Abstract
Background
Oral pre-exposure prophylaxis (PrEP) is effective for HIV prevention and PrEP delivery studies are investigating ways to deliver PrEP with high adherence. However, in many settings with high HIV burden, intimate partner violence (IPV) is reported often and could be a barrier to effective PrEP use. We examined the association between IPV and interruptions in PrEP use.
Methods
We analyzed data from 1,013 serodiscordant heterosexual couples enrolled in a large PrEP demonstration project in Kenya and Uganda, the Partners Demonstration Project. At quarterly study visits, HIV-negative participants receiving PrEP were asked about interruptions in their PrEP use and experiences with IPV. The association between IPV and PrEP interruptions was analyzed using multivariable generalized estimating equations.
Results
At baseline and follow-up there were 53 visits with reports of abuse by 49 HIV-negative partners, including physical, economic, and verbal IPV. Interruptions in PrEP use were reported at 328 visits (7.1% of all visits) by 249 people. The median length of PrEP interruption was 28 days (interquartile range [IQR]: 7–45). The frequency of PrEP interruptions among those reporting IPV was 23.8% and those without IPV was 6.9%. PrEP interruption was significantly associated with IPV after adjustment for age and frequency of sexual intercourse (adjusted OR=2.6, 95% CI 1.2–6.0).
Conclusion
IPV was more likely to be reported at visits when PrEP interruptions were also reported, which may have implications for sustained adherence to PrEP. Within PrEP delivery programs, there may be opportunities to assess individual safety and well-being in order to bolster adherence.
Keywords: Intimate Partner Violence, Pre-exposure prophylaxis, HIV, adherence, serodiscordant couples, Africa
Introduction
Oral pre-exposure prophylaxis (PrEP) is an effective HIV prevention tool that is being rolled out for use in many settings, including Kenya.1,2,3 In one demonstration project evaluating delivery models for PrEP, high PrEP adherence was observed among high-risk heterosexual HIV serodiscordant couples among whom an integrated PrEP and ART delivery model reduced HIV incidence by 96%.1 Demonstration projects in other populations, such as female sex workers, men who have sex with men, adolescents, transgender women, people who inject drugs, are ongoing and exploring delivery strategies to foster high adherence.4
In some settings where PrEP delivery is being brought to scale, intimate partner violence (IPV) is also common. For example, according to the Kenyan 2014 Demographic Health Survey, spousal violence in Kenya was reported by 39% of women and 9% of men who had ever been married.5 In Uganda, spousal violence was reported by 60% of women and 40% of men according to the Uganda 2012 Demographic and Health Survey.6 IPV increases risk for HIV though limited safe sex negotiation, forced sex with discordant partner and increased sexual risk taking behaviors.7,8 Furthermore, IPV is associated with a higher risk of HIV infection in sub-Saharan Africa,9,10,8 with up to a 55% increased risk of HIV acquisition due to IPV among women in Uganda11 and a 12% population attributable fraction of HIV infection due to IPV among South African women.12 Women living with HIV are also more likely to have experienced IPV than their HIV-negative female counterparts.13,14,15
Given these associations between IPV and HIV acquisition, it is critical to effectively delivery PrEP to individuals at risk for and experiencing IPV. One study examining predictors of effective PrEP use, found that experiences with verbal, physical, and economic IPV were associated with low PrEP adherence among heterosexual serodiscordant couples participating in a randomized clinical trial of PrEP efficacy.16 The objective of this paper was to examine whether there is an association between IPV and self-reported interruptions in PrEP use. Our study was conducted in the context of an open-label demonstration project, lending itself to provide recommendations for scalable PrEP delivery strategies within public health facilities for high-risk individuals.
