Abstract
Debate remains as to the relative HIV transmission contributions from individuals who are recently HIV infected and individuals who have long-term infections. In this study, we examine the relationship between new HIV seroconversions occurring among young Black men who have sex with men (YBMSM) and network proximity to recently or long-term HIV infected individuals.
A cohort of YBMSM (N = 618) was generated through respondent driven sampling across three waves. A recent HIV infection was defined as either: 1) a confirmed seroconversion ≤9 months prior to interview date or 2) a laboratory confirmed acute infection; long-term HIV infected individuals were defined as a diagnosis date ≥9 months prior to interview date. RDS-weighted logistic regression was utilized to examine network proximity of HIV transmission events to HIV infected individuals in the network.
Within the cohort, 343 (55.5%) participants were identified as HIV seronegative at baseline. Of these, 33 (9.6%) seroconverted during the study period. The odds of seroconversion increased significantly with each additional recent HIV infected individual in one’s network (AOR = 12.96; 95% CI: 5.69–29.50), but were not significantly altered by the number of long-term infected individuals in one’s network. Additionally, for each member of one’s network who used PrEP, the odds of seroconversion decreased significantly (AOR = 0.44; 95% CI: 0.20–0.96).
Early diagnosis and treatment is a critical first step in the HIV care continuum and together with PrEP awareness and use are critical targets for disrupting the transmission of HIV through most at risk networks.
Introduction
High viremia during acute or recent HIV infection may translate into an increased probability of disease transmission 1 and has been shown to have a disproportionate effect on HIV transmission among heterosexual networks.2 There is still debate, however, as to whether new HIV infections are more likely to be a result of closer network proximity to those who are recently infected or those with long-term HIV infection, but are unsuppressed. Previous research demonstrates that one-third of HIV-infected adults have not reached sustained viral suppression3 and that there is continued, albeit limited, risk of transmission even among those who are on antiretrovirals for >6 months.4 Long-term infected individuals may also be contributing to ongoing transmission risk by increasing the number of people already infected in a stable epidemic. To appropriately identify HIV transmission risk and accurately target interventions within defined networks, it is necessary to determine how transmission events are positioned within these networks with proximity to recently and long-term HIV infected individuals.
Risk of HIV acquisition is not limited to behaviors exhibited solely by the individual but rather are influenced by both individual- and network-level behaviors.5–7 Previous research has demonstrated that individual-level intervention among people who inject drugs (PWID) can reduce HIV risk among the overall network.8 A review of networks of men who have sex with men (MSM) has found that the risk of HIV acquisition among black MSM (BMSM) increases among networks with high density and high racial homogeneity.9 In addition larger social support networks are associated with reduced engagement in risk-related sexual behaviors such as condomless sex and exchange sex.10,11 Further, past work has demonstrated that network-level behaviors within YBMSM networks are associated with individual-level behaviors6 and that network-level HIV prevention approaches are feasible when attempting to engage BMSM, who are at increased risk of HIV acquisition.12
In this analysis, we focus on Black MSM, a group at disproportionate risk for HIV infection. The majority (2 in 3) of new HIV diagnoses occur among MSM13 and some have estimated an overall lifetime risk of HIV infection of 1 in 2.14 Because network phenomena are more likely to concentrate HIV and other STIs within racially homogeneous groups15, examination of transmission processes within large cohorts of Black MSM is critical to limiting ongoing infection in this community. We use data from the uConnect Cohort (2013–2016) to explore the relative extent to which new HIV infections among young Black MSM are associated with proximity to recently infected persons or those with long-term infections.
Methods
Setting, sampling and visit procedures have been described previously.16 In brief, uConnect is a longitudinal population-based cohort study designed to determine factors associated with HIV risk and transmission within a sample of YBMSM primarily residing on the South Side of Chicago including adjacent southern suburbs, one of the largest contiguous Black community areas in the United States.17 Participants were enrolled at wave one and followed-up at waves two and three, no new participants were enrolled after wave one.
Eligibility Criteria
Study respondents were eligible to participate if they 1) self-identified as African American or Black, 2) were assigned male at birth, 3) were between 16 and 29 years of age (inclusive), 4) reported oral or anal sex with a male within the past 24 months, 5) spent the majority of their time on the Southside of Chicago, and 6) were willing and able to provide informed consent at the time of the study visit.
