Abstract
Background
HIV preexposure prophylaxis (PrEP) using daily oral tenofovir-disoproxil-fumarate/emtricitabine (TDF/FTC) is effective for preventing HIV acquisition, but concerns remain about its potential kidney toxicity. This study examined kidney function in individuals using PrEP in real-world clinical settings.
Setting
Demonstration project in two sexually transmitted infection clinics and a community health center.
Methods
We evaluated kidney function among men who have sex with men and transgender women taking TDF/FTC PrEP for up to 48 weeks. Serum creatinine and urine dipstick for protein were obtained at 12-week intervals. Kidney function was estimated using creatinine clearance (CrCl) (Cockcroft-Gault) and estimated glomerular filtration rate (eGFR) (CKD-EPI).
Results
From October 2012 to January 2014, we enrolled 557 participants (median age 33). Mean creatinine increased from baseline to week 12 by 0.03 mg/dL (4.6%) (p<0.0001); mean CrCl decreased by 4.8 mL/min (3.0%) (p<0.0001). These changes remained stable through week 48 (p=0.81, p=0.71 respectively). There were 75/478 (15.7%) participants who developed worsening proteinuria at week 12 compared to baseline (p<0.0001), and this percent remained stable through week 48 (p=0.73). Twenty-five participants (5.1%) developed new-onset eGFR <70 mL/min/1.73m2; independent predictors of this outcome were age ≥40 years (OR 3.79, 95% CI 1.43–10.03) and baseline eGFR<90 mL/min/1.73m2 (OR 9.59, 3.69–24.94).
Conclusions
In a demonstration setting, daily TDF/FTC PrEP leads to reduced CrCl and eGFR; however, these eGFR changes are based on very small changes in serum creatinine and appear to be nonprogressive after the first 12 weeks. Future studies are needed to understand the prognostic significance of these small changes.
Keywords: preexposure prophylaxis, HIV prevention, kidney function, tenofovir disoproxil fumarate, men who have sex with men
INTRODUCTION
Multiple randomized clinical trials have demonstrated the efficacy of preexposure prophylaxis (PrEP) using oral tenofovir-disoproxil-fumarate/emtricitabine (TDF/FTC) in preventing HIV infection.1–3 Based on the evidence from these trials, the United States (US) Food and Drug Administration (FDA) approved the use of TDF/FTC for PrEP in 2012, and the Centers for Disease Control and Prevention (CDC) recommended PrEP for HIV-uninfected adults at high risk for HIV infection.4 While PrEP has been proven to be effective in reducing the incidence of HIV, the side effects of TDF—including kidney toxicity—have been raised as concerns,5 particularly when PrEP is delivered in real-world settings. Studies among HIV-infected patients have shown evidence of kidney impairment in those treated with TDF-containing regimens, as evidenced by declining creatinine-based eGFR.6, 7 Among HIV-uninfected individuals, clinical trials of daily oral TDF/FTC for PrEP found small but statistically significant elevations in serum creatinine and/or reductions in eGFR.8–10 Recently published results from the iPrEx open-label extension (OLE) demonstrated a small but statistically significant decline in creatinine clearance (CrCl) (mean decline of 2.9%) over 18 months of use, and that both increasing age and lower CrCl at baseline predicted a reduction of CrCl to ≤70 mL/min at least once during the study.11
Studies to date assessing the effects of TDF/FTC on kidney function among HIV-negative individuals have focused on those enrolled in clinical trials. Due to more restricted inclusion criteria, these participants may not be representative of the general population. Thus, it is important to evaluate the effect of TDF/FTC on kidney function in more heterogeneous populations, including those with comorbid conditions (e.g. diabetes mellitus, hypertension) or concurrent use of nephrotoxic medications (e.g. nonsteroidal anti-inflammatory drugs) that put them at increased risk of kidney injury.12
The current study examines individuals provided PrEP in “real-world” settings, specifically to a diverse group of men who have sex with men (MSM) and transgender women (TGW) presenting to sexually transmitted disease clinics and a community health center in three US cities. In this cohort, we monitored serum creatinine and urine protein every three months, as well as adherence to PrEP in a subset of participants, as measured by TDF/FTC drug levels. This study aims to provide important safety information and identify potential risk factors for the development of kidney impairment in the setting of expanding PrEP roll-out.
