, such as forelimb reaching. Spine plasticity also involves both L2/3 (Peters et al., 2014) and L5 pyramidal neurons (Xu et al., 2009; Yang et al., 2009). New spines associated with these tasks are clustered on the same dendritic branch (Fu et al., 2012), and acquisition of distinct skills leads to clustering on distinct dendritic branches (Yang et al., 2014; Figure 4B).
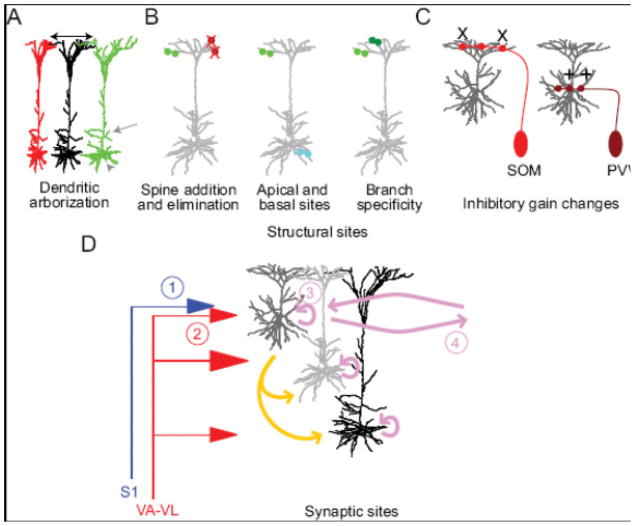
Figure 4. Structural and synaptic sites of plasticity in M1 during motor skill learning.
A range of sites for cortical plasticity have been examined. Structural sites include (A) changes in the size of the dendritic arbor (black arrows) of pyramidal neurons in the topographic region where plasticity occurs (Greenough et al., 1985; Withers and Greenough, 1989). Gray arrow and arrowhead in (A) indicate addition and elimination of dendritic surface possible during plasticity. (B) Changes in spine addition (green and cyan), elimination (red X), and stabilization (Xu et al., 2009; Yang et al., 2009) occur, including differences in apical and basal locations (Peters et al., 2014) and branch specificity or clustering (dark green versus light green; Fu et al., 2012; Yang et al., 2014). (C) Structural changes in interneuron axons, including addition (+) or loss (X) in an interneuron subtype-specific manner (Chen et al., 2015) are also reported. (D) We illustrate here the putative changes in local, horizontal, and long-range inputs as a result of motor skill learning. Long-range inputs that may change include (1) S1 inputs to L2/3 neurons and (2) VL inputs to L2/3 neurons (Baranyi and Feher, 1981; Iriki et al., 1989; Kaneko et al., 1994; Sakamoto et al., 1987), albeit with distinct learning rules for corticocortical and thalamocortical plasticity. Changes within motor cortex include L2/3 (3) local (Aroniadou and Keller, 1995) and (4) long-range horizontal connections (Hess et al., 1996; Hess and Donoghue, 1994; Rioult-Pedotti et al., 2000; Rioult-Pedotti et al., 1998). Other intralaminar (pink) and translaminar (gold) local connections are also putative sites of synaptic changes.
It is of interest then, to identify the presynaptic partners of these new contacts and from the range of inputs seen in Figure 3, and understand how they are potentiated. For example, both S1 and motor thalamus (VL) projections to M1 have inputs whose responses are plastic in response to patterned stimulation or motor learning (Baranyi and Feher, 1981; Sakamoto et al., 1987). The suggested learning rule is that S1 inputs to L2/3 pyramidal neurons undergo synapse-specific plasticity, while inputs from thalamus require paired stimulation with S1 inputs (Iriki et al., 1989; Kaneko et al., 1994). Interestingly, these changes in synaptic strength are restricted to a subset of neurons (Baranyi and Feher, 1978): the M1 neurons involved in the movement (Biane et al., 2016). Local excitatory circuit changes in vitro are also implicated in the neural substrate of motor skill learning (Aroniadou and Keller, 1995), such as changes in synaptic strength of L2/3 horizontal connections. Skilled reaching results in increased amplitude of evoked field excitatory post-synaptic potentials (fEPSPs) in pyramidal cells of the forelimb area of L2/3 rat M1 compared to untrained controls (Rioult-Pedotti et al., 1998). In a follow up study, evoked long term potentiation in trained rats was smaller, and evoked LTD was larger, indicating that the larger fEPSPs were due to LTP induction (Rioult-Pedotti et al., 2000). Furthermore, this plasticity is regulated by inhibitory transmission (Hess et al., 1996; Hess and Donoghue, 1994). This data suggests that strengthening of local connections within M1 is a result of motor skill learning.