RDS Sampling
Our goal was to purposively recruit a diverse group of YBMSM to serve as seeds who would be interested and willing to participate in the study and who would be able to recruit others like themselves. While ten seeds were recruited via connections to the House/Ball community, the majority of the seeds were recruited via avenues that were less specific (e.g., via web sites, Facebook postings, community events, college campuses etc.) There was a total of 62 seeds with no additional seeds having been recruited past baseline. Each participant was invited and given instructions about recruiting other eligible MSM they knew using six coupons with unique ID numbers. The distribution of successful referrals were 0 (6.4%), 1 (44.3%), 2 (24.4%), 3 (15.1%), 4 (5.9%), 5 (2.5%) and 6 (1.4%). This was repeated for each new participant whether they were a seed or a sprout. Each respondent, seed and recruit, were offered $60 for their participation in the interview and were told they would receive an additional $20 for each recruit who participated.
Interview
Between August 2013 and January of 2016, YBMSM were recruited in Chicago using respondent-driven sampling (RDS) across three waves, each separated by nine months. All interviews were conducted using Computer Aided Personal Interviewing (CAPI) with some portions of the interview self-administered. The interview itself involved different types of questions and activities: background and socio-demographic questions, self-administered scales of substance use, HIV care continuum measures, and collection of dried blood spots for HIV related testing.
As part of data collection at each wave, participants were asked detailed information regarding sexual partners and confidants, including names, if known. Entity resolution18 was conducted across all participants and waves for network members reported in order to identify unobserved ties which may exist. The algorithm used to complete entity resolution was verified by two separate analysts and any new matches were confirmed manually; this process has been described in detail elsewhere 19, and is described in Supplementary Materials 1.0.
Measures
Our main outcome indicated whether an HIV seronegative individual at baseline seroconverted during the study period and was treated as a binary variable. HIV infection status (including acute infection) was determined by 4th generation HIV immunoassay (Abbott ARCHITECT HIV Ag/Ab Combo assay), HIV-1/-2 Ab differentiation (Bio-Rad Multispot HIV-1/-2 Rapid Test) and HIV RNA testing (Abbott ReaLTime HIV-1 assay) applied to samples eluted from dry blood spots (DBS)20. Chicago Department of Public Health (CDPH) HIV surveillance data were utilized to determine date of HIV diagnosis. Acute infections were defined as individuals who had 4th gen antigen positivity in the absence of antibody confirmed via HIV RNA. These individuals and those found to have HIV antibody or HIV RNA in the absence of antibody at prior waves of the study were categorized as seroconverted participants. Separately, individuals identified as HIV-positive at baseline were stratified to either recently infected or long-term infected. A recent HIV infection was defined as either: 1) a confirmed seroconversion ≤9 months prior to interview date; or 2) a laboratory confirmed acute infection. Long-term HIV infected individuals at baseline were defined as those who were HIV-positive with a diagnosis date ≥9 months prior to interview date. Analyses included only those individuals who had completed all three waves of the study.
Separate network typologies were examined in order to remove the effects of individual network size from any observed association between infection type and seroconversion. These included each individual’s total network size defined as the summation of non-repetitive ties to non-sexual confidant network and sexual network parnters (referred to herein as “total network”). Participants were able to self-report up to six sexual partners in the past six months and five confidants. Only named partners who were also study participants were included in this analysis as these were the only individuals on who we had laboratory confirmed HIV status. The confidant and sexual networks are not mutually exclusive; however, duplicate ties were removed when analyzing the total network. The network typology included entity resolution18 across waves and were operationalized as a continuous variable, network degree. Network degree is defined as the number of connections each individual has to all other individuals in the network. Similar to total network degree, we also separately assessed, and included, the number of ties to HIV-negative individuals in one’s network.
To examine whether seroconversions were more likely to be associated with the number of recent or long-term HIV-infected individuals in one’s network, we determined the number of recent and long-term infected individuals in each individual’s total network. Recent network degree was defined as the number of ties to those with a recent HIV infection. Likewise, long-term network degree was defined as the number of ties to those with a long-term HIV infection.
Demographic and Risk Characteristics
Demographic and risk characteristics are operationalized as in previous work.21,22 Employment was categorized as not employed, part-time employment (<30 hours/week), or full-time employment (>30 hours/week). Health coverage was defined as having any health insurance public or private plan. Student status was defined as being an active high school or post-secondary school student. Relationship status was defined as currently in a main or casual relationship or single. Sexual identity was categorized as gay, bisexual, or other. Criminal justice involvement was defined as any arrest, detention, or parole in the respondent’s lifetime. Housing stability was defined as being homeless at any point during the past 12 months. Risk behaviors included condomless sex and group sex and were each defined as at least one occurrence in the past 12 months. Due to the high usage of marijuana in this population it was treated as a separate variable with use of marijuana categorized as never, intermittent (up to and including multiple times per week), and heavy (at least once per day). All other drugs were combined into a single, binary variable (including: ecstasy, E or molly (MDMA); poppers (volatile nitrates); crack (cocaine); acid, LSD, mushrooms, G or GHB, K or special K, PCP (psychedelics); oxycodone, Vicodin, T3, etc (prescription pain killers)). Other drug use was defined as any use in the past 12 months. Alcohol use was categorized as never, one to two drinks per year, one to two drinks per month, one per week, and greater than one per week. Number of PrEP users in each participant’s network was utilized as a continuous variable (“Have you ever used PrEP?”). Having a profile on the social networking site Grindr© or Jack’d© was utilized as a binary variable.
