Abstract
BACKGROUND/OBJECTIVES
Prenatal growth, which is widely marked by birthweight, may play a pivotal role in affecting the lifelong risk of cardiometabolic disorders; however, comprehensive evaluation of its relations with childhood cardiometabolic risk patterns and the ethnic and gender disparities in national representative populations is still lacking. The aim of this study was to evaluate the associations between birthweight and comprehensive patterns of cardiometabolic risk in a nationally representative sample of children and adolescents.
SUBJECTS/METHODS
Prospective analyses were performed using data from 28,153 children 0 to 15 years in the National Health and Nutrition Examination Survey (NHANES) from 1999 through 2014. We defined childhood cardiometabolic disorders using standard definitions for obesity, high blood pressure, hyperglycemia, and dyslipidemia.
RESULTS
Five birthweight categories <2.5, 2.5–3.0, 3.0–3.5, 3.5–4.2, and ≥4.2 kg accounted for 8.2%, 17.9%, 35.7%, 27.9%, and 10.4% of the population, respectively. In all children, with increasing birthweight, we observed significantly increasing trends of the risk of general and central obesity (p for trend < 0.01), and significantly decreasing trends of the risk of high SBP, high HbA1c, and low HDL-C (p for trend < 0.05). The associations were independent of current BMI. In addition, we found that the relations of birthweight with high waist circumference in black children showed U-shape, as well as high SBP in Mexican and Hispanic children. Moreover, we found that the associations of low birthweight with high SBP and low HDL-C appeared to more prominent significant in boys, while the inverse association with high HbA1c was more evident in girls.
CONCLUSIONS
Our data indicate that birthweight is significantly related to childhood cardiometabolic risk, independent of current BMI; and the associations exhibit race and gender-specific patterns.
Keywords: birthweight, childhood cardiometabolic risks, multiracial children
INTRODUCTION
Compelling evidence has shown that prenatal growth may play a pivotal role in affecting fetal metabolism and organ structure, and subsequently influence health after birth.1–4 In epidemiological studies, low birthweight (LBW), a widely used indicator for prenatal growth retardation caused by intrauterine malnutrition or other stress, has been consistently associated with increased risk of a variety of cardiometabolic disorders such as type 2 diabetes (T2D) and cardiovascular disease (CVD) in adults.5–9
Adulthood cardiometabolic risks have their origin in childhood; and childhood obesity, high blood pressure, hyperglycemia, and dyslipidemia have been found to predict cardiometabolic diseases in adults.10–12 In the U.S., the prevalence of obesity and cardiometabolic disorders has been rapidly increasing in children and adolescents.13 However, few studies have comprehensively assessed the relations between birthweight and overall cardiometabolic risk patterns in children from nationally representative populations. In addition, previous studies in adults suggest that the effects of birthweight may be partly through affecting body weight after birth; it remains unclear whether birthweight is independently related to childhood cardiometabolic risk.14,15
In this study, we performed prospective analyses on associations between birthweight and comprehensive patterns of cardiometabolic risk in a nationally representative sample of children and adolescents from the National Health and Nutrition Examination Survey (NHANES). We particularly compared the association patterns across different race groups, and between girls and boys.
MATERIALS AND METHODS
Study design and data sources
The NHANES is a nationally representative cross-sectional survey of civilian non-institutionalized U.S. population. The NHANES includes two phases: personal interviews at home on a variety of health topics and demographic information, and standardized physical examinations and laboratory tests at a mobile examination center (MEC). Signed informed consent was obtained from all participants attended both phases, and data are publicly available. This study was deemed exempt from human subjects approval by the Institutional Review Board of the School of Public Health and Tropical Medicine at the Tulane University. Data were obtained from the public-release data sets of the most recent eight NHANES circles (NHANES survey conducted every two years since 1999), with NHANES 1999–2000 as the first and NHANES 2013–2014 the most recent. We included subjects who were both interviewed at home and received an MEC examination (N=78,518). Among them, only children aged 0 to 15 years were with available birthweight information (N=29,758). After an exclusion of individuals with missing data on birthweight or for whom birthweight was under 1.00 pound or above 13 pounds, a total number of 28,679 children were included in the current study. The demographic characteristics are shown in Table S1.
Birthweight
Information about birthweight in NHANES 1999–2014 was collected through an in-home interview, children 0 to 15 years of age at the time of examination. Participants were asked to report their birthweight in pounds and ounces. For those who were too young or unable to answer, birthweight was responded by their parents or guardians. We converted birthweight into kilograms, and generated birthweight groups with five categories (<2.5, 2.5–3.0, 3.0–3.5, 3.5–4.2, and ≥4.2 kg), given <2.5kg as a LBW group and ≥4.2 kg as high birthweight (HBW) group.16 Extreme birthweight were excluded (<0.45 kg or ≥5.90 kg).
Covariates
Demographic information were all self-reported or responded by parents or guardians during the in-home interviews, including age at screening, gender (male or female), race (Non-Hispanic White; Non-Hispanic Black; Mexicans and Hispanics; Other race including Multi-Racial), and the ratio of family income to poverty (calculated by dividing family income to family size, ranging from 0.00 to 5.00). Additionally, maternal smoking status was also included, defined as (1) no smoking during the entire pregnancy; (2) quit smoking in the first trimester of pregnancy; (3) quit smoking in the second trimester of pregnancy; (4) quit smoking in the third trimester of pregnancy; (5) kept smoking throughout the entire pregnancy.
Childhood cardiometabolic disorders
Eleven measured variables related to known cardiometabolic risks were examined in children, with the definition of abnormal values shown in Table 1. Detailed information about physical examination and laboratory procedures can be found with online documentary manuals of “Laboratory Methods” and “Examination and Laboratory Procedures” of every NHANES circles from 1999 to 2014, from the official website (http://www.cdc.gov/nchs/nhanes.htm).