METHODS
Study Design and Study Population
This analysis was conducted among participants enrolled in the US PrEP Demonstration Project, which has been described in detail previously.13–14 In brief, this open-label prospective cohort study assessed PrEP uptake and use among HIV-negative, at-risk MSM and TGW at three sites: two sexually transmitted disease clinics in San Francisco and Miami and a community health center in Washington, DC. Participants underwent screening and were eligible to participate if their baseline CrCl (using the Cockcroft-Gault equation)15 was ≥60 mL/min and their urine dipstick was either negative or trace for protein. Participants enrolled in the study were given up to 48 weeks of TDF/FTC to use daily as HIV PrEP.
Procedures
Enrolled participants were asked to return at weeks 4, 12, 24, 36 and 48 for clinical monitoring, including measurement of serum creatinine and urine protein (by dipstick) every 12 weeks. Kidney function was estimated by calculating the CrCl by the Cockcroft-Gault equation, as recommended by CDC guidelines.4 To determine adherence to PrEP, tenofovir diphosphate (TFV-DP) was measured in dried blood spot (DBS) samples obtained at all follow-up visits for a subset of participants. We estimated adherence to PrEP by categorizing TFV-DP levels of <350, 350–699, and ≥700 fmol/punch as taking <2 doses/week, 2–3 doses/week, and 4–7 doses/week, respectively, based on prior pharmacokinetic modeling and confirmed in a directly-observed dosing study.16.17
Statistical Analysis
We evaluated the mean change from baseline in creatinine and CrCl at each follow-up visit using a linear mixed-effects model. We also evaluated the proportion of participants with an increase in serum creatinine ≥0.2 mg/dL as well as a CrCl decline by >25% as potentially clinically significant reductions in kidney function.18 For participants with TFV-DP, we used a linear regression model to evaluate the association between TFV-DP levels and changes in creatinine and CrCl from baseline. In addition, we evaluated the cumulative incidence of CrCl <60, the recommended threshold for discontinuing TDF/FTC PrEP,4 and cumulative incidence of <70 mL/min, an endpoint used in prior studies.11, 19 Glomerular filtration rate (eGFR) was also estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, as this is considered a more accurate measure of GFR than CrCl or eGFR calculated using other equations, particularly at higher GFR.20
We conducted bivariate analyses using logistic regression to identify baseline correlates of incident eGFR<70 mL/min/1.73m2, including enrollment site, age, race/ethnicity, diabetes mellitus, hypertension (based on self-report and/or blood pressure ≥140/90 measured in clinic), use of anti-hypertensive or diabetic medications, use of nonsteroidal anti-inflammatory drugs (NSAIDs), use of recreational drugs, and baseline CrCl/eGFR. Covariates associated with the outcome at a p-value <0.10 in bivariate analysis were included in a multivariable logistic model. In an additional bivariate analysis of TFV-DP and emtricitabine triphosphate (FTC-TP) in DBS as time-dependent predictors of eGFR<70 mL/min/1.73m2 at each follow-up visit, we used robust standard errors to account for within-participant clustering of the repeated outcomes.
For the analysis of urine dipstick protein, we defined “worsening proteinuria” as an increase in proteinuria from the baseline value (e.g. increase from trace to 1+). We evaluated worsening proteinuria across study visits using a random effects logistic model. Finally, we correlated worsening proteinuria with change in creatinine and CrCl using the Kruskall-Wallis test, and TFV-DP level with worsening proteinuria at the final visit using Fisher’s exact tests, and in the case of repeated measures, generalized linear mixed logistic models.
Ethics
The study was approved by the local institutional review boards at each site and written informed consent was obtained from all participants.
RESULTS
Baseline Characteristics
From October 2012 to January 2014, we enrolled 557 HIV-negative MSM and TGW. The median age was 33 (range: 18–65). At enrollment, there were 11.3% of participants with hypertension, 1.6% with diabetes mellitus, and 13.8% who reported use of NSAIDs. At baseline, the median serum creatinine was 0.92 mg/dL, and the median CrCl and eGFR were 124 mL/min and 108 mL/min/1.73m2, respectively. No participant had a CrCl of <70 mL/min at baseline. Additional baseline characteristics are shown in Table 1. Further details about the cohort and retention have been described previously.13–14
Table 1.