Statistical Analyses
Unadjusted and adjusted RDS-weighted logistic regression models were utilized to examine characteristics associated with odds of serconversion over the study period. Individuals who were HIV-positive did not contribute to the regression analysis. All covariates identified as statistically significant at the p ≤ .05 level, using the log-rank test, or known confounders were included in the multivariable regression model.
All analyses were performed in Stata v14.0.23
Results
Sample characteristics
The final analytic sample included 343 respondents identified as HIV seronegative at baseline. These respondents were generated through RDS chains of up to 13 waves in length and with a median of 2 recruits per participant. Sample characteristics for respondents are presented in Table 1 and are stratified by seroconversion during the study period. Our sample contained 310 (90.4%) individuals who remained HIV-negative throughout the study and 33 (9.6%) who seroconverted during the study period. Among those who were HIV-positive at baseline, and who had an available date of diagnosis (n = 131), 54 (41.2%) were recent infections and 77 (58.7%) were long-term infections. At baseline, the majority of total respondents were employed at least part-time (184, 53.6%), had health coverage (180, 52.5%), had condomless sex at least once in the past 12 months (195, 56.9%), and used marijuana at least intermittently (241, 70.3%). We found that in the past 12 months, 60 (17.5%) participants used the following substances: MDMA (ecstasy, E, or molly) 28 (8.2%), prescription pain killers (oxycodone, Vicodin, T3, etc.) 20 (5.8%), alkly nitrates (poppers) 17 (5.0%), cocaine and/or crack 7 (2.0%), psychoactive drugs (acid, LSD, mushrooms, G or GHB, K or special K, PCP) 3 (0.9%), heroin 4 (1.2%), and methamphetamines 2 (0.6%), (data not shown).
Table 1.
Sample characteristics stratified by seroconversion among young Black MSM who were confirmed as seronegative at baseline via laboratory analyses in Chicago, uConnect (N = 343 persons)
| Characteristic | Total | Seronegative | Seroconverted1 | p-value2 | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. | % | No. | % | No. | % | ||
| Total | 343 | 100 | 310 | 90.4 | 33 | 9.6 | |
| Demographics, n(%) | |||||||
| Employment | 0.250 | ||||||
| Unemployed | 159 | 46.4 | 140 | 45.2 | 19 | 57.6 | |
| < 30 hours/week | 93 | 27.1 | 84 | 27.1 | 9 | 27.3 | |
| > 30 hours/week | 91 | 26.5 | 86 | 27.7 | 5 | 15.1 | |
| Housing Instability3 | 85 | 24.8 | 78 | 25.2 | 7 | 21.2 | 0.611 |
| Have health coverage | 180 | 52.5 | 162 | 52.3 | 18 | 54.5 | 0.384 |
| Current student | 126 | 36.7 | 110 | 35.5 | 16 | 48.5 | 0.141 |
| In a relationship | 128 | 37.3 | 116 | 37.4 | 12 | 36.4 | 0.840 |
| Risk behaviors,3 n(%) | |||||||
| Condomless sex | 195 | 56.9 | 176 | 56.7 | 19 | 57.6 | 0.930 |
| Group sex | 57 | 16.8 | 49 | 16.0 | 8 | 24.2 | 0.226 |
| Drug and alcohol use,3 n(%) | |||||||
| Marijuana use4 | 0.341 | ||||||
| Never | 102 | 29.7 | 91 | 29.4 | 11 | 33.3 | |
| Intermittent | 141 | 41.1 | 125 | 40.3 | 16 | 48.5 | |
| Heavy | 100 | 29.2 | 94 | 30.3 | 6 | 18.2 | |
| Other Substance Use3,5 | 60 | 17.5 | 52 | 16.8 | 8 | 24.2 | 0.283 |
| Alcohol use | 0.453 | ||||||
| Never | 47 | 13.7 | 41 | 13.2 | 6 | 18.2 | |
| ≤ Once per day | 217 | 63.3 | 195 | 62.9 | 22 | 66.7 | |
| At least once per day | 79 | 23.0 | 74 | 23.9 | 5 | 15.2 | |
| Network degree measures, mean (range) | |||||||
| Total network | 10.0 | 1–24 | 9.8 | 1–24 | 11.6 | 7–20 | 0.028 |
| Recent HIV | 0.6 | 0–13 | 0.4 | 0–11 | 2.2 | 0–13 | <0.001 |
| Long-term HIV | 1.3 | 0–11 | 1.2 | 0–7 | 2.0 | 0–11 | 0.001 |
| HIV-negative | 9.45 | 1–24 | 9.44 | 1–24 | 9.64 | 4–18 | 0.799 |
Defined as HIV seropositive and HIV seronegative in a prior wave.
