Abstract
Oleic acid is a common pharmaceutical excipient that has been widely used in various dosage forms. Gas chromatography (GC) has often been used as the quantitation method for fatty acids normally requiring a derivatization step. The aim of this study was to develop a simple, robust, and derivatization-free GC method that is suitable for routine analysis of all the major components in oleic acid USP-NF (United States Pharmacopeia-National Formulary) material. A gas chromatography–flame ionization detection (GC–FID) method was developed for direct quantitative analysis of oleic acid and related fatty acids in oleic acid USP-NF material. Fifteen fatty acids were separated using a DB-FFAP (nitroterephthalic acid modified polyethylene glycol) capillary GC column (30 m×0.32 mm i.d.) with a total run time of 20 min. The method was validated in terms of specificity, linearity, precision, accuracy, sensitivity, and robustness. The method can be routinely used for the purpose of oleic acid USP-NF material analysis.
Keywords: Oleic acid, Fatty acids, Gas chromatography, Method development, Derivatization-free
1. Introduction
Oleic acid USP-NF (United States Pharmacopeia-National Formulary) material is a common pharmaceutical excipient that has been widely used in various dosage forms. It serves as an emulsion agent in topical pharmaceutical formulations, a penetration enhancer in transdermal formulations, a solubility enhancer for gastrointestinal (GI) tract delivery formulations, and has various other applications in pharmaceutical formulations. The fatty acid profile in oleic acid USP-NF material has been found to be associated with product stability and functionality [1]. United State Pharmacopeia (USP36/NF31) requires the identification and quantitation of oleic acid and related fatty acids in oleic acid USP-NF material [2]. A variety of analytical methods have been developed for determination of oleic acid and fatty acids in various types of samples. The most commonly employed techniques are high performance liquid chromatograph (HPLC) and gas chromatography (GC) methods [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15].
HPLC is the most commonly used tool for the analysis of pharmaceutical products, but it is less successful in the quantitation of fatty acids due to the absence of chromophores or fluorescent functional groups [3]. As a result, a majority of HPLC methods in the literature require a derivatization process prior to analysis [4]. Guo et al. [5] presented an HPLC method equipped with an evaporative light-scattering detector (ELSD) for direct quantification of un-derivatized fatty acids. Four un-derivatized fatty acids in an oil matrix were separated and quantified by an Eclipse XDB C18 column with a mobile phase of methanol/water/acetic acid (88/11/1, v/v/v). Compared with the standard GC method, this method achieved acceptable separation and precision, but had poor sensitivity.
GC is the most commonly used technology for the analysis of fatty acids. In the literature, most fatty acids analyses by GC require derivatization due to the high boiling points of fatty acids, which are difficult to evaporate and have a low FID response [6]. The European Pharmacopoeia (Ph.Eur, 7.0) and United States Pharmacopeia (USP36/NF31) describe similar GC methods for identifying and quantifying oleic acid and fatty acids in oleic acid USP-NF material. Both methods require a methylation process prior to GC analysis. In recent years, many derivatization techniques for fatty acids have been reported [8], [9], [10], [11], [12], [13]. In analyses of fatty acids from herbs, Tong et al. [14] described a technique for extraction of fatty acids from the herbs using petroleum ether; the extracts were then methylated using 0.5% (w/v) sodium hydroxide in methanol. In this report, nine fatty acids from the herbs including oleic acid were identified and quantified. Tomato seeds are a source of vegetable oil containing a lot of saturated and unsaturated fatty acids. To quantify the fatty acid amount in tomato seed oil, Botinestean et al. [15] described a method for analysis of oleic acid and fatty acids. Tomato seed oil was first esterified by methanol/boron trifluoride, followed by GC analysis. In this report, eight fatty acid esters, including oleic acid ester, were identified and accurately quantified. In addition to the use of the aforementioned chemicals for fatty acid derivatization, anaerobic digestion can also be used for fatty acid derivatization. Neves et al. [16] presented a method for analysis of long chain fatty acids in biomass samples. In this study, samples were equally homogenized with dichloromethane and digested at 100 °C for 3.5 h. After digestion, the samples were further vortex-mixed for 30 min and analyzed by GC–FID. Mean fatty acid recoveries above 90% were obtained. Although discussions about the necessity of the derivatization process have lasted for decades, many contradictory opinions are still presented. Regardless of the derivatization process used, the derivatization is always a laborious, tedious and time consuming process, which often leads to lower accuracy and precision [17]. In addition, some interactions between derivatizing agents and active drug compounds are unknown; therefore, further research has been recently carried out on direct determination of fatty acids in various sources without the use of derivatization steps. Meng et al. [18] developed a derivatization-free GC–FID method with fast temperature programing and a micro-bore short capillary column. Ten fatty acids (C12:0–C26:0, even numbers only) were well separated with satisfactory recoveries and reproducibility. However, it did not include the separation of the odd numbered fatty acids. Hua et al. [19] reported the evaluation of un-derivatized fatty acids in plasma of diabetic nephropathy (DN) patients. After extraction of fatty acids from plasma, a highly polar CP-Wax 58 (FFAP) CB capillary column was employed to directly quantitate fatty acids. Fifteen fatty acids (C10:0 to C22:0) were detected and separated. This method facilitated the assay of saturated fatty acids in medical laboratories. Brooks et al. [20] described a method for direct quantitation of oleic acid and related fatty acids in pharmaceutical aerosol products. In this method, a GC–FID equipped with DB-FFAP (acidified polyethylene glycol) fused silica column was used for obtaining an impurity profile of oleic acid. Sixteen fatty acids (C10:0 to C18:0) were detected and separated. The results showed that the method had sufficient selectivity and sensitivity for fatty acids, but the analysis time was too long and needed at least 80 min to complete the separation. Despite the aforementioned advancement in fatty acids analysis, there is still room for improvement. All of the methods have drawbacks such as low sensitivity, poor resolution or a long analysis time.
