Abstract
Background/Objectives
Growing evidence suggests antibiotic use is associated with childhood body mass index (BMI), potentially via mechanisms mediated by gut microbiome alterations. Less is known on the potential role of prenatal antimicrobial use in offspring obesity risk. We examined if prenatal antibiotic or antifungal use was associated with BMI at age 2 years in 527 birth cohort participants.
Methods/Subjects
Antimicrobial use was obtained from the prenatal medical record. Height and weight were measured at age 2 years. Overweight/obesity was defined as a BMI≥85th percentile.
Results
A total of 303 (57.5%) women used antibiotics and 101 (19.2%) used antifungals during pregnancy. Prenatal antifungal use was not associated with child BMI at age 2 years. In the fully-adjusted model, prenatal antibiotic use was associated with a 0.20±0.10 (P=0.046) higher mean BMI Z-score at age 2 years. Associations between prenatal antibiotic use and childhood BMI varied by trimester of exposure, with 1st or 2nd trimester exposure more strongly associated with larger BMI at age 2 years for both BMI Z-score (interaction P=0.032) and overweight/obesity (interaction P=0.098) after covariate adjustment.
Conclusions
Prenatal antibiotic, but not antifungal, use is associated with larger BMI at age 2 years; associations were stronger for antibiotic exposures in earlier trimesters. Future studies examining if these associations are due to alterations in the maternal and/or infant microbiome are necessary. Children who are overweight at age 2 years are at higher risk for overweight as they age; prenatal antibiotic use is a potentially modifiable exposure that could reduce childhood obesity.
Introduction
Rates of childhood overweight and obesity continue to rise in the United States.1 Rates of medication use in pregnancy have also increased, with antibiotics being among the most commonly administered prescription drugs.2 In the Project Women and Infants Starting Healthy Study conducted in San Francisco between 2001 and 2002, over half of all women were prescribed a prescription drug during pregnancy, with 62% of those prescriptions being antibiotics.3
Because of the established impact of antimicrobials on the gut microbiome,4 and murine studies demonstrating that administration of sub-therapeutic concentrations increases adiposity,5 their administration during pregnancy has the potential to be obesogenic.6 Antibiotics have been used in animal husbandry for over 6 decades to increase livestock weight gain, with administration to young, but not mature animals, resulting in increased weight.6–8 Antibiotic exposure in infancy is associated with increased childhood body mass index (BMI) and/or risk of overweight/obesity.9, 10 There is limited, and conflicting evidence exploring prenatal antibiotic use with childhood obesity. Two studies have shown positive associations between prenatal antibiotic use and overweight/obesity at age 7 years11 or ages 7–16 years12; in contrast, in another study, prenatal antibiotic orders were not associated with child BMI Z-score at age 3 years.13
Fungal species also impact gut symbiosis.14 However, in contrast to antibiotics, antifungals have not been shown to impact weight in animals.8 Antifungal medications are commonly used by pregnant women, most often for vaginal candidiasis.15 In non-pregnant, reproductive age women, topical antifungal treatment of Candida infection alters the bacterial vaginal microbiome,16 suggesting that use of these agents during pregnancy could potentially alter offspring’s risk of obesity via vertical transmission of an altered vaginal microbiome during the birthing process.
To our knowledge, however, no study has examined the association of prenatal antimicrobial use (antibiotic or antifungal use, separately) with BMI in early childhood, overall and within each trimester. We examined the association of prenatal antimicrobial use with childhood BMI at age 2 years in the Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS) birth cohort.17, 18
Materials/Subjects and Methods
WHEALS recruited pregnant women who delivered from September 2003 through December 2007, and who were seeing a Henry Ford Health System (HFHS) obstetrics practitioner at one of five clinics.17, 18 All women were in their second trimester or later, age 21–49 years, and lived in a predefined geographic area that was selected to encourage racial and socioeconomic diversity (city of Detroit and surrounding suburban areas). All participants provided written, informed consent. Study protocols were approved by the Institutional Review Board at HFHS.
Definition of Antimicrobial Use
As part of a study specifically examining racial (i.e., black-white) differences in vitamin D, maternal prenatal and delivery medical records were abstracted for data including antimicrobial use. Antibiotic use was defined as systemic antibiotic use (ingestion, intravenous, intramuscular) at any time during pregnancy. Antifungal use was defined as use of a systemic (ingested) or vaginally applied antifungal medication any time during pregnancy.18 We defined use of antimicrobials by trimester as follows: 1st trimester if used between 0 and <14 weeks gestational age; 2nd trimester if used 14–<27 weeks gestational age; and 3rd trimester if used 27 weeks gestational age through, and including, delivery. Number of prenatal antibiotics used was categorized as 0, 1, 2–3, or 4+.19 Antibiotics were classified based on structure/mode of action into the following groups: aminoglycosides, cephalosporins, lincosamides, quinolones, macrolides, nitroimidazoles, nitrofurantoins, penicillins, vancomycins, and sulfonamide/ trimethoprims.20–22
Covariate Measurement
Maternal date of birth, race, marital status, household income, education, number of previous births and breastfeeding at one month were self-reported. Medical records were abstracted to obtain height and weight at first prenatal care visit, prenatal antiviral use, mode of delivery, gestational age at delivery and infant birthweight. Gender- and gestational-age adjusted birthweight Z-scores were calculated using the US population as reference.23 Child medical records were abstracted to obtain information on antibiotic use before 1 month and 6 months of age.
