Abstract
Mist1 was recently shown to identify a discrete population of stem cells within the isthmus of the oxyntic gland within the gastric corpus. Chief cells at the base of the gastric corpus also express Mist1. The relevance of Mist1 expression as a marker of specific cell populations within the antral glands of the distal stomach, however, is unknown. Using Mist1-CreERT mice, we revealed that Mist1+ antral cells, distinct from the Mist1+ population in the corpus, comprise long-lived progenitors that reside within the antral isthmus above Lgr5+ or CCK2R+ cells. Mist1+ antral progenitors can serve as an origin of antral tumors induced by loss of Apc or MNU treatment. Mist1+ antral progenitors, as well as other antral stem/progenitor population, express Cxcr4, and are located in close proximity to Cxcl12 (the Cxcr4 ligand)-expressing endothelium. During antral carcinogenesis, there is an expansion of Cxcr4+ epithelial cells as well as the Cxcl12+ perivascular niche. Deletion of Cxcl12 in endothelial cells or pharmacological blockade of Cxcr4 inhibits antral tumor growth. Cxcl12/Cxcr4 signaling may be a potential therapeutic target.
Keywords: gastric cancer, stem cell, mist1, cxcr4, cxcl12
INTRODUCTION
Gastric cancer is one of the leading causes of cancer death worldwide, and the prognosis for patients with advanced disease remains poor [1, 2]. Gastric cancer contains multiple histological subtypes with distinct molecular signatures, but dominant oncogenic mutations are generally less frequent in gastric cancers than in other gastrointestinal cancers [3], limiting opportunities for targeted therapy in the disease. Cancer growth is influenced greatly by interactions between cancer stem cells and the tumor microenvironment, which has emerged as a promising therapeutic target [4, 5]. Cancer stem cells are believed to arise from normal stem or progenitor cells [6]. Stem cells are defined by the properties of self-renewal and multi-potency, or the ability to give rise to more than one lineage, which are modulated to some extent by the surrounding microenvironment or niche [7, 8].
The mouse stomach is comprised of three major parts, forestomach, corpus, and antrum. Forestomach consists of squamous epithelium and thus is in many aspects more similar to esophageal epithelium. The proximal part corpus and distal part antrum have columnar epithelium and are collectively referred to the glandular stomach. We recently showed that relatively quiescent Mist1+ gastric corpus stem cells located in the isthmus, where the corpus gland narrows near the upper third position of glands, can serve as the cellular origin of all epithelial lineages, as well as gastric cancer. In the corpus, Mist1+ stem cells are regulated by Cxcl12+ endothelial cells and Cxcr4+ innate lymphoid cells (ILCs), and the Cxcl12/Cxcr4 niche is required for progression to diffuse-type gastric cancer [9]. During inflammation, the Cxcl12/Cxcr4 niche expands and supports cancer cell growth by paracrine release of growth factors such as Wnt5a. However, the gastric corpus and antrum are two different organs, in terms of not only anatomical and functional differences but also their stem cell biology and contribution to carcinogenesis. Moreover, intestinal-type gastric cancer is typically more common in Helicobacter pylori-related human gastric cancers than diffuse-type. Therefore, the contribution of Cxcl12/Cxcr4 in antral stem cells and other form of gastric cancer has not been fully elucidated.
In the gastric antrum, several stem/progenitor cell markers have been identified such as CCK2R, Sox2, eR1, Villin, Axin2, and Lgr5 [10–15]. Lgr5+ cells are present at the base of antral glands, whereas other stem/protenitor cells reside within the antral isthmus, where gland narrows just above Lgr5+ cells. Although Wnt and Notch signaling have been suggested as important modulators of gastrointestinal stem cells, other critical niche signals that regulate antral stem cells, and that might contribute to the development of cancer, have not been fully explored. Here, we identify Mist1 expression in antral isthmus progenitors, and define their contribution to the antral lineages and to gastric cancer. Finally, we demonstrate a role for Cxcl12/Cxcr4 signaling in antral tumorigenesis.
RESULTS
Mist1 is expressed in long-lived isthmus progenitors in the antrum
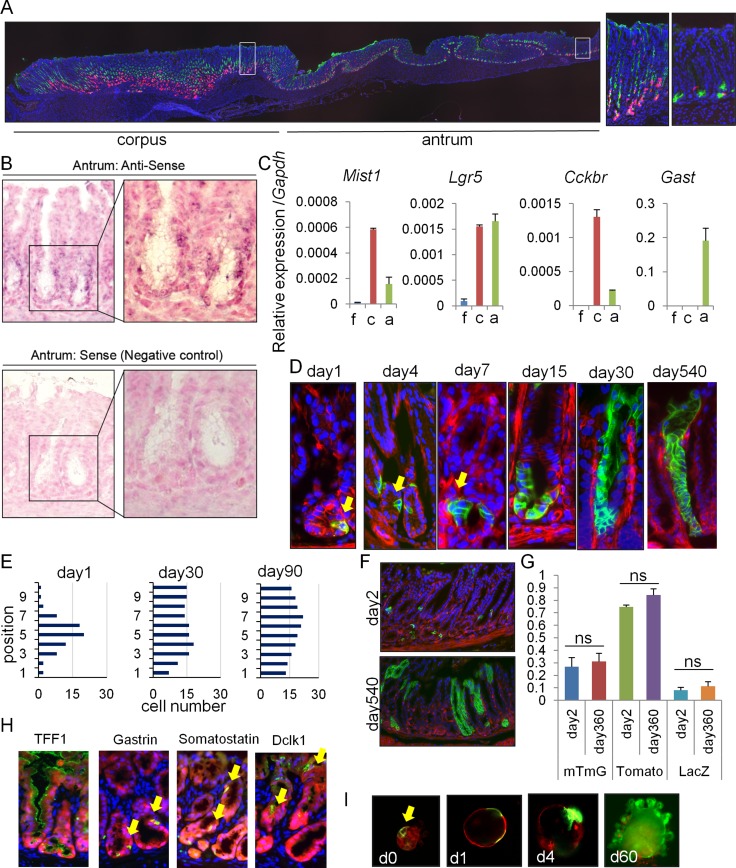
Although Mist1 expression has been reported in the gastric corpus [16], we recently found abundant Cre recombination in the antrum of Mist1-CreERT mice, and that Mist1 antral lineages appeared to contribute to tumor development [17]. To clarify Mist1 expression in the stomach in more detail, we looked at an entire longitudinal section of Mist1-CreERT; R26-TdTomato mice 5 days after tamoxifen (TAM) induction (Figure 1A). As reported previously [9], there are abundant TdTomato+ chief cells at the corpus gland base, while there are TdTomato+ stem cells above GSII+ mucous neck region, some of which start to lineage trace from the corpus isthmus. In addition, we observed scattered, but solid TdTomato expression within the antral isthmus where stem/progenitors are thought to reside, in contrast to previous reports [16]. These cells reside adjacent to GSII-expressing deep antral mucous cells, but most of them do not overlap. In addition, these TdTomato+ cells are negative for other differentiated cell markers found in this region, including Dclk1, somatostatin, and gastrin (Supplementary Figure 1A). In situ hybridization confirmed Mist1 mRNA expression in cells at this position (Supplementary Figure 1B–1C), while Mist1 protein was not detected by immunohistochemical staining (not shown). We performed RT-PCR using mRNA extracted from different parts of the stomach including forestomach, corpus, and antrum, and confirmed that Mist1 is expressed in the antrum, at a lower level compared to the corpus, but a higher level compared to the forestomach, where no TdTomato+ cells are seen (Supplementary Figure 1C, 1D). A gastrin receptor gene Cckbr, which is expressed in differentiated cells in the corpus and stem cells in the antrum, is also expressed in these parts similar to Mist1. Lgr5 expression is equivalent between corpus and antrum, as reported previously [18]. Thus, Mist1 expression level well correlates with recombination rate in each part of the stomach of Mist1-CreERT mice.
