Abstract
Ezetimibe, which selectively inhibits cholesterol absorption across the intestinal wall and is used as an antihyperlipidemic agent, is synthesized for commercial use as a drug substance in highly pure form. During the synthetic process development studies of ezetimibe, an impurity was detected in the final product at levels ranging from 0.05% to 0.15% in reverse phase gradient high performance liquid chromatography (HPLC) method and its molecular weight was determined by LC–MS analysis. The impurity was identified as (3R,4S)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)-1-phenylazetidin-2-one which is called desfluoro ezetimibe (lactam-related) impurity, synthesized and characterized, the mechanism of its formation was discussed in detail. After all standardization procedures, it was used as a reference standard during validation of HPLC method and routine analyses. In addition, content of Eze-1 desfluoro impurity in Eze-1 intermediates was specified as 0.10% to keep the formation of desfluoro ezetimibe impurity under control and the related substances HPLC method was validated accordingly.
Keywords: Ezetimibe, Desfluoro ezetimibe, Synthesis, Characterization, NMR, HPLC
1. Introduction
There are two recognized sources of cholesterol in the serum: biosynthesis in the liver and absorption of dietary cholesterol in the small intestine [1], [2]. Statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the catalyst of the rate-limiting step of cholesterol biosynthesis in the liver, and have been prescribed as the predominant class of cholesterol-lowering agents since 1980s [3]. Ezetimibe, (3R,4S)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)azetidin-2-one (Scheme 1), has become an increasingly important choice for reducing serum cholesterol level and it is the only marketed example of this new class of anticholesterolemia drugs [4], [5]. Ezetimibe is a potent, metabolically stable cholesterol absorption inhibitor [6], which strongly blocks the absorption of biliary and dietary cholesterol from the small intestine without affecting the absorption of fat-soluble vitamins, triglycerides or bile acids [7]. It may be used alone or together with statins (e.g. ezetimibe/simvastatin) for the treatment of primary hypercholesterolemia, homozygous sitosterolemia, homozygous familial hypercholesterolemia, and mixed hyperlipidemia [8], [9], and reduces the risk of coronary heart disease [10].
Scheme 1.
Synthesis of ezetimibe and desfluoro ezetimibe impurity.
Organic and pharmaceutical chemists were attracted by the novel structure and potent biological activity of ezetimibe, which led to the development of several syntheses for this active pharmaceutical ingredient (API) [11], [12], [13], [14], [15], [16], [17]. Few analytical methods have been reported in the literature for the determination of ezetimibe including studies on its pharmaceutical dosage forms and degradation studies [18], [19], [20], [21]. In recent years, studies related to identification, synthesis and characterization of process and degradation related impurities, including stereoisomers of ezetimibe, have been reported [22], [23], [24], [25], [26], [27], [28].
During high performance liquid chromatograph (HPLC) analyses of the drug substance ezetimibe, synthesized according to the process given in Scheme 1 [16], one process related impurity was observed consistently in the range of 0.05–0.15%. As per the general guidelines recommended to qualify the drug substance by International Conference on Harmonisation (ICH) [29], [30], the acceptable level for a known and unknown related compounds (impurity) should be less than 0.15% and 0.10%, respectively; and the impurities present in the drug substance must be identified and characterized. In recent years, the impurity profile of a drug substance becomes more important for marketing approval and this work is done as part of a drug development process [31], [32], [33], [34], [35].
The impurity observed on HPLC analyses of ezetimibe was identified by LC–MS studies as desfluoro ezetimibe, (3R,4S)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)-1-phenylazetidin-2-one and pathway of its formation was clarified after thorough evaluation of the ezetimibe synthetic route. The same pathway was used for synthesis of desfluoro impurity and its related intermediates. All compounds synthesized in the scope of this study were characterized by MS, IR and NMR (1H, 13C, DEPT, 19F). To the best of our knowledge, desfluoro ezetimibe impurities were mentioned only in a few reports [36], [37], [38], [39] and there is no report in the literature on the identification, synthesis and characterization of the desfluoro impurity. As complementary of this work, HPLC related substances methods for ezetimibe (Eze) and its intermediate Eze-1 were validated and reported herein.
2. Experimental
2.1. Chemicals, reagents and samples
Ezetimibe samples were taken from commercial batches produced by Deva Holding A.Ş. (Tekirdağ, Turkey), or synthesized in the R&D laboratories of Deva. Ezetimibe standard was supplied by a specialized team on standardization of reference standards for analytical use in Deva. Synthetic and analytical reagents and solvents were supplied from different chemical companies such as Merck KGaA (Darmstadt, Germany), J.T. Baker (Phillipsburg, USA), Sigma-Aldrich (St. Louis, MO, USA), Lab-Scan (Gliwice, Poland), Acros Organics (Geel, Belgium), Akkim (Yalova, Turkey), Sodas Sodyum Sanayi A.S. (Izmir, Turkey), and Emekcioglu Tuz A.S. (Ankara, Turkey). All non-aqueous reactions were performed in dry glassware under an atmosphere of dry nitrogen. Deionized water was prepared using MilliQ plus purification system (Millipore, Bedford, MA, USA). Deuterated solvents (dimethylsulfoxide-d6 and deuterated chloroform) were purchased from Merck KGaA (Darmstadt, Germany).
2.2. Mass spectrometry
The mass spectra were recorded on Waters LC–MS ZQ 2000/4000 system (Waters Corporation, Milford, MA, USA). The fragmentation profile of the samples was established by carrying out MS studies in the positive or negative electrospray ionization (ESI) mode. For LC–MS identification of the impurity, the HPLC method given infra for ezetimibe related substances was used. The samples of synthesized compounds were directly infused using a syringe at a concentration of 1 mg/mL in acetonitrile.
2.3. NMR spectroscopy
1H, 13C and DEPT NMR experiments were performed on a 300 MHz NMR Spectrometer (Varian NMR Instruments, Oxford, UK; later acquired by Agilent Technologies, Santa Clara, CA, USA) using deuterated dimethylsulfoxide (DMSO-d6) and deuterated chloroform (CDCl3) as a solvent and tetramethylsilane (TMS) as an internal standard.
2.4. FT-IR spectroscopy and melting range
Samples were measured as neat by Attenuated Total Reflectance (ATR) on Shimadzu FTIR Spectrometer IR Prestige-21 (Shimadzu Corporation, Kyoto, Japan) in the range of 600–4000 cm−1 with 20 scans and 2 cm−1 resolution. Melting ranges of the samples were determined on an Electrothermal 9100 digital melting point instrument (Thermo Fisher Scientific, Essex, UK).
2.5. HPLC
Chromatographic separations were performed on HPLC system with Waters Alliance 2695 separation module equipped with a Waters 996 photodiode array detector and an Empower-pro data handling system (Waters Corporation, Milford, MA, USA).
The related substances analysis of ezetimibe was carried out on Zorbax Rx C8 (0.25 m×4.6 mm, 5 µm) column at 5 °C sample and 35 °C column temperatures. The injection volume and detection wavelength were fixed at 10 µL and 220 nm, respectively. Data was acquired during 50 min by using gradient elution system flowing at a rate of 1.3 mL/min. Buffer solution was prepared by dissolving 2.71 g of potassium dihydrogen phosphate in 1000 mL of water and pH was adjusted to 3.0±0.05 with 10% phosporic acid. Mixture of buffer solution and acetonitrile (80:20) was used as mobile phase A; and acetonitrile as diluent and mobile phase B. Test solution was prepared by dissolving 25.0 mg of sample in 25.0 mL of acetonitrile (1.0 mg/mL). Reference solution was prepared by dissolving 25.0 mg of ezetimibe reference standard (RS) in 25.0 mL of acetonitrile, followed by dilution of 1.0 mL of this solution to 100.0 mL with acetonitrile, then dilution of 1.0 mL of the resulting solution to 10.0 mL with acetonitrile (0.10% with respect to the test solution, 0.001 mg/mL). Test solutions were injected freshly and reference solution was stable up to 48 h at room temperature. The separation was employed using gradient elution based on the following program: time (min)/% of mobile phase B: 0/12, 5/12, 25/62, 40/62, 41/12, 50/12. This analysis method was also used during standardization of desfluoro ezetimibe.
The related substances analysis of Eze-1 was carried out on Kromasil (or ACE) C18 (0.25 m×4.6 mm, 5 µm) column with 10 µL injection volume at a wavelength of 225 nm by using gradient elution flowing at a rate of 1.1 mL/min during 25 min. Column and sample temperatures were 30 °C and 25 °C, respectively. Buffer solution was prepared by dissolving 2.72 g of potassium dihydrogen phosphate in 1000 mL of water and pH was adjusted to 7.5±0.05 with 10% potassium hydroxide. Mixture of buffer solution and acetonitrile (60:40) was used as mobile phase A; and acetonitrile as diluent and mobile phase B. Test solution was prepared by dissolving 20.0 mg of the sample in 50.0 mL of acetonitrile (0.4 mg/mL). Reference solution was prepared by dissolving 20.0 mg of Eze-1 RS in 50.0 mL of acetonitrile, followed by dilution of 1.5 mL of this solution to 50.0 mL with acetonitrile, then dilution of 1.0 mL of the resulting solution to 10.0 mL with acetonitrile (0.30% with respect to the test solution; 0.0012 mg/mL). Reference and test solutions were found stable up to 48 h at room temperature. The separation was employed using gradient elution based on the following program: time (min)/% of mobile phase B: 0/0, 2/0, 15/35, 18/35, 20/0, 25/0. This analysis method was also used during standardization of desfluoro Eze-1.
The related substances analysis of Eze-3 was carried out on Zorbax Rx C8 (0.25 m×4.6 mm, 5 µm) column with 20 µL injection volume at a wavelength of 220 nm by using gradient elution flowing at a rate of 1.3 mL/min during 40 min. Column and sample temperatures were 35 °C and 20 °C, respectively. Mobile phase A was prepared by dissolving 2.74 g of potassium dihydrogen phosphate in 1000 mL of water and pH was adjusted to 3.0±0.05 with 10% phosphoric acid. Acetonitrile was used as diluent and mobile phase B. Test solution was prepared by dissolving 10.0 mg of the sample in 10.0 mL of acetonitrile (1.0 mg/mL) and reference solution by dissolving 10.0 mg of Eze-3 RS in 50.0 mL of acetonitrile. Reference and test solutions were found stable up to 96 h at room temperature. The separation was employed using gradient elution based on the following program: time (min)/% of mobile phase B: 0/30, 5/30, 30/70, 32/30, 40/30.
The related substances analysis of Eze-4 was carried out on Zorbax Rx C8 (0.25 m×4.6 mm, 5 µm) column with 10 µL injection volume at a wavelength of 220 nm by using gradient elution flowing at a rate of 1.3 mL/min during 55 min. Column and sample temperatures were 35 °C and 25 °C, respectively. Mobile phase A was prepared by dissolving 2.74 g of potassium dihydrogen phosphate in 1000 mL of water and pH was adjusted to 7.5±0.05 with 10% potassium hydroxide. Acetonitrile was used as diluent and mobile phase B. Test solution was prepared by dissolving 30.0 mg of the sample in 10.0 mL of acetonitrile (3.0 mg/mL) and reference solution by dissolving 10.0 mg of Eze-4 RS in 50.0 mL of acetonitrile (0.2 mg/mL). Reference and test solutions were found stable up to 96 h at room temperature. The separation was employed using gradient elution based on the following program: time (min)/% of mobile phase B: 0/35, 2/35, 28/75, 46/75, 47/35, 55/35.
