Abstract
In this work we report the effect of the hard block dianhydride structure on the overall properties of partially biobased semiaromatic polyimides. For the study, four polyimides were synthesized using aliphatic fatty dimer diamine (DD1) as the soft block and four different commercially available aromatic dianhydrides as the hard block: 4,4′-(4,4′-isopropylidenediphenoxy) bis(phthalic anhydride) (BPADA), 4,4′-oxidiphthalic anhydride (ODPA), 4,4′-(Hexafluoroisopropylidene) diphthalic anhydride (6FDA), and 3,3′,4,4′-biphenyltetracarboxylic dianhydride (BPDA). The polymers synthesized were fully organo-soluble thermoplastic branched polyimides with glass transition temperatures close to room temperature. The detailed analysis took into account several aspects of the dianhydrides structure (planarity, rigidity, bridging group between the phtalimides, and electronic properties) and related them to the results obtained by differential scanning calorimetry, rheology, fluorescence and broadband dielectric spectroscopy. Moreover, the effects of physical parameters (crystallization and electronic interactions) on the relaxation behavior are discussed. Despite the presence of the bulky branched soft block given by the dimer diamine, all polyimides showed intermolecular charge transfer complexes, whose extent depends on the electronic properties of the dianhydride hard block. Furthermore, the results showed that polyimides containing flexible and bulky hard blocks turned out fully amorphous while the more rigid dianhydride (BPDA) led to a nanophase separated morphology with low degree of crystallinity resulting in constrained segmental relaxation with high effect on its mechanical response with the annealing time. This work represents the first detailed report on the development and characterization of polyimides based on a biobased fatty dimer diamine. The results highlight the potential of polymer property design by controlled engineering of the aromatic dianhydride blocks.
Keywords: Polyimides, Fatty dimer diamine, Alkyl branches, Aromatic dianhydrides, Structure–polymer properties
Short abstract
Effect of the hard block architecture on the physical properties of a set of side-branched semiaromatic polyimides comprising biobased fatty dimer diamine is discussed.
Introduction
Due to increased concerns regarding environmental sustainability, highly directed by growing and demanding ecological regulations, renewable resources are increasingly receiving both industrial and academic attention.1−3 Among the different renewable sources vegetable oils are generally considered the most important class due to their availability and versatility. Vegetable oils are used to synthesize different types of polymers4 such as polycarbonates, polyurethanes, polyesters, polyethers, polyamides, and polyolefins5 for paints, adhesives, composites, and biomedical applications.3 Various thermoset and thermoplastic polymers can be obtained, resulting in miscellaneous characteristics to meet many different industrial requirements, thereby confirming vegetable-oil based polymers’ potential as alternatives to petroleum-based ones.6,7
Vegetable oils are composed of different triglycerides which are the esterification products of glycerol with various fatty acids. The heterogeneity and variability of the triglycerides, which is due to the statistical distribution of fatty acids per triglyceride, makes a clear and reproducible correlation between the material properties and the monomer structures difficult. However, utilizing difunctional fatty acid-based monomers (diols, diamines, and diacids) with a well-defined molecular structure can lead to biobased polymers with better tunable properties.8 Fatty dimers have been used in the past to prepare polymers with innovative molecular architectures, such as supramolecular polymers with hydrogen bonding9 or reversible ionic interactions10 and PU–acrylate coatings.11 They are reportedly being utilized for suppressing H-bonding, inducing phase segregation and tuning viscosity, crystallinity, and thermo-mechanical properties in renewable polyurethanes and polyamides12,13 and their biocomposites.14,15 More recently biobased fatty dimer diamines with long alkyl branches (Priamine) were reported as useful building blocks to develop room temperature self-healing polyimides with high mechanical properties.16,17 In that previous work we reported16 the effect of the stoichiometric offset of the ODPA/DD1 ratio on the dynamic mechanical and self-healing properties of the resulting polymers. However, the role of the dianhydride architecture on the overall properties of these fatty dimer diamine based polyimides was not examined, leaving room for further dedicated research.
Polyimides are known for their capability of forming intra- and interchain electronic interactions called charge-transfer complexes (CTC), which are the reason behind their high thermo-mechanical properties and deep coloration.18 However, most of the aromatic polyimides are insoluble in any organic solvent. That, in addition to very high transition temperatures, often higher than their decomposition temperatures, limits their usefulness for many applications.19 Flexible and bulky linkage groups are traditionally being introduced between aromatic rings in the dianhydride monomer20,21 to enhance solubility, transparency, and processing. The goal of these modifications is to either disrupt their aromaticity (to prevent intramolecular conjugation) or to make the chains pack less efficiently (to prevent intermolecular CTC formation). Naturally, modification of the molecular architecture of PIs influences their mechanical properties and molecular dynamics as well.22−24
In this work we report the effect of the aromatic dianhydride structure on the thermal, optical, and mechanical properties and relaxation behavior of a set of polyimides based on a biobased aliphatic dimer diamine (DD1). The results are discretized with regards to several aspects: planarity, rigidity, bridging group between the phtalimides, electronic properties, and the combinations thereof. Moreover, the effect of different physical constraints (crystal formation and electronic interactions) on the relaxation behavior is discussed in detail. Despite their partially aliphatic and densely branched architectures, it was found that the synthesized polyimides are still able to form intermolecular charge transfer complexes, playing an important role in the overall properties of these semiaromatic thermoplastic polyimides. To the best of our knowledge, this is the first time that the effect of fatty dimer diamine is explored in a set of different polyimides with varying dianhydride architecture.