Table 1.
Sample Size and Definition of Abnormal Values for Cardiometabolic Risk-Factor Variables.*
| Variable | Age Range (yrs.) | Sample Size (n, %) | Definition of Abnormal Value |
|---|---|---|---|
| Glycemic profile | |||
| FBG | 12 – 15 | 2410 (8.6) | ≥100 mg/dL |
| HbA1c | 12 – 15 | 6077 (21.6) | >5.7% |
| Blood Pressure | |||
| SBP | 8 – 15 | 12199 (43.3) | ≥95th percentile# |
| DBP | 8 – 15 | 12200 (43.3) | ≥95th percentile# |
| Blood Lipids | |||
| TG | 3 – 15 | 3208 (11.4) | ≥150 mg/dL |
| TC | 3 – 15 | 14409 (51.2) | ≥200 mg/dL |
| HDL-C | 3 – 15 | 14407 (51.2) | <35 mg/dL |
| LDL-C | 3 – 15 | 3193 (11.3) | ≥100 mg/dL |
| Body Mass Measurements | |||
| BMI | 2 – 15 | 22495 (79.9) | ≥95th percentile§ |
| Waist | 2 – 15 | 21633 (76.8) | ≥90th percentile§ |
| Weight | 0 – 15 | 28153 (100%) | ≥90th percentile§ |
Data are shown for children under 16 years old. FBG, fasting blood glucose; HbA1c, glycated hemoglobin; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, fasting blood triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, fasting blood low-density lipoprotein cholesterol; BMI, body mass index; Whites, Non-Hispanic White; Blacks, Non-Hispanic Black; Mex & His, Mexicans and Other Hispanics.
Age-, gender- and height-specific cutoff value.
Age- and gender-specific cutoff value.
Obesity
Waist circumference (WC) was measured at the time of the physical examination for all children (n=28,153), whereas standing height for children was measured only in those two years of age or over. We defined abnormal values of body mass index (BMI) as any value that was ≥ 95th percentile specific for age and gender, derived from the Clinical Growth Charts from Centers for Disease Control and Prevention (CDC).17 The abnormal values of WC and weight were defined as ≥ 90th percentile specific for age and gender, in accord with the recommendations by the International Diabetes Federation (IDF).18
High blood pressure
Up to three consecutive blood pressure measurements were recorded on all examinees aged eight years and older. Blood pressure was calculated as the mean value of up to three consecutive measurements in the MEC (86% of the children had three measurements, 8% had two, and 6% had one). Abnormal values of both systolic blood pressure (SBP) and diastolic blood pressure (DBP) were defined as any value that was ≥ 95th percentile specific for age, gender, and height derived from the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents.19
Hyperglycemia
Glycemic profile was obtained with two laboratory variables, including glycated hemoglobin (HbA1c) for participants aged 12 years and older and fasting blood glucose (FBG) in the subgroup who examined in the morning examination session. Any participant with a history of diabetes or antidiabetic medication was removed from the analysis of HbA1c and FBG, and an additional requirement for participants with fasting hours between 8 hours and 24 hours was applied to the analysis of FBG. Hyperglycemia in children and adolescents were defined as high FBG (≥ 100 mg/dL) and high HbA1c (> 5.7%), according to the recommendation from ADA (American Diabetes Association)20, AHA (American Heart Association),21 IDF,22 NHLBI (National Heart, Lung, and Blood Institute),23 and AAP (American Academy of Pediatrics).24
Dyslipidemia
Complete lipid profile was obtained, including non-fasting total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) on participants three years of age or over. Fasting triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) were available in the subgroup of who attended the morning examination session in NHANES 1999–2012. Participants whose fasting hours less than 8.5 hours or exceeding 24 hours were excluded from the analysis of TG and LDL-C. Dyslipidemia was defined as high TG (≥ 150 mg/dL), high TC (≥ 200 mg/dL), low HDL-C (< 35 mg/dL), or high LDL-C (≥ 130 mg/dL).21–24
Each cardiometabolic risk factor variable varies due to specialized sampling frame and examination requirements in NHANES; detailed information of analysis sample size is shown for all children, boys, and girls by race in Table S3.
Statistical Analysis
In the current study, all reported results and statistical tests were accounted for the NHANES survey design (the primary sampling units and strata) and sample weights (two-year sample weights for participants who both interviewed and MEC examined) for the unequal probabilities of selection, nonresponse and adjustment to independent population controls. Stratified analysis was performed by gender, by race/ethnicity, and by gender and race group. The estimated prevalence of each cardiometabolic disorder was calculated with 95% confident intervals (95% CI) in five ordinal birthweight categories. For further adjustments, we performed survey-weighted generalized linear models to estimate odds ratios (ORs) for each cardiometabolic disorders by birthweight categories, controlling for age, gender, race/ethnicity, the ratio of family income to poverty, maternal smoking status and the NHANES circles (model 1). Besides covariates mentioned above, we subsequently explored whether the associations of birthweight with each cardiometabolic disorders were affected through current BMI (model 2). Linear and non-linear trends were tested by introducing an ordinal variable of birthweight categories and the quadratic term into the model, respectively. All data management and analysis were conducted using R version 3.2.3 (Wooden Christmas-Tree). For analysis of complex survey samples, the R package named “Survey” was introduced to produce nationally representative estimates, with inverse-probability weighting and design-based standard errors.25–27 Statistical significance was assessed at the 2-sided α=0.05 level.