2.6. Ultra performance liquid chromatography (UPLC)
Chromatographic separations for Eze-5, 6, 7 and their related substances were performed on Waters Acquity UPLC system equipped with Waters TUV detector and Empower-pro data handling system (Waters Corporation, Milford, MA, USA). The analysis was carried out on ChromPEARL SH C18 (0.15 m×2.0 mm, 1.7 μm) column with 2 µL injection volume at a wavelength of 220 nm by using gradient elution flowing at a rate of 0.4 mL/min during 33 min. Column and sample temperatures were 40 °C and 5 °C, respectively. Buffer solution was prepared by dissolving 1.36 g of potassium dihydrogen phosphate in 1000 mL of water and pH was adjusted to 7.5±0.05 with 10% potassium hydroxide. Mixture of buffer solution and acetonitrile (80:20) was used as mobile phase A, and acetonitrile as diluent and mobile phase B. Test solution was prepared by dissolving 25.0 mg of the sample in 25.0 mL of acetonitrile (1.0 mg/mL). Reference solution was prepared by dissolving 25.0 mg of related reference standard in 25.0 mL of acetonitrile, followed by dilution of 0.5 mL of this solution to 50.0 mL with acetonitrile (1.0% with respect to the test solution; 0.01 mg/mL). Reference and test solutions were found stable up to 48, 24 and 72 h at 5 °C, respectively. The separation was employed using gradient elution based on the following program: time (min)/% of mobile phase B: 0/38, 12/53, 15/63, 25/88, 30/38, 33/38. Analyses of desfluoro Eze-5, 6, and 7 were carried out by using this method.
2.7. Synthesis of ezetimibe and desfluoro ezetimibe impurity
2.7.1. Synthesis of Eze-1 and desfluoro Eze-1
4-Hydroxybenzaldehyde (1.0 equiv.) was dissolved in isopropanol at 60 °C under stirring. 4-Fluoroaniline or aniline (1.0 equiv.) was added to the resulting solution and the mixture was stirred at 60 °C for 3 h. The solution was allowed to cool to ambient, during which the expected imine crystallized. The crystals were filtered out, washed with isopropanol and dried at room temperature to obtain 4-((4-fluorophenylimino)methyl)phenol (Eze-1) or 4-((phenylimino)methyl)phenol (desfluoro Eze-1) as yellowish crystalline powder with 82% and 85% yield, respectively.
2.7.2. Synthesis of Eze-4
Mixture of 3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-(S)-phenyloxazolidin-2-one (Eze-3) (1.0 equiv.), methanol, trimethylorthoformate (2.0 equiv.) and catalytic amount of p-toluene sulfonic acid monohydrate (pTSA) was heated and stirred until no trace of starting material (Eze-3) was observed in the reaction mixture. After completion of the reaction, the mixture was concentrated to a defined volume, quenched by addition of N,N-diisopropylethylamine (DIPEA) and followed by cooling for crystallization. The product crystals were filtered, washed with methanol and dried under vacuum to obtain Eze-4 as white to slightly yellowish crystalline powder (83%).
2.7.3. Synthesis of Eze-5 and desfluoro Eze-5
Mixture of DIPEA (4.0 equiv.), benzyl chloroformate (CbzCl, 1.3 equiv.) and toluene was added into a cooled suspension of Eze-1 or desfluoro Eze-1 (1.2 equiv.) in methylene chloride. In a separate reaction vessel, titanium complex was prepared by mixing titanium tetrachloride (0.9 equiv.) and titanium tetraisopropoxide (0.3 equiv.) in methylene chloride under cooling. Solution of Eze-4 (1.0 equiv.) in methylene chloride was added into the cooled Eze-2 (Cbz-protected Eze-1) or desfluoro-Eze-2 (Cbz-protected desfluoro Eze-1) solution at −15 °C and it was followed by additional cooling to −45 °C. The titanium complex mixture was added into this solution at −45 °C and stirred for reaction. The reaction was quenched by addition of acetic acid and aqueous solution of citric acid, respectively. The synthesis was followed by warming up the reaction mixture to 25 °C, work-up and crystallization processes to obtain Eze-5 or desfluoro Eze-5 as white to slightly yellowish crystalline powder with 49% and 35% yield, respectively.
2.7.4. Synthesis of Eze-6 and desfluoro Eze-6
Dimethyl sulfide borane (1 M in methylene chloride, BH3.Me2S) and (R)-2-Me-CBS oxazaborolidine (1 M in toluene) Corey–Bakshi–Shibata catalyst mixture was prepared in methylene chloride. Solution of Eze-5 or desfluoro Eze-5 (1.0 equiv.) in methylene chloride was added into the catalyst mixture. After completion of the reaction, it was quenched by addition of methanol and aqueous solution of hydrochloric acid (1.0 M), respectively. The synthesis was followed by work-up and crystallization processes to obtain Eze-6 or desfluoro Eze-6 as white to slightly yellowish crystalline powder with 79% and 82% yield, respectively.
2.7.5. Synthesis of Eze-7 and desfluoro Eze-7
Lithium perchlorate (0.1 equiv.) and hexamethyldisilazane (HMDS, 0.9 equiv.) were added into the solution of Eze-6 or desfluoro Eze-6 (1.0 equiv.) in methylene chloride at room temperature. After stirring for a defined time period and completion of the reaction, it was quenched by addition of water. The synthesis was followed by work-up and crystallization processes to obtain Eze-7 or desfluoro Eze-7 as white to slightly yellowish crystalline powder with 90% and 94% yield, respectively.
2.7.6. Synthesis of ezetimibe and desfluoro ezetimibe
Solution of Eze-7 or desfluoro Eze-7 (1.0 equiv.) in tetrahydrofuran (THF) was cooled to −20 °C. N,O-bis(trimethylsilyl)acetamide (BSA, 2.1 equiv.) and catalytic amount of tetrabutylammonium hydroxide (TBAH, 50% in methanol) were added into this mixture and stirred for a defined time. After completion of the reaction, it was quenched by addition of acetic acid and concentrated by distillation. Methanol, acetic acid and catalytic amount of Pd/C catalyst (5%) were added into the residue and stirred in the presence of hydrogen (H2) gas. After completion of the reaction, the mixture was filtered, concentrated to a defined volume and crystallized to obtain crude ezetimibe. The synthesis was followed by recrystallization and drying processes to obtain ezetimibe or desfluoro ezetimibe in highly pure form as white to off-white crystalline powder with 60% and 48% yield, respectively.
3. Results and discussion
3.1. Ezetimibe and desfluoro ezetimibe syntheses
Ezetimibe is a β-lactam type hypolipidemic agent bearing three chiral centers. Of these, two are located on the β-lactam ring while the last one (alcohol) is present in the side chain. The molecule is manufactured as a single enantiomer with absolute stereochemistry (2R,11S,5S). The enantioselective determination of the opposite OH-enantiomer, which is likely to be detected in the final API, is an integral part of the ezetimibe specification. A validated chiral HPLC method was used to control this enantiomer with the acceptance limit of not more than 0.15%.
The stereochemistry issues are important for ensuring the production of diastereo as well as enantiopure final API. Ezetimibe and desfluoro ezetimibe were synthesized according to the reaction steps given in Scheme 1. Ketone functionality of Eze-3 was protected by using trimethyl orthoformate and catalytic amount of p-toluenesulfonic acid to form dimethyl ketal Eze-4. Corresponding Eze-1 imines (Schiff base) were prepared by reaction of 4-hydroxybenzaldehyde and 4-fluoroaniline or aniline and then protected by reaction with benzyl chloroformate under basic conditions to obtain Eze-2 or desfluoro Eze-2 intermediates, respectively. Highly exothermic coupling reaction of protected imines (Eze-2 or desfluoro Eze-2) and Eze-4 was done under strong cooling by using titanium complex (Ti(OiPr)Cl3) prepared by mixing titanium tetrachloride and titanium tetraisopropoxide with the ratio of 3:1. The reaction was quenched by acid addition, and ketal deprotection was done by further acid treatment in situ to obtain crystalline Eze-5 or desfluoro Eze-5.
Chiral centers C-2 and C-11 were created by diastereoselective addition of optically active titanium enolate of Eze-4 to C=N double bond of Cbz-protected imine Eze-2. Chirality of oxazolidide-ketone Eze-4 induces chirality of both C-2 and C-11 chiral centers during the coupling reaction of Eze-2 and Eze-4 intermediates. Thus, it is highly important to have this intermediate with high optical purity. For this reason, optical purity of Eze-3 was controlled and a limit for content of its enantiomer was set as not more than 0.10% and reference standard of Eze-3 enantiomer was prepared. Oxazolidide-acetal Eze-4 was treated with a strong base DIPEA to generate an enolate which by action of Ti(OiPr)Cl3 formed Ti(IV)-enolate of Eze-4. The latter exists due to steric bulkiness of phenyl group on oxazolidinone ring exclusively in (Z)-configuration. Imine Eze-2 is (E)–configured and binds to Ti-enolate, thus forming essentially sterically less hindered and sterically more hindered complexes. In both cases, imine is located below the plane of enolate, which defines absolute configuration at C-2. Accordingly, only two diastereoisomeric products were formed in this reaction step with highly prevailing as expected (6–8:1). Both primary products were hydrolyzed in situ by acids forming ketone Eze-5 and its diastereoisomer. Luckily, minor and unwanted diastereoisomer of Eze-5 is much more soluble and single crystallization was sufficient to reduce its content.
The chiral center bearing hydroxyl group (C-5) was created by applying Corey–Bakshi–Shibata (CBS) protocol to Eze-5 intermediates to obtain the corresponding Eze-6 alcohols, namely reduction of ketone with borane dimethyl sulfide complex in the presence of (R)-2-Me-CBS oxazaborolidine ((R)-hexahydro-1-methyl-3,3-diphenylpyrrolo[1,2]-c[1,3,2]oxazaborole) as a chirality inductor. The enantioselective reduction of Eze-5 provided highly predominating alcohol Eze-6 accompanied by minor amounts of epimeric alcohol epi-Eze-6 with the amount of maximum 4%. The content of the latter compound was set to not more than 3.0% and reference standard was prepared by CBS-reduction of Eze-5 in the presence of (S)-2-Me-CBS oxazaborolidine. By using solubility differences of epimers, content of minor epimeric alcohol was further decreased by recrystallization of Eze-6 in methanol. Of course, diastereomeric impurity of ketone Eze-5 also underwent reduction to corresponding alcohols. However, the content of such alcohols, which was already low, was further decreased by crystallization of the reaction product Eze-6.
In the following step, TMS protection of alcohol moiety of Eze-6 intermediates was done by reaction with HMDS under catalysis of lithium perchlorate to form corresponding Eze-7 intermediates and purified by crystallization which enhanced the stereochemical purity further. In the last complex step, trans-β-lactam ring was closed first by the action of BSA and catalytic amount of TBAH upon Eze-7 intermediates. The formation of cis-β-lactam that would originate from silyl-ether originating from epimeric Eze-5 was much more sluggish. This implies that the formation of measurable quantities of ezetimibe stereoisomer with 2R,11R,5S configuration as an impurity in final API is highly improbable. The formed 2-azetidinone intermediates reacted without isolation further for Cbz- and TMS-deprotections by Pd/C catalyzed hydrogenation under acidic conditions to obtain crude ezetimibe or desfluoro ezetimibe. Furthermore, products were crystallized so that the content of such an impurity with 2R,11R,5S configuration, if any formed, was further reduced. 2,11-epimer and enantiomer of ezetimibe with 2S,11R,5S and 2S,11R,5R configurations, respectively, would be only hypothetical products of CBS-reduction of very improbable diastereomeric form of ketone Eze-5. 5-Epimer of ezetimibe with 2R,11S,5R configuration and called ezetimibe OH-isomer was more likely to be detected in manufactured API as it was formed from epimer of Eze-6 via epimer of Eze-7. Accordingly, this stereoisomer was prepared and routinely tested for its presence in final API (limit not more than 0.15%). Ezetimibe and desfluoro ezetimibe were finally recrystallized to reach pharmaceutically acceptable purity and highly pure product to be used as reference standard during validation of related substances HPLC method of ezetimibe, respectively. All the facts discussed above clearly demonstrate that the production of stereochemically highly pure final products was ensured.