Experimental Section
Synthesis
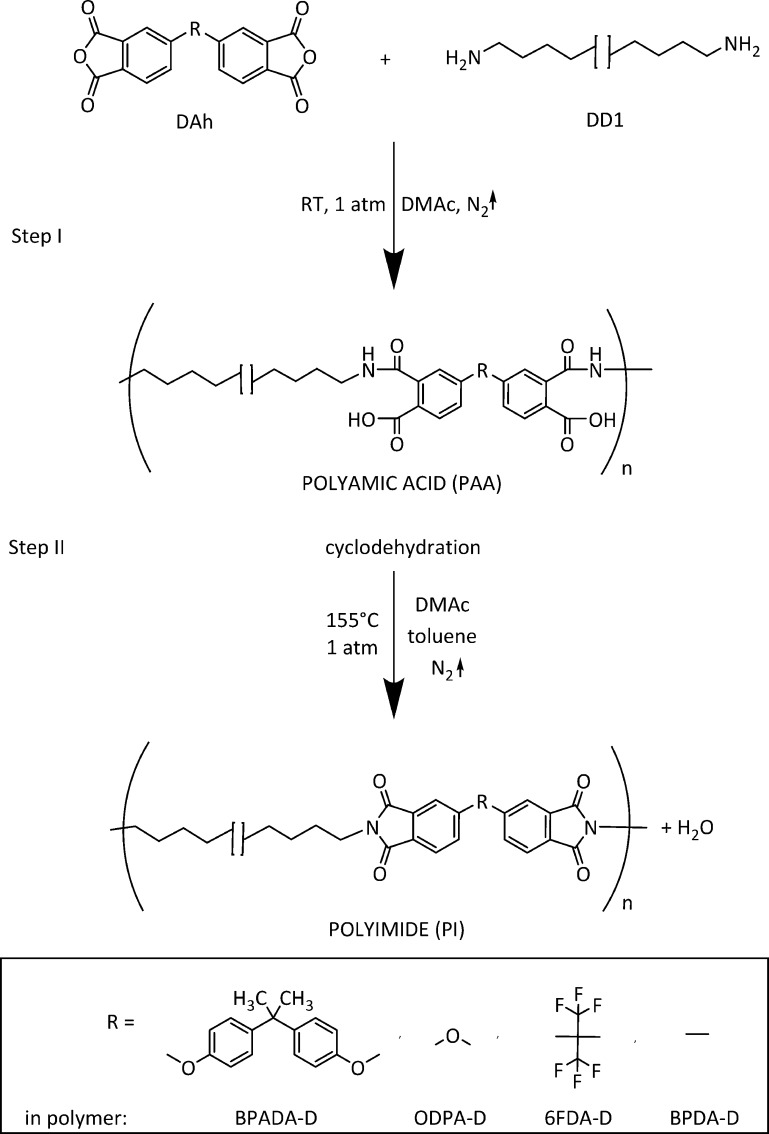
Four aromatic dianhydrides were used as hard aromatic block (Scheme 1): 4,4′-(4,4′-isopropylidenediphenoxy) bis(phthalic anhydride) (BPADA; 97%, Sigma-Aldrich), 4,4′-oxidiphthalic anhydride (ODPA; 98%, TCI Europe N.V.), 4,4′-(hexafluoroisopropylidene) diphthalic anhydride (6FDA; 98%, TCI Europe N.V.), 3,3′,4,4′-biphenyltetracarboxylic dianhydride (BPDA; 98%, TCI Europe N.V.). The soft block was in all cases a fatty dimer diamine derived from vegetable oil (Priamine 1075, here named DD1; Croda Nederland B.V.) with the structure as shown in Scheme 1. DD1 appears as a light yellow viscous liquid with melting point at −30°C and 100% renewable carbon content. Theoretical stoichiometric ratios, calculated according to the molecular weights of the monomers (MWBPADA = 520.49 g/mol, MWODPA = 310.20 g/mol, MW6FDA = 444.24 g/mol, MWBPDA = 294.22 g/mol, and MWDD1 = 536.80 g/mol) and assuming all chemicals are 100% difunctional, were used. The synthesis was conducted in N,N-dimethylacetamide (DMAc, 99.5% extra dry, Acros Organics) polar aprotic solvent with total solids (monomers) content of 20 wt %. Using a two-step polymerization process as described in Scheme 2, four polymers were obtained: BPADA-D, ODPA-D, 6FDA-D, and BPDA-D. The details of the synthesis procedure in Scheme 2 can be found elsewhere.16
Scheme 1. Structures of the Monomers Used in the Polyimides Synthesis.
The left side shows four dianhydrides used (BPADA, ODPA, 6FDA, and BPDA) and the right side shows a generalized structure of the dimer diamine (DD1).
Scheme 2. Schematic Representation of the Thermal Imidization Reaction (Cyclodehydration of Polyamic Acid into a Polyimide).
Characterization Methods
Infrared Spectroscopy
Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy was employed in order to follow reaction completion. Each IR spectrum was recorded as an average of two scans in the wavenumber range 4000–500 cm–1.
Gel Permeation Chromatography (GPC)
Molecular weight distributions of synthesized polymers were determined by GPC on a Shimadzu Prominence GPC system equipped with a Shodex LF-804 column and a refractive index detector. The flow rate of the eluent tetrahydrofuran (THF) was 1 mL/min, and polystyrene was used as the standard. Polymer solutions were prepared in THF at 1 mg/mL and the samples were filtered (0.45 μm syringe filter) prior to use.
Thermal Analysis
Thermal properties were evaluated by PerkinElmer Pyris Diamond TG/DTA thermogravimetric analysis (TGA) and PerkinElmer Sapphire differential scanning calorimetry (DSC). All measurements were performed under nitrogen. TGA was run from room temperature to 500 °C at 10 °C/min. DSC measurements were carried out at 10 °C/min following this procedure: (1) heating from −50 to +200 °C; (2) holding at 200 °C for 2 min; (3) cooling to −50 °C, and (4) repeat steps 1 to 3. The glass transition temperature (Tg) was determined from the second heating cycle.
Density Determination
The density of the polymers was determined by hydrostatic weighing method coupled with an analytical laboratory scale with a precision of 0.1 mg.
Rheological Measurements
The linear viscoelastic properties of the PIs were investigated by the Haake Mars III rheometer, using the parallel plate geometry, with plate diameter of 8 mm. Preliminary strain amplitude sweeps at 1 Hz were performed at the highest and the lowest tested temperatures, from 0.001% to 10% strain to evaluate the extension of the linear viscoelastic region for the different polymers. Based on these results, a shear strain of 0.5% for all polymers was used to ensure the tests were performed in the linear viscoelastic region. Temperature sweep experiments were performed at 1 Hz in a cooling ramp from 50 to 5 °C. The tan δ curves from the temperatures sweeps were used to determine Tg from the maximum of the tan δ peak (Tg = T(tan δMAX)). For the aim of activation energies, Ea, calculations, the frequency sweep experiments from 10 to 0.1 Hz were performed at temperatures between 110 and 10 °C, in steps of 5 °C. Tests were repeated twice-showing high reproducibility. The rheological mastercurves at the reference temperature of the previously determined Tg = T(tan δMAX) = Tref were constructed from the obtained data applying the time–temperature superposition principle (TTS) using the dedicated Rheowin software. From the shift factors, aT, the Arrhenius plots (ln aT vs 1000/T) were constructed and the activation energies were calculated from the slopes of the linear fit,25 as shown in the Supporting Information (S-6).
Broadband Dielectric Spectroscopy (BDS)
BDS measurements were performed on an ALPHA high resolution dielectric analyzer (Novocontrol Technologies GmbH). All samples were mounted in the dielectric cell between two parallel gold-plated electrodes. The complex permittivity ε*(ω) = ε′(ω) – iε″(ω) of each sample was measured by performing consecutive isothermal frequency sweeps over a frequency window of 10–1 < f (Hz) < 106 (where f = w/2π is the frequency of the applied electric field) in the temperature range from 0 to 100 °C in steps of 5 °C. The resulting relative error of each parameter is less than 3%.
Tensile Properties
Tensile mechanical tests were performed on an Instron model 3365 universal testing systems equipped with a 1 kN load cell, using dog-bone specimens according to the ASTM D1708 standard (thickness, t = 2 ± 0.5 mm) at 80 mm/min crosshead speed and ambient temperature (23 ± 2 °C).
Fluorescence Spectroscopy
The fluorescence spectra of polyimides were obtained using a PTI QuantaMaster Photoluminescence Spectrometer equipped with 75 W Xe lamp and using a PMT voltage of 1000 V. The same specimen geometry as for the tensile test was used. First the excitation scan was run in the range 280–400 nm, collecting emission at 410 nm. From this, the fluorescent peak was determined at 360 nm. Further, the samples were excited at that particular wavelength and monitored over the range 370–700 nm using an aperture slit width of 2 nm with 1 nm step size and 0.1 s integration time during monitoring. Each measurement was repeated three times, showing good reproducibility. The sample thickness was found not to have an effect on the measured peak.
Results and Discussion
Effect of the Dianhydride Structure on the PIs Properties
The imidization of PAA into PI can be confirmed by IR spectroscopy using the disappearance of the amic acid peaks typically visible at 1716, 1640, and 1550 cm–1 in PAA spectra and the appearance of the characteristic peaks of imide bonds at 1770, 1710, 1360, and 745 cm–1 in PI spectra. The imidization reaction was confirmed for all of the polymers (Figure 1) and supported by 1H NMR analysis (Figure S1, Supporting Information). The percentage yields were calculated by the standard approach shown in the SI (Page S-2). Yields of 83% for BPADA-D, 89% for ODPA-D, 74% for 6FDA-D, and 73% for BPDA-D were obtained.