Methods
Study Population
The Partners Demonstration Project was an open-label prospective implementation study at 4 sites in Uganda and Kenya1. In brief, daily, oral pre-exposure prophylaxis (PrEP) was offered to HIV-negative partners in high-risk HIV serodiscordant heterosexual relationships until antiretroviral treatment (ART) was initiated and sustained for at least 6 months by the partner living with HIV.1,17 Of the 1,013 HIV-negative participants (334 women and 667 men), 985 initiated PrEP during the study. During periods when PrEP was dispensed to the HIV-negative partners, PrEP uptake, continuation and adherence were high.1
Study procedures
At enrollment, HIV-negative partners had normal kidney function and women were not pregnant and partners living with HIV had not yet initiated ART. Following enrollment, participants completed quarterly study visits for up to 24 months and couples were encouraged to attend together when feasible and desired. At each visit, HIV-negative partners were tested for HIV, screened for acute HIV infection based on recent history of symptoms, and provided PrEP refills and adherence counseling. Partners living with HIV were monitored for CD4 count and viral load on a 6-monthly basis. Partners living with HIV were also advised to initiate ART as soon as they met national ART initiation guidelines and participants chose to access ART at the study clinic or HIV clinic of their choice.
Data Collection
Data on demographics, sexual behavior, partnership stability, symptoms, and side effects were collected through standardized interviewer-administered questionnaires in the participant’s preferred language (English, Kiswahili, Kikuyu, Luo, or Luganda). Partnership stability was assessed by inquiring about marital status, cohabitation, and length of partnership, number of children, and time known to be HIV discordant. Scales screening for depression18 and stigma were administered at enrollment and annual study visits. The depression screening was done using the 16-item Hopkins Symptoms Checklist for Depression scale and stigma18–20 was assessed using the Internalized AIDS-Related Stigma Scale21.
Self-reported PrEP interruption
At enrollment and quarterly visits, participants were counselled on PrEP use and adherence. Prior and during their use of PrEP they were counselled to align their PrEP use with their HIV risk and encouraged to disclose information regarding to their reasons to stop PrEP. At quarterly visits, participants receiving PrEP reported whether there had been any interruptions in their PrEP use during the past 3 months using the following question: “Since the last study visit, was there any period of time when you deliberately decided to take a break from taking PrEP?” If the participant responded affirmatively, additional questions followed to define the length of the longest PrEP interruption and the reason for the PrEP interruption. The form used to collect details on PrEP interruption was introduced shortly after project initiation, and thus data on PrEP interruption were missing for 1.6% of all follow-up visits. Apart from self-report, additional measures of adherence to PrEP medication included pill counts, recordings of pill bottle openings by a medication event monitoring system (MEMS), and quantification of tenofovir in archived plasma for a subset of participants. This analysis is limited to self-reported PrEP interruption as the primary outcome because we wanted to assess deliberate PrEP interruptions that participants recognized as substantial enough to report.
Intimate partner violence
At baseline and each quarterly study visit, participants were asked if they had been verbally, physically, or economically abused by their study partner in the last three months. This question was not interviewer-administered but asked in culturally appropriate manner in the context of a counselling session to foster trust and to encourage disclosure of abuse. Affirmative responses were followed by discussion with the study counselor to yield more details, including the type of abuse, (whether economic, physical, verbal, or other), and to determine a care plan for the participant. This is the same procedures that we have followed in previous studies of HIV serodiscordant couples.22,23
Data analysis
The relationship between experiencing intimate partner violence and self-reporting a PrEP interruption was examined using a generalized estimating equation (GEE) extension to logistic regression with an exchangeable correlation structure to account for correlation among observations from the same person. Based on current literature, a number of demographic, sexual behavior, and medical factors were considered potential confounders,16 including partner stability and side effects. The final model included adjustment for factors that substantially changed the odds ratio (by 10% or more). Statistical analysis was conducted using STATA version 14.2.
Ethics statement
The study protocol was approved by the University of Washington Human Subjects Division and ethics review committees at each of the study sites. All participants provided written informed consent in English or their local language.
Results
Participant characteristics at enrollment
A total of 1,013 HIV-1 serodiscordant heterosexual couples were enrolled and this analysis was restricted to the HIV-negative members of the couple, 67% of whom were male (Table 1). Approximately half of the HIV-negative women (55%) and men (47%) were age 29 years or younger. Over 95% of couples reported being married to each other and over 97% lived together. The median length of partnership was nearly 5 years for couples with an HIV-negative woman and 2 years for couples with HIV-negative man. Female participants reported a median of 2 children and male participants reported a median of 1 child. Twenty-seven percent of women and 34% of men reported experiencing stigma. Probable depression was prevalent in 12% of women and 9% of men and 3% of women and 13% of men reported having 3 or more alcoholic drinks per week. One female participant and 2 male participants reported experiencing abuse from their study partner in the past 3 months.
