Using new enabling technologies, we document behavioral patterns and susceptibility to light pollution never previously seen.
Abstract
Light is a major cue for nearly all life on Earth. However, most of our knowledge concerning the importance of light is based on organisms’ response to light during daytime, including the dusk and dawn phase. When it is dark, light is most often considered as pollution, with increasing appreciation of its negative ecological effects. Using an Autonomous Surface Vehicle fitted with a hyperspectral irradiance sensor and an acoustic profiler, we detected and quantified the behavior of zooplankton in an unpolluted light environment in the high Arctic polar night and compared the results with that from a light-polluted environment close to our research vessels. First, in environments free of light pollution, the zooplankton community is intimately connected to the ambient light regime and performs synchronized diel vertical migrations in the upper 30 m despite the sun never rising above the horizon. Second, the vast majority of the pelagic community exhibits a strong light-escape response in the presence of artificial light, observed down to 100 m. We conclude that artificial light from traditional sampling platforms affects the zooplankton community to a degree where it is impossible to examine its abundance and natural rhythms within the upper 100 m. This study underscores the need to adjust sampling platforms, particularly in dim-light conditions, to capture relevant physical and biological data for ecological studies. It also highlights a previously unchartered susceptibility to light pollution in a region destined to see significant changes in light climate due to a reduced ice cover and an increased anthropogenic activity.
INTRODUCTION
At any given moment in time, half Earth’s surface is in the dark. Although darkness prevails, processes induced by solar illumination are considered to be inactive. When it is dark, light is often considered as pollution that potentially affects light-controlled rhythms and behavior. However, darkness is a relative state, and even small natural changes in ambient solar and lunar light may have an effect on marine organisms (1, 2). Light-induced responses on animal behavior during dark or dim conditions have been documented for a variety of environments and organisms. For instance, lunar illumination affects zooplankton and micronekton, as well as their predators in tropical, subtropical, and Arctic waters (1, 3–6). In addition, changes in ambient light can affect the vertical distribution of deep scattering layers down to 1000 m in deep-sea environments (7). Despite an increased awareness that small changes in natural light affect the behavior of marine organisms in naturally dim environments, we are only starting to understand how and why organisms respond to changes in light that occur on scales below what most commercial sensors can detect (1, 2, 8, 9).
Artificial light pollution is widespread in the marine environment, with increased shipping and light fishing that introduce a substantial source of disturbance in an otherwise dim environment (8). However, the effects and behavioral responses to this light pollution are still poorly understood (8), not the least in the marine Arctic characterized by constant daylight during summer and prevailing darkness during the polar night. Toward the poles, the period of total winter darkness (and summer midnight sun) increases gradually (Fig. 1) (1, 2). At its extreme, the entire year is simply one long day and one long night—each 6 months in duration at the North Pole. However, although illumination at summer solstice does not change radically with latitude, the polar night not only becomes longer but also crucially darker along a latitudinal gradient (Fig. 1). At the same time, and because of the fact that sea ice reduces light penetration into the underlying water by up to 99%, the region destined to see the greatest relative change in its light climate following both a retreat of the ice cover and increased human activities is the central Arctic Ocean during the polar night (Fig. 1). In this marine environment, increased shipping and anthropogenic presence may introduce a substantial source of disturbance in an otherwise dim environment. Because biological responses to small variations in both solar and lunar illumination have been documented (1, 9, 10), we hypothesize that the susceptibility to light pollution is at its extreme in this region.
Fig. 1. The light-climate regions of the Arctic.
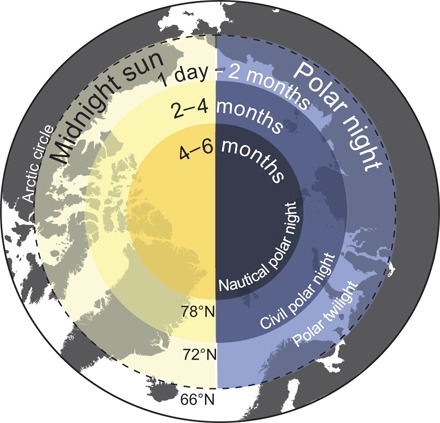
Circles of yellow (left) indicate duration of the midnight sun period (3). Circles of blue (right) indicate duration of the polar night period with illumination at winter solstice defined as polar twilight (outer), civil polar night (middle), and nautical polar night (inner).
Among biological responses are diel vertical migrations (DVMs) or cyclic patterns of vertical movement synchronized with variations in irradiance that are commonly observed in zooplankton (11, 12) and fish (13, 14) across all aquatic habitats. DVM is triggered by light (15) but ultimately driven by predator-prey interactions (16) and the need to avoid depths with light intensities sufficient for visual predators. On acoustic echograms, a DVM pattern is seen as one or more sound scattering layers (SSLs) migrating vertically according to a diel cycle in illumination (16). At high latitudes, DVMs have been considered to be less prominent (17, 18), because the absence of day/night cycles during extended periods of either midnight sun or polar night leaves organisms without temporal refuge. However, recent studies have documented that zooplankton, particularly krill, carry out DVM during the polar night and in particular during periods of civil twilight (solar altitude not exceeding 0° to −6°) (19, 20). Although preliminary studies also reported DVM during nautical polar night (solar altitude not exceeding −6° to −12°) (19), and in response to lunar light (1), no study has unequivocally described DVM in response to the diel solar irradiance cycle during nautical polar night. We hypothesize that this is partly due to research vessels introducing an artificial light field, biasing measurements of both natural ambient light and light-dependent ecological processes (10, 21–25). If present, then DVM during the darkest period of the polar night is likely restricted to surface layers and temporally centered on the very short period of elevated light intensity at solar noon due to small variations in ambient light that can only be detected by organisms near the surface (9). Other sampling issues apart from light pollution may bias DVM detections under low solar irradiance conditions. Towed zooplankton nets generally have a coarse vertical sampling resolution (>10 m), whereas bottom-moored and hull-mounted echo sounders generally fail to provide data from the upper 5 to 15 m of the water column due to the conical shape of the acoustic beam, strong reflection at the water-air interface, and/or to the ship’s draft that adds to the near-field region (26). Thus, using these traditional sampling methods during dark periods, such as the Arctic polar night, we potentially miss crucial processes occurring in the upper water column.