RESULTS
Baseline characteristics
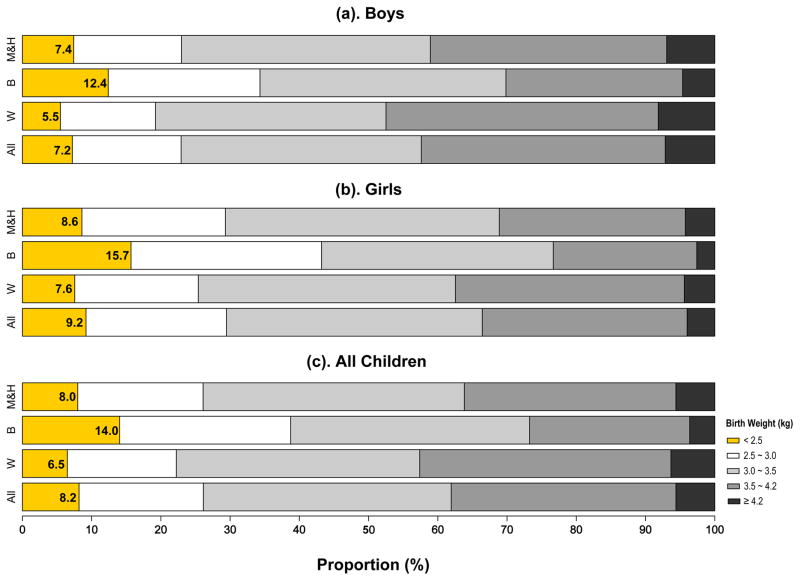
Among 28,679 children 0 to 15 years of age (50.9% were boys; 57.2% were Non-Hispanic Whites), 8.2% were born at LBW (Figure 1), with the highest proportion of LBW in Blacks (14.0%) and the lowest in Whites (6.5%). The mean birthweight was 3.32±0.61 kg in all children, 3.13±0.66 kg in Non-Hispanic Black children, 3.31±0.61 kg in Mexicans & Hispanics, and 3.32±0.62 kg in Whites (Figure S1). On average, boys were born 0.1 kg heavier than girls (Figure S1 and Table S2). In NHANES, ten cardiometabolic traits (except for body weight) were available in sub-samples (8.6~79.9% of the whole sample) with varying age ranges (Table 1).
Figure 1. Proportion of Low birth weight in boys and girls, and all children by three different race/ethnic groups.
W, Non-Hispanic Whites; B, Non-Hispanic Blacks; Mex & His, Mexicans and Other Hispanics.
Cardiometabolic risk patterns by five birthweight categories
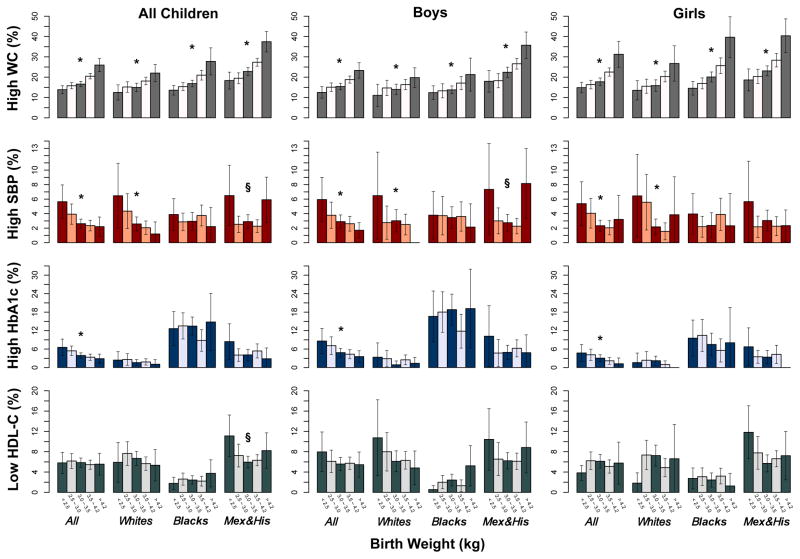
In all children, boys and girls (Figure 2), we observed consistently and significantly increasing trends of prevalence of high WC, whereas significantly decreasing trends of prevalence of high SBP and high HbA1c with increasing birthweight. Of note, in Mexican and Hispanic children, the prevalence and ORs of high SBP, and low HDL-C showed U-shaped relationships with birthweight. As WC, BMI, and weight were highly correlated obesity markers, we found the similar prevalence trends and association patterns of birthweight with BMI and weight (Figure S2–S4, and Table S4 and S6). No clear trends were observed for prevalence of high DBP, high FBG, high TG, high TC, and high LDL-C according to birthweight in the whole population, in each race groups, and in boys and girls (Figure S2–S4, and Table S8, S9, S11, S12, and S14).
Figure 2. Prevalence of Four Cardiometabolic risk factors by birth weight in all children, boys and girls by three different race/ethnic groups*.
P for linear trend <0.05; §, P for non-linear trend <0.05; W, Non-Hispanic Whites; B, Non-Hispanic Blacks; Mex & His, Mexicans and other Hispanics.