In contrast to excitatory connectivity, synaptic changes in inhibitory circuitry during motor skill learning have received less attention (though note Hess et al., 1996; Hess and Donoghue, 1994). The local connectivity of interneurons in L5 of M1 is described (Apicella et al., 2012), though a comprehensive map across all layers is lacking. As inhibition is critical in regulating plasticity in sensory areas (see below), we hypothesize that inhibition plays a comparable role in regulating M1 plasticity. Interneurons in cortex do not have spines, but changes in their synaptic output can be monitored by imaging their axons and quantifying changes in axonal bouton density and persistence. For example, while learning a lever press task, axonal boutons of somatostatin-positive (SOM+) neurons in M1 were generally eliminated during the early sessions of task learning (Chen et al., 2015). These neurons are low-threshold spiking cells whose axons typically target distal dendrites of pyramidal neurons. In contrast, axonal boutons of fast-spiking parvalbumin-positive (PV+) neurons are generally added during the early phases of skill learning, only to later be eliminated. This suggests that plasticity in multiple interneuron classes might play a role in regulating M1 plasticity, though distal dendritic spines (in similar laminae to SOM+ axons) show greater dynamics during learning than proximal (perisomatic) ones (Chen et al., 2015). SOM dynamics may be part of the neural instantiation of motor skill learning in M1, while PV+ interneurons may be more involved in maintaining homeostatic plasticity (Chen et al., 2015), synchronizing inputs across structures (Bartos et al., 2007), or facilitating ongoing network consolidation (Ognjanovski et al., 2017).
PLASTICITY IN SENSORY CORTICES
How do the specific synaptic changes in M1 associated with motor skill learning compare to plasticity in other cortical areas as a result of learning? It is well established that plasticity in sensory cortex occurs during specific windows in early life called critical periods (Hensch, 2004). Is cortical plasticity in M1 also subject to a critical period during development, or is plasticity in M1 able to be turned on and off throughout the lifetime of the animal?
In either case, we can potentially learn a great deal about plasticity in M1 by studying the mechanisms of plasticity in sensory areas. The mechanisms of cortical plasticity have received great attention in sensory areas, especially primary visual and somatosensory areas. In part, this is due to the ease of developing extreme modulations of incoming sensory activity for these areas, including those designed to (1) block most of the incoming sensory information, as by visual deprivation or whisker trimming, as well as (2) those designed to establish an activity-dependent competition between sources of input, such as monocular deprivation or single whisker trimming (Hubel and Wiesel, 1970; Simons and Land, 1987). Although the early work in these systems was done in a range of mammals, such as cat, ferret, primate, and rodent, studies are now converging on mice, as the genetic tools for cell-type specific recording and manipulation proliferate in this model, and advantages remain in the accessibility for imaging, recording, and injection. The developmental critical periods are well-defined in primary visual (V1) and somatosensory (S1) cortices across a variety of species (Hensch, 2004). These have not been as well defined for motor skills, though there is a convincing relationship between expertise and the age at which skill learning begins (Ericsson, 1993). Indeed, it is interesting to speculate whether or not M1 has an analogous critical period. Our speculation is that the developmental timing of circuit plasticity in M1 may be of longer duration, perhaps throughout the lifespan of the animal. Furthermore, the degree of plasticity that can be evoked may differ across motor areas from sensory areas.
As in M1, the large numbers of cortical circuit elements and interconnections raises the possibility that changes at many synapses underlie the plasticity associated with sensory deprivation. Within V1 and S1, these include three major feedforward excitatory connections (the principal thalamocortical inputs to L4, the ascending L4->L3 connections, and the descending L2/3->L5 projection), three major within-layer connections (horizontal connections of L4, L2/3, and L5), and the ascending projection from L5 to L2/3 (Binzegger et al., 2004; Douglas and Martin, 2004; Hooks et al., 2011; Lefort et al., 2009). These connections are proposed as possible loci of changes in synaptic weights.