3.2. Detection and identification of desfluoro impurity
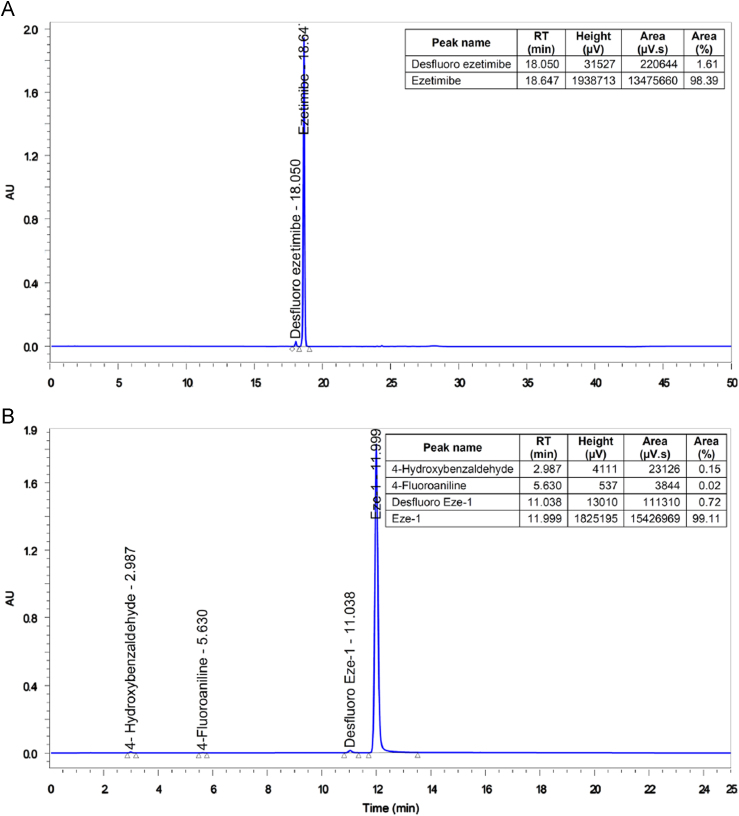
A typical analytical HPLC chromatogram of a production batch of ezetimibe bulk drug spiked with desfluoro impurity (system suitability solution) is shown in Fig. 1A. The impurity was detected in the final drug substance during process development studies and its identification was done by LC–MS. The ESI mass spectrum of the impurity observed at 18.05 min (RRT 0.97) exhibited a molecular ion at m/z 392 in positive ion mode, indicating molecular weight of 391, which was less by 18 amu than that of ezetimibe. The difference relates to a missing fluorine (−19) and addition of a hydrogen (+1) instead. There are two possibilities to form such an impurity in the final product. One is potential Eze-3 desfluoro impurity and the second one is Eze-1 desfluoro impurity. After our analytical studies, it was found that Eze-1 contained the related desfluoro impurity and Eze-3 did not. So, the related desfluoro impurity was subsequently synthesized to obtain sufficient quantity for full characterization and further analytical studies. The synthesized impurity was co-injected with ezetimibe to confirm its identity based on the retention time matching. The impurity at 18.05 min (RRT 0.97) was well resolved from ezetimibe and the HPLC method was validated accordingly.
Fig. 1.
HPLC chromatogram of (A) ezetimibe spiked with desfluoro ezetimibe impurity (system suitability solution) and (B) Eze-1 spiked with desfluoro Eze-1 impurity and other related impurities (system suitability solution).
3.3. Formation of desfluoro impurity and structure elucidations
Solubility of desfluoro ezetimibe impurity is very similar to that of ezetimibe and it was not possible to remove this impurity by recrystallization processes. That is why its formation should be kept under control from the beginning of the ezetimibe production process. The source of this impurity is desfluoro Eze-1, which is found in the intermediate Eze-1. As shown in Scheme 1, Eze-1 and desfluoro Eze-1 and their corresponding intermediates are similarly reacting under the same synthetic conditions. So, to keep the amount of desfluoro impurity within the specified known impurity limits (0.15%) in the final product, the amount of desfluoro Eze-1 should be kept below 0.15% in Eze-1. To be on the safe side, the limit of desfluoro Eze-1 was specified as 0.10% in Eze-1; an HPLC method for related substances was developed and validated accordingly. Chromatogram of system suitability solution where Eze-1 was spiked with its related substances is given in Fig. 1B.
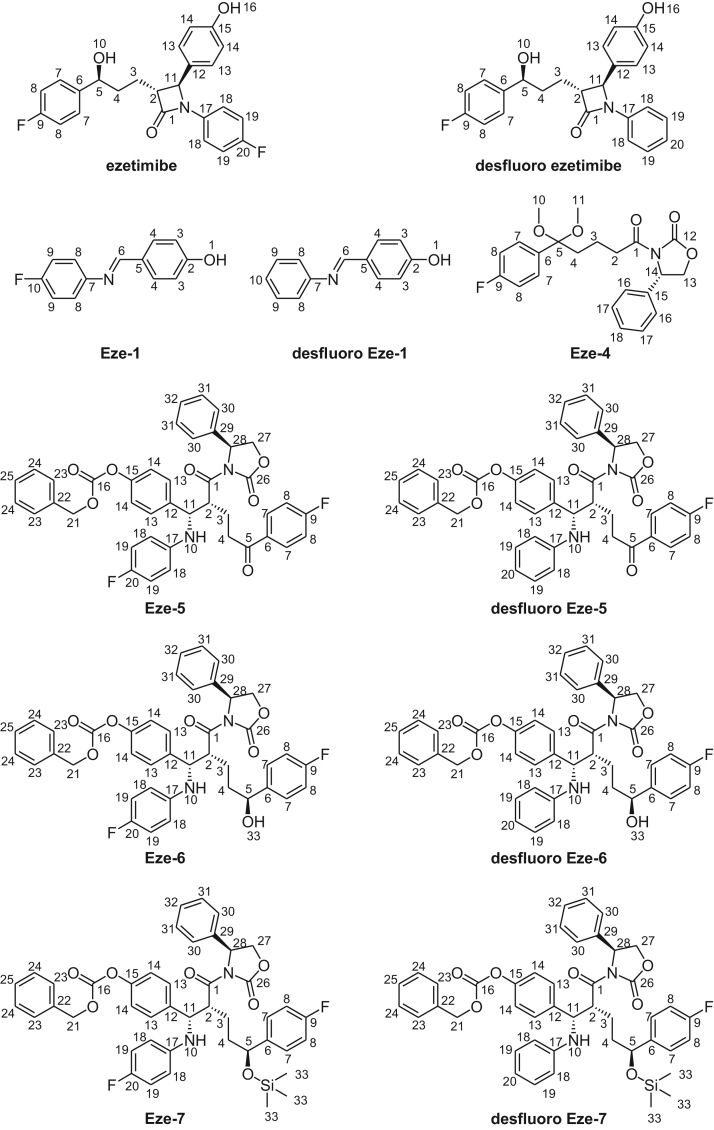
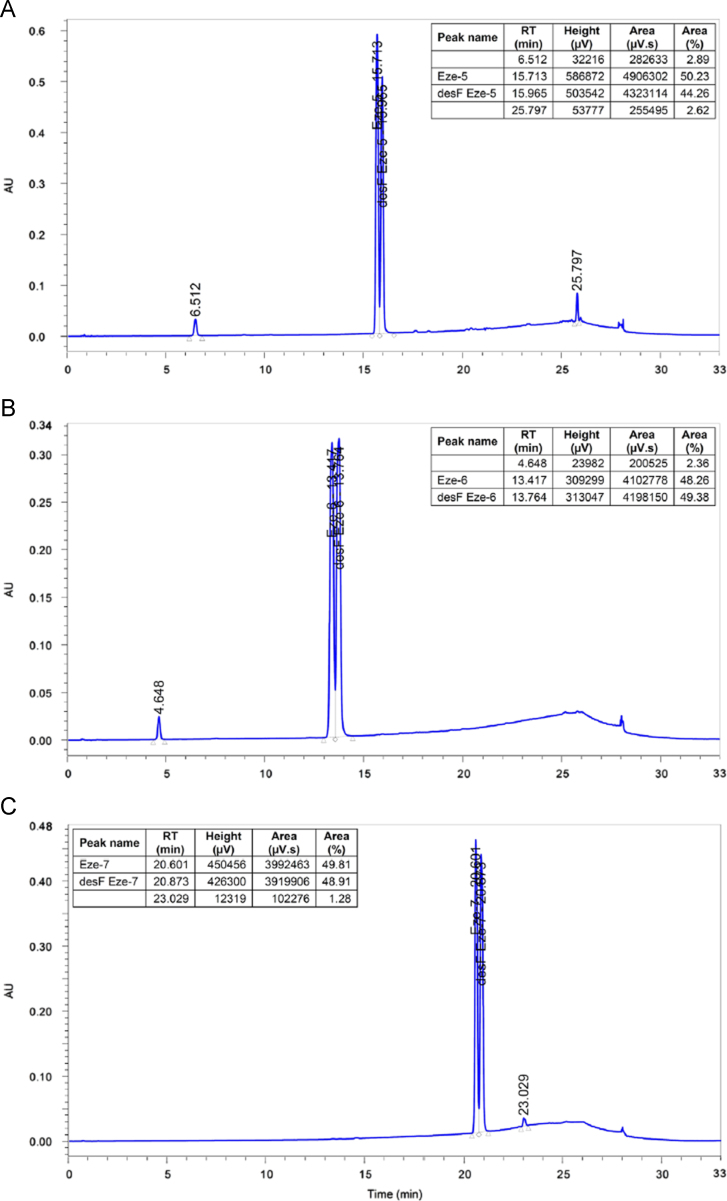
Synthesis of ezetimibe was done by using 0.08% desfluoro Eze-1 containing Eze-1 intermediate, resulting in formation of 0.05% of desfluoro ezetimibe impurity. Purity, melting range, IR and mass data of the synthesized compounds (Fig. 2) are listed in Table 1. Also, UPLC chromatograms of Eze-5, Eze-6 and Eze-7 spiked with their related desfluoro impurities are shown in Fig. 3.
Fig. 2.
Structures of ezetimibe, desfluoro impurity and intermediates.
Table 1.