Autonomous Surface Vehicles (ASVs) provide new opportunities for marine studies (27). These vehicles do not require facilities to provide an operator or helmsman with working light, safety, and comfort, which results in smaller and more efficient platforms without artificial light, particularly beneficial in the Arctic where heavy logistics is necessary for human presence and security. The automated behavior of these systems not only provides increased accuracy and repeatability of maneuvering during data collection over larger temporal and spatial scales (28) but also offers a unique opportunity to autonomously sample in an undisturbed environment without introducing artificial light (29), an aspect critical to this study.
Here, we test the use of the Jetyak, a purpose-built ASV fitted with a multifrequency echo sounder and a spectroradiometer to study the distribution and potential DVM of zooplankton during the polar night in Kongsfjorden, a high Arctic fjord on Svalbard (fig. S1). By comparing the data provided from the ASV with those from manned research vessels, we also investigate the impact of artificial light on the pelagic community and the ability of traditional sampling platforms to provide data that represent the natural state of the ecosystem in the upper 50 to 100 m of the water column during dark periods.
RESULTS
Variations in diffuse skylight irradiance
During the Jetyak deployments over three consecutive days (21 to 23 January 2016), the sun remained below the horizon with inclination angles at solar noon of −8.9°, −8.7°, and −8.4°, respectively. The waxing moon (full on 24 January) was above the horizon all day at inclination angles of 6° to 10° during the times of deployment. During the 21 January deployment, scattered clouds resulted in diffuse skylight irradiance (Ed) at the water’s surface initially decreasing early in the deployment (minimum at ~0.012 μW m−2 or 5.5 × 10−9 μmol photons m−2 s−1) before increasing around the time of solar noon (1123 GMT) (maximum at ~0.017 μW m−2 or 8.0 × 10−9 μmol photons m−2 s−1) followed by decreasing irradiance at the end of the deployment (fig. S2). Longer deployments on 22 and 23 January provided a more complete measurement of the solar cycle with increasing and decreasing Ed about solar noon (1124 GMT) (Fig. 2A and fig. S2). Minimum irradiances for deployments on 22 and 23 January were 0.0031 and 0.0017 μW m−2 (1.6 × 10−8 and 8.8 × 10−9 μmol photons m−2 s−1), respectively. Maximum irradiances were 0.0091 and 0.0084 μW m−2 (4.9 × 10−8 and 4.6 × 10−8 μmol photons m−2 s−1).
Fig. 2. Volume backscattering (Sv) echograms.
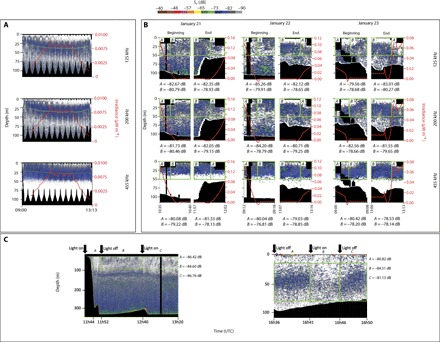
(A) Acoustic Zooplankton Fish Profiler (AZFP; ASL Environmental Science Inc.) echograms at 125 (dominated by euphausiids), 200 (dominated by chaetognaths), and 455 kHz (dominated by copepods) on 23 January. The red line (and second y axis) indicates the irradiance (μW m−2) when away from the ship. The green line indicates the median depth of the SSL at each frequency. Note that a time-varied threshold of −122 dB was applied at 455 kHz. (B) AZFP echograms at 125, 200, and 455 kHz at the beginning and at the end of each survey. The red line indicates the irradiance, and the green boxes indicate acoustic backscatter data collected in the top 50 m (A) close to the ship and (B) away from the ship. Mean Sv within each box is indicated below the echograms. (C) Responses of zooplankton to ambient versus artificial light. The RV Helmer Hanssen EK60 echogram was recorded at 120 kHz in Kongsfjorden on 9 January 2017 (left) and the AZFP echogram at 125 kHz was recorded from a small boat in Kongsfjorden on 21 January 2017 (right). Black arrows indicate when the lights were turned off and on, and mean Sv in the green boxes (calculated in the linear form) for each period is indicated by the echograms. Note that the sequence of artificial light on/off is reversed in the left and right panels, and the SSL responds accordingly with avoidance during the “Light on” period. In all echograms, black areas mask the near-field region, noise, and areas below the seafloor.
SSLs and DVMs
An SSL was present in the top 50 m during all three deployments (fig. S2). The SSL was slightly deeper in shallow than in deep areas, which explains the bottom-related oscillation within the echograms, as the Jetyak was cruising back and forth from deep to shallow areas (fig. S2). The median depth of the SSL significantly increased with irradiance (Spearman’s rank correlation; P ≤ 0.001; Fig. 2A and fig. S2). This correlation indicates the occurrence of a small-scale (6 to 8 m) light-triggered DVM within the top 50 m during the polar night. The acoustic backscatter was stronger at 455 kHz than at 200 and 125 kHz for 58% of the echo integration cells within this SSL on 21 January, 60% on 22 January, and 67% on 23 January, indicating that copepods dominated the assemblage (30). Copepods, mainly Calanus spp., Pseudocalanus spp., and Oithona similis, also dominated the zooplankton assemblage from two multinet (180-μm mesh size) deployments in Kongsfjorden during the survey, particularly in the 20- to 0-m strata (fig. S4). The speeds of ascent and descent measured at 455 kHz (that is, the frequency that better detects copepods) varied from 0.06 to 0.72 cm s−1 and from 0.07 to 1.33 cm s−1, respectively (table S1).