Table 1.
Participant demographics at enrollment (N=1013)
| Female N (%) or Median (IQR) N=334 |
Male N (%) or Median (IQR) N=679 |
|
|---|---|---|
| Age | ||
| 18–24 | 91 (27.3) | 116 (17.1) |
| 25–29 | 93 (27.8) | 205 (30.2) |
| 30–34 | 65 (19.5) | 140 (20.6) |
| 35+ | 85 (25.5) | 218 (32.1) |
| Education (years) | ||
| ≤8 | 210 (62.9) | 354 (52.1) |
| >8 | 124 (37.1) | 325 (47.9) |
| Sexual Behavior | ||
| Sex acts per month, with study partner | 4.5 (3.0–8.0) | 6.0 (3.0–12.0) |
| Number of other sex partners | ||
| None | 329(98.5) | 600(88.4) |
| 1 or more | 5 (1.5) | 79 (11.6) |
| Medical History | ||
| Circumcision at enrollment (men) | N/A | 462 (68.0) |
| Effective contraceptive use* (women) | 107 (32.0) | N/A |
| Alcohol abuse | ||
| Number of alcoholic drinks/week | ||
| 0 | 286 (85.6) | 489 (72.0) |
| 1–2 | 37 (11.1) | 97 (14.3) |
| 3 or more | 11(3.3) | 93 (13.7) |
| Mental Health | ||
| Probable depression** | 39 (11.7) | 64 (9.4) |
| Social support mean *** | 3.6 (3.2–3.9) | 3.7 (3.2–4.0) |
| Experienced stigma**** | 90 (27.0) | 228 (33.6) |
| Characteristics of HIV+ partner | ||
| Age | ||
| 18–24 | 292 (43.0) | 25 (7.5) |
| 25–29 | 194 (28.5) | 56 (16.8) |
| 30–34 | 95 (14.0) | 71 (21.3) |
| 35+ | 98 (14.4) | 182 (54.5) |
| CD4 Count (copies/μL) | ||
| ≥500 | 293 (43.1) | 127 (38.0) |
| 200–499 | 292 (43.0) | 140 (41.9) |
| <200 | 94 (13.8) | 67 (20.0) |
| Viral Load (copies/mL) | ||
| ≥50,000 | 235 (34.6) | 196 (58.7) |
| 10,000–49,999 | 223 (32.8) | 78 (23.3) |
| <10,000 | 221 (32.5) | 60 (18.0) |
| WHO stage | ||
| Clinical Stage 1 | 503 (74.1) | 205 (61.4) |
| Clinical Stage 2 | 176 (25.9) | 129 (38.6) |
| Characteristics of Couple | ||
| Cohabitating | 326 (97.6) | 657 (96.8) |
| Married to each other | 325 (97.3) | 647 (95.2) |
| Length of Partnership (years) | ||
| <1 | 46 (14.1) | 201 (30.6) |
| 1–5 | 128 (39.3) | 297 (45.2) |
| >5 | 152 (46.6) | 159 (24.2) |
| Number of Children | 2(1–3) | 1 (0–3) |
| Time discordant (years) | 0.08 (0.08–0.5) | 0.08 (0.0–0.1) |
| Partnerships satisfaction scale***** | 31(27–34) | 32 (28–35) |
| Experienced IPV in the last 3 months | 1 (0.3) | 2(0.3) |
Prevalence of IPV
At baseline and follow-up, there were 53 visits reports of intimate partner violence by 49 HIV-negative partners inflicted by the study partner, including physical, economic, verbal, other types of abuse, or combinations of types of abuse (Figure 1). Over 50% of reports described verbal abuse, 25% were physical, and 22% were economic. Fifty-three percent of reports were made by women and 47% were made by men. Men and women reported verbal abuse at similar frequencies while the majority of physical abuse reports were made by women.
Figure 1. Types of IPV reports in visits 53 by 49 women and men during the entire study.