Child Body Size Measurement
At the study’s 2-year research clinic visit, trained field staff measured child height in stocking feet with a wall stadiometer; child weight was measured with the child in light clothing using a balance beam physician scale. BMI Z-scores and percentiles were calculated according to the 2000 Centers for Disease Control and Prevention age- and gender-specific growth charts. Overweight was defined as BMI between the 85th and 95th percentile and obesity as BMI≥95th percentile.
Statistical Analysis
Maternal, newborn and child characteristics were compared by prenatal antibiotic or antifungal exposure using a chi-square test of independence for discrete characteristics and a Student’s t-test for continuous characteristics.
Logistic regression was used to examine the association between prenatal antibiotic or antifungal use and overweight/obesity (BMI≥85th percentile); linear regression was used for BMI Z-score. Models were fit unadjusted and adjusted for infant gender, mode of delivery (cesarean section or vaginal), ever breastfed, maternal age, maternal education, maternal race (white or black), birthweight Z-score, child age (in months) at the 2 year visit and maternal BMI at first prenatal care visit. To examine if associations varied by mode of delivery, child gender, maternal race or ever breastfed, multiplicative interaction terms were fit. Similar models were fit to determine if type of antibiotic (ever use of cephalosporin, ever use of macrolide, ever use of nitroimidazole, ever use of nitrofurantoin, ever use of penicillin, or ever use of other antibiotic (aminoglycoside, lincosamide, quinolone, vancomycin, or sulfonamide/trimethoprim) was associated with BMI at age 2 years.
To test for differences in timing of antibiotic or antifungal exposure across pregnancy, multiple informant models were used, which allowed us to test differences in an exposure-outcome relationship across time windows while maintaining the effect estimates that would be obtained fitting separate regression models for each time point.24 A score test of the equality of all the trimester-specific parameter estimates was used to evaluate differences across trimesters.24 A generalized estimating equation approach with linear and logistic regression was utilized to fit the multiple informant models for BMI Z-score and overweight/obesity, respectively.
Not all WHEALS children completed a 2-year clinic visit.17 To account for loss-to-follow-up, we calculated inverse probability weights (IPW) for complete follow-up.25 IPW were calculated by fitting a logistic regression model for complete follow-up at age 2 years with the following baseline maternal factors: age, race, insurance type, income (including whether or not the question was answered), education, smoking status, prenatal alcohol use, urban residence, marital status, history of asthma/allergies, and mode of delivery and obtaining the predicted probability of successful follow-up. Data were reanalyzed using the IPW as model weights to adjust for bias due to incomplete follow-up. SAS version 9.4 (SAS Institute, Cary, NC) was used for all analysis.
Results
A total of 1,258 children were originally included in WHEALS; 659 of these children completed a 2-year clinic visit with height and weight measurement. We excluded 108 children where the maternal chart was not abstracted and 24 children where maternal antimicrobial use was unknown. We compared maternal and child characteristics between the 527 included in the sample to the 731 excluded from the analytic sample; compared to those excluded, those included tended to have slightly older maternal age at delivery (30.1 vs. 29.2 years), to be black (69.3% vs. 56.5%) and to be slightly larger at birth (3349 vs 3270 g) (all P<0.05).
In our study, 303 (57.5%) women had antibiotic use and 101 (19.2%) had antifungal use during pregnancy. There were 577 antibiotic courses and 131 antifungal courses in the 527 women, giving a total of 1.09 antibiotic and a total of 0.25 antifungal courses per pregnancy in our cohort. Nearly all 538/577 (93.2%) the antibiotic courses were systemic (commonly oral administration); 24 (4.2%) antibiotic courses were delivered vaginally and only 15 (2.6%) were delivered topically. Compared to women not exposed to antibiotics, those with antibiotic exposure were slightly younger, were more likely to be black, had lower household income, lower education levels, had greater prenatal antiviral exposure and were less likely to ever breastfeed (Table 1). Black women, those with lower household income, those exposed to an antiviral prenatally and women carrying a male child were more likely to have prenatal antifungal use (Table 1). There was no association of prenatal antibiotic or antifungal use with child use of antibiotic before 1 or 6 months of age (all P>0.14).
Table 1.