Purity, melting range, FTIR and mass spectral data.
| Compounda | Purity (%) | Melting range (°C) | IR (neat, cm−1) | MS m/z |
|---|---|---|---|---|
| Eze-1 | 99.5b | 183±3 | 3048 (w), 2913 (w), 2872 (w), 2794 (w), 2735 (w), 2674 (w), 2593 (w), 2480 (w), 1605 (m), 1593 (s), 1575 (s), 1515 (m), 1500 (s), 1444 (s), 1287 (s), 1236 (s), 1186 (s), 1163 (s), 1151 (s), 1098 (m), 980 (m), 842 (s), 821 (s), 763 (s). | 216 (+) |
| Desfluoro-Eze-1 | 99.2 | 194±3 | 3047 (w), 2914 (w), 2860 (w), 2795 (w), 2731 (w), 2673 (w), 2586 (w), 2476 (w), 1602 (m), 1575 (s), 1515 (m), 1484 (s), 1443 (s), 1284 (s), 1241 (s), 1189 (s), 1163 (s), 1151 (s), 1111 (w), 978 (m), 840 (s), 758 (s), 690 (s). | 198 (+) |
| Eze-4 | 99.4 | 82±3 | 2959 (w), 2832 (w, C–H), 1800 (m), 1778 (s), 1703 (s, C=O), 1385 (s), 1329 (m), 1275 (s), 1215 (s), 1198 (m), 1155 (m), 1130 (m), 1115 (m), 1088 (m), 1061 (s), 1038 (s), 953 (s), 831 (s), 756 (s), 698 (s). | 400 (−) |
| Eze-5 | 99.4 | 176±3 | 3327 (w, N–H), 2960 (w, C–H), 1787 (s), 1748 (s), 1737 (s, C=O), 1687 (s), 1679 (s), 1597 (m), 1513 (s), 1506 (s), 1369 (m), 1322 (m), 1264 (s), 1222 (s), 1214 (s), 1199 (s), 1156 (m), 1100 (s), 1084 (m), 1065 (m), 1039 (m), 980 (m), 845 (m), 828 (s), 758 (s), 707 (s). | 705 (+) |
| Desfluoro-Eze-5 | 86.8 | 135±3 | 3382 (w, N–H), 2910 (w, C–H), 1780 (s), 1755 (s, C=O), 1680 (s), 1599 (m), 1506 (s), 1382 (m), 1326 (m), 1317 (m), 1247 (s), 1228 (s), 1209 (s), 1164 (m), 1102 (m), 1064 (m), 748 (s), 698 (s). | 687 (+) |
| Eze-6 | 99.6 | 163±3 | 3536 (w, O–H), 3346 (w, N–H), 3064 (w), 2964 (w), 2918 (w, C–H), 1777 (s), 1754 (s, C=O), 1683 (s), 1511 (s), 1390 (m), 1377 (s), 1289 (s), 1261 (s), 1209 (s), 1102 (m), 1072 (m), 1060 (s), 841 (s), 829 (s), 690 (s). | 707 (+) |
| Desfluoro-Eze-6 | 94.8 | 124±3 | 3564 (w, O–H), 3382 (w, N–H), 2918 (w, C–H), 1764 (s), 1756 (s, C=O), 1683 (s), 1603 (m), 1508 (s), 1384 (m), 1325 (m), 1239 (s), 1206 (s), 1105 (m), 1063 (m), 836 (m), 748 (s), 699 (s). | 689 (+) |
| Eze-7 | 99.7 | 184±3 | 3345 (w, N–H), 2964 (w), 2927 (w), 2864 (w, C–H), 1773 (s, C=O), 1684 (m), 1507 (s), 1390 (m), 1378 (m), 1253 (s), 1216 (s), 1206 (s), 1105 (m), 1090 (m), 983 (m), 885 (m), 848 (m), 828 (s), 776 (m), 762 (m), 751 (m), 738 (s), 693 (s). | 779 (+) |
| Desfluoro-Eze-7 | 97.1 | 174±3 | 3382 (w, N–H), 3036 (w), 2964 (w), 2900 (w, C–H), 1780 (s), 1768 (s), 1761 (s, C=O), 1687 (s), 1602 (m), 1507 (s), 1377 (m), 1327 (m), 1262 (m), 1236 (s), 1224 (s), 1208 (s), 1172 (m), 1106 (s), 1081 (s), 1036 (m), 982 (m), 887 (m), 843 (s), 833 (s), 747 (s), 691 (s). | 761 (+) |
| Ezetimibe | 99.9c | 163±3 | 3455 (br), 3273 (br, O–H), 2927 (w, C–H), 1727 (s, C=O), 1596 (w), 1509 (s), 1448 (w), 1399 (m), 1353 (m), 1224 (s), 1212 (s), 1172 (s), 1161 (s), 1154 (s), 1140 (m), 1101 (m), 1007 (s), 937 (m), 829 (s), 822 (s). | 410 (+) |
| Desfluoro-ezetimibe | 97.9 | 131±3 | 3418 (br, O–H), 3036 (w), 2909 (w, C–H), 1714 (s, C=O), 1599 (m), 1503 (s), 1403 (s), 1394 (m), 1226 (s), 1170 (m), 1157 (m), 1072 (m), 1009 (w), 834 (s), 748 (s), 687 (s). | 392 (+) |
Copies of spectra are presented in Appendix A.
0.08% desfluoro Eze-1 content.
0.05% desfluoro ezetimibe content.
Fig. 3.
UPLC chromatograms of (A) Eze-5, (B) Eze-6, and (C) Eze-7 spiked with their related desfluoro impurities.
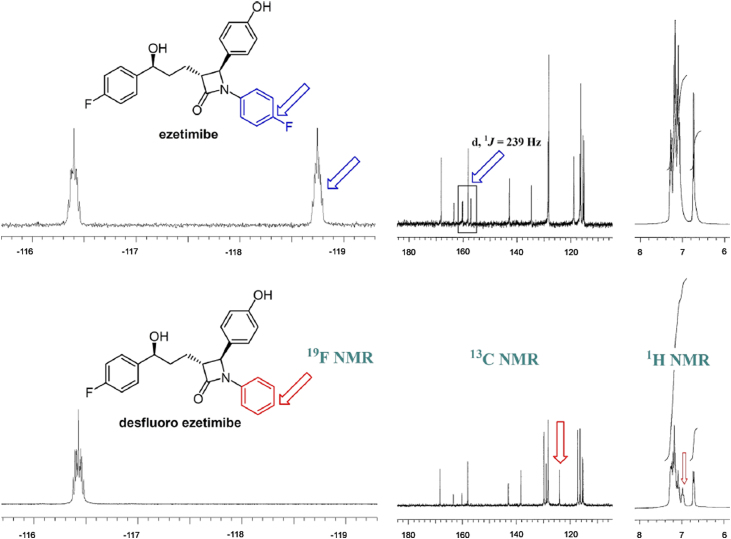
1H and 13C NMR assignments for the structures given in Fig. 2 are shown in Table 2, Table 3, Table 4, Table 5. Compared with ezetimibe and its intermediates, 1H NMR spectra of desfluoro compounds showed one more proton peak in the aromatic region. Also, a very dramatic difference could be seen on 13C NMR shifts where fluorine splitting was clearly seen on eight different aromatic carbons and in the case of desfluoro compounds only four of them were observed and others did not split due to a regular C–H bonding. 19F NMR spectrum of ezetimibe showed two multiplets at −116.40 and −118.75 ppm corresponding to the fluorine atoms on C-9 and C-20 (Fig. 4), respectively. As expected, desfluoro ezetimibe showed one multiplet at −116.43 ppm corresponding to the fluorine atom on C-9. All spectral data (Supplementary Appendix A) confirmed the structures of desfluoro impurities synthesized in scope of this study.
Table 2.
1H and 13C NMR assignments for Eze-1 and desfluoro Eze-1.
| Positiona |
1H–δ ppm |
13C–δ ppm (DEPT) |
||
|---|---|---|---|---|
| Eze-1b | Desfluoro Eze-1b | Eze-1b | Desfluoro Eze-1b | |
| 1 | 10.15 (br, OH) | 10.13 (br, OH) | – | – |
| 2 | – | – | 161.3 (C) | 161.3 (C) |
| 3 | 6.87 (d, J=8.5 Hz, 2H) | 6.87 (dd, J=8.4, 1.8 Hz, 2H) | 116.3 (2CH) | 116.3 (2CH) |
| 4 | 7.74 (d, J=8.5 Hz, 2H) | 7.75 (dd, J=8.4, 1.8 Hz, 2H) | 131.4 (2CH) | 131.4 (2CH) |
| 5 | – | – | 128.1 (C) | 128.2 (C) |
| 6 | 8.43 (s, 1H) | 8.43 (s, 1H) | 160.8 (CH) | 160.8 (CH) |
| 7 | – | – | 149.0 (d, 4J=2.6 Hz, C) | 152.7 (C) |
| 8 | 7.15–7.26 (m, 4H) | 7.36 (dd, J=8.1, 7.5 Hz, 2H) | 123.3 (d, 3J=8.4 Hz, 2CH) | 121.6 (2CH) |
| 9 | 7.17 (d, J=7.8 Hz, 2H) | 116.5 (d, 2J=22 Hz, 2CH) | 129.8 (2CH) | |
| 10 | – | 7.18 (t, J=6.3 Hz, 1H) | 160.8 (d, 1J=242 Hz, C) | 126.0 (CH) |
Assignments: s: singlet; d: doublet; t: triplet; m: multiplet; br: broad singlet. Mean values used for coupled signals.
Numbering of all compounds shown in Fig. 2 and copies of NMR spectra are presented in Appendix A.
Solvent is DMSO-d6.
Table 3.
1H and 13C NMR assignments for Eze-4, ezetimibe and desfluoro ezetimibe.
| Positiona |
1H-δ ppm |
13C-δ ppm (DEPT) |
||||
|---|---|---|---|---|---|---|
| Eze-4c | Ezetimibeb | Desfluoro ezetimibeb | Eze-4c | Ezetimibeb | Desfluoro ezetimibeb | |
| 1 | – | – | – | 172.5 (C) | 168.1 (C) | 168.3 (C) |
| 2 | 2.81 (dt, J=7.5, 3.0 Hz, 2H) | 3.04–3.08 (m, 1H) | 3.03 (br, 1H) | 35.4 (CH2) | 60.1 (CH) | 59.9 (CH) |
| 3 | 1.22–1.33 (m, 2H) | 1.60–1.83 (m, 2H) | 1.60–1.83 (m, 2H) | 18.3 (CH2) | 25.2 (CH2) | 25.3 (CH2) |
| 4 | 1.83–1.90 (m, 2H) | 1.60–1.83 (m, 2H) | 1.60–1.83 (m, 2H) | 36.5 (CH2) | 37.1 (CH2) | 37.1 (CH2) |
| 5 | – | 4.47 (m, 1H) | 4.45 (br, 1H) | 103.2 (C) | 71.8 (CH) | 71.7 (CH) |
| 6 | – | – | – | 136.6 (d, 4J=3.2 Hz, C) | 142.9 (d, 4J=2.9 Hz, C) | 142.9 (d, 4J=2.9 Hz, C) |
| 7 | 7.25 (dd, J=8.1, 1.8 Hz, 2H) | 7.07–7.31 (m, 2H) | 7.06–7.29 (m, 2H) | 129.0 (d, 3J=7.7 Hz, 2CH) | 128.2 (d, 3J=6.2 Hz, 2CH) | 128.2 (d, 3J=7.7 Hz, 2CH) |
| 8 | 7.00 (dt, J=8.7, 1.8 Hz, 2H) | 6.74 (d, J=8.4 Hz, 2H) | 6.72 (d, J=8.1 Hz, 2H) | 115.1 (d, 2J=21 Hz, 2CH) | 115.4 (d, 2J=21 Hz, 2CH) | 115.4 (d, 2J=21 Hz, 2CH) |
| 9 | – | – | – | 162.5 (d, 1J=245 Hz, C) | 161.7 (d, 1J=241 Hz, C) | 161.7 (d, 1J=240 Hz, C) |
| 10 | 3.11 (s, 3H) | 5.28 (d, J=4.5 Hz, 1H) | 5.26 (br, 1H) | 48.8 (CH3) | – | – |
| 11 | 3.09 (s, 3H) | 4.79 (d, J=2.1 Hz, 1H) | 4.77 (br, 1H) | 48.8 (CH3) | 60.3 (CH) | 60.0 (CH) |
| 12 | – | – | – | 154.0 (C) | 128.6 (C) | 128.8 (C) |
| 13 | 4.25 (dd, J=9.0, 3.6 Hz, 1H); 4.65 (t, J=9.0 Hz, 1H) | 7.07–7.31 (m, 4H) | 7.06–7.29 (m, 4H) | 70.2 (CH2) | 128.25 (2CH) | 128.19 (2CH) |
| 14 | 5.36 (dd, J=8.4, 3.6 Hz, 1H) | 57.7 (CH) | 116.4 (2CH) | 116.4 (2CH) | ||
| 15 | – | – | – | 139.3 (C) | 158.1 (C) | 158.0 (C) |
| 16 | 7.32–7.42 (m, 5H) | 9.53 (s, 1H) | 9.50 (s, 1H) | 126.1 (2CH) | – | – |
| 17 | – | – | 129.4 (2CH) | 134.7 (d, 4J=2.0 Hz, C) | 138.2 (C) | |
| 18 | 7.07–7.31 (m, 4H) | 7.06–7.29 (m, 4H) | 129.0 (CH) | 119.0 (d, 3J=7.7 Hz, 2CH) | 117.3 (2CH) | |
| 19 | – | – | 116.6 (d, 2J=22 Hz, 2CH) | 129.7 (2CH) | ||
| 20 | – | – | 6.98 (t, J=7.2 Hz, 1H) | – | 158.7 (d, 1J=239 Hz, C) | 124.1 (CH) |
Assignments: s: singlet; d: doublet; t: triplet; m: multiplet; br: broad singlet. Mean values used for coupled signals.