Using chi-square analysis or T-test
In the past 12 months
Intermittent use is defined as less than and including weekly use; heavy use is defined as at least once per day.
Includes the use of ecstasy/molly/E, poppers, cocaine/crack, heroin, psychedelics, methamphetamines, prescriptions.
Among study respondents who seroconverted during the study period, the majority were unemployed (27, 81.8%), had never been incarcerated (21, 63.6%), had health coverage (18, 54.5%), had condomless sex during the past 12 months (19, 57.6%), and used marijuana at least intermittently (24, 72.7%). Compared to those who did not seroconvert (mean = 0.2; range: 0–2), those who seroconverted (mean = 1.7; range: 0–5) had a significantly higher number of recent HIV infected individuals in their network (p < 0.001). There was no significant difference between those who did and did not seroconvert with reference to the number of long-term HIV infected individuals in their network nor those with concurrent partners. Nor were there any significant differences in the total, confidant, or sexual network sizes between those who seroconverted and those who did not.
Logistic regression analysis
In the adjusted RDS-weighted logistic regression models (Table 2), we found that the rate of seroconversion increased significantly with each additional recent HIV infected individual in one’s network (Adjusted Odds Ratio [AOR] = 12.96; 95% CI: 5.69–29.50). Total network size significantly increased the odds of seroconversion (Adjusted Odds Ratio [AOR] = 1.22; 95% CI: 1.01–1.46). We also found that the rate of seroconversion increased significantly among those who had health coverage (AOR = 4.15; 95% CI: 1.11–15.53), compared to those who did not have health coverage. Heavy marijuana users, compared to those who never use marijuana, were significantly less likely to seroconvert (AOR = 0.12; 95% CI: 0.02–0.72). Additionally, use of at least one substance other than marijuana, compared to those who do not use any other substance, significantly increased the odds of seroconversion (AOR = 5.63; 95% CI: 1.44–22.02).
Table 2.
Adjusted RDS-weighted logistic regression models of selected characteristics with odds of seroconversion,1 uConnect
| Characteristic | Network Model | PrEP Model | ||
|---|---|---|---|---|
|
| ||||
| (n = 326) | (n = 304) | |||
|
| ||||
| OR | 95% CI | OR | 95% CI | |
| Recent network members | 12.96** | 5.69–29.50 | - | - |
| Long-term network members | 0.74 | 0.14–3.82 | - | - |
| Total network members | 1.22* | 1.01–1.46 | 8.91** | 4.53–17.52 |
| HIV-negative network members | - | - | 0.14** | 0.07–0.26 |
| PrEP users in network | - | - | 0.44* | 0.20–0.96 |
| Condomless sex2 | ||||
| Never | Ref | - | Ref | - |
| ≥ 1 time | - | - | 0.51 | 0.16–1.63 |
| Employment | ||||
| >30 hours | Ref | - | Ref | - |
| <30 hours | 1.37 | 0.23–8.19 | 1.87 | 0.27–13.00 |
| Unemployed | 3.25 | 0.76–16.38 | 5.27* | 1.19–23.41 |
| Have health coverage | ||||
| No | Ref | - | Ref | - |
| Yes | 4.15* | 1.11–15.53 | 2.64 | 0.85–8.16 |
| Current student | ||||
| No | Ref | - | Ref | - |
| Yes | 0.77 | 0.31–1.92 | 0.76 | 0.37–1.54 |
| In a relationship2 | ||||
| No | Ref | - | Ref | - |
| Yes | 0.79 | 0.24–2.65 | 0.76 | 0.25–2.36 |
| Marijuana use3 | ||||
| Never | Ref | - | Ref | - |
| Intermittent | 0.42 | 0.08–2.40 | 0.42 | 0.10–1.77 |
| Heavy | 0.12* | 0.02–0.72 | 0.08** | 0.02–0.41 |
| Other Substance Use4 | ||||
| None | Ref | - | Ref | - |
| ≥ One other substance | 5.63* | 1.44–22.02 | 4.96* | 1.33–18.54 |
Abbreviations: OR = odds ratio; CI = confidence interval; PrEP = pre-exposure prophylaxis;
Defined as HIV seropositive with previous HIV seronegative in a prior wave.
