Abstract
Invariant natural killer T (iNKT) cells develop into three subsets (NKT1, NKT2, and NKT17) expressing a distinct transcription factor profile, which regulates cytokine secretion upon activation. iNKT cell development in the thymus is modulated by signaling lymphocytic activation molecule family (SLAMF) receptors. In contrast to other SLAMF members, Ly9 (SLAMF3) is a non-redundant negative regulator of iNKT cell development. Here, we show that Ly9 influences iNKT cell lineage differentiation. Ly9-deficient mice in a BALB/c background contained a significantly expanded population of thymic NKT2 cells, while NKT1 cells were nearly absent in BALB/c.Ly9−/− thymus. Conversely, the number of peripheral NKT1 cells in BALB/c.Ly9−/− mice was comparable to that in wild-type mice, indicating that the homeostasis of the different iNKT cell subsets may have distinct requirements depending on their tissue localization. Importantly, Ly9 absence also promoted NKT2 cell differentiation in the NKT1-skewed C57BL/6 background. Furthermore, treatment of wild-type mice with an agonistic monoclonal antibody directed against Ly9 impaired IL-4 and IFN-γ production and reduced by half the number of spleen iNKT cells, with a significant decrease in the proportion of NKT2 cells. Thus, anti-Ly9 targeting could represent a novel therapeutic approach to modulate iNKT cell numbers and activation.
Graphical Abstract
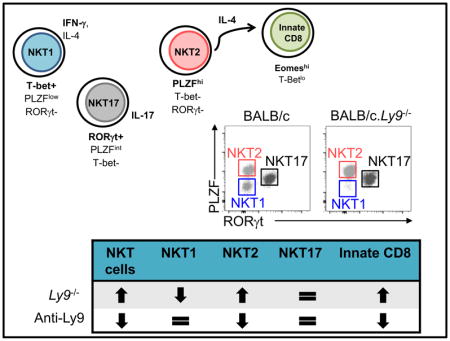
Ly9 (SLAMF3) cell-surface receptor is a negative regulator of iNKT development. Here we show that Ly9 absence favors the differentiation of NKT2 cells over NKT1 cells, leading to increased numbers of innate CD8+ cells. Furthermore, in vivo targeting of Ly9 with monoclonal antibodies diminishes splenic NKT cell numbers and activation.
Introduction
Invariant natural killer T (iNKT) cells constitute an innate-like T cell subset that recognize lipid antigens presented by the nonclassical major histocompatibility complex (MHC) I-like molecule CD1d [1]. Three major lineages of iNKT cells have been identified in naïve mice: NKT1, NKT2 and NKT17 cells, which can be distinguished by their phenotype, transcription factor profile and cytokine production [2]. Following in vitro stimulation, thymic NKT1, NKT2 and NKT17 cells produce interferon-γ (IFN-γ), interleukin 4 (IL-4) and IL-17, respectively [2]. Similarly, intravenous (i.v.) administration of the CD1d-binding glycolipid α-galactosylceramide (αGalCer), which specifically activates iNKT cells, promotes a rapid iNKT cell response and systemic production of IFN-γ and IL-4 [3,4]. NKT2 cells expressing high levels of the transcription factor promyelocytic leukemia cell finger (PLZF) also produce high amounts of IL-4 in the steady state, which drives acquisition of a ‘memory-like’ phenotype in bystander CD8+ single-positive (SP) cells [2,5]. Memory-like CD8+ T cells, characterized by the abundant expression of the transcription factor eomesodermin (Eomes) [6], can arise in absence of antigenic exposure in the thymus (innate CD8+ T cells) or in the periphery (virtual memory (VM) CD8+ T cells) [5,7].
Recent studies support the idea that the distinct iNKT cell subsets represent diverse lineages that arise from a common progenitor able to give rise to either NKT1, NKT2, or NKT17 cells depending on the interplay of T-Bet, GATA-3 and RORγt [2]. However, the molecular factors that condition this cell fate decision are only partially understood.
Homotypic interactions between signaling lymphocytic activation molecule (SLAM) family receptors are required for the positive selection of the precursors that will enter the developmental program of iNKT cells [8]. Ly9 (SLAMF3) acts as a non-redundant negative regulator for iNKT cell development and cytokine secretion [9]. In this study, we show that Ly9 negatively regulates thymic NKT2 cell differentiation independently of mice genetic background. Moreover, in vivo Ly9 targeting with an agonistic antibody significantly reduced peripheral iNKT cell numbers and activation.