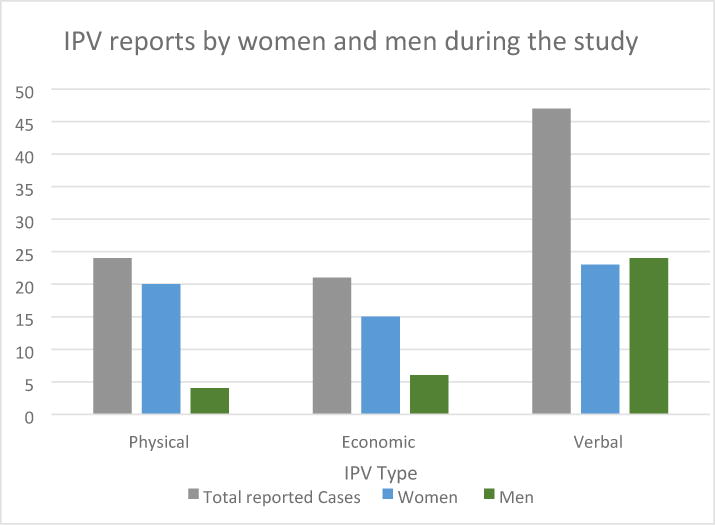
* There was 1 additional case that could not be categorized as economic/verbal/physical
Prevalence of self-reported PrEP interruption
249 participants reported intentional interruptions in PrEP use at a total of 328 visits that were subsequent to PrEP dispensing (7.1% of all follow-up visits), 65% were from men. Of the 249 participants reporting a PrEP interruption, 192 reported an interruption only once during their visits. Only 1 person reported 7 interruptions throughout their visits. The median length of PrEP interruption was 28 days (interquartile range [IQR]: 7–45). Of the 328 PrEP interruptions reported, 235 visits had a PrEP interruption longer than 7 days (Table 2). The most common reasons reported for an interruption in PrEP use included: breaking up with the study partner living with HIV (27.0%), experiencing side effects or being fearful of side effects (24.4%), not being at home or having a partner who was not home (17.0%), running out of pills (7.3%), and getting tired of taking pills (7.0%).
Table 2.
Prevalence of PrEP interruption among 249 people following PrEP dispensation in 328 visits
| Months since study enrollment | Any self-reported PrEP interruption N (% of all visits) |
Self-reported PrEP Interruption >7 days N (% of all visits) |
|---|---|---|
| Between enrollment and month 3 | 55/689 (8.0) | 32/689(4.6) |
| 3 months | 58/754 (7.7) | 35/754 (4.6) |
| 6 months | 59/774 (7.6) | 44/774 (5.7) |
| 9 months | 50/715 (7.0) | 39/715 (5.4) |
| 12 months | 39/536 (7.3) | 35/536 (6.5) |
| 15 months | 12/405 (3.0) | 10/405 (2.5) |
| 18 months | 25/315 (7.9) | 17/315 (5.4) |
| 21 months | 17/246 (6.9) | 14/246 (5.4) |
| 24 months | 13/193 (6.7) | 9/193 (4.7) |
| Total during all follow-up | 328/4,631 (7.1) | 235/4,631 (5.1) |
Intimate partner violence and self-reported PrEP interruption
PrEP interruptions were reported during 23.8% of visits when any IPV was also reported and 6.9% of visits when IPV was not reported (odds ratio [OR] 3.8, 95% confidence interval [CI] =1.8–8.0, Table 3). After adjusting for age and frequency of sex, the association between PrEP interruption and IPV remained significant (adjusted OR=2.6, 95% CI= 1.2–6.0). Additional correlates of self-reported PrEP interruption were considered and included being married to the study partner (OR= 2.4, 95% CI= 1.5–3.9), intestinal symptoms (OR=2.3, 95% CI= 1.8–3.2), and having probable depression (OR=2.1, 95% CI=1.5–3.2) were significantly associated in univariate analysis but these were no longer significant in the multivariable model.
Table 3.
