Summary
Gaucher’s disease (GD) is a rare disease characterized by a β-glucocerebroside accumulation in the reticulo-endothelial system. Patients may refer to the clinic with complaints of bone pain, hepatosplenomegaly, anemia, thrombocytopenia, growth retardation, interstitial pulmonary disease, pulmonary hypertension, and skeletal disorders. Skeletal system involvement is observed commonly in Gaucher patients and a significant cause of morbidity. Our patient was followed for several years as a glass child - osteogenesis imperfecta and he had joint deformities due to skeletal fractures. We wanted to present this case to raise awareness of GD’s skeletal involvement and effects of late diagnosis.
Keywords: Gaucher Disease, glass-child, β-glucocerebroside, osteogenesis imperfecta, fracture
Introduction
Gaucher’s disease (GD) is a rare disease characterized by a β-glucocerebroside accumulation in the reticulo-endothelial system cells due to a lack of glucocerebrosidase, which is a lysosomal enzyme. It has a prevalence of 1/30,000 to 1/100,000. Exhibiting an autosomal recessive inheritance pattern, the Gaucher’s disease is more prevalent among Ashkenazi Jews (1).
The most frequently observed sub-type is Type 1. Patients may refer to the clinic with complaints of bone pain, hepatosplenomegaly, anemia, thrombocytopenia, growth retardation, interstitial pulmonary disease, pulmonary hypertension, and skeletal disorders. We would like to present the case of a 34-year old patient who had experienced bone fractures for eight times since childhood and followed up with osteogenesis imperfecta despite splenomegaly. This case is significant as it represents an example for a late-diagnosed Gaucher’s disease.
Case report
A 34-year-old female patient referred to our hospital for a health certificate. The patient had had a normal physical development until ten years old, after which she had a fracture history at arms and legs. Her parents were cousins. Back in her adolescence, the patient had referred to a hospital with complaints of fatigue, stomach pain, and asymmetric enlargement of the stomach in the external appearance. However, the patient was not diagnosed or treated. Following that period, the patient was diagnosed with osteogenesis imperfecta, which is colloquially known as brittle bone or glass-bone disease, as she had a history of eight spontaneous fractured bones at upper and lower extremities due to simple traumas.