The aim of this study was to develop a simple and derivatization-free method for the simultaneous analysis of oleic acid and related fatty acids in a single run. The developed method should be accurate, robust and suitable to be used in a quality control lab for oleic acid USP-NF material analysis.
2. Experimental
2.1. Materials
Oleic acid USP-NF materials (animal and vegetable origin) were purchased from Croda Inc., (Mill Hall, Pennsylvania, USA). Oleic acid reference standard (C18:1, purity>99%) and fifteen individual fatty acids, including lauric acid (C12:0), myristic acid (C14:0), pentadecylic acid (C15:0), palmitic acid (C16:0), palmitoleic acid (C16:1), margaric acid (C17:0), stearic acid (C18:0), linoleic acid (C18:2), linolenic acid (C18:3), nonadecylic acid (19:0), arachidic acid (C20:0), heneicosylic acid (C21:0), behenic acid (C22:0), tricosylic acid (C23:0) and lignoceric acid (C24:0), were purchased from Sigma Aldrich Co. (St. Louis, Missouri, USA). Isopropanol (IPA) was of GC grade from Thermo Fisher Scientific (Fair Lawn, New Jersey, USA). Milli-Q water was obtained from a Millipore Direct-Qultra-pure water system (Billerica, Massachusetts, USA). All other reagents were of pharmaceutical grade and used as received.
2.2. Instruments
An Agilent 6890N gas chromatographic system (Agilent Technologies, Santa Clara, California, USA) equipped with a flame ionization detector and an automated liquid sampler was used for method development and method validation.
2.3. Sample preparation
2.3.1. Standard stock solutions
Isopropanol was used as the diluent for oleic acid and the related fatty acids due to their solubility. Oleic acid and related fatty acid stock solutions were prepared by referring to the USP36/NF31 specifications (Table 1). Oleic acid reference standard was dissolved in isopropanol to obtain a stock solution at the concentration of approximately 1800 µg/mL. Eight related fatty acids were individually dissolved in volumetric flasks with isopropanol to obtain stock solutions with a concentration range of 1000–4400 µg/mL (Table 2).
Table 1.
Specification limit of fatty acid profile in oleic acid NF material.
| Name | Bond | Composition (%) |
|---|---|---|
| Myristic acid | C14:0 | ≤5.0 |
| Palmitic acid | C16:0 | ≤16.0 |
| Palmitoleic acid | C16:1 | ≤8.0 |
| Margaric acid | C17:0 | ≤4.0 |
| Stearic acid | C18:0 | ≤6.0 |
| Linoleic acid | C18:2 | ≤18.0 |
| Linolenic acid | C18:3 | ≤4.0 |
| Arachidic acid | C20:0 | ≤4.0 |
| Oleic acid | C18:1 | 65.0–88.0 |
Table 2.
Preparation of oleic acid and fatty acid stock solutions.
| Name | Weight (mg) | Flask volume (mL) | Approximate stock concentration (µg/mL) |
|---|---|---|---|
| Myristic acid | 70±3.5 | 50 | 1400 |
| Palmitic acid | 200±10 | 50 | 4000 |
| Palmitoleic acid | 100±5 | 50 | 2000 |
| Margaric acid | 50±2.5 | 50 | 1000 |
| Stearic acid | 70±3.5 | 50 | 1400 |
| Linoleic acid | 220±11 | 50 | 4400 |
| Linolenic acid | 50±2.5 | 50 | 1000 |
| Arachidic acid | 50±2.5 | 50 | 1000 |
| Oleic acid | 180±9 | 100 | 1800 |
2.3.2. Fatty acid mixture solution
A fatty acid mixture solution was prepared by mixing 8.0 mL of each of the individual fatty acid stock solutions into a 100 mL of volumetric flask, excluding oleic acid, and diluted to volume with sample solvent.
2.3.3. System suitability samples
System suitability samples were freshly prepared by pipetting 10 mL of oleic acid stock solution and 1 mL each of palmitic acid, stearic acid, and linoleic acid stock solutions into a 100 mL volumetric flask, mixed well and diluted to volume with sample solvent.
2.3.4. Linearity and accuracy samples
Linearity and range samples were prepared in triplicate by serial dilution in isopropanol, covering the ranges of quantitation limit (QL) to at least 125% of each component assay level. Three levels (50%, 100% and 150%) of oleic acid accuracy samples were prepared in triplicate by pipetting 2.5, 5.0 and 7.5 mL of oleic acid stock solutions (1800 µg/mL) into 50 mL volumetric flasks and spiking with 1.0 mL of fatty acid mixture solution. Three levels (50%, 100% and 150%) of individual fatty acid accuracy samples were prepared in triplicate by pipetting 3.0, 6.0 and 9.0 mL of fatty acid mixture solutions into 50 mL volumetric containers, respectively, and spiking with 1.0 mL of oleic acid stock solution.
2.3.5. Assay samples
An oleic acid USP-NF material assay sample was prepared by weighing (20±2.0) mg of oleic acid NF material into a 100 mL volumetric flask and diluted to volume with isopropanol (approximately 200 µg/mL).
3. Results and discussion
3.1. Method development
The aim of this study was to develop a simple, robust and derivatization-free method for the analysis of fatty acids in the oleic acid USP-NF material. A systematic method development strategy was utilized to optimize the parameters/conditions for the analysis of oleic acid and related fatty acids, including sample solvent, inlet temperature, column temperature and temperature program, stationary phase, inlet type, sample size and injection technique.