Figure 1.
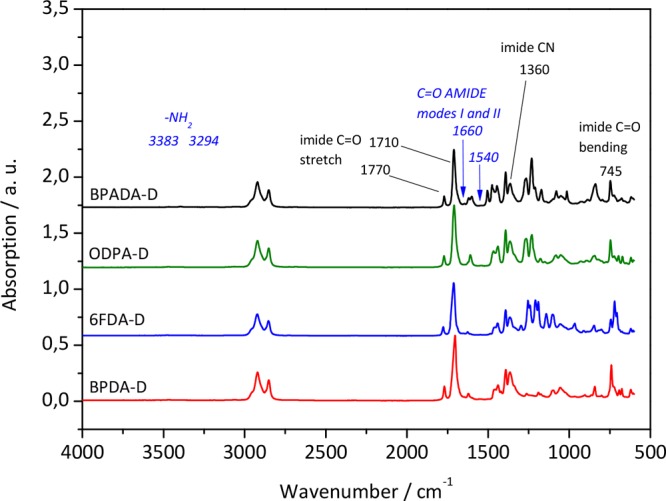
IR spectra of four PIs with different dianhydrides showing characteristic imide peaks (labeled black) and absence of prepolymer (amide) peaks (labeled italic blue).
As can be seen from the TGA curves (Figure S2, Supporting Information), all polymers show a high thermal stability independent of the dianhydride architecture with values for the onset degradation temperature (2% weight loss) at 330–380 °C (Table 1) similar to those of traditional commercial polyimides such as LaRC-IA. All samples showed only a small weight loss up to 0.6% until 200 °C suggesting that almost no solvent (toluene or DMAc) was entrapped during the imidization and that the monomers were fully reacted. The resulting polymers were fully soluble in common organic solvents, such as toluene, THF, and chloroform, which facilitates their processing (for example, in coatings, adhesives and thin films applications), as opposed to commercial polyimides.19 The GPC results are presented in Table 1. The small differences in the molecular weights might be due to various reasons, such as impurities or side-reactions but also differences in the electron affinities of the aromatic dianhydrides. As the susceptibility of the nucleophilic attack increases with the electrophilicity of the dianhydride group, the reactivity of the dianhydride monomer is related to its electron affinity: higher values indicate higher reactivity of the dianhydride. According to the literature,18,26 the electron affinities of the dianhydrides used in this work increase in the order BPADA-D < ODPA-D < BPDA-D < 6FDA-D.
Table 1. Effect of the DAh type on Mw, Mn, and PDI as Calculated from the Major Peak Obtained in GPCa.
| polymer | Mw (g/mol) | Mn (g/mol) | PDI | DSC-Tg (°C) | rheology-Tg (°C) | BDS-Tgd (°C) | TGA T (2% weight loss) (°C) | density (g/cm3) |
|---|---|---|---|---|---|---|---|---|
| BPADA-D | 29k | 18k | 1.6 | 24 | 36 | 20 | 360 | 1.05 |
| ODPA-D | 32k | 16k | 2.0 | 13 | 25 | 11 | 380 | 1.05 |
| 6FDA-D | 41k | 20k | 2.0 | 25 | 40 | 21 | 330 | 1.12 |
| BPDA-D | 37k | 20k | 1.9 | 22 | 33e, 46e | 18 | 350 | 1.05 |
Tg obtained from DSC, rheology, and BDS and temperatures for 2% weight loss obtained from TGA. Samples were tested as produced.
Tg was calculated from the 2nd heating curve, 10 °C/min.
Tg was taken as the maximum of the peak in the tan δ curve from the temperature sweeps, performed in cooling ramp, 1 °C/min.
Tg is obtained from the broadband dielectric spectroscopy (BDS) measurements, by extrapolating the VFT fit to the temperature at which τmax is equal to 100 s (see Figure S5, Supporting Information).
Polymer BPDA-D exhibits two Tg peaks [Tg (I) and Tg (II)] in rheological temperature sweep plots, which is shown and discussed later in the article.
The flexibility of the dianhydride moieties is reflected in the value of the Tg, where three polymers (BPADA-D, 6FDA-D, and BPDA-D) exhibit a similar Tg, ranging from 22 to 25 °C and ODPA-D showing a lower value of 13 °C. The Tg values of these PIs are incomparable to those of fully aromatic commercial PIs (200 < Tg < 400 °C),27 which naturally excludes the possibility for their use in high-temperature applications. However, their Tg’s are higher than the ones of other reported polymers that contain fatty acid dimer as the building block that remained significantly below room temperature. The ionic supramolecular networks prepared from fatty acid dimer by Aboudzadeh et al. exhibit their Tg’s in the range −29 <Tg < 10 °C.10 The fatty acid dimer based polyamides from Hablot at al. showed −17 < Tg < −5 °C,12 while the values in the range −10 < Tg < −0.9 °C were obtained in the fatty acid dimer based polyamides synthesized by van Velthoven et al.13
The ODPA and BPADA dianhydrides have a very flexible oxygen linker between the two phtalic anhydride parts of the molecule (Scheme 1). Comprising two oxygen linkers, BPADA may appear the most flexible out of the four dianhydrides used here, yet it does not exhibit the lowest Tg. This is likely to be caused by the increase in aromatic content compared to ODPA-D, where the extra flexibility of the ether linkage is counterbalanced by the increased number of aromatic rings. Despite the structural differences, BPDA-D and 6FDA-D display a similar Tg. The expected increase in backbone flexibility of the 6FDA-D polymer may be compensated by the extra bulkiness of the CF3 groups. A similar effect of dianhydride structure on the Tg of nonbranched fully20 and partially aromatic28 polyimides was reported in the literature, with the exception of BPADA. A detailed DSC analysis (Figure S3, Supporting Information) shows the absence of melting or crystallization peaks thereby reflecting the amorphous nature of all of the polyimides in their state just after synthesis.
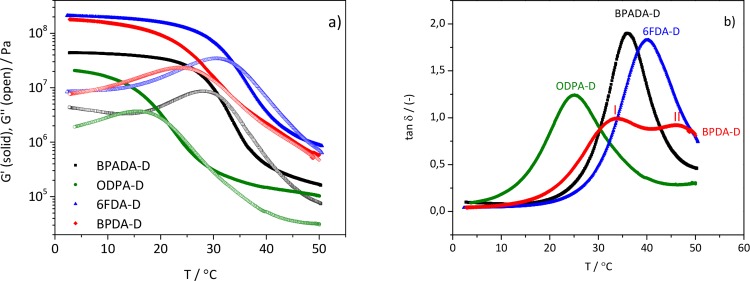
Rheological temperature sweeps were performed at a very slow cooling rate (1 °C/min), and the results are shown in Figure 2. Figure 2a shows the values of the storage modulus (G′) and loss modulus (G″) while damping factors (tan δ) versus temperature at 1 Hz for all of the samples studied are shown in Figure 2b.
Figure 2.
(a) Storage (G′) and loss (G′’) moduli and (b) tan δ curves from the rheological temperature sweeps experiments, showing distinct Tg relaxations of the four PIs.