Baseline Characteristics of Cohort of All Participants Enrolled in Study
| Participant Characteristics at Baseline (N=557) | N or Median (% or IQR) |
|---|---|
| Study Site | |
| San Francisco | 300 (53.9%) |
| Miami | 157 (28.2%) |
| Washington, DC | 100 (18.0%) |
|
| |
| Age (years) | |
| 18–29 | 205 (36.8%) |
| 30–39 | 181 (32.5%) |
| 40+ | 171 (30.7%) |
|
| |
| Gender | |
| Male | 548 (98.4%) |
| Transgender women | 7 (1.3%) |
| Other | 2 (0.4%) |
|
| |
| Race/ethnicity | |
| White | 266 (47.8%) |
| Latino | 192 (34.5%) |
| Black/African-American | 40 (7.2%) |
| Asian | 26 (4.7%) |
| Other | 32 (5.8%) |
|
| |
| Hypertension | |
| No | 494 (88.7%) |
| Yes | 63 (11.3%) |
|
| |
| Diabetes mellitus | |
| No | 548 (98.4%) |
| Yes | 9 (1.6%) |
|
| |
| Anti-hypertensive or diabetic medication use | |
| No | 520 (93.4%) |
| Yes | 37 (6.6%) |
|
| |
| Recreational drug use | |
| No | 133 (26.7%) |
| Yes | 366 (73.4%) |
|
| |
| Nonsteroidal anti-inflammatory drug use | |
| No | 480 (86.2%) |
| Yes | 77 (13.8%) |
|
| |
| Weight (kg) | 76 (70–88) |
|
| |
| Baseline serum creatinine (mg/dL) | 0.92 (0.83–1.01) |
| Baseline CrCl (mL/min) | 124 (108–144) |
| ≥90 | 504 (91.5%) |
| ≥70 to <90 | 47 (8.5%) |
| Baseline eGFR (mL/min/1.73m2) | 108 (96–119) |
| ≥90 | 463 (83.1%) |
| ≥70 to <90 | 85 (15.3%) |
| ≥60 to <70 | 7 (1.3%) |
| <60 | 2 (0.4%) |
CrCl = creatinine clearance, calculated using the Cockcroft-Gault equation; eGFR = estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration equation.
Changes in Creatinine, CrCl, and eGFR
Mean creatinine increased from baseline to week 12 by 0.03 mg/dL (95% CI 0.02–0.05, p <0.0001) corresponding to an increase of 4.6% (95% CI 3.4–5.8%), and mean CrCl decreased by 4.8 mL/min (95% CI 3.3–6.3, p<.0001), corresponding to decrease of 3.0% (95% CI 1.8–4.2). Both creatinine (p=0.81) and CrCl (p=0.71) remained stable thereafter through week 48 (Figure 1). The results for eGFR were similar, demonstrating a mean decrease from baseline to week 12 of 3.2 mL/min/1.73m2 (95% CI 2.1–4.3), which is a relative decline of 2.3% (95% CI 1.2–3.5).
Figure 1.