Depth limit of Calanus light detection
To independently assess whether the DVM of 6- to 8-m amplitude observed in the acoustics could be in response to changes in the ambient light field at depth, we modeled how diffuse skylight irradiance measured from the Jetyak propagated through the water column. The resulting quantity of light [scalar irradiance, Eo(λ)] was calculated over time at 1-m depth intervals on 23 January. Eo(λ) represents light in all directions and is therefore ecologically relevant to visual systems capable of detecting light from a broad range of angles, as is the case for copepods (31). Furthermore, we weighted Eo(λ) by the light capture capabilities of Calanus spp. copepods, which dominated the assemblage, yielding a measure of light in terms of “Calanus-utilized light” that most closely represents the light available to copepods. Spectral irradiance calculated this way was maximal at 500 nm throughout the measurement period and across depth. According to Båtnes et al. (32), an isolume of 5 × 10−8 μmol photons m−2 s−1 (as Calanus-utilized light) represents a photo-behavioral irradiance threshold for Calanus spp. On 23 January, we calculated this isolume to be located at 51.5-m depth at 9:30, descending to a maximum of 60-m depth at 12:00, and then shoaling to 53-m depth at 13:00 (Fig. 3). Thus, on the basis of the underwater light field and Calanus spp. sensitivity to light, we predict DVM of ~8 m for these copepods during the Jetyak deployments.
Fig. 3. Modeled depth of a constant light level (isolume) set to be the depth limit of copepod light-mediated behavior in Kongsfjorden.

Underwater spectral scalar irradiance [Eo(λ), μmol photons m−2 s−1] was weighted by the spectral response of C. finmarchicus to yield a photometric quantity of Calanus-utilized light that is shown in colored contours as a function of depth at 30-min intervals on the 23 January Jetyak deployment. White horizontal lines show the depth of an isolume at 5 × 10−8 μmol photons m−2 s−1 of Calanus-utilized light. UTC, coordinated universal time.
Artificial light affecting the zooplankton community
Diffuse skylight irradiance increased by 15 times when the Jetyak departed or returned to the research vessel (that is, at the beginning and end of all deployments) (Fig. 2B). Irradiance increased from <0.01 μW m−2 (~5 × 10−9 μmol photons m−2 s−1) away from the RV Helmer Hanssen to over 0.15 μW m−2 (~7 × 10−7 μmol photons m−2 s−1) in its vicinity. The mean volume acoustic backscattering strength (Sv, calculated in the linear form before being transformed in dB re 1 m−1) in the top 50 m was consistently lower close to the ship compared to areas not affected by artificial light sources (Fig. 2B). This impact had a horizontal footprint varying from 22 to 188 m from the boat, depending on the deployment. The ship draft (~7 m) and the near field of the EK60 echo sounder (3.3 m at 120 kHz) resulted in a 10.3-m blind zone below the RV Helmer Hanssen, which prevented the detection of vertical migrations just below the surface (fig. S3). In comparison, the blind zone of the Jetyak-mounted AZFP was ~2 m.
In January 2017, further testing in Kongsfjorden with the RV Helmer Hanssen (Fig. 2C, left) and an 8-m-long Polarcirkel boat (Fig. 2C, right) confirmed that zooplankton in the SSL avoided artificial light and that the effect can reach depths of >80 m. Zooplankters rapidly came back to their normal distribution once the lights were turned off (Fig. 2C).
DISCUSSION
The DVM of zooplankton is the most widespread and synchronized movement of biomass on the planet (33) and thus is one of the most important factors to consider for understanding marine food-web interactions and ecosystem structures (16). In the high Arctic, this behavior has been frequently observed during autumn and spring when the day/night cycle is pronounced [(2, 34) and references therein], whereas a number of studies have failed to find any coordinated vertical migration during periods of continuous light or darkness (18, 35). Despite this, Berge et al. (19) detected a synchronized DVM of zooplankton from two fjords in Svalbard (78° and 80°N), even during the darkest period of the polar night, using moored acoustic instruments. These behaviors have biogeochemical significance. Vertical transport to depth of millions of tons of organic carbon through zooplankton DVM contributes to ocean uptake and sequestration of CO2 and represents an important process for the mitigation of the planetary greenhouse effect causing climate change (30, 36). However, this process is poorly quantified at high latitudes, partly due to a lack of quantitative measurements of polar night DVM (20). Our results are the first to unequivocally show that short-ranged DVMs, with an amplitude of only 8 m, occur under natural light conditions. This DVM is restricted to the upper 20 m of the water column (Fig. 4). Furthermore, our results suggest that artificial light from both a large research vessel and headlamps on a small open vessel introduce light pollution that induce an avoidance response in the pelagic community down to more than 80-m depth. Consequently, any attempt to quantify DVMs and their biogeochemical impact in the upper water column during polar night or any other low solar irradiance condition will be insufficient if traditional sampling methods are used.
Fig. 4. Conceptual model of the behavioral response of zooplankton to natural solar light, light pollution, and lunar light.
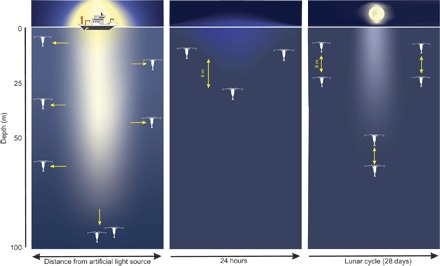
(A) Light escape response (vertical and horizontal arrows) from light pollution from a ship detected down to 100 m and up to 180 m on each side of the ship. (B) DVM of zooplankton in response to natural ambient light during the polar night. Centered around noon, organisms perform a DVM with a amplitude of 8 m within the upper 20 m of the water column. (C) In response to lunar light, zooplankton and fish perform DVM but at different depths depending on the moon phase.