3.1.1. Sample solvent selection
Development of this GC method was started with selection of a sample solvent that dissolves oleic acid and related fatty acids. Fatty acids are carboxylic acids with long aliphatic chains. As the aliphatic chain length increases, the solubility of the fatty acids in water decreases very rapidly. Methanol, ethanol and isopropanol are all good solvents for oleic acids (at least >11.6 g/mL), but methanol and ethanol are more polar alcohols than isopropanol [21]. GC injection using methanol and ethanol as sample solvents showed broad peak shapes for some long chain fatty acids due to the polarity mismatch between the sample solvents and the stationary phase. The peak shapes of all fatty acids under study were significantly improved by using isopropanol as sample solvent. Therefore, isopropanol was selected as sample solvent (diluent).
3.1.2. Determination of sample concentration
Three sample concentrations (100, 200 and 300 µg/mL) were evaluated for two types of oleic acid USP-NF materials (vegetable origin and animal origin). Both materials exhibited similar chromatographic profiles.
However, the sample at the concentration of approximately 100 µg/mL showed a low peak response for the fatty acids with a carbon chain length greater than C18; and the sample at the concentration of approximately 300 µg/mL appeared to be too concentrated and caused significant residual carry-over. Therefore, the sample concentration at about 200 µg/mL was chosen in the final validated method.
3.1.3. GC column screenings
The polarity of the column stationary phase plays a critical role in a successful separation for fatty acids. To improve peak resolution, the polarity of the column stationary phase should closely match the polarity of the fatty acids. Oleic acid and related fatty acids are polar compounds in nature; therefore, four types of columns having a similar polarity as the related fatty acids were selected for column screening. A DB-FFAP column (30 m×0.32 mm i.d., 0.25 µm film thickness, Agilent, Santa Clara, USA) consists of a polyethylene glycol (PEG) bonded nitroterephthalic acid phase. The phase is designed for the analysis of volatile free fatty acids. In the experiment, DB-FFAP column easily separated fifteen fatty acids within 20 min with most of the fatty acid peaks exhibiting excellent peak symmetry. It also demonstrated that the DB-FFAP column chemistry closely matched the polarity of fatty acids being analyzed.
Another column that was evaluated, a ZB FFAP (30 m×0.25 mm i.d., 0.25 µm film thickness, Phenomenex, Torrance, USA), also consists of a polyethylene glycol bonded nitroterephthalic acid phase. A ZB FFAP column was able to separate fifteen fatty acids with a broad concentration range of 8.17–183 µg/mL (Table 3). However, some peak broadening was observed due to a high concentration of fatty acids injected on a narrow column (0.25 mm i.d.) resulting in column overloading.
Table 3.
Linearity sample concentrations and results.
| Name | Conc. range (µg/mL) |
Regression equation (Y=ax+b) | Correlation coefficient (r) | ||||
|---|---|---|---|---|---|---|---|
| QL | 50% | 75% | 100% | 125% | |||
| Myristic acid | 0.27 | 5.33 | 8.53 | 10.70 | 12.8 | Y=9591.4x−617.5 | 0.9998 |
| Palmitic acid | 0.80 | 16.1 | 25.7 | 32.1 | 38.6 | Y=9001.4x+747.2 | 0.9998 |
| Palmitoleic acid | 0.40 | 8.20 | 13.1 | 16.3 | 19.6 | Y=8751.6x−1071.9 | 0.9999 |
| Margaric acid | 0.51 | 4.07 | 6.57 | 8.17 | 9.80 | Y=8636.6x−673.47 | 0.9998 |
| Stearic acid | 0.72 | 5.73 | 9.17 | 11.5 | 13.8 | Y=8253.7x+2674.9 | 0.9978 |
| Linoleic acid | 0.89 | 17.9 | 28. 7 | 35.8 | 43.0 | Y=7883.9x+37.508 | 0.9996 |
| Linolenic acid | 1.03 | 4.13 | 6.60 | 8.23 | 9.87 | Y=7830.1x−528.23 | 0.9999 |
| Arachidic acid | 0.84 | 4.20 | 6.70 | 8.37 | 10.0 | Y=7276.3x−976 | 0.9995 |
| Oleic acid | 1.10 | 91.5 | 146 | 183 | 220 | Y=8647.3x−10,075 | 0.9990 |
A Stabilwax-DA column (30 m×0.32 mm i.d., 0.25 µm film thickness, Restek, Bellefonte, USA) with a stationary phase bonded with polyethylene glycol having an acidic functionality incorporated into the polymer structure was the third column selected for this study. In the experiment, only 12 fatty acids were separated and the resolution of stearic acid (C18:0), oleic acid (C18:1) and linoleic acid (C18:2) was poor. Some of the separated fatty acids exhibited peak fronting in the chromatograms. Most likely, the peak fronting can be attributed to the polarity mismatch between the column stationary phase and the fatty acids.
The final column assessed for this experiment was a DB-23 (30 m×0.25 mm i.d., 0.25 µm film thickness, Agilent, Santa Clara, USA ) consisting of a highly polar (50% cyanopropyl)-methylpolysiloxane stationary phase and is designed for the separation of fatty acids and esters. In a preliminary experiment, the DB-23 column only separated 12 fatty acids with poor resolution among stearic acid (C18:0), oleic acid (C18:1) and linoleic acid (C18:2). Based on the above column screening data, the DB-FFAP column was found to be the best column for analysis of oleic acid and its related free fatty acids. As such, the Agilent DB-FFAP column was selected for further study.