The differences between the Tg values determined from the maximum of the tan δ peak (25 °C < Tg < 40 °C) arise from the effect of different aromatic dianhydride structures on the glass transition processes. The width of the curves indicate the breadth of the temperature range over which the glass transition occurs but also the polymer structural heterogeneity.29 The values of tan δ of these polymers (especially BPADA-D and 6FDA-D with values close to 2) are remarkably high over a broad range of near-room temperatures, which makes them great candidates in applications where high damping properties are required at ambiental conditions (noise or vibration insulating materials, shock absorbers, and sealants). In general, damping materials with tan δ>0.5 are considered for outdoor or machinery applications.30 (As seen in Figure 2b, BPADA-D and 6FDA-D are displaying the narrowest tan δ peaks. A somewhat broader curve was obtained with ODPA-D and the broadest with BPDA-D. Moreover, the BPDA-D system exhibits stepwise glass transition (shown in Figure 2b as (I) and (II)), as opposed to the other three (BPADA-D, ODPA-D, and 6FDA-D), which show single Tg.
Similar observations could be made from the broadband dielectric spectroscopy (BDS) data. The spectra corresponding to all four polymers over a wide range of frequencies at different temperatures are shown in Figure S4, Supporting Information. Tg was calculated from the temperature dependence of the segmental relaxation times (τmax). When this dependence follows a Vogel–Fulcher–Tammann (VFT) behavior, Tg is obtained by extrapolating the VFT fit to the temperature at which τmax is equal to 100 s (see the SI).31 The Tg’s calculated following this approach (Table 1) are similar to the values obtained by DSC and follow the same trend of increase among the four polymers investigated (ODPA-D < BPDA-D < BPADA-D < 6FDA-D), as seen by DSC and rheology. The temperature dependence of the relaxation times in the whole region of the segmental relaxation further supports the fact that the ODPA-D polymer has the least restricted dynamics (see the SI).
In addition to a third estimate of Tg, BDS gave some further insights on the structure and polymer architecture of the studied PIs.
By plotting the normalized dielectric loss vs normalized frequency, a clearer view on how the shape (symmetry and broadness) of the relaxation spectra varies is obtained. Figure 3 shows the normalized plots at a selected temperature (T = 50 °C) at which the dielectric relaxation is clearly observable as a well-resolved peak in the frequency window for all polymers. Depending on the nature of the dianhydride incorporated into the polymer, clear differences in the shape of the spectra can be noticed. Schönhals and Schlosser32 phenomenological model proposes that the shape of the normalized dielectric loss peak is related to the behavior of the polymer at low and high frequencies, controlled by inter- and intramolecular interactions, respectively. The application of such a model to the studied polyimides suggests that the differences on the low frequency side may be related to the changes in the dynamics of the main chain segments due to the contribution/restriction imposed by the different dianhydrides, while the variations on the high frequency side can be attributed to the influence of the dangling side chains.
Figure 3.
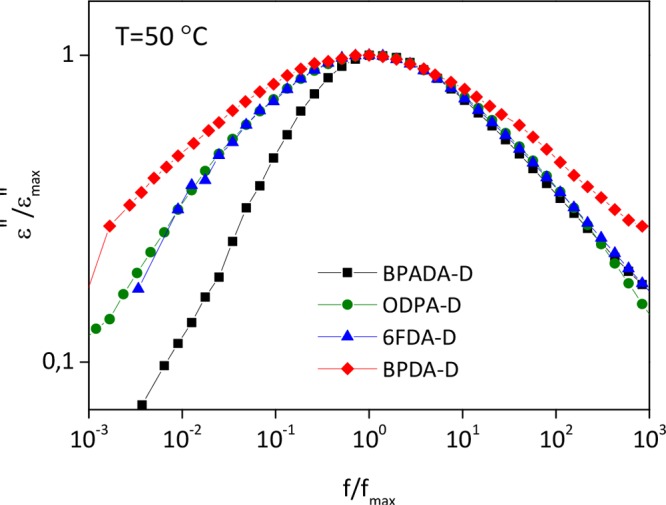
Normalized dielectric loss log(ε″) vs normalized frequency log(f) of PI samples with different dianhydrides at T = 50 °C.
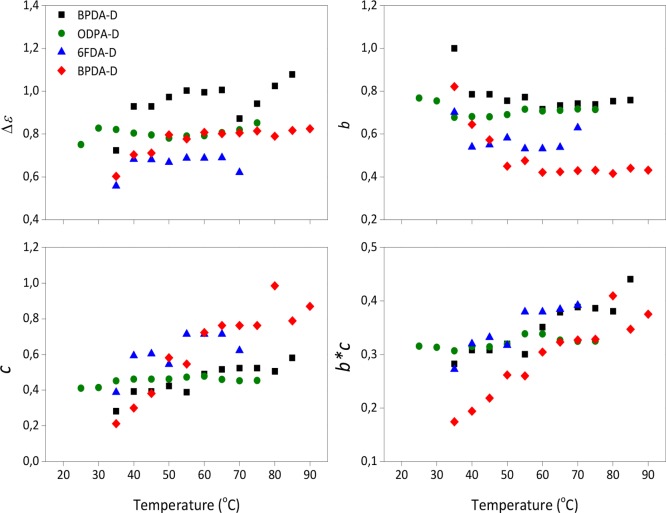
In order to quantify these differences, the dielectric strength (Δε) and the shape parameters b and c derived from the Havriliak–Negami (HN) fitting function (see the SI) were calculated and shown in Figure 4. The b and c parameters characterize the symmetric and asymmetric broadening of the relaxation time distribution, respectively. The term b*c is also shown in the same figure. In a log(ε″) vs log(f) plot, just as in Figure 3, the shape parameters b and b*c correspond to the low- and high-frequency slopes of the relaxation function respectively, with regards to the position of the maximal loss.33
Figure 4.
Dielectric parameters derived from the HN fitting function in the temperature range of the segmental relaxation.
A detailed analysis of the plots in Figure 4 gives better insight into the effect of different dianhydrides on the resulting polymer architectures. An increase in dielectric strength (Δε) can be understood in terms of an increasing number of mobile dipoles (increasing fraction of polar molecules) involved in the relaxation, indirectly reflecting stronger molecular interactions. We can therefore infer that the BPADA-D system has higher dipole interactions than the rest of the PI systems with other dianhydrides. Recently, research on the relaxation behavior of thermo-reversible elastomer networks based on 2-ureido-4-pyrimidinone (UPy) dimers has been published by Luo et al.34 In their work they report on the appearance of a new relaxation, seen as a shoulder close to the segmental relaxation, ascribed to the dissociation dynamics of dimer complexes within the network. They conclude that this new relaxation is related to the local UPy dimers dynamics resulting from the dissociation and reformation of the complexes. They also report an increase of Δε due to the increased number of dissociated UPy units. In our work, there is no evidence of a new relaxation probably because they take place within the temperature range within the glass transition; therefore, any weaker relaxation is hidden by the strong segmental relaxation.
Second, the fact that the b parameter is higher for the BPADA-D system reflects a narrower and more symmetrical loss peak. In terms of the polymer architecture, this would suggest a more homogeneous structure formed by polymer chains with similar large scale motions. On the contrary, the lowest b value for the BPDA-D system suggests chain segments with different dynamics and thus a higher degree of structural heterogeneity, implying regions of distinct mobility. This hypothesis is confirmed by the presence of a stepwise glass transition, as reported by rheological measurements (Figure 2b). Moreover, the lowest b*c parameter for BPDA-D is further confirming heterogeneous dynamics of this sample with a more visible temperature dependence. As the temperature increases, the b*c parameter tends to increase, presuming the distinct heterogeneous mobility regions become more homogeneous, especially above 50 °C. As will be discussed later on, the increase on b*c values above 50 °C could be related to local ordering present in the BPDA-D sample appearing at around 50 °C.