Avoidance of artificial light from ships at night by marine organisms has been previously documented for euphausiids down to 60 m on the Nova Scotia continental shelf (37). Furthermore, artificial light is known to affect the distribution of fish by either attracting or repulsing them (38, 39). In addition, a recent study using data sets from across the Arctic, including Kongsfjorden, documented moonlight effects on the vertical distribution of zooplankton down to around 50 m (1). Here, we demonstrated that most of the zooplankton community avoided artificial light from research vessels during the polar night, biasing measurements of abundance, vertical distribution, and DVM. Copepods dominated the zooplankton assemblage and the acoustic signal, and they were most likely performing DVM under ambient conditions and avoiding artificial light. However, the difference in Sv in the proximity and away from the ship was generally greater at 125 than at 455 kHz (Fig. 2B), indicating that zooplankton larger than copepods also avoided artificial light.
Each time the Jetyak approached the RV Helmer Hanssen, the acoustic signal showed that zooplankton reacted by avoiding the artificial light field of the vessel (that is, the acoustic backscatter decreased) rather than slowly descending as when ambient irradiance was changing gradually. This effect could be seen up to ~190 m away from the ship. Change in light intensity is a well-known cue for initiating zooplankton DVM and other predator-avoidance behavior (15). However, and although our data do not allow for a quantification of swimming speed, the observed response appeared immediate and faster than any previously reported average swimming speeds. The reported swimming speed for euphausiids (for example, Thysanoessa spp.) is in the range of 10 to 45 mm s−1 (40, 41) and 2 to 30 mm s−1 with an escape speed of up to 120 to 400 mm s−1 for Calanus spp. (42, 43). With an acoustic beam angle of 7°, the maximum distance to exit the beam is 1.2 m at 20-m depth, 3.1 m at 50-m depth, and 6.1 m at 100-m depth. Applying a combination of the fastest swimming speeds and escape speeds reported, the escape time for these organisms would range in the order of 0.5 to 2 min, depending on depth. In addition, a synchronized change in orientation relative to the light and acoustic beam could explain part of the reduction in observed Sv values. Further surveys should focus on identifying the main functional groups reacting to artificial light; however, we conclude that the observed and sudden reduction in Sv values following the introduction of artificial light (Fig. 2) could plausibly be explained by the reported swimming and escape speeds of Calanus spp. and Thysanoessa spp. We also suggest that the sudden and sustained increase in irradiance in an otherwise dim environment caused by the bright artificial illumination of the ship, as well as the seemingly insignificant illumination caused by researcher headlamps on an open boat (Fig. 2C), resulted in zooplankters rapidly moving down and/or laterally to return to their ambient illumination conditions (Fig. 4A).
Using an ASV capable of both measuring ambient light in a natural environment and taking acoustic profiles of zooplankton in the water column, we provide evidence of DVM synchronized with background illumination of the sun even during the polar night, when the sun remains over 8° below the horizon. The low-amplitude (6 to 8 m) DVM behavior included a slow but significant descent centered around solar noon within the upper 30 m of the water column (Fig. 4B). This is in line with the results presented by Last et al. (1) who reported a mass escape from the upper 50 m of the water column during periods of full moon during the polar night (Fig. 4C). The vertical distribution of biomass follows the change in ambient irradiance and is hence indicative of the proximate role of light as a cue for vertical movement of zooplankton in the SSL in the middle of the polar night. In particular, the observed magnitude of SSL migration matches the ~8-m shift in the depth of the modeled isolume over the 23 January deployment (Fig. 3). Although the lower SSL limit is near the isolume depth, the discrepancy between median SSL depth at 455 kHz (15 to 33 m) and isolume depth (51.5 to 60 m) is likely due in part to the choice of ambient irradiance that we tracked (5 × 10−8 μmol photons m−2 s−1 of Calanus-utilized light). This value approximates the absolute photo-behavioral threshold of Calanus spp. (32), and it is probable that the median photosensitivity for copepods in SSLs is not that low. Although copepods dominated the assemblage within the SSL, a slight difference (<10 m) between the median depth of the SSL at 125, 200, and 455 kHz (Fig. 2A and fig. S2) suggests that other functional groups also conducted DVM, which could partly explain the discrepancy between median SSL depth and isolume depth. Polar night DVMs have previously been reported on the basis of moored acoustic instruments (19, 30); however, it has never been possible to quantitatively sample nor detect this behavior using traditional nets or ship-borne acoustics (20). In light of the data presented herein, this is likely due to the avoidance of zooplankton in the upper 100 m of the water column when exposed to artificial light from research vessels (Fig. 4A). The short vertical extent of the DVM behavior also provides an explanation as to why polar night DVM has often remained undetected by moored acoustic surveys (34), and on the basis of the reported observations herein, we postulate that the vertical bin size used in previous acoustic analyses (generally 4 m) has usually been too deep to detect such a pattern.
Recognition of artificial light effects on DVM is not new (44); however, our work highlights a particular vulnerability for zooplankton in the Arctic during the polar night period, which extends up to 6 months. Artificial light is poised to increase in this region with sea ice loss facilitating new shipping routes and opportunities for oil and gas exploration and production, activities that lead to light pollution and cascading ecological consequences (23, 45). Light transmits well in the optically clear water before the spring bloom (9, 46), with the potential to affect organisms far deeper than in more turbid water. This combination of high susceptibility to light pollution and the predicted increased anthropogenic activity in high Arctic seas has the potential to severely affect natural rhythms and processes at a local scale.
Our results suggest that ship-based acoustic measurements or net sampling conducted under dim-light conditions result in abundance estimates lower than reality. Thus, studies of light-dependent behaviors in organisms during polar night or at night in optically clear water need to be devoid of any artificial light to ensure ecologically sound measurements. Together, these findings suggest that certain aspects of the polar marine ecosystems are extremely sensitive to potential light pollution and that traditional sampling techniques are insufficient to study them. Although this study was carried out in the high Arctic and during the polar night, similar effects are to be expected for nighttime processes in other parts of the globe. ASVs, such as the Jetyak, could represent better instrument-carrying platforms than research vessels and small manned boats for detecting vertical migrations of zooplankton and nekton near the surface under dim-light conditions due to (i) a smaller acoustic blind zone and (ii) the absence of artificial light that results in ship avoidance of the animals. If larger vessels are used, then artificial light sources from the ship need to be turned off when sampling zooplankton at night. In any case, biological sampling during the polar night needs to be reshaped around small-scale migrations and strong light avoidance behavior.