3.1.4. Injection parameter determination
Considering the low level of oleic acid and fatty acids to be loaded onto the column, a splitless injection mode was chosen to increase the method sensitivity. To avoid overloading the column, the injection volume of 1 μL was selected. The boiling points of oleic acid and related fatty acids are quite high with broad ranges of 162–383 °C. The inlet temperature must balance the quick fatty acid evaporation and the generation of a good peak shape. Three inlet temperatures (210, 230 and 250 °C) were evaluated. At the low inlet temperature of 210 °C, some fatty acids with high boiling points could not be eluted due to lack of energy to vaporize. At the high inlet temperature of 250 °C, the recoveries of saturated fatty acids were better than 210 °C, but the peak areas of some unsaturated fatty acids were decreased due to the thermal degradation. Hence, the inlet temperature of 230 °C was chosen for further study. No thermal degradation was observed at the inlet temperature of 230 °C.
Three commercially available inlet liners were assessed. An Agilent straight inlet liner for splitless injection model made of borosilicate glass and deactivated to prevent adsorption of active compounds was assessed. However, the peak areas of oleic acid using this inlet liner were generally lower, most likely due to poor evaporation.
The Restek Sky inlet liner for splitless injection model is also made of borosilicate glass packed with quartz wool. The reproducibility of the sample injection was poor using this liner, most likely due to the solute absorbance on quartz wool, especially when fibers are broken. A Restek siltek deactivated cyclo-double gooseneck liner consisting of a borosilicate glass spiral, which significantly increased vaporization space, was the final liner for further study. This inlet liner yielded better precision (repeatability); therefore, a 4 mm ID Restek deactivated cyclo double gooseneck liner was chosen for further study.
3.1.5. Column temperature programing
During the preliminary study for column temperature programing, the initial column temperatures were set at 115 °C in order to obtain prolonged retention for early eluting peaks. However, a longer analysis time was required; therefore, the initial column temperature was increased to 120 °C while maintaining acceptable retention for early eluting peaks. The variable column temperature gradient rates (27–33 °C/min) were evaluated and had no impact on the fatty acid elution profiles. The final column temperature was evaluated. At a lower final column temperature (230 °C), the fatty acids with carbon chain length greater than C18 eluted at a retention time ranging from 7.8 to 17.5 min with relatively small peak heights.
When the final column temperature was set to 250 °C, the retention time of the fatty acids with carbon chain length greater than C18 shortened and the peak intensity was larger. Unfortunately, the maximum allowable column temperature of DB-FFAP is 250 °C as recommended by the vendor. Therefore, the final column temperature was set at 245 °C, 5 °C below the column maximum temperature to extend column life. In summary, a slope of 30 °C along with an initial temperature of 120 °C and a final temperature of 245 °C at a flow rate of 2.8 mL/min gave optimal separation. Oleic acid eluted at retention time of 7.2 min.
3.2. Validation
The proposed method was validated for specificity, linearity, accuracy, precision, sensitivity and robustness as per the ICH method validation guidelines [22].
3.2.1. Specificity
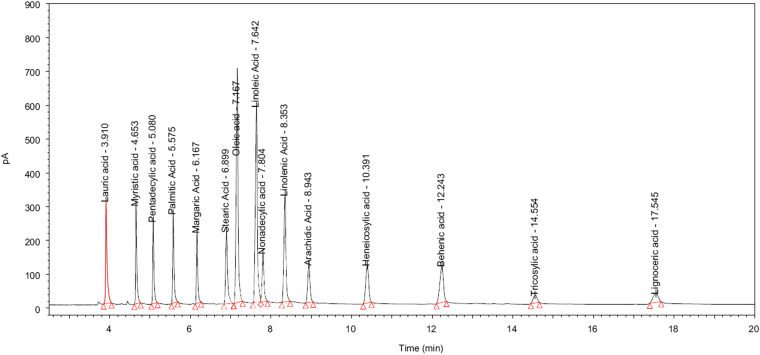
No interfering peaks were found at the retention time of oleic acid and related fatty acids while injecting the diluent (isopropanol) into the system. The retention times of oleic acid and related fatty acids were confirmed by comparing their retention times with those obtained from each co-injected individual fatty acid. All fatty acids were adequately resolved from each other. Though the method was only validated for 9 fatty acids based on the compendia requirement, the method provided baseline separation for all 15 fatty acids as shown in Fig. 1.
Fig. 1.
Identification chromatogram of 15 fatty acids.
3.2.2. System
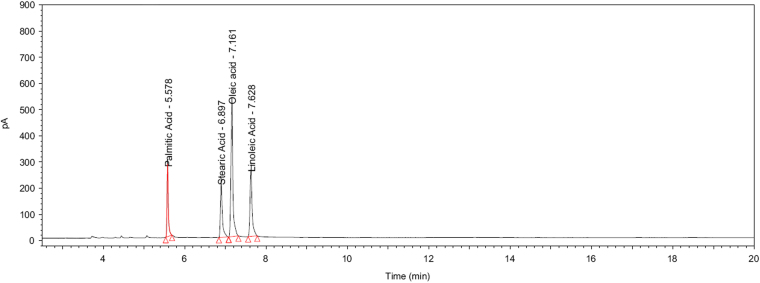
The average relative standard deviations (RSDs) of peak area ratio were 0.68% for palmitic acid/oleic acid, 0.31% for stearic acid/oleic acid and 0.37% for linoleic acid/oleic acid. The resolution (Rs) of each pair of fatty acid peaks was greater than 2.0 and the tailing of oleic acid and the three fatty acid peaks was less than 2.0. A typical system suitability chromatogram is shown in Fig. 2.
Fig. 2.
System suitability chromatogram.
3.2.3. Linearity and range
Linearity and range samples were serially diluted from each oleic acid and fatty acid stock solution to obtain five concentration levels, covering QL to at least 125% of the assay level. The peak areas at each level of oleic acid and each individual fatty acid were calculated to assess the method linearity. Graphs of each peak area versus each corresponding concentration of fatty acid were plotted. The correlation coefficient of the regression line for each fatty acid standard ranged from 0.9998 (C14:0) to 0.9995 (C20:0). The detailed results are summarized in Table 3.