Aromatic polyimides are well-known for forming charge transfer complexes (CTCs), which are widely claimed to be the reason behind their great mechanical and thermal properties, as well as their characteristic colors,18 due to their absorption characteristics tailings in the visible region caused by the intra and/or intermolecular charge transfer (CT) interactions of the PI backbones. Moreover, CTCs have a major effect on the chain packing.27,35 In order to investigate the origin of the different coloration, the polymer optical properties were tested by fluorescence spectroscopy as typically used in polyimide research to identify the formation of CTCs.18,35−39 The CTCs existence has been previously confirmed for not only fully aromatic PIs but also in the semiaromatic ones.40 Our results showed that CTCs in the branched polyimides are identified by a long-wavelength absorption at λ > 330 nm, similar to those reported in literature.37,38 As can be seen in Figure 5, the four polyimides developed in this work are fluorescent in the CTC-region (400 nm < λem < 450 nm).
Figure 5.
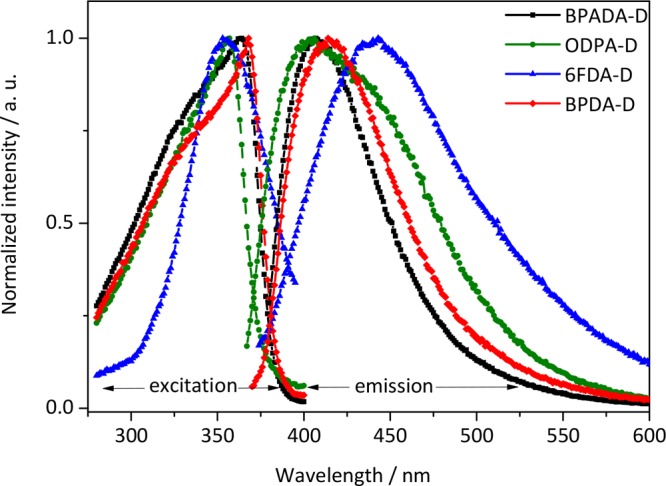
Optical properties of the four PIs: fluorescence excitation (left) and emission (right) spectra, normalized by maximum. λexc = 360 nm.
Effect of the Low Temperature Annealing on Local Ordering
Another important factor affecting the physical properties of polyimides is the aggregation state of polymer chains, i.e., macromolecular packing. Many have shown that imidization temperature affects the aggregation state of PIs,38 but the postimidization thermal treatment (i.e., annealing) does as well.41 In order to further explore the origin of physical constraints on the properties of the given set of DD1 based PIs, they were subjected to low temperature annealing, Tann = T(tan δMAX) = Tg (see Table 2), for 1, 5, and 11 days. Their thermal, optical, and mechanical properties were then evaluated as a function of the annealing time. The DSC scans of all four polymers show no change in their Tg values.
Table 2. Annealing Temperatures Taken as Maxima of the tan δ Peak (Tg) of the Rheological Temperature Sweep Curves (Figure 2b).
| polymer | Tann = T(tan δMAX) = Tg/°C |
|---|---|
| BPADA-D | 36 |
| ODPA-D | 25 |
| 6FDA-D | 40 |
| BPDA-D | 33 |
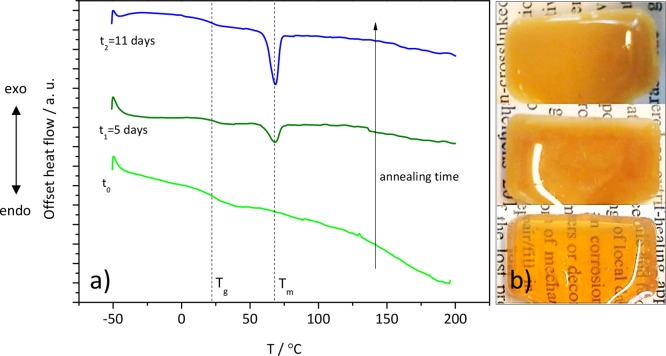
However, as opposed to the other three polymers, a change in opacity with time was noticed for BPDA-D, turning from translucent to fully turbid after 5 and 11 days at Tann. DSC scans of turbid BPDA-D show an appearance of a melting peak at 68 °C (yet no change in the Tg value). The melt enthalpy increases from 5 J/g (for 5 days annealed) to 11 J/g (for 11 days annealed) with no change in Tm (Figure 6). As seen by polarized microscopy (Figure S7, Supporting Information), the birefringence increases with annealing time as well. These observations can be explained by the rigidity and planarity of the BPDA monomer which result in the chain regularity and hence efficient chain packing, allowing the BPDA-D polymer to crystallize.28 At this stage it is not possible to unambiguously state which segments of the molecule crystallize. Nevertheless, the increase of order in chain packing with annealing time in BPDA-D polymer was confirmed by small-angle X-ray scattering measurements, SAXS (Figure S8, Supporting Information) by the appearance of two additional peaks at annealing times 5 and 11 days, with respect to 1 day. The peak ratio is q1:q2:q3 = 1:2:3, which was previously reported to be related to the nanophase separation in lamellar morphology.42 Taking in account that Tm is rather low (68 °C) and considering reported similar range of Tm values for other partially aromatic polymers with alkyl side branches in the literature,43,44 we suggest that crystals might be formed within the nanodomains of the alkyl side branches. As Beiner and Huth suggested, this may be a result of a confinement of the alkyl side chains by less-mobile main chains.45 However, further research is necessary to confirm this hypothesis.
Figure 6.
(a) DSC traces from the second heating curve with Tg and Tm indicated by the dashed lines and (b) corresponding images showing increase in opacity of BPDA-D polymer with annealing time. Tann = T(tan δMAX) = Tg.
On the contrary, the polymers with the other three dianhydrides remain fully amorphous with the annealing time. Even though rigid, the bulky hexafluoroisopropylidene spacer of 6FDA dianhydride causes distortions of backbone symmetry which prevents the parallel alignment of the chains. On the other hand, the ether linkages of BPADA and ODPA are not bulky but offer high rotational freedom and flexibility which reduces the rigidity of the backbone, introduces kinks which hinder the coplanarity and thus prevent any efficient chain packing.22,27,28 The same dianhydride effect on crystallization ability was noticed in fully aromatic rigid rod polyimides and copolyimides, comprising various diamines. For example, Arnold et al. prepared nine polyimides using six different dianhydrides, including ODPA, 6FDA, and BPDA among others. Only two polymers with different diamines were able to crystallize during drawing or/and annealing and both of them comprised BPDA as the dianhydride.46
While the rheological temperature sweeps of the polymers BPADA-D, ODPA-D, and 6FDA-D do not show any change with the annealing time, the BPDA-D polymer undergoes significant change in the glass transition region, as can be seen in Figure 7.
Figure 7.
