Abstract
Background
Unhealthy alcohol use may be particularly detrimental among individuals living with HIV and/or HCV, and is often under-reported. Direct biomarkers of alcohol exposure may facilitate improved detection of alcohol use.
Methods
We evaluated the association of alcohol exposure determined by both self-report (Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]) and a direct biomarker (phosphatidylethanol [PEth]), with mortality among HIV-infected and uninfected in the VACS-Biomarker Cohort. We considered PEth <8 ng/mL to represent no alcohol use. Alcohol exposure by AUDIT-C scores (0, 1–3/1–2 (men/women), 4–7/3–7 (men/women), 8–12) and PEth (<8, ≥8) were combined into categories to model the relationship of alcohol with mortality. Participants were followed from blood collection date for five years or until death within five years.
Results
The sample included 2344 (1513 HIV+; 831 uninfected) individuals, 95% men. During a median follow-up of five years, 13% died. Overall, 36% were infected with HCV (40% HIV+/HCV+, 27% HIV−/HCV+). Overall, 43% (1015/2344) had AUDIT-C=0 (abstinence). Of these, 15% (149/1015) had PEth ≥8 suggesting recent alcohol exposure. Among those with AUDIT-C=0, HCV+ individuals were more likely to have PEth ≥8. After controlling for age, sex, race, HIV, HCV and HIV viral suppression, those with AUDIT-C=0 but PEth ≥8 had the highest risk of mortality (adjusted hazard ratio 2.15, 95%CI: 1.40, 3.29).
Conclusions
PEth in addition to self-report may improve detection of alcohol use in clinical settings, particularly among those at increased risk of harm from alcohol use. Individuals infected with HCV were more likely to under-report alcohol use.
Keywords: HIV, HCV, Alcohol, Mortality, AUDIT-C, Phosphatidylethanol
INTRODUCTION
Unhealthy alcohol use carries a risk of adverse health and social consequences and is a major public health issue.1,2 It is common among individuals infected with HIV (HIV+) and/or hepatitis C virus (HCV+) and may be particularly detrimental in these populations.3–6 The potentially high susceptibility to harm from unhealthy alcohol use may be related to its known association and negative impact on HIV medication adherence,4 disease progression,7,8 risk of hepatic disorders,3,9 and exacerbation of other effects of HIV infection.7,10 Unhealthy alcohol use is linked to harmful health consequences and mortality.9–12 A clear dose-dependent relationship between levels of heavy drinking and all-cause mortality has been reported.12,13
Despite the known health impacts of unhealthy alcohol use, accurate characterization of the spectrum of alcohol exposure is challenging.14,15 To assess alcohol consumption among patients and study participants, health care providers and researchers typically rely on self-reported measures. However, likely due to social desirability bias, alcohol consumption is frequently under-reported, especially among populations such as HIV+ or HCV+ individuals for whom alcohol use is discouraged.16,17 Reliance on self-report is an important limitation in many alcohol studies;14,18 however, it remains the standard method for characterizing exposure in clinical settings.14 The Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) questionnaire is widely used to screen for self-reported alcohol consumption19,20 and has been validated in multiple settings.19–21
To address the biases associated with self-report, several alcohol biomarkers have been evaluated to objectively assess alcohol use.22 Phosphatidylethanol (PEth) is a direct metabolite of alcohol consumption formed from phosphatidylcholine by the action of the enzyme phospholipase D, and can detect alcohol exposure up to three weeks following consumption.22–25 PEth performs relatively well compared to other established alcohol biomarkers because it is both highly sensitive (ranging from 85% to 99%) and specific (almost 100%).26,27 It does not appear to be affected by age, sex, other ingested substances or non-alcohol-associated diseases.24 However, it can vary between persons consuming the same amount of alcohol, likely due to differences in alcohol metabolism.28 In settings in which under-reported alcohol use is common, the high specificity of both PEth and self-report have been used in combination (considered positive if positive on either measure) to increase sensitivity.29
Our primary objective was to evaluate the association between alcohol consumption, determined using a combination of AUDIT-C and PEth, and all-cause mortality, among a cohort of HIV-infected and uninfected comparators. The secondary objectives were to: 1) evaluate the level of agreement between the alcohol measures; and 2) determine whether HIV or HCV status were associated with under-reporting of alcohol.
METHODS
Study Design, Setting and Participants
We used data from the Veterans Aging Cohort Study (VACS), an ongoing longitudinal cohort study with the aim of understanding the role of alcohol use on health. All HIV+ individuals receiving care in the Veterans Affairs (VA) Healthcare System are included plus an age-, race-, and site-of-care matched control group without HIV infection. In addition to full electronic health record data, a sub-set of consented patients also provide survey data on alcohol use and other behaviors. Detailed information about VACS has been reported elsewhere.30,31 The Institutional Review Boards of the participating VA sites and the coordinating center approved the VACS.
The present study was restricted to 2344 VACS survey participants (1513 HIV+ and 831 uninfected) who consented to be included in a tissue repository sub-study (VACS-Biomarker Cohort [VACS-BC]), provided blood specimen between 2005–2007, as previously described,32,33 and for whom PEth assay was available. Analyses were performed only among participants for whom complete AUDIT-C survey data characterizing self-reported alcohol consumption in the past year were available. Participant baseline was defined as the date of collection of the blood specimen.
Main Outcome
The primary outcome was all-cause five-year mortality. Participants were followed for five years after their baseline date or until death within five years. Deaths during follow-up were ascertained from the VA vital status file which uses multiple sources including: (i) the Patient Treatment File, which records hospital deaths in the VA healthcare system; (ii) the Beneficiary Identification Records Locating System, which tracks VA death benefits; (iii) the Medicare Vital Status File, containing vital statistics records including death information on all Medicare beneficiaries; and (iv) the Social Security Death Master File, containing information on deceased persons that is typically collected in connection with filing of benefits by family members.