MATERIALS AND METHODS
The ASV Jetyak
The Jetyak ASV was first developed by the Woods Hole Oceanographic Institution based on a commercially available polyethylene single-person kayak (47). The vessel’s shape was similar to that of a river kayak, but it was fitted with a petrol engine driving a water jet unit at the aft. The vehicle was 3 m long and 0.9 m wide, weighed 160 kg, and had an operational range of 8 hours at a speed of 7 to 11 knots. The onboard control system allowed the vehicle to follow preprogrammed transect lines or to operate in a remotely controlled mode. Batteries provided power for the control systems as well as navigation and scientific instruments. A radio frequency modem provided low-bandwidth communication for the Jetyak at ranges up to 20 km.
Study area and design
The Jetyak was deployed three consecutive days in Kongsfjorden in 2016, on the west coast of the Svalbard archipelago, and a total of 54.7 km of transects was ran (Table 1). Kongsfjorden is 26 km long, and the inlet was approximately 10 km wide, with the deepest point at approximately 370 m depth (fig. S1). Ice floes and growlers from the glaciers Kronebreen and Kongsbreen were encountered during the survey. The Jetyak was deployed from the RV Helmer Hanssen and programmed to patrol a predefined transect while continuously measuring diffuse atmospheric light intensities and acoustic backscatter from zooplankton before and after solar noon. Manual remote control was used during the launch and recovery. On the basis of the results obtained in 2016, acoustic measurements with and without light were conducted from RV Helmer Hanssen and from an 8-m-long Polarcirkel boat in Kongsfjorden in January 2017 to test the potential light avoidance by the zooplankton community.
Table 1. Details of the ASV deployments.
Time is local time (GMT + 1 hour).
| Date | Number of transects | Duration | Start time | End time | Platform | Distance (km) |
| 21 January 2016 | 2 | 00:47 | 10:42 | 11:29 | ASV Jetyak | 4.5 |
| 22 January 2016 | 21 | 03:30 | 09:22 | 12:52 | ASV Jetyak | 23.9 |
| 23 January 2016 | 23 | 03:45 | 09:10 | 12:55 | ASV Jetyak | 26.2 |
| 09 January 2017 | 1 | 01:36 | 12:44 | 14:20 | Helmer Hanssen | 0 |
| 21 January 2017 | 1 | 00:14 | 17:36 | 17:50 | Polarcirkel | 0 |
| Sum | 09:50 | 54.7 |
Sampling and processing of hydroacoustic data
A downward-looking AZFP was mounted in the sea chest of the Jetyak and recorded hydroacoustic data at 125, 200, 455, and 769 kHz during transects. Because the range of the 769-kHz transducer is limited to a few meters below the instrument, only data from the three lower frequencies were considered in this study. Vertical spatial resolution was 1 m, pulse duration was 300 μs, source level was 210 dB (reference 1 μPa at 1 m), and ping rate was 1 ping 2 s−1 (0.5 Hz). The AZFP was calibrated by the manufacturer (±1 dB) before deployment. In parallel, acoustic data were continuously recorded from a ship-based multifrequency (18, 38, and 120 kHz) Simrad EK60 echo sounder calibrated before departure and located approximately 1 to 2 km from the Jetyak throughout its missions.
Acoustic data were processed with EchoView 6.1. Noise and the near-field region (48) were excluded from the analysis. Conductivity-temperature-depth (CTD) profiles provided the average sound speed and coefficient of absorptions. A time-varied threshold ranging from −118 to −122 dB at 1 m was added to the 455-kHz echogram to offset noise amplification at depth by the time varied gain (49). The echograms were divided into 1-m-vertical by 1-min-horizontal echo-integration cells, and the mean volume acoustic backscattering strength (Sv in decibel reference 1 m−1) within each cell was exported at each frequency. For each cell, the mean Sv at each frequency was compared to determine the dominant scattering zooplankton group. Following the model described in Darnis et al. (30) for Kongsfjorden, cells with Sv125kHz > Sv200kHz < Sv455kHz were assumed to be dominated by euphausiids, cells with Sv125kHz < Sv200kHz < Sv455kHz by copepods, and cells with Sv125kHz < Sv200kHz > Sv455kHz by chaetognaths. To eliminate the effects of bathymetry on the depth of the SSL, the echograms were divided into deep (that is, in the middle of transects) and shallow (that is, at the end of transects) echograms (fig. S2). For each of these echograms, the depths of the top and bottom edges of the SSL at each frequency were identified by the −82 dB backscatter contour, and the median depth of the SSL was exported. The median SSL depths from the deep and shallow echograms were then averaged to obtain the median depth of the whole SSL. To verify the potential effects of artificial light from the ship on the abundance of acoustic scatterers during each deployment, the mean Sv within the SSL when the Jetyak was near the ship was compared to the mean Sv away from the vessel, that is, the zone with ambient polar night light conditions not affected by light pollution from human activity.
Sampling and processing of zooplankton net samples
Zooplankton was sampled by vertical hauls (towing speed, 0.5 m s−1) from close to the seafloor to the surface using a multiple opening/closing net (Multinet, Hydrobios; mouth opening, 0.25 m2; mesh size, 180 μm). Five depth strata (320, 200, 100, 50, 20, and 0 m) were sampled in mid-fjord (78°57ʹN, 11°57ʹE; bottom depth, 340 m) in January 2016 and January 2017, and three depth strata (60, 50, 20, and 0 m) were sampled in the inner fjord in January 2016 (78°53ʹN, 12°26ʹE; bottom depth, 80 m). Samples were preserved in a 4% formaldehyde-in-seawater solution and later analyzed under a Leica stereomicroscope. Samples were examined by subsampling with aliquots obtained with 5-ml automatic pipette, with the pipette tip cut at a diameter of 5-mm to allow free collection of mesozooplankton. Large (total length, >5 mm) organisms were removed before taking subsamples and identified and counted. The number of subsamples analyzed was chosen so that at least 100 individual of Calanus and 300 other copepods were counted. Samples with low abundance were examined in their entirety.