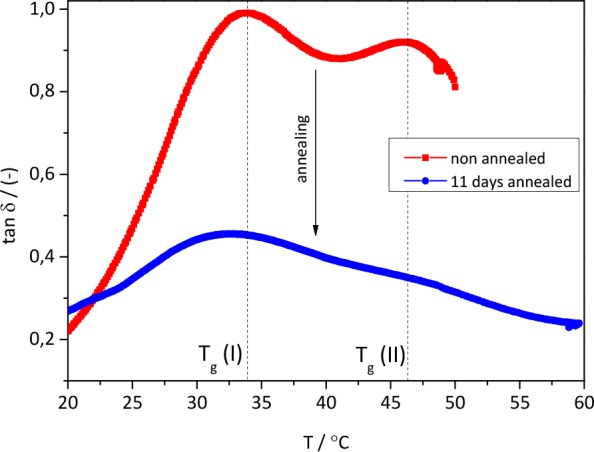
Rheological T-sweep curves. Effect of annealing on the glass transition relaxation behavior of the BPDA-D polymer. Red curve represents the nonannealed polymer and the blue curve shows the polymer annealed for 11 days at Tann = T(tan δMAX) = Tg.
After 11 days of annealing, the tan δ decreases over the whole range of temperatures tested. Furthermore, the curve broadens and the two peaks are less pronounced, which is expected, as the content of the amorphous phase decreases with the increase in the crystalline fraction. This multiple-Tg relaxation behavior is characteristic for multiphase structures and incompatible blends. However, the behavior noticed in the BPDA-D polymer is peculiar because of the (consistently) reverse magnitudes of the two peaks (Tg(I), at the lower temperature and Tg(II), at the higher temperature). This can be expressed in terms of activation energies as well. Activation energies, Ea, are calculated from the Arrhenius plots (Figure S6, Supporting Information), as described in the Supporting Information. The peak at the lower temperature, Tg(I), exhibits higher Ea than the one at higher temperature, Tg(II) (see Table S1, Supporting Information). Such an observation can be explained by assuming a less mobile phase present at the crystal–amorphous phase interface.47 Thus, based on the literature and the DSC evidence that the BPDA-D polymer is the only semicrystalline (Figure 6) polymer out of the four here investigated, we could explain the multiple tan δ peaks as follows: the relaxation occurring at about 33 °C is presumed to be the relaxation of the major part of the amorphous phase, a Tg per se. The relaxation occurring at a higher temperature of 46 °C can be attributed to the restricted amorphous phase located in the interfacial region.
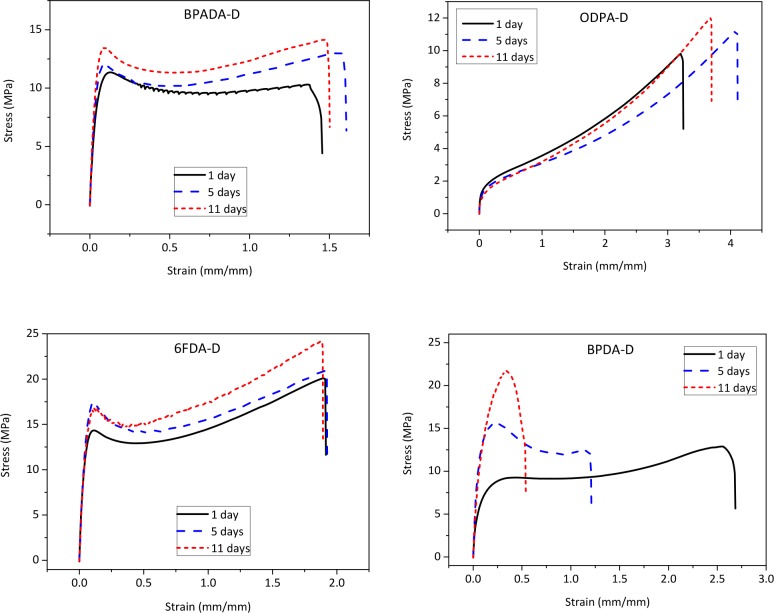
The effect of the aromatic dianhydride architecture on the mechanical properties was also evaluated. Figure 8 shows representative strain–stress curves of the four PIs obtained at ambient temperature (Ttest = 23 ± 2 °C). The mechanical performance changes significantly with varying the dianhydride architecture. ODPA-D polymer shows elastomeric behavior, which is as expected because its’ Tg is below Ttest, as opposed to the other three polymers that are in their glassy state (Tg > Ttest).
Figure 8.
Effect of annealing time on the stress–strain behavior of the four PIs. Tann = T(tan δMAX) = Tg.
The stress at break values (σb) increase in the order ODPA-D < BPADA-D < BPDA-D < 6FDA-D and the strain at break (εb) values in the following order BPADA-D < 6FDA-D < BPDA-D < ODPA-D. The 6FDA-D system shows the highest toughness and strain hardening while BPADA-D exhibits strain softening, having yield stress higher than its stress at break value. Young’s moduli are in a broad range, from 90 MPa (ODPA-D) to 430 MPa (6FDA-D), comparable to the fatty acid dimer based polyamides of Hablot at al.12 When compared to commercial fully aromatic PIs, these materials are roughly 1 order of magnitude lower in strength.48
The effect of annealing on the tensile properties is also shown in Figure 8. The three amorphous polymers (BPADA-D, ODPA-D, and 6FDA-D) show minor improvement of the mechanical properties with annealing time, while the effect on the semicrystalline BPDA-D is more evident. The stress at break value increases from 11 to 20 MPa, and the strain at break value decreases from 260% to 35%, despite the nonrelevant variation in Tg. The repeated fluorescence spectra after annealing the samples did not show any shifts in fluorescence peaks wavelengths.
Conclusions
A series of partially bio based semiaromatic polyimides varying in their aromatic dianhydride structure was synthesized in stoichiometric ratio with the fatty dimer diamine and their thermal, mechanical, and optical properties were investigated. The polymers obtained were fully organo-soluble thermoplastic branched polyimides with glass transition temperatures close to room temperature. Their solubility, amorphous thermoplasticity, and high thermal stability makes them easily processable and recyclable without deterioration of mechanical properties. The results have shown that the dianhydrides comprising a flexible ether spacer (BPADA and ODPA), as well as the one with rigid and bulky hexafluoroisopropylidene spacer (6FDA), yielded fully amorphous polymers. However, the dianhydride with no spacer (BPDA) provided a semicrystalline polymer structure with the annealing time, which lead to a constrained segmental relaxation. Despite their partially aliphatic and densely branched architectures, these polyimides were able to form the intermolecular charge transfer complexes, whose extent was dependent on the dianhydride electronic properties and evident from their fluorescence spectra and normalized dielectric loss. The increase in crystallinity with annealing time was shown to have a great effect on the tensile properties of the BPDA-D polymer despite no significant Tg variation was measured. The values of tan δ of these polymers (especially BPADA-D and 6FDA-D with values close to 2) were remarkably high over a broad range of near-room temperatures, which makes them great candidates in applications where high damping properties are required at ambiental conditions (noise or vibration insulating materials, shock absorbers, and sealants).
Acknowledgments
We thank Professor Sybrand van der Zwaag for his continued interest in the work and valuable comments on the manuscript during its construction. The authors acknowledge the financial support from the Dutch IOP program on self-healing materials under Grant No. IOP-SHM-012036. We acknowledge our industrial partner Croda Nederland BV, especially the sustained support and valuable discussions with Dr. Angela Smits (Croda). Many thanks to Dr. Brian Richard Pauw (Bundesanstalt für Materialforschung und–prüfung, Berlin) for performing the SAXS measurements.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.7b03026.
Additional characterization of all of the polymers from the main text. (PDF)
Author Present Address
† Institute of Polymer Science and Technology (ICTP-CSIC), Juan de la Cierva, 3, 28006 Madrid, Spain.