Primary Explanatory Variable
Our primary explanatory variable was alcohol exposure, which was characterized using two different alcohol exposure measures, separately and combined: (i) self-reported AUDIT-C; and (ii) PEth. AUDIT-C data were collected as part of a confidential self-administered survey conducted at VACS enrolment and during annual follow-ups. PEth assays were performed on dried blood spot samples by United States Drug Testing Laboratories (USDTL), in Des Plaines, IL, using a previously reported method.34 A positive PEth (measuring phosphatidylethanol species 16:0/18:1) indicates alcohol exposure for up to approximately 21 days prior to sample collection.22,35
Alcohol Exposure Categories
AUDIT-C consists of the first 3-items on the 10-item AUDIT survey that asks about quantity and frequency of alcohol consumption as well as the frequency of heavy episodic drinking. Details of the questions and possible responses have been described previously.14 Responses to each of the 3-items are assigned 0 to 4 points. Summing points for the 3-items in AUDIT-C results in a score total ranging from 0 to 12, with higher scores reflecting greater severity of alcohol use.36 Since the recommended AUDIT-C threshold for unhealthy alcohol use differs between men (≥ 4) and women (≥ 3),20 we categorized self-reported alcohol consumption in the past year according to AUDIT-C using the following cutoffs: 0 (no alcohol use/“abstinence”); 1–3 for men/1–2 for women (lower risk drinking); 4–7 for men/3–7 for women (at-risk drinking); and 8–12 (high risk drinking).
There is currently no established PEth level for acceptable lower risk alcohol intake,24,26 although a level of ≥8 ng/mL, the lower limit of quantitation is commonly used as an indicator of any alcohol use in the prior 21 days.16,37–39 As PEth 8 ng/mL represents the lower threshold at which the assay can reliably quantify alcohol exposure,34 we considered a PEth <8 ng/mL to represent no alcohol use and created two PEth levels (<8 versus ≥8 ng/mL) accordingly. We also considered a PEth variable with 3 levels (<8, 8–49, and ≥50), as PEth of ≥50 has been considered to indicate unhealthy alcohol use.
We further categorized alcohol exposure combining AUDIT-C and PEth measures. We show characteristics and mortality rates by all AUDIT-C/PEth categories in tabular form. In our approach to modeling, we prioritized any alcohol use based on self-reported AUDIT-C over PEth because AUDIT-C assesses alcohol use over the past year whereas PEth detects alcohol use for only up to 3 weeks. We therefore used a combined AUDIT-C/PEth measure only among individuals who self-reported abstinence (AUDIT-C= 0). The final alcohol exposure categories were: (i) AUDIT-C = 0 and PETH <8 (self-reported abstinence with negative PEth); (ii) AUDIT-C = 0 and PETH ≥8 (self-reported abstinence with positive PEth); (iii) AUDIT-C = 1–3 for men/1–2 for women (lower risk drinking); (iv) AUDIT-C = 4–7 for men/3–7 for women (at-risk drinking); and (v) AUDIT-C = 8–12 (high risk drinking).
Covariates
Primary covariates included age, sex, race/ethnicity, HIV, HCV antibody status, and HIV viral suppression. Age at blood draw was used as both a categorical (< 50, 50–64, and ≥ 65 years) and a continuous variable. Race was classified as African-American, White, and Hispanic/Other (includes other race not already classified). HIV+ status was confirmed during enrolment at the VA sites. HCV infection was defined based on a positive HCV antibody test, detectable HCV RNA or at least one inpatient and/or two outpatient ICD-9 code.40 Plasma HIV RNA of ≤ 500 copies/mL was used to define viral suppression. Additional covariates included smoking status (never/past/current), injection drug use in the past year (yes/no), and having at least some college education (yes/no), all of which were collected on the VACS surveys.
Statistical Analysis
Sample characteristics were assessed descriptively using chi-square tests for categorical variables and t tests or Wilcoxon rank-sum tests for continuous variables. We compared agreement between self-report and PEth-detected alcohol exposure by summarizing PEth (proportion ≥8 ng/mL, mean and median) by AUDIT-C for HIV+ and uninfected. Of those self-reporting abstinence (AUDIT-C=0), we compared the proportion with positive PEth by HIV and HCV status, using chi-square tests.
We calculated cumulative incidence of mortality over the 5-year follow-up period and compared across alcohol measures. We fit Cox proportional hazards models for time to death and estimated mortality hazard ratios with 95% confidence intervals (CI) first using AUDIT-C as the only alcohol metric, adjusted for age, sex, race/ethnicity, HIV, HCV, and viral suppression (these variables were identified a priori and are commonly associated with mortality). We used the lower risk drinking category (AUDIT-C = 1–3 for men/1–2 for women) as the reference group as this has been suggested to be an ideal comparison group for studying the effect of alcohol on health outcomes.41 We then refit the model using the combined AUDIT-C/PEth alcohol exposure categories, and used the likelihood ratio test to determine whether model fit improved. We further adjusted for smoking status, injection drug use in the past year, and having at least some college education.
To address potential concern about differences in timing between the PEth blood draw date and the AUDIT-C date, we restricted the dataset to 682 individuals with blood draw on the same day or within 21 days prior to the AUDIT-C date and re-ran all analyses on this restricted sample.
All statistical analyses were performed using Stata, version 14.2 (StataCorp, College Station, Texas).
RESULTS
Demographics and Sample Characteristics
Of 2656 VACS-BC participants (1721 HIV+; 935 uninfected) with PEth data (1529 HIV+; 836 uninfected), a total of 2344 participants (1513 HIV+ and 831 uninfected) also had complete AUDIT-C data and were included in the final sample of this study. The median age at blood draw was 52 and 53 years among HIV+ and uninfected individuals, respectively. Over half the sample were aged 50 to 64 years, 95% were men, 70% had at least some college education, 36% had hepatitis C infection, 27% were current smokers, and 9% reported using IV drugs in the past year (Table 1). Approximately two-thirds of the 1513 HIV+ participants had suppressed viral load (≤ 500 copies/mL) at blood collection date. Characteristics of the restricted sample were very similar to those of the full sample. The median time between blood draw (PEth measurement) and AUDIT-C survey date was 46 days (25th, 75th percentile: 0, 222) for the entire sample (n= 2344). For the restricted sample (n=682), the median time between dates was 0 days (25th, 75th percentile: 0, 0), and 91% had a blood draw and AUDIT-C survey on the same day.