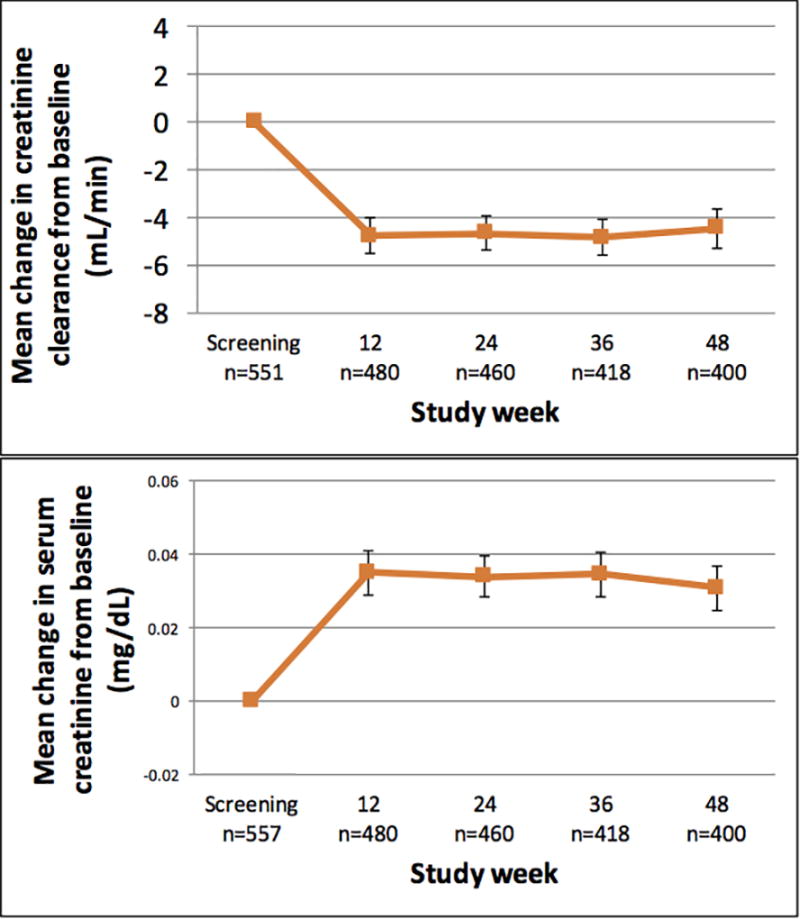
Mean change in creatinine clearance (top panel) and serum creatinine (bottom panel) from baseline to 48 weeks in a demonstration project of HIV preexposure prophylaxis among men who have sex with men using daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC). Number of participants with available data at each study visit is represented by n. Bars represent standard errors. Results were similar when including only participants who returned for all four quarterly follow-up visits.
There were 94/499 (18.8%) participants with an increase of serum creatinine ≥0.2 mg/dL, with 18 confirmed on a consecutive follow-up visit. There were 31/499 (6.2%) participants who had a >25% loss in CrCl from baseline. Among the 25/31 participants who had repeat testing of creatinine, only six were confirmed and only one persisted through the last follow-up visit. TDF/FTC was held in three participants due to elevated serum creatinine, which were not confirmed on repeat testing, and PrEP was restarted in these individuals without further interruptions. Tabulations of changes in serum creatinine versus decline in CrCl/eGFR are presented in Supplementary Tables 1 and 2.
A subset of 294 participants had TFV-DP measured at least once during the study. At week 12, 7.5%, 10.6%, and 81.9% of participants had TFV-DP suggestive of taking <2, 2–3, and 4–7 doses/week, respectively. The mean change in CrCl from baseline to week 12 was +5.0 mL/min (+5.6%), −4.4 mL/min (−3.1%), and −6.1 mL/min (−4.1%) in participants with blood levels consistent with taking <2, 2–3, and 4–7 doses/week, respectively (Figure 2). There was a statistically significant difference in the mean CrCl change for those whose TFV-DP were consistent with <2 versus 4–7 doses/week (p=0.011), but not for <2 versus 2–3 doses/week (p=0.084) or 2–3 versus 4–7 doses/week (p=0.64). This pattern was similar when using eGFR instead of CrCl and when using changes in creatinine (Figure 2) to represent kidney function.
Figure 2.