Results and Discussion
Ly9 absence promotes a selective expansion of NKT2 versus NKT1 cells in the thymus
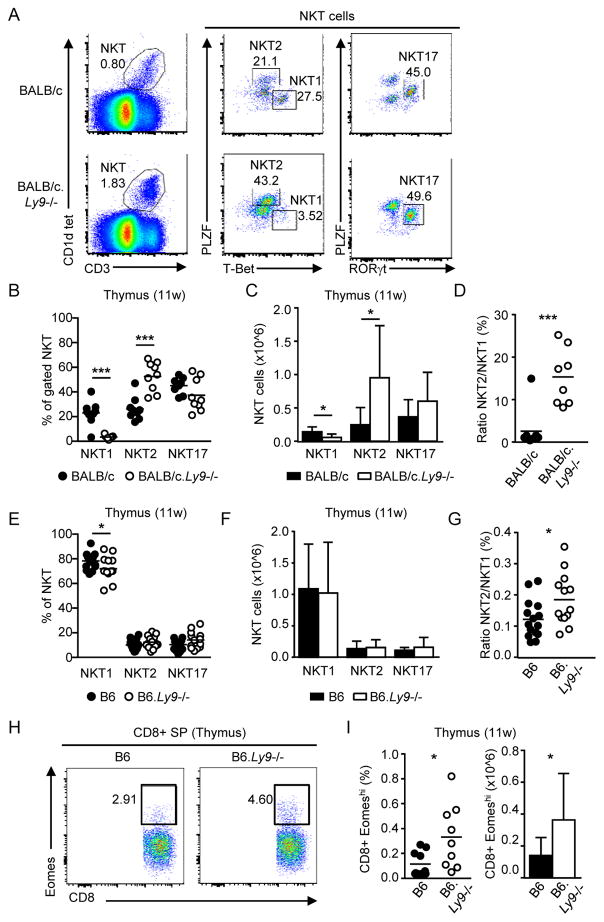
We previously reported that Ly9 deficiency leads to a marked increase in the numbers of thymic iNKT cells [9]. To better understand the factors driving this expansion, we further analyzed the proportion of NKT1, NKT2 and NKT17 subsets (Fig. 1A). BALB/c.Ly9−/− mice displayed a significant increase in both the proportion and total number of thymic NKT2 cells compared to wild-type controls, while NKT1 cells were severely reduced in Ly9 absence (Fig. 1B and 1C), leading to a dramatic increase in the NKT2/NKT1 cell ratio (Fig. 1D). Alternative iNKT cell staining with the surface markers CD122, CD4, and CD27 [2] confirmed that CD1d tetramer+ CD122+ cells (NKT1) were barely detectable in the thymus of 11-week-old BALB/c.Ly9−/− mice (Supporting Information Fig. 1A and 1B). Young (4-week-old) BALB/c.Ly9−/− mice already displayed a significantly reduced number of NKT1 cells, concomitant with an increased frequency of NKT2 cells (Supporting Information Fig. 2A–C).
Figure 1.
Ly9 absence results in a preferential expansion of NKT2 cells in the thymus. (A) Gating strategy for NKT1, NKT2, and NKT17 cells. Plots show the expression of PLZF, T-Bet and RORγt in thymic iNKT cells (CD1d tetramer+ CD3+) from 11-week-old BALB/c and BALB/c.Ly9−/− mice. Numbers in plots indicate the percentage of each subset over total iNKT cells. (B) Frequency and (C) absolute number of iNKT cell subsets and (D) NKT2/NKT1 ratio in the thymus of 11-week-old BALB/c (n=10) or BALB/c.Ly9−/− (n=9) mice. (E) Frequency and (F) absolute number of iNKT cell subsets and (G) NKT2/NKT1 ratio in the thymus of 11-week-old B6 (n=16) or B6.Ly9−/− (n=14) mice. (H) Representative staining of innate CD8+ T cells (Eomeshi) in gated CD8 SP thymocytes from B6 or B6.Ly9−/− mice. (I) Percentage (left) and absolute number (right) of innate CD8+ T cells in B6 or B6.Ly9−/− mice (n=9/group). (B, D, E, G, I) Each dot represents an individual mouse. Small horizontal lines indicate the mean. (C, F, I) Results are expressed as mean ± SD. *p <0.05; **p <0.01; ***p <0.001 (unpaired two-tailed t-test). Data are pooled from at least two independent experiments with 3–6 mice per group.