Association of reported IPV and self-reported PrEP interruption*
| N (%) with no self-reported time off PrEP | N (%) with self-reported time off PrEP | OR (95% CI) p-value |
Adjusted OR (95% CI) p-value |
|
|---|---|---|---|---|
| Partner violence | ||||
| Reported IPV | 32 (76.2) | 10 (26.8) | 3.8 (1.8–8.0) p<0.0001 |
2.6 (1.2–6.0) p=0.02 |
| No IPV report | 4,263 (93.1) | 315 (6.9) | REF | REF |
| Sex acts per month with study partner | ||||
| 0–1 | 982 (86.7) | 150 (13.3) | REF | REF |
| 2–5 | 1,735 (94.9) | 93 (5.1) | 0.37 (0.3–0.5) p<0.0001 |
0.39 (0.3–0.5) p<0.0001 |
| 6 or more | 1,586 (94.9) | 85 (5.1) | 0.37 (0.3–0.5) p<0.0001 |
0.36(0.3–0.5) p<0.0001 |
| Age | ||||
| 18–24 | 864 (91.5) | 80 (8.5) | REF | REF |
| 25–29 | 1,239 (92.3) | 102 (7.6) | 0.88 (0.6–1.3) p=0.48 |
0.87 (0.6–1.2 p=0.45 |
| 30–34 | 888 (93.8) | 59 (6.2) | 0.67 (0.4–1.0) p=0.06 |
0.72 (0.4–1.1) p=0.13 |
| 35+ | 1,312 (93.8) | 87 (6.2) | 0.66 (0.4–0.9) p=0.03 |
0.68 (0.4–0.9) p=0.04 |
any PrEP interruption in the last 3 months
Discussion
In this study, HIV-negative participants in a known serodiscordant partnership who reported any IPV were significantly more likely to report an interruption in their oral PrEP use. To our knowledge only one published study has examined the association between IPV and PrEP interruption.16 That study did not identify mechanisms on the pathway which we believe could be mental health challenges, lack of support, partnership conflict and partnership dissolution. Participant reports of IPV were rare; but the most common type was verbal abuse, followed by physical and economic abuse. Reports of PrEP interruptions among people who had been dispensed drug were also rare, and they were most often related to partner break up and actual or perceived side effects of the PrEP medication.
Interruptions in PrEP use are important to understand, especially for women for whom missed PrEP doses may be less forgiving than men.24 Resumption of PrEP after interruption may coincide with resumption of sexual risk. This scenario is relevant because oral PrEP resumption may provide partial protection until tenofovir is activated and achieves a steady state.25 An analysis within a blinded, placebo-controlled oral PrEP clinical trial of heterosexual serodiscordant couples, found that women reporting IPV in the past three months had an increased risk of low adherence by pill count (adjusted RR=1.5, 95% CI=1.2–1.9) and plasma tenofovir levels (adjusted RR=1.5, 95% CI=1.1–2.2).16 The current open-label study provides data in a context where PrEP use was encouraged to be aligned with HIV risk, thus adherence may have varied over time more than in placebo-controlled clinical trials. In our study, participants were also encouraged to disclose gaps in PrEP adherence without judgement from study staff or consequence to their research participation so that counseling messages could be tailored to reflect true risk. While there is still likely to be underreporting of IPV and PrEP interruptions in our data, the strength of the correlation that we observed suggests that violence can undermine the use of PrEP and leave people vulnerable to HIV acquisition.