Table 1.
Baseline Characteristics of the Study Population
| Full Sample | Restricted Sample | ||||
|---|---|---|---|---|---|
|
| |||||
| Characteristics | |||||
| HIV+ N (%) |
Uninfected N (%) |
HIV+ N (%) |
Uninfected N (%) |
||
| N | 1513 | 831 | 432 | 250 | |
| Age (years) | |||||
| < 50 | 585 (39) | 274 (33) | 188 (44) | 94 (38) | |
| 50–64 | 846 (56) | 460 (55) | 226 (52) | 135 (54) | |
| ≥ 65 | 82 (5) | 97 (12) | 18 (4) | 21 (8) | |
| Sex, female | 42 (3) | 79 (10) | 13(3) | 28(11) | |
| Race | |||||
| White | 283 (19) | 174 (21) | 80 (18)) | 54 (22) | |
| African-American | 1041 (69) | 554 (67) | 297 (69) | 163 (65) | |
| Hispanic/Other* | 189 (12) | 103 (12) | 55 (13) | 33 (13) | |
| At least some college education | 1055 (70) | 563 (69) | 313 (73) | 179 (72) | |
| Hepatitis C infection | 611 (40) | 228 (27) | 156 (36) | 60 (24) | |
| Smoking status | |||||
| Never | 360 (24) | 193 (23) | 118 (27) | 59 (24) | |
| Past | 758 (50) | 394 (48) | 207 (49) | 124 (50) | |
| Current | 395 (26) | 242 (29) | 1075 (25) | 67 (27) | |
| IV drug use | 60 (9) | 36 (10) | 14 (7) | 9 (10) | |
| Viral load ≤ 500 copies/mL | 1002 (66) | N/A | 289 (67) | N/A | |
| AUDIT-C** | |||||
| 0 | 642 (42) | 373 (45) | 180 (42) | 111 (44) | |
| 1–3 (men), 1–2 (women) | 528 (35) | 248 (30) | 145 (34) | 72 (29) | |
| 4–7 (men), 3–7 (women) | 272 (18) | 154 (19) | 80 (18) | 43 (17) | |
| ≥ 8 | 71 (5) | 56 (7) | 27 (6) | 24 (10) | |
| PEth (ng/mL)*** | |||||
| < 8 | 965 (64) | 514 (62) | 277 (64) | 155 (62) | |
| Aug-59 | 296 (20) | 145 (17) | 85 (20) | 44 (18) | |
| ≥50 | 252 (17) | 172 (21) | 70 (16) | 51 (20) | |
| AUDIT-C | PEth | ||||
| 0 | <8 | 549 (36) | 317 (38) | 159 (37) | 99 (40) |
| ≥8 | 93 (6) | 56 (7) | 21 (5) | 12 (5) | |
| 1–3/1–2 | <8 | 349 (23) | 157 (19) | 95 (22) | 45 (18) |
| ≥8 | 179 (12) | 91 (11) | 50 (12) | 27 (11) | |
| 4–7/ 3–7 | <8 | 59 (4) | 31 (4) | 17 (3) | 5 (2) |
| ≥8 | 213 (14) | 123 (15) | 63 (15) | 38 (15) | |
| ≥ 8 | <8 | 8 (1) | 9 (1) | 6 (1) | 6 (2) |
| ≥8 | 63 (4) | 47 (6) | 21 (5) | 18 (7) | |
Note: Numbers in parenthesis represent column percent
, Includes other/unknown race;
, Represents self-report alcohol consumption in the past year as determined by AUDIT-C with categories defined as follows: 0 (deny any alcohol use), 1–3 [men]/1–2 [women] (lower risk drinking), 4–7 [men]/3–7 [women] (at-risk drinking), and 8–12 (high risk drinking). Note: we used different AUDIT-C threshold to determine unhealthy alcohol use in men and women;
, Captures recent alcohol exposure for up to three weeks as determined by blood PEth with categories defined as follows: < 8 ng/mL (low to no alcohol users), ≥ 8 ng/mL (medium to high alcohol users);
AUDIT-C, Alcohol Use Disorders Identification Test-Consumption; N/A, Not applicable; PEth, Phosphatidylethanol
For both HIV+ and uninfected, AUDIT-C characterized a higher proportion of individuals as alcohol exposed (AUDIT-C >0) compared to PEth (≥8 ng/mL) (58% vs. 36% for HIV+; 55% vs. 38% for uninfected). Additionally, AUDIT-C characterized a higher proportion of individuals as drinking unhealthy levels of alcohol (AUDIT-C ≥4 for men/3 for women) compared to (PEth ≥50) (23% vs. 17% for HIV+; 26% vs. 21% for uninfected) (Table 1). Comparing AUDIT-C and PEth, the proportion with positive PEth (i.e. ≥8 ng/mL) increased as AUDIT-C increased. Among those who reported alcohol use (AUDIT-C>0), median PEth increased with increasing AUDIT-C (Table 2). Approximately 43% (1015/2344) of participants self-reported past-year abstinence (AUDIT-C=0). However, 15% (149/1015) of these individuals had PEth ≥8 ng/mL suggesting very recent alcohol consumption (Table 2). Similarly, in the restricted sample 43% (291/682) reported abstinence, and 12% of these (33/291) had PEth ≥8 (Table 2), thus corroborating results from the full sample.
Table 2.