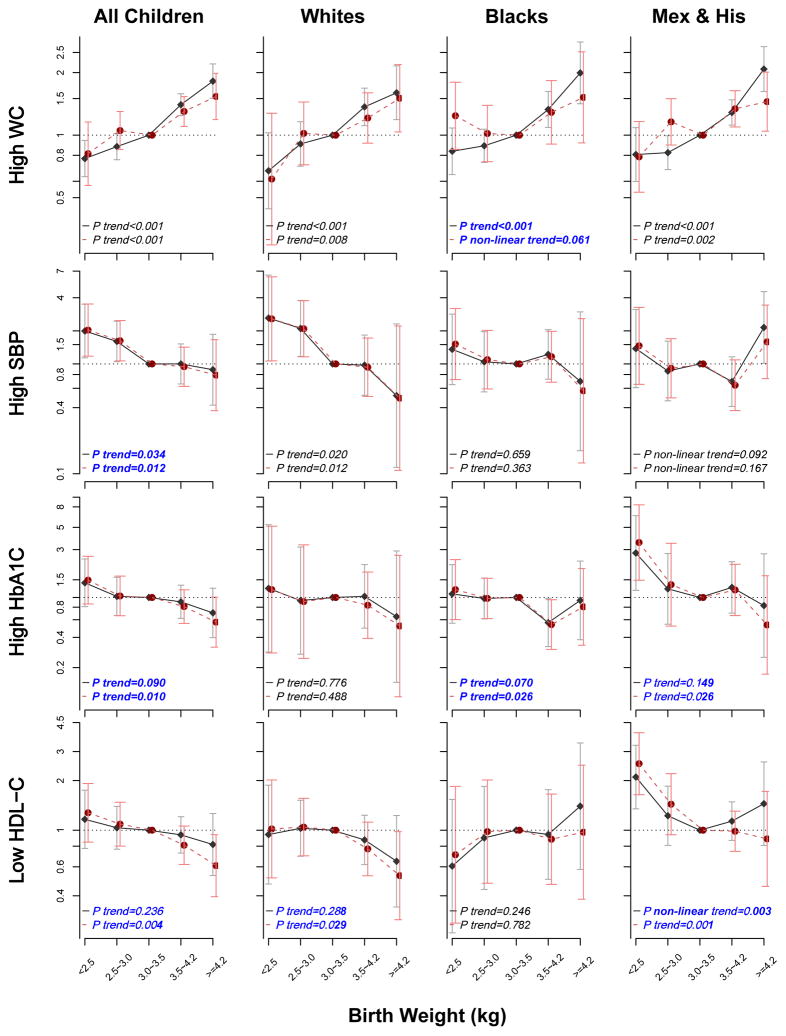
Difference in association patterns with and without adjustment for current BMI
We further found that the risk patterns of four cardiometabolic disorders (high WC, SBP, and HbA1C and low HDL-C) with birthweight groups showed significant difference with and without adjustment for current BMI (Figure 3). Consistent with prevalence association patterns in Figure 2, Figure 3 showed similar linear trend of ORs for the associations of birthweight with high WC (P<0.001) and high SBP (P=0.034), but not with high HbA1c (P=0.090) and low HDL-C (P=0.236) in models without adjustment for BMI in all children. We found that additional adjustment for current BMI significantly improved the inverse associations of birthweight with high HbA1c and HDL-C (P=0.010 and 0.004, respectively); while the associations with high WC and SBP remained significant. In addition, the sensitivity analyses, by limiting our study sample into children with a normal birthweight of 2.5~4.2 kg (data not shown), also showed similar associations between birthweight (kg) and cardiometabolic risks (ORs: 1.35, 0.59, 0.79, and 0.76 for high WC, high SBP, high HbA1c, and low HDL-C, respectively).
Figure 3. Odds Ratios of Four Cardiometabolic risk factors by birth weight in all children, and three race groups.
Model 1 with solid line, adjusted for survey-weighted logistic regression models that controlled for age, ethnic group, gender, NHANES circles, family income, and maternal smoking status; Model 2 with dotted line, adjusted for BMI and covariates in Model 1. P trend represented the significance of linear trend test.
Ethnical difference in cardiometabolic risk patterns by five birthweight categories
In figure 2 and 3, significant ethnical difference were shown and tested (all p<0.05) in the association patterns of cardiometabolic risk patterns with birthweight, we then performed similar analyses in each race group. Of interest, after adjustment for current BMI, the linear association between birthweight and high WC turned into a U-shape in black children (Ptrend<0.001 vs. Pnon-linear trend=0.061), whereas the U-shaped relation of birthweight with low HDL-C turned into a linear pattern in Mexican & Hispanic children (Pnon-linear trend=0.003 vs. Ptrend=0.001).
Gender difference in cardiometabolic risk patterns by five birthweight categories
We also analyzed the associations in boys and girls separately (Table 2). Except for high WC, the inverse trends of ORs were not significant in models without adjustment for current BMI among both boys and girls. After adjustment for current BMI, the inverse associations of birthweight with high SBP and low HDL-C became significant in boys, similar with the trends of ORs for high HbA1c in girls. Adjustment for current BMI did not appreciably change the relation between birthweight and high WC.
Table 2.