The major manipulation used to induce plasticity in V1, monocular deprivation (MD), establishes competition between the inputs of the deprived and non-deprived eye in regions of V1 where they overlap. MD then causes a shift in the degree to which a given V1 neuron responds to the deprived and non-deprived eye, generally with a gain of responsiveness to the non-deprived eye, while losing responsiveness to the deprived eye. This shift is called ocular dominance (OD) plasticity. OD plasticity is much greater during a developmental critical period (Hubel and Wiesel, 1970). OD plasticity onsets rapidly (<1 day of deprivation) in layers extragranular layers (L2/3 and L5/6; Trachtenberg et al., 2000). Depression of deprived eye responses is the most rapid (Mrsic-Flogel et al., 2007), while potentiation of the non-deprived eye responses occur over several days (Frenkel and Bear, 2004). LTD of inputs in both L4 and L2/3 is occluded in V1 following visual deprivation (Heynen et al., 2003), though evidence connecting these long-term changes in synaptic strength to specific presynaptic inputs is not yet conclusive. At least in part, this due to reorganization of connectivity within L2/3 pyramidal cells, which show significant increases in connectivity between neurons with similar orientation preference in the two weeks following eye opening (Ko et al., 2013; Ko et al., 2011), and this connectivity can be manipulated by visual deprivation (Ko et al., 2014). Lateral connectivity in L2/3 is also implicated in barrel cortex plasticity (reviewed in Fox, 2002). Visual deprivation also alters excitatory synaptic strength between L4 neurons, though only early in development (Maffei et al., 2006; Maffei et al., 2004). Changes in thalamocortical input do occur (Antonini et al., 1999; Antonini and Stryker, 1993), but these gross changes occur more slowly than changes in local circuit connectivity. Long-range corticocortical connectivity between higher visual areas and V1 (Glickfeld et al., 2014; Wang and Burkhalter, 2007) is beginning to be described, and it will be exciting to see how plasticity in these long-range pathways contributes to V1 plasticity.
One of the most exciting findings in V1 plasticity is that the triggering of the critical period for plasticity is dependent on the development of GABAergic inhibition (Fagiolini and Hensch, 2000; Hensch et al., 1998). Specifically, enhancement of early GABAergic development or developmental delays could manipulate the opening and subsequent closure of the critical period. Consistent with this, visual deprivation strengthens inhibitory connections between fast-spiking (presumed PV+) interneurons and L4 cells (Maffei et al., 2006). In vivo observations during deprivation suggest the change in responsiveness is due to a reduction in local excitatory input to PV+ interneurons (Kuhlman et al., 2013). Coincidentally, plasticity in inhibitory output from PV+ neurons, but not SOM+ cells, is also activity regulated (Xue et al., 2014). Thus, there is considerable reason to believe that GABAergic circuitry will play an important role in M1 plasticity as well (Hess and Donoghue, 1994). An important question to address is, ‘How does GABAergic connectivity and processing in M1 differ from that in V1 such that new motor skills can be learned throughout life?’ Alternately, during perceptual learning, ‘Is there an analogous increase in the computational output from V1 as demonstrated by increased representation in L5, as is seen in M1?’
FUTURE PROSPECTS FOR UNDERSTANDING THE CIRCUIT MECHANISMS OF MOTOR CORTEX PLASTICITY
There is a substantial gap between our current knowledge and what we seek to understand about the mechanisms of plasticity underlying motor skill learning in M1. In the future, we would like to understand how plasticity is regulated at each node of the circuit across different timescales of skill learning, possibly involving independent mechanisms across varying brain structures. For clinical reasons, this might be useful in helping patients recover function following a stroke or lesion. In other disorders, such as focal motor dystonias, aberrant sensorimotor plasticity may contribute to the dysfunction (Hamani et al., 2006; Lenz et al., 1999). This understanding might more generally provide insight into the means by which the cortex retains plasticity in the adult, since our experience suggests that humans learn new motor skills long past the critical period of V1.
So, what questions can be answered in the near term? We need 1) to use motor skills such as dexterous reaching that highlight the unique contribution of M1 and the descending corticospinal tract, 2) to identify synaptic changes in M1 responsible for within-session versus across-session learning and 3) to use mapping approaches that examine multiple loci simultaneously. First, we focus on tasks of sufficient skill that M1 is required for rodents to learn and execute them. This may more closely approximate the situation in humans, who suffer profound deficits following M1 stroke late in life. Second, it would be best to know these changes for a given point in the learning curve, starting with the mechanisms responsible for across-session improvements in performance, as these are thought to be more permanent. Similar methodologies might later be helpful in elucidating transient changes during within-session improvements, as might occur during an initial training session. It is of general interest how performance varies throughout a training session, as understanding the neural basis of performance as a function of time would be valuable in a number of competitive athletic endeavors. Third, we need mapping approaches that examine multiple loci – the inputs from defined presynaptic populations to defined postsynaptic targets. In mice, Cre-driver lines make an excellent start for interneurons (Hippenmeyer et al., 2005; Taniguchi et al., 2011) as well as pyramidal cells (Gerfen et al., 2013). But more rapid methods for quantification of synaptic strength would be desirable to test multiple inputs simultaneously. A means of tracking synaptic strength changes over time to a given neuron would be at the top of the neuroscience tools wish list. Furthermore, although genetic specificity is helpful, it would be ideal to know about changes in connectivity to neurons with defined responses, as has been accomplished in challenging experiments in visual areas (Bock et al., 2011; Ko et al., 2011).