The patient experienced limitations of movement because of the multiple fractures. The physical examination revealed an extreme kyphosis and remarkably triangular face. An abdominal examination detected hepatomegaly and splenomegaly that expanded into the left inguinal region.
The hemogram presented a pancytopenia (Leukocyte: 3.5 K/uL, Hb: 10.1 g/dl, Plt: 31 10e3/uL). Biochemistry and thyroid function test results were normal. Serologic examinations revealed negative anti-smooth muscle antibody (ASMA), anti-microsomal antibody (AMA), and liver kidney microsomal antibody, whereas the anti-nuclear antibody (ANA) was positive (+) in granular type. Hepatitis test results were normal (Table 1).
Table 1.
Laboratory findings.
| Laboratory | Reference range | |
|---|---|---|
| WBC | 3.5 K/uL | 4.5–10.3 K/uL |
| Hemoglobin | 10.1 g/dl | 13.6–17.2 g/dl |
| Platelets | 31×10e3/uL | 156–373×10e3/uL |
| Sedimentation | 14 mm | 0–20 mm |
| Creatinine | 0.3 mg/dl | 0.3 – 0.7 mg/dl |
| Calcium | 8.8 mg/dl | 8.3–10.6 md/dl |
| Phosphorus | 3.8 mg/dl | 2.4–5.1 mg/dl |
| Albumin | 4.2 gr/dl | 3.2–4.8 gr/dl |
| Alkaline Phosphatase | 69 u/L | 45–129 u/L |
| Alanine Transaminase | 22 u/L | 10–49 u/L |
| Lactate Dehydrogenase | 127 u/L | 120–246 U/L |
| Acid Phosphatase | 11,1 IU/L | 0.5–1.9 IU/L |
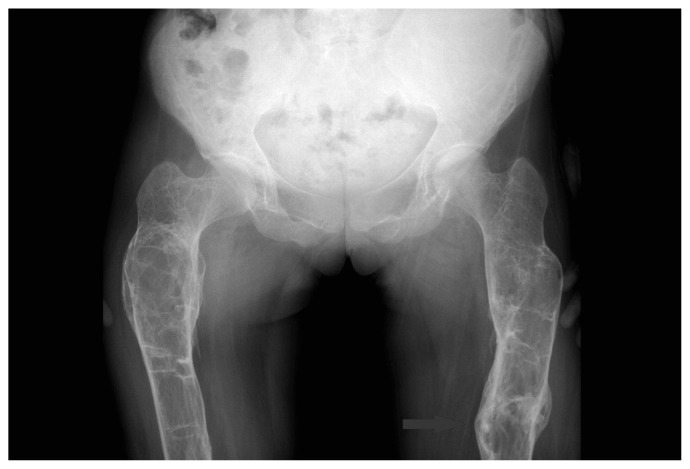
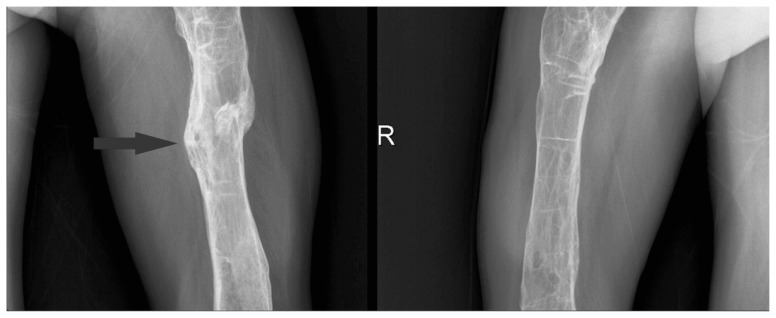
Direct radiographies drew attention to numerous organized fractures at upper and lower extremities and lytic areas (Figures 1, 2).
Figure 1.
Pelvic junction.
Figure 2.
Right and left femur.
The patient, who clearly had an anatomic disorder, underwent an ecocardiography due to orthopnea and paroxysmal nocturnal dyspnea. The Ejection Fraction was measured as 25%. There was no heart valve pathology in echocardiography. Right-sided atrioventricular cavities exhibited marked dilatation. The patient started to receive right-sided heart failure treatment.
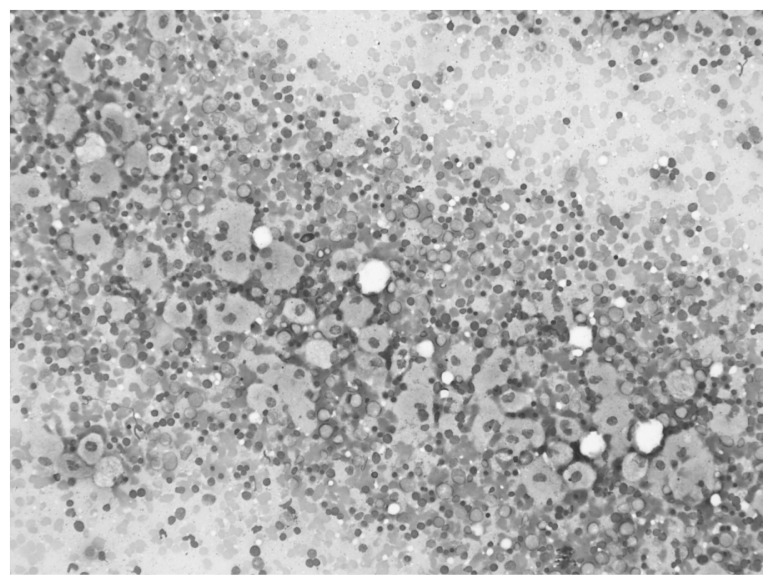
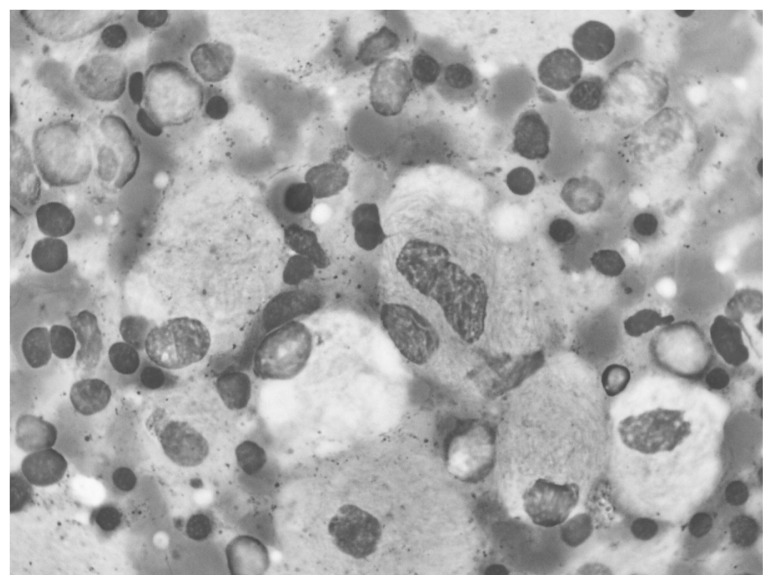
The bone marrow aspiration and biopsy operations performed due to the pancytopenia and hepatosplenomegaly revealed Gaucher cells of 10–80 μ, with single or multiple small, dark nucleus localized in the periphery, similar to wrinkled silk in appearance, and a blue-stained cytoplasm (Figures 3, 4).