Proportion with PEth ≥8 ng/mL, and Median PEth Among HIV-infected and Uninfected
| Full Sample | Restricted Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV+ (n = 1513) | Uninfected (n = 831) | HIV+ (n =432) | Uninfected (n =250) | |||||||||
|
|
|
|
|
|||||||||
| PEth ≥8** | Range of detectable Peth (≥ 8ng/mL) | PEth ≥8** | Range of detectable Peth (≥8ng/mL) | PEth ≥8** | Range of detectable Peth (≥8ng/mL) | PEth ≥8** | Range of detectable Peth (≥8ng/mL) | |||||
|
|
|
|
|
|||||||||
| AUDIT-C* | % | Median(Q1, Q3) | Max. | % | Median(Q1, Q3) | Max. | % | Median(Q1, Q3) | Max. | % | Median(Q1, Q3) | Max. |
| 0 | 14.5 | 28 (14, 74) | 745 | 15.0 | 40 (10, 107) | 1238 | 11.7 | 23 (15, 38) | 163 | 10.8 | 44 (9, 119) | 390 |
| 1–3 (men), 1–2 (women) | 33.9 | 24 (14, 58) | 1283 | 36.7 | 41 (14, 103) | 776 | 34.5 | 29 (13, 87) | 586 | 37.5 | 40 (15, 98) | 776 |
| 4–7 (men), 3–7 (women) | 78.3 | 64 (31, 161) | 868 | 79.9 | 80 (36, 188) | 865 | 78.8 | 76 (40, 143) | 458 | 88.4 | 54 (18, 179) | 635 |
| 8–12 | 88.7 | 110 (42, 252) | 1093 | 83.9 | 114 (41, 229) | 882 | 77.8 | 62 (28, 231) | 926 | 75.0 | 175 (56, 534) | 882 |
| Overall | 36.2 | 42 (19, 118) | 1283 | 38.1 | 60 (22, 168) | 1238 | 35.9 | 42 (21, 103) | 926 | 38.0 | 52 (18, 179) | 882 |
Note: numbers in parenthesis represent column percent, except for the 25th percentile (Q1) and 75th percentile (Q3) accompanying the median detectable PEth value
Represents self-report alcohol consumption in the past year as determined by AUDIT-C with categories defined as follows: 0 (deny any alcohol use), 1–3 [men]/1–2 [women] (lower risk drinking), 4–7 [men]/3–7 [women] (at-risk drinking), and 8–12 (high risk drinking. Note: we used different AUDIT-C threshold to determine unhealthy alcohol use in men and women;
Captures recent alcohol exposure for up to three weeks as determined by blood PEth with categories defined as follows: < 8 ng/mL (low to no alcohol users), ≥ 8 ng/mL (medium to high alcohol users);
AUDIT-C, Alcohol Use Disorders Identification Test-Consumption;
PEth, Phosphatidylethanol
Q1, 25th percentile;
Q3, 75th percentile.
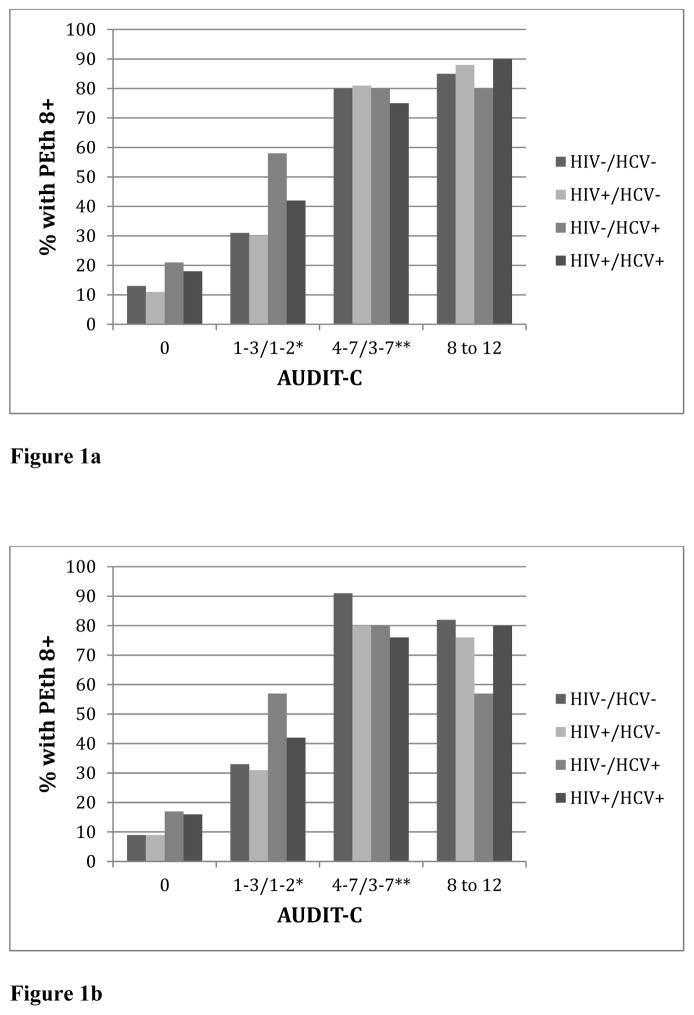
Patterns of AUDIT-C and PEth by HIV and HCV status are shown in Figure 1. Among those who self-reported abstinence, the proportion with PEth ≥8 ng/mL was similar by HIV status (14.5% for HIV+; 15.0% for uninfected), but differed by HCV status; a higher proportion of those with HCV had PEth ≥8 ng/mL compared to those without HCV (18% vs. 11%, P=.009 for HIV+ and 21% vs. 13%, P=.039 for uninfected) (Figure 1a). Results were similar in the restricted sample, though not statistically significant. Among those who self-reported abstinence, a higher proportion of those with HCV had PEth ≥8 ng/mL compared to those without HCV (16% vs. 9% for HIV+ and 17% vs. 9% for uninfected) (Figure 1b).