Author Contributions
All authors contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Williams C. K.; Hillmyer M. A. Polymers from Renewable Resources: A Perspective for a Special Issue of Polymer Reviews. Polym. Rev. 2008, 48 (1), 1–10. 10.1080/15583720701834133. [DOI] [Google Scholar]
- Bozell J. J. Feedstocks for the Future – Biorefinery Production of Chemicals from Renewable Carbon. Clean: Soil, Air, Water 2008, 36 (8), 641–647. 10.1002/clen.200800100. [DOI] [Google Scholar]
- Adekunle K. F. A Review of Vegetable Oil-Based Polymers: Synthesis and Applications. Open J. Polym. Chem. 2015, 05 (03), 34–40. 10.4236/ojpchem.2015.53004. [DOI] [Google Scholar]
- Jain J. P.; Sokolsky M.; Kumar N.; Domb A. J. Fatty Acid Based Biodegradable Polymer. Polym. Rev. 2008, 48 (1), 156–191. 10.1080/15583720701834232. [DOI] [Google Scholar]
- Miao S.; Wang P.; Su Z.; Zhang S. Vegetable-Oil-Based Polymers as Future Polymeric Biomaterials. Acta Biomater. 2014, 10 (4), 1692–1704. 10.1016/j.actbio.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Xia Y.; Larock R. C. Vegetable Oil-Based Polymeric Materials: Synthesis, Properties and Applications. Green Chem. 2010, 12 (11), 1893–1909. 10.1039/c0gc00264j. [DOI] [Google Scholar]
- Meier M. A.; Metzger J. O.; Schubert U. S. Plant Oil Renewable Resources as Green Alternatives in Polymer Science. Chem. Soc. Rev. 2007, 36 (11), 1788–1802. 10.1039/b703294c. [DOI] [PubMed] [Google Scholar]
- Maisonneuve L.; Lebarbe T.; Grau E.; Cramail H. Structure-Properties Relationship of Fatty Acid-Based Thermoplastics as Synthetic Polymer Mimics. Polym. Chem. 2013, 4 (22), 5472–5517. 10.1039/c3py00791j. [DOI] [Google Scholar]
- Cordier P.; Tournilhac F.; Soulie-Ziakovic C.; Leibler L. Self-Healing and Thermoreversible Rubber from Supramolecular Assembly. Nature 2008, 451 (7181), 977–980. 10.1038/nature06669. [DOI] [PubMed] [Google Scholar]
- Aboudzadeh A.; Fernandez M.; Muñoz M. E.; Santamaría A.; Mecerreyes D. Ionic Supramolecular Networks Fully Based on Chemicals Coming from Renewable Sources. Macromol. Rapid Commun. 2014, 35 (4), 460–465. 10.1002/marc.201300732. [DOI] [PubMed] [Google Scholar]
- Lutz A.; van den Berg O.; Van Damme J.; Verheyen K.; Bauters E.; De Graeve I.; Du Prez F. E.; Terryn H. A Shape-Recovery Polymer Coating for the Corrosion Protection of Metallic Surfaces. ACS Appl. Mater. Interfaces 2015, 7 (1), 175–183. 10.1021/am505621x. [DOI] [PubMed] [Google Scholar]
- Hablot E.; Donnio B.; Bouquey M.; Avérous L. Dimer Acid-Based Thermoplastic Bio-Polyamides: Reaction Kinetics, Properties and Structure. Polymer 2010, 51 (25), 5895–5902. 10.1016/j.polymer.2010.10.026. [DOI] [Google Scholar]
- van Velthoven J. L. J.; Gootjes L.; Noordover B. A. J.; Meuldijk J. Bio-Based, Amorphous Polyamides with Tunable Thermal Properties. Eur. Polym. J. 2015, 66, 57–66. 10.1016/j.eurpolymj.2015.01.040. [DOI] [Google Scholar]
- Boumbimba R. M.; Wang K.; Hablot E.; Bahlouli N.; Ahzi S.; Avérous L. Renewable Biocomposites Based on Cellulose Fibers and Dimer Fatty Acid Polyamide: Experiments and Modeling of the Stress–Strain Behavior. Polym. Eng. Sci. 2017, 57 (1), 95–104. 10.1002/pen.24390. [DOI] [Google Scholar]
- Reulier M.; Boumbimba R. M.; Walsh Korb Z.; Vaudemont R.; Avérous L. Thermomechanical and Cyclic Behavior of Biocomposites Based on Renewable Thermoplastics from Dimer Fatty Acids. J. Appl. Polym. Sci. 2017, 134 (12), 44610. 10.1002/app.44610. [DOI] [Google Scholar]
- Susa A.; Bose R. K.; Grande A. M.; van der Zwaag S.; Garcia S. J. Effect of the Dianhydride/Branched Diamine Ratio on the Architecture and Room Temperature Healing Behavior of Polyetherimides. ACS Appl. Mater. Interfaces 2016, 8 (49), 34068–34079. 10.1021/acsami.6b10433. [DOI] [PubMed] [Google Scholar]
- van der Kooij H. M.; Susa A.; García S. J.; van der Zwaag S.; Sprakel J. Imaging the Molecular Motions of Autonomous Repair in a Self-Healing Polymer. Adv. Mater. 2017, 29 (26), 1701017. 10.1002/adma.201701017. [DOI] [PubMed] [Google Scholar]
- Ando S.; Matsuura T.; Sasaki S. Coloration of Aromatic Polyimides and Electronic Properties of Their Source Materials. Polym. J. 1997, 29 (1), 69–76. 10.1295/polymj.29.69. [DOI] [Google Scholar]
- Ayala D.; Lozano A. E.; De Abajo J.; De La Campa J. G. Synthesis and Characterization of Novel Polyimides with Bulky Pendant Groups. J. Polym. Sci., Part A: Polym. Chem. 1999, 37, 805–814. . [DOI] [Google Scholar]
- Hegde M.; Shahid S.; Norder B.; Dingemans T. J.; Nijmeijer K. Gas Transport in Metal Organic Framework–Polyetherimide Mixed Matrix Membranes: The Role of the Polyetherimide Backbone Structure. Polymer 2015, 81, 87–98. 10.1016/j.polymer.2015.11.002. [DOI] [Google Scholar]
- Ogieglo W.; Madzarevic Z. P.; Raaijmakers M. J. T.; Dingemans T. J.; Benes N. E. High-Pressure Sorption of Carbon Dioxide and Methane in All-Aromatic Poly(etherimide)-Based Membranes. J. Polym. Sci., Part B: Polym. Phys. 2016, 54 (10), 986–993. 10.1002/polb.24001. [DOI] [Google Scholar]
- Ragosta G.; Abbate M.; Musto P.; Scarinzi G. Effect of the Chemical Structure of Aromatic Polyimides on Their Thermal Aging, Relaxation Behavior and Mechanical Properties. J. Mater. Sci. 2012, 47 (6), 2637–2647. 10.1007/s10853-011-6089-0. [DOI] [Google Scholar]
- Li F.; Ge J. J.; Honigfort P. S.; Fang S.; Chen J.-C.; Harris F. W.; Cheng S. Z. D. Dianhydride Architectural Effects on the Relaxation Behaviors and Thermal and Optical Properties of Organo-Soluble Aromatic Polyimide Films. Polymer 1999, 40 (18), 4987–5002. 10.1016/S0032-3861(98)00721-6. [DOI] [Google Scholar]