Figure 3.
Gaucher cells, eccentric small nucleus and wrinkled silk like storages in blue cytoplasm.
Figure 4.
Gaucher cells, striated and fibrillary like cytoplasm with small dense nucleus.
We checked the glucocerebroside (β-glucosidase) enzyme level for a final diagnosis. With a 5.1% enzyme level (reference level > 20), the case was diagnosed with Gaucher’s disease. Glucocerebrosidase enzyme (Cerezyme 60 U/kg every 2 weeks) and bisphosphonate were started.
Discussion
Gaucher’s disease is the most prevalent one among lysosomal storage diseases. The disease follows an autosomal recessive inheritance pattern. Due to a series of mutations at the GBA (Glucosylceramide Beta) gene, which is localized at the chromosome 1q21., patients develop a lack of β-glucocerebroside (acid-β-glucosidase) enzyme coded by the gene. Because of this lack, patients exhibit an excessive accumulation of lypid glucocerebroside at the lysosomes of their macrophages (2, 3).
The disease is divided into three clinical types. Type 1 can be observed in patients of all ages, and ethnic differences are demonstrated. On average, patients are diagnosed with hepatosplenomega and splenomegaly at their late twenties.
A literature review presented by Essebar et al. with 11 cases found average age of diagnosis as 4 (between 3 months and 14 years). Nine patients were Type 1, with an average age of diagnosis of 6 years (ranging from 2 to 14 years) (3). This patient had also referred to a hospital with complaints of splenomegaly at 10 years old; however, she was diagnosed after 24 years.
The Gaucher’s disease appears as a multi-systemic disease, which is associated with the progressive growth of macrophages with glucocerebrosidase accumulation in different organs. Type 1 has the highest prevalence (94%). Although neurological involvement is not observed, the disease exhibits a large-scale involvement that includes the spleen, liver, bone marrow, lung, bones, and kidneys. Untreated patients frequently exhibit hematological complications associated with splenomegaly and bone marrow involvement (4). Patients referred to clinic with symptoms associated with anemia and thrombocytopenia. The reported patient also suffered from pancytopenia associated with splenomegaly and deteriorated marrow hematopoiesis when she referred to a hospital for the first time.
The rarity of the disease and non-specific symptoms may cause the diagnosis of the disease to be delayed. Patients can be diagnosed with viral infection or hematological malignancy because of hepatosplenomegaly. The cases can be followed by Niemann pick disease who has hepatosplenomegaly and neurologic degeneration. The presentation with a bone crisis quite often leads to the misdiagnosis of bacterial osteomyelitis. Also, in children, Legg-Calve-Perthes disease, an avascular necrosis of the femoral head could be confused with GD diagnostic delays led to complications that are potentially preventable (5). Our patient watched with osteogenesis imperfecta for a long time, before diagnosis of GD.
The skeletal involvement is a notable cause of morbidity that results in a serious limitation of movement. It has a wide range of complications associated with the skeletal system. Children may exhibit symptoms such as growth retardation, osteopenia, lytic lesions, osteonecrosis, infarct of the cortical-medullar bone, pathological fracture, and failure of regeneration (6, 7). Nevertheless, some patients are asymptomatic. On a global scale, a study demonstrated that 62% of the patients with GD exhibited a radiological finding of bone involvement while 43% reported pain (8). The Erlenmeyer flask deformity, which is a significant radiological finding, starts in the pre-puberty period and is observed in 80% of the adults. It is frequently found in the distal femur and other tubular bones; and its course includes expanding metaphysis regions and narrowing diaphysis (9). Our case had a history of eight fractures. The patient was diagnosed with the glass-bone disease as she frequently experienced bone fractures. Pre-diagnosis radiographies revealed extensive sequel findings associated with the fractures, whereas a physical examination detected deformities causing disability. In fact, osteogenesis imperfecta is not characterized with a combination of splenomegaly and bone fractures. Therefore, the physicians should have noticed the situation with the splenomegaly combined with fractures and pancytopenia.