Figure 1.
Figure 1a. Percent with PEth ≥8 by AUDIT-C, HIV and HCV Status Among the Full Sample (N = 2344)
Figure 1b. Percent with PEth ≥8 by AUDIT-C, HIV and HCV Status
Among the Restricted Sample (N = 682)
* AUDIT-C summary score 1–3 for men and 1–2 for women
** AUDIT-C summary score 4–7 for men and 3–7 for women
Association of Alcohol Exposure with Mortality According to AUDIT-C and PEth
During a mean of 4.7 years of follow-up time, 13% of the sample died, for cumulative incidence of 2.71, 95% CI: 2.42, 3.03 per 100 person years (PY). Mortality was higher among HIV+ (3.13 per 100 PY, 95% CI: 2.74, 3.57) compared to uninfected individuals (1.96 per 100PY, 95% CI: 1.57, 2.45) (Table 3). Mortality among HIV+ individuals self-reporting abstinence (3.68 per 100 PY, 95% CI: 3.05, 4.44) was higher than that of those with lower risk (2.05 per 100 PY, 95% CI: 1.56, 2.70) and at-risk drinking (3.44 per 100 PY, 95% CI: 2.55, 4.64), but lower than of those with high risk drinking (5.35 per 100 PY, 95% CI: 3.33, 8.61) (Table 3). A similar pattern was observed among uninfected individuals, except where power was limited; the lowest mortality was among those with high risk drinking (2 deaths with wide confidence intervals). In both HIV+ and uninfected individuals, mortality rates were higher among individuals with PEth ≥ 8 ng/mL compared to those with PEth < 8 ng/mL; and highest among those with PEth ≥ 50 ng/mL compared to those with PEth <8 and PEth 8–49 (Table 3). Among HIV+, the highest mortality was among those reporting abstinence but with PEth ≥8 ng/mL (5.69 per 100 PY, 95% CI 3.78–8.56) and among those with high risk AUDIT-C and PEth ≥8 ng/mL (6.12 per 100 PY, 95% CI 3.82–9.85). Among the uninfected, those reporting abstinence but having PEth ≥8 ng/mL had the highest mortality rate (3.03 per 100 PY, 95% CI 1.52–6.07). Patterns are similar among the restricted sample (Table 3).
Table 3.
Five-year Mortality Rates by AUDIT-C and PEth Among HIV-infected and Uninfected
| Characteristics | Full Sample | Restricted Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HIV+ (n = 1513) | Uninfected (n = 831) | HIV+ (n = 432) | Uninfected (n=250) | ||||||
|
| |||||||||
| Deaths | Rate per 100 PY (95% CI) | Deaths | Rate per 100 PY (95% CI) | Deaths | Rate per 100 PY (95% CI) | Deaths | Rate per 100 PY (95% CI) | ||
| Mortality during follow-up* | 220 | 3.13 (2.74, 3.57) | 78 | 1.96 (1.57, 2.45) | 60 | 2.97 (2.30, 3.82) | 15 | 1.24 (0.75, 2.06) | |
| AUDIT-C | |||||||||
| 0 | 108 | 3.68 (3.05, 4.44) | 39 | 2.18 (1.60, 2.99) | 30 | 3.59 (2.51, 5.14) | 9 | 1.68 (0.88, 3.24) | |
| 1–3 (men), 1–2 (women) | 52 | 2.05 (1.56, 2.70) | 23 | 1.96 (1.30, 2.94) | 13 | 1.87 (1.08, 3.22) | 3 | 0.86 (0.28, 2.66) | |
| 4–7 (men), 3–7 (women) | 43 | 3.44 (2.55, 4.64) | 14 | 1.90 (1.13, 3.21) | 13 | 3.57 (2.07, 6.15) | 3 | 1.46 (0.47, 4.54) | |
| ≥ 8 | 17 | 5.35 (3.33, 8.61) | 2 | 0.73 (0.18, 2.92) | 4 | 3.14 (1.18, 8.38) | 0 | N/A | |
| PEth (ng/mL) | |||||||||
| < 8 | 125 | 2.77 (2.32, 3.30) | 44 | 1.78 (1.32, 2.39) | 36 | 2.77 (2.00, 3.84) | 7 | 0.93 (0.44, 1.94) | |
| Aug-49 | 43 | 3.13 (2.32, 4.22) | 14 | 2.03 (1.20, 3.43) | 13 | 3.27 (1.90, 5.63) | 3 | 1.41 (0.45, 4.37) | |
| 50+ | 53 | 4.44 (3.47, 5.97) | 20 | 2.48 (1.60, 3.84) | 11 | 3.26 (1.87, 6.09) | 5 | 2.08 (0.87, 5.00) | |
| AUDIT-C | PEth | ||||||||
| 0 | <8 | 85 | 3.36 (2.72–4.15) | 31 | 2.04 (1.43–2.90) | 26 | 3.54 (2.41, 5.20) | 6 | 1.25 (0.56, 2.79) |
| ≥8 | 23 | 5.69 (3.78–8.56) | 8 | 3.03 (1.52–6.07) | 4 | 4.02 (1.51, 10.70) | 3 | 5.36 (1.73, 17.0) | |
| 1–3/1–2 | <8 | 30 | 1.78 (1.25–2.54) | 12 | 1.59 (0.90–2,79) | 7 | 1.53 (0.73, 3.21) | 1 | 0.45 (0.06, 3.19) |