Studies already completed have suggested these changes will occur at many loci, and thus, knowing the details of the molecular mechanisms underlying them will be useful, especially if they occur at neuron types with specific genetic subtypes that offer the chance for manipulation. In the near future, the synaptic changes in which we are most interested are those involving specific interneuron subtypes. Since GABAergic cells are already established as crucial determinants of the onset and closure of plasticity in S1, it will be interesting to learn if they play a similar role in agranular M1, or whether the role of GABAergic activity in M1 is fundamentally different. Furthermore, as it is not clear that M1 has a sharply defined critical period closure, it will also be interesting to learn how plasticity in M1 might be differentially developmentally regulated. As some of the technology and models available for circuit investigation include those for targeting GABAergic neurons, the next decade of work may illuminate this issue.
The highlights of this work include.
Description of motor learning tasks and the timecourse of acquisition
Description of the major descending pathways involved in fine motor control
Description of local and long-range connectivity of primary motor cortex
Identification of loci where motor cortex circuitry may change during learning
Comparison of motor cortex plasticity to critical period plasticity in sensory areas
Acknowledgments
We thank Jay Couey for comments on the manuscript. This work was supported by a NARSAD Young Investigator Award to BMH and by National Institute of Neurological Disorders and Stroke, National Institutes of Health (R01 NS103993).
ABBREVIATIONS
- AAV
Adeno-associated virus
- C2
Second cervical (level of spinal cord)
- ChR2
ChannelRhodopsin-2 (light-sensitive ion channel)
- fEPSP
Field Excitatory Post-Synaptic Potential
- GABA
gamma-Aminobutyric Acid (an inhibitory neurotransmitter)
- HCN
Hyperpolarization-activated cyclic nucleotide-gated (a class of ion channels)
- IT
Intratelencephalic-type pyramidal neuron (One subtype of L5 pyramidal neuron)
- L2/3
Cortical layer 2/3 (also L4, L5A, L5B, L6)
- LTD
Long-term depression
- LTP
Long-term potentiation
- ms
Millisecond
- M1
Primary motor cortex
- M2
Secondary motor cortex (a frontal cortical area)
- MD
Monocular deprivation
- MvdM
Medullary reticular formation (ventral part)
- NMDA
N-methyl-D-aspartate (a subset of ionotropic glutamate receptors)
- OC
Orbital cortex (a frontal cortical area)
- OD
Ocular dominance
- PO
Posterior thalamic nucleus (higher order somatosensory thalamus)
- PT
Pyramidal tract-type pyramidal neuron (One subtype of L5 pyramidal neuron)
- PV
Parvalbumin (a marker for a subset of cortical fast-spiking GABAergic interneurons)
- RSC
Retrosplenial cortex
- S1
Primary somatosensory cortex
- SOM
Somatostatin (a marker for a subset of cortical regular-spiking GABAergic interneurons)
- Th1
First thoracic (level of spinal cord)
- TMS
Transcranial Magnetic Stimulation
- V1
Primary visual cortex
- VA
Ventral anterior thalamic nucleus (motor thalamus)
- VL
Ventrolateral thalamic nucleus (motor thalamus)
- VM
Ventromedial thalamic nucleus (motor thalamus)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaverdashvili M, Whishaw IQ. Motor cortex stroke impairs individual digit movement in skilled reaching by the rat. Eur J Neurosci. 2008;28:311–322. doi: 10.1111/j.1460-9568.2008.06315.x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci. 2012;35:559–578. doi: 10.1146/annurev-neuro-062111-150527. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 2004;91:1832–1839. doi: 10.1152/jn.00820.2003. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Pettersson LG. Skilled reaching and grasping in the rat: lacking effect of corticospinal lesion. Front Neurol. 2014;5:103. doi: 10.3389/fneur.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Sheets PL, Kiritani T, Shepherd GM. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nature neuroscience. 2010;13:739–744. doi: 10.1038/nn.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Fagiolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- Apicella AJ, Wickersham IR, Seung HS, Shepherd GM. Laminarly orthogonal excitation of fast-spiking and low-threshold-spiking interneurons in mouse motor cortex. J Neurosci. 2012;32:7021–7033. doi: 10.1523/JNEUROSCI.0011-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou VA, Keller A. Mechanisms of LTP induction in rat motor cortex in vitro. Cerebral cortex. 1995;5:353–362. doi: 10.1093/cercor/5.4.353. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014;508:357–363. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi A, Feher O. Conditioned changes of synaptic transmission in the motor cortex of the cat. Exp Brain Res. 1978;33:283–298. doi: 10.1007/BF00238066. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Feher O. Synaptic facilitation requires paired activation of convergent pathways in the neocortex. Nature. 1981;290:413–415. doi: 10.1038/290413a0. [DOI] [PubMed] [Google Scholar]