3.2.4. Accuracy/recovery
The recovery experiment was carried out to evaluate the accuracy of the method. Response of the three levels (50%, 100% and 150%) of spiked oleic acid samples in triplicate yielded a mean recovery of 99.6% with an RSD(n=9) of 0.50%. Recoveries of three levels (50%, 100% and 150%) each of eight of spiked fatty acids were 85.6%–114.1% with RSD(n=9) from 0.83% to 7.43%. The variations in accuracy/recoveries were calculated from the response of each individual fatty acid on FID detection. The results demonstrated that the method had sufficient capability for the accurate quantification of oleic acid and fatty acids in oleic acid USP-NF samples. Detailed accuracy/recovery results are summarized in Table 4.
Table 4.
Accuracy results.
| Name | Average recovery (%) | RSD(n=9) (%) |
|---|---|---|
| Myristic acid | 114.1 | 1.78 |
| Palmitic acid | 108.7 | 2.21 |
| Palmitoleic acid | 104.3 | 0.83 |
| Margaric acid | 102.7 | 1.43 |
| Stearic acid | 104.7 | 7.43 |
| Linoleic acid | 95.6 | 2.69 |
| Linolenic acid | 93.2 | 1.22 |
| Arachidic acid | 85.6 | 2.30 |
| Oleic acid | 99.6 | 0.64 |
3.2.5. Precision
Method precision was evaluated at three levels: repeatability, intermediate precision and reproducibility. The intra-day precision (repeatability) was evaluated using the results of six preparations each containing nine fatty acids. The RSD of oleic acid was less than 3.0% and the RSDs of fatty acids (individual) were found to be 2.0%–6.6% (Table 5). The intermediate precision was evaluated by data generated on different days. The difference of the mean from two days ranged from −0.14% to 2.90% (Table 6). The reproducibility was evaluated by comparing data from two different laboratories (Lab A and Lab B). The percentage of oleic acid in oleic acid USP-NF materials (vegetable origin) was measured as 87.39% by Lab A and 87.57% by Lab B. The RSD of oleic acid measurements in the two laboratories was found to be 0.14%, indicating the validated test method is reproducible. Furthermore, the method was also evaluated by comparing the data obtained using this validated test method to the vendor׳s data using the Ph. Eur. method. The results in Table 7 demonstrated that the data were comparable.
Table 5.
Precision (repeatability) results.
| Injections (#) | Myristic acid | Palmitic acid | Palmitoleic acid | Margaric acid | Stearic acid | Oleic acid | Linoleic acid | Linolenic acid | Arachidic acid |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 21,846 | 60,080 | 28,658 | 14,736 | 21,885 | 1,877,924 | 63,107 | 12,915 | 11,342 |
| 2 | 20,488 | 59,562 | 29,129 | 14,717 | 21,738 | 1,919,905 | 65,356 | 12,763 | 11,657 |
| 3 | 20,856 | 61,201 | 29,665 | 14,542 | 22,321 | 1,952,613 | 63,770 | 12,802 | 12,228 |
| 4 | 22,623 | 64,583 | 30,178 | 15,719 | 25,316 | 1,983,366 | 66,062 | 13,714 | 11,992 |
| 5 | 20,329 | 58,928 | 28,659 | 14,497 | 23,039 | 1,881,780 | 62,814 | 12,553 | 11,403 |
| 6 | 20,626 | 59,985 | 29,275 | 14,309 | 21,085 | 1,917,685 | 62,661 | 12,374 | 11,880 |
| Average | 21,128 | 60,723 | 29,261 | 14,753 | 22,564 | 1,922,212 | 63,962 | 12,854 | 11,750 |
| SD | 908.2 | 2032.5 | 591.2 | 498.4 | 1495.9 | 40,708.8 | 1423.5 | 463.7 | 346.3 |
| RSD (%) | 4.30 | 3.35 | 2.02 | 3.38 | 6.63 | 2.12 | 2.23 | 3.61 | 2.95 |
Table 6.
Precision (intermediate) results.
| Name | Conc. (µg/mL) | Fatty acid spiking solution injected on day 1 |
Fatty acid spiking solution injected on day 2 |
Difference (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area 1 | Area 2 | Area 3 | Average | Area 1 | Area 2 | Area 3 | Average | |||
| Myristic acid | 5.4 | 50,492 | 48,875 | 49,381 | 49,583 | 52,106 | 50,437 | 51,104 | 51,216 | 1.62 |
| Palmitic acid | 16.1 | 1,49,664 | 144,244 | 143,700 | 145,869 | 149,179 | 148,805 | 149,305 | 149,096 | 1.09 |
| Palmitoleic acid | 8.1 | 65,366 | 69,281 | 69,882 | 68,176 | 68,705 | 71,316 | 71,958 | 70,660 | 1.79 |
| Margaric acid | 4.1 | 32,823 | 34,879 | 33,760 | 33,821 | 35,279 | 35,820 | 35,068 | 35,389 | 2.27 |
| Stearic acid | 5.7 | 59,699 | 52,841 | 51,907 | 54,816 | 55,778 | 54,432 | 53,764 | 54,658 | −0.14 |
| Linoleic acid | 17.9 | 1,46,164 | 146,038 | 139,584 | 143,929 | 147,434 | 148,488 | 143,640 | 146,521 | 0.89 |
| Linolenic acid | 4.2 | 29,394 | 32,056 | 30,838 | 30,763 | 31,869 | 32,602 | 32,296 | 32,256 | 2.37 |
| Arachidic acid | 4.1 | 23,338 | 29,298 | 29,101 | 27,246 | 27,006 | 29,123 | 30,490 | 28,873 | 2.90 |
| Oleic acid | 108.2 | 883,693 | 892,062 | 868,135 | 881,297 | 848,260 | 912,798 | 898,706 | 886,588 | 0.30 |
Table 7.