Numbering of all compounds shown in Fig. 2 and copies of NMR spectra are presented in Appendix A.
Solvent is DMSO-d6.
Solvent is CDCl3.
Table 4.
1H NMR assignments for intermediates of ezetimibe and desfluoro ezetimibe impurity.
| Positiona |
δ ppm |
|||||
|---|---|---|---|---|---|---|
| Eze-5b | Desfluoro Eze-5b | Eze-6b | Desfluoro Eze-6b | Eze-7b | Desfluoro Eze-7b | |
| 2 | 4.19 (dd, J=8.7, 3.6 Hz, 1H) | 4.14–4.17 (m, 1H) | 4.18 (dd, J=8.7, 3.3 Hz, 1H) | 4.15 (dd, J=8.7, 3.6 Hz, 1H) | 4.24 (dd, J=8.7, 3.3 Hz, 1H) | 4.26 (dt, J=8.4, 3.0 Hz, 1H) |
| 3 | 1.79–1.90 (m, 1H); 2.16–2.28 (m, 1H) | 1.85–1.96 (m, 1H); 2.19–2.30 (m, 1H) | 1.45–1.54 (m, 1H); 1.58–1.82 (m, 1H) | 1.48–1.60 (m, 1H); 1.61–1.81 (m, 1H) | 1.44–1.53 (m, 1H); 1.58–1.74 (m, 1H) | 1.42–1.59 (m, 1H); 1.60–1.78 (m, 1H) |
| 4 | 2.90 (dt, J=7.1, 1.2 Hz, 2H) | 2.85–2.97 (m, 2H) | 1.58–1.82 (m, 2H) | 1.61–1.81 (m, 2H) | 1.58–1.74 (m, 2H) | 1.60–1.78 (m, 2H) |
| 5 | – | – | 4.51–4.60 (m, 1H) | 4.51–4.59 (m, 1H) | 4.54 (t, J=5.7 Hz, 1H) | 4.50–4.62 (m, 1H) |
| 7 | 7.88 (dd, J=8.7, 5.4 Hz, 2H) | 7.86–7.90 (m, 2H) | 7.16–7.27 (m, 2H) | 6.96–7.08 (m, 2H) | 7.04 (t, J=8.7 Hz, 2H) | 7.12–7.21 (m, 2H) |
| 8 | 6.75 (t, J=8.7 Hz, 2H) | 6.96–7.19 (m, 2H) | 6.76 (t, J=8.7 Hz, 2H) | 6.96–7.08 (m, 2H) | 6.83 (t, J=8.7 Hz, 2H) | 7.06 (dt, J=8.4, 2.4 Hz, 2H) |
| 10 | 5.06 (d, J=9.9 Hz, 1H) | 5.18 (d, J=8.1 Hz, 1H) | 5.05 (d, J=10.2 Hz, 1H) | 5.21 (d, J=9.9 Hz, 1H) | 5.08 (d, J=9.9 Hz, 1H) | 5.27 (d, J=7.8 Hz, 1H) |
| 11 | 4.47 (t, J=8.7 Hz, 1H) | 4.55–4.66 (m, 1H) | 4.40 (t, J=9.3 Hz, 1H) | 4.49 (t, J=7.8 Hz, 1H) | 4.44 (t, J=9.9 Hz, 1H) | 4.73 (t, J=8.4, 2.4 Hz, 1H) |
| 13 | 7.37–7.46 (m, 2H) | 7.38–7.43 (m, 2H) | 7.39–7.48 (m, 2H) | 7.36–7.49 (m, 2H) | 7.44–7.54 (m, 2H) | 7.47–7.56 (m, 2H) |
| 14 | 7.02–7.26 (m, 2H) | 6.96–7.19 (m, 2H) | 7.01 (t, J=8.7 Hz, 2H) | 7.16–7.25 (m, 2H) | 7.09–7.27 (m, 2H) | 7.23–7.28 (m, 2H) |
| 18 | 7.37–7.46 (m, 2H) | 6.47 (d, J=6.9 Hz, 2H) | 7.39–7.48 (m, 2H) | 6.44 (d, J=7.8 Hz, 2H) | 7.44–7.54 (m, 2H) | 6.55 (d, J=6.6 Hz, 2H) |
| 19 | 6.38 (dd, J=8.7, 4.2 Hz, 2H) | 6.61–6.68 (m, 2H) | 6.38 (dd, J=9.0, 4.5 Hz, 2H) | 6.96–7.08 (m, 2H) | 6.45 (dd, J=9.0, 4.5 Hz, 2H) | 7.12–7.21 (m, 2H) |
| 20 | – | 6.96–7.19 (m, 1H) | – | 6.63 (t, J=7.2 Hz, 1H) | – | 6.73 (t, J=7.5 Hz, 1H) |
| 21 | 5.27 (s, 2H) | 5.27 (s, 2H) | 5.28 (s, 2H) | 5.26 (s, 2H) | 5.34 (s, 2H) | 5.36 (s, 2H) |
| 23–25 | 7.02–7.26 (m, 5H) | 6.96–7.19 (m, 5H) | 7.05–7.12 (m, 5H) | 6.96–7.08 (m, 5H) | 7.09–7.27 (m, 5H) | 7.12–7.21 (m, 5H) |
| 27 | 4.55–4.62 (m, 1H); 4.67 (t, J=8.7 Hz, 1H) | 4.55–4.66 (m, 2H) | 4.51–4.60 (m, 1H); 4.64 (t, J=8.7 Hz, 1H) | 4.51–4.59 (m, 1H); 4.62 (t, J=8.7 Hz, 1H) | 4.54 (t, J=5.7 Hz, 1H); 4.69 (t, J=8.7 Hz, 1H) | 4.50–4.62 (m, 2H) |
| 28 | 5.44 (dd, J=8.4, 3.3 Hz, 1H) | 5.41 (m, 1H) | 5.40 (dd, J=8.1, 1.8 Hz, 1H) | 5.37 (dd, J=8.1, 2.7 Hz, 1H) | 5.45 (dd, J=8.4, 2.4 Hz, 1H) | 5.47 (d, J=8.1 Hz, 1H) |
| 30 | 7.02–7.26 (m, 5H) | 7.38–7.43 (m, 2H) | 7.16–7.27 (m, 4H) | 7.36–7.49 (m, 2H) | 7.33 (d, J=8.7 Hz, 2H) | 7.47–7.56 (m, 2H) |
| 31 | 7.24–7.28 (m, 2H) | 7.16–7.25 (m, 2H) | 7.09–7.27 (m, 3H) | 7.34–7.37 (m, 2H) | ||
| 32 | 6.96–7.19 (m, 1H) | 7.05–7.12 (m, 1H) | 7.13 (dt, J=7.2, 2.1 Hz, 1H) | 7.12–7.21 (m, 1H) | ||
| 33 | – | – | 2.15 (d, J=3.3 Hz, 1H) | 2.04 (d, J=2.4 Hz, 1H) | 0.00 (s, 9H) | 0.01 (s, 9H) |
Assignments: s: singlet; d: doublet; t: triplet; m: multiplet; br: broad singlet. Mean values used for coupled signals.
Numbering of all compounds shown in Fig. 2 and copies of NMR spectra are presented in Appendix A.
Solvent is CDCl3.
Table 5.
13C NMR chemical shifts for intermediates of ezetimibe and desfluoro ezetimibe impurity.