- Barth AL, Gerkin RC, Dean KL. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J Neurosci. 2004;24:6466–6475. doi: 10.1523/JNEUROSCI.4737-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Biane JS, Takashima Y, Scanziani M, Conner JM, Tuszynski MH. Thalamocortical Projections onto Behaviorally Relevant Neurons Exhibit Plasticity during Adult Motor Learning. Neuron. 2016;89:1173–1179. doi: 10.1016/j.neuron.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi L, Viaro R, Lodi E, Canto R, Bonifazzi C, Franchi G. Complex movement topography and extrinsic space representation in the rat forelimb motor cortex as defined by long-duration intracortical microstimulation. J Neurosci. 2013;33:2097–2107. doi: 10.1523/JNEUROSCI.3454-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk JA, Farrell JS, Palmer LA, Singleton AC, Pittman QJ, Teskey GC. HCN channels segregate stimulation-evoked movement responses in neocortex and allow for coordinated forelimb movements in rodents. J Physiol. 2017;595:247–263. doi: 10.1113/JP273068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Localization in the Cerebral Cortex (Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues) London: Smith-Gordon; 1909. [Google Scholar]
- Brown AR, Teskey GC. Motor cortex is functionally organized as a set of spatially distinct representations for complex movements. J Neurosci. 2014;34:13574–13585. doi: 10.1523/JNEUROSCI.2500-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, Brownstone RM. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron. 2013;78:191–204. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, Cheng SY, Ko KW, Jones TA, Nishiyama H. The long-term structural plasticity of cerebellar parallel fiber axons and its modulation by motor learning. J Neurosci. 2013;33:8301–8307. doi: 10.1523/JNEUROSCI.3792-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: A rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75:309–320. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Chen SX, Kim AN, Peters AJ, Komiyama T. Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nature neuroscience. 2015;18:1109–1115. doi: 10.1038/nn.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestek CA, Batista AP, Santhanam G, Yu BM, Afshar A, Cunningham JP, Gilja V, Ryu SI, Churchland MM, Shenoy KV. Single-neuron stability during repeated reaching in macaque premotor cortex. J Neurosci. 2007;27:10742–10750. doi: 10.1523/JNEUROSCI.0959-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38:819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: reciprocity versus parity. Brain Res Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nature neuroscience. 1999;2:563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Kornysheva K. Motor skill learning between selection and execution. Trends Cogn Sci. 2015;19:227–233. doi: 10.1016/j.tics.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Doyon JU, LG . Functional anatomy of motor skill learning. In: LRS, Squire DL, editors. Neuropsychology of Memory. New York: Guilford Press; 2002. pp. 225–238. [Google Scholar]
- Driskell JE, Copper C, Moran A. Does Mental Practice Enhance Performance? Journal of Applied Psychology. 1994;79:481–492. [Google Scholar]
- Ericsson KA, Krampe RT, Tesch-Romer C. The Role of Deliberate Practice in the Acquisition of Expert Performance. Psychological Review. 1993;100:363–406. [Google Scholar]
- Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fosque BF, Sun Y, Dana H, Yang CT, Ohyama T, Tadross MR, Patel R, Zlatic M, Kim DS, Ahrens MB, et al. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science. 2015;347:755–760. doi: 10.1126/science.1260922. [DOI] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Reid RC, Andermann ML. A mouse model of higher visual cortical function. Curr Opin Neurobiol. 2014;24:28–33. doi: 10.1016/j.conb.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Brecht M, Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. J Neurosci. 2005;25:8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Kalambogias J, Yoshioka S, Han W, Li Z, Kawasawa YI, Pochareddy S, Li Z, Liu F, Xu X, et al. Control of species-dependent cortico-motoneuronal connections underlying manual dexterity. Science. 2017;357:400–404. doi: 10.1126/science.aan3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JZ, Graves AR, Guo WW, Zheng J, Lee A, Rodriguez-Gonzalez J, Li N, Macklin JJ, Phillips JW, Mensh BD, et al. Cortex commands the performance of skilled movement. Elife. 2015;4 doi: 10.7554/eLife.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haibach PRG, Collier D. Motor Learning and Development. Champaign, IL: Human Kinetics; 2011. [Google Scholar]
- Hamani C, Dostrovsky JO, Lozano AM. The motor thalamus in neurosurgery. Neurosurgery. 2006;58:146–158. doi: 10.1227/01.neu.0000192166.62017.c1. discussion 146–158. [DOI] [PubMed] [Google Scholar]