In the past 12 months
Intermittent use is defined as less than and including weekly use; heavy use is defined as at least once daily.
Includes the use of ecstasy/molly/E, marijuana, cocaine/crack, heroin, psychedelics, methamphetamines, prescription drugs
p <0.05;
p <0.001
We found that the rate of seroconversion decreased significantly with each additional network member who used PrEP (AOR = 0.44; 95% CI: 0.20–0.96). We also found that the rate of seroconversion decreased for each additional HIV-negative member in one’s network (AOR = 0.14; 95% CI: 0.07–0.26). We continued to find that an increase in one’s overall network size significantly increased the odds of serconversion (AOR = 8.91; 95% CI: 4.53–17.52). We found that the odds of seroconversion increased significantly among those who were unemployed (AOR = 5.27; 95% CI: 1.19–23.41) and those who used at least one other substance (AOR = 4.96; 95% CI: 1.33–18.54), compared to those employed full-time and those did not use any other substances, respectively.
Discussion
To date, little research has characterized the relative contributions of network members with recent and long-term HIV infection on seroconversion. In this study, we present new findings regarding the role of both recent and long-term HIV infected network members. First, we found that 10% of our population-based sample of YBMSM who were negative at baseline seroconverted during the 18-month study period and none of the individual level behaviors or demographics were associated with this seroconversion. Second, we found that an increase in the number of recently HIV infected individuals in a participant’s network significantly increased the rate of seroconversion. Finally, we found that for each additional member of a participant’s network who is using PrEP there was a significant decrease in the odds of seroconverting.
Network composition plays a key role in predicting HIV risk among young black men who have sex with men. While past research has shown that members of the same network typically share HIV risk behaviors,6,9 our study demonstrates that the timing of HIV infection among network members itself, either recent or long-term, may also play a role in risk of HIV acquisition. These findings are similar to past research which utilized both the Duke-UNC-Emory Acute HIV Consortium data as well as data available on long-term HIV infections from previously published studies. Study findings have demonstrated that men are hyperinfectious during acute HIV infection, and that there is a decreased odds of transmission per coital act among those with long-term HIV infection, likely due to an increased probability of antiretroviral use.2 Further, past research has shown that probability of transmission of HIV among long-term HIV infected is insufficient to sustain an epidemic.24 Currently, there is no research examining the probability of HIV transmission among recent or long-term HIV infected BMSM. Our findings suggest that, in this young population, recent infection seems to be playing a larger role in the risk of HIV transmission. We also observed that differences in network composition may alter an individual’s risk of HIV acquisition and may be key in targeting future interventions, particularly among those who have a recent HIV infection.25
Prior work has demonstrated that many MSM spend a significant amount of time using multiple mobile apps such as Grindr© and Jack’d© and that the age at which respondents first used these apps was significantly associated with age at the first instance of either insertive or receptive anal intercourse.26 The New York Community Healthcare Network reported that, among users of hook-up apps, 80.9% of users knew that HIV was transmitted through “unprotected anal sex, vaginal sex, and – less frequently – oral sex”, yet almost half of the respondents (46.6%) reported condomless anal intercourse,27 a finding also found among MSM in Los Angeles.28 While past research has demonstrated that hook-up sites significantly increase the risk of HIV acquisition, we did not find this to be a significant predictor of seroconversion in this study. However, one recent study found that young MSM express a willingness to participate in HIV prevention programs via these platforms29, particularly among non-White MSM,30 making them a potential key source for HIV prevention given the high rate of use observed in this cohort. Further research is necessary to investigate whether HIV prevention programs administered via these platforms can reduce the risk of HIV infection.
Access to health care and the use of marijuana may each play a significant role in acquisition of HIV, particularly with regards to network typology. We observed that those with health coverage were significantly more likely to seroconvert during the study period. This finding is consistent with past research suggesting that health coverage increases the rate of HIV testing (and thus the rate of diagnoses31), particularly among high-risk populations.32 Past research has suggested that marijuana use, either recreationally or in the context of sex, is an important risk factor for the transmission of both HIV and STIs among high-risk populations.33 Other research has also shown that those who use marijuana either recreationally or as a sex-drug are less likely to be aware of their HIV-positive status22 and more likely to participate in HIV risk behaviors, such as group sex and condomless sex, respectively.33,34 Our findings, however, suggest that the role of heavy marijuana use may be more complicated than previously thought. We found that heavy marijuana users were less likely to seroconvert during the study period, compared to those who never use marijuana. Considering this finding in the context of past research, it is possible that the network context in which an individual uses marijuana may play a key role risk of HIV acquisition. Further research should be conducted to assess these conflicting findings, particularly in the context of network-level behaviors.