Sampling and processing of irradiance data
Diffuse sky spectral irradiance, Eλ at 0.4 nm spectral resolution, was measured from the Jetyak synchronously with hydroacoustics. Spectral irradiance was captured by a QE Pro fiber optic spectrometer (Ocean Optics) calibrated for absolute irradiance measurement with a 200-μm entrance slit and 1000-μm optical fiber (9). The spectrometer was held within the hull compartment of the Jetyak in a watertight insulated box, with its internal charge-coupled device array detector thermoelectrically cooled by Peltier element to reduce noise (dark current). A spectrally neutral (350 to 730 nm) Spectralon reflectance standard plate (SRT-99-050, Labsphere) was mounted to a mast on the Jetyak and faced upward collecting light, which was sampled by the optical fiber attached to spectrometer. In this way, the plate collected and reflected 99% of diffuse skylight at each wavelength. The spectrometer’s optical fiber was positioned on an articulating arm clamped to the mast and pointed downward at ~45° such that the fiber’s field of view contained only reflected skylight from plate. Spectra from 350 to 730 nm were captured at 10-s intervals, corresponding to the integration time of the instrument. In this configuration, the acceptable detection limit for the spectrometer was ~1 × 10−7 μW m−2 nm−1 across the calibrated wavelength range (~4 × 10−10 μmol photons m−2 s−1 as EPAR). For irradiance time series to compare to hydroacoustic data, spectral irradiance at each time point (μW m−2 nm−1) was integrated (350 to 730 nm; μW m−2) and smoothed with a 5-min Savitzky-Golay filter in Matlab.
Light modeling
To determine whether the movement of SSLs captured by the AZFP was consistent with changes in isolume depths that would be expected if light were serving as a cue for migrating zooplankton (15), we used the radiative transfer model HydroLight 5.2 (41) to characterize the underwater light field for one of our transects (23 January from 9:30 to 13:00) with model runs at 30-min intervals. Light input to the model was diffuse downwelling atmospheric spectral irradiance measured from the Jetyak, with additional inclusion of Raman scattering, chlorophyll a fluorescence (emitted light) at 0.06 μg liter−1 over the whole water column, and spectral coefficients for absorption, scattering, and beam attenuation (50) collected near that location in Kongsfjorden in January 2015 [additional parameter and model details are described in the study of Cohen et al. (9)]. Because the genus Calanus spp. is among the most abundant copepods in Kongsfjorden in January and contributes to the SSL [(20); this study], we weighted the underwater spectral scalar irradiance generated from the model [Eo (λ); 360 to 720 nm at 10-nm resolution; μmol photons m−2 s−1 nm−1] by the spectral response of Calanus finmarchicus photo-behavior (51). Thus, model runs yielded a photometric quantity of Calanus-utilized light; that is, only the spectral bandwidth that Calanus was capable of detecting (blue-green part of the visible spectrum). Model results were in 1-m-depth bins from just below the surface to 99-m depth, at intervals of 30 min for a 4-hour duration centered on solar noon.
Supplementary Material
Acknowledgments
We thank the students on the University Centre on Svalbard course AB334 “Underwater robotics and polar night biology” for help and efforts during the field campaign, and the crew onboard RV Helmer Hanssen. We also thank the employees of Kings Bay AS for hospitality and help during the campaigns. Funding: The work was co-funded by The Norwegian Research Council through project numbers 195160, 223254, 226417, and 244319. Author contributions: M.L., J.B., M.G., J.H.C., A.J.S., and G.J. planned and executed the work; M.L., J.B., M.G., J.H.C., A.J.S., M.D., and G.J. analyzed the data and wrote the text; P.R.D.L.T., S.M.N., and H.S. provided expertise using the autonomous platform and sensors. Competing interest: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/1/eaap9887/DC1
fig. S1. Map of the study area in Kongsfjorden.
fig. S2. AZFP echograms from ASV.
fig. S3. EK60 echograms from RV Helmer Hanssen.
fig. S4. Vertical distribution of zooplankton.
table S1. Ascent and descent rates of the SSL at 455 kHz in January 2016.