Figure 1. Mist1 marks long-lived, multipotent isthmus progenitors in the antrum.
(A) Longitudinal stomach section of Mist1-CreERT; R26-TdTomato mice stained with GS-II (green). Areas indicated by white boxes in the corpus and antrum are enlarged in right. Macroscopic cut line of the section is shown by blue line in S1D. (B) In situ hybridization of Mist1 in the antrum. (C) Relative gene expression per Gapdh in each part of the stomach (n = 3). (D–F) Lineage tracing in Mist1-CreERT; R26-mTmG mice from days 1-540. Arrows indicate Mist1+ cells and their progeny. Quantification of the Mist1-traced cell position is shown in (E). A total of 50 glands were analyzed at each time point. (G) Lineage tracing frequency in Mist1-CreERT; R26-mTmG, Mist1-CreERT; R26-TdTomato, and Mist1-CreERT; R26-LacZ mice at day 1 and 360. (H) Immunofluorescence of the indicated markers (green) in Mist1-CreERT; R26-TdTomato mice 12 months after TAM induction. (I) Antral gland culture of TAM-induced Mist1-CreERT; R26-mTmG mice. The arrow indicates Mist1+ cells.
We next performed a detailed time course of lineage tracing in Mist1-CreERT; R26-mTmG mice. The Mist1-CreERT; R26-mTmG mice showed isolated recombined GFP+ cells in the lower third of antral glands at 1 day after TAM induction (Figure 1D–1E). Mist1+ cells were present at average of 1-2 cells/gland, ranging from position 1 to 7 with a peak at position +5. After TAM induction, the Mist1+ lineage expanded gradually with a doubling time of ∼4 days, and entire antral glands were labeled within 30 days (Figure 1D–1E). Similar lineage tracing pattern in antral glands was also observed in Mist1-CreERT; R26-LacZ and Mist1-CreERT; R26-TdTomato mice (Supplemantary Figure 1E–1F). Lineage tracing in these mice persisted beyond 18 months, and the Mist1 lineage contained all cell types including TFF1+ surface pit cells, gastrin+ G cells, somatostatin+ D cells, and Dclk1+ tuft cells. (Figure 1H). The frequency of lineage tracing was consistent throughout the observation period, depending on the reporter strain used (Figure 1G). We did not observe any tamoxifen-induced epithelial injury in the antrum during the time course, as reported previously [19]. Lineage tracing during in vitro organoid culture supported an expansion of the Mist1+ lineage (Figure 1I). Together, these results suggest that Mist1-expressing antral cells contain long-lived, multipotent progenitors in the isthmus.
Mist1+ cells take up BrdU more rapidly than Lgr5+ cells in the antrum
Given that Lgr5 expression has also been associated in the antrum with long-lived, self-renewing stem cells [10], we examined possible overlap between Mist1 and Lgr5 using Mist1-CreERT; Lgr5-DTR-GFP; R26-TdTomato mice. Although Mist1+ cells and Lgr5+ cells are often located in close proximity, Mist1+ cells were again located slightly higher up in the antral glands, and the vast majority (e.g. > 95%) of Mist1+ cells were found to be Lgr5-negative by microscopic and FACS analysis (Figure 2A–2B). We sorted Lgr5-high and Lgr5-low expressing cells separately, and confirmed that Mist1 mRNA is expressed in Lgr5-low cells, but not in Lgr5-high cells (Figure 2C). We then ablated Lgr5+ cells by administration of diphtheria toxin (DT), and found that lineage tracing of Mist1+ cells still occurred with unchanged kinetics (Figure 2D). Furthermore, the Lgr5+ cells reappeared by 30 days within the Mist1+ lineage, indicating that Mist1+ stem cells can give rise to Lgr5+ cells. Taken together, Mist1+ antral stem cells are mostly distinct from Lgr5+ cells.
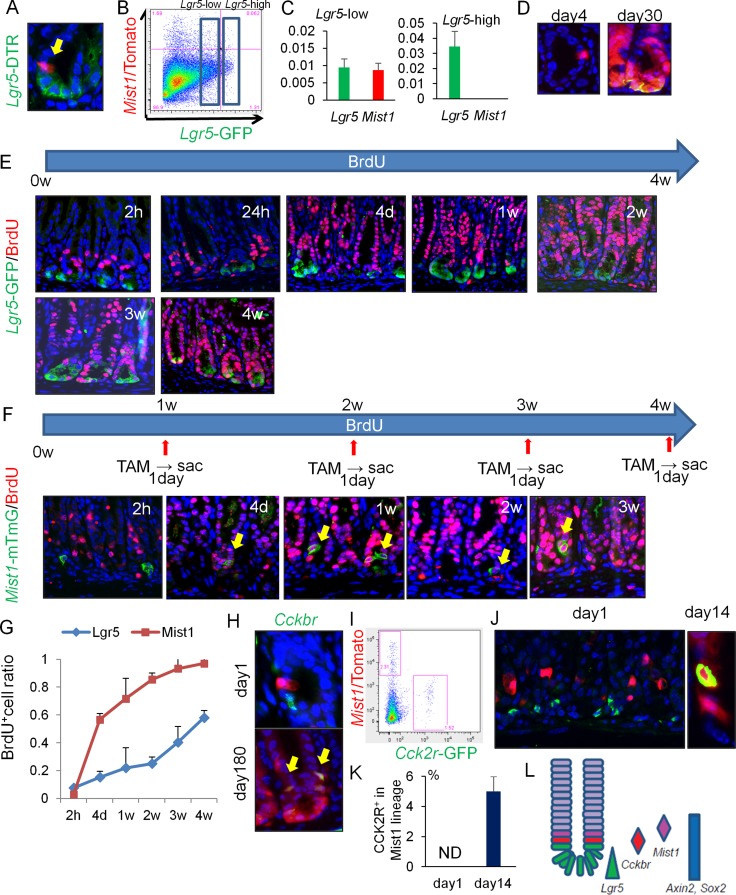
Figure 2. Mist1+ cells take up BrdU more rapidly than Lgr5+ cells.