Odds Ratios for Four Cardiometabolic risk factors by birth weight in boys and girls by Birth Weight Category.*
| Birth Weight Category | Boys
|
Girls
|
||
|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | |
| High WC | ||||
| <2.5 | 0.75 (0.56, 1.01) | 0.76 (0.50, 1.16) | 0.76 (0.58, 1.00) | 0.84 (0.52, 1.36) |
| 2.5~3.0 | 0.88 (0.71, 1.10) | 1.08 (0.83, 1.40) | 0.86 (0.72, 1.03) | 1.03 (0.76, 1.39) |
| 3.0~3.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 3.5~4.2 | 1.43 (1.19, 1.72) | 1.40 (1.13, 1.72) | 1.43 (1.20, 1.71) | 1.23 (0.97, 1.54) |
| ≥4.2 | 1.77 (1.38, 2.27) | 1.36 (0.95, 1.97) | 2.17 (1.58, 2.99) | 2.04 (1.36, 3.06) |
| P<0.001 | P= 0.002 | P<0.001 | P= 0.003 | |
| High SBP | ||||
| <2.5 | 2.08 (1.13, 3.84) | 2.18 (1.21, 3.91) | 1.79 (0.82, 3.94) | 1.83 (0.83, 4.03) |
| 2.5~3.0 | 1.30 (0.68, 2.49) | 1.33 (0.70, 2.56) | 1.85 (0.99, 3.46) | 1.86 (0.99, 3.48) |
| 3.0~3.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 3.5~4.2 | 1.15 (0.66, 2.02) | 1.04 (0.59, 1.84) | 0.86 (0.48, 1.52) | 0.84 (0.47, 1.48) |
| ≥4.2 | 0.72 (0.32, 1.61) | 0.62 (0.28, 1.38) | 1.30 (0.40, 4.29) | 1.20 (0.36, 4.01) |
| P= 0.126 | P= 0.037 | P= 0.087 | P= 0.063 | |
| High HbA1c | ||||
| <2.5 | 1.67 (0.85, 3.28) | 1.73 (0.89, 3.38) | 1.17 (0.57, 2.39) | 1.27 (0.63, 2.57) |
| 2.5~3.0 | 1.08 (0.59, 1.99) | 1.09 (0.58, 2.06) | 1.04 (0.54, 2.01) | 1.05 (0.54, 2.05) |
| 3.0~3.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 3.5~4.2 | 1.05 (0.64, 1.71) | 0.95 (0.58, 1.58) | 0.75 (0.38, 1.49) | 0.68 (0.34, 1.35) |
| ≥4.2 | 0.90 (0.50, 1.62) | 0.84 (0.46, 1.53) | 0.38 (0.08, 1.89) | 0.20 (0.04, 1.00) |
| P= 0.265 | P= 0.119 | P= 0.123 | P= 0.018 | |
| Low HDL-C | ||||
| <2.5 | 1.66 (0.90, 3.03) | 1.84 (0.99, 3.42) | 0.71 (0.46, 1.11) | 0.80 (0.52, 1.23) |
| 2.5~3.0 | 1.01 (0.64, 1.62) | 1.11 (0.69, 1.79) | 1.03 (0.73, 1.44) | 1.06 (0.74, 1.51) |
| 3.0~3.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 3.5~4.2 | 1.03 (0.74, 1.44) | 0.89 (0.62, 1.28) | 0.86 (0.61, 1.22) | 0.71 (0.47, 1.07) |
| ≥4.2 | 0.73 (0.41, 1.33) | 0.59 (0.32, 1.08) | 0.95 (0.45, 1.99) | 0.67 (0.32, 1.38) |
| P= 0.171 | P= 0.020 | P= 0.927 | P= 0.085 | |
P value was given for the statistical significance of the linear trend test of odds ratios among five ordinal birthweight categories.
Model 1, survey-weighted logistic regression models that controlled for age, ethnic group, NHANES circles, family income, and maternal smoking status.
Model 2 = Model 1 + BMI.
DISCUSSION
In this prospective study of a nationally representative children and adolescent sample, we found that birthweight, a widely-used marker of prenatal growth, was significantly and positively associated with a higher risk of general and central obesity, while significantly and negatively associated with cardiometabolic disorders including high SBP, high HbA1c, and low HDL-C, independent of current body size. The relations of birthweight with childhood cardiometabolic disorders showed different patterns by race and gender.
Our findings are in line with previous findings in children and adolescents. A meta-analysis28 showed a 1.76 (1.65–1.87) fold higher risk of overweight with high birthweight (>4.0 kg) among 643,902 participants under 18. Two systematic reviews by Huxley29 and Law30 noted that a lower birthweight was significantly associated with an higher SBP in adolescence. Birthweight has been consistently related to cardiometabolic risk in adults, but with relatively smaller effect sizes on obesity and SBP compared with in children and adolescents.28–30 Growing evidence revealed that people who were born with LBW had a higher risk of T2D in a later life.7,9 Nevertheless, regarding the association of birthweight with specific glycemic and lipid profiles, the existing data appeared to be conflicting in both children and adults.5,15,31 Two previous studies32,33 reported that there was no difference in HbA1c levels between LBW and non-LBW group, but both studies were based on analyses among childhood-onset T2D patients. A lower HDL-C level was observed in LBW group in two adults based studies;34,35 however, such difference did not reach significant levels, probably due to insufficient power. Additionally, the thus-far largest genome-wide association study36 demonstrated that genetic factors were the major contributor to the negative association between birthweight and future cardiometabolic risk. Our data suggested that higher birthweight might particularly improve SBP, HbA1c, and HDL-C levels, regardless elevated adiposity, in children.