| ≥8 | 22 | 2.59 (1.71–3.93) | 11 | 2.62 (1.45–4.74) | 6 | 2.52 (1.13, 5.60) | 2 | 1.58 (0.39, 6.30) | |
| 4–7/3–7 | <8 | 10 | 3.79 (2.04–7.05) | 1 | 0.66 (0.09–4.71) | 3 | 3.96 (1.28, 12.29) | 0 | N/A |
| ≥8 | 33 | 3.34 (2.38–4.70) | 13 | 2.22 (1.29–3.83) | 10 | 3.47 (1.87, 6.44) | 3 | 1.67 (0.54, 5.16) | |
| ≥ 8 | <8 | 0 | N/A | 0 | N/A | 0 | N/A | 0 | N/A |
| ≥8 | 17 | 6.12 (3.82–9.85) | 2 | 0.87 (0.21–3.49) | 4 | 4.11(1.54,10.96) | 0 | N/A | |
, The mortality rate in the entire sample was 2.71 (95% CI: 2.42, 3.03) per 100 person-years;
, Includes other/unknown race; AUDIT-C, Alcohol Use Disorders Identification Test-Consumption;
PEth, Phosphatidylethanol; PY, Person years
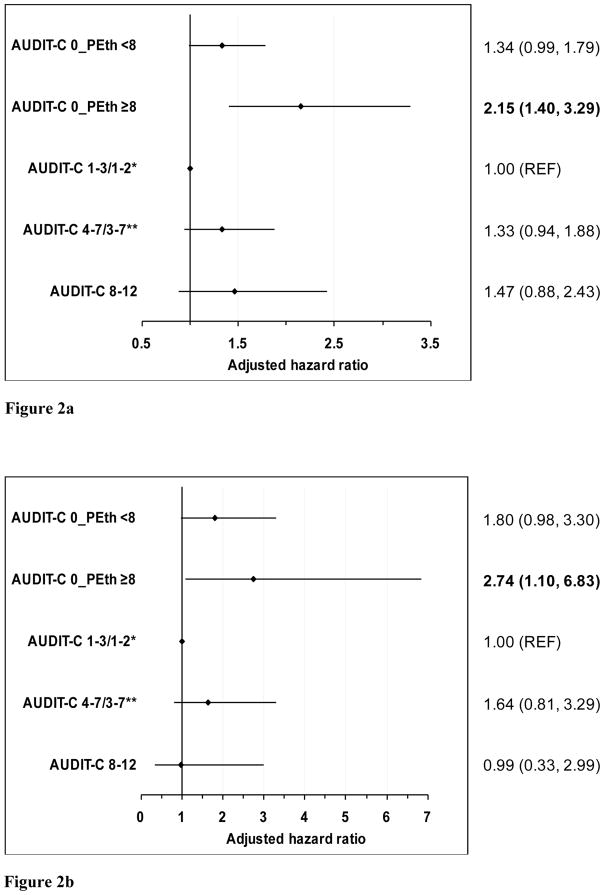
The association between alcohol exposure and mortality persisted after adjusting for age, sex, race, HIV status, HCV and viral suppression in Cox models. First, using only AUDIT-C alcohol characterization, individuals self-reporting abstinence had higher mortality (adjusted hazard ratio [aHR] 1.45, 95% CI: 1.10, 1.92) compared to those with lower risk drinking (reference group). Adding PEth improved model fit based on the likelihood ratio test (p=.027). Compared to those with lower risk drinking, individuals self-reporting abstinence but with PEth ≥8 ng/mL had higher mortality (aHR 2.15, 95% CI: 1.40, 3.29) (Figure 2a). Those with both self-report and PEth suggesting abstinence, at-risk and high risk drinking groups also had increased mortality risk but the association was not statistically significant. Further adjustment for smoking, injection drug use, and education results minimally attenuated the association: (aHR 2.04, 95% CI: 1.33, 3.12) comparing individuals self-reporting abstinence but with PEth ≥8 ng/mL to those with lower risk drinking (data not otherwise shown). Results were similar in the restricted sample; specifically, compared to those with lower risk drinking, significantly higher mortality was observed only among those individuals with self-reported abstinence but with a PEth ≥8 ng/mL (aHR: 2.74, 95 % CI: 1.10, 6.83) (Figure 2b).
Figure 2.
Figure 2a. Association of Alcohol Exposure with Five-Year Mortality Among HIV+ and Uninfected Individuals Among the Full Sample (N= 2344)
Figure 2b. Association of Alcohol Exposure with Five-Year Mortality Among HIV+ and Uninfected Individuals Among the Restricted Sample (N = 682)
Note: model adjusted for age, sex, race/ethnicity, HIV, HCV and HIV viral suppression. We used different AUDIT-C threshold to determine unhealthy alcohol use in men and women.
* AUDIT-C summary score 1–3 for men and 1–2 for women
** AUDIT-C summary score 4–7 for men and 3–7 for women
AUDIT-C, Alcohol Use Disorders Identification Test-Consumption
PEth, Phosphatidylethanol
DISCUSSION
To our knowledge, this is the first study to evaluate the association between alcohol use determined via a combined self-report/biomarker-detected measure, and risk of mortality among a cohort of HIV+ and uninfected individuals. Several important findings emerged from our study. Likely due to the difference in the encompassed time period, more individuals overall were characterized as using alcohol and having unhealthy alcohol use with AUDIT-C than with PEth. However a substantial proportion of those self-reporting abstinence in the past year had biomarker evidence of alcohol consumption in the past 3 weeks. These individuals appear to be at highest risk of mortality. Additionally, individuals infected with HCV including those co-infected with HIV were more likely to under-report exposure and may be at particularly high risk.