(A) Lgr5 (green) and Mist1 (red) expression in Mist1-CreERT; Lgr5-DTR-GFP; R26-TdTomato mice 1 day after TAM induction. (B) FACS plot of Mist1-CreERT; Lgr5-DTR-GFP; R26-TdTomato mice antrum 1 day after TAM induction. Boxes indicate Lgr5-low and Lgr5-high expressing cell populations. (C) Relative mRNA expression/Gapdh of Lgr5 and Mist1 in Lgr5-high cells and Lgr5-low cells from the Mist1-CreERT; Lgr5-DTR-GFP; R26-TdTomato mice antrum 1 day after TAM induction. N.D. means “not detected”. N = 3. (D) Lineage tracing of DT-treated (day4 and day30 after tamoxifen induction) Mist1-CreERT; Lgr5-DTR-GFP; R26-TdTomato mice. DT was given at 1 day after tamoxifen. (E) Immunofluorescence of GFP (green) and BrdU (red) in Lgr5-EGFP-IRES-CreERT mice given BrdU continuously by drinking water (1.0 mg/ml). Mice were sacrificed at indicated time points. (F) Immunofluorescence of GFP (green) and BrdU (red) in Mist1-CreERT; R26-mTmG mice given BrdU continuously by drinking water. Mice were sacrificed at the indicated time points (1 day after TAM induction). (G) BrdU+ cell ratio of Lgr5+ cells and Mist1+ cells. A total of 300 cells from three mice were analyzed at each time point. (H) Antral images of Mist1-CreERT; R26-TdTomato mice crossed to Cckbr-EGFP mice 1 and 180 days after TAM induction. Arrows indicate Tomato and EGFP double-positive cells. (I) FACS plot of Mist1-CreERT; R26-TdTomato; Cckbr-EGFP mice 1 day after tamoxifen. (J–K) Immunofluorescence images showing CCK2R staining (green) in Mist1-CreERT; R26-TdTomato mice 1 day and 14 days after TAM induction. CCK2R+ cells/Mist1+ cells are quantified in (K). A total of 300 cells from three mice were analyzed. (L) Schematic model of antral stem/progenitor cells.
To further analyze the differences between Mist1+ and Lgr5+ antral cells, we administered bromodeoxyuridine (BrdU) to Lgr5-EGFP-IRES-CreERT mice and Mist1-CreERT; R26-mTmG mice continuously through their drinking water. Lgr5-high cells at the base of glands (positions +1 to +3) failed to label with BrdU in the first few weeks, while Lgr5-low cells in the isthmus occasionally took up BrdU and then such labeling expanded bi-directionally (Figure 2E). However, it required more than 4 weeks to label all basal Lgr5+ cells with continuous BrdU administration, suggesting that many of these cells are either quiescent or post-mitotic. Indeed, using a FucciG1 transgenic mouse line where cells in G1 cell cycle state turns into red and cells in post-mitotic cells show bright red signal due to accumulation of fluorescent protein after cell cycle exit [20], we realized that most of Lgr5-high cells in the antrum of Lgr5-EGFP-IRES-CreERT; FucciG1 mice display bright red nuclei thus they are likely post-mitotic (Supplementary Figure 2A).
In contrast, while Mist1+ cells did not take up BrdU immediately, consistent with their doubling time of 4 days, they could be uniformly labeled with BrdU within two weeks (Figure 2F, 2G). Thus, these data suggest marked differences in cellular kinetics between Mist1+ and Lgr5+ cells, with the Mist1+ cells labeling with BrdU well before Lgr5-high cells.
To investigate possible overlap between Mist1+ cells and Sox2+ or CCK2R+ antral stem cells, we generated Mist1-CreERT; Sox2-EGFP; R26-TdTomato mice and Mist1-CreERT; Cckbr-EGFP; R26-TdTomato mice [11, 12]. One day after TAM induction, Mist1+ cells were distinct from Cckbr-GFP+ and Sox2-GFP+ cells based on immunofluorescent and FACS analysis (Figure 2H–2I, Supplementary Figure 2B–2C). Immunohistochemistry confirmed that CCK2R+ cells were located at the +4 position, just below Mist1+ cells (Figure 2J–2K). Axin2 protein, a recently reported antral stem cell marker, is expressed in the isthmus, but there is no overlap with Mist1+ cells (Supplementary Figure 2D). Mist1+ cells were able to generate Sox2+ and CCK2R+ cells and indeed all epithelial lineages within the antral gland, suggesting Mist1 also labels bona fide antral stem cells, although interconversion between these various states cannot be excluded (Figure 2H, 2J–2L, Supplementary Figure 2C).
Antral Mist1+ cells serve as a cellular origin of cancer
Given that Mist1+ antral cells function as stem cells in the normal stomach, we investigated whether Mist1+ stem cells could be a cell-of-origin for antral gastric metaplasia and cancer. We generated Mist1-CreERT; LSL-KrasG12D mice, which were previously shown to rapidly develop gastric metaplasia of the corpus [9]. At day 14 after induction of mutant Kras in Mist1+ cells, we found rapid expansion from the antral gland base of mucous-producing cells that were Alcian Blue+, and which populated entire glands in a gland-by-gland fashion (Figure 3A). The expansion of Kras-activated, p-ERK+ cells was accompanied by an increase in CD44 and GSII expression, a marker of gastric preneoplasia [13] (Figure 3B–3C). Thus, Kras activation in Mist1+ antral stem cells causes antral hyperplasia with expansion of preneoplastic cells.
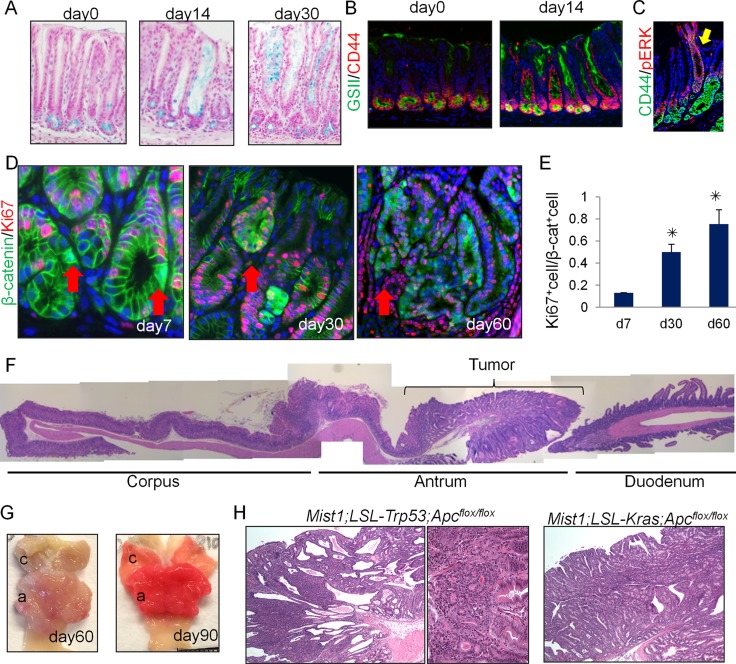
Figure 3. Antral Mist1+ cells serve as a cellular origin of cancer.
(A) Alcian blue (blue) staining in Mist1-CreERT; LSL-KrasG12D mice at the indicated time points. (B) GSII (green) and CD44 (red) staining in Mist1-CreERT; LSL-KrasG12D mice at the indicated time points. (C) CD44 (green) and p-ERK (red) staining in Mist1-CreERT; LSL-KrasG12D mice at day 30 after tamoxifen. (D-E) Immunofluorescence for β-catenin (green) and Ki67 (red) in Mist1-CreERT; Apcflox/flox mice on days 7, 30, and 60 after TAM induction (D). The arrows indicate the nuclear β-catenin+ cells. Ki67+ cell ratio in total nuclear β-catenin+ cells is quantified (E). A total of 300 cells from three mice are analyzed at each time point. (F) Longitudinal H&E stained section of Mist1-CreERT; Apcflox/flox mouse stomach 60 days after TAM induction. (G) Gross picture Mist1-CreERT; Apcflox/flox mice 60 and 90 days after TAM induction. (H) H&E staining of Mist1-CreERT; LSL-Trp53R172H; Apcflox/flox mice and Mist1-CreERT; LSL-KrasG12D; Apcflox/flox mice 150 days after TAM induction.