Descriptive characteristics of the WHEALS participants, stratified by prenatal antibiotic use and prenatal antifungal use
| Antibiotic Use | Antifungal Use | |||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| N1 (%) | 303 (57.5%) | 224 (42.5%) | 101 (19.2%) | 426 (80.8%) |
| Maternal characteristic | ||||
| Age (years) | 29.5 ± 5.3** | 31.0 ± 4.9** | 29.6 ± 5.1 | 30.2 ± 5.2 |
| Race | ||||
| White | 76 (25.1%)** | 86 (38.4%)** | 22 (21.8%)** | 140 (32.9%)** |
| Black | 227 (74.9%) | 138 (61.6%) | 79 (78.2%) | 286 (67.1%) |
| BMI at first prenatal care visit (kg/m2) | 31.0 ± 8.2 | 29.8 ± 7.6 | 31.3 ± 7.3 | 30.3 ± 8.1 |
| Household Income | ||||
| <$20k | 39 (15.4%)** | 17 (8.5%)** | 16 (19.3%)** | 40 (10.8%)** |
| 20k–<$40k | 73 (28.7%) | 40 (19.9%) | 15 (18.1%) | 98 (26.3%) |
| 40k–<$80k | 80 (31.5%) | 60 (29.9%) | 33 (39.8%) | 107 (28.8%) |
| 80k–<100k | 25 (9.8%) | 46 (22.9%) | 13 (15.7%) | 58 (15.6%) |
| >=100k | 37 (14.6%) | 38 (18.9%) | 6 (7.2%) | 69 (18.6%) |
| Highest Level Education | ||||
| < High-school Diploma | 10 (3.3%)** | 11 (4.9%)** | 4 (4.0%) | 17 (4.0%) |
| High-school Diploma/Equivalent | 61 (20.1%) | 18 (8.0%) | 16 (15.8%) | 63 (14.8%) |
| Some College | 148 (48.8%) | 100 (44.6%) | 52 (51.5%) | 196 (46.0%) |
| >=Bachelor’s Degree | 84 (27.7%) | 95 (42.4%) | 29 (28.7%) | 150 (35.2%) |
| Antiviral Use | ||||
| No | 281 (92.7%)** | 218 (97.3%)** | 410 (96.2%)** | 89 (88.1%)** |
| Yes | 22 (7.3%) | 6 (2.7%) | 16 (3.8%) | 12 (11.9%) |
| Neonate characteristic | ||||
| Cesarean section delivery | 105 (34.7%) | 85 (38.0%) | 43 (42.6%) | 147 (34.5%) |
| Birth Weight (grams) | 3309.1 ± 595.9 | 3403.1 ± 588.4 | 3447.3 ± 548.8 | 3327.5 ± 602.0 |
| Birth Weight Z-score | −0.09 ± 1.0 | 0.05 ± 1.0 | 0.09 ± 1.0 | −0.06 ± 1.0 |
| Male gender | 153 (50.5%) | 120 (53.6%) | 67 (66.3%)** | 206 (48.4%)** |
| Ever breastfed | 224 (76.2%)** | 179 (83.6%)** | 72 (73.5%) | 331 (80.7%) |
| Ever used antibiotic before 1 month visit | 6 (3.2%) | 8 (6.7%) | 3 (5.5%) | 11 (4.4%) |
| Ever used antibiotic before 6 month visit | 30 (19.5%) | 28 (27.5%) | 8 (15.4%) | 50 (24.5%) |
| Child characteristic at 2 year visit | ||||
| Age (years) | 2.2 ± 0.3 | 2.2 ± 0.2 | 2.2 ± 0.3 | 2.2 ± 0.2 |
| BMI (kg/m2) | 16.8 ± 1.7** | 16.5 ± 1.5** | 16.8 ± 1.8 | 16.6 ± 1.6 |
| BMI Z-score | 0.17 ± 1.1** | −0.04 ± 1.1** | 0.15 ± 1.1 | 0.06 ± 1.1 |
| BMI category | ||||
| Underweight (BMI<5th percentile) | 20 (6.6%) | 15 (6.7%) | 6 (5.9%) | 29 (6.8%) |
| Normal Weight (BMI≥5th and <85th percentile) | 231 (76.2%) | 171 (76.3%) | 75 (74.3%) | 327 (76.8%) |
| Overweight (BMI≥85th and <95th percentile | 21 (6.9%) | 24 (10.7%) | 14 (13.9%) | 31 (7.3%) |
| Obese (BMI≥95th percentile) | 31 (10.2%) | 14 (6.3%) | 6 (5.9%) | 39 (9.2%) |
| Prenatal Antimicrobial characteristics | ||||
| Timing of medication exposure (trimester) | ||||
| 1st ONLY | 60 (19.8%) | N/A | 26 (25.7%) | N/A |
| 2nd ONLY | 35 (11.6%) | N/A | 27 (26.7%) | N/A |
| 3rd ONLY | 100 (33.0%) | N/A | 39 (38.6%) | N/A |
| 1st and 2nd | 21 (6.9%) | N/A | 3 (3.0%) | N/A |
| 1st and 3rd | 36 (11.9%) | N/A | 1 (1.0%) | N/A |
| 2nd and 3rd | 34 (11.2%) | N/A | 4 (4.0%) | N/A |
| All 3 | 17 (5.6%) | N/A | 1 (1.0%) | N/A |
| Number of medication exposures | ||||
| 1 | 157 (51.8%) | N/A | 84 (83.2%) | N/A |
| 2–3 | 116 (38.2%) | N/A | 14 (13.9%) | N/A |
| 4+ | 30 (9.9%) | N/A | 3 (3.0%) | N/A |
| Route of antifungal exposure | ||||
| Oral only | N/A | N/A | 38 (37.6%) | N/A |
| Vaginal only | N/A | N/A | 53 (52.5%) | N/A |
| Vaginal and Oral | N/A | N/A | 10 (9.9%) | N/A |
BMI at first prenatal care visit (N = 520), Household Income (N= 455), Birth Weight (N = 504), Birth Weight Z-score (N = 490), Ever breastfed (N = 508), Infant antibiotic use before age 1 month (N=307), Infant antibiotic use before age 6 months (N=256).