Although women more frequently reported experiencing physical IPV in our study, men also reported experiencing IPV, particularly verbal violence. Data documenting IPV among men are uncommon, but it is important to document violence experienced by men, as well as women, and consider the adoption of gender-based interventions. Reports of physical violence were more frequent in women than in men, consistent with the findings of other studies in the region where women were mainly the victims of physical abuse.26
There are several strengths and limitations of this study. Key strengths include the use of longitudinal data from a prospective cohort study with >90% retention through follow-up visits, and collection of data related to men as victims of IPV. A limitation is the reliance on self-report of IPV events, an inherent limitation of all studies of this nature. Underreporting of IPV could have occurred since there are certain societal expectations and stigma associated with IPV.27 Additionally, our questions may not have been sufficiently comprehensive to elicit all reports of IPV. We asked about three types of abuse, provided examples, and counselors were trained in non-judgmental counselling techniques about collecting social harm information. However, the examples did not describe every possible experience of abuse and participants might not have been able to recognize their experience among the listed categories. In addition, IPV is a difficult subject to disclose, especially to study staff, than to close family members and friends.28,29 In our study, IPV was reported less frequently than the 2014 Kenya Demographic and Health Survey where 39% of women and 9% of men reported IPV and the 2010 Uganda Demographic and Health Survey where 60% of women and 40% of men reported IPV.5,6 Another limitation is the use of self-reported PrEP interruption as our measure of PrEP adherence. The study used MEMS to capture daily adherence data but we limited this analysis to the self-reported time off PrEP because we wanted to focus on deliberate interruptions that participants recognized as substantial enough to report. Adherence counseling for PrEP in this open-label study focused on PrEP use being aligned with HIV risk, and thus participants were encouraged to feel comfortable describing periods when they did not take their pills.describing periods when they did not take Nonetheless, participants may not have wanted to disclose PrEP interruptions due to social desirability or difficulties with recall and we are unable to assess whether the degree of accuracy in PrEP recall differed among people experiencing and not experiencing IPV. Future studies should use innovative strategies, including qualitative techniques and motivational interviewing to encourage more disclosure.
The results of this study highlight the potential for IPV to impact oral PrEP use and therefore its effectiveness. Adherence support for persistent PrEP use has been recognized as being an important facet of a comprehensive HIV prevention program.30,31 Within PrEP delivery programs, there may opportunities to identify people experiencing IPV and dovetail IPV interventions with biomedical HIV prevention services for people that are using PrEP, as had been done in other HIV prevention intervention studies. The SHARE intervention in Uganda, a combination of IPV prevention and HIV prevention services significantly reduced reports of women’s physical and sexual IPV and was associated with a lower HIV incidence.32 The IMAGE study in South Africa, combined microfinancing with participatory training on subjects such as domestic abuse. The authors found that the risk of partner violence levels in the past year was reduced by more than half through women’s economic and social empowerment.33 Programs like this may lend themselves to being settings to deliver PrEP or market PrEP for women.
Overcoming the barriers to PrEP adherence faced by women and men is an important objective for programs delivering oral PrEP for HIV prevention. PrEP delivery involves health providers interacting with individuals at established intervals, providing a valuable opportunity for IPV interventions, which could reduce IPV, as well as HIV risk and interruptions in PrEP use. Integrating IPV interventions within PrEP delivery may help optimize PrEP use and increase the degree to which providers are aware and empowered to intervene on violent situations.
Acknowledgments
We thank the couples who participated in this study for their motivation and dedication and the referral partners, community advisory groups, institutions, and communities that supported this work.
Funding: The Partners Demonstration Project was funded by the Bill & Melinda Gates Foundation (OPP1056051), the National Institute of Mental Health of the US National Institutes of Health (NIH, R01 MH095507) and the United States Agency for International Development (USAID, JAID-OAA-A-12-00023). This work is made possible by the generous support of the American people through USAID; the contents are the responsibility of the authors and do not necessarily reflect the views of USAID, NIH, or the United States Government.
Partners Demonstration Project Team
Coordinating Center (University of Washington) and collaborating investigators (Harvard Medical School, Johns Hopkins University, Massachusetts General Hospital): Jared Baeten (protocol chair), Connie Celum (protocol co-chair), Renee Heffron (project director), Deborah Donnell (statistician), Ruanne Barnabas, Jessica Haberer, Harald Haugen, Craig Hendrix, Lara Kidoguchi, Mark Marzinke, Susan Morrison, Jennifer Morton, Norma Ware, Monique Wyatt
Project sites:
Kabwohe, Uganda (Kabwohe Clinical Research Centre): Stephen Asiimwe, Edna Tindimwebwa
Kampala, Uganda (Makerere University): Elly Katabira, Nulu Bulya
Kisumu, Kenya (Kenya Medical Research Institute): Elizabeth Bukusi, Josephine Odoyo
Thika, Kenya (Kenya Medical Research Institute, University of Washington): Nelly Rwamba Mugo, Kenneth Ngure
Data Management was provided by DF/Net Research, Inc. (Seattle, WA). PrEP medication was donated by Gilead Sciences.