We next treated the Mist1-CreERT; R26-mTmG mice with the chemical carcinogen, N-nitroso-N-methylurea (MNU). One week after the 5 weekly cycles of MNU, the ratio of Ki67+ cells/Mist1+ cells was increased in the stomachs of MNU-treated mice compared to untreated controls, suggesting that MNU treatment activates relatively quiescent Mist1+ cells (4% are Ki67+ at baseline) to become more proliferative (28% are Ki67+ after MNU) cells (Supplementary Figure 3A). In addition, at 40 weeks MNU-derived tumors showed robust lineage tracing from Mist1+ cells (Supplementary Figure 3B) in 50 % of mice analyzed (3/6), showing that MNU-induced antral tumor is at least in part derived from Mist1+ cells.
We then crossed Mist1-CreERT mice with Apcflox/flox mice and generated Mist1-CreERT; Apcflox/flox mice in order to examine the effect of Apc loss in Mist1+ cells. As shown in Supplementary Figure 3C, 2 days after TAM induction, there was strong nuclear translocation of β-catenin evident in cells within the antral isthmus. The number of cells positive for nuclear β-catenin increased gradually, with small dysplastic nodules positive for β-catenin widely present at 14 - 30 days after the TAM induction. Apc-deleted Mist1+ lineages were initially Ki67-negative (day 7, 14), but later became Ki67+ after the formation of large dysplasia (day 30 or after) (Figure 3D–3E). Sixty days after TAM induction, ten of 10 Mist1-CreERT; Apcflox/flox mice (100 %) exhibited large antral tumors, as shown previously in different Cre driver mice [21] (Figure 3E, 3F). Notably, macroscopic tumors in these mice are confined to the antrum (the incidence of corpus tumor is 0 % (10/10)), while the normal corpus is sometimes displaced proximally towards the forestomach when the tumors enlarge at later stage. Although Mist1 is also expressed in corpus stem cells and Brunner glands in the duodenum, these tissues were unaffected by the loss of Apc in Mist1+ cells. Thus, in the upper GI tract, antral Mist1+ cells appeared uniquely susceptible to Apc/β-catenin-driven tumorigenesis.
Histologically, high-grade, intraepithelial neoplasia/carcinoma in situ were seen in the antrum of Mist1-CreERT; Apcflox/flox mice around 60 days after tamoxifen induction, which expanded further by day 120 but remained intra-mucosal without invasion (Supplementary Figure 3D). Antral organoids of Mist1-CreERT; Apcflox/flox mice can grow without Wnt3a and R-spondin 1 in culture media only when recombination is induced by tamoxifen, suggesting that the tumor development in these mice is cell-autonomous effect (Supplementary Figure 3E, 3F). The combination of Apc loss and Trp53 mutation led to a higher dysplastic grade, compared to Apc loss alone, but still remained intra-mucosal without invasion (Figure 3H). The addition of KrasG12D mutation to Apc loss led to severe metaplasia and expansion of the neoplastic process both in the antrum to the corpus, but again submucosal invasion was not observed. The combination of Apc loss, Kras mutation, and Trp53 mutation in Mist1 lineage also generated dysplastic lesion resembling the tumors with Apc knockout and Kras mutation both in the antrum and corpus in 1 month, but all the mice died within a month due to pancreatic tumor formation (not shown). Overall, these findings indicate that Mist1+ cells can serve as an origin of antral intestinal-type cancer.
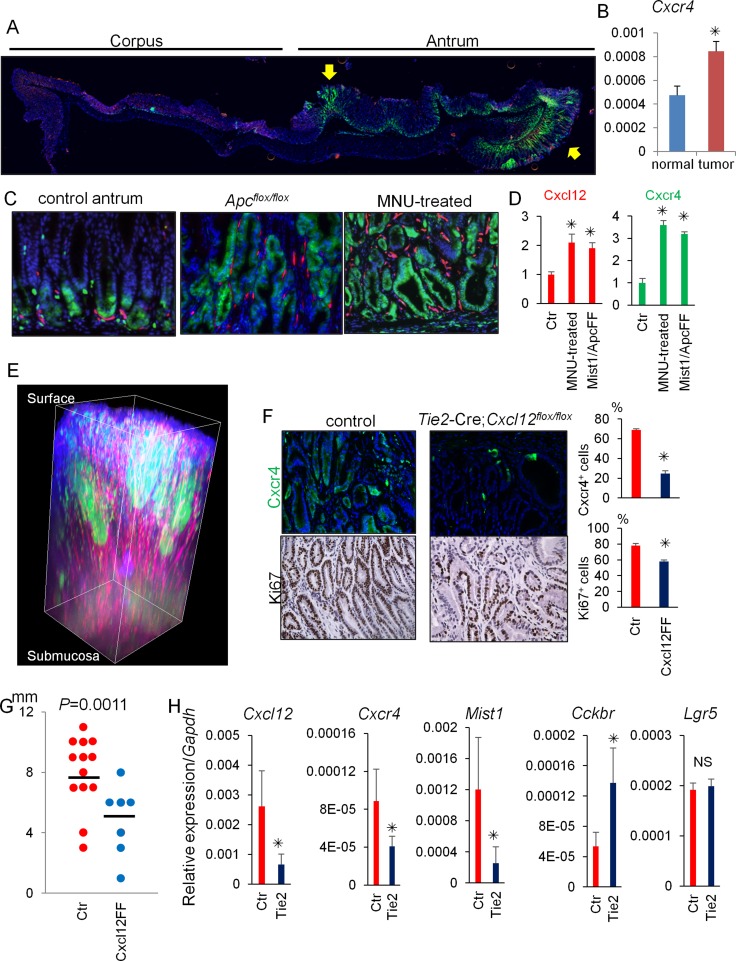
Cxcl12/Cxcr4 axis contributes to antral stem cell niche
We previously showed that the Cxcl12+ endothelium and Cxcr4+ ILCs regulated gastric corpus stem cells and also contributed strongly to diffuse-type gastric cancer development [9]. When we observed Cxcl12/Cxcr4 expression in the entire stomach of Cxcl12-dsRED; Cxcr4-GFP mice, Cxcr4+ epithelial cells were abundant at the lower half of the antral glands (positions 1-10), while Cxcr4+ epithelial cells were absent and instead rare Cxcr4+ immune cells is present in the corpus as previously reported [9, 22] (Supplementary Figure 4A). In order to visualize distinct expression pattern between these two parts more clearly, we utilized tissue decolorization method and performed 3D reconstitution of full mucosal layers (Figure 4B, 4C) [23]. Although examination of a 5-μm section does not immediately provide clear information of cell localization, 3D images demonstrate that in the corpus, Cxcr4+ immune cells and Cxcl12+ expression in the endothelium are predominantly located in the isthmus region, and that in the antrum, there are 2 distinct Cxcr4+ cells including basal epithelial cells and immune cells throughout the mucosa, both of which are surrounded by Cxcl12+ endothelial network. Immunohistochemical staining confirmed that Cxcl12+ cells were CD31+ endothelial cells, and not podoplanin+ lymphatic cells nor α-SMA+ myofibroblasts (Supplementary Figure 4B).