P<0.05 comparing women with and without the specific antimicrobial exposure
BMI, body mass index
Prenatal antimicrobial use and BMI at age 2 years
Mean BMI of the WHEALS children at age 2 years was 16.6±1.7 kg/m2 and mean BMI Z-score was 0.08±1.1; 90 (17.1%) WHEALS children were overweight/obese at age 2 years. There was no evidence that prenatal antibiotic use was associated with overweight/obesity risk and there was no evidence prenatal antifungal medication use was associated with BMI Z-score or overweight/obesity at age 2 years (Table 2). Prenatal antibiotic use was positively associated with BMI Z-score at age 2 years (P=0.046); after covariate adjustment, children born to mothers who used antibiotics in the prenatal period had an estimated 0.20±0.10 unit higher BMI Z-score at age 2 years compared to children born to mothers who did not use prenatal antibiotics (Table 2). There was no evidence that the association between prenatal antibiotic or antifungal medication use with child BMI varied by mode of delivery, child gender, maternal race, or breastfeeding status (all interaction P>0.11).
Table 2.
Association of overall prenatal antibiotic or antifungal use with body mass index (BMI) Z-score and child overweight/obese at age 2 year visit
| BMI Z-score | Overweight/Obese | |||
|---|---|---|---|---|
| β (se) | P | OR (95 % CI OR) | P | |
| Any Prenatal Antibiotic Use | ||||
| Model 1 | 0.21 (0.10) | 0.033 | 1.01 (0.64,1.61) | 0.953 |
| Model 2 | 0.20 (0.10) | 0.046 | 1.04 (0.62, 1.74) | 0.885 |
| Any Prenatal Antifungal Use | ||||
| Model 1 | 0.09 (0.12) | 0.464 | 1.26 (0.72, 2.18) | 0.419 |
| Model 2 | −0.13 (0.13) | 0.323 | 0.99 (0.53, 1.87) | 0.984 |
Model 1: Unadjusted; Model 2: adjusted for infant gender, delivery mode, ever breastfed, maternal age, maternal race, maternal education, birthweight Z-score, child age at 2-year visit and BMI at first prenatal care visit
Although there was no evidence of a prenatal antibiotic-by-delivery mode interaction with childhood BMI, given our a priori hypothesis that the maternal microbiome may mediate associations between prenatal antibiotic use and childhood obesity, we refit our models stratified by delivery mode. In the fully-adjusted model, prenatal antibiotic use was not statistically significantly associated with BMI Z-score at age 2 years (0.17±0.18; P=0.35) in offspring of women who delivered via C-section; in contrast, the association of prenatal antibiotic use with BMI Z-score at age 2 years (0.23±0.13; P=0.064) approached statistical significance in women who delivered vaginally.
Trimester-specific antimicrobial use and BMI at age 2 years
Trimester of prenatal antibiotic exposure was associated with BMI Z-score and overweight/obesity at age 2 years (Table 3). In the fully adjusted model, there was evidence for a trimester-specific effect of antibiotic use with BMI Z-score (interaction P=0.032); 1st trimester antibiotic use was associated with a 0.21±0.12 unit higher BMI Z-score (P=0.071), 2nd trimester antibiotic use was associated with a statistically significant 0.28±0.13 unit higher BMI-score (P=0.028), but 3rd trimester use was not associated with BMI Z-score (P=0.691). Results were similar for odds of overweight/obesity; in the fully-adjusted model, there was borderline suggestive significant evidence of a trimester-specific effect of antibiotic use (interaction P=0.098). Children born to mothers who used antibiotics in the 1st trimester had 1.78 (95% CI OR: 1.07, 2.98) times greater odds of being overweight/obese at age 2 years (P=0.028); there was no association of 2nd or 3rd trimester antibiotic use and odds of overweight/obesity at age 2 years. There was no evidence for a trimester-specific effect of antifungal use on childhood BMI at age 2 years.
Table 3.
Trimester-specific association of antibiotic or antifungal use with body mass index (BMI) Z-score and child overweight/obese at age 2 year visit; evidence for a trimester-specific effect is indicated with the interaction P term
| BMI Z-score | Overweight/Obese | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Antibiotic Use | β±se (P) | β±se (P) | OR (95 % CI OR) (P) | OR (95 % CI OR) (P) |