References
- 1.Baeten JM, Heffron R, Kidoguchi L, et al. Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1–Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda. In: Siegfried N, editor. PLOS Med. 8. Vol. 13. 2016. p. e1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya. 2016. MINISTRY OF HEALTH; http://www.faces-kenya.org/wp-content/uploads/2016/07/Guidelines-on-Use-of-Antiretroviral-Drugs-for-Treating-and-Preventing-HI….pdf. Accessed May 9, 2017. [Google Scholar]
- 4.AVAC. (AVAC), AIDS Vaccine Advocate Coalition. Ongoing and Planned PrEP Demonstration and Implementation Studies. 2016 Dec; http://www.avac.org/resource/ongoing-and-planned-prep-demonstration-and-implementation-studies. Published 2016. Accessed April 18, 2017.
- 5.Kenya National Bureau of Statistics (KNBS) Kenya 2014 Demographic and Health Survey. Rockville, MD: 2015. http://evaw-global-database.unwomen.org/-/media/files/unwomen/vaw/vawsurvey/1kenyadhs2014.pdf. Accessed April 19, 2017. [Google Scholar]
- 6.Bureau of Statistics U, International ICF. Uganda Demographic and Health Survey. Kampala, Uganda: 2012. http://evaw-global-database.unwomen.org/-/media/files/unwomen/vaw/vawsurvey/ugandavawsurvey.pdf. Accessed April 20, 2017. [Google Scholar]
- 7.Maman S, Campbell J, Sweat MD, Gielen AC. The intersections of HIV and violence: directions for future research and interventions. Soc Sci Med. 2000;50(4):459–478. doi: 10.1016/s0277-9536(99)00270-1. http://www.ncbi.nlm.nih.gov/pubmed/10641800. Accessed September 14, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Marshall CM, Rees HC, Nunez A, Ezeanolue EE, Ehiri JE. Intimate partner violence and HIV infection among women: a systematic review and meta-analysis. J Int AIDS Soc. 2014;17(1):18845. doi: 10.7448/ias.17.1.18845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durevall D, Lindskog A. Intimate partner violence and HIV in ten sub-Saharan African countries: what do the Demographic and Health Surveys tell us? Lancet Glob Heal. 2015;3(1):e34–e43. doi: 10.1016/S2214-109X(14)70343-2. [DOI] [PubMed] [Google Scholar]
- 10.Kouyoumdjian FG, Findlay N, Schwandt M, Calzavara LM, Villarruel A. A Systematic Review of the Relationships between Intimate Partner Violence and HIV/AIDS. In: Stephenson R, editor. PLoS One. 11. Vol. 8. 2013. p. e81044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouyoumdjian FG, Calzavara LM, Bondy SJ, et al. Intimate partner violence is associated with incident HIV infection in women in Uganda. AIDS. 2013;27(8):1331–1338. doi: 10.1097/QAD.0b013e32835fd851. [DOI] [PubMed] [Google Scholar]
- 12.Jewkes RK, Dunkle K, Nduna M, Shai N, Banks A, Allen S. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet (London, England) 2010;376(9734):41–48. doi: 10.1016/S0140-6736(10)60548-X. [DOI] [PubMed] [Google Scholar]
- 13.Eloff I, Forsyth B, Finestone M, et al. Intervention groups for HIV-infected women: the need for additional services. South African J Psychol. 2011;41(1):38–51. [Google Scholar]
- 14.Were E, Curran K, Delany-Moretlwe S, et al. A prospective study of frequency and correlates of intimate partner violence among African heterosexual HIV serodiscordant couples. AIDS. 2011;25(16):2009–2018. doi: 10.1097/QAD.0b013e32834b005d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson KS, Deya R, Masese L, et al. Prevalence and correlates of intimate partner violence in HIV-positive women engaged in transactional sex in Mombasa, Kenya. Int J STD AIDS. 2016;27(13):1194–1203. doi: 10.1177/0956462415611514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts ST, Haberer J, Celum C, et al. Intimate Partner Violence and Adherence to HIV Pre-exposure Prophylaxis (PrEP) in African Women in HIV Serodiscordant Relationships. JAIDS J Acquir Immune Defic Syndr. 2016;73(3):313–322. doi: 10.1097/QAI.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahle EM, Hughes JP, Lingappa JR, et al. An Empiric Risk Scoring Tool for Identifying High-Risk Heterosexual HIV-1–Serodiscordant Couples for Targeted HIV-1 Prevention. JAIDS J Acquir Immune Defic Syndr. 