- Harrison TC, Ayling OG, Murphy TH. Distinct cortical circuit mechanisms for complex forelimb movement and motor map topography. Neuron. 2012;74:397–409. doi: 10.1016/j.neuron.2012.02.028. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–338. doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helduser S, Gunturkun O. Neural substrates for serial reaction time tasks in pigeons. Behav Brain Res. 2012;230:132–143. doi: 10.1016/j.bbr.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. Laminar organization of thalamic projections to the rat neocortex. Science. 1980;207:532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 1994;71:2543–2547. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nature neuroscience. 2003;6:854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63:508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. Physiological aspects of sport training and performance. Champaign, IL: Human Kinetics; 2002. Warm-up and Flexibility; pp. 155–168. [Google Scholar]
- Hooks BM, Hires SA, Zhang YX, Huber D, Petreanu L, Svoboda K, Shepherd GM. Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol. 2011;9:e1000572. doi: 10.1371/journal.pbio.1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Mao T, Gutnisky DA, Yamawaki N, Svoboda K, Shepherd GM. Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J Neurosci. 2013;33:748–760. doi: 10.1523/JNEUROSCI.4338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori N, Carp JS, Carpenter DO, Akaike N. Corticospinal transmission to motoneurons in cervical spinal cord slices from adult rats. Life Sci. 2002;72:389–396. doi: 10.1016/s0024-3205(02)02279-8. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. 2011;31:2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation in the motor cortex. Science. 1989;245:1385–1387. doi: 10.1126/science.2551038. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Whishaw IQ. On the origin of skilled forelimb movements. Trends Neurosci. 2000;23:372–376. doi: 10.1016/s0166-2236(00)01618-0. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975;249:617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Spencer WA. Cellular neurophysiological approaches in the study of learning. Physiol Rev. 1968;48:65–134. doi: 10.1152/physrev.1968.48.1.65. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. II. Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol. 1994;345:172–184. doi: 10.1002/cne.903450203. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai R, Markman T, Poddar R, Ko R, Fantana AL, Dhawale AK, Kampff AR, Olveczky BP. Motor cortex is required for learning but not for executing a motor skill. Neuron. 2015;86:800–812. doi: 10.1016/j.neuron.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritani T, Wickersham IR, Seung HS, Shepherd GM. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J Neurosci. 2012;32:4992–5001. doi: 10.1523/JNEUROSCI.4759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002a;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002b;934:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB, Mrsic-Flogel TD. The emergence of functional microcircuits in visual cortex. Nature. 2013;496:96–100. doi: 10.1038/nature12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Mrsic-Flogel TD, Hofer SB. Emergence of feature-specific connectivity in cortical microcircuits in the absence of visual experience. J Neurosci. 2014;34:9812–9816. doi: 10.1523/JNEUROSCI.0875-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto E, Furuta T, Nakamura KC, Unzai T, Hioki H, Kaneko T. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cerebral cortex. 2009;19:2065–2077. doi: 10.1093/cercor/bhn231. [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Ohno S, Furuta T, Unzai T, Tanaka YR, Hioki H, Kaneko T. Ventral medial nucleus neurons send thalamocortical afferents more widely and more preferentially to layer 1 than neurons of the ventral anterior-ventral lateral nuclear complex in the rat. Cerebral cortex. 2015;25:221–235. doi: 10.1093/cercor/bht216. [DOI] [PubMed] [Google Scholar]
- Landgren S, Phillips CG, Porter R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon’s hand and forearm. J Physiol. 1962;161:91–111. doi: 10.1113/jphysiol.1962.sp006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Park IS, Kim H, Greenough WT, Pak DT, Rhyu IJ. Motor skill training induces coordinated strengthening and weakening between neighboring synapses. J Neurosci. 2013;33:9794–9799. doi: 10.1523/JNEUROSCI.0848-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Landau W, Tutssel D, Lawrence DG. Lawrence and Kuypers 1968a, b) revisited: copies of the original filmed material from their classic papers in Brain. Brain. 2012;135:2290–2295. doi: 10.1093/brain/aws037. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Jaeger CJ, Seike MS, Lin YC, Reich SG, DeLong MR, Vitek JL. Thalamic single neuron activity in patients with dystonia: dystonia-related activity and somatic sensory reorganization. J Neurophysiol. 1999;82:2372–2392. doi: 10.1152/jn.1999.82.5.2372. [DOI] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Li W, Ma L, Yang G, Gan WB. REM sleep selectively prunes and maintains new synapses in development and learning. Nature neuroscience. 2017;20:427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FY, Moret V, Wiesendanger M, Rouiller EM. Corticomotoneuronal connections in the rat: evidence from double-labeling of motoneurons and corticospinal axon arborizations. J Comp Neurol. 1991;311:356–366. doi: 10.1002/cne.903110306. [DOI] [PubMed] [Google Scholar]