| 1st trimester | 0.23±0.11 (P=0.043) | 0.21±0.12 (P=0.071) | 1.71 (1.05, 2.77) (P=0.032) | 1.78 (1.07, 2.98) (P=0.028) |
| 2nd trimester | 0.24±0.12 (P=0.05) | 0.28±0.13 (P=0.028) | 1.44 (0.85, 2.45) (P=0.175) | 1.34 (0.74, 2.46) (P=0.337) |
| 3rd trimester | −0.001±0.10 (P=0.990) | −0.04±0.10 (P=0.691) | 0.74 (0.45, 1.21) (P=0.234) | 0.72 (0.42, 1.22) (P=0.225) |
| Interaction P | 0.070 | 0.032 | 0.094 | 0.098 |
| Antifungal Use | β±se (P) | β±se (P) | OR (95 % CI OR) (P) | OR (95 % CI OR) (P) |
| 1st trimester | 0.35±0.21 (P=0.090) | 0.02±0.22 (P=0.915) | 1.18 (0.47, 2.96) (P=0.729) | 0.63 (0.22, 1.85) (P=0.403) |
| 2nd trimester | −0.02±0.20 (P=0.907) | −0.08±0.20 (P=0.687) | 1.23 (0.52, 2.91) (P=0.635) | 1.18 (0.48, 2.89) (P=0.714) |
| 3rd trimester | −0.10±0.18 (P=0.560) | −0.23±0.18 (P=0.201) | 0.89 (0.38, 2.05) (P=0.777) | 0.88 (0.40, 1.96) (P=0.763) |
| Interaction P | 0.221 | 0.578 | 0.943 | 0.787 |
Model 1: Unadjusted; Model 2: adjusted for infant gender, delivery mode, ever breastfed, maternal age, maternal race, maternal education, birthweight Z-score, child age at 2-year visit and BMI at first prenatal care visit
Dose-response relationship with antibiotic use
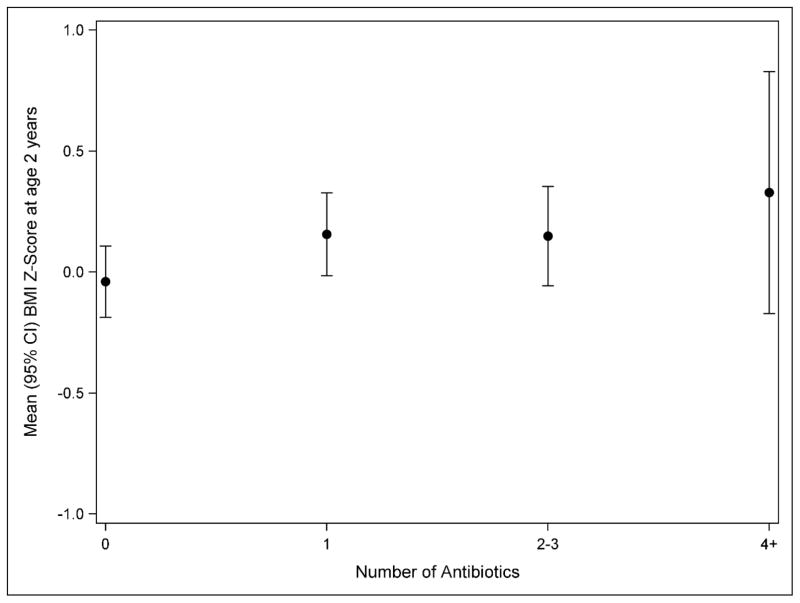
Most women who used antibiotics had one exposure over pregnancy (n=157; 51.8%) (Table 1). Figure 1 shows the relationship between number of prenatal antibiotics used and mean BMI Z-score at age 2 years, with the highest BMI Z-score seen in children with the greatest number of prenatal exposures to antibiotics, however this was not statistically significant (P-trend=0.144). There was no association of number of antibiotics used with overweight/obesity. Only 17 women had multiple uses of antifungal medications (Table 1), thus we did not evaluate potential dose-response relationships for antifungal use.
Figure 1.
Relationship between number of prenatal antibiotic exposures and mean BMI Z-score at age 2 years
Antibiotic Type and Body Mass Index
Antibiotic use fell into the following groupings: ever penicillin use (n=189 uses); ever nitroimidazole use (n=112 uses); ever cephalosporin use (n=87 uses); ever nitrofurantoin use (n=72 uses); ever macrolide use (n=62 uses); and ever use of other antibiotic (n=55 uses). After covariate adjustment, compared to children born to women without antibiotic exposure, children born to a mother ever exposed to macrolides had a statistically significantly higher BMI Z-score (0.37±0.18; P=0.039). No other antibiotic type was associated with BMI Z-score. Antibiotic type was also not associated with overweight/obesity.
Sensitivity Analysis
Model inferences were similar after accounting for IPW. In the fully adjusted model, the association of use of prenatal antibiotics with BMI Z-score was slightly attenuated (β=0.19±0.11; P=0.069). There remained evidence for a trimester-specific effect of prenatal antibiotic use on BMI Z-score (interaction P=0.035) and overweight/obesity (interaction P=0.069). First-trimester antibiotic use was associated with a mean increase in BMI Z-score of 0.21±0.12 (P=0.069) and 2nd trimester antibiotic use was associated with a mean increase in BMI Z-score of 0.29±0.13 (P=0.023). First trimester antibiotic use was also associated with a 1.85 (95% CI: 1.09, 3.16; P=0.023) times increased odds of overweight/obesity.
Discussion
In the current study, prenatal antibiotic, but not antifungal, exposure was associated with increased risk of offspring having a larger BMI Z-score at age 2 years. We provide new evidence suggesting that this association varies by timing of the antibiotic exposure, with antibiotic exposure in the 1st or 2nd trimester, but not the 3rd trimester, associated with greater BMI Z-score at age 2 years. Similarly, exposures to antibiotics in the 1st trimester were associated with overweight/obesity at age 2 years. Even at early ages, children who are obese are at higher risk of becoming obese adolescents and adults;26 prenatal antibiotic use may be one modifiable exposure that could reduce the current rates of childhood obesity, particularly if we are able to reduce unnecessary antibiotic use.