Results obtained from different labs.
| Name | Composition obtained (%) |
|||
|---|---|---|---|---|
| Validated method in Merck Lab A | Validated method in Merck Lab B | Eur. method by the manufacturer | Eur. method by a CRO | |
| Myristic acid | ND | 0.13 | 0.00 | 0.00 |
| Palmitic acid | 1.62 | 1.36 | 1.21 | 1.30 |
| Palmitoleic acid | ND | ND | 0.00 | ND |
| Margaric acid | ND | 0.11 | 0.10 | n/a |
| Stearic acid | 2.89 | 2.73 | 2.96 | 2.70 |
| Oleic acid | 87.39 | 87.57 | 87.11 | 87.30 |
| Linoleic acid | 6.84 | 5.85 | 5.97 | 6.10 |
| Linolenic acid | ND | ND | 0.00 | ND |
| Fatty acid chain length great than C18 | 0.68 | 0.61 | 0.94 | 0.10 |
ND — not detected.
3.2.6. Detection limit (DL) and quantification limit (QL)
Method sensitivity was assessed by DL and QL. The concentration of QL was about 6% of the individual fatty acid assay level and injected into GC system to obtain a signal to noise ratio greater than 10. The DL was estimated based on a QL injection to obtain a signal to noise ratio greater than 3.0 (Table 8).
Table 8.
Detection limit (DL) and quantification limit (QL).
| Name | Boiling point (°C) | DL (µg/mL) | QL (µg/mL) |
|---|---|---|---|
| Myristic acid | 250 | 0.13 | 0.27 |
| Palmitic acid | 215 | 0.40 | 0.80 |
| Palmitoleic acid | 162 | 0.20 | 0.40 |
| Margaric acid | 227 | 0.25 | 0.51 |
| Stearic acid | 383 | 0.36 | 0.72 |
| Linoleic acid | 230 | 0.45 | 0.89 |
| Linolenic acid | 232 | 0.50 | 1.03 |
| Arachidic acid | 328 | 0.42 | 0.84 |
| Oleic acid | 360 | 0.55 | 1.10 |
3.2.7. Robustness
Robustness testing was performed by varying the operational parameters, one at a time, such as flow rate, ramp temperature, inlet temperature, oven temperature, and detector temperature. The variability of the percentage of peak areas in fatty acid samples is summarized in Table 9. Results showed that the percentage differences (absolute) of the individual fatty acid content found in the spiking solution using each of the altered GC conditions and the procedural GC conditions were all within ±0.5% of that found from the procedural conditions. The oleic acid content found in spiking solution obtained from each of the altered GC conditions was within an absolute difference of ±1.0% of that found from the procedural conditions. These low percentage variation values (absolute) revealed that the proposed method is robust.
Table 9.
Robustness test results.
| Parameters | % Peak compositions (% differences, absolute) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Myristic acid |
Palmitic acid |
Palmitoleic acid |
Margaric acid |
Stearic acid |
Oleic acid |
Linoleic acid |
Linolenic acid |
Arachidic acid |
|
| Procedural | 3.56 | 10.44 | 5.09 | 2.50 | 3.54 | 57.03 | 9.37 | 2.29 | 2.18 |
| Flow rate (±0.3 mL/min) | |||||||||
| 2.5 | 3.48 (−0.08) | 10.29 (−0.15) | 4.99 (−0.10) | 2.46 (−0.04) | 3.48 (−0.06) | 57.46 (0.43) | 9.51 (0.14) | 2.21 (−0.08) | 2.17 (−0.01) |
| 3.1 | 3.39 (−0.17) | 10.24 (−0.20) | 5.01 (−0.08) | 2.48 (−0.02) | 3.54 (0.00) | 57.22 (0.19) | 9.69 (0.32) | 2.27 (−0.02) | 2.17 (−0.01) |
| Ramp temp. (±3 °C) | |||||||||
| 27 | 3.44 (−0.12) | 10.32 (−0.12) | 5.03 (−0.06) | 2.48 (−0.02) | 3.50 (−0.04) | 57.47 (0.44) | 9.54 (0.17) | 2.25 (−0.04) | 2.13 (−0.05) |
| 33 | 3.48 (−0.08) | 10.37 (−0.07) | 5.06 (−0.03) | 2.48 (−0.02) | 3.55 (0.01) | 57.51 (0.48) | 9.67 (0.30) | 2.22 (−0.07) | 2.05 (−0.13) |
| Inlet temp. (±10 °C) | |||||||||
| 220 | 3.58 (0.02) | 10.43 (−0.01) | 5.12 (0.03) | 2.48 (−0.02) | 3.49 (−0.05) | 57.52 (0.49) | 9.59 (0.22) | 2.21 (−0.08) | 2.03 (−0.15) |
| 240 | 3.45 (−0.11) | 10.33 (−0.11) | 5.04 (−0.05) | 2.48 (−0.02) | 3.52 (−0.02) | 57.80 (0.77) | 9.67 (0.30) | 2.20 (−0.09) | 2.04 (−0.14) |
| Final oven temp. (±5 °C) | |||||||||
| 240 | 3.53 (−0.03) | 10.39 (−0.05) | 5.09 (0.00) | 2.48 (−0.02) | 3.52 (−0.02) | 57.85 (0.82) | 9.71 (0.34) | 2.16 (−0.13) | 1.97 (−0.21) |
| 250 | 3.47 (−0.09) | 10.29 (−0.15) | 5.05 (−0.04) | 2.47 (−0.03) | 3.51 (−0.03) | 57.32 (0.29) | 9.47 (0.10) | 2.16 (−0.13) | 1.96 (−0.22) |
| Detector temp. (±10 °C) | |||||||||
| 270 | 3.54 ( −0.02) | 10.43 (−0.01) | 5.11 (0.02) | 2.51 (0.01) | 3.51 (−0.03) | 57.87 (0.84) | 9.79 (0.42) | 2.12 (−0.17) | 1.94 (−0.24) |
| 290 | 3.43 (−0.13) | 10.13 (−0.31) | 4.96 (−0.13) | 2.40 (−0.10) | 3.46 (−0.08) | 57.09 (0.06) | 9.87 (0.50) | 2.03 (−0.26) | 1.75 (−0.43) |
3.2.8. Solution stability
Solution stability studies were carried out using fatty acid spiking samples and oleic acid USP-NF material samples on different days. Both stability samples were stored either at ambient temperature (approximately 20 °C) or refrigerated (2–8 °C). The chromatographic profile of the aged solution was comparable with that obtained at the initial point. The percentage differences of the individual fatty acid tested at initial time point and at each of the storage conditions and storage time points were within ±0.5% of the initial value (absolute). The percentage changes of the oleic acid content of the aged time points were less than ±3.0% of the initial value (relative). Detailed results are recorded in Table 10. The low percentage values of stability sample changes confirmed that the solutions of oleic acid and related fatty acids are stable for at least 7 days, stored either at ambient temperature (approximately 20 °C) or refrigerated (2–8 °C).