- McCreight K. W.; Ge J. J.; Guo M.; Mann I.; Li F.; Shen Z.; Jin X.; Harris F. W.; Cheng S. Z. D. Phase Structures and Transition Behaviors in Polymers Containing Rigid Rodlike Backbones with Flexible Side Chains. V. Methylene Side-Chain Effects on Structure and Molecular Motion in a Series of Polyimides. J. Polym. Sci., Part B: Polym. Phys. 1999, 37 (14), 1633–1646. . [DOI] [Google Scholar]
- Wood-Adams P.; Costeux S. Thermorheological Behavior of Polyethylene: Effects of Microstructure and Long Chain Branching. Macromolecules 2001, 34 (18), 6281–6290. 10.1021/ma0017034. [DOI] [Google Scholar]
- Ghosh A.; Mistri E. A.; Banerjee S.. Fluorinated Polyimides: Synthesis, Properties, and Applications. In Handbook of Specialty Fluorinated Polymers; Banerjee S., Ed.; William Andrew Publishing: Amsterdam, 2015; pp 97–185. [Google Scholar]
- Acar H. Y.; Ostrowski C.; Mathias L. J.. Investigation of Structure-Property Relationships in Aromatic Polyimides and Polyamides. In Polyimides and Other High Temperature Polymers: Synthesis, Characterization and Applications; Mittal K. L., Ed.; VSP: Zeist, 2001; Vol. 1, pp 3–18. [Google Scholar]
- Wakita J.; Sekino H.; Sakai K.; Urano Y.; Ando S. Molecular Design, Synthesis, and Properties of Highly Fluorescent Polyimides. J. Phys. Chem. B 2009, 113 (46), 15212–15224. 10.1021/jp9072922. [DOI] [PubMed] [Google Scholar]
- Szczepanski C. R.; Pfeifer C. S.; Stansbury J. W. A New Approach to Network Heterogeneity: Polymerization Induced Phase Separation in Photo-Initiated, Free-Radical Methacrylic Systems. Polymer 2012, 53 (21), 4694–4701. 10.1016/j.polymer.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-f.; Akiyama S. Enhancement of Damping Performance of Polymers by Functional Small Molecules. Chin. J. Polym. Sci. 2002, 20 (2), 119–127. [Google Scholar]
- Angell C. A. Relaxation in Liquids, Polymers and Plastic Crystals — Strong/Fragile Patterns and Problems. J. Non-Cryst. Solids 1991, 131, 13–31. 10.1016/0022-3093(91)90266-9. [DOI] [Google Scholar]
- Schönhals A.; Schlosser E. Dielectric Relaxation in Polymeric Solids Part 1. A New Model for the Interpretation of the Shape of the Dielectric Relaxation Function. Colloid Polym. Sci. 1989, 267 (2), 125–132. 10.1007/BF01410350. [DOI] [Google Scholar]
- Schönhals A.Molecular Dynamics in Polymer Model Systems. In Broadband Dielectric Spectroscopy; Kremer F., Schönhals A., Eds.; Springer-Verlag: Berlin, 2003; pp 248–251. [Google Scholar]
- Luo M.-C.; Zhang X.-K.; Zeng J.; Gao X.-X.; Huang G.-S. Enhanced Relaxation Behavior below Glass Transition Temperature in Diene Elastomer with Heterogeneous Physical Network. Polymer 2016, 91, 81–88. 10.1016/j.polymer.2016.03.083. [DOI] [Google Scholar]
- Hasegawa M.; Horie K. Photophysics, Photochemistry, and Optical Properties of Polyimides. Prog. Polym. Sci. 2001, 26 (2), 259–335. 10.1016/S0079-6700(00)00042-3. [DOI] [Google Scholar]
- Tang H.; Feng H.; Luo H.; Dong L.; Feng Z. The Aggregation State of Polyimide. Eur. Polym. J. 1997, 33 (4), 519–523. 10.1016/S0014-3057(96)00210-8. [DOI] [Google Scholar]
- Wachsman E. D.; Frank C. W. Effect of Cure History on the Morphology of Polyimide: Fluorescence Spectroscopy as a Method for Determining the Degree of Cure. Polymer 1988, 29 (7), 1191–1197. 10.1016/0032-3861(88)90043-2. [DOI] [Google Scholar]
- Hasegawa M.; Kochi M.; Mita I.; Yokota R. Molecular Aggregation and Fluorescence Spectra of Aromatic Polyimides. Eur. Polym. J. 1989, 25 (4), 349–354. 10.1016/0014-3057(89)90148-1. [DOI] [Google Scholar]
- Hasegawa M.; Mita I.; Kochi M.; Yokota R. Charge-Transfer Emission Spectra of Aromatic Polyimides. J. Polym. Sci., Part C: Polym. Lett. 1989, 27 (8), 263–269. 10.1002/pol.1989.140270804. [DOI] [Google Scholar]
- García M. G.; Marchese J.; Ochoa N. A. Aliphatic–Aromatic Polyimide Blends for H2 Separation. Int. J. Hydrogen Energy 2010, 35 (17), 8983–8992. 10.1016/j.ijhydene.2010.06.038. [DOI] [Google Scholar]
- Luo L.; Yao J.; Wang X.; Li K.; Huang J.; Li B.; Wang H.; feng Y.; Liu X. The Evolution of Macromolecular Packing and Sudden Crystallization in Rigid-Rod Polyimide via Effect of Multiple H-Bonding on Charge Transfer (CT) Interactions. Polymer 2014, 55 (16), 4258–4269. 10.1016/j.polymer.2014.06.080. [DOI] [Google Scholar]
- Yokoyama H. Small Angle X-ray Scattering Studies of Nanocellular and Nanoporous Structures. Polym. J. 2013, 45 (1), 3–9. 10.1038/pj.2012.205. [DOI] [Google Scholar]
- Prosa T. J.; Winokur M. J.; McCullough R. D. Evidence of a Novel Side Chain Structure in Regioregular Poly(3-alkylthiophenes). Macromolecules 1996, 29 (10), 3654–3656. 10.1021/ma951510u. [DOI] [Google Scholar]
- Pankaj S.; Beiner M. Confined Dynamics and Crystallization in Self-Assembled Alkyl Nanodomains. J. Phys. Chem. B 2010, 114 (47), 15459–15465. 10.1021/jp1072999. [DOI] [PubMed] [Google Scholar]
- Beiner M.; Huth H. Nanophase Separation and Hindered Glass Transition in Side-Chain Polymers. Nat. Mater. 2003, 2 (9), 595–599. 10.1038/nmat966. [DOI] [PubMed] [Google Scholar]
- Arnold F. E.; Bruno K. R.; Shen D.; Eashoo M.; Lee C. J.; Harris F. W.; Cheng S. Z. D. The Origin of β Relaxations in Segmented Rigid-Rod Polyimide and Copolyimide Films. Polym. Eng. Sci. 1993, 33 (21), 1373–1380. 10.1002/pen.760332102. [DOI] [Google Scholar]
- Dechter J. J.; Axelson D. E.; Dekmezian A.; Glotin M.; Mandelkern L. An Analysis of the β Transition of Linear and Branched Polyethylenes by Carbon-13 NMR. J. Polym. Sci., Polym. Phys. Ed. 1982, 20 (4), 641–650. 10.1002/pol.1982.180200407. [DOI] [Google Scholar]
- Yu X.; Liang W.; Cao J.; Wu D. Mixed Rigid and Flexible Component Design for High-Performance Polyimide Films. Polymers 2017, 9 (9), 451. 10.3390/polym9090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.