Because PEth detects alcohol use only in the 3 weeks prior to blood draw, we also conducted analysis in a subsample restricted to those with PEth blood draw within 21 days prior to or on the same day that AUDIT-C was measured, thus ensuring complete overlap of the 3 weeks covered by PEth measurement with the 1 year encompassed by AUDIT-C. This analysis yielded results consistent with our findings in the larger sample. It is important to emphasize that patients who self-reported abstinence are likely a heterogeneous group, which could include those with lifetime abstinence, those who are currently abstinent but previously consumed alcohol, and those who do not abstain but report non-drinking due to social desirability bias or other unknown factors. Taken together, these results underscore the need for further research evaluating health outcomes among patients who self-report abstinence. This will help to better understand the drivers of under-reporting alcohol exposure as well as to fully elucidate the mortality risk associated with this behavior, especially among HCV+ and HIV+/HCV+ co-infected individuals who are prone to greater risk of harm from unhealthy alcohol use.3–5
Consistent with prior findings of under-reporting alcohol exposure among HIV+ individuals,16,17,38,39,42,43 about 15% of HIV+ individuals self-reporting abstinence in our sample had positive PEth. Three studies among HIV+ individuals in Uganda reported higher level (25%, 25%, 27%) of disagreement between AUDIT-C and PEth among those self-reporting abstinence.16,38,43 These studies assessed self-report alcohol use in the last 3016 and 90 days.38,43 Another study among young people in San Francisco who inject drugs reported positive PEth results among 6% of those self-reporting abstinence.42 Our findings corroborate those from other settings,16,29,38,42,43 that combining AUDIT-C and PEth can improve detection of alcohol exposure. Our work extends prior findings by demonstrating an association between under-report and increased risk of mortality.
Our results should be interpreted in light of some limitations. First, our sample is predominantly male, so our results may not generalize to women. Secondly, one should note that AUDIT-C assesses drinking over the past year whereas PEth can only detect alcohol exposure only over approximately 3 weeks.44 As observed, despite reporting alcohol use ranging from lower risk to high risk drinking, some individuals self-reporting alcohol use had PEth <8 ng/mL in both the full sample and the sample restricted to the PEth time window. This may be a reflection of the difference in the timing between PEth measurement and AUDIT-C survey date, but may also be because PEth is not 100% sensitive, especially for drinking below cutoffs for excessive use.25 In the restricted sample, positive PEth results could not be attributed to alcohol use that occurred after AUDIT-C survey date. In these analyses, a similar proportion of patients with self-reported abstinence had positive PEth as in the full sample (15% vs. 12%) and this group also showed significantly increased risk of mortality, corroborating results from the full sample. Additionally, previous research using AUDIT-C trajectory models indicates that self-report of alcohol use varies little over time in this population.45 We were also limited by sample size in our ability to use the combined alcohol measure in the modeling of mortality risk among patients who self-reported any alcohol use. Finally, due to the observational nature of our study, we cannot establish causality.
Conclusions
Due to the known adverse effects of unhealthy alcohol use on liver-related and all-cause morbidity and mortality, it is critical to accurately measure the spectrum of alcohol consumption. Even though PEth measures alcohol use in a shorter timeframe, we found that it enhanced detection of alcohol use when used in conjunction with AUDIT-C. In high-risk groups such as those infected with HCV or HIV+/HCV+ (co-infected), a group in which our data suggest greater likelihood of under-report of alcohol use, using PEth in combination with AUDIT-C can improve alcohol detection.
Acknowledgments
Source of Funding: This work was supported by the National Institutes of Health: National Institute on Alcohol Abuse and Alcoholism (U10-AA013566, U24-AA020794, U01-AA020790); National Institute on Aging (R01-AG029154); National Heart, Lung and Blood Institute (R01-HL095136; R01-HL090342; RCI-HL100347); and National Institute of Allergy and Infectious Diseases (U01-A1069918). Oghenowede Eyawo is supported by a Canadian Institutes of Health Research Doctoral Award (GSD-129177), and a Canada Graduate Scholarship - Michael Smith Foreign Study Supplements Award (FSS-143008).
We acknowledge the veterans who participate in the Veterans Aging Cohort Study and the study coordinators and staff at each of our sites and at the West Haven Coordinating Center. Without the commitment and care of these individuals, this research would not be possible. We would also like to acknowledge the substantial in-kind support we receive from the Veterans Affairs Healthcare System.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.World Health Organization (WHO) [Accessed December 17, 2016];Global status report on alcohol and health. 2014 Available at: ; http://www.who.int/substance_abuse/publications/global_alcohol_report/en/
- 2.Saitz R. Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352(6):596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 3.Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, Justice AC Veterans Aging Cohort 3-Site S. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J Acquir Immune Defic Syndr. 2003;33(4):521–525. doi: 10.1097/00126334-200308010-00014. [DOI] [PubMed] [Google Scholar]
- 4.Braithwaite RS, Bryant KJ. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Health. 2010;33(3):280–287. [PMC free article] [PubMed] [Google Scholar]
- 5.Peters MG, Terrault NA. Alcohol use and hepatitis C. Hepatology. 2002;36(5 Suppl 1):S220–225. doi: 10.1053/jhep.2002.36811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res. 2016;40(10):2056–2072. doi: 10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. Journal of acquired immune deficiency syndromes (1999) 2007;46(2):194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349(9055):825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 9.Muga R, Sanvisens A, Fuster D, et al. Unhealthy alcohol use, HIV infection and risk of liver fibrosis in drug users with hepatitis C. PLoS One. 2012;7(10):e46810. doi: 10.1371/journal.pone.0046810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rentsch C, Tate JP, Akgun KM, et al. Alcohol-Related Diagnoses and All-Cause Hospitalization Among HIV-Infected and Uninfected Patients: A Longitudinal Analysis of United States Veterans from 1997 to 2011. AIDS Behav. 