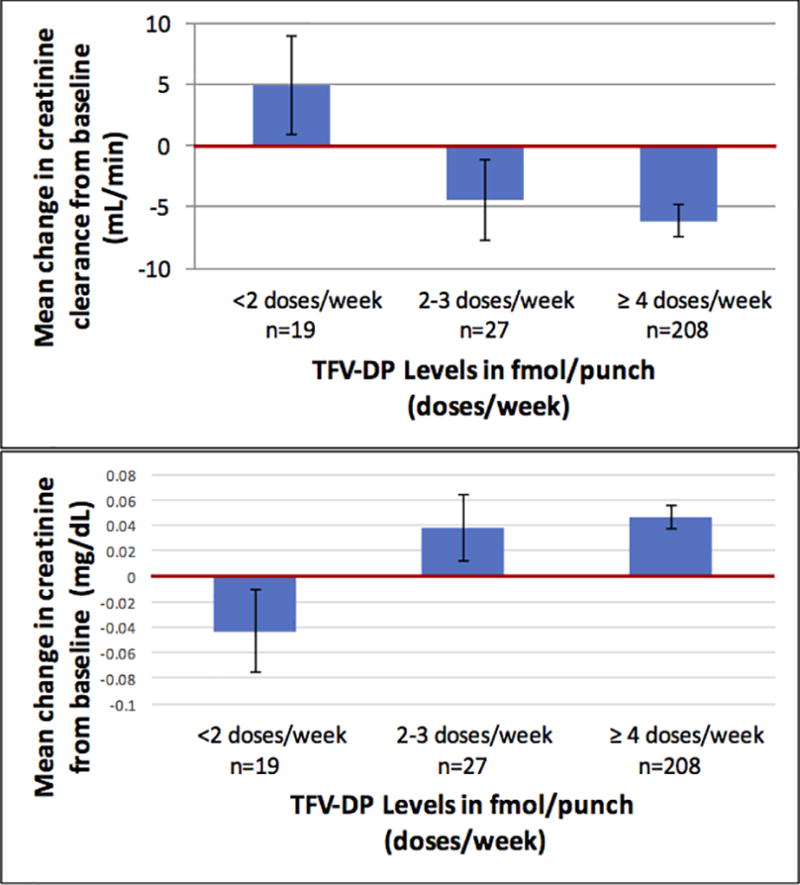
Mean change in creatinine clearance (top panel) and serum creatinine (bottom panel) from baseline to week 12 by tenofovir disoproxil level (TFV-DP) in dried blood spots among men who have sex with men in a demonstration project of HIV preexposure prophylaxis using daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC). Number of participants in each category is represented by n. Bars represent standard errors.
While three participants had a CrCl that fell below 60 mL/min during the study period, none were confirmed on repeat testing performed within two weeks. Only 10/499 (2.0%) had new onset of CrCl that fell below 70 mL/min. However, using eGFR, 11/497 (2.2%) participants had new onset eGFR <60 mL/min/1.73m2, and 25/491 (5.1%) participants had new onset eGFR <70 mL/min/1.73m2 on a least one follow-up visit, 11 which were confirmed on repeat testing at the next visit. In bivariate analyses, age ≥40 years (p =0.0001), use of NSAIDs (p=0.027) or recreational drugs (p=0.028), and baseline eGFR <90 mL/min/1.73m2 (p<0.0001) were associated with new onset eGFR <70 mL/min/1.73m2. The association between increasing weight by quartile and new onset eGFR <70 mL/min/1.73m2 did not reach statistical significance but was included in the multivariable model (p for trend=0.07). Neither TFV-DP level (p for trend=.40) nor detectable FTC-TP (p=0.90) was significantly associated with the likelihood of developing an eGFR <70 mL/min/1.73m2. Other variables examined, including enrollment site, race/ethnicity, hypertension, and use of anti-hypertensive or diabetic drugs, were not significantly associated with a new onset eGFR <70 mL/min/1.73m2. In the multivariable analysis, ≥40 years of age (OR 3.79, 1.43–10.03) and baseline eGFR <90 mL/min/1.73m2 (OR 9.59, 3.69–24.94) remained significant predictors of new onset eGFR <70 mL/min/1.73m2. Among 344 participants who were both <40 years of age and had an eGFR ≥90 mL/min/1.73m2 at baseline, only two had an eGFR fall below 70 mL/min/1.73m2 during the study, which were not confirmed on repeat testing.
Urine Protein
Six participants were excluded from enrollment because they had ≥1+ proteinuria at both screening and enrollment. At baseline, 19/557 (3.4%) participants had ≥1+ proteinuria on the initial urine dipstick tests, but all repeat tests were trace or negative for protein. The distribution of proteinuria across study visits is displayed in Figure 3. There were 75/478 (15.7%) participants who developed worsening proteinuria at week 12 compared to baseline (p<0.0001), and this percent remained stable through week 48 (p=0.73). New onset proteinuria at week 12 was not associated with race/ethnicity, baseline weight, diabetes, hypertension, use of NSAIDS or antihypertensive/diabetic drugs, recreational drug use, or baseline eGFR, after adjustment for site. Approximately half (37/75, 49.3%) of participants with worsening proteinuria at week 12 had this finding confirmed at their next visit. One incident each of 4+ proteinuria at week 36 and 3+ proteinuria at week 24 occurred; however, repeat urine dipsticks for confirmation were trace for protein. Those participants who had worsening proteinuria at their last follow-up visit compared to baseline had larger increases in serum creatinine (0.06 mg/dL) at their final visit compared to those who did not have worsening proteinuria at their final visit (0.02 mg/dL, Kruskal-Wallis p=.017), corresponding to a decrease in CrCl of 8.9 mL/min (−6.3%) versus 3.6 mL/min (−2.4%), respectively (Kruskal-Wallis p=0.009). This relationship was also consistent when looking at worsening proteinuria and the corresponding change in creatinine (p=0.0011) and CrCl (p<0.0005) at repeated visits. Higher TFV-DP levels (but not detectable FTC-TP levels) at the final visit were associated with concurrent proteinuria (Fisher’s exact p=0.03), but this association was not significant when evaluated as repeated visits (p=0.19). No participants stopped FTC/TDF due to development of proteinuria.