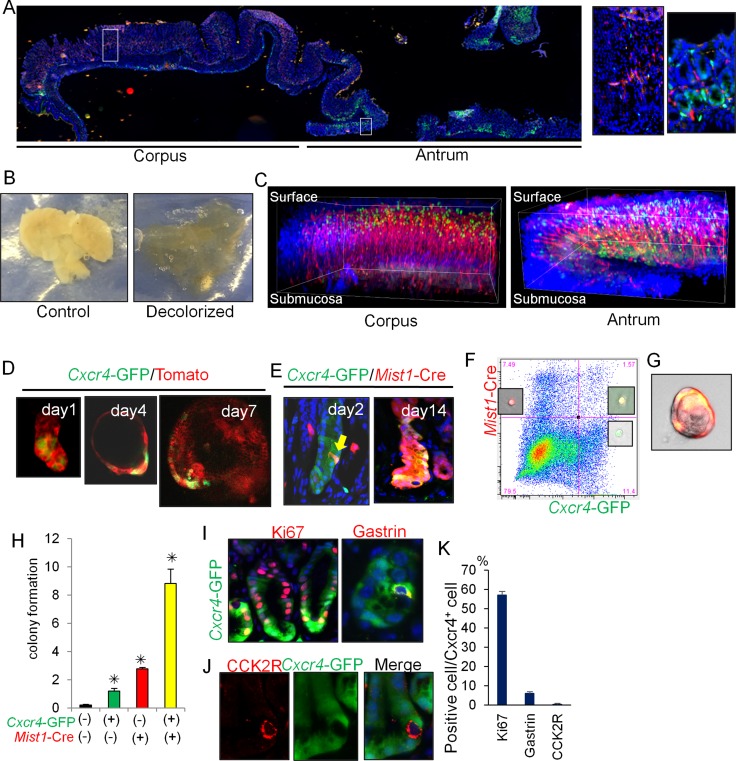
Figure 4. Cxcl12/Cxcr4 axis contributes to antral stem cell niche.
(A) Longitudinal stomach section of Cxcr4-EGFP; Cxcl12-dsRED mice stained with DAPI. Areas indicated by white boxes in the corpus and antrum are enlarged in right. (B) Representative gross images of control and decolorized stomachs. (C) 3D reconstructed images of Cxcr4-EGFP; Cxcl12-dsRED mouse corpus and antrum stained with DAPI. (D) Antral gland culture of Cxcr4-EGFP; R26-mTmG mice. (E) Lineage tracing of Mist1-CreERT; R26-TdTomato; Cxcr4-EGFP mice 2 and 14 days after TAM induction. Arrow indicates double positive cells. (F) FACS plot of antral cells from Mist1-CreERT; Cxcr4-EGFP; R26-TdTomato mice 1 day after TAM induction. Representative images of each cellular population are shown. (G-H) Single cell culture images (G) and relative colony formation efficacy of sorted cells (H). Double-negative cells, Cxcr4+ cells (green), Mist1+ cells (red), and double-positive cells (yellow) were analyzed. (I–K) Immunofluorescence staining of Ki67 and gastrin (I, red), and CCK2R (J, red) in Cxcr4-EGFP mice. Ki67+, Gastrin+, and CCK2R+ cell ratio in Cxcr4+ cells are quantified in (K). A total of 300 cells from three mice were analyzed.
Cxcr4+ antral epithelial cells can exist in cultured organoids, with a gradual expansion during the time course (Figure 4D). We utilized Mist1-CreERT; R26-TdTomato; Cxcr4-EGFP mice to investigate possible overlap between Cxcr4+ and Mist1+ epithelial cells. Two days after TAM induction, the Tomato signal (Mist1+ cell) and the GFP signal (Cxcr4+ cells) were found to overlap in isolated cells near the +5 position (Figure 4E). On day 14 after TAM induction, most of the Cxcr4+ cells were clearly derived from the Mist1+ progeny. FACS analysis of antral cells from Mist1-CreERT; R26-TdTomato; Cxcr4-EGFP mice confirmed the presence of double-positive (Mist1+Cxcr4+) cells (Figure 4F), while there was no double-positive cells in the corpus [9]. Single cell culture analysis revealed that Mist1+Cxcr4+ double-positive cells showed a higher rate of colony formation compared to single-positive or double-negative populations (Figure 4G–4H).
Approximately 50% of Cxcr4-EGFP+ cells expressed Ki67 in the lower half of glands, and thus presumably included many of the active stem/progenitor cells in this region such as Lgr5+ cells (Figure 4I). Interestingly, CCK2R+ stem cells in the antrum were negative for Cxcr4-EGFP (Figure 4J–4K). Some of the gastrin-expressing (G) cells also expressed Cxcr4-EGFP, whereas there was no overlap at baseline between Cxcr4-EGFP and Dclk1+ tuft cells or somatostatin+ D-cells (not shown). We found that Cxcr4+CD45+ immune cells present in the antrum were primarily Lin-CD90.2+Cxcr4+ ILCs (Supplementary Figure 4C-4D). Thus, these data indicate that that Mist1+ antral progenitors are localized within the Cxcl12/Cxcr4 perivascular niche.
Cxcl12/Cxcr4 axis contributes to antral tumor growth
We next investigated the contribution of the Cxcl12/Cxcr4 axis to antral gastric tumorigenesis using two different mouse models: conditional Apc knockout in Mist1+ cells and MNU-induced antral tumorigenesis. While Cxcr4+ epithelial cells are normally at the gland base surrounded by Cxcl12+ endothelial cells, there was marked expansion of Cxcr4+ epithelial cells to within the antral tumor (Figure 5A–5D). Cxcr4 gene expression is upregulated in tumors than in normal antrum. In addition, there was a corresponding expansion of Cxcl12+ stromal cells. Tissue decolorization and 3D reconstitution successfully emphasized remarkable expansion of Cxcl12/Cxcr4 expressing cells within antral tumor (Figure 5E). Interestingly, there are strong Cxcr4-GFP expressing clusters within the tumor, suggesting the clonal expansion of Cxcr4+ cells in dysplastic glands.
Figure 5. Cxcl12/Cxcr4 axis contributes to antral tumor growth.
(A) Longitudinal stomach section of Mist1-CreERT; Apcflox/flox; Cxcr4-EGFP; Cxcl12-dsRED mice 60 days after tamoxifen stained with DAPI. Arrows indicate dysplastic lesions. (B) Relative gene expression per Gapdh in normal antrum and antral tumors of Mist1-CreERT; Apcflox/flox mice 60 days after tamoxifen. (C–D) Cxcl12-dsRED; Cxcr4-EGFP mouse antrum without (control antrum) and after MNU treatment (MNU-treated), and Mist1-CreERT; Cxcl12-dsRED; Cxcr4-EGFP; Apcflox/flox mice 6 weeks after TAM induction (Apcflox/flox). Cxcl12+ and Cxcr4+ areas were measured in (D). A total of 30 high power fields (HPF) from three mice were analyzed. (E) 3D reconstructed images of Mist1-CreERT; Apcflox/flox; Cxcr4-EGFP; Cxcl12-dsRED mouse antrum 60 days after tamoxifen stained with DAPI. (F) GFP and Ki67 staining of Cxcr4-EGFP; Cxcl12flox/flox (control) mice and Tie2-Cre; Cxcr4-EGFP; Cxcl12flox/flox mice 40 weeks after the start of 5 cycles of MNU treatment. Cxcr4+ and Ki67+ epithelial cell ratio of Cxcr4-EGFP; Cxcl12flox/flox (control) mice and Tie2-Cre; Cxcr4-EGFP; Cxcl12flox/flox mice were quantified. The total 1500 cells from three mice are analyzed. (G) Macroscopic antral tumor size was measured in Cxcl12flox/flox (control, N = 13) mice and Tie2-Cre; Cxcl12flox/flox mice (N = 7) 40 weeks after the start of 5 cycles of MNU treatment. (H) Relative mRNA expression/Gapdh of the indicated genes from the MNU-induced tumor tissues in Cxcl12flox/flox mice (Ctr) and Tie2-Cre; Cxcl12flox/flox mice (Tie2).