- Linkenhoker BA, Knudsen EI. Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature. 2002;419:293–296. doi: 10.1038/nature01002. [DOI] [PubMed] [Google Scholar]
- Linkenhoker BA, von der Ohe CG, Knudsen EI. Anatomical traces of juvenile learning in the auditory system of adult barn owls. Nature neuroscience. 2005;8:93–98. doi: 10.1038/nn1367. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM. Stages of motor skill learning. Mol Neurobiol. 2005;32:205–216. doi: 10.1385/MN:32:3:205. [DOI] [PubMed] [Google Scholar]
- MacKay-Lyons M. Central pattern generation of locomotion: a review of the evidence. Phys Ther. 2002;82:69–83. doi: 10.1093/ptj/82.1.69. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nature neuroscience. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Makino H, Hwang EJ, Hedrick NG, Komiyama T. Circuit Mechanisms of Sensorimotor Learning. Neuron. 2016;92:705–721. doi: 10.1016/j.neuron.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Molina-Luna K, Hertler B, Buitrago MM, Luft AR. Motor learning transiently changes cortical somatotopy. Neuroimage. 2008;40:1748–1754. doi: 10.1016/j.neuroimage.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11:471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Mosberger AC, de Clauser L, Kasper H, Schwab ME. Motivational state, reward value, and Pavlovian cues differentially affect skilled forelimb grasping in rats. Learn Mem. 2016;23:289–302. doi: 10.1101/lm.039537.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognjanovski N, Schaeffer S, Wu J, Mofakham S, Maruyama D, Zochowski M, Aton SJ. Parvalbumin-expressing interneurons coordinate hippocampal network dynamics required for memory consolidation. Nat Commun. 2017;8:15039. doi: 10.1038/ncomms15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Kuramoto E, Furuta T, Hioki H, Tanaka YR, Fujiyama F, Sonomura T, Uemura M, Sugiyama K, Kaneko T. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cerebral cortex. 2012;22:2840–2857. doi: 10.1093/cercor/bhr356. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Li CS, Bizzi E. Neuronal activity in the supplementary motor area of monkeys adapting to a new dynamic environment. J Neurophysiol. 2004;91:449–473. doi: 10.1152/jn.00876.2002. [DOI] [PubMed] [Google Scholar]
- Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nature neuroscience. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Liu X, MacDonald CJ, Moffa A, Zhou J, Redondo RL, Tonegawa S. Activating positive memory engrams suppresses depression-like behaviour. Nature. 2015;522:335–339. doi: 10.1038/nature14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci U S A. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. The Mind within the Brain: How we make decisions and how those decisions go wrong. New York, NY: Oxford University Press; 2013. [Google Scholar]
- Reep RL, Goodwin GS, Corwin JV. Topographic organization in the corticocortical connections of medial agranular cortex in rats. J Comp Neurol. 1990;294:262–280. doi: 10.1002/cne.902940210. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nature neuroscience. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Porter LL, Asanuma H. Long-lasting potentiation of synaptic potentials in the motor cortex produced by stimulation of the sensory cortex in the cat: a basis of motor learning. Brain research. 1987;413:360–364. doi: 10.1016/0006-8993(87)91029-8. [DOI] [PubMed] [Google Scholar]
- Savidan J, Kaeser M, Belhaj-Saif A, Schmidlin E, Rouiller EM. Role of primary motor cortex in the control of manual dexterity assessed via sequential bilateral lesion in the adult macaque monkey: A case study. Neuroscience. 2017;357:303–324. doi: 10.1016/j.neuroscience.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Schmidt EM, Bak MJ, McIntosh JS. Long-term chronic recording from cortical neurons. Exp Neurol. 1976;52:496–506. doi: 10.1016/0014-4886(76)90220-x. [DOI] [PubMed] [Google Scholar]
- Scott SH. A Functional Taxonomy of Bottom-Up Sensory Feedback Processing for Motor Actions. Trends Neurosci. 2016;39:512–526. doi: 10.1016/j.tins.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets PL, Suter BA, Kiritani T, Chan CS, Surmeier DJ, Shepherd GM. Corticospinal-specific HCN expression in mouse motor cortex: I(h)-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. J Neurophysiol. 2011;106:2216–2231. doi: 10.1152/jn.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14:278–291. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GMG, Stepanyants A, Bureau I, Chklovskii DB, Svoboda K. Geometric and functional organization of cortical circuits. Nature neuroscience. 2005;8:782–790. doi: 10.1038/nn1447. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. 2. Cambridge, Mass: MIT Press; 2006. [Google Scholar]