2015 doi: 10.1007/s10461-015-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braithwaite RS, Conigliaro J, Roberts MS, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS care. 2007;19(4):459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 13.Plunk AD, Syed-Mohammed H, Cavazos-Rehg P, Bierut LJ, Grucza RA. Alcohol consumption, heavy drinking, and mortality: rethinking the j-shaped curve. Alcohol Clin Exp Res. 2014;38(2):471–478. doi: 10.1111/acer.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Justice AC, McGinnis KA, Tate JP, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016 doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner BJ, McLellan AT. Methodological challenges and limitations of research on alcohol consumption and effect on common clinical conditions: evidence from six systematic reviews. J Gen Intern Med. 2009;24(10):1156–1160. doi: 10.1007/s11606-009-1072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajunirwe F, Haberer JE, Boum Y, 2nd, et al. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in southwestern Uganda. PLoS One. 2014;9(12):e113152. doi: 10.1371/journal.pone.0113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hormes JM, Gerhardstein KR, Griffin PT. Under-reporting of alcohol and substance use versus other psychiatric symptoms in individuals living with HIV. AIDS care. 2012;24(4):420–423. doi: 10.1080/09540121.2011.608795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaziano JM, Gaziano TA, Glynn RJ, et al. Light-to-moderate alcohol consumption and mortality in the Physicians’ Health Study enrollment cohort. Journal of the American College of Cardiology. 2000;35(1):96–105. doi: 10.1016/s0735-1097(99)00531-8. [DOI] [PubMed] [Google Scholar]
- 19.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Archives of internal medicine. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 20.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 21.Aertgeerts B, Buntinx F, Ansoms S, Fevery J. Screening properties of questionnaires and laboratory tests for the detection of alcohol abuse or dependence in a general practice population. Br J Gen Pract. 2001;51(464):206–217. [PMC free article] [PubMed] [Google Scholar]
- 22.Wurst FM, Thon N, Yegles M, Schruck A, Preuss UW, Weinmann W. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res. 2015;39(11):2060–2072. doi: 10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]
- 23.Winkler M, Skopp G, Alt A, et al. Comparison of direct and indirect alcohol markers with PEth in blood and urine in alcohol dependent inpatients during detoxication. Int J Legal Med. 2013;127(4):761–768. doi: 10.1007/s00414-012-0812-5. [DOI] [PubMed] [Google Scholar]
- 24.Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788–14812. doi: 10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn JA, Anton RF, Javors MA. The Formation, Elimination, Interpretation, and Future Research Needs of Phosphatidylethanol for Research Studies and Clinical Practice. Alcohol Clin Exp Res. 2016;40(11):2292–2295. doi: 10.1111/acer.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41(4):431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- 27.Hahn JA, Dobkin LM, Mayanja B, et al. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcohol Clin Exp Res. 2012;36(5):854–862. doi: 10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Javors MA, Hill-Kapturczak N, Roache JD, Karns-Wright TE, Dougherty DM. Characterization of the Pharmacokinetics of Phosphatidylethanol 16:0/18:1 and 16:0/18:2 in Human Whole Blood After Alcohol Consumption in a Clinical Laboratory Study. Alcohol Clin Exp Res. 2016;40(6):1228–1234. doi: 10.1111/acer.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn JA, Emenyonu NI, Fatch R, et al. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction (Abingdon, England) 2016;111(2):272–279. doi: 10.1111/add.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Medical care. 2006;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conigliaro J, Madenwald T, Bryant K, et al. The Veterans Aging Cohort Study: observational studies of alcohol use, abuse, and outcomes among human immunodeficiency virus-infected veterans. Alcohol Clin Exp Res. 2004;28(2):313–321. doi: 10.1097/01.alc.0000113414.73220.21. [DOI] [PubMed] [Google Scholar]
- 32.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55(1):126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.So-Armah KA, Tate JP, Chang CC, et al. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? J Acquir Immune Defic Syndr. 2016;72(2):206–213. doi: 10.1097/QAI.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones J, Jones M, Plate C, Lewis D. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Analytical Methods. 2011;3(5):1101–1106. [Google Scholar]
- 35.United States Drug Testing Laboratories Inc. [Accessed December 9, 2016];PEth Testing. 2016 Available at: ; http://www.usdtl.com/testing/peth-alcohol-test-labs.
- 36.Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res. 2013;37(8):1380–1390. doi: 10.1111/acer.12092. [DOI] [PubMed] [Google Scholar]
- 37.Stewart SH, Koch DG, Willner IR, Anton RF, Reuben A. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38(6):1706–1711. doi: 10.1111/acer.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asiimwe SB, Fatch R, Emenyonu NI, et al. Comparison of Traditional and Novel Self-Report Measures to an Alcohol Biomarker for Quantifying Alcohol Consumption Among HIV-Infected Adults in Sub-Saharan Africa. Alcohol Clin Exp Res. 2015;39(8):1518–1527. doi: 10.1111/acer.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papas RK, Gakinya BN, Mwaniki MM, et al. Associations Between the Phosphatidylethanol Alcohol Biomarker and Self-Reported Alcohol Use in a Sample of HIV-Infected Outpatient Drinkers in Western Kenya. Alcohol Clin Exp Res. 2016;40(8):1779–1787. doi: 10.1111/acer.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19(Suppl 3):S99–105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 41.Rehm J, Irving H, Ye Y, Kerr WC, Bond J, Greenfield TK. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am J Epidemiol. 2008;168(8):866–871. doi: 10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain J, Evans JL, Briceno A, Page K, Hahn JA. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49(5):520–524. doi: 10.1093/alcalc/agu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn JA, Fatch R, Kabami J, et al. Self-Report of Alcohol Use Increases When Specimens for Alcohol Biomarkers Are Collected in Persons With HIV in Uganda. J Acquir Immune Defic Syndr. 2012;61(4):e63–64. doi: 10.1097/QAI.0b013e318267c0f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Justice AC, McGinnis KA, Tate JP, et al. Validating Harmful Alcohol Use as a Phenotype for Genetic Discovery Using Phosphatidylethanol and a Polymorphism in ADH1B. Alcohol Clin Exp Res. 2017;41(5):998–1003. doi: 10.1111/acer.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall BDL, Operario D, Bryant KJ, Cook RL, Edelman EJ, Gaither JR, et al. Drinking trajectories among HIV-Infected Men who Have Sex ith Men: A Cohort Study of United States Veterans. DAD. 2015;148:69–76. doi: 10.1016/j.drugalcdep.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]