Figure 3.
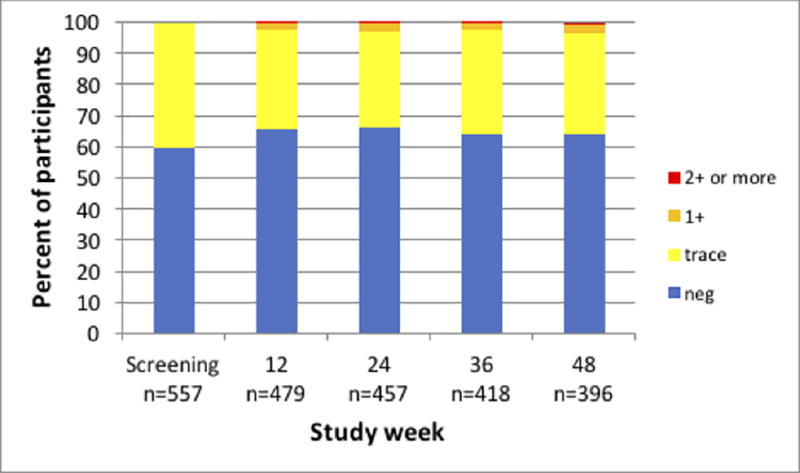
Urine protein level from results of urine dipstick by study week among men who have sex with men in a demonstration project of preexposure prophylaxis using daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC). Number of participants in each category is represented by n. Results were similar when including only participants who returned for all four quarterly follow-up visits.
DISCUSSION
Our results, in a demonstration setting, confirm prior controlled studies showing that after starting PrEP with daily TDF/FTC, small but statistically significant increases in serum creatinine are observed, corresponding to small reductions in CrCl and eGFR. These changes appear to stabilize after an initial creatinine rise at 12 weeks. Those who had the highest blood levels of TFV-DP had larger elevations in creatinine and reductions in CrCl compared to those who had the lowest TFV-DP. While few participants had an eGFR that fell below <60 mL/min/1.73m2, those ≥40 years old and those who had an eGFR<90 mL/min/1.73m2 at baseline were more likely to reach a threshold that might be considered indicative of kidney impairment (<70 mL/min/1.73m2).11, 19
Similar to the study by Gandhi et al. (2016),11 we found an association between lower baseline kidney function and eGFR falling to <70 mL/min/1.73m2 while on PrEP. We also found a statistically significant association between age ≥40 years and eGFR <70 mL/min/1.73m2, consistent with previous studies reporting that older age (≥30,9 ≥40,11 and ≥4521 years of age) predicts worse kidney outcomes among patients taking PrEP. The CDC currently recommends checking creatinine at six-month intervals in those on PrEP who do not have additional risk factors for chronic kidney disease.4 Our data suggest that testing kidney function every six months or even annually may be sufficient for most; however, for those who are at increased risk for kidney impairment, particularly those with a baseline eGFR <90 mL/min/1.73m2 and those who are age 40 years or older, more frequent monitoring may be indicated.
Our study showed that approximately 16% of participants developed worsening proteinuria from baseline, and the proportion with worsening proteinuria remained stable from week 12 to 48. This pattern mirrors the small increase in creatinine throughout the study and may indicate a potential link between both measures. In addition, greater adherence as measured by exposure to TFV-DP was associated with higher likelihood of worsening proteinuria at the end of follow-up. However, the implications of these findings are unclear because the dipstick is an imprecise measure of urine protein and does not indicate the specific proteins that are appearing in the urine of PrEP users. The CDC PrEP guidelines do not recommend regular screening for urine protein prior to PrEP initiation or during follow-up,4 and our findings do not suggest substantial benefit of tracking proteinuria in addition to monitoring creatinine.