Birthweight is fundamentally determined by in utero environment. According to the theory of the developmental origins of health and disease (DOHaD),3,37 the life-long risk of major adult-onset cardiometabolic diseases has its origin in prenatal development. Previous studies in adults consistently support detrimental effects of LBW that indicates impaired intrauterine growth.9,38,39 Our data indicates that such adverse effects of LBW may start from early life. Impaired intrauterine growth may affect “metabolic programming”40,41 and structure of organs and tissues,4,42,43 which result in irreversible long-term adverse effects on cardiometabolic health3,41. For example, LBW could contribute to an elevated blood pressure via affecting the hypothalamic– pituitary–adrenal (HPA) axis regulation, renal injury, oxidative stress and inflammation, nutrition, and glucocorticoids.3,44,45 Furthermore, LBW infants are subject to leptin resistance and insulin resistance, which may lead to defects in glycemic homeostasis such as elevated HbA1c.1,4,46 Nevertheless, our data and sensitivity analyses suggested participants born with a higher birthweight may carry the cardiometabolic risk associated with general and abdominal obesity, but may be protected against insulin resistance and dyslipidemia, probably owing to a greater lean mass47, undisturbed glucose homeostasis48, or an appropriate gestational age49, compared with LBW. The divergent associations of birthweight with obesity and other cardiometabolic disorders including high SBP, high HbA1c, and low HDL-C suggest that the effects of birthweight on these cardiometabolic disorders may be independent of adiposity. An alternative hypothesis is that LBW infants experience “catch-up” growth in the first months after birth when a “mismatch” between the prenatal and postnatal environment occurs,4 whereas HBW infants might experience growth deceleration. Such differences during the critical stage of development may have lasting influence on cardiometabolic health.4,49
Intriguingly, we noted the U-shaped relations of birthweight with high WC and high SBP in black and Mexican & Hispanic children, respectively. We speculated the combination of LBW and rapid postnatal catch-up growth contributed to an increased risk of abdominal obesity in black children50, due to a well-documented lower birthweight compared with whites, as well as diet-related disparities, a less nutritious, calorie-dense food environment because of a lower family incomes and poverty.51 Though the underlined mechanism is not well-defined, excessive glucocorticoid exposure, rapid postnatal growth, and enhanced insulin secretion/action in LBW children were considered to participate in the increased visceral/abdominal fat.52–54 Additionally, the significant “J” or “U” shaped relations between birthweight and cardiometabolic disorders were more frequently observed in non-European populations.8,28,55–58 These observations, together with ours, suggest that the effects of birthweight on cardiometabolic risk patterns may differ according to racial background. We assume that diversity in genetic predispositions and varying environment among different racial populations may partly account for such disparities, though more studies are warranted to explore the potential mechanisms.
In addition, we found the cardiometabolic risk associated with birthweight showed gender-specific patterns, boys born lighter appeared to have an elevated SBP and lower HDL-C level, while girls born at HBW showed a higher risk of adulthood obesity.58 Such gender-specific relations were also previously reported in both children and adults. A cross-sectional study of 5,141 children aged 9–11 years conducted in 12 countries found that a positive association of birthweight and the odds of childhood obesity was noted in girls, whereas a U-shaped relation seen in boys.59 Similarly, our stratified analyses showed a significant U-shaped relationship of WC with birthweight only in the black boys after adjusting for BMI (p=0.035), but not in girls (Table S5). This founding might indicate that, African American boys, compared with girls, are more vulnerable to prenatal growth retardation for its later adverse impact on the accumulation of visceral/abdominal adiposity. Results from a prospective analysis on 197,954 adults from 20 Nordic cohorts showed a significant inverse linear trend of SBP with birthweight in men, while a non-linear trend in women.60 Sex hormones may play a critical role in “programming” the gender-specific response to abnormal fetal growth45 and subsequent cardiometabolic risk, whereas other mechanisms are also likely to be involved.
In the current study, we used a nationally representative sample to evaluate the association between birthweight and multiple cardiometabolic disorders in childhood. To our knowledge, this study including eight circles of NHANES since 1999 had thus far the largest sample size of children and adolescent to study the effects of birthweight on cardiometabolic risk patterns, Further, because NHANES had kept a high response rate, strict quality control, and data cross-validation, potential bias due to population selection and measurement errors were minimized. Even though, several limitations merit discussion. First, information about birth weight was collected through interview of participants or their parents or guardians, not from the birth records. Second, small sample size in some subgroups resulted in wide confidence intervals and missings coefficients due to little cases. Third, although we had carefully controlled for covariates in our analyses, information on some potential confounders was unavailable (such as gestation period, breastfeeding duration, gestational BMI and weight gain, childhood puberty, dietary intake, and physical activity), making us unable to control for their influence on the associations. In addition, childhood high blood pressure and dyslipidemia were measured in certain age range of study samples, which might limited the generaliztion of what we found.
In summary, birthweight was significantly associated with overall cardiometabolic risk patterns including obesity, high SBP, high HbA1c, and low HDL-C in childhood and adolescence, and such associations showed race- and gender-specific patterns. Our findings reemphasize the point that adulthood cardiometabolic risk associated with prenatal growth retardation may have its origin in childhood and highlight the importance of life-course prevention on cardiometabolic disorders.
Supplementary Material
Acknowledgments
Funding Source
The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States–Israel Binational Science Foundation Grant2011036. Dr. Qi was a recipient of the American Heart Association Scientist Development Award (0730094N).
Dianjianyi Sun: Dr. Sun conceptualized and designed the study, contributed to data cleanning and the statistical analysis, drafted the initial manuscript, and approved the final manuscript as submitted.
Tiange Wang, Yoriko Heianza, Tao Huang, Jun Lv: Dr. Wang, Dr. Heianza, Dr. Huang, and Dr. Lv contributed to data cleanning and the statistical analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Xiaoyun Shang: Dr. Shang contributed to the 2nd round revision and approved the final manuscript as submitted.
Shengxu Li, Emily Harville, Wei Chen, and Vivian Fonseca: Dr. Li, Dr. Harville, Dr. Chen, and Dr. Fonseca coordinated and supervised the project, critically reviewed the manuscript, and approved the final manuscript as submitted.