In contrast to BALB/c mice, B6 mice are skewed toward NKT1 cells at all ages [2,4,10], although the precise molecular factors that drive such interstrain differences remain unknown. Absolute numbers of thymic NKT1, NKT2 and NKT17 cells were not altered in B6.Ly9−/− mice (Fig. 1E and 1F), indicating that Ly9 ablation was not sufficient to overcome other genetic contributions to NKT1 skewing in the B6 background. Nevertheless, the NKT2/NKT1 cell ratio in B6.Ly9−/− mice was higher than in wild-type controls (Fig. 1G), and this shift was accompanied by increased numbers of thymic innate CD8+ T cells in B6.Ly9−/− mice (Fig. 1H and 1I), similar to the increase of these cells observed in the thymus of BALB/c.Ly9−/− mice [9]. The development of innate CD8+ T cells is highly dependent on NKT2-derived IL-4, and there is a positive correlation between the frequency of innate CD8+ T cells and that of NKT2 cells [2]. Altogether, our data suggests that, even in a ‘NKT1 cell skewed’ environment, Ly9 deficiency favors the development of NKT2 cells.
Importantly, the generation of NKT2 cells is tightly dependent on SAP-elicited signals. SAP is critically required for iNKT cell development in both humans and mice [11–13]. However, forced expression of the Vα14 Jα18 TCR transgene in SAP-deficient (Sh2d1a−/−) mice allows a partial rescue of the iNKT cell pool [14] with cell differentiation of NKT1 and NKT17 subsets, but not NKT2 cells [15]. Lack of SAP in iNKT cells significantly reduces the expression of the NKT2 cell-associated transcription factors GATA-3 and PLZF [15]. This phenotype is opposite to that observed in Ly9-deficient mice, which display increased numbers of NKT2 cells. Thus, our data support the idea that Ly9 signaling restricts NKT2 cell development, and this is consistent with a previously proposed model where Ly9 could decrease SAP availability in the immunological synapse [9].
The non-redundant negative role of Ly9 in iNKT cell development and NKT2 differentiation distinguishes this receptor from the rest of the SLAM family members. Thus, single-deficient mice of other SLAM family receptors display mild or no alterations in iNKT cell development. Concomitant homophilic interactions of CD150 (SLAMF1) and Ly108 (SLAMF6) are required for iNKT cell expansion and differentiation after thymic selection [8]. Generation of triple-deficient Slamf1,5,6-knockout mice has confirmed that the combined absence of these three receptors reduces iNKT cell numbers [16–18]. In line with these findings, a recent report shows that deletion of all SLAMF receptors causes a 90% reduction in thymic iNKT cell numbers [19]. In addition, the study by Chen et al. demonstrates that the positive CD150 and Ly108 signaling required for iNKT cell development is mediated by SAP, since these receptors negatively regulate iNKT cell development in SAP-deficient mice [19]. Here we describe that, in contrast to CD150 and Ly108, Ly9 exerts an inhibitory role in iNKT development and lineage differentiation also in SAP-sufficient cells.