The small but significant increase in creatinine found among PrEP users in our study and in other studies8–11 is of unclear clinical significance. No participant in our study reached the threshold of CrCl <60 mL/min, the criterion for stopping TDF/FTC PrEP. In addition, while small rises in creatinine are observed rapidly after initiating TDF/FTC, they remain stable over time, and they appear to be reversible, as demonstrated in two large PrEP clinical trials.8, 22 The pattern of acute elevation and then stabilization of creatinine suggests alternative explanations to acute and reversible glomerular damage. Approximately 10% of creatinine elimination is via active secretion by proximal tubule cells, which are known to be the site of TDF toxicity.23 The adverse effects of TDF on the proximal tubule cell, reported to be via mitochondrial toxicity,24 may lead both to wasting of low molecular weight proteins in the urine and to reduced secretion of creatinine. These combined manifestations of TDF’s impact on the proximal tubule cell could explain the correlation of small acute rises in both serum creatinine and proteinuria in the months after TDF initiation, and the stability of both findings over the subsequent 36 weeks.
A limitation of our study was the relatively short period of follow up of 48 weeks. Studies that have followed HIV-positive individuals on TDF-containing regimens over many years have demonstrated an increased risk of kidney impairment, including chronic kidney disease,19, 25 which was not initially evident in clinical trials of HIV treatment.6, 7 While our study had the advantage of studying a more heterogeneous population than those enrolled in clinical trials, the possibility that those on PrEP for longer intervals will experience significant kidney toxicity cannot be ruled out, and more long-term studies of PrEP use are needed. Furthermore, this study was relatively underpowered to assess the effect of PrEP on groups known to be at higher risk of kidney impairment, including those with underlying comorbidities, and larger studies specifically recruiting these groups may be informative. Finally, as this study was an open-label demonstration project of daily TDF/FTC PrEP, changes in creatinine and CrCl associated with PrEP initiation were observational in this cohort and could not be compared to a placebo group.
In conclusion, our study demonstrates that, in terms of its effect on the kidney, PrEP taken for a year was relatively safe among a diverse cohort of MSM. PrEP was associated with small changes in serum creatinine and proteinuria that were stable after the initial 12 weeks of follow-up. Our findings suggest that those with reduced eGFR at baseline and those ≥40 years old may warrant more frequent monitoring of creatinine relative to those <40 years old with eGFR >90 mL/min/1.73m2 at baseline. Together with evidence from participants in clinical trials, our results provide relevant safety information as PrEP continues to expand as an important modality for preventing HIV infection in the real world. Future studies, including those using novel biomarkers of kidney injury,26 should evaluate the clinical relevance and pathophysiology of the kidney changes observed in our study.
Supplementary Material
Acknowledgments
Conflicts of Interests and Source of Funding:
Potential conflicts of interest. SEC, AYL, and SPB have led and participated in studies in which Gilead Sciences has donated study drug. RE has served as a consultant to and provided expert testimony for Gilead, and received payment from Gilead for lectures and development of educational presentations; he has also served as a consultant to ViiV and received payment for lectures from Jannsen. PLA has received research support from Gilead Sciences, paid to his institution.
Sources of Support. This work was supported by the National Institute of Allergy and Infectious Diseases [grant number UM1AI069496]; the National Institute of Mental Health [grant number R01MH095628]; the National Institutes of Health (Miami Center for AIDS Research) [grant number P30AI073961]; and the National Institutes of Health (Gladstone Institute of Virology and Immunology–University of California, San Francisco, Center for AIDS Research) [grant number P30AI027763]. The study drug and support for pharmacokinetic and resistance testing were provided by Gilead Sciences.
Footnotes
Meeting Presentations:
Part of this data has been previously presented at the Conference on Retroviruses and Opportunistic Infections (CROI) in February 2016 in Boston, MA [abstract 867].
The remaining authors have no conflicts of interest to declare.
Contributor Information
Eric C. Tang, Residency Program in Internal Medicine, Kaiser Permanente San Francisco Medical Center; Residency Program in General Preventive Medicine and Public Health, University California, San Francisco; San Francisco, USA.