| Positiona |
δ ppm (DEPT) |
|||||
|---|---|---|---|---|---|---|
| Eze-5b | Desfluoro Eze-5b | Eze-6b | Desfluoro Eze-6b | Eze-7b | Desfluoro Eze-7b | |
| 1 | 175.0 (C) | 175.0 (C) | 175.2 (C) | 175.2 (C) | 174.9 (C) | 175.0 (C) |
| 2 | 47.6 (CH) | 47.5 (CH) | 48.1 (CH) | 48.0 (CH) | 48.2 (CH) | 48.1 (CH) |
| 3 | 25.5 (CH2) | 25.6 (CH2) | 27.1 (CH2) | 27.1 (CH2) | 27.2 (CH2) | 27.3 (CH2) |
| 4 | 36.6 (CH2) | 36.6 (CH2) | 36.5 (CH2) | 36.5 (CH2) | 38.2 (CH2) | 38.2 (CH2) |
| 5 | 198.0 (C) | 198.0 (C) | 73.6 (CH) | 73.6 (CH) | 74.0 (CH) | 74.0 (CH) |
| 6 | 133.1 (d, 4J=3.2 Hz, C) | 133.1 (d, 4J=3.2 Hz, C) | 140.1 (d, 4J=3.2 Hz, C) | 140.0 (d, 4J=3.2 Hz, C) | 140.6 (d, 4J=2.9 Hz, C) | 140.6 (d, 4J=3.2 Hz, C) |
| 7 | 131.0 (d, 3J=9.2 Hz, 2CH) | 131.0 (d, 3J=9.5 Hz, 2CH) | 127.8 (d, 3J=8.0 Hz, 2CH) | 127.8 (d, 3J=8.0 Hz, 2CH) | 127.4 (d, 3J=7.7 Hz, 2CH) | 127.5 (d, 3J=8.0 Hz, 2CH) |
| 8 | 116.1 (d, 2J=22 Hz, 2CH) | 116.0 (d, 2J=22 Hz, 2CH) | 115.9 (d, 2J=22 Hz, 2CH) | 115.6 (d, 2J=22 Hz, 2CH) | 115.6 (d, 2J=22 Hz, 2CH) | 115.1 (d, 2J=21 Hz, 2CH) |
| 9 | 166.0 (d, 1J=253 Hz, C) | 166.0 (d, 1J=253 Hz, C) | 162.5 (d, 1J=244 Hz, C) | 162.4 (d, 1J=244 Hz, C) | 162.0 (d, 1J=243 Hz, C) | 162.0 (d, 1J=243 Hz, C) |
| 11 | 61.2 (CH) | 59.8 (CH) | 61.1 (CH) | 59.7 (CH) | 61.4 (CH) | 60.1 (CH) |
| 12 | 138.4 (C) | 138.3 (C) | 138.4 (C) | 138.3 (C) | 138.3 (C) | 138.2 (C) |
| 13 | 129.3 (2CH) | 129.4 (2CH) | 129.3 (2CH) | 129.4 (2CH) | 129.0 (2CH) | 129.2 (2CH) |
| 14 | 121.6 (2CH) | 121.5 (2CH) | 121.6 (2CH) | 121.5 (2CH) | 121.3 (2CH) | 121.2 (2CH) |
| 15 | 154.6 (C) | 154.5 (C) | 154.7 (C) | 154.6 (C) | 154.5 (C) | 154.4 (C) |
| 16 | 150.6 (C) | 150.5 (C) | 150.6 (C) | 150.5 (C) | 150.4 (C) | 150.3 (C) |
| 17 | 142.7 (d, 4J=1.7 Hz, C) | 146.4 (C) | 142.8 (d, 4J=1.7 Hz, C) | 146.4 (C) | 142.5 (d, 4J=1.7 Hz, C) | 146.2 (C) |
| 18 | 115.1 (d, 3J=7.2 Hz, 2CH) | 113.9 (2CH) | 115.2 (d, 3J=7.4 Hz, 2CH) | 113.9 (2CH) | 115.0 (d, 3J=7.1 Hz, 2CH) | 113.8 (2CH) |
| 19 | 115.9 (d, 2J=22 Hz, 2CH) | 129.3 (2CH) | 115.7 (d, 2J=22 Hz, 2CH) | 129.3 (2CH) | 115.1 (d, 2J=22 Hz, 2CH) | 129.0 (2CH) |
| 20 | 156.4 (d, 1J=234 Hz, C) | 118.0 (CH) | 156.3 (d, 1J=234 Hz, C) | 118.0 (CH) | 156.1 (d, 1J=234 Hz, C) | 117.8 (CH) |
| 21 | 70.3 (CH2) | 70.3 (CH2) | 70.3 (CH2) | 70.2 (CH2) | 70.1 (CH2) | 70.0 (CH2) |
| 22 | 134.9 (C) | 134.9 (C) | 134.9 (C) | 134.9 (C) | 134.8 (C) | 134.8 (C) |
| 23 | 128.9 (2CH) | 128.8 (2CH) | 128.9 (2CH) | 128.8 (2H) | 128.7 (2CH) | 128.7 (2CH) |
| 24 | 129.0 (2CH) | 129.0 (2CH) | 129.0 (2CH) | 129.0 (2CH) | 128.8 (2CH) | 128.8 (2CH) |
| 25 | 129.1 (CH) | 129.1 (CH) | 129.1 (CH) | 129.1 (CH) | 128.9 (CH) | 128.9 (CH) |
| 26 | 153.7 (C) | 153.7 (C) | 153.8 (C) | 153.8 (C) | 153.5 (C) | 153.5 (C) |
| 27 | 70.7 (CH2) | 70.6 (CH2) | 70.7 (CH2) | 70.6 (CH2) | 70.4 (CH2) | 70.4 (CH2) |
| 28 | 58.4 (CH) | 58.3 (CH) | 58.4 (CH) | 58.3 (CH) | 58.2 (CH) | 58.1 (CH) |
| 29 | 138.5 (C) | 138.6 (C) | 138.7 (C) | 138.9 (C) | 138.6 (C) | 138.7 (C) |
| 30 | 125.6 (2CH) | 125.5 (2CH) | 125.6 (2CH) | 125.5 (2CH) | 125.3 (2CH) | 125.3 (2CH) |
| 31 | 128.2 (2CH) | 128.3 (2CH) | 128.3 (2CH) | 128.2 (2CH) | 128.1 (2CH) | 128.1 (2CH) |
| 32 | 128.6 (CH) | 128.6 (CH) | 128.6 (CH) | 128.5 (CH) | 128.4 (CH) | 128.3 (CH) |
| 33 | – | – | – | – | 0.0 (3CH3) | 0.0 (3CH3) |
Numbering of all compounds shown in Fig. 2 and copies of NMR spectra are presented in Appendix A. Mean values used for coupled signals.
Solvent is CDCl3.
Fig. 4.
Comparison of 1H, 13C and 19F NMRs of ezetimibe and desfluoro ezetimibe impurity.
3.4. Validation of HPLC methods
Methods were validated according to ICH Q2 (R1) guideline [40].
3.4.1. Validation of Eze-1 related substances method
Related substances limits for Eze-1 are specified as follows: 4-hydroxy benzaldehyde not more than 0.50%, 4-fluoroaniline not more than 0.50%, desfluoro Eze-1 not more than 0.10%, any other impurity not more than 0.30% and total impurities not more than 1.0% and validated accordingly. Purities of reference standards used during the validation study are as follows: Eze-1 99.7%, 4-hydroxybenzaldehyde 99.8%, 4-fluoroaniline 99.8%, and desfluoro Eze-1 99.7%.
The system suitability was conducted throughout the validation study by using 1.2 μg/mL of Eze-1 reference solution and evaluated by making six replicate injections. The system was deemed to be suitable for use as the relative standard deviation (RSD) of the areas was found below 5.0%, resolution above 2.0 (4.6) and symmetry factor below 1.5 (1.1). Average of the areas was found as 44,117 with standard deviation (SD) 459 and RSD was calculated as 1.0%, confirming the injection repeatability of the developed method.
The following injections were done for specificity test: two diluents, two from each impurity at specification limit, two from mixture of impurities at specification limit, two test solutions (0.4 mg/mL Eze-1), and two test solutions spiked with impurities at specification limit. No interference was observed in the chromatogram of diluent where Eze-1 and its impurities peaks elute. Purity angles of Eze-1 and all impurities peaks (0.25, 0.46, 1.04, and 1.76, respectively) were found less than purity thresholds (0.85, 0.65, 1.23, and 1.97, respectively). Thus, the method was found specific for determination of the related substances in Eze-1.
Stability of solutions was evaluated by injection of two reference solutions and two test solutions freshly and then each 24 h during two days. Reference and test solutions were accepted as stable for a time period during which the absolute differences between fresh and different period results were below 0.10% and 0.05%, respectively. This difference did not exceed 0.01% and 0.04%, respectively for both solutions with RSD value of 0.7% and they were found stable up to 48 h. At longer time periods, amounts of 4-hydroxybenzaldehyde and 4-fluoroaniline impurities were increased due to the dissociation of Eze-1 to its starting materials.
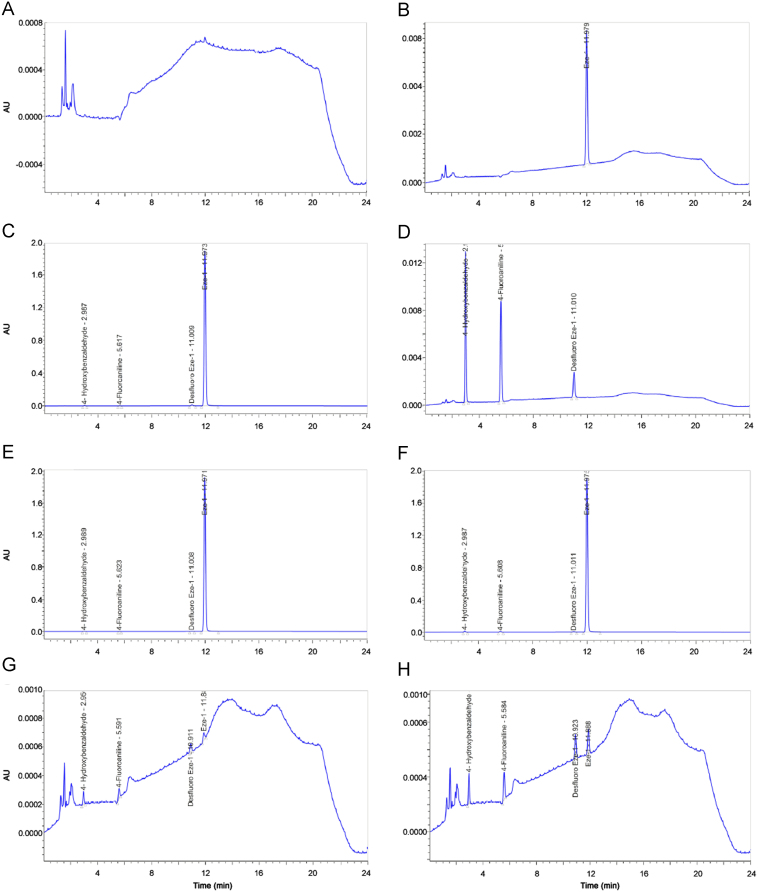
Limit of detection (LOD) and limit of quantitation (LOQ) values were calculated according to the following equations: LOD=3×(BN/H)×C; LOQ=10×(BN/H)×C, where BN is baseline-noise (mV), H is height (mV) and C is concentration of the reference solution (µg/mL). Six replicate diluent injections were done for baseline noise calculations, and solution containing Eze-1 RS and impurities at 1.2 µg/mL concentrations was injected six times for calculation of peak heights. LOD and LOQ values for Eze-1, 4-hydroxybenzaldehyde, 4-fluoroaniline and desfluoro Eze-1 were found to be 0.003% (0.013 µg/mL), 0.003% (0.010 µg/mL), 0.004% (0.014 µg/mL), 0.003% (0.011 µg/mL), and 0.011% (0.042 µg/mL), 0.008% (0.032 µg/mL), 0.012% (0.046 µg/mL), 0.010% (0.038 µg/mL), respectively. LOQ values were below the reported level 0.05% with RSD of 0.3%, 0.3%, 0.2% and 0.2%, respectively. Signal to noise ratios at LOD and LOQ concentrations were found below 3 and 10, respectively, and met the validation criterion. Precision at LOQ level was done by six replicate injections of Eze-1 RS and impurities solution prepared at LOQ concentration. Averages of the areas were found as 1357, 1225, 1399 and 1343 with standard deviation of 61, 60, 94, and 83, and RSD of 4.5%, 4.9%, 6.7%, and 6.2%, respectively, which were below the acceptance limit of 10.0%. Correction response factors (CRF) were calculated according to the following equation: CRF=(A×Ci)/(Ai×C), where A is peak area of Eze-1, Ai is peak area of the impurity, C is concentration of Eze-1 (µg/mL), and Ci is concentration of the impurity (µg/mL). CRFs for 4-hydroxybenzaldehyde, 4-fluoroaniline and desfluoro Eze-1 were found as 1.1, 1.3, and 0.9, respectively. Chromatograms of diluent, system suitability solution, reference and test solutions, mixture solution of all impurities and test solution spiked with all impurities at specification limits, LOD and LOQ solutions are given in Fig. 5.
Fig. 5.
Chromatograms of (A) diluent, (B) reference solution, (C) system suitability solution, (D) solution of impurities at specification limit, (E) test solution, (F) test solution spiked with impurities at specification limit, (G) LOD solution, and (H) LOQ solution of Eze-1 related substances HPLC method validation.
Linearity test solutions of Eze-1 RS and its impurities were prepared at five concentration levels ranging from disregard limit (DL) to 150% of the specification limit and each sample was injected three times. The peak area versus concentration data was analyzed with least squares linear regression. Correlation coefficient (r) and y-intercept ratio to the response of target concentration were found as 1.0000, 0.9999, 0.9993, 0.9993 (≥0.99) and 0.24%, 0.95%, 0.45%, 0.41% (≤5.0%), respectively, indicating the good linearity of the method. Solutions at level 1 (DL) and level 5 (150% of specification limit) were injected six times, and RSD values of areas were calculated for range study, and found to be 0.7%, 1.3%, 1.3%, 0.8% for level 1 and 0.5%, 0.6%, 0.4%, 0.8% for level 5, respectively, below the acceptance limits of 7.0% and 5.0%.