Ly9 influences peripheral iNKT cell subsets and cytokine production
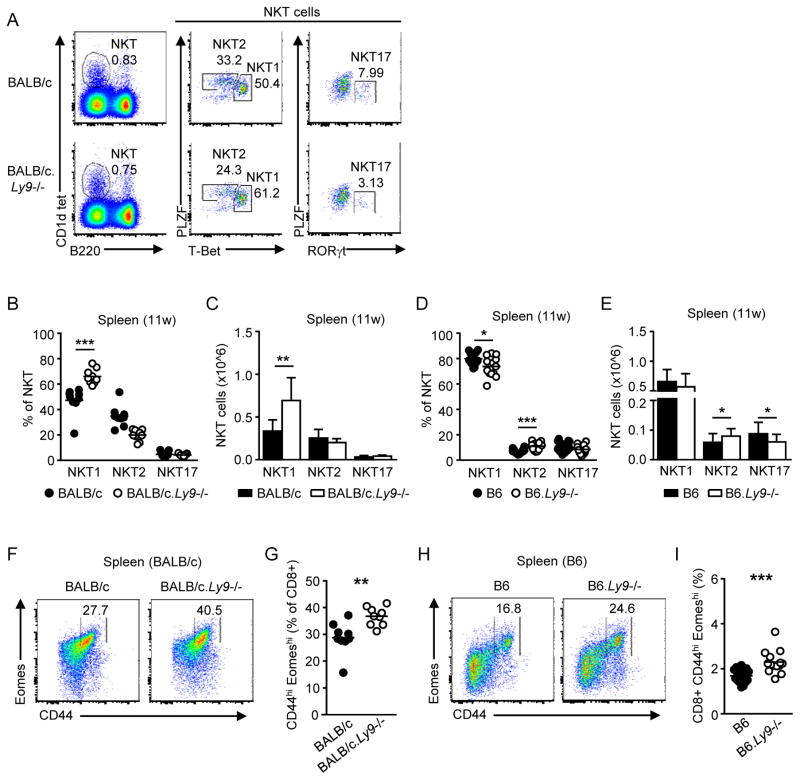
We next determined the frequency of NKT1, NKT2 and NKT17 subsets in the spleen of BALB/c.Ly9−/− mice (Fig. 2A). The proportion and number of NKT2 cells was increased in the spleen of young (4-week-old) BALB/c.Ly9−/− mice compared to wild-type mice (Supporting Information Fig. 2D). However, we found that the peripheral distribution of iNKT subsets in Ly9-deficient mice varied with age and did not mirror that of the thymus. Young Ly9-deficient mice contained a considerable proportion of NKT1 cells in the spleen (Supporting Information Fig. 2D and 2E); moreover, the splenic NKT1 cell pool in 11-week-old BALB/c.Ly9−/− mice was significantly enlarged compared to BALB/c controls (Fig. 2B and 2C). In contrast, B6.Ly9−/− mice displayed an increased proportion and number of NKT2 cells in the spleen (Fig. 2D and 2E), which was consistent with the increased NKT2/NKT1 cell ratio observed in the thymus.
Figure 2.
Ly9 regulates the size of the peripheral NKT1 cell pool. (A) Intracellular staining of PLZF, RORγt and T-Bet in spleen iNKT cells (CD1d tetramer+ B220-) from 11-week-old BALB/c or BALB/c.Ly9−/− mice. Numbers indicate the frequency of each subset among total iNKT cells in the spleen. (B) Percentage and (C) number of splenic iNKT subsets in 11-week-old BALB/c or BALB/c.Ly9−/− mice (n=10/group). (D) Percentage and (E) absolute number of splenic NKT1, NKT2, and NKT17 subsets in 11-week-old B6 (n=16) or B6.Ly9−/− (n=14) mice. (F) Representative staining of virtual memory (VM) CD8+ T cells (CD44hi Eomeshi) in gated CD3+ CD8+ splenocytes from BALB/c or BALB/c.Ly9−/− mice. (G) Percentage of VM CD8+ T cells in spleens from 11-week-old BALB/c or BALB/c.Ly9−/− mice (n=9/group). (H) Representative staining of VM CD8+ T cells (CD44hi Eomeshi) in gated CD3+ CD8+ splenocytes from B6 or B6.Ly9−/− mice. (I) Percentage of VM CD8+ T cells in spleens from 11-week-old B6 (n=16) or B6.Ly9−/− (n=14) mice. (B, D, G, I) Each dot represents an individual mouse. Small horizontal lines indicate the mean. (C, E) Results are expressed as mean ± SD. **p <0.01; ***p <0.001 (unpaired two-tailed t-test). Data are pooled from at least two independent experiments with 3–6 mice per group.
The presence of NKT1 cells in the spleen of BALB/c.Ly9−/− mice may be explained by their immediate thymic egress once they are generated in Ly9 absence, since the trafficking of NKT1, NKT2 and NKT17 lineages is differentially influenced by the thymic microenvironment [20]. Another possibility is that, after leaving the thymus, iNKT cells retain a certain degree of plasticity that allows the transdifferentiation of NKT2 into NKT1 cells. It has been elegantly demonstrated that non-IL-4-producing NKT2 cells can leave the thymus and give rise to T-Bet+ NKT1 cells in the spleen [2]. Hence, thymus-derived NKT2 cells could be the source of a splenic NKT1 cell pool, although further experiments will be required to confirm if this is the case in a Ly9 deficiency setting.