We observed that network-level use of PrEP significantly reduced individual-level HIV acquisition risk, supporting the findings of past research. Khan et al. (2013) tested a “firewall effect” theory, suggesting that HIV infected individuals with low infectivity may prevent the spread of the virus through the network, a theory confirmed by later work.35 Our findings also support this theory and suggest that one’s risk environment plays an important role in individual risk of HIV acquisition; in this study, an increase in the network-level use of PrEP may establish a firewall, essentially shielding segments of the network from onwards transmission. Encouragingly, recent work utilizing data from the National HIV Behavioral Surveillance study has found overall knowledge regarding PrEP and PEP to be on the rise between 2011 to 2014.36 Further, recent increases in PrEP interest and knowledge have been found among MSM using a sexual networking site, however, uptake of PrEP use among those who would benefit most was not observed.37
While we found several important factors associated with HIV seroconversion, there are some important limitations to our study. First, our study utilizes baseline characteristics to predict future HIV seroconversion and thus does not allow for causal inference. We also aimed to collect as much network data as possible, however, as with many network studies we are not observing the complete network of individuals nor were we able to determine HIV serostatus of unobserved network members. We defined long-term HIV infection as those infected in the past nine months, as opposed to six months in other past research, in order to stay consistent with the same length of time between study visits; however, utilizing a definition of six months did not alter our findings. Finally, we were only able to infer potential relationships between risk of seroconversion and recent or long-term infection among network members based upon network proximity and were not able to determine whether a transmission occurred or the direction of transmission utilizing our data.
In the context of our study limitations, we have shown that an increase in the number of acute/recent HIV infected individuals in one’s network is associated with an increased rate of seroconversion. We also demonstrated that the odds of seroconversion are significantly reduced by an increase in the number of network members who use PrEP and marijuana use. Early diagnosis and treatment is a critical first step in the HIV care continuum and together with PrEP awareness and use are critical targets for disrupting the transmission of HIV through high risk networks.
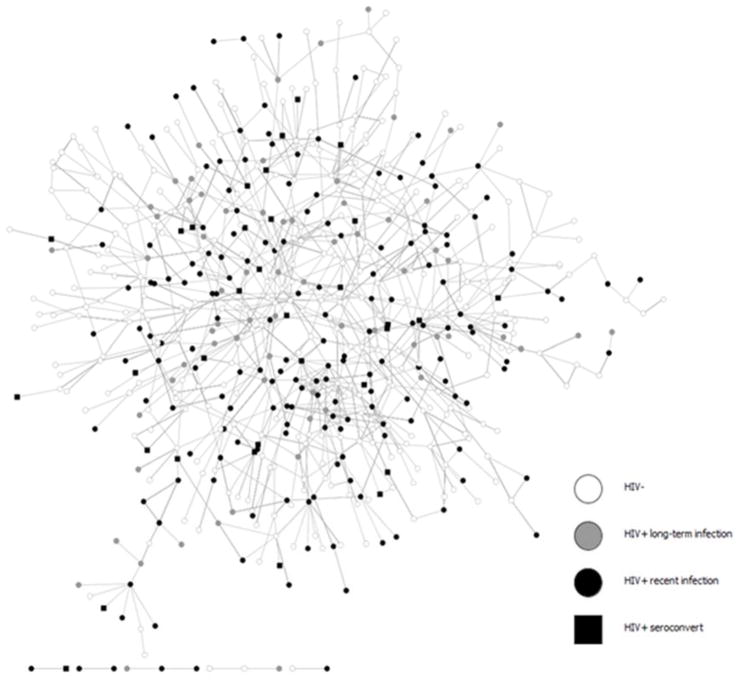
Figure 1.
Total network, including social and sexual ties, among participants who were HIV seronegative- at baseline (n = 606).
1A recent HIV infection at baseline was defined as either: 1) a new diagnosis ≤9 months prior to interview date or 2) a laboratory confirmed acute infection at baseline.
2Long-term HIV infected individuals at baseline were defined as those who were HIV-positive with a diagnosis date ≥9 months prior to interview date
Acknowledgments
Sources of Funding: This work was supported by NIH grants R21MH098768, R01DA033875 and R01DA039934. The dried blood spot assay development and validation was supported by NIH grants P30-AI- 027757 and UM1-AI-68636 and -06701.
We would like to thank all study participants for the time and effort required to recruit their network members and take part in the interview.