Antibiotic use during pregnancy is associated with alterations in the vaginal microbiome at gestational week 36, with significantly increased Staphylococcus species in women treated with oral antibiotics.27 Particularly in vaginally-born infants, the infant gut, skin and oral microbiome resembles the maternal vaginal microbiome.28 Children with greater Staphylococcus aureus counts in fecal samples from 6 or 12 months of age are statistically significantly more likely to be overweight at age 7 years.29 Thus, if prenatal antibiotic exposure increases vaginal Staphylococcus aureus numbers, this could explain the increased risk of larger BMI at age 2 years detected in our study. In animal models, maternal antibiotic treatment is associated with alterations in the offspring gut microbiome.30 While intra-partum maternal antibiotic use in Group B Streptococcus positive mothers is associated with alterations in the infant gut microbiome,31, 32 to our knowledge, data from humans examining if prenatal antibiotic use over various gestational ages is associated with infant gut microbiome are lacking. Antibiotic use during the last month of pregnancy was not associated with the infant gut microbiome at age 1 month,33 which is consistent with our finding that 3rd trimester antibiotic exposure is not associated with childhood BMI at age 2 years. Given the microbial dynamics that occur normally in the maternal gut and vaginal microbiome over pregnancy,34–36 which are postulated to play a role in priming fetal development, it is plausible that antibiotic-associated maternal microbiome perturbation may impact post-natal infant microbiome development, and, subsequently, child health measures. Our results, which showed a stronger, albeit only near statistically significant association of maternal antibiotic use with BMI Z-score at age 2 in children delivered vaginally compared to by C-section further suggests that alterations in the microbiome may be a mechanism linking maternal antibiotic use with childhood BMI.
In addition to mechanisms linked to the microbiome, differential timing of antibiotics over gestational development could directly impact the developing endocrine system, and thus prime a fetus for future risk of obesity. Development of the human endocrine system commences at approximately gestational week 4, involving hormone production related to subsequent fetal growth in gestational weeks 4–10.37 In contrast, fetal adipose tissue deposition primarily occurs throughout the latter 3rd of gestation.38 Hence exposures at distinct periods of gestation may have a differential impact on fetal development. For instance, data from several studies conducted on pregnancies that occurred during famines suggest that timing of the exposure, that is, under-nutrition in early pregnancy, may be important for increased risk of offspring obesity.39 Maternal antibiotic use may have a similar trimester-specific effect on the fetal endocrine system and development.
Alternative mechanisms by which prenatal antibiotic exposure may impact childhood BMI are possible. Antibiotic exposure could impact the development, implantation or function of the placenta, subsequently affecting development of the fetus and future growth. Limited data exists describing the impact of antibiotics on the placenta, however, in a recent study examining amniotic membrane samples from a single placenta, antibiotic exposure damaged the cell membrane and resulted in loss of microvillia.40 The placenta undergoes rapid development during the first trimester of pregnancy41 and itself has a distinct microbiome that resembles the oral microbiome42; antibiotic exposure early in pregnancy may similarly impact the placental microbiome at a critical stage of fetal development. Data from the Project Viva cohort demonstrated a trimester-specific effect of antibiotic exposure on leptin, a hormone involved in energy balance and appetite suppression; exposures in the 1st and 2nd trimester were associated with non-statistically significant lower levels of cord blood leptin, whereas exposures in the 3rd trimester were associated with statistically significant higher levels of cord blood leptin.43 Lower cord blood leptin levels are associated with higher BMI at age 3 years.44 Thus, although the associations were not statistically significant, the finding of lower leptin in cord blood following 1st or 2nd trimester antibiotic exposure is consistent with our finding that earlier pregnancy antibiotic exposure is associated with larger BMI at age 2 years. Finally, prenatal exposure to antibiotics could increase risk of antibiotic use in early childhood, which is known to increase risk of obesity;45 however, in our cohort, prenatal antibiotic use was not associated with early childhood antibiotic use, suggesting that this was not acting as a confounder in our study. However, each of these potential mechanisms requires further study.
To our knowledge, few previous studies specifically examined prenatal antibiotic use and childhood BMI and none have examined antifungal use. In the Northern Manhattan Mothers and Children study, antibiotic exposure in the 2nd or 3rd trimester was associated with risk of obesity and BMI Z-score at age 7.11 In contrast to our findings at age 2 years, 1st trimester antibiotic use was not associated with childhood BMI at age 7 years. Similarly, in a study of Danish schoolchildren ages 7–16 years, prenatal antibiotic exposure was associated with overweight and obesity risk.12 In a study conducted in Geisinger Clinic data, however, prenatal antibiotic orders were not associated with BMI Z-score at age 3 years.13 There are several differences in these studies which may explain some of these varying results. Antibiotic use in WHEALS was defined based on medical chart review, compared to self-report during a 3rd trimester interview in the Northern Manhattan study; women may have difficulty self-reporting medication use, particularly early in pregnancy. The prevalence of antibiotic use in WHEALS in the 2nd and 3rd trimester was 32.1% compared to 16% in the Northern Manhattan study, 33% in the Danish study, and 60.4% of women in the Geisinger study were ordered antibiotics prenatally. While WHEALS is comprised of primarily black and white women, the Northern Manhattan study is comprised primarily of black and Dominican women, the Danish study consisted of Dutch women, and the Geisinger study was nearly all white. Finally, the age at childhood BMI measurement (age 2 in WHEALS vs. age 7 years in the Northern Manhattan Study, age 7–16 years in the Danish study and age 3 years in the Geisinger study) differed. Electronic health record data was used to define child BMI at age 3 years in the Geisinger study, compared to measurements obtained by research staff in WHEALS. Overall, there is evidence to support a role for prenatal antibiotic use and childhood BMI; future studies that address BMI trajectories throughout childhood are needed.