Similar to innate CD8+ T cells found in the thymus, the generation and maintenance of peripheral VM CD8+ T cells in BALB/c mice is highly dependent on IL-4 secreted by NKT2 cells in the steady state [2,21]. Interestingly, the frequency of VM CD8+ T cells was increased in both BALB/c.Ly9−/− and B6.Ly9−/− mice compared to their wild-type counterparts (Fig. 2F–I).
Ly9 antibody targeting reduces peripheral iNKT cell numbers and impairs their cytokine secretion
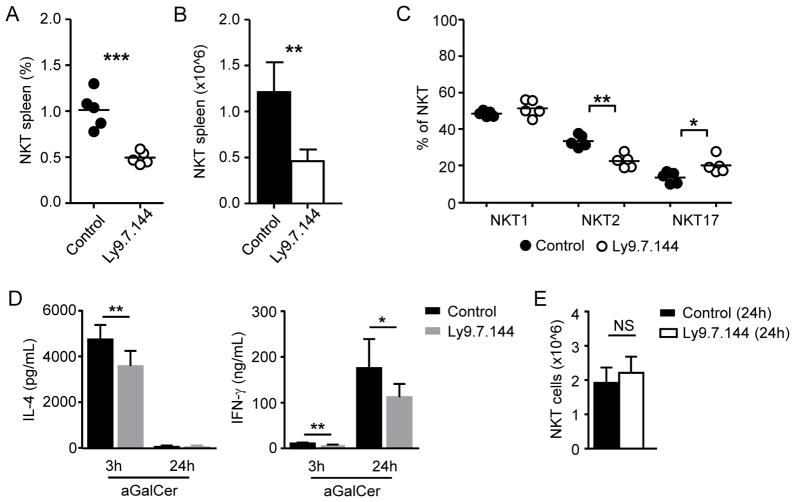
The phenotype of Ly9-deficient mice revealed that Ly9 influences iNKT cell development. To assess if Ly9 targeting could modulate iNKT cell homeostasis and activation, we treated BALB/c mice with an agonistic monoclonal antibody directed against Ly9 (Ly9.7.144) [22]. A single anti-Ly9 dose (250 μg/ mouse) reduced threefold the number of iNKT cells in the spleen (Fig. 3A and 3B). Ly9.7.144 treatment reduced the absolute numbers of both NKT1 and NKT2 cells (data not shown), although Ly9 targeting selectively reduced the proportion of NKT2 cells (Fig. 3C). The different susceptibility of peripheral NKT1 and NKT2 cells to Ly9 targeting was not due to different levels of Ly9 expression on the surface of these subsets (Table S1). Peripheral iNKT cell depletion following Ly9.7.144 treatment was not immediate but gradual, being appreciated from day 9 after antibody injection and maintained up to day 35 (data not shown).
Figure 3.
Treatment with a monoclonal antibody against Ly9 (Ly9.7.144) reduces peripheral iNKT cell numbers and impairs their cytokine production. (A) Frequency and (B) absolute number of total spleen iNKT cells (gated as CD1d tetramer+ B220-) in BALB/c mice 26 days after receiving a single dose (250 μg, i.p.) of Ly9.7.144 or an isotype-matching control. (C) Frequency of NKT1, NKT2, and NKT17 subsets in BALB/c mice 26 days after treatment with Ly9.7.144 or an isotype control antibody as detailed in A. (D) Serum levels of IL-4 (left) and IFN-γ (right) after in vivo challenge with αGalCer. BALB/c mice received 250 μg i.p. of Ly9.7.144 or an isotype control antibody. 24 hours later, mice were injected i.v. with 6 μg αGalCer. Serum samples were tested 3 and 24 hours after αGalCer administration. (E) Number of spleen iNKT cells in BALB/c mice 24 hours after treatment with Ly9.7.144 or control antibody. (A, C) Each dot represents an individual mouse. Small horizontal lines indicate the mean. (B, D, E) Results are expressed as mean ± SD. NS: non-significant (p > 0.05); *p < 0.05; **p < 0.01; ***p < 0.001 (unpaired two-tailed t-test). Data are representative of at least three independent experiments with 4 to 6 mice/group.
We next sought to determine the impact of anti-Ly9 administration in iNKT cell function. Mice were treated with a single dose of Ly9.7.144 or isotype control and 24 hours later were challenged with αGalCer i.v. Treatment with anti-Ly9 significantly reduced the production of both IL-4 and IFN-γ after αGalCer challenge (Fig. 3D). Remarkably, this effect was not due to a reduced iNKT cell pool after treatment (Fig. 3E), which is only observed at longer time points (≥9 days) after Ly9.7.144 administration (Fig. 3A). In conclusion, our data suggest that signaling through Ly9 receptor limits the number and activation of peripheral iNKT cells.