This work was supported by NIH grants R21MH098768, R01DA033875 and R01DA039934. The dried blood spot assay development and validation was supported by NIH grants P30-AI- 027757 and UM1-AI-68636 and -06701. We would like to thank Audrey Wong, Jose Ortega, Eleanor Espinosa, Carol Gallardo, Corey Scherrer, and Glenda Daza for laboratory technical support.
References
- 1.Brenner BG, Roger M, Routy J, et al. High Rates of Forward Transmission Events after Acute/Early HIV-1 Infection. J Infect Dis. 2007;195(7):951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 2.Pilcher CD, Tien HC, Eron JJ, Jr, et al. Brief but Efficient: Acute HIV Infection and the Sexual Transmission of HIV. J Infect Dis. 2004;189(10):1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 3.Tie Y, Frazier E, Skarbinski J. IDWeek. San Diego, CA: IDSA; 2015. [Accessed October 11, 2016]. Effectiveness of Antiretroviral Therapy in HIV-infected Adults Receiving Medical Care in the United States. https://idsa.confex.com/idsa/2015/webprogram/Paper50208.html. [Google Scholar]
- 4.Supervie V, Viard J-P, Costagliioloa D, Breban R. Heterosexual Risk of HIV Transmission per Sexual Act Under Combined Antiretroviral Therapy: Systematic Review and Bayesian Modeling. Clin Infect Dis2. 2014;59(1):115–122. doi: 10.1093/cid/ciu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SR, Neaigus A, Jose B, et al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997;87(8):1289–1296. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider JA, Cornwell B, Ostrow D, et al. Network mixing and network influences most linked to HIV infection and risk behavior in the HIV epidemic among black men who have sex with men. Am J Public Heal. 2013;103(1):e28–36. doi: 10.2105/AJPH.2012.301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related Mechanisms May Help Explain Long-term HIV-1 Seroprevalence Levels That Remain High but Do Not Approach Population-Group Saturation. Am J Epidemiol. 2000;152(10):913–922. doi: 10.1093/aje/152.10.913. [DOI] [PubMed] [Google Scholar]
- 8.Latkin CA, Donnell D, Metzger D, et al. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Soc Sci Med. 2009;68(4):740–748. doi: 10.1016/j.socscimed.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amirkhanian YA. Social Networks, Sexual Networks and HIV Risk in Men Who Have Sex with Men. Curr HIV/AIDS Rep. 2014;11(1):81–92. doi: 10.1007/s11904-013-0194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapadia F, Siconolfi DE, Barton S, Olivieri B, Lombardo L, Halkitis PN. Social support network characteristics and sexual risk taking among a racially/ethnically diverse sample of young, urban men who have sex with men. AIDS Behav. 2013;17(5):1819–1828. doi: 10.1007/s10461-013-0468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauby JL, Marks G, Bingham T, et al. Having supportive social relationships is associated with reduced risk of unrecognized HIV infection among black and Latino men who have sex with men. AIDS Behav. 2012;16(3):508–515. doi: 10.1007/s10461-011-0002-3. [DOI] [PubMed] [Google Scholar]
- 12.Kelly JA, Amirkhanian YA, Seal DW, et al. Levels and Predictors of Sexual HIV Risk in Social Networks of Men who Have Sex with Men in the Midwest. AIDS Educ Prev. 2010;22(6):483–495. doi: 10.1521/aeap.2010.22.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas. [Accessed March 29, 2016];HIV Surveillance Reports. http://www.cdc.gov/hiv/library/reports/surveillance/2013/surveillance_Report_vol_25.html. Published 2013.