2013;62(3):339–347. doi: 10.1097/QAI.0b013e31827e622d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psaros C, Haberer JE, Boum Y, et al. The Factor Structure and Presentation of Depression Among HIV-Positive Adults in Uganda. AIDS Behav. 2015;19(1):27–33. doi: 10.1007/s10461-014-0796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez P, Andia I, Emenyonu N, et al. Alcohol Use, Depressive Symptoms and the Receipt of Antiretroviral Therapy in Southwest Uganda. AIDS Behav. 2008;12(4):605–612. doi: 10.1007/s10461-007-9312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolton P. Cross-cultural validity and reliability testing of a standard psychiatric assessment instrument without a gold standard. J Nerv Ment Dis. 2001;189(4):238–242. doi: 10.1097/00005053-200104000-00005. http://www.ncbi.nlm.nih.gov/pubmed/11339319. Accessed September 14, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009;21(1):87–93. doi: 10.1080/09540120802032627. [DOI] [PubMed] [Google Scholar]
- 22.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celum C, Wald A, Lingappa JR, et al. Acyclovir and Transmission of HIV-1 from Persons Infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottrell ML, Yang KH, Prince HMA, et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis. 2016;214(1):55–64. doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JC, Baty ML, Ghandour RM, Stockman JK, Francisco L, Wagman J. The intersection of intimate partner violence against women and HIV/AIDS: a review. Int J Inj Contr Saf Promot. 2008;15(4):221–231. doi: 10.1080/17457300802423224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan KL. Gender differences in self-reports of intimate partner violence: A review. Aggress Violent Behav. 2011;16(2):167–175. doi: 10.1016/j.avb.2011.02.008. [DOI] [Google Scholar]
- 28.Okenwa LEE, Lawoko S, Jansson B. Factors associated with disclosure of intimate partner violence among women in Lagos, Nigeria. J Inj Violence Res. 2009;1(1):37–47. doi: 10.5249/jivr.v1i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilika AL. Women’s perception of partner violence in a rural Igbo community. Afr J Reprod Health. 2005;9(3):77–88. http://www.ncbi.nlm.nih.gov/pubmed/16623192. Accessed May 9, 2017. [PubMed] [Google Scholar]
- 30.Braksmajer A, Senn TE, Mcmahon J. The Potential of Pre-Exposure Prophylaxis for Women in Violent Relationships. doi: 10.1089/apc.2016.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haberer JE, Baeten JM, Campbell J, et al. Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa. In: Siegfried N, editor. PLoS Med. 9. Vol. 10. 2013. p. e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagman JA, King EJ, Namatovu F, et al. Combined Intimate Partner Violence and HIV/AIDS Prevention in Rural Uganda: Design of the SHARE Intervention Strategy. Health Care Women Int. 2016;37(3):364–387. doi: 10.1080/07399332.2015.1061526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JC, Watts CH, Hargreaves JR, et al. Understanding the impact of a microfinance-based intervention on women’s empowerment and the reduction of intimate partner violence in South Africa. Am J Public Health. 2007;97(10):1794–1802. doi: 10.2105/AJPH.2006.095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai AC, Tomlinson M, Comulada WS, Rotheram-Borus MJ. Food insufficiency, depression, and the modifying role of social support: Evidence from a population-based, prospective cohort of pregnant women in peri-urban South Africa. Soc Sci Med. 2016;151:69–77. doi: 10.1016/j.socscimed.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Med Care. 1988;26(7):709–723. doi: 10.1097/00005650-198807000-00006. http://www.ncbi.nlm.nih.gov/pubmed/3393031. Accessed April 23, 2017. [DOI] [PubMed] [Google Scholar]
- 36.Ware NC, Wyatt MA, Haberer JE, et al. What’s love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59(5):463–468. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]