Concluding remarks
Here, we describe a non-redundant negative role of Ly9 in iNKT cell development and NKT2 differentiation, which distinguishes this receptor from the rest of the SLAM family members. Our observation that Ly9 critically regulates NKT2 cell homeostasis is further reinforced by the fact that Ly9 absence leads to increased numbers of innate-like CD8+ T cells in both the thymus and the periphery, regardless of mice genetic background. Moreover, in vivo targeting of Ly9 with a monoclonal antibody dramatically reduces the number of iNKT cells in the periphery. This report also describes how targeting of a single SLAM family receptor alters iNKT cell subsets, paving the way for the use of anti-Ly9 monoclonal antibodies as novel therapeutic tools able to modulate iNKT cell numbers and function.
Materials and methods
Mice
Female 4- to 12-week-old BALB/c and C57BL/6 (B6) mice were obtained from Charles River Laboratories (Saint-Aubin-lès-Elbeuf, France). BALB/c.Ly9−/− and B6.Ly9−/− mice have been previously described [9]. Mice were maintained under specific pathogen-free (SPF) conditions. All mice experiments were performed according to the European Community Directive 2010/63/EU and Spanish legislation (Real Decreto 53/2013, BOE BOE-A-2013-1337) regulating the protection and usage of laboratory animals. Experimental procedures were approved by the Ethics Committee for Animal Experiments (CEEA) of the University of Barcelona.
Cell isolation
Cell suspensions of lymphocytes from thymus and spleen were treated with RBC lysis buffer (0.15M NH4Cl, 0.01M Tris HCL), washed, and incubated in 20% heat-inactivated rabbit serum before being stained with fluorophore-labeled antibodies. Cell counts were determined by using PerfectCountTM microspheres (Cytognos).
Flow cytometry and antibodies
The anti-mouse monoclonal antibodies Eomes (Dan11mag), PLZF (Mags.21F7), CD27 (LG.7F9), CD122 (TM-b1) and T-Bet (eBio4B10) were purchased from eBioscience; RORγt (Q31-378), CD8a (53-6.7), and CD44 (IM7) were from BD Pharmingen; B220 (RA3-6B2), Ly9 (Ly9.ab3) and CD4 (GK1.5) were purchased from BioLegend; CD3 (145-2C11) was from Tonbo Bioscience, and PBS57-loaded mCD1d tetramer was kindly provided by the NIH Tetramer Core Facility. Data were acquired with either FACSCanto II or LSRII Fortessa flow cytometers (BD Biosciences).
In vivo cytokine response to αGalCer
For in vivo stimulation of iNKT cells, mice were injected intravenously with 6μg α-galactosylceramide (KRN7000, Funakoshi). Blood samples were collected 3 and 24h later, and centrifuged (10 min, 4°C, 2000xg) to obtain serum.
ELISA of cytokines in serum
Mouse IL-4 and IFN-γ ELISA MAX kit (BioLegend) were used for quantification of serum cytokines following manufacturer’s instructions.
Treatment with Ly9.7.144
BALB/c mice were injected intraperitoneally with 250μg anti-Ly9 monoclonal antibody (Ly9.7.144) [9], or 250μg of isotype control antibody (IgG1), diluted in sterile PBS.
Statistical analysis
Differences between group means were assessed by unpaired two-tailed Student t tests. Two-tailed p values <0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism software version 6.01.
Supplementary Material
Acknowledgments
The authors thank the NIH tetramer Core Facility for provision of PBS-57-loaded mCD1d tetramers. We thank A. Lázaro and M. Balada for technical assistance. This work was supported by Ministerio de Economia y Competitividad Grant SAF2015-69829-R (to P.E.), NIH Grant PO1 AI 065687 (to C.T. and P.E.), and Ministerio de Educación, Cultura y Deporte Grant AP2010-1754 (to M.C.).