To elucidate the functional role of the Cxcl12/Cxcr4 axis in antral tumorigenesis, we generated Tie2-Cre; Cxcl12flox/flox mice with targeted deletion of Cxcl12 in endothelial cells, and used these animals in the MNU tumor model. In untreated mice at baseline, the expression of Cxcr4 and Ki67 in antral epithelial cells of Tie2-Cre; Cxcr4-EGFP; Cxcl12flox/flox mice and Cxcr4-EGFP; Cxcl12flox/flox mice (control littermates) was comparable (Supplementary Figure 5A). However, while MNU treatment caused marked proliferation (increased Ki67+ cells) and expansion of Cxcr4+ epithelial cells in control mice, MNU-treated Tie2-Cre; Cxcr4-EGFP; Cxcl12flox/flox mice showed much smaller changes, with significantly decreased Cxcr4+ epithelial cells and Ki67+ cells compared to MNU treated controls (Figure 5F).
To assess the contribution of Cxcr4+ ILCs in antral tumor development, we treated MNU-treated mice with an anti-CD90.2 antibody. While treatment with the anti-CD90.2 antibody efficiently depleted CD90.2+ ILCs, MNU-induced tumor development was not inhibited in this protocol (Supplementary Figure 5B–5D). Thus, these data suggest that the expansion of the Cxcl12+ endothelium may contribute to antral tumorigenesis in this model predominantly through activation of Cxcr4+ epithelial stem/progenitors, rather than through regulation of ILCs.
The macroscopic tumor size was significantly smaller in the MNU-treated Tie2-Cre; Cxcr4-EGFP; Cxcl12flox/flox mice compared to controls (Figure 5G). RT-PCR analysis revealed that deletion of Cxcl12 in the endothelium downregulated gene expression of Cxcr4 as well as Mist1, while Cckbr was upregulated in Tie2-Cre; Cxcl12flox/flox mice compared to control mice (Figure 5H). Lgr5 gene expression was not altered by conditional Cxcl12 knockout. These results suggest that knockout of Cxcl12 in the endothelium inhibited expansion of the Mist1+Cxcr4+ cell population, but the loss of Mist1+ cells may have been partially compensated by CCK2R+ stem cell expansion.
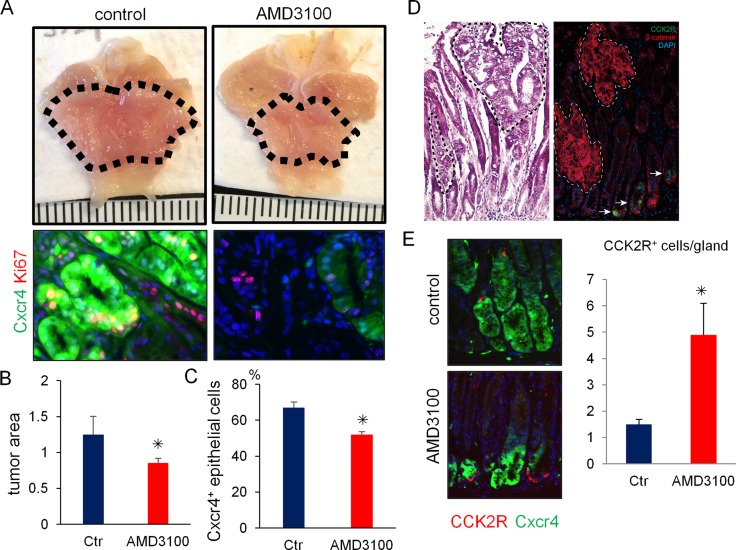
Pharmacological blockade of Cxcr4 inhibits antral tumor growth
Finally, to test the possible therapeutic utility of pharmacologic inhibition of the Cxcl12/Cxcr4 axis on antral tumor development, we treated Mist1-CreERT; Cxcr4-EGFP; Apcflox/flox mice with AMD3100, a specific inhibitor of CXCR4. AMD3100 significantly reduced the number of Cxcr4+ epithelial cells in the gastric antrum and decreased macroscopic tumor size (Figure 6A–6C). In the Mist1-CreERT; Apcflox/flox mice, most dysplastic cells demonstrated nuclear translocation of β-catenin. However, CCK2R+ cells in these murine tumors did not show nuclear expression of β-catenin (Figure 6D). Thus, the majority of Apc-deleted Mist1+ cells do not interconvert to CCK2R+ cells during this rapid tumor formation. Furthermore, the numbers of CCK2R+ cells were increased when mice were treated with AMD-3100 (Figure 6E), suggesting again that in this tumorigenic setting, CCK2R+ cells may behave as a compensatory lineage, distinct from Mist1+ cells.
Figure 6. Pharmacological blockade of Cxcr4 inhibits antral tumor growth.
(A–C) Gross picture (A, top) and immunofluorescence images (A, bottom) of GFP (green) and Ki67 (red) in Mist1-CreERT; Cxcr4-EGFP; Apcflox/flox mice 6 weeks after TAM induction with or without 2 weeks AMD3100 treatment. The dashed-line indicates tumor area. Macroscopic antral tumor area was quantified in (B) (N = 4 /group). The percentage of Cxcr4+ epithelial cells per total Ki67+ cells was quantified in (C). A total of 1500 cells from three mice were analyzed. (D) H&E staining and immunofluorescence of CCK2R (green) and β-catenin (red) in Mist1-CreERT2; Apcflox/flox mice 6 weeks after TAM induction. (E) Immunofluorescence of GFP (green) and CCK2R (red) in Mist1-CreERT2; Apcflox/flox mice 6 weeks after TAM induction with or without AMD3100 treatment. CCK2R+ cells/gland were counted. A total 150 glands from three mice were analyzed.
DISCUSSION
In the current study, we identified Mist1+ long-lived progenitor cells in the gastric antrum that are distinct from other reported gastric stem cell populations, including Lgr5+ cells and CCK2R+ cells. In addition, we have shown that antral Mist1+ cells can serve as an origin of gastric tumors in a different way from the corpus Mist1+ stem cells. Mist1+ progenitors overlap in the antrum with Cxcr4+ antral stem/progenitor cells, which appear to be supported by Cxcl12 secreted from endothelial cells within a perivascular niche. Activation of Cxcl12/Cxcr4 signaling appears to be needed for antral tumor growth.