- Shiotsuki H, Yoshimi K, Shimo Y, Funayama M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S, Hattori N. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 2010;189:180–185. doi: 10.1016/j.jneumeth.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc Lond B Biol Sci. 2005;360:797–814. doi: 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987;326:694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Sorensen AT, Cooper YA, Baratta MV, Weng FJ, Zhang Y, Ramamoorthi K, Fropf R, LaVerriere E, Xue J, Young A, et al. A robust activity marking system for exploring active neuronal ensembles. Elife. 2016;5 doi: 10.7554/eLife.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL. Cortical projections of the feline thalamic nucleus ventralis lateralis. Brain Res. 1970;20:130–134. doi: 10.1016/0006-8993(70)90162-9. [DOI] [PubMed] [Google Scholar]
- Suter BA, Shepherd GM. Reciprocal interareal connections to corticospinal neurons in mouse M1 and S2. J Neurosci. 2015;35:2959–2974. doi: 10.1523/JNEUROSCI.4287-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ. Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Methods. 1996;65:1–17. doi: 10.1016/0165-0270(95)00131-x. [DOI] [PubMed] [Google Scholar]
- Tolias AS, Sultan F, Augath M, Oeltermann A, Tehovnik EJ, Schiller PH, Logothetis NK. Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron. 2005;48:901–911. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C, Stryker MP. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science. 2000;287:2029–2032. doi: 10.1126/science.287.5460.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer M, Kurth-Nelson Z, Redish AD. Information processing in decision-making systems. Neuroscientist. 2012;18:342–359. doi: 10.1177/1073858411435128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–1255. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Conner JM, Rickert J, Tuszynski MH. Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci U S A. 2011;108:2545–2550. doi: 10.1073/pnas.1014335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Burkhalter A. Area map of mouse visual cortex. J Comp Neurol. 2007;502:339–357. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sporns O, Burkhalter A. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J Neurosci. 2012;32:4386–4399. doi: 10.1523/JNEUROSCI.6063-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wildes CP, Pattarabanjird T, Sanchez MI, Glober GF, Matthews GA, Tye KM, Ting AY. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat Biotechnol. 2017 doi: 10.1038/nbt.3909. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler N, Wood L, Yu J, Solla SA, Shepherd GM. Top-down laminar organization of the excitatory network in motor cortex. Nature neuroscience. 2008;11:360–366. doi: 10.1038/nn2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny B, Kolb B, Tetzlaff W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav Brain Res. 1993;56:59–76. doi: 10.1016/0166-4328(93)90022-i. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny BP, Pellis VC. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav Brain Res. 1991;42:77–91. doi: 10.1016/s0166-4328(05)80042-7. [DOI] [PubMed] [Google Scholar]
- Withers GS, Greenough WT. Reach training selectively alters dendritic branching in subpopulations of layer II–III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27:61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF. Experience, cortical remapping, and recovery in brain disease. Neurobiol Dis. 2010;37:252–258. doi: 10.1016/j.nbd.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G. Attention and Motor Skill Learning. Champaign, IL: Human Kinetics; 2007. [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Borges K, Suter BA, Harris KD, Shepherd GM. A genuine layer 4 in motor cortex with prototypical synaptic circuit connectivity. Elife. 2014;3:e05422. doi: 10.7554/eLife.05422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Radulovic J, Shepherd GM. A Corticocortical Circuit Directly Links Retrosplenial Cortex to M2 in the Mouse. J Neurosci. 2016;36:9365–9374. doi: 10.1523/JNEUROSCI.1099-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Shepherd GM. Synaptic circuit organization of motor corticothalamic neurons. J Neurosci. 2015;35:2293–2307. doi: 10.1523/JNEUROSCI.4023-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HW, Lemon RN. An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: lack of evidence for cortico-motoneuronal synapses. Exp Brain Res. 2003;149:458–469. doi: 10.1007/s00221-003-1393-9. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature neuroscience. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Tomonaga M. Interactional synchrony in chimpanzees: Examination through a finger-tapping experiment. Sci Rep. 2015;5:10218. doi: 10.1038/srep10218. [DOI] [PMC free article] [PubMed] [Google Scholar]