Abbreviations used in this study
- αGalCer
α-galactosylceramide
- DP
Double positive
- IFN-γ
Interferon gamma
- IL-17
Interleukin 17
- IL-4
Interleukin 4
- i.v
Intravenous
- iNKT
Invariant Natural killer T cell
- PLZF
Promyelocytic leukemia zinc finger
- SAP
SLAM-associated protein
- SLAM
Signaling lymphocytic activation molecule
- VM
virtual memory
Footnotes
Conflict of interest Disclosure
The authors declare no competing financial interests.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellicci DG, Uldrich AP, Kyparissoudis K, Crowe NY, Brooks AG, Hammond KJ, Sidobre S, et al. Intrathymic NKT cell development is blocked by the presence of alpha-galactosylceramide. Eur J Immunol. 2003;33:1816–1823. doi: 10.1002/eji.200323894. [DOI] [PubMed] [Google Scholar]
- 4.Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, Hogquist KA. Tissue-Specific Distribution of iNKT Cells Impacts Their Cytokine Response. Immunity. 2015;43:566–578. doi: 10.1016/j.immuni.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurzweil V, LaRoche A, Oliver PM. Increased peripheral IL-4 leads to an expanded virtual memory CD8+ population. J Immunol. 2014;192:5643–5651. doi: 10.4049/jimmunol.1301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sintes J, Cuenca M, Romero X, Bastos R, Terhorst C, Angulo A, Engel P. Cutting edge: Ly9 (CD229), a SLAM family receptor, negatively regulates the development of thymic innate memory-like CD8+ T and invariant NKT cells. J Immunol. 2013;190:21–26. doi: 10.4049/jimmunol.1202435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 11.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquier B, Yin L, Fondanèche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 14.Cen O, Ueda A, Guzman L, Jain J, Bassiri H, Nichols KE, Stein PL. The adaptor molecule signaling lymphocytic activation molecule-associated protein (SAP) regulates IFN-gamma and IL-4 production in V alpha 14 transgenic NKT cells via effects on GATA-3 and T-bet expression. J Immunol. 2009;182:1370–1378. doi: 10.4049/jimmunol.182.3.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel ML, Lenoir C, Massot B, Diem S, Pasquier B, Sawa S, Rignault-Bricard R, et al. SLAM-associated protein favors the development of iNKT2 over iNKT17 cells. Eur J Immunol. 2016;46:2162–2174. doi: 10.1002/eji.201646313. [DOI] [PubMed] [Google Scholar]
- 16.De Calisto J, Wang N, Wang G, Yigit B, Engel P, Terhorst C. SAP-Dependent and -Independent Regulation of Innate T Cell Development Involving SLAMF Receptors. Front Immunol. 2014;5:186. doi: 10.3389/fimmu.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang B, Gomez-Rodriguez J, Preite S, Garrett LJ, Harper UL, Schwartzberg PL. CRISPR-Mediated Triple Knockout of SLAMF1, SLAMF5 and SLAMF6 Supports Positive Signaling Roles in NKT Cell Development. PLoS One. 2016;11:e0156072. doi: 10.1371/journal.pone.0156072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu JK, Crampton JC, Locci M, Crotty S. CRISPR-Mediated Slamf1Δ/Δ Slamf5Δ/Δ Slamf6Δ/Δ Triple Gene Disruption Reveals NKT Cell Defects but Not T Follicular Helper Cell Defects. PLoS One. 2016;11:e0156074. doi: 10.1371/journal.pone.0156074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Cai C, Li Z, Liu G, Wang Y, Blonska M, Li D, et al. Dissection of SAP-dependent and SAP-independent SLAM family signaling in NKT cell development and humoral immunity. J Exp Med. 2017;214:475–489. doi: 10.1084/jem.20161312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drennan MB, Govindarajan S, De Wilde K, Schlenner SM, Ware C, Nedospasov S, Rodewald HR, et al. The thymic microenvironment differentially regulates development and trafficking of invariant NKT cell sublineages. J Immunol. 2014;193:5960–5972. doi: 10.4049/jimmunol.1401601. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi P, Morris SC, Perkins C, Sholl A, Finkelman FD, Hildeman DA. IL-4 and IL-15 promotion of virtual memory CD8(+) T cells is determined by genetic background. Eur J Immunol. 2016;46:2333–2339. doi: 10.1002/eji.201646404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuenca M, Romero X, Sintes J, Terhorst C, Engel P. Targeting of Ly9 (CD229) Disrupts Marginal Zone and B1 B Cell Homeostasis and Antibody Responses. J Immunol. 2016;196:726–737. doi: 10.4049/jimmunol.1501266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.