Table 10.
Stability study results.
| Name | % Peak compositions (% differences, absolute) |
||||||
|---|---|---|---|---|---|---|---|
| Initial | Stored at ambient temperature (approximately 20 °C) |
Stored in refrigerator temperature (<4 °C) |
|||||
| Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | ||
| Myristic acid | 2.50 | 2.65 (0.15) | 2.63 (0.13) | 2.78 (0.28) | 2.68 (0.18) | 2.60 (0.10) | 2.80 (0.30) |
| Palmitic acid | 6.95 | 7.19 (0.24) | 7.06 (0.11) | 7.00 (0.05) | 7.41 (0.46) | 7.11 (0.16) | 7.39 (0.44) |
| Margaric acid | 3.47 | 3.76 (0.29) | 3.70 (0.23) | 3.49 (0.02) | 3.57 (0.10) | 3.54 (0.07) | 3.74 (0.27) |
| Palmitoleic acid | 1.59 | 1.58 (−0.01) | 1.53 (−0.06) | 1.45 (−0.14) | 1.61 (0.02) | 1.58 (−0.01) | 1.61 (0.02) |
| Stearic acid | 2.43 | 2.21 (−0.22) | 2.12 (−0.31) | 2.32 (−0.11) | 2.76 (0.33) | 2.33 (−0.10) | 2.43 (0.0) |
| Linoleic acid | 6.23 | 5.94 (−0.29) | 6.18 (−0.05) | 6.32 (0.09) | 5.92 (−0.31) | 6.17 (−0.06) | 6.00 (−0.23) |
| Linolenic acid | 1.29 | 1.12 (−0.17) | 1.07 (−0.22) | 0.86 (−0.43) | 1.14 (−0.15) | 1.11 (−0.18) | 1.00 (−0.29) |
| Arachidic acid | 1.13 | 0.92 (−0.21) | 0.85 (−0.28) | 0.64 (−0.49) | 1.11 (−0.02) | 1.03 (−0.10) | 1.02 (−0.11) |
| Oleic acid (% changes, relative) | 77.59 | 77.79 (0.26) | 77.81 (0.28) | 76.86 (−0.94) | 77.59 (0.0) | 77.58 (−0.01) | 77.56 (−0.04) |
3.3. Applications
The analytical method was successfully applied to simultaneous determination of oleic acid and related fatty acids in oleic acid samples of different origins (vegetable and animal).
The contents of these components in oleic acid USP-NF materials are summarized in Table 11. From the results presented in Table 11, it was found that the contents of oleic acid and related fatty acids in both oleic acid NF materials were all within the USP specification listed in Table 1. Results proved this method was accurate and effective to assess the quality of oleic acid USP-NF material by simultaneous quantification of the major fatty acids.
Table 11.
Contents of oleic acid and related fatty acids in oleic acid USP-NF material samples.
| Name | Retention time (min) (RT) | Relative retention time (RRT) | Content (%) (animal origin) (K-00250) | Content (%) (vegetable origin) (H-H07913) |
|---|---|---|---|---|
| Myristic acid | 4.7 | 0.7 | 3.77 | n/a |
| Palmitic acid | 5.6 | 0.8 | 4.02 | 1.62 |
| Margaric acid | 6.2 | 0.9 | n/a | n/a |
| Stearic acid | 6.9 | 1.0 | 0.95 | 2.89 |
| Oleic acid | 7.2 | 1.0 | 77.59 | 87.39 |
| Linoleic acid | 7.2 | 1.1 | 5.48 | 6.84 |
| Linolenic acid | 8.4 | 1.1 | n/a | n/a |
| Arachidic acid | 8.9 | 1.2 | 0.66 | 0.68 |
4. Conclusion
A GC–FID method was developed for simultaneous analysis of oleic acid and related fatty acids in oleic acid USP-NF material. The sample preparation procedure was simple and straightforward with no derivatization required. Fifteen fatty acids were separated using a DB-FFAP (nitroterephthalic acid modified polyethylene glycol) capillary GC column with a total run time of 20 min. The method is free of interference from the diluent used in oleic acid USP-NF material preparation and demonstrates specificity for all 15 fatty acids. The sensitivity shows that the method is applicable for the analysis of oleic acid USP-NF material. In addition, the precision for the assay of each related fatty acid is acceptable.