The accuracy of the method was evaluated on test solutions spiked with Eze-1 impurities in triplicate at three concentration levels of 50%, 100% and 150% of the specification limit. Good-to-excellent recoveries of the impurities (98.63–104.2%) at each level were achieved within the limit range of 90.0–110.0% (Table 6). RSDs of recoveries were found below 5.0%, as 2.0%, 2.6%, and 2.0%, respectively.
Table 6.
Accuracy and precision studies of the HPLC method for determination of Eze-1 related substances.
| Compound | Accuracy (n=3) |
Precision |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Intra-day (n=6) |
Inter-day (n=6) |
||||||||
| Amount in test soln. (%) | Amount theo. (Mean, %) | Amount found (Mean±SD, %) | RSD (%) | Recovery (%) | Amount found (Mean±SD, %) | RSD (%) | Amount found (Mean±SD, %) | RSD (%) | |
| 4-Hydroxy benzaldehyde | 0.094 | 0.242 | 0.245±0.008 | 3.2 | 100.9 | 0.10±0.004 | 4.0 | 0.10±0.004 | 4.0 |
| 0.492 | 0.495±0.009 | 1.8 | 100.5 | ||||||
| 0.735 | 0.740±0.009 | 1.2 | 100.7 | ||||||
| 4-Fluoroaniline | 0.014 | 0.261 | 0.263±0.003 | 1.2 | 100.9 | 0.02±0.00 | 0.0 | 0.02±0.00 | 0.0 |
| 0.513 | 0.521±0.007 | 1.3 | 101.5 | ||||||
| 0.756 | 0.787±0.031 | 3.9 | 104.2 | ||||||
| Desfluoro Eze-1 | 0.077 | 0.077a | 0.076±0.001 | 1.5 | NA | 0.08±0.00 | 0.0 | 0.08±0.00 | 0.0 |
| 0.097 | 0.096±0.0006 | 0.60 | 98.63 | ||||||
| 0.146 | 0.149±0.001 | 0.67 | 102.1 | ||||||
Not spiked. NA: not applicable.
The precision of the method was investigated by injecting six individual test solutions of Eze-1 (0.4 mg/mL). The same procedure was applied for the inter-day precision by a different analyst using different batch column and different instrument located within the same laboratory. The system suitability results for the intra-day and inter-day precision studies were obtained as RSD 3.0% and 0.2%, resolution 4.6 and 3.7, symmetry factor 1.1 and 1.2, respectively. Results obtained for RSD were below 5.0% in both studies (Table 6) and difference between mean of results of two studies was found below 0.05% (absolute value), and the method was found to be sufficiently precise since no significant variation in the found amounts was observed on any day.
Robustness of the method was tested by changing column, flow rate by ±10% (1.1±0.1 mL/min), buffer pH by ±0.2 units (7.5±0.2), column temperature by ±3 °C (30±3 °C), and acetonitrile content of mobile phase A by ±5% (40±5%). System suitability parameters were fulfilled in all of the above varied chromatographic conditions. Difference between the results of normal and altered conditions did not exceed 0.01% (<0.05%), indicating the robustness of the method.
Requirements for each stage of the validation study were fulfilled. Thus, our method was found precise, linear, accurate, sensitive, selective and robust, and could be used for related substances analysis of Eze-1. This method was used in routine productions of ezetimibe to control impurities in the intermediate Eze-1 and keep the drug substance impurity content in acceptable range.
3.4.2. Validation of ezetimibe related substances method
Related substances limits for ezetimibe are specified as follows: desfluoro ezetimibe not more than 0.15%, any impurity individually not more than 0.10% and total impurities not more than 0.5% and validated accordingly. Purities of reference standards used during the validation study are as follows: ezetimibe 99.9% and desfluoro ezetimibe 97.9%.
System suitability was conducted throughout the validation study by using ezetimibe standard solution in concentration of 1.0 µg/mL and evaluated by making six replicate injections. The system was accepted to be suitable for use as RSD of areas was below 5.0%, symmetry factor in the range of 0.8–1.5, and the resolution between peaks of ezetimibe and desfluoro ezetimibe was below 2.0. Average of the areas was found as 14,165 with standard deviation of 411. RSD of areas, symmetry factor and resolution were found as 2.90%, 1.0 and 3.5, respectively, confirming the system suitability of the developed method.
Specificity test was carried out by injections of three diluents, and two from each of the following solutions: test, desfluoro ezetimibe at specification limit and test solution spiked with desfluoro ezetimibe at specification limit (0.10%). No peak was observed on diluent chromatogram at the retention time of ezetimibe and desfluoro ezetimibe. Purity angles of ezetimibe and desfluoro ezetimibe peaks were found less than purity thresholds. Stability of reference solution was evaluated by injection of two different reference solutions freshly and then each 24 h during two days. Stability of test solutions was evaluated by injection of two different sample solutions freshly and then each 3 h during 24 h. Solutions were accepted as stable for a time period during which the difference between fresh and different period results was below 0.05%. The reference and test solutions were found stable up to 48 h and 24 h, respectively.
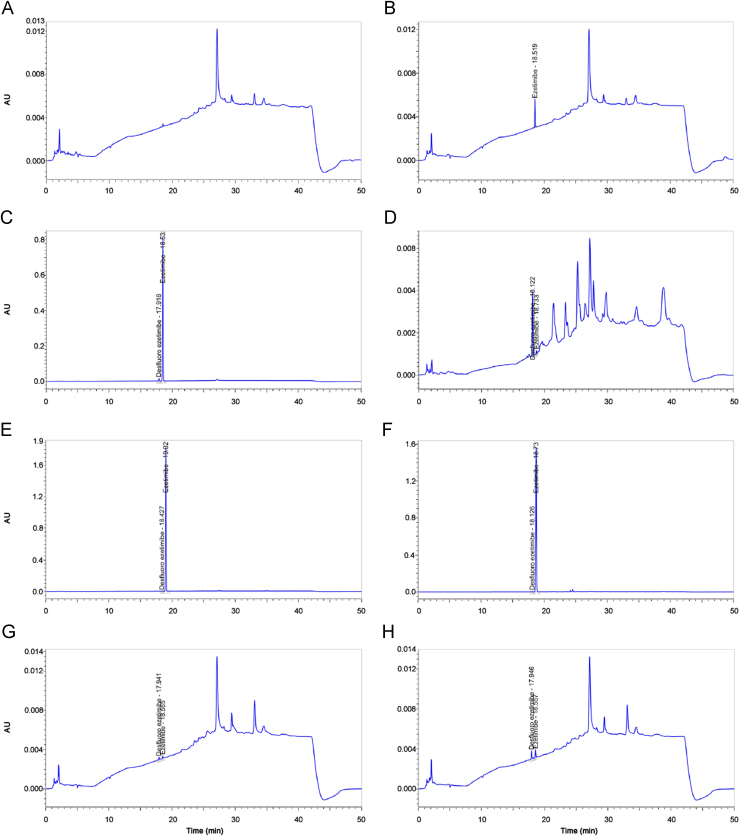
The LOD and LOQ for ezetimibe and desfluoro ezetimibe were estimated at a signal-to-noise ratio of 3:1 and 10:1, respectively, by injecting six replicate diluent and solution containing ezetimibe and desfluoro ezetimibe at 1 μg/mL concentration. The LOD and LOQ for ezetimibe and desfluoro ezetimibe were found 0.012% (0.12 μg/mL), 0.013% (0.13 μg/mL) and 0.041% (0.41 μg/mL), 0.043% (0.43 μg/mL), respectively. LOD and LOQ signal to noise ratios were found to be 3.4, 3.1 (≥3) and 10.7, 10.4 (≥10), respectively. LOQ was found below the reported level 0.05% and met the validation criterion. Precision at the LOQ level was done by injecting six replicates of solution containing ezetimibe and desfluoro ezetimibe at LOQ concentration. Average of the areas for ezetimibe and desfluoro ezetimibe was found as 4879 and 5171 with standard deviation of 65 and 52, and RSD value of 1.3% and 1.0%, which were below the acceptance limit of 10.0%. CRF was calculated according to the following equation: CRF=(A×Ci)/(Ai×C), where A is peak area of ezetimibe, Ai is peak area of desfluoro impurity, C is concentration of ezetimibe (1 µg/mL), and Ci is concentration of desfluoro impurity (1 µg/mL). RSD of the peak areas was found to be 1.7% and CRF for desfluoro impurity was calculated as 1.01. Chromatograms of diluent, system suitability solution, reference and test solutions, desfluoro impurity and test solution spiked with desfluoro impurity at specification limit (0.15%), LOD and LOQ solutions are given in Fig. 6.
Fig. 6.
Chromatograms of (A) diluent, (B) reference solution, (C) system suitability solution, (D) solution of desfluoro ezetimibe impurity at specification limit, (E) test solution, (F) test solution spiked with desfluoro ezetimibe impurity at specification limit (0.15%), (G) LOD solution (ezetimibe 0.12 μg/mL (0.012%) and desfluoro impurity 0.13 μg/mL (0.013%)), and (H) LOQ solution (ezetimibe 0.41 μg/mL (0.041%) and desfluoro impurity 0.43 μg/mL (0.043%)) of ezetimibe related substances HPLC method validation.
Linearity test solutions were prepared at five concentration levels ranging from DL to 120% of the specification limit (0.15%) and each sample was injected three times. The peak area versus concentration data was analyzed with least squares linear regression. Correlation coefficient (r) and y-intercept ratio to the response of target concentration for ezetimibe and desfluoro impurity were found as 0.9930, 0.9998 (above 0.99) and 0.94%, 1.72% (below 5.0%), respectively, indicating the good linearity of the method. Solutions at level 1 and level 5 were injected six times and RSDs of ezetimibe and desfluoro ezetimibe peak areas were calculated for range study, and found as 1.8%, 1.0% and 0.6%, 0.4%, respectively, below the acceptance limit of 10.0% and 5.0%.
The accuracy of the method was evaluated by injection of six desfluoro ezetimibe solutions at specification limit, six different test solutions for determination of desfluoro impurity content, and three test solutions spiked with desfluoro ezetimibe at concentration levels of 80%, 100% and 120% of the specification limit in triple. Good-to-excellent recoveries (100.4%–100.8%) at each level were achieved within the limit range of 90.0%–110.0% (Table 7). RSD of recoveries and RSD of desfluoro ezetimibe peak areas for six replicate injections were found as 1.4% (below 5.0%) for both.
Table 7.
Accuracy and precision studies of the HPLC method for determination of ezetimibe related substances.
| Compound | Accuracy (n=3) |
Precision |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intra-day (n=6) |
Inter-day (n=6) |
|||||||||
| Amount in test soln. (%) | Spiked conc. (µg/mL) | Amount theo. (Mean, %) | Amount found (Mean±SD, %) | RSD (%) | Recovery (%) | Amount found (Mean±SD, %) | RSD (%) | Amount found (Mean±SD, %) | RSD (%) | |
| Desfluoro ezetimibe | 0.028 | 0.1882 | 0.120 | 0.121±0.003 | 2.2 | 100.8 | 0.09±0.00 | 0.0 | 0.09±0.00 | 0.0 |
| 0.4690 | 0.147 | 0.148±0.003 | 2.0 | 100.7 | ||||||
| 0.7499 | 0.174 | 0.174±0.001 | 0.66 | 100.4 | ||||||
The method precision of an analytical procedure expresses the close agreement between single results of the method applied repeatedly by the same analyst, using the homogeneous sample, the same instrumentation and same reagents. The precision of the method was investigated by injecting six individual test solutions. The inter-day precision was conducted according to the same procedure on a different day by a different analyst using different batch column and different instrument located in the same laboratory. No difference was found between the results of intra-day and inter-day precision studies and the amount of desfluoro impurity was found to be 0.09% with RSD of 0.0% on both days (Table 7), indicating the precision of the method.