REFERENCES AND NOTES
- 1.Last K. S., Hobbs L., Berge J., Brierley A. S., Cottier F., Moonlight drives ocean-scale mass vertical migration of zooplankton during the Arctic winter. Curr. Biol. 26, 244–251 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Berge J., Renaud P. E., Darnis G., Cottier F., Last K., Gabrielsen T. M., Johnsen G., Seuthe L., Weslawski J. M., Leuc E., Moline M., Nahrgang J., Søreide J. E., Varpe Ø., Lønne O. J., Daase M., Falk-Petersen S., In the dark: A review of ecosystem processes during the Arctic polar night. Prog. Oceanogr. 139, 258–271 (2015). [Google Scholar]
- 3.Hernández-León S., Almeida C., Yebra L., Arístegui J., Lunar cycle of zooplankton biomass in subtropical waters: Biogeochemical implications. J. Plankton Res. 24, 935–939 (2002). [Google Scholar]
- 4.Benoit-Bird K. J., Au W. W. L., Wisdom D. W., Nocturnal light and lunar cycle effects on diel migration of micronekton. Limnol. Oceanogr. 54, 1789–1800 (2009). [Google Scholar]
- 5.Drazen J. C., De Forest L. G., Domokos R., Micronekton abundance and biomass in Hawaiian waters as influenced by seamounts, eddies, and the moon. Deep Sea Res. Part I Oceanogr. Res. Pap. 58, 557–566 (2011). [Google Scholar]
- 6.Rubolini D., Maggini I., Ambrosini R., Imperio S., Paiva V. H., Gaibani G., Saino N., Cecere J. G., The effect of moonlight on Scopoli’s shearwater Calonectris diomedea colony attendance patterns and nocturnal foraging: A test of the foraging efficiency hypothesis. Ethology 121, 284–299 (2015). [Google Scholar]
- 7.Aksnes D. L., Røstad A., Kaartvedt S., Martinez U., Duarte C. M., Irigoien X., Light penetration structures the deep acoustic scattering layers in the global ocean. Sci. Adv. 3, e1602468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies T. W., Duffy J. P., Bennie J., Gaston K. J., The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 12, 347–355 (2014). [Google Scholar]
- 9.Cohen J. H., Berge J., Moline M. A., Sørensen A. J., Last K., Falk-Petersen S., Renaud P. E., Leu E. S., Grenvald J., Cottier F., Cronin H., Menze S., Norgren P., Varpe Ø., Daase M., Darnis G., Johnsen G., Is ambient light during the high Arctic polar night sufficient to act as a visual cue for zooplankton? PLOS ONE 10, e0126247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin H. A., Cohen J. H., Berge J., Johnsen G., Moline M. A., Bioluminescence as an ecological factor during high Arctic polar night. Sci. Rep. 6, 36374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cushing D. H., The vertical migration of planktonic crustacea. Biol. Rev. Camb. Philos. Soc. 26, 158–192 (1951). [DOI] [PubMed] [Google Scholar]
- 12.Ringelberg J., Van Gool E., On the combined analysis of proximate and ultimate aspects in diel vertical migration (DVM) research. Hydrobiologia 491, 85–90 (2003). [Google Scholar]
- 13.Clark C. W., Levy D. A., Diel vertical migrations by juvenile sockeye salmon and the antipredation window. Am. Nat. 131, 271–290 (1988). [Google Scholar]
- 14.Benoit D., Simard Y., Gagné J., Geoffroy M., Fortier L., From polar night to midnight sun: Photoperiod, seal predation, and the diel vertical migrations of polar cod (Boreogadus saida) under landfast ice in the Arctic Ocean. Polar Biol. 33, 1505–1520 (2010). [Google Scholar]
- 15.J. H. Cohen, R. B. Forward, Zooplankton diel vertical migration—A review of proximate control, in Oceanography and Marine Biology: An Annual Review, Vol 47, R. N. Gibson, R. J. A. Atkinson, J. D. M. Gordon, Eds. (CRC Press, 2009), vol. 47, pp. 77–109. [Google Scholar]
- 16.Hays G. C., A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503, 163–170 (2003). [Google Scholar]
- 17.Longhurst A., Sameoto D., Herman A., Vertical distribution of Arctic zooplankton in summer: Eastern Canadian Archipelago. J. Plankton Res. 6, 137–168 (1984). [Google Scholar]
- 18.Blachowiak-Samolyk K., Kwasniewski S., Richardson K., Dmoch K., Hansen E., Hop H., Falk-Petersen S., Mouritsen L. T., Arctic zooplankton do not perform diel vertical migration (DVM) during periods of midnight sun. Mar. Ecol. Prog. Ser. 308, 101–116 (2006). [Google Scholar]
- 19.Berge J., Cottier F., Last K. S., Varpe Ø., Leu E., Søreide J., Eiane K., Falk-Petersen S., Willis K., Nygård H., Vogedes D., Griffiths C., Johnsen G., Lorentzen D., Brierley A. S., Diel vertical migration of Arctic zooplankton during the polar night. Biol. Lett. 5, 69–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grenvald J. C., Callesen T. A., Daase M., Hobbs L., Darnis G., Renaud P. E., Cottier F., Nielsen T. G., Berge J., Plankton community composition and vertical migration during polar night in Kongsfjorden. Polar Biol. 39, 1879–1895 (2016). [Google Scholar]
- 21.Longcore T., Rich C., Ecological light pollution. Front. Ecol. Environ. 2, 191–198 (2004). [Google Scholar]
- 22.Depledge M. H., Godard-Codding C. A. J., Bowen R. E., Light pollution in the sea. Mar. Pollut. Bull. 60, 1383–1385 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Davies T. W., Coleman M., Griffith K. M., Jenkins S. R., Night-time lighting alters the composition of marine epifaunal communities. Biol. Lett. 11, 20150080 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaston K. J., Visser M. E., Hölker F., The biological impacts of artificial light at night: The research challenge. Philos. Trans. R. Soc. B Biol. Sci. 370, 20140133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamir R., Lerner A., Haspel C., Dubinsky Z., Iluz D., The spectral and spatial distribution of light pollution in the waters of the northern Gulf of Aqaba (Eilat). Sci. Rep. 7, 42329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scalabrin C., Marfia C., Boucher J., How much fish is hidden in the surface and bottom acoustic blind zones? ICES J. Mar. Sci. 66, 1355–1363 (2009). [Google Scholar]