The method was validated and proved to be specific, precise and accurate for analysis of oleic acid and related fatty acids. Applications of this method to oleic acid NF materials indicated that the method can be used as a quality control method for oleic acid NF materials.
Acknowledgments
The authors would like to acknowledge Yu-Chien Wei, Hui Liu, Joy Jin, Tina Masiuk, Maureen Marsales, Justin Pennington and Brent Donovan for productive discussions.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
References
- 1.Golightly L.K., Smolinske S.S., Bennett M.L. Pharmaceutical excipients. Adverse effects associated with inactive ingredients in drug products. Med. Toxicol. Advers. Drug Exp. 1988;2:128–165. [PubMed] [Google Scholar]
- 2.United States Pharmacopeia/National Formulary, in: Proceedings of 36th United States Pharmacopeia Commission, Rockville, USA, 2013, pp. 2111–2112.
- 3.Tsuyama Y., Uchiro T., Goto T. Analysis of un-derivatized C12–C18 fatty acids by reversed-phase ion-pair high-performance liquid chromatography with conductivity detection. J Chromatogr. A. 1992;596:181–184. [Google Scholar]
- 4.Makahleh A., Saad B., Siang G.H. Determination of un-derivatized long chain fatty acids using RP-HPLC with capacitively coupled contactless conductivity detection. Talanta. 2010;81:20–24. doi: 10.1016/j.talanta.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Guo H., Hu C., Qian J. Determination of un-derivatized long chain fatty acids using HPLC with an evaporative light-scattering detector. J. Am. Oil Chem. Soc. 2012;89:183–187. [Google Scholar]
- 6.Laakso T.S., Laakso I., Hiltunen R. Analysis of fatty acids by gas chromatography, and its relevance to research on health and nutrition. Anal. Chim. Acta. 2002;465:39–62. [Google Scholar]
- 7.European Pharmacopoeia (Ph Eur.), European Pharmacopoeia Commission, 7.0, Strasbourg, France, 2005, p. 2610.
- 8.Christie W.W. Equivalent chain-lengths of methyl ester derivatives of fatty acids on gas chromatography. J Chromatogr. A. 1988;447:305–314. [Google Scholar]
- 9.Metcalfe L.D., Schmitz A.A. The rapid preparation of fatty acid esters for gas chromatographic analysis. Anal. Chem. 1961;33:363–364. [Google Scholar]
- 10.Eder K. Gas chromatographic analysis of fatty acid methyl esters. J. Chromatogr. B. 1995;671:113–131. doi: 10.1016/0378-4347(95)00142-6. [DOI] [PubMed] [Google Scholar]
- 11.Ulberth F., Gabernig R.G., Schrammel F. Flame-ionization detector response to methyl, ethyl, propyl, and butyl esters of fatty acids. J. Am. Oil Chem. Soc. 1999;76:263–266. [Google Scholar]
- 12.Ulberth F., Gabernig R.G., Schrammel F. Accurate quantitation of short-, medium-, and long-chain fatty acid methyl esters by split-injection capillary gas–liquid chromatography. J. Chromatogr. A. 1995;704:455–463. [Google Scholar]
- 13.Lverson J.L., Sheppard A.J. Determination of fatty acids in butter fat using temperature-programmed gas chromatography of the butyl esters. Food Chem. 1986;21:223–234. [Google Scholar]
- 14.Tong L., Zhang L., Yu S.H. Analysis of the fatty acids from Periploca sepium by GC–MS and GC–FID. Asian J. Tradit. Med. 2007;2:110–114. [Google Scholar]
- 15.Botinestean C., Hadaruga N.G., Hadarura D.I. Fatty acids composition by gas chromatography–mass spectrometry (GC–MS) and most important physical–chemical parameters of tomato seed oil. J. Agroaliment. Process Technol. 2012;18(1):89–94. [Google Scholar]
- 16.Neves L., Perira M.A., Mota M. Detection and quantification of long chain fatty acids in liquid and solid samples and its relevance to understand anaerobic digestion of lipids. Bioresour. Technol. 2009;100:91–96. doi: 10.1016/j.biortech.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Mizumoto M., Shimokita E., Ona T. Rapid and direct characterization of total fatty acids in wood by thermochemolysis–gas chromatography–flame ionization detector/mass spectrometer with tetrabutylammonium hydroxide. J. Anal. Appl. Pyrolysis. 2011;87:163–167. [Google Scholar]
- 18.Meng Z., Wen D., Sun D. Rapid determination of C12–C26 non-derivatized fatty acids in human serum by fast gas chromatography. J. Sep. Sci. 2007;30:1537–1543. doi: 10.1002/jssc.200600344. [DOI] [PubMed] [Google Scholar]
- 19.Hua H., Liang Q., Chen J. Development of a derivatization-free GC–FID method for evaluation of free fatty acid levels in plasma of diabetic nephropathy patients. Chem. Res. 2011;27(4):578–583. [Google Scholar]
- 20.Brooks S.T., Spreen R.C., Xuk P.E. Analysis of un-derivatized oleic acid and related impurities by capillary gas chromatography. J. High Resolut. Chromatogr. 1990;13:287–289. [Google Scholar]
- 21.Hoerr C.W., Harwood H.J. The solubilities of oleic acids in common organic solvents. J. Phys. Chem. 1952;56(9):1068–1073. [Google Scholar]
- 22.ICH Guideline, Q2B, Validation of analytical procedures: methodology, in: Proceedings of the International Conference on Harmonization, 1996.