Earlier studies using electron microscopy and autoradiography suggested that antral stem cells with an undifferentiated, granule-free appearance reside within the isthmus, and supply daughter cells bidirectionally towards the top and the base of the glands [24, 25]. Several markers have been reported for actively cycling antral stem cells, but until now clear relationship between these markers has not been well established [10–12]. In the paper describing Villin+ progenitors [14], the authors used Villin-LacZ and Villin-Cre mice and found rare Villin+ cells in the isthmus of antral glands which can show traced glands only when mice are treated with interferon (INF). The phenotype of INF-dependent tracing definitely suggests that Villin+ cells are not stem or progenitor cells in normal state and distinct from Mist1+ cells. Sox2 was initially reported to be expressed in rare stem cells within the corpus and antrum isthmus in the adult stomach [11]. More recently Sox2 was found to be expressed in broader transit-amplifying cells in the antral isthmus, which contribute to tumor development in the antrum with loss of Apc [21]. The enhancer element eR1 is recently reported corpus/antral stem and progenitor cell marker [13], but the expression is scattered and its biology and contribution to cancers are not fully determined. Axin2 is also expressed in both broad isthmus progenitor cells and basal Lgr5+ cells, and likely overlaps with eR1 or other markers. Finally, CCK2R+ antral stem cells which were identified by our group [12] reside at +4 position and appear to show quite close proximity to Mist1+ antral cells. Our current findings using fluorescent imaging and FACS analysis failed to demonstrate the evident overlap between Mist1 and other markers. Nonetheless, we do not exclude the possibility that some of reported stem/progenitor cell markers (e.g., eR1, Sox2, or Axin2) may contain Mist1+ cells when using reported CreERT lines. It should be noted that Mist1 expression is more restricted to the isthmus region than other markers, however, it may be possible that Mist1 marks heterogeneous isthmus progenitors that are long-lived.
We show here that Mist1+ cells can serve as the cell-of-origin for antral gastric tumors. It has long been suspected that cancer arises from genetic alterations in stem cells, given their longevity and capacity for self-renewal [26]. Indeed, Lgr5+ and Sox2+ cells can also give rise to dysplasia or tumors with loss of the Apc gene [10, 21]. We have shown here that relatively quiescent Mist1+ cells can give rise to cancer after exposure to the carcinogen (MNU) or loss of Apc. In the distal stomach, intestinal-type gastric cancer is the more common histologic type, and is typically associated with activation of the Wnt/β-catenin signaling pathway [10, 27]. Indeed, the frequency of Apc mutation in human intestinal-type gastric cancer is significantly higher than that in diffuse-type [28]. The fact that Apc mutation models in the stomach resulted only in antral tumors without corpus tumor may suggest that the susceptibility to cancer in response to certain oncogenes and carcinogens is different between the antrum and corpus. In humans, MSI+ cancers are more common in antral cancers, while Tp53-mutated CIN+ tumors are found more often in proximal cancers. Thus, it may be crucial to understand the molecular mechanism in both antral and corpus carcinogenesis separately.
In previous studies, we found that the development of diffuse-type corpus cancer was highly dependent on an endothelial niche, with Cxcl12 signaling from endothelial cells to Cxcr4-expressing ILCs, which then activate Mist1+ corpus stem cells through Wnt5a secretion. In the current study, we observed a similar juxtaposition of Cxcl12-expressing endothelial cells adjacent to Mist1+ antral cells. In addition, we previously reported that Cxcl12/Cxcr4 signaling is involved in activation and recruitment of cancer-associated fibroblasts during gastric carcinogenesis, and that aberrant expression of Cxcl12 accelerates gastric cancer development [22, 29]. Here, we show clear Cxcr4 expression in the Mist1+ antral cells, and in response to carcinogen exposure, there was a marked expansion of Cxcr4+ epithelial cells that eventually encompassed almost the entire gland. Thus, Cxcr4 signal may broadly affect gastric carcinogenesis through multiple mechanisms.
Interestingly, while Cxcr4 inhibition leads to suppression of Mist1-derived tumor growth, there is compensatory activation of the CCK2R lineage, which we showed was negative for Cxcr4 expression. Thus, in the absence of Cxcl12/Cxcr4 signaling and reduction in the Mist1 lineage, there is an upregulation of CCK2R+ cells (but interestingly not Lgr5+ cells), suggesting that other stem cell populations can compensate for loss of the Mist1 lineage. Given this observation, it may be interesting to consider simultaneous blockade of the Cxcr4 axis and inhibition of CCK2R+ cells may achieve better tumor suppression.
MIST1 expression in human gastric antrum was recently described [30], and epithelial Cxcr4 expression in human gastric cancer tissue has been also reported [31, 32]. In addition, a meta-analysis reported that Cxcr4 expression in primary human gastric cancer tissues was positively associated with tumor progression and disease prognosis, including vascular invasion [33]. Thus, we believe that Cxcr4+ epithelial cells, which include the Mist1+ antral progenitors, contribute to human gastric cancer progression, and that the Cxcr4/Cxcl12 axis may still be a promising therapeutic target against broad spectrum of gastric cancers. While targeting the endothelium using antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) has shown benefit in some tumors in clinical trials [34, 35], the role of Cxcl12 secretion in the response has not been explored. The current study provides further support for the existence of Cxcl12 endothelium as a representing key niche for gastrointestinal stem cells.
MATERIALS AND METHODS
Mice
Mist1-CreERT mice [36] and Cxcl12-dsRED mice [37] were described previously. Cxcr4-EGFP mice were provided from Richard J. Miller (North-western University Medical School). LSL-KrasG12D and LSL-Trp53R172H mice were provided by Dr. Kenneth Olive (Columbia University). Cckbr-GFP BAC transgenic mice were purchased from MMRRC (GENSAT project [38]). Lgr5-DTR-GFP mice were provided by Genentech. Apcflox/flox mice were obtained from the National Cancer Institute. Lgr5-EGFP-IRES-CreERT, Sox2-EGFP, R26-mTmG, R26-TdTomato, R26-LacZ, Cxcl12flox/flox, and Tie2-Cre mice were purchased from the Jackson Laboratory. FucciG1 mice were obtained from RIKEN BRC. Cre recombinase was activated by oral administration of tamoxifen (TAM, Sigma, 3 mg/0.2 ml corn oil). All animal studies and procedures were approved by the ethics committees at Columbia University and the University of Tokyo. All mice were bred under specific pathogen free conditions. Comparisons were made with age- and sex- matched control animals.
Treatment
For Lgr5+ cell ablation, diphtheria toxin (DT, Sigma) was administered intraperitoneally (20mg/kg). N-nitroso-N-methylurea (MNU, Sigma) was dissolved in distilled water at a concentration of 240 ppm and administered in drinking water. Mice (8-week-old) were given drinking water containing MNU on alternate weeks for five cycles [12]. Mice were analyzed 50 weeks after the beginning of the MNU treatment. Mice were treated with CD90.2 mAb (30H12) (BioXcell) intraperitoneally at a dose of 250 µg/mouse for 4 weeks. AMD3100 (Tocris) was administered at a dose of 5mg/kg/day by implanted subcutaneous osmotic pumps (Alzet 2004) for 2 weeks.
Statistical analysis
The difference between the means was compared by either Student’s t-test or the Wilcoxon test. p values < 0.05 were considered to indicate statistical significance. Other detailed information is described in Supplementary methods.
SUPPLEMENTARY MATERIALS FIGURES
Acknowledgments
In Memoriam, Dr. Xiaowei Chen. Our co-author Sean was a great researcher, and we respect his enormous contribution, his tremendous achievement, and his wonderful life. We offer our deepest condolences to Sean and his family.
We thank Dr. Kenneth Olive and Dr. Richard J. Miller for providing the mice, Ms. Kristie Gordon for assisting the fluorescence-activated cell sorting (FACS) analysis, Ms. Theresa Swayne for taking the three-dimensional images, Dr. Rani Sellers, Ms. Barbara Cannella, and Ms. Supreet Kainth for assisting with the in situ hybridization.