Lu Qi: Dr. Qi conceptualized and designed the study, contributed to the statistical analysis, critically reviewed the manuscript, and approved the final manuscript as submitted.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Abbreviations list
- BMI
body mass index
- DBP
diastolic blood pressure
- DOHaD
the developmental origins of health and disease
- FBG
fasting blood glucose
- HbA1c
glycated hemoglobin A1c
- HDL-C
high-density lipoprotein cholesterol
- LBW
low birthweight
- LDL-C
fasting blood low-density lipoprotein cholesterol
- SBP
systolic blood pressure
- TC
total cholesterol
- TG
fasting blood triglyceride
- WC
waist circumference
Footnotes
Financial Disclosure
All the authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest
All the authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. American journal of physiology. Endocrinology and metabolism. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 2.Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77:726–730. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- 3.Inadera H. Developmental origins of obesity and type 2 diabetes: molecular aspects and role of chemicals. Environmental health and preventive medicine. 2013;18:185–97. doi: 10.1007/s12199-013-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluckman PD. Living with the Past: Evolution, Development, and Patterns of Disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 5.Newsome CA, Shiell AW, Fall CHD, Phillips DIW, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism?--A systematic review. Diabetic medicine : a journal of the British Diabetic Association. 2003;20:339–48. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 6.Benyshek DC, Martin JF, Johnston CS. A reconsideration of the origins of the type 2 diabetes epidemic among Native Americans and the implications for intervention policy. Medical anthropology. 2001;20:25–64. doi: 10.1080/01459740.2001.9966186. [DOI] [PubMed] [Google Scholar]
- 7.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. American journal of epidemiology. 2007;165:849–57. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 8.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ (Clinical research ed) 1994;308:942–5. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–97. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 10.Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. The New England journal of medicine. 2011;364:1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International journal of obesity (2005) 2011;35:891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 12.Baker JL, Olsen LW, S⊘rensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. The New England journal of medicine. 2007;357:2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden CL, Flegal KM, Carroll MD, Johnson CL, FX P-S, WH D, et al. Prevalence and Trends in Overweight Among US Children and Adolescents, 1999–2000. JAMA. 2002;288:1728. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 14.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet (London, England) 2002;360:659–65. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 15.Huxley R, Owen CG, Whincup PH, Cook DG, Colman S, Collins R. Birth weight and subsequent cholesterol levels: exploration of the ‘fetal origins’ hypothesis. JAMA. 2004;292:2755–64. doi: 10.1001/jama.292.22.2755. [DOI] [PubMed] [Google Scholar]
- 16.Watson P, Wall C. Essentials of human nutrition. Vol. 41. OXFORD UNIVERSITY PRESS; 2003. p. 99. [Google Scholar]
- 17.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics. Series 11, Data from the national health survey. 2002:1–190. at < http://www.ncbi.nlm.nih.gov/pubmed/12043359>. [PubMed]
- 18.Zimmet P, Alberti KGM, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatric diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 19.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 20.Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes care. 2016;39(Suppl 1):S4–5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 21.Kavey REW, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107:1562–1566. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]
- 22.Alberti SG, Zimmet P. The IDF Consensus definition of the Metablic Syndrome in Children and Adolescents. International Diabetes Federation. 2007;24 2-930229-49-7. [Google Scholar]
- 23.Daniels Tephen, Benuc Irwin, Christakis Da, Dennison Ba. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. 2012;216 doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels SR, Greer FR Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 25.Levy PS, Lemeshow S. Sampling of Populations: Methods and Applications. John Wiley & Sons; 2013. at < https://books.google.com/books?id=XU9ZmLe5k1IC&pgis=1>. [Google Scholar]
- 26.Yeo D, Mantel HJ, Liu T-P. Bootstrap variance estimation for the National Population Health Survey. JSM 1999 Proceedings of the Survey Research Methods Section. 1999:778–785. at < http://www.amstat.org/sections/srms/proceedings/papers/1999_136.pdf>.
- 27.Shao J, Tu D. The Jackknife and Bootstrap. Springer Science & Business Media; 2012. at < https://books.google.com/books?id=VO3SBwAAQBAJ&pgis=1>. [Google Scholar]
- 28.Schellong K, Schulz S, Harder T, Plagemann A, King D, Mokdad A, et al. Birth Weight and Long-Term Overweight Risk: Systematic Review and a Meta-Analysis Including 643,902 Persons from 66 Studies and 26 Countries Globally. PLoS ONE. 2012;7:e47776. doi: 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. Journal of hypertension. 2000;18:815–31. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 30.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. Journal of hypertension. 1996;14:935–41. [PubMed] [Google Scholar]
- 31.Lauren L, Järvelin M-R, Elliott P, Sovio U, Spellman A, et al. Group the E-BS. Relationship between birthweight and blood lipid concentrations in later life: evidence from the existing literature. International Journal of Epidemiology. 2003;32:862–876. doi: 10.1093/ije/dyg201. [DOI] [PubMed] [Google Scholar]
- 32.Mokhashi MH, Mercante D, Simonsen N, Vargas A, Chalew SA. Low birth weight is not associated with type 2 diabetes in African American children in New Orleans. The Journal of the Louisiana State Medical Society : official organ of the Louisiana State Medical Society. 163:44–7. [PubMed] [Google Scholar]