The robustness of an analytical method is a measure of the effect of variable conditions on analytical results in the process of analysis. For the determination of the method’s robustness, flow rate, pH of the buffer and column temperature were varied within realistic range and the effect of variations on analytical procedure was examined and reported. Robustness of the method was tested by changing flow rate (1.3±0.13 mL/min), buffer pH (3.0±0.3), and column temperature (35±3.5 °C) by ±10%. In all of the above varied chromatographic conditions, RSD and symmetry factors were found in the range of 1.5%–2.8% and 0.8–1.0, respectively. No difference was found between the results of normal and altered conditions, suggesting the robustness of the method.
System suitability parameters were fulfilled in each stage of the validation study and the results showed that our developed method is specific, linear, precise, accurate and robust, and it could be used for related substances analyses of ezetimibe manufacturing batches. This method was used in routine productions and stability testing of ezetimibe drug substance. The mount of desfluoro impurity in ezetimibe was found below the specified limit and as expected it was not affected during the stability studies since it was not a degradation product.
4. Conclusion
In conclusion, a process related impurity of ezetimibe, produced according to the synthetic route given in Scheme 1, was identified, synthesized and characterized. Structural elucidations of all synthesized compounds were done by using NMR (1H, 13C, DEPT, 19F), IR and MS spectral data. Thus, the regulatory requirement was fulfilled by characterizing this impurity and the prepared impurity standard was used during analytical method validation studies. This work also supported the optimization stage of the process development and enabled us to see and control the critical points of the process. The knowledge of the impurity source (Eze-1) helped us to control the amount of desfluoro Eze-1 impurity by a newly developed and validated HPLC method, which allowed us to reduce the desfluoro ezetimibe impurity successfully even below 0.10% in the final drug substance.
Acknowledgments
The authors are thankful for the management of Deva Holding A.S., Istanbul, Turkey, and also former owner of the company Zentiva–part of Sanofi-aventis, Tekirdağ, Turkey for supporting this work and the Scientific and Technological Research Council of Turkey (TUBITAK-TEYDEB Project No: 3080507) for the financial support.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2015.04.002.
Appendix A. Supplementary materials
Supplementary data
References
- 1.Burnett D.A. β-Lactam cholesterol absorption inhibitors. Curr. Med. Chem. 2004;11:1873–1887. doi: 10.2174/0929867043364865. [DOI] [PubMed] [Google Scholar]
- 2.Turley S.D., Dietschy J.M. Sterol absorption by the small intestine. Curr. Opin. Lipidol. 2003;14:233–240. doi: 10.1097/00041433-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Earl J., Kirkpatrick P. Ezetimibe. Nat. Rev. Drug Discovery. 2003;2:97–98. doi: 10.1038/nrd1015. [DOI] [PubMed] [Google Scholar]
- 4.Gagne C., Bays H.E., Weiss S.R. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am. J. Cardiol. 2002;90:1084–1091. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 5.Clader J.W. Ezetimibe and other azetidinone cholesterol absorption inhibitors. Curr. Top. Med. Chem. 2005;5:243–256. doi: 10.2174/1568026053544498. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblum S.B., Huynh T., Afonso A. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J. Med. Chem. 1998;41:973–980. doi: 10.1021/jm970701f. [DOI] [PubMed] [Google Scholar]
- 7.van Heek M., Davis H. Pharmacology of ezetimibe. Eur. Heart J. Suppl. 2002;4(Suppl. J):J5–J8. [Google Scholar]
- 8.Patel J., Sheehan V., Gurk-Turner C. Ezetimibe (Zetia): a new type of lipid-lowering agent. BUMC Proc. 2003;16:354–358. doi: 10.1080/08998280.2003.11927928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clader J.W. The discovery of ezetimibe: a view from outside the receptor. J. Med. Chem. 2004;47:1–9. doi: 10.1021/jm030283g. [DOI] [PubMed] [Google Scholar]
- 10.Catapano A.L. Ezetimibe: a selective inhibitor of cholesterol absorption. Eur. Heart J. Suppl. 2001;3(Suppl. E):E6–E10. [Google Scholar]
- 11.Castaner R.M., Sorbera L.A., Castaner J., Ezetimibe Drugs. Future. 2000;25:679–685. [Google Scholar]
- 12.Burnett D.A. Asymmetric synthesis and absolute stereochemistry of cholesterol absorption inhibitor, SCH 48461. Tetrahedon Lett. 1994;35:7339–7342. [Google Scholar]
- 13.Wu G., Wong Y., Chen X. a novel one-step diastereo- and enantioselective formation of trans-azetidinones and its application to the total synthesis of cholesterol absorption inhibitors. J. Org. Chem. 1999;64:3714–3718. doi: 10.1021/jo990428k. [DOI] [PubMed] [Google Scholar]
- 14.S´nieżek M., Stecko S., Panfil I. Total synthesis of ezetimibe, a cholesterol absorption inhibitor. J. Org. Chem. 2013;78:7048–7057. doi: 10.1021/jo400807c. [DOI] [PubMed] [Google Scholar]
- 15.Michalak M., Stodulski M., Stecko S. A formal synthesis of ezetimibe via cycloaddition/rearrangement cascade reaction. J. Org. Chem. 2011;76:6931–6936. doi: 10.1021/jo2010846. [DOI] [PubMed] [Google Scholar]
- 16.M. Slavikova, H. Stepankova, J. Zezula, et al., Method of producing (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)hydroxypropyl]-4-(4-hydroxyphenyl)-2-azetidinone, Zentiva K.S., Czech Republic, PCT Pat. Appl. WO 2009140932 A2 (Nov 26, 2009).
- 17.M. Adiyaman, E. Bellur Atici, Method for preparation of (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one (ezetimibe) and intermediates thereof, Zentiva Kimyasal Urunler San. ve Tic. A.S., Turkey, TR Pat. Appl. TR 201000116 A1 (Jul 21, 2011).
- 18.Sistla R., Tata V.S.S.K., Kashyap Y.V. Development and validation of a reversed-phase HPLC method for the determination of ezetimibe in pharmaceutical dosage forms. J. Pharm. Biomed. Anal. 2005;39:517–522. doi: 10.1016/j.jpba.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Singh S., Singh B., Bahuguna R. Stress degradation studies on ezetimibe and development of a validated stability-indicating HPLC assay. J. Pharm. Biomed. Anal. 2006;41:1037–1040. doi: 10.1016/j.jpba.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Chimalakonda K.R., Gudala V., Gutta M. Development and validation of chiral HPLC method for identification and quantification of (R)-enantiomer in ezetimibe. Am. J. Anal. Chem. 2012;3:478–483. [Google Scholar]
- 21.Beludari M.I., Prakash K.V., Mohan G.K. RP-HPLC method for simultaneous estimation of rosuvastatin and ezetimibe from their combination tablet dosage form. Int. J. Chem. Anal. Sci. 2013;4:205–209. [Google Scholar]
- 22.Filip K., Bankowski K., Sidoryk K. Physicochemical characterization of ezetimibe and its impurities. J. Mol. Struct. 2011;991:162–170. [Google Scholar]
- 23.Ren Y., Li R.J., Deng Y. First synthesis and characterization of SRR/RSS-ezetimibe. Tetrahedron Lett. 2013;54:6443–6446. [Google Scholar]
- 24.Raman B., Sharma B.A., Butala R. Structural elucidation of a process-related impurity in ezetimibe by LC/MS/MS and NMR. J. Pharm. Biomed. Anal. 2010;52:73–78. doi: 10.1016/j.jpba.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Santa Z., Koti J., Szoke K. Structure of the major degradant of ezetimibe. J. Pharm. Biomed. Anal. 2012;58:125–129. doi: 10.1016/j.jpba.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 26.Guntupalli S., Ray U.K., Murali N. Identification, isolation and characterization of process related impurities in ezetimibe. J. Pharm. Biomed. Anal. 2014;88:385–390. doi: 10.1016/j.jpba.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Ren Y., Duan Y.J., Li R.J. First synthesis and characterization of key stereoisomers related to ezetimibe. Chin. Chem. Lett. 2014;25:1157–1160. [Google Scholar]
- 28.Chimalakonda K., Kamani V., Gutta M. Isolation and characterization of R-enantiomer in ezetimibe. Am. J. Anal. Chem. 2013;4:488–495. [Google Scholar]
- 29.Guidance for industry Q3A (R2), Impurities in new drug substances, in: International Conference on Harmonisation, 2006.
- 30.Guidance for industry Q3B (R2), Impurities in new drug products, in: International Conference on Harmonisation, 2006.
- 31.Zhang D., Song X., Su J. Isolation, identification and characterization of novel process-related impurities in flupirtine maleate. J. Pharm. Biomed. Anal. 2014;90:27–34. doi: 10.1016/j.jpba.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Dousa M., Srbek J., Rádl S. Identification, characterization, synthesis and HPLC quantification of new process-related impurities and degradation products in retigabine. J. Pharm. Biomed. Anal. 2014;94:71–76. doi: 10.1016/j.jpba.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 33.Darcsi A., Tóth G., Kökösi J. Structure elucidation of a process-related impurity of dapoxetine. J. Pharm. Biomed. Anal. 2014;96:272–277. doi: 10.1016/j.jpba.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Thomas S., Paul S.K., Joshi S.C. Identification, synthesis and characterization of an unknown process related impurity in eslicarbazepine acetate active pharmaceutical ingredient by LC/ESI-IT/MS, 1H, 13C and 1H-1H COSY NMR. J. Pharm. Anal. 2014;4:339–344. doi: 10.1016/j.jpha.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellur Atici E., Karlıg˘a B. Identification, synthesis and characterization of process related impurities of benidipine hydrochloride, stress-testing/stability studies and HPLC/UPLC method validations. J. Pharm. Anal. 2015 doi: 10.1016/j.jpha.2015.02.001. 10.1016/j.jpha.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.V.B.R. Uppala, P.R. Vaddadi, V.V. Sunkara, et al., Preparation of ezetimibe, U.S. Pat. Appl. US 20070049748 A1 (Mar 01, 2007).
- 37.Gajjar A.K., Shah V.D. Impurity profiling: a case study of ezetimibe. Open Conf. Proc. J. 2011;2:108–112. [Google Scholar]
- 38.Regalado E.L., Zhuang P., Chen Y. Chromatographic resolution of closely related species in pharmaceutical chemistry: dehalogenation impurities and mixtures of halogen isomers. Anal. Chem. 2014;86:805–813. doi: 10.1021/ac403376h. [DOI] [PubMed] [Google Scholar]
- 39.Regalado E.L., Dermenjian R.K., Joyce L.A. Detection of dehalogenation impurities in organohalogenated pharmaceuticals by UHPLC-DAD-HRESIMS. J. Pharm. Biomed. Anal. 2014;92:1–5. doi: 10.1016/j.jpba.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 40.ICH Q2 (R1), Validation of analytical procedures, in: International Conference on Harmonisation, 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data