We found evidence to suggest that macrolide antibiotic use in pregnancy was associated with higher BMI Z-score at age 2 years. One recent study of antibiotic exposure in the first year of life showed that each class of antibiotic studied (narrow spectrum, broad spectrum and macrolides) was associated with weight gain though age 8 years.45 Saari et al (2015) found that macrolide use in infancy had the greatest impact on weight gain in children through age 24 months; these authors attributed this to pharmacokinetic properties of macrolides, which are excreted in bile and thus may have direct contact with microbiota in the colon, compared to the other classes of antibiotics which are eliminated by the kidneys.9 In a study of 142 Finnish children ages 2–7 years, macrolide, but not penicillin use was associated with a persistent change in the composition and function of the gut microbiome; macrolide use was also positively associated with BMI Z-score.22 Findings were similar in a mouse model; tylosin, a veterinary-medicine macrolide, given shortly after weaning, increased both total and lean mass of mice, compared to only lean mass increases in mice exposed to amoxicillin.46 Further, tylosin was also associated with microbiota composition changes that persisted longer than changes in amoxicillin-exposed mice.46 Together, these studies support a potential microbiome-mediated role linking certain antibiotics with increases in BMI. Given that a number of women in our study used multiple antibiotic classes over pregnancy, however, these findings will require replication in a larger study that will permit exploration of the different combinations of antibiotics typically used over pregnancy.
We found no evidence that prenatal antifungal use was associated with offspring obesity risk. Although treatment of Candida infection alters the bacterial vaginal microbiome,16 there is limited existing data on the type of changes that occur in the vaginal microbiome as a result of antifungal use and how those changes are transmitted to offspring. Given the propensity for microbiota re-assembly following perturbation, topical antifungal treatment of vaginal infection, which was the most common route of exposure in our study, may only be pertinent in the period immediately prior to birth.
There are several limitations to the current investigation. We assigned antimicrobial use to a specific trimester based on pregnancy dating information; inaccurate reporting of last menstrual period for instance may have resulted in incorrect assignment of exposure time. Although information on antimicrobial use was obtained from the medical record and thus is not subject to recall bias, we do not have information on whether or not the woman took the medication as prescribed. We do not have comprehensive information on the symptoms or specific illnesses that led to the use of prenatal antibiotics, thus, we cannot rule out that the associations detected are a result of an underlying infection. The infectious agents themselves, rather than the subsequent antimicrobial use, could impact child growth.47 A recent longitudinal birth cohort study from Kaiser Permanente Northern California suggested that childhood infection, rather than overall early-childhood antibiotic use, was associated with childhood obesity; however, consistent with our overall hypothesis, in this study, early infancy antibiotic use (first 6 months of life) was associated with childhood obesity.48 To our knowledge, however, similar studies have not been conducted during pregnancy, and thus future work to examine prenatal infection with and without antibiotic use, may be needed.
Conclusions
We found evidence suggesting that prenatal antibiotic use, but not antifungal use, is associated with childhood BMI at age 2 years. Higher BMI Z-score and risk of overweight/obesity were greatest for children with maternal exposures during the 1st or 2nd trimester. Inappropriate antibiotic use during pregnancy is common.49 While there are clear indications for antibiotic use during pregnancy, inappropriate maternal antibiotic use (such as for upper respiratory infection of probable viral origin50) may contribute to the childhood obesity epidemic. Additional studies examining if changes in the gut microbiome (both maternal and offspring) mediate associations between prenatal antibiotic use and childhood BMI are warranted.
Acknowledgments
Funding Source: This study was supported by the National Institutes of Health (R01 AI050681, R01 HL113010, R01 HD082147, and P01 AI089473) and the Fund for Henry Ford Hospital.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Skinner AC, Perrin EM, Skelton JA. Prevalence of obesity and severe obesity in US children, 1999–2014. Obesity. 2016;24(5):1116–23. doi: 10.1002/oby.21497. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51.e1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley EH, Fuentes-Afflick E, Jackson RA, Escobar GJ, Brawarsky P, Schreiber M, et al. Correlates of prescription drug use during pregnancy. J Women health. 2005;14(5):401–9. doi: 10.1089/jwh.2005.14.401. [DOI] [PubMed] [Google Scholar]
- 4.Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212–8. doi: 10.1172/JCI72333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–21. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley LW, Raphael E, Faerstein E. Obesity in the United States - dysbiosis from exposure to low-dose antibiotics? Front Public Health. 2013;1:69. doi: 10.3389/fpubh.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis. 2013;13(10):889–99. doi: 10.1016/S1473-3099(13)70179-8. [DOI] [PubMed] [Google Scholar]
- 8.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–26. doi: 10.1542/peds.2014-3407. [DOI] [PubMed] [Google Scholar]
- 10.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatrics. 2014;168(11):1063–9. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- 11.Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes. 2015;39(4):665–70. doi: 10.1038/ijo.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mor A, Antonsen S, Kahlert J, Holsteen V, Jorgensen S, Holm-Pedersen J, et al. Prenatal exposure to systemic antibacterials and overweight and obesity in Danish schoolchildren: a prevalence study. Int J Obes. 2015;39(10):1450–5. doi: 10.1038/ijo.2015.129. [DOI] [PubMed] [Google Scholar]