The skeletal system involvement is treated with enzyme replacement and bisphosphonates. Patients treated with bisphosphonates supplemented by enzyme replacement, Vitamin D and calcium therapies exhibited clearly better results in terms of increases in bone densitometer (10). We included the bisphosphonate therapy in our patient’s treatment. Treatment in the childhood considerably mitigates the osteonecrosis risk in adults. Reduced prevalence of late complications is found clearly related to early treatment (11). In our case, perhaps the patient would have never developed a skeletal deformity if she were diagnosed with the Gaucher’s disease at an earlier time.
Conclusion
Skeletal system involvement is observed commonly in Gaucher patients and a significant cause of morbidity. Daily physical activity of patients are affected by skeletal system involvement. It can be seen as pathologic fractures, joint shape changes, lytic lesions. In contrast, it may be asymptomatic. Good response can be achieved with early treatment. Therefore, GD should be considered in patients who have hematologic findings, hepatosplenomegaly, pathologic skeletal system findings with visceral involvement such as pulmonary hypertension for early diagnosis. Our patient was followed for several years as a glass child - osteogenesis imperfecta and he had joint deformities due to skeletal fractures. We wanted to present this case to raise awareness of GD’s skeletal involvement and effects of late diagnosis.
References
- 1.Mehta A. Epidemiology and natural history of Gaucher’s disease. Eur J Intern Med. 2006 Nov;17(Suppl):S2–5. doi: 10.1016/j.ejim.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Nagral A. Gaucher Disease. Journal of Clinical and Experimental Hepatology. 2014:37–50. doi: 10.1016/j.jceh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essabar L, Meskini T, Lamalmi N, Ettair S, et al. Gaucher’s disease: report of 11 cases with review of literature. Pan Afr Med J. 2015 Jan 7;20:18. doi: 10.11604/pamj.2015.20.18.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dandana A, Ben Khelifa S, Chahed H, Miled A, Ferchichi S. Gaucher Disease: Clinical, Biological and Therapeutic Aspects. Pathobiology. 2016;83(1):13–23. doi: 10.1159/000440865. [DOI] [PubMed] [Google Scholar]
- 5.Marcucci G, Zimran A, Bembi B, Kanis J, et al. Gaucher Disease and Bone Manifestations. Calcif Tissue Int. 2014;95:477–494. doi: 10.1007/s00223-014-9923-y. [DOI] [PubMed] [Google Scholar]
- 6.Simpson WL, Hermann G, Balwani M. Imaging of Gaucher disease. World J Radiol. 2014 Sep 28;6(9):657–68. doi: 10.4329/wjr.v6.i9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikosch P, Hughes D. An overview on bone manifestations in Gaucher disease. Wien Med Wochenschr. 2010 Dec;160(23–24):609–24. doi: 10.1007/s10354-010-0841-y. [DOI] [PubMed] [Google Scholar]
- 8.Mucci JM, Rozenfeld P. Pathogenesis of Bone Alterations in Gaucher Disease: The Role of Immune System. J Immunol Res. 2015;2015:192761. doi: 10.1155/2015/192761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masi L, Brandi ML. Gaucher disease: The role of the specialist on metabolic bone diseases. Clin Cases Miner Bone Metab. 2015 May-Aug;12(2):165–9. doi: 10.11138/ccmbm/2015.12.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuffrida G, Cingari MR, Parrinello N, Romano A, et al. Bone turnover markers in patients with type 1 Gaucher disease. Hematol Rep. 2012 Nov 19;4(4):e21. doi: 10.4081/hr.2012.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deegan PB, Pavlova E, Tindall J, Stein PE, et al. Osseous manifestations of adult Gaucher disease in the era of enzyme replacement therapy. Medicine (Baltimore) 2011 Jan;90(1):52–60. doi: 10.1097/MD.0b013e3182057be4. [DOI] [PubMed] [Google Scholar]