- 33.Sugihara S, Sasaki N, Amemiya S, Kohno H, Tanaka T, Matsuura N. Analysis of weight at birth and at diagnosis of childhood-onset type 2 diabetes mellitus in Japan. Pediatric diabetes. 2008;9:285–90. doi: 10.1111/j.1399-5448.2008.00402.x. [DOI] [PubMed] [Google Scholar]
- 34.Mogren I, Högberg U, Stegmayr B, Lindahl B, Stenlund H. Fetal exposure, heredity and risk indicators for cardiovascular disease in a Swedish welfare cohort. International journal of epidemiology. 2001;30:853–62. doi: 10.1093/ije/30.4.853. [DOI] [PubMed] [Google Scholar]
- 35.Mzayek F, Cruickshank JK, Amoah D, Srinivasan S, Chen W, Berenson GS. Birth weight was longitudinally associated with cardiometabolic risk markers in mid-adulthood. Annals of Epidemiology. 2016;26:643–647. doi: 10.1016/j.annepidem.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horikoshi M, Beaumont RN, Day FR, Warrington NM, Kooijman MN, Fernandez-Tajes J, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538:248–252. doi: 10.1038/nature19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benyshek DC. The developmental origins of obesity and related health disorders--prenatal and perinatal factors. Collegium antropologicum. 2007;31:11–17. [PubMed] [Google Scholar]
- 38.Rich E, GAC, MJS, WCW, MWG, CHH, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Annals of Internal Medicine. 1999;130:278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 39.Wang T, Huang T, Li Y, Zheng Y, Manson JE, Hu FB, et al. Low birthweight and risk of type 2 diabetes: a Mendelian randomisation study. Diabetologia. 2016 doi: 10.1007/s00125-016-4019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiological reviews. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 41.Pinney SE, Simmons RA. Metabolic programming, epigenetics, and gestational diabetes mellitus. Current diabetes reports. 2012;12:67–74. doi: 10.1007/s11892-011-0248-1. [DOI] [PubMed] [Google Scholar]
- 42.Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, et al. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. The Journal of endocrinology. 2003;177:235–41. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJP. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eriksson J, Forsen T. Unravelling the fetal origins hypothesis. The Lancet. 2002;360 doi: 10.1016/s0140-6736(02)11951-9. [DOI] [PubMed] [Google Scholar]
- 45.Nuyt AM, Alexander BT. Developmental programming and hypertension. Current opinion in nephrology and hypertension. 2009;18:144–52. doi: 10.1097/MNH.0b013e328326092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker D, Eriksson J, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 47.Morrison KM, Ramsingh L, Gunn E, Streiner D, Van Lieshout R, Boyle M, et al. Cardiometabolic Health in Adults Born Premature With Extremely Low Birth Weight. PEDIATRICS. 2016;138:e20160515–e20160515. doi: 10.1542/peds.2016-0515. [DOI] [PubMed] [Google Scholar]
- 48.Kuhle S, Maguire B, Ata N, MacInnis N, Dodds L. Birth Weight for Gestational Age, Anthropometric Measures, and Cardiovascular Disease Markers in Children. The Journal of Pediatrics. 2017;182:99–106. doi: 10.1016/j.jpeds.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 49.Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, et al. Birthweight and mortality in adulthood: A systematic review and meta-analysis. International Journal of Epidemiology. 2011;40:647–661. doi: 10.1093/ije/dyq267. [DOI] [PubMed] [Google Scholar]
- 50.Cho WK, Suh B-K. Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr. 2016;59:1–7. doi: 10.3345/kjp.2016.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J, et al. Youth Risk Behavior Surveillance: United States 2015. MMWR. Surveillance Summaries. 2016;65:1–174. doi: 10.15585/mmwr.ss6506a1. [DOI] [PubMed] [Google Scholar]
- 52.Uthaya S, Thomas EL, Hamilton G, Doré CJ, Bell J, Modi N. Altered adiposity after extremely preterm birth. Pediatric Research. 2005;57:211–215. doi: 10.1203/01.PDR.0000148284.58934.1C. [DOI] [PubMed] [Google Scholar]
- 53.Ibáñez L, Suárez L, Lopez-Bermejo A, Díaz M, Valls C, de Zegher F. Early Development of Visceral Fat Excess after Spontaneous Catch-Up Growth in Children with Low Birth Weight. The Journal of Clinical Endocrinology & Metabolism. 2008;93:925–928. doi: 10.1210/jc.2007-1618. [DOI] [PubMed] [Google Scholar]
- 54.Stansfield BK, Fain ME, Bhatia J, Gutin B, Nguyen JT, Pollock NK. Nonlinear Relationship between Birth Weight and Visceral Fat in Adolescents. The Journal of pediatrics. 2016;174:185–92. doi: 10.1016/j.jpeds.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tam CHT, Wang Y, Luan J, Lee HM, Luk AOY, Tutino GE, et al. Non-linear relationship between birthweight and cardiometabolic risk factors in Chinese adolescents and adults. Diabetic medicine : a journal of the British Diabetic Association. 2015;32:220–5. doi: 10.1111/dme.12630. [DOI] [PubMed] [Google Scholar]
- 56.Ruiz-Narváez EA, Palmer JR, Gerlovin H, Wise LA, Vimalananda VG, Rosenzweig JL, et al. Birth weight and risk of type 2 diabetes in the black women’s health study: does adult BMI play a mediating role? Diabetes care. 2014;37:2572–8. doi: 10.2337/dc14-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nightingale CM, Rudnicka AR, Owen CG, Newton SL, Bales JL, Donin AS, et al. Birthweight and risk markers for type 2 diabetes and cardiovascular disease in childhood: the Child Heart and Health Study in England (CHASE) Diabetologia. 2015;58:474–84. doi: 10.1007/s00125-014-3474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rockenbach G, Luft VC, Mueller NT, Duncan BB, Stein MC, Vigo Á, et al. Sex-specific associations of birth weight with measures of adiposity in mid-to-late adulthood: The Brazilian longitudinal study of adult health (ELSA-Brasil) International journal of obesity (2005) 2016 doi: 10.1038/ijo.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiao Y, Ma J, Wang Y, Li W, Katzmarzyk P, Chaput J-P, et al. Birth weight and childhood obesity: a 12-country study. International Journal of Obesity Supplements. 2015;5:74–79. doi: 10.1038/ijosup.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, Bengtsson C, et al. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. American journal of epidemiology. 2007;166:634–45. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.