- 13.Poulsen MN, Pollak J, Bailey-Davis L, Hirsch AG, Glass TA, Schwartz BS. Associations of prenatal and childhood antibiotic use with child body mass index at age 3 years. Obesity. 2017;25(2):438–444. doi: 10.1002/oby.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parfrey LW, Walters WA, Knight R. Microbial eukaryotes in the human microbiome: ecology, evolution, and future directions. Front Microbiol. 2011;2:153. doi: 10.3389/fmicb.2011.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Headley J, Northstone K, Simmons H, Golding J. Medication use during pregnancy: data from the Avon Longitudinal Study of Parents and Children. Eur J Clin Pharmacol. 2004;60(5):355–61. doi: 10.1007/s00228-004-0775-7. [DOI] [PubMed] [Google Scholar]
- 16.Liu MB, Xu SR, He Y, Deng GH, Sheng HF, Huang XM, et al. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS One. 2013;8(11):e79812. doi: 10.1371/journal.pone.0079812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassidy-Bushrow AE, Wegienka G, Havstad S, Levin AM, Lynch SV, Ownby DR, et al. Does Pet-Keeping Modify the Association of Delivery Mode with Offspring Body Size? Matern Child Health J. 2015;19(6):1426–33. doi: 10.1007/s10995-014-1649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wegienka G, Havstad S, Zoratti EM, Kim H, Ownby DR, Johnson CC. Combined effects of prenatal medication use and delivery type are associated with eczema at age 2 years. Clin Exp Allergy. 2015;45(3):660–8. doi: 10.1111/cea.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stensballe LG, Simonsen J, Jensen SM, Bonnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013;162(4):832–838. e3. doi: 10.1016/j.jpeds.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Etebu EAI. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. IJAMBR. 2016;4(6):90–101. [Google Scholar]
- 21.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8(6):423–35. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410. doi: 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119(3):409–415. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10 Supl 2):S103–7. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 26.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature Prev Med. 1993;22(2):167–77. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 27.Stokholm J, Schjorring S, Eskildsen CE, Pedersen L, Bischoff AL, Folsgaard N, et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin Microbiol Infect. 2014;20(7):629–35. doi: 10.1111/1469-0691.12411. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 30.Fak F, Ahrne S, Molin G, Jeppsson B, Westrom B. Microbial manipulation of the rat dam changes bacterial colonization and alters properties of the gut in her offspring. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G148–54. doi: 10.1152/ajpgi.00023.2007. [DOI] [PubMed] [Google Scholar]
- 31.Aloisio I, Mazzola G, Corvaglia LT, Tonti G, Faldella G, Biavati B, et al. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol. 2014;98(13):6051–60. doi: 10.1007/s00253-014-5712-9. [DOI] [PubMed] [Google Scholar]
- 32.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983–93. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 33.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 34.Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7(6):e36466. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–80. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter TE. Development and Function of the Fetal Endocrine System. In: Bazer FW, editor. Endocrinology of Pregnancy. Humana Press; Totowa, NJ: 1998. pp. 387–405. [Google Scholar]
- 38.Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol. 2003;179(3):293–9. doi: 10.1677/joe.0.1790293. [DOI] [PubMed] [Google Scholar]
- 39.Delisle H. Evidence and implications for policy and intervention strategies. Suiza: World Health Organization; 2002. Programming of chronic disease by impaired fetal nutrition. [Google Scholar]
- 40.Aykut V, Celik U, Celik B. The destructive effects of antibiotics on the amniotic membrane ultrastructure. Int Ophthalmol. 2015;35(3):381–5. doi: 10.1007/s10792-014-9959-z. [DOI] [PubMed] [Google Scholar]
- 41.Konkel L. Lasting Impact of an Ephemeral Organ: The Role of the Placenta in Fetal Programming. Environ Health Perspect. 2016;124(7):A124–9. doi: 10.1289/ehp.124-A124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller NT, Rifas-Shiman SL, Blaser MJ, Gillman MW, Hivert MF. Association of prenatal antibiotics with foetal size and cord blood leptin and adiponectin. Pediatr Obes. 2017;12(2):129–36. doi: 10.1111/ijpo.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123(2):682–9. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerber JS, Bryan M, Ross RK, et al. Antibiotic exposure during the first 6 months of life and weight gain during childhood. JAMA. 2016;315(12):1258–1265. doi: 10.1001/jama.2016.2395. [DOI] [PubMed] [Google Scholar]
- 46.Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Communic. 2015;6:7486. doi: 10.1038/ncomms8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turta O, Rautava S. Antibiotics, obesity and the link to microbes - what are we doing to our children? BMC medicine. 2016;14(1):57. doi: 10.1186/s12916-016-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li DK, Chen H, Ferber J, Odouli R. Infection and antibiotic use in infancy and risk of childhood obesity: a longitudinal birth cohort study. Lancet Diabetes Endocrinol. 2017;5(1):18–25. doi: 10.1016/S2213-8587(16)30281-9. [DOI] [PubMed] [Google Scholar]
- 49.Blaser M. Antibiotic overuse: Stop the killing of beneficial bacteria. Nature. 2011;476(7361):393–394. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 50.Martinez de Tejada B. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Environ Res Public Health. 2014;11(8):7993–8009. doi: 10.3390/ijerph110807993. [DOI] [PMC free article] [PubMed] [Google Scholar]