Abbreviations
- ILCs
innate lymphoid cells
- MNU
N-nitroso-N-methylurea
- TAM
tamoxifen
- DT
diphtheria toxin
- BrdU
bromodeoxyuridine
Author contributions
K.S. and Y.H. contributed equally to the design of the experiments, performance of the experiments, and analysis of the data. A.R.S., H.D., and Z.N. conducted the pathological evaluation. H.D., H.A., N.S., S.I., H.K., M.K., S.O., W.K., T.T., H.L., X.C., and Y.T. performed various portions of the experiments. K.S., Y.H., D.L.W., and T.C.W. wrote the manuscript. K.S., Y.H., J.G.F., S.F.K., H.O., Y.H., S.A., K.K., and T.C.W. contributed to the study supervision and coordination.
CONFLICTS OF INTEREST
The authors disclose no conflicts.
FUNDING
This research was supported by National Institute of Health grants U54CA126513, R01CA093405, R01CA120979, R01DK052778, and by the Clyde Wu Family Foundation (T.C.W.), JSPS Research Fellowship for Young Scientists and Mitsukoshi health and Welfare Foundation (K.S.), the Project for Cancer Research And Therapeutic Evolution (P-CREATE) from the Japan Agency of Medical Research and Development, AMED, the KAKENHI Grant-in-Aid for Young Scientist Start-up and (A) (16H06749 and 17H05081), the Kobayashi Foundation for Cancer Research, the Mochida Memorial Foundation for Medical and Pharmacological Research, the Mitsubishi Foundation, Natural Sciences, the Advanced Research and Development Programs for Medical Innovation (PRIME) and the Tokyo Society of Medical Sciences (Y.H.), Foundation of Jiangxi Educational Committee (20151BAB215008, 20151BBG70200) (H.D.), and China Scholarship Council (Z.N.).
Editorial note
This paper has been accepted based in part on peer-review conducted by another journal and the authors' response and revisions as well as expedited peer-review in Oncotarget.
REFERENCES
- 1.Lordick F, Janjigian YY. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat Rev Clin Oncol. 2016;13:348–60. doi: 10.1038/nrclinonc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan P, Yeoh KG. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology. 2015;149:1153–62. doi: 10.1053/j.gastro.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 5.Brungs D, Aghmesheh M, Vine KL, Becker TM, Carolan MG, Ranson M. Gastric cancer stem cells: evidence, potential markers, and clinical implications. J Gastroenterol. 2016;51:313–26. doi: 10.1007/s00535-015-1125-5. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa Y, Fox JG, Wang TC. The Origins of Gastric Cancer From Gastric Stem Cells: Lessons From Mouse Models. Cellular and Molecular Gastroenterology and Hepatology. 2017;3:331–338. doi: 10.1016/j.jcmgh.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao XT, Gumucio DL. Current molecular markers for gastric progenitor cells and gastric cancer stem cells. J Gastroenterol. 2011;46:855–65. doi: 10.1007/s00535-011-0413-y. [DOI] [PubMed] [Google Scholar]
- 8.Ishimoto T, Sawayama H, Sugihara H, Baba H. Interaction between gastric cancer stem cells and the tumor microenvironment. J Gastroenterol. 2014;49:1111–20. doi: 10.1007/s00535-014-0952-0. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, Chen X, Takemoto Y, Kim W, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell. 2015;28:800–14. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–29. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa Y, Jin G, Wang H, Chen X, Westphalen CB, Asfaha S, Renz BW, Ariyama H, Dubeykovskaya ZA, Takemoto Y, Lee Y, Muley A, Tailor Y, et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–53. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo J, Kimura S, Yamamura A, Koh CP, Hossain MZ, Heng DL, Kohu K, Chih-Cheng Voon D, Hiai H, Unno M, Yan So JB, Zhu F, Srivastava S, et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology. 2016;152:218–231. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, Samuelson LC, Gumucio DL. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–98. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigal M, Logan CY, Kapalczynska M, Mollenkopf HJ, Berger H, Wiedenmann B, Nusse R, Amieva MR, Meyer TF. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature. 2017;548:451–5. doi: 10.1038/nature23642. [DOI] [PubMed] [Google Scholar]
- 16.Nam KT, Lee HJ, Sousa JF, Weis VG, O’Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–37. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, Jiang Z, Tanaka T, Dubeykovskaya ZA, et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell. 2017;31:21–34. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, et al. Differentiated troy(+) chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–68. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa Y, Jin G, Wang H, Chen X, Westphalen CB, Asfaha S, Renz BW, Ariyama H, Dubeykovskaya ZA, Takemoto Y, Lee Y, Muley A, Tailor Y, et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–53. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–98. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar A, Huebner AJ, Sulahian R, Anselmo A, Xu X, Flattery K, Desai N, Sebastian C, Yram MA, Arnold K, Rivera M, Mostoslavsky R, Bronson R, et al. Sox2 Suppresses Gastric Tumorigenesis in Mice. Cell Rep. 2016;16:1929–41. doi: 10.1016/j.celrep.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Shibata W, Ariyama H, Westphalen CB, Worthley DL, Muthupalani S, Asfaha S, Dubeykovskaya Z, Quante M, Fox JG, Wang TC. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut. 2013;62:192–200. doi: 10.1136/gutjnl-2011-301824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekitani T, Yokota T, Kuribara K, Kaltenbrunner M, Fukushima T, Inoue Y, Sekino M, Isoyama T, Abe Y, Onodera H, Someya T. Ultraflexible organic amplifier with biocompatible gel electrodes. Nat Commun. 2016;7:11425. doi: 10.1038/ncomms11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985;172:205–24. doi: 10.1002/aja.1001720304. [DOI] [PubMed] [Google Scholar]
- 25.Lee ER. Dynamic histology of the antral epithelium in the mouse stomach: I. Architecture of antral units. Am J Anat. 1985;172:187–204. doi: 10.1002/aja.1001720303. [DOI] [PubMed] [Google Scholar]
- 26.Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ, Wang TC. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer. 2016;16:305–18. doi: 10.1038/nrc.2016.24. [DOI] [PubMed] [Google Scholar]
- 27.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 28.Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. 2002;8:787–91. doi: 10.3748/wjg.v8.i5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi E, Roland JT, Barlow BJ, Neal R, Rich AE, Nam KT, Shi C, Goldenring JR. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711–20. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda T, Nakashima Y, Ando K, Yoshinaga K, Saeki H, Oki E, Morita M, Oda Y, Maehara Y. Nuclear expression of chemokine receptor CXCR4 indicates poorer prognosis in gastric cancer. Anticancer Res. 2014;34:6397–403. [PubMed] [Google Scholar]
- 32.Satomura H, Sasaki K, Nakajima M, Yamaguchi S, Onodera S, Otsuka K, Takahashi M, Muroi H, Shida Y, Ogata H, Okamoto K, Kato H. Can expression of CXCL12 and CXCR4 be used to predict survival of gastric cancer patients? Anticancer Res. 2014;34:4051–7. [PubMed] [Google Scholar]
- 33.Han M, Lv S, Zhang Y, Yi R, Huang B, Fu H, Bian R, Li X. The prognosis and clinicopathology of CXCR4 in gastric cancer patients: a meta-analysis. Tumour Biol. 2014;35:4589–97. doi: 10.1007/s13277-013-1603-4. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 35.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 36.Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–78. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–25. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.