Eric Vittinghoff, Department of Epidemiology and Biostatistics, University of California, San Francisco; San Francisco, USA.
Peter L. Anderson, Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus; Aurora, USA.
Stephanie E. Cohen, San Francisco Department of Public Health, University of California San Francisco; San Francisco, USA.
Susanne Doblecki-Lewis, Division of Infectious Diseases, University of Miami Miller School of Medicine; Miami, USA.
Oliver Bacon, San Francisco Department of Public Health; Division of HIV, Infectious Diseases and Global Medicine, University of California, San Francisco; San Francisco, USA.
Megan E. Coleman, Whitman-Walker Health; Washington, DC, USA.
Wairimu Chege, Prevention Sciences Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, USA.
Susan P. Buchbinder, Bridge HIV, San Francisco Department of Public Health; Departments of Medicine, Epidemiology and Biostatistics, University of California, San Francisco; San Francisco, USA.
Michael A. Kolber, Department of Medicine, University of Miami Miller School of Medicine; Miami, USA.
Richard Elion, Providence Hospital Department of Infectious Disease, George Washington University School of Medicine, Washington, DC, USA.
Michael Shlipak, Kidney Health Research Collaborative, San Francisco VA Medical Center, University of California, San Francisco; San Francisco, USA.
Albert Y. Liu, Bridge HIV, San Francisco Department of Public Health; Department of Medicine, University of California, San Francisco; San Francisco, USA.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. [Accessed June 16, 2017];Preexposure prophylaxis for the prevention of HIV in the United States - 2014 clinical practice guideline. Available at http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf.
- 5.Tetteh RA, Yankey BA, Nartey ET, et al. Pre-exposure prophylaxis for HIV prevention: safety concerns. Drug Saf. 2017;40:273–283. doi: 10.1007/s40264-017-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallant JE, Parish MA, Keruly JC, et al. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis. 2005;40:1194–1198. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 7.Cooper RD, Wiebe N, Smith N, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 8.Solomon MM, Lama JR, Glidden DV, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014;28:851–859. doi: 10.1097/QAD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin M, Vanichseni S, Suntharasamai P, et al. Renal function of participants in the Bangkok tenofovir study--Thailand, 2005–2012. Clin Infect Dis. 2014;59:716–724. doi: 10.1093/cid/ciu355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175:246–254. doi: 10.1001/jamainternmed.2014.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi M, Glidden DV, Mayer K, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV. 2016;3:e521–e528. doi: 10.1016/S2352-3018(16)30153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocroft A, Ryom L. The benefits and risks of PrEP and kidney function. Lancet HIV. 2016;3:e501–e502. doi: 10.1016/S2352-3018(16)30148-5. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SE, Vittinghoff E, Bacon O, et al. High interest in preexposure prophylaxis among men who have sex with men at risk for HIV infection: baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015;68:439–448. doi: 10.1097/QAI.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176:75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29:384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson PL, Liu AY, Castillo-Mancilla J, et al. Presented at: Conference on Retroviruses and Opportunistic Infections. Seattle, WA: 2017. TFV-DP in dried blood spots (DBS) following directly observed therapy: DOT-DBS study [419] [Google Scholar]
- 18.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocroft A, Lundgren JD, Ross M, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3:e23–e32. doi: 10.1016/S2352-3018(15)00211-8. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mugwanya KK, Heffon R, Wyatt C, et al. Presented at: XXI International AIDS Conference. Durban; South Africa: 2016. Optimizing the frequency of kidney safety monitoring in HIV-uninfected persons using daily oral tenofovir disoproxil fumarate pre-exposure prophylaxis [FRAE0106LB] [Google Scholar]
- 22.Mugwanya KK, Wyatt C, Celum C, et al. Reversibility of glomerular renal function decline in HIV-uninfected men and women discontinuing emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2016;71:374–380. [Google Scholar]
- 23.Hall AM, Hendry BM, Nitsch D, et al. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57:773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009;89:513–519. doi: 10.1038/labinvest.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jotwani V, Scherzer R, Abraham A, et al. Association of urine α1-microglobulin with kidney function decline and mortality in HIV-infected women. Clin J Am Soc Nephrol. 2015;10:63–73. doi: 10.2215/CJN.03220314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