- 14.Centers for Disease Control and Prevention. Lifetime risk of HIV diagnosis in the United States. 2016 Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2016; [Accessed March 7, 2016]. http://www.cdc.gov/nchhstp/newsroom/2016/croi-2016.html#Graphics2. [Google Scholar]
- 15.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26(5):250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Khanna AS, Michaels S, Skaathun B, et al. Preexposure Prophylaxis Awareness and Use in a Population-Based Sample of Young Black Men Who Have Sex With Men. JAMA Intern Med. 2015:1–3. doi: 10.1001/jamainternmed.2015.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United States Census Bureau. 2005–2009 American Community Survey 5-Year Estimates. 2011. [Google Scholar]
- 18.Getoor L, Machanavajjhala A. Entity Resolution for Big Data. 19th ACM SIGKDD Conference on Knowledge Discovery and Data Mining; Chicago, IL. 2013. [Google Scholar]
- 19.Skaathun B, Voisin D, Lauderdale DS, Schneider JA. Environmental Factors and Network Dynamics among YBMSM in Chicago. 2016. [Google Scholar]
- 20.Chang M, Daza G, Dragavon J, Hart S, Seilie M, Murphy S. Application of a 4th generation HIV diagnostic testing algorithm using finger-prick dried blood spot cards. Annual Clinical Virology Symposium; Daytona Beach. 2014. [Google Scholar]
- 21.Khanna AS, Michaels S, Skaathun B, Morgan E, Green K, Young LE. Low preexposure prophylaxis (PrEP) awareness & uptake in a population-based sample of young Black MSM in Chicago. International AIDS Society; Vancouver, Canada. 2015. [Google Scholar]
- 22.Morgan E, Khanna AS, Skaathun B, et al. Marijuana use among young black men who have sex with men and the HIV Care Continuum: Findings from the uConnect Cohort. Subst Use Misuse. 2016 doi: 10.1080/10826084.2016.1197265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.StataCorp. Stata Statistical Software: Release 14. 2015. [Google Scholar]
- 24.Pinkerton SD, Abramason P, Kalichman SC, Catz S, Johnson-Masotti A. Secondary HIV transmission rates in a mixed-gender sample. Int J STD AIDS. 2000;11:38–44. doi: 10.1258/0956462001914887. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SR, Downing MJ, Smyrnov P, et al. Socially-integrated transdisciplinary HIV prevention. AIDS Behav. 2014;18(10):1821–1834. doi: 10.1007/s10461-013-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goedel WC, Duncan DT. Geosocial-Networking App Usage Patterns of Gay, Bisexual, and Other Men Who Have Sex With Men: Survey Among Users of Grindr, A Mobile Dating App. JMIR public Heal Surveill. 2015;1(1):e4. doi: 10.2196/publichealth.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Community Healthcare Network. [Accessed September 14, 2016];Community Healthcare Network Releases New Report on Attitudes, Knowledge About HIV/AIDS Among Gay, Bisexual Men Who Use Social Networking Apps. 2013 http://www.chnnyc.org/wp-content/files/Final-Grindr-PR-FINAL-1-232.pdf.
- 28.Landovitz RJ, Tseng C-H, Weissman M, et al. Epidemiology, Sexual Risk Behavior, and HIV Prevention Practices of Men who Have Sex with Men Using GRINDR in Los Angeles, California. J Urban Heal. 2013;90(4):729–739. doi: 10.1007/s11524-012-9766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holloway IW, Rice E, Gibbs J, Winetrobe H, Dunlap S, Rhoades H. Acceptability of smartphone application-based HIV prevention among young men who have sex with men. AIDS Behav. 2014;18(2):285–296. doi: 10.1007/s10461-013-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun CJ, Stowers J, Miller C, Bachmann LH, Rhodes SD. Acceptability and feasibility of using established geosocial and sexual networking mobile applications to promote HIV and STD testing among men who have sex with men. AIDS Behav. 2015;19(3):543–552. doi: 10.1007/s10461-014-0942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mannheimer S, Wang L, Wilton L, et al. Infrequent HIV Testing and Late HIV Diagnosis Are Common Among A Cohort of Black Men Who Have Sex with Men (BMSM) in Six US Cities. J Acquir Immune Defic Syndr. 2014;67(4):438–445. doi: 10.1097/QAI.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood N, Wagner Z, Wu Y. The Impact of Insurance on HIV Testing. Am J Heal Econ. 2015;1(4):515–536. [Google Scholar]
- 33.Hendershot C, Magnan RE, Bryan A. Associations of marijuana use and sex-related marijuana expectancies with HIV/STD risk behavior in high-risk adolescents. Psychol Addict Behav. 2010;24(3):404–414. doi: 10.1037/a0019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan E, Skaathun B, Michaels S, et al. Marijuana Use as a Sex-Drug is Associated with HIV Risk Among Black MSM and Their Network. AIDS Behav. 2015 doi: 10.1007/s10461-015-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan B, Dombrowski K, Saad M, McLean K, Friedman S. Network Firewall Dynamics and the Subsaturation Stabilization of HIV. Discret Dyn Nat Soc. 2013;2013:1–16. doi: 10.1155/2013/720818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan E, Skaathun B, Lancki N, et al. Trends in HIV Risk, Testing and Treatment among MSM in Chicago 2004–2014: Implications for HIV Elimination Planning. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer KH, Oldenburg C, Novak DS, Krakower D, Mimiaga MJ. Differences in PrEP Knowledge and Use in U.S. MSM Users of a Popular Sexual Networking Site Surveyed in August 2013 and January 2014. AIDS Res Hum Retroviruses. 2014;30(S1):A91–A92. doi: 10.1089/aid.2014.5168a.abstract. [DOI] [Google Scholar]