- 27.Schofield O., Ducklow H. W., Martinson D. G., Meredith M. P., Moline M. A., Fraser W. R., How do polar marine ecosystems respond to rapid climate change? Science 328, 1520–1523 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Nilssen I., Ødegård Ø., Sørensen A. J., Johnsen G., Moline M. A., Berge J., Integrated environmental mapping and monitoring, a methodological approach to optimise knowledge gathering and sampling strategy. Mar. Pollut. Bull. 96, 374–383 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Ludvigsen M., Sørensen A. J., Towards integrated autonomous underwater operations for ocean mapping and monitoring. Annu. Rev. Control 42, 145–157 (2016). [Google Scholar]
- 30.Darnis G., Hobbs L., Geoffroy M., Grenvald J. C., Renaud P. E., Berge J., Cottier F., Kristiansen S., Daase M., Søreide J. E., Wold A., Morata N., Gabrielsen T., From polar night to midnight sun: Diel vertical migration, metabolism and biogeochemical role of zooplankton in a high Arctic fjord (Kongsfjorden, Svalbard). Limnol. Oceanogr. 62, 1586–1605 (2017). [Google Scholar]
- 31.Elofsson R., The frontal eyes of crustaceans. Arthropod Struct. Dev. 35, 275–291 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Båtnes A. S., Miljeteig C., Berge J., Greenacre M., Johnsen G., Quantifying the light sensitivity of Calanus spp. during the polar night: Potential for orchestrated migrations conducted by ambient light from the sun, moon, or aurora borealis? Polar Biol. 38, 51–65 (2015). [Google Scholar]
- 33.Tarling G. A., Johnson M. L., Satiation gives krill that sinking feeling. Curr. Biol. 16, R83–R84 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Wallace M. I., Cottier F. R., Berge J., Tarling G. A., Griffiths C., Brierley A. S., Comparison of zooplankton vertical migration in an ice-free and a seasonally ice-covered Arctic fjord: An insight into the influence of sea ice cover on zooplankton behavior. Limnol. Oceanogr. 55, 831–845 (2010). [Google Scholar]
- 35.Fischer J., Visbeck M., Seasonal variation of the daily zooplankton migration in the Greenland Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 40, 1547–1557 (1993). [Google Scholar]
- 36.Ducklow H. W., Steinberg D. K., Buesseler K. O., Upper ocean carbon export and the biological pump. Oceanography 14, 50–58 (2001). [Google Scholar]
- 37.Sameoto D., Cochrane N. A., Herman A. W., Response of biological acoustic backscattering to ships’ lights. Can. J. Fish. Aquat. Sci. 42, 1535–1543 (1985). [Google Scholar]
- 38.Marchesan M., Spoto M., Verginella L., Ferrero E. A., Behavioural effects of artificial light on fish species of commercial interest. Fish. Res. 73, 171–185 (2005). [Google Scholar]
- 39.B. Nightingale, T. Longcore, C. A. Simenstad, Artificial night lighting and fishes, in Ecological Consequences of Artificial Night Lighting, C. Rich, T. Longcore, Eds. (Island Press, 2006), pp. 257–276. [Google Scholar]
- 40.Price H. J., Swimming behavior of krill in response to algal patches: A mesocosm study. Limnol. Oceanogr. 34, 649–659 (1989). [Google Scholar]
- 41.De Robertis A., Schell C., Jaffe J. S., Acoustic observations of the swimming behavior of the euphausiid Euphausia pacifica Hansen. ICES J. Mar. Sci. 60, 885–898 (2003). [Google Scholar]
- 42.J. Mauchline, Advances Marine Biology: The Biology of Calanoid Copepods (Academic Press, 1998), vol. 33, pp. 1–710. [Google Scholar]
- 43.Fields D. M., Shema S. D., Browman H. I., Browne T. Q., Skiftesvik A. B., Light primes the escape response of the calanoid copepod, Calanus finmarchicus. PLOS ONE 7, e39594 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.M. V. Moore, S. M. Pierce, H. M. Walsh, S. K. Kvalvik, J. D. Lim, Urban light pollution alters the diel vertical migration of Daphnia, in Proceedings of the International Association of Theoretical and Applied Limnology, W. D. Williams, Ed. (2001), vol. 27, pp. 779–782. [Google Scholar]
- 45.Cordes E. E., Jones D. O. B., Schlacher T. A., Amon D. J., Bernardino A. F., Brooke S., Carney R., DeLeo D. M., Dunlop K. M., Escobar-Briones E. G., Gates A. R., Génio L., Gobin J., Henry L.-A., Herrera S., Hoyt S., Joye M., Kark S., Mestre N. C., Metaxas A., Pfeifer S., Sink K., Sweetman A. K., Witte U., Environmental impacts of the deep-water oil and gas industry: A review to guide management strategies. Front. Environ. Sci. 4, 58 (2016). [Google Scholar]
- 46.Volent Z., Johnsen G., Sigernes F., Kelp forest mapping by use of airborne hyperspectral imager. J. Appl. Remote Sens. 1, 011503 (2007). [Google Scholar]
- 47.P. Kimball, J. Bailey, S. Das, R. Geyer, T. Harrison, C. Kunz, K. Manganini, K. Mankoff, K. Samuelson, T. Sayre-McCord, F. Straneo, P. Traykovski, H. Singh, The WHOI Jetyak: An autonomous surface vehicle for oceanographic research in shallow or dangerous waters, in 2014 IEEE/OES Autonomous Underwater Vehicles (AUV) (IEEE, 2014), pp. 1–7. [Google Scholar]
- 48.J. Simmonds, D. N. MacLennan, Fisheries Acoustics: Theory and Practice (Blackwell Science Ltd., ed. 2, 2008). [Google Scholar]
- 49.Geoffroy M., Cottier F. R., Berge J., Inall M. E., AUV-based acoustic observations of the distribution and patchiness of pelagic scattering layers during midnight sun. ICES J. Mar. Sci. 74, 2342–2353 (2016). [Google Scholar]
- 50.Twardowski M. S., Sullivan J. M., Donaghay P. L., Zaneveld J. R. V., Microscale quantification of the absorption by dissolved and particulate material in coastal waters with an ac-9. J. Atmos. Oceanic Tech. 16, 691–707 (1999). [Google Scholar]
- 51.Buskey E. J., Swift E., Behavioral responses of oceanic zooplankton to simulated bioluminescence. Biol. Bull. 168, 263–275 (1985). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/1/eaap9887/DC1
fig. S1. Map of the study area in Kongsfjorden.
fig. S2. AZFP echograms from ASV.
fig. S3. EK60 echograms from RV Helmer Hanssen.
fig. S4. Vertical distribution of zooplankton.
table S1. Ascent and descent rates of the SSL at